Abstract
The off-the-shelf t-Branch device (Cook Medical, Bloomington, Ind) significantly advanced the endovascular treatment of ruptured thoracoabdominal aortic aneurysms. Improved techniques for expeditious implantation of the t-Branch may improve clinical outcomes for this emergent procedure. Currently, implantation is described using axillary and femoral access. We describe the repair of a ruptured thoracoabdominal aortic aneurysm exclusively through femoral access aided by a steerable sheath and newer generation, low-profile bridging stents.
Keywords: Thoracoabdominal aneurysm, Aneurysm rupture, t-Branch
Ruptured thoracoabdominal aortic aneurysm (TAAA) represents a high-mortality vascular catastrophe. Branched endografts for elective TAAA repair in high-risk patients show satisfactory results.1, 2 Fabrication delay limits their emergent use. The four-branched t-Branch (Cook Medical, Bloomington, Ind) is designed for emergent use in patients not suitable for surgery. Side-branch locations allow compatible anatomy in up to 58% of TAAA patients.3 Current implantation techniques require upper limb access to catheterize branches.4, 5 Upper extremity access may lengthen procedures and contribute to complications and, potentially, stroke rate.5 We report a single-femoral access, ruptured TAAA repair using retrograde side-branch cannulation with the Aptus Heli-FX Guide (Medtronic, Santa Rosa, Calif). The patient's consent was obtained for this report.
Case report
A 78-year-old man presented with sudden-onset back pain. History included hypertension, chronic atrial fibrillation, and stroke. Imaging demonstrated a ruptured 7.7-cm type II TAAA with hemothorax (Fig 1). Image fusion guided procedural navigation (Discovery IGS 740; GE Healthcare, Buc, France). A cerebrospinal fluid drainage catheter was used. In the right common femoral artery, open 18F access was used. In the left common femoral artery, 5F access was percutaneous. Two Zenith Alpha (Cook) thoracic endografts were implanted; a 42- × 225-mm endograft was placed distal to the left subclavian artery, and a 38- × 217-mm endograft was placed 15 mm above the celiac trunk.
Fig 1.
Preoperative computed tomography (CT) of ruptured thoracoabdominal aortic aneurysm (TAAA). A, Three-dimensional volume rendering. B, Hemothorax. C, Posterior rupture. D, TAAA.
The t-Branch endograft was introduced and angiography performed to verify that the branches were positioned above their target vessels before deployment. A Valiant thoracic stent graft (Medtronic) was implanted in the distal abdominal aorta (28 ×100 mm). Achieving a distal seal without a bifurcated component allowed single-femoral artery, large-bore access. Sealing zones and component overlaps were dilated with a Coda balloon.
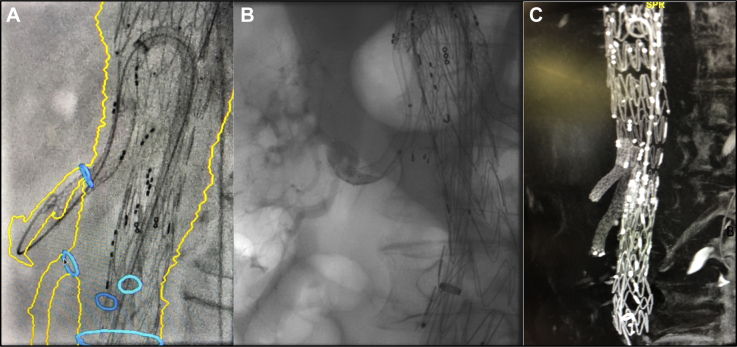
The 16F steerable sheath (22-mm deflected tip, 62-cm length) was advanced within the t-Branch. Endograft branches and target vessels were catheterized using a 100-cm 5F BER II catheter and hydrophilic guidewire, then Rosen wire. Bridging stents included BeGraft Plus (Bentley Innomed, Hechingen, Germany) for the celiac (9 × 57 mm) and left renal artery (8 × 57 mm) and Covera (Bard, Tempe, Ariz) for the superior mesenteric (9 × 80 mm) and right renal artery (7 × 80 mm). The overlap between the branches and stents was reinforced with BeGraft stents. Both the BeGraft Plus and Covera are double-layer polytetrafluoroethylene, low-profile stents with high conformability. At 10-mm diameter, these stents are compatible with 8F access. Selective side-branch and completion angiography and cone beam computed tomography (CT) confirmed aneurysm exclusion and target vessel patency (Fig 2). Procedure duration was 98 minutes. Radiation dose was 646 mGy. Contrast material dose was 120 mL.
Fig 2.
Transfemoral t-Branch procedure. A, Side-branch and target vessel cannulation and stenting aided by image fusion and steerable sheath. B, Selective side-branch angiography. C, Completion noncontrast-enhanced cone beam computed tomography (CT).
The patient remained hemodynamically stable intraoperatively and postoperatively. His cerebrospinal fluid catheter was removed on postoperative day (POD) 2. Paraplegia occurred on POD 3. Partial motor and full sensory function returned with permissive hypertension. Thoracoscopic hemothorax evacuation was performed on POD 17. CT scans performed on POD 3 and POD 15 confirmed successful TAAA exclusion without endoleak and patent target vessels (Fig 3).
Fig 3.
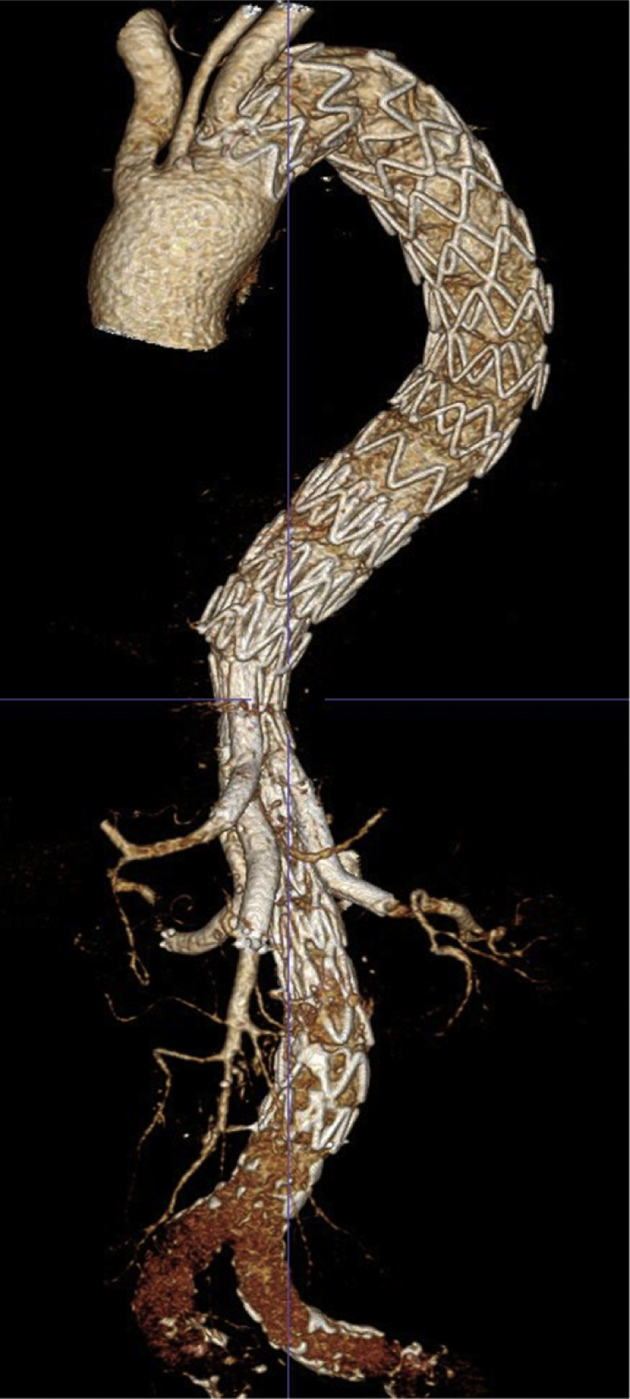
Computed tomography (CT) scan done on postoperative day 3 (POD 3) demonstrating aneurysm exclusion, patent side-branch vessels, and no endoleak.
Discussion
We report the endovascular repair of a ruptured TAAA using a t-Branch endograft by a femoral approach. The procedure was performed in 1.5 hours, and the patient was discharged with successful aneurysm exclusion and patent target visceral vessels. The use of the 16F Aptus Heli-FX steerable guide sheath was instrumental to technical success. Steerable sheaths are routinely used in cardiac interventional procedures, such as trans-septal access and endocardial ablation.6 In addition, the support afforded by steerable sheaths allows delivery and stable positioning of large, bulky structural heart devices, such as transcatheter heart valves. Although it was originally designed to deliver the Aptus Heli-FX Aortic EndoAnchor System, use of the steerable guide to avoid supra-aortic access has been reported.7 We find the ease of use and provided support to be beneficial in performing complex endovascular procedures. In this case, a steerable sheath allowed quick and accurate cannulation of side branches and target vessels. To advance bridging stents to their respective target vessels from a femoral approach, latest generation covered stents with 7F or 8F profiles are required. The combination of low-profile bridging stents with the use of a stable, steerable sheath (16F) is imperative to this technique. Additional factors aiding success in this case were relative hemodynamic stability and the use of CT-fluoroscopy fusion. Stability and adequate resuscitation can be important for device sizing. Image fusion greatly aids in cannulating target vessels (Fig 2), whether from above or below.
An all-retrograde approach excludes the need for axillary or brachial artery access. Access complications can lengthen the postoperative course of these frail patients.8 A key advantage of this approach is the absence of aortic arch crossing with endovascular tools, minimizing the risk of aortic atheroma mobilization and possible cerebral embolization. In addition, access from above is often performed in a nonergonomic working position in which the surgeon is not well protected from radiation. The most significant advantage of femoral-only access in this case was the speed of t-Branch implantation.
TAAA rupture remains one of the most lethal and morbid pathologic processes in vascular surgery. The t-Branch device has proved to be a successful therapy but still carries a 6% to 14% 30-day mortality and a 6% to 21% rate of spinal cord ischemia.4, 5 When it is used to treat aortic rupture, the success of the t-Branch device depends on an expeditious operative time. In the recent experience of Spanos et al5 reporting t-Branch use in urgent TAAA, mean operative time of experienced users was 407 minutes and as high as 497 minutes in technically challenging cases. The ability to cannulate branch vessels expeditiously from a single access reduced the operative time to 98 minutes in our case. Approach to and difficulty in cannulating target vessels is highly dependent on the patient's anatomy. Despite a large experience with the t-Branch and cannulation from above, we were surprised at the ease of use of this novel approach. Although we also have significant experience with steerable sheaths,9 this approach may prove easier than upper extremity access in certain anatomies4, 10, 11 and improve rates of technical success, currently at 82% to 93%.4, 5 Potential anatomic limitations to this technique include a narrowed aortic lumen at the origin of side branches, stenosed side branches, and high tortuosity in the iliac arteries or infrarenal aorta. When it is done with a bifurcated abdominal component, adequate bilateral iliac access is necessary.
Conclusions
This case demonstrates that ruptured TAAA repair can be achieved swiftly with a t-Branch through an all-retrograde, unifemoral approach using a large steerable sheath and latest generation bridging stents. Transfemoral access for side-branch cannulation with the t-Branch may serve as a useful and expeditious adjunct to current techniques. A larger experience and careful follow-up are necessary to confirm similar technical success and branch vessel patency compared with upper extremity access.
Footnotes
Author conflict of interest: S.H. is a consultant for Cook Medical, Bentley, and GE Healthcare.
The editors and reviewers of this article have no relevant financial relationships to disclose per the Journal policy that requires reviewers to decline review of any manuscript for which they may have a conflict of interest.
References
- 1.Verhoeven E.L., Katsargyris A., Bekkema F., Oikonomou K., Zeebregts C.J., Ritter W. Editor’s choice—ten-year experience with endovascular repair of thoracoabdominal aortic aneurysms: results from 166 consecutive patients. Eur J Vasc Endovasc Surg. 2015;49:524–531. doi: 10.1016/j.ejvs.2014.11.018. [DOI] [PubMed] [Google Scholar]
- 2.Greenberg R., Eagleton M., Mastracci T. Branched endografts for thoracoabdominal aneurysms. J Thorac Cardiovasc Surg. 2010;140:S171–S178. doi: 10.1016/j.jtcvs.2010.07.061. [DOI] [PubMed] [Google Scholar]
- 3.Gasper W.J., Reilly L.M., Rapp J.H., Grenon S.M., Hiramoto J.S., Sobel J.D. Assessing the anatomic applicability of the multibranched endovascular repair of thoracoabdominal aortic aneurysm technique. J Vasc Surg. 2013;57:1553–1558. doi: 10.1016/j.jvs.2012.12.021. [DOI] [PubMed] [Google Scholar]
- 4.Gallitto E., Gargiulo M., Freyrie A., Pini R., Mascoli C., Ancetti S. Off-the-shelf multibranched endograft for urgent endovascular repair of thoracoabdominal aortic aneurysms. J Vasc Surg. 2017;66:696–704.e5. doi: 10.1016/j.jvs.2016.12.129. [DOI] [PubMed] [Google Scholar]
- 5.Spanos K., Kölbel T., Theodorakopoulou M., Heidemann F., Rohlffs F., Debus E.S. Early outcomes of the t-Branch off-the-shelf multibranched stent-graft in urgent thoracoabdominal aortic aneurysm repair. J Endovasc Ther. 2018;25:31–39. doi: 10.1177/1526602817747282. [DOI] [PubMed] [Google Scholar]
- 6.Ullah W., Hunter R.J., McLean A., Dhinoja M., Earley M.J., Sporton S. Impact of steerable sheaths on contact forces and reconnection sites in ablation for persistent atrial fibrillation. J Cardiovasc Electrophysiol. 2015;26:266–273. doi: 10.1111/jce.12573. [DOI] [PubMed] [Google Scholar]
- 7.Oberhuber A., Duran M., Ertaş N., Simon F., Schelzig H. Implantation of an iliac branch device after EVAR via a femoral approach using a steerable sheath. J Endovasc Ther. 2015;22:610–612. doi: 10.1177/1526602815590972. [DOI] [PubMed] [Google Scholar]
- 8.Wooster M., Powell A., Back M., Illig K., Shames M. Axillary artery access as an adjunct for complex endovascular aortic repair. Ann Vasc Surg. 2015;29:1543–1547. doi: 10.1016/j.avsg.2015.05.024. [DOI] [PubMed] [Google Scholar]
- 9.Le Houerou T., Fabre D., Alonso C.G., Brenot P., Bourkaib R., Angel C. In situ antegrade laser fenestrations during endovascular aortic repair. Eur J Vasc Endovasc Surg. 2018;56:356–362. doi: 10.1016/j.ejvs.2018.05.014. [DOI] [PubMed] [Google Scholar]
- 10.Wolosker N., Fioranelli A., Ferreira M., Tachibana A., Lembrança L., Oliveira C. Endovascular repair of ruptured thoracoabdominal aortic aneurysm with an off-the-shelf endoprosthesis. Ann Vasc Surg. 2017;43:312.e1–312.e4. doi: 10.1016/j.avsg.2017.02.013. [DOI] [PubMed] [Google Scholar]
- 11.Tsilimparis N., Fiorucci B., Debus E.S., Rohlffs F., Kölbel T. Technical aspects of implanting the t-Branch off-the-shelf multibranched stent-graft for thoracoabdominal aneurysms. J Endovasc Ther. 2017;24:397–404. doi: 10.1177/1526602817690730. [DOI] [PubMed] [Google Scholar]