Abstract
Background:
This study aimed to evaluate the efficacy of memantine in the acute treatment of geriatric with bipolar disorder (BD) hospitalized for mania.
Materials and Methods:
This study conducted on 70 patients older than 60 years with BD in the acute phase of mania. Oral sodium valproate was prescribed in both groups. The intervention group received memantine tablet and the placebo group received a placebo tablet based on a same procedure. Severity of mania, cognitive changes, and quality of life (QoL) were assessed and recorded 4 and 8 weeks after the beginning of the study. The collected data were analyzed with SPSS (version 20) using independent samples t-test, analysis of variance in repeated observations, Chi-squared test, and Fisher's exact test.
Results:
Mania severity score had no significant difference at the beginning of the study, but 4 and 8 weeks after the intervention, it was reduced significantly in both groups (P < 0.001) that was higher in memantine group (P = 0.038). The mean increase in score of cognitive variations was 6.74 in the memantine group and 3.62 in the placebo group with a nonsignificant difference (P = 0.125). The scores of each dimension of QoL in the two groups showed that in all four dimensions, the patient's physical, psychological, social, and environmental status increased significantly by time (P < 0.001).
Conclusions:
According to the results of this study, memantine as an adjuvant to administration of sodium valproate may have a significant effect on decreasing the intensity of mania in the long run.
Keywords: Bipolar disorder, geriatrics, mania, memantine
Introduction
Mood disorders are one of the leading causes of morbidity, disability, and premature mortality,[1] contributing for about 50% of the nonfatal burden of mental disorders.[2] Bipolar disorder (BD) has a lifetime prevalence of approximately 5%. Eighty-three percent of BD cases are classified as “seriously severe” and 17.1% as “moderately severe.”[3]
The 1-year prevalence of BD among adults aged 65 and older is 0.4%, significantly lower than in younger adults (1.4%).[3,4] BD is highly recurrent, with 85% to 100% of patients experiencing a recurrence after the initial episode.[4]
Despite the scientific advances, the exact mechanism causing BD has not yet been clearly identified, thus various drugs have been approved by the US Food and Drug Administration for treating. In this regard, despite the advances, recent studies have shown that about 50% of BD patients are not fully treated and as a result, the illness is often needed for long-term treatment.[5]
Selected drugs for treatment and prevention of this disorder include lithium carbonate, anticonvulsants (such as carbamazepine, valproic acid, and lamotrigine), antipsychotics (such as olanzapine, risperidone, aripiprazole, ziprasidone, and quetiapine) and other drugs. Certainly, the drugs may be associated with severe complications, long-term intolerance (irreversible and disabling neurodegenerative), and lack of proper response to treatment.[6,7,8,9,10,11,12]
Here, considering that the current medications have no significant and uncomplicated effects on the treatment of elderly patients with BD, researchers are still looking for a more safe and effective treatment in this regard, and for this reason, the antagonists have attracted some attention. Memantine, a low-to-moderate-affinity, noncompetitive N-methyl-D-aspartate (NMDA)-receptor antagonist, is commonly used in the treatment of moderate-to-severe Alzheimer's disease.[13] Antidepressant-like effects of noncompetitive NMDA receptor antagonists have been shown in animal models. These data can use to predict the antidepressant activity of these drugs.[14,15] The antimanic effects of NMDA antagonists have been demonstrated in animal models, and case reports suggested that they may also be effective in controlling the manic and mixed phases of BD.[16,17] Although there are still no definitive findings in this regard, some studies have shown the efficacy of memantine as a strong strategy and even memantine monotherapy for acute manic/mixed episodes.[18] Memantine is a less complicated drug and is usually well tolerated;[19] therefore, it has been considered as an adjunctive strategy to treat BD.
In this regard, recent studies have shown that by the addition of memantine to the usual therapeutic regimen of these patients in a 12-month follow-up period[20] or a 3-year follow-up period[21] causes to facilitate the control of mania symptoms and helps to stabilize their mood.
Here, considering the large number of elderly patients with BD (due to the chronic nature of the disease) and given that drug metabolism, renal and hepatic excretion of drug, etc., in the elderly patients differ from young patients, and most of the commonly used medications to treat BD can have excessive side effects and there is a possibility of arbitrary cessation of drug by the patient due to its side effects; therefore, the need for innovation and replacing adjunctive drugs to treat BD in the elderly patients (to respond to better and faster treatment) is essential and useful. Moreover, elderly patients usually use multiple drugs per day due to and other concomitant diseases/disorders; therefore, the need for finding uncomplicated or less complicated adjunctive drugs is important in this group of patients with respect to the importance of drug interaction.[8,22,23,24]
For this purpose, in this study, the results of the efficacy of memantine as an adjunctive treatment in elderly patients with acute manic phase of BD have been compared with placebo group.
Materials and Methods
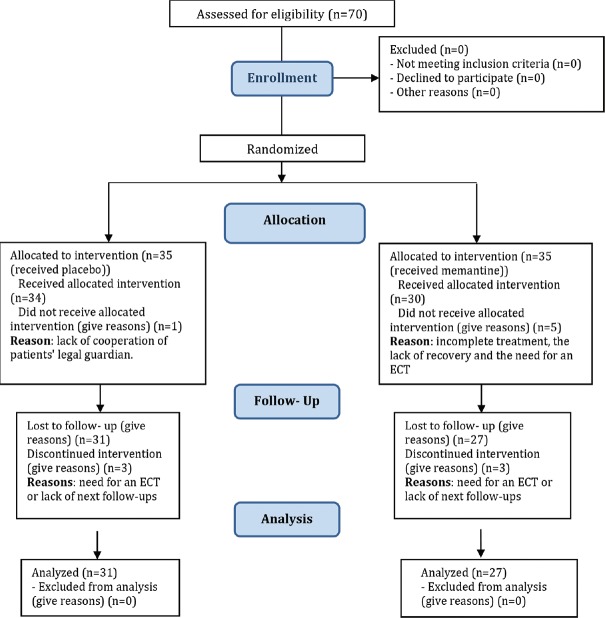
This study was a double-blind randomized clinical trial conducted on population included all patients older than 60 years with BD in the acute phase of mania (with a minimum Young's score of 20) admitted to the Emergency Department and Psychiatric Clinics of Khorshid Hospital in Isfahan from March 2016 to March 2017. The sample size of 35 patients in each group was estimated based on a confidence level of 95%, a statistical power of the test of 80% and symptoms improvement in the memantine group and the placebo group equal 73% and 40% respectively and the level of error equal 0.05.[24] Using convenient sampling method, 70 elderly patients of more than 60 years old with acute phase of mania BD were included in this study. The inclusion criteria were a minimum score of 20 in Young's questionnaires and considering the lack of dependence or abuse of alcohol and any other drugs during the 3 months before admission (except for nicotine or caffeine), lack of comorbidity major psychiatry, lack of proven kidney and liver disease, lack of using memantine during 3 months before admission, no history of side effects and drug sensitivity with sodium valproate and memantine, and satisfaction and cooperation in the process of implementation of the plan after providing adequate explanations by researcher. The exclusion criteria included lack of cooperation of patients’ legal guardian or incomplete treatment, exacerbation of the symptoms of the disease and the need for an electroconvulsive therapy (fortunately, no patient encountered this problem in the present study), or lack of next follow-ups. Out of all participants, 4 participants in the control group and 8 participants in the memantine group were excluded [Figure 1].
Figure 1.
Consort flow diagram
After obtaining the ethics code from the Ethics Committee of Isfahan University of Medical Sciences (IR.MUI.REC.1396.332) and obtaining written consent, the patients were randomly divided into two groups of memantine and placebo. At the beginning of the study, their demographic data such as age, gender, marital status, job, and education level and severity of their mania were assessed and recorded using the Young Mania Rating Scale questionnaire. In addition, for assessing their cognitive changes, Neuropsychiatry Unit Cognition Assessment (NUCOG) questionnaire was used and for assessing the quality of life (QoL), WHOQOL-26 QoL Questionnaire was used and the results were recorded.
The Young Mania questionnaire was designed by Young in 1978 to assess the severity of mania symptoms and has a total validity of 0.96, reliability of 0.92, and Cronbach's alpha of 0.72.[23] It was also normalized in Iran in 2007.[24] This scale has 11 items, which seven items are scored from 0 to 4 and four items are scored from 0 to 8 based on clinical interview with the patient. The general score is from 0 to 60.
The NUCOG is a cognitive test that was first introduced in 2003 by Walterfang et al. In the previous studies, the validity of this test was confirmed in the Persian translated version and the reliability coefficient of Cronbach's alpha was calculated to be 0.919.[25,26] The total score of this questionnaire varies from 0 to 100, and a score more than 85 indicates a normal cognitive function. The QoL questionnaire with 26 items has been used (WHOQOL-26) to assess the patients’ QoL. Its validity and reliability have been reported more than 0.70.[27,28] This questionnaire has four dimensions of physical health, mental health, social relationships, and environmental health. It is scored from 0 to 100. To treat the acute phase of mania in both groups, oral sodium valproate was prescribed at a dose of 500-750 mg daily. The intervention group received a memantine tablet at a dose of 5 mg/day for 10 days at the beginning of the treatment. Then, they received 10 mg of memantine tablet per day for 10 days. Then, they received 20 of memantine tablet daily for 60 days.[20]
The placebo group also received a placebo tablet. It was prescribed for the placebo group based on a procedure similar to treatment group. It should be noted that memantine hydrochloride drug manufactured by Sobhan Pharmaceutical Company in the form of tablet and placebo was also used using similar tablets of memantine (in terms of color, appearance, and odor) and without any drug effect. As this study was double-blind research, the patients were not aware of the content of packages, except for manufacturer of drug, which did not have role in drug or placebo prescription. Mania severity and cognitive changes and QoL were assessed and recorded 4 and 8 weeks after the beginning of the study.
Finally, the collected data were entered into SPSS software (version 20; SPSS Inc., Chicago, Ill., USA). Accordingly, Kolmogorov–Smirnov's test and based on the normal distribution of data, parametric tests such as independent t-test, analysis of variance in repeated observations, Chi-square test, and Fisher's exact test were used and in all analyses and the significance level was considered to be <0.05.
Results
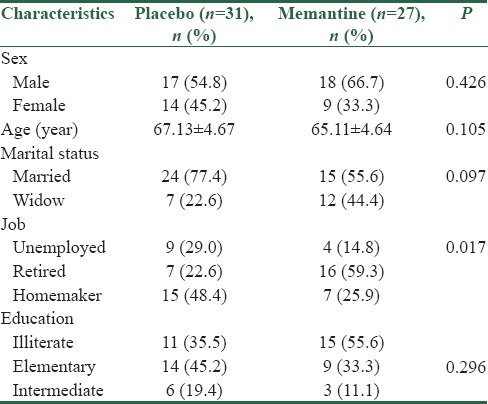
In this study, 58 elderly peoples with acute phase of mania in bipolar mood disorder participated. Out of all participants, 4 participants in the control group and 8 participants in the memantine group were excluded [Figure 1]. According to Table 1, there was no significant difference between two groups in terms of demographic characteristics (P > 0.05).
Table 1.
Demographic characteristics of the elderly in two groups of the study
Evaluation of mania severity score among the two groups indicated that at the beginning of the study, both groups did not have a significant difference in the score of mania severity, but 4 and 8 weeks after the intervention, it was found that the severity of mania was reduced significantly in both groups (P < 0.001), in addition, this reduction was higher in the memantine group than the placebo group (P = 0.038) [Table 2].
Table 2.
The mean score of mania severity before, 4 weeks, and 8 weeks after intervention between two memantine and placebo groups

Moreover, increase in the mean scores of cognitive variations was significant (P < 0.001). The mean increase in score of cognitive variations was 6.74 in the memantine group and 3.62 in the placebo group, which did not show a significant difference between the two groups (P = 0.125) [Table 3].
Table 3.
The mean score of cognitive variations before, 4 weeks, and 8 weeks after intervention between two memantine and placebo groups

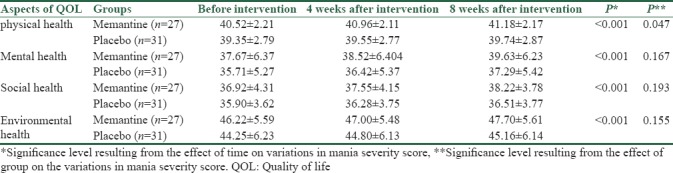
The evaluation of variations in the scores of each dimension of QoL in the two groups showed that in all four dimensions, the patient's physical, psychological, social and environmental status increased significantly by passage of time since the beginning of the study to 8-week follow-up after the intervention (P < 0.001). In addition, the improvement in the physical status of the elderly in the memantine group with mean of 0.66 was higher than the placebo group with a mean of 0.39 (P = 0.047), but the effect of the study group was not significant on variations of other QoL variables (P > 0.05) [Table 4].
Table 4.
The mean scores of each dimension of the quality of life of the elderly before, 4 weeks, and 8 weeks after the intervention between the memantine group and placebo group
Discussion
In the present study, there were 58 BD patients in both groups and maximum patients of both groups were male and their mean age was >60 years. In other words, according to our study population, most of the elderly people with BD aged over 60 years were male. Although in this study, the two groups were matched for demographic characteristics of patients such as age, sex, education level, and married.
On the other hand, at time of entering the study, the severity of mania did not differ significantly between the two groups, but after the 4– 8-week intervention, there was a significant decrease in the severity of mania. In addition, after the 8-week intervention, the severity of mania was significantly lower in the memantine group than placebo group.
In line with the current study, in their study, Koukopoulos et al. showed that by the addition of memantine to the usual therapeutic regimen of elderly patients with BD can facilitate the control of mania symptoms, and this drug would help to stabilize their mood. Although in this study patients were followed for 12 months, their drug regimen was not the same, and the control group was not considered for comparing the use of memantine with placebo.[20]
In addition, a study conducted by Serra et al. suggested that the by the addition of memantine to the drug regimen of patients who did not well respond to the usual treatments could significantly reduce the duration and recurrence of periods of depression or mania over 3-year follow-up period. In this study, the drug regimen of patients was not homogeneous and there was no control group to compare the results.[21]
In this regard, perhaps, it might be said that having a control group was considered as one of advantages of our study and two groups were matched for demographic characteristics. However, our study was not also checked the patients’ diet.
In this study, the results from evaluation of average score of cognitive changes in these patients showed that two groups were matched at time of entering the study. However, after the 4–8-week treatment, a significant increase in their cognitive change scores was observed. However, it is worth considering that there were no statistical significant differences between the two groups in terms of cognitive change score after the 8-week intervention. In other words, in both groups, treatment has led to an increase in cognitive change scores of patients, but it is not possible to distinguish between the effects of the added memantine to oral sodium valproate compared to oral sodium valproate given alone on cognitive changes in these patients.
In 2003–2004, it has been found that the sensitization of dopamine receptors induced by imipramine is followed, after imipramine withdrawal, by a desensitization of these receptors associated with a depressive-like behavior assessed in the forced swimming test. The dopamine receptor sensitization can be prevented by MK-801, an NMDA receptor antagonist, but not by currently used mood stabilizers (lithium, carbamazepine, and valproate). These observations led to suggest and later confirm with preliminary clinical observations that memantine may have an acute antimanic and a long-lasting mood-stabilizing effect in treatment of resistant BD patients. Now, it has been shown that memantine prevents not only the dopamine receptor sensitization induced by imipramine, as observed with MK-801, but also the ensuing desensitization and the associated depressive-like behavior observed after antidepressant withdrawal.[29]
Growing evidence show that memantine might be effective at preventing recurrences of both phases of BD and in reducing the manic-like symptomatology associated with several neurological and psychiatric conditions.[21,30,31] Memantine monotherapy was reported to show evidence of antimanic effects at well-tolerated daily doses (20–50 mg) in a 3-week open-label trial in 33 acutely manic patients.[18]
Memantine as a monotherapy also has been reported to show beneficial effects in a few individual patients with BD, including after discontinuation of lithium treatment.[32,33,34] Another short-term study found memantine to be more effective than placebo when added to lamotrigine for 4 weeks to treat acute bipolar depression in a randomized, controlled trial, but this effect was no longer significant at 8 weeks.[35] A recent 12-week trial found little overall difference in effects of small doses of memantine (5 mg/d; n = 62) versus placebo added to valproate in bipolar II disorder patients for 12 weeks.[36] Finally, we just reported the results of a 3-year naturalistic study of adding memantine to 30 treatment-resistant bipolar patients.[21] In this study, memantine showed a long-term and progressive ability to prevent depressive and mania/hypomania recurrences, in patients who had been resistant to standard treatments for more than 3 years. Memantine decreases the duration of illness, the duration of new episodes, recurrence frequency, and symptomatology severity.
In a review study by Sani et al., it has been reported that the effects of memantine in anxiety disorders have been poorly investigated, but data indicate that the use of the drug in obsessive-compulsive disorder and posttraumatic stress disorder holds promise, while findings relating to generalized anxiety disorder are rather disappointing. Results in eating disorders, catatonia, impulse control disorders (pathological gambling), substance and alcohol abuse/dependence, and attention-deficit hyperactivity disorder are inconclusive. In most psychiatric non-Alzheimer's disease conditions, the clinical data fail to support the usefulness of memantine as monotherapy or add-on treatment. However, recent preclinical and clinical findings suggest that add-on memantine may show antimanic and mood-stabilizing effects in treatment-resistant BD.[31]
Finally, the assessment of QoL among elderly people in four domains of physical, psychological, environmental, and social health indicated that in both groups, treatment can be effective in improving the quality of these patients in all four domains, but the use of memantine as an adjunctive drug with oral sodium valproate compared to the use of oral sodium valproate given alone could not have any effect on improving the QoL of the elderly in terms of psychological, social, and environmental health. Although the use of the adjunctive drug in the treatment process of these patients could not be improved their physical state significantly more than the placebo group.
Depp et al. showed that patients in remission from BD had QWB scores that were worse than those of normal comparison subjects. Greater severity of psychotic and depressive symptoms and cognitive impairment were associated with lower HRQoLF. BD was associated with substantial disability in this sample of older adults, similar in severity to schizophrenia. Remission of BD was associated with significant but incomplete improvement in functioning, whereas psychotic and depressive symptoms and cognitive impairment seemed to contribute to lower HRQoLF.[37]
In a systematic literature review conducted to identify BD studies of HRQoL, functioning, work impairment, and health-care utilization and costs, it was revealed that for all HRQoL instruments used, BD patients’ HRQoL was rated similarly to that of unipolar depression patients and equal to or lower compared with patients with other chronic nonmental illnesses. Current treatments have been shown to improve HRQoL and physical and social functioning; some data indicate that management may improve self-reported work impairment and absenteeism. It has also shown that BD patients have been found to utilize health-care services more than do patients with depression or chronic medical conditions. Inpatient costs are the largest cost contributor; treatment to prevent recurrence has been shown to be the most effective way to reduce costs.[38]
In this regard, many studies have pointed out to reduce the QoL in BD patients, so that BD can affect mood swings, irritability, dangerous behaviors and interpersonal relationships among people, and in some cases, it can be led to suicide.[5,39] Here, given that elderly persons may have poor QoL due to aging, the effect of reducing the QoL in people with this type of disorder would be double, thus, it is important to pay attention to this issue.
Finally, it should be noted that in the current study, the age of involvement with disease was not exactly defined, because some elderly people have referred to physicians after months or years of the disease onset and then it would be diagnosed. Therefore, this was one of the limitations of the study that could not be avoided. In addition, since our study population consists of elderly people, the majority of them had very low literacy and the scores of their cognitive changes were low and the minor changes can be due to their lack of literacy as filling a part of the questionnaire is related to the level of literacy and the ability of reading and writing. However, the strength of the present study is paying attention to the elderly and to study on these patients, because this part of the society is less considered in the community, while they are more vulnerable to mental risks and disorders.
Conclusions
According to the results of this study, the use of memantine as an auxiliary drug with oral sodium valproate in the long term (8 weeks) can be effective in reducing the severity of mania in the elderly. In addition, this therapeutic approach has made it possible to improve the elders’ QoL in terms of physical health; although it has no significant effect in the aspects of psychological, social, and environmental health as well as the cognitive changes in these patients. Therefore, it seems that this drug can play a significant role in reducing the severity of mania in patients with long-term treatment.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Ferrari AJ, Norman RE, Freedman G, Baxter AJ, Pirkis JE, Harris MG, et al. The burden attributable to mental and substance use disorders as risk factors for suicide: Findings from the global burden of disease study 2010. PLoS One. 2014;9:e91936. doi: 10.1371/journal.pone.0091936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Whiteford HA, Degenhardt L, Rehm J, Baxter AJ, Ferrari AJ, Erskine HE, et al. Global burden of disease attributable to mental and substance use disorders: Findings from the Global Burden of Disease Study 2010. Lancet. 2013;382:1575–86. doi: 10.1016/S0140-6736(13)61611-6. [DOI] [PubMed] [Google Scholar]
- 3.Kessler RC, Demler O, Frank RG, Olfson M, Pincus HA, Walters EE, et al. Prevalence and treatment of mental disorders, 1990 to 2003. N Engl J Med. 2005;352:2515–23. doi: 10.1056/NEJMsa043266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Trinh NH, Forester B. Bipolar disorder in the elderly: Differential diagnosis and treatment. Psychiatr Times. 2007;24:38–43. [Google Scholar]
- 5.Shiffer HH. Glutamate receptor genes: Susceptibility factors in schizophrenia and depressive patients. Mol Biol. 2002;25:191–212. doi: 10.1385/MN:25:2:191. [DOI] [PubMed] [Google Scholar]
- 6.Goodwin FK. Rationale for long-term treatment of bipolar disorder and evidence for long-term lithium treatment. J Clin Psychiatry. 2002;63(Suppl 10):5–12. [PubMed] [Google Scholar]
- 7.Rapoport SI, Basselin M, Kim HW, Rao JS. Bipolar disorder and mechanisms of action of mood stabilizers. Brain Res Rev. 2009;61:185–209. doi: 10.1016/j.brainresrev.2009.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sajatovic M, Gyulai L, Calabrese JR, Thompson TR, Wilson BG, White R, et al. Maintenance treatment outcomes in older patients with bipolar I disorder. Am J Geriatr Psychiatry. 2005;13:305–11. doi: 10.1176/appi.ajgp.13.4.305. [DOI] [PubMed] [Google Scholar]
- 9.Veronese N, Solmi M, Luchini C, Lu RB, Stubbs B, Zaninotto L, et al. Acetylcholinesterase inhibitors and memantine in bipolar disorder: A systematic review and best evidence synthesis of the efficacy and safety for multiple disease dimensions. J Affect Disord. 2016;197:268–80. doi: 10.1016/j.jad.2016.03.034. [DOI] [PubMed] [Google Scholar]
- 10.Tohen M, Greil W, Calabrese JR, Sachs GS, Yatham LN, Oerlinghausen BM, et al. Olanzapine versus lithium in the maintenance treatment of bipolar disorder: A 12-month, randomized, double-blind, controlled clinical trial. Am J Psychiatry. 2005;162:1281–90. doi: 10.1176/appi.ajp.162.7.1281. [DOI] [PubMed] [Google Scholar]
- 11.Mitchell PB, Malhi GS, Ball JR. Major advances in bipolar disorder. Med J Aust. 2004;181:207–10. doi: 10.5694/j.1326-5377.2004.tb06238.x. [DOI] [PubMed] [Google Scholar]
- 12.Bahrami Kalashgaran S, Maroufi A, Rezaei F, Ghaderi E, Hassanzadeh K. Efficacy of memantine as an adjuvant therapy in bipolar disorder; a double blind, randomized, clinical trial. Sci J Kurdistan Univ Med Sci. 2016;21:1–10. [Google Scholar]
- 13.Reisberg B, Doody R, Stöffler A, Schmitt F, Ferris S, Möbius HJ. Memantine in moderate-to-severe Alzheimer's disease. N Engl J Med. 2003;348:1333–41. doi: 10.1056/NEJMoa013128. [DOI] [PubMed] [Google Scholar]
- 14.Réus GZ, Fries GR, Stertz L, Badawy M, Passos IC, Barichello T, et al. The role of inflammation and microglial activation in the pathophysiology of psychiatric disorders. Neuroscience. 2015;300:141–54. doi: 10.1016/j.neuroscience.2015.05.018. [DOI] [PubMed] [Google Scholar]
- 15.Rogóz Z, Skuza G, Maj J, Danysz W. Synergistic effect of uncompetitive NMDA receptor antagonists and antidepressant drugs in the forced swimming test in rats. Neuropharmacology. 2002;42:1024–30. doi: 10.1016/s0028-3908(02)00055-2. [DOI] [PubMed] [Google Scholar]
- 16.Gao Y, Payne RS, Schurr A, Hougland T, Lord J, Herman L, et al. Memantine reduces mania-like symptoms in animal models. Psychiatry Res. 2011;188:366–71. doi: 10.1016/j.psychres.2010.12.030. [DOI] [PubMed] [Google Scholar]
- 17.Agarwal V, Tripathi A. Memantine in the management of a clinically challenging case of bipolar disorder. Indian J Psychiatry. 2009;51:137–8. doi: 10.4103/0019-5545.49455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Keck PE, Jr, Hsu HA, Papadakis K, Russo J., Jr Memantine efficacy and safety in patients with acute mania associated with bipolar I disorder: A pilot evaluation. Clin Neuropharmacol. 2009;32:199–204. doi: 10.1097/WNF.0b013e318184fae2. [DOI] [PubMed] [Google Scholar]
- 19.Pelton GH, Harper OL, Roose SP, Marder K, D’Antonio K, Devanand DP, et al. Combined treatment with memantine/es-citalopram for older depressed patients with cognitive impairment: A pilot study. Int J Geriatr Psychiatry. 2016;31:648–55. doi: 10.1002/gps.4375. [DOI] [PubMed] [Google Scholar]
- 20.Koukopoulos A, Serra G, Koukopoulos AE, Reginaldi D, Serra G. The sustained mood-stabilizing effect of memantine in the management of treatment resistant bipolar disorders: Findings from a 12-month naturalistic trial. J Affect Disord. 2012;136:163–6. doi: 10.1016/j.jad.2011.09.040. [DOI] [PubMed] [Google Scholar]
- 21.Serra G, Koukopoulos A, De Chiara L, Koukopoulos AE, Tondo L, Girardi P, et al. Three-year, naturalistic, mirror-image assessment of adding memantine to the treatment of 30 treatment-resistant patients with bipolar disorder. J Clin Psychiatry. 2015;76:e91–7. doi: 10.4088/JCP.13m08956. [DOI] [PubMed] [Google Scholar]
- 22.Serra G, Demontis F, Serra F, De Chiara L, Spoto A, Girardi P, et al. Memantine: New prospective in bipolar disorder treatment. World J Psychiatry. 2014;4:80–90. doi: 10.5498/wjp.v4.i4.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Young RC, Biggs JT, Ziegler VE, Meyer DA. A rating scale for mania: Reliability, validity and sensitivity. Br J Psychiatry. 1978;133:429–35. doi: 10.1192/bjp.133.5.429. [DOI] [PubMed] [Google Scholar]
- 24.Barakatain M, Tavakkoli M, Molavi H, Maroofi M, Salehi M. Standardization, validity and reliability of young mania rating scale in Iran. J Psychol. 2007;2:150–66. [Google Scholar]
- 25.Barekatain M, Behdad M, Tavakkoli M, Mahvari J, Maracy MR, Walterfang M, et al. Psychometric Properties of the Persian Version of the Neuropsychiatry Unit Cognitive Assessment Tool (NUCOG) in Patients with Dementia. Iranian Journal of Psychiatry and Clinical Psychology. 2010;16(1):14–20. [Google Scholar]
- 26.Walterfang M, Velakoulis D, Gibbs A, Lloyd J. The NUCOG: Construction and piloting of a cognitive screening instrument in a neuropsychiatric unit. Australas Psychiatry. 2003;11:325–9. [Google Scholar]
- 27.World Health Organization. Geneva: World Health Organization; 1996. WHOQOL-BREF: Introduction, Administration, Scoring and Generic Version of the Assessment: Field Trial Version. [Google Scholar]
- 28.Jahanlou AS, Karami NA. WHO quality of life-BREF 26 questionnaire: Reliability and validity of the Persian version and compare it with Iranian diabetics quality of life questionnaire in diabetic patients. Prim Care Diabetes. 2011;5:103–7. doi: 10.1016/j.pcd.2011.02.001. [DOI] [PubMed] [Google Scholar]
- 29.Demontis F, Falconi M, Canu D, Serra G. Memantine prevents “bipolar-like” behavior induced by chronic treatment with imipramine in rats. Eur J Pharmacol. 2015;752:49–54. doi: 10.1016/j.ejphar.2015.01.041. [DOI] [PubMed] [Google Scholar]
- 30.Zdanys K, Tampi RR. A systematic review of off-label uses of memantine for psychiatric disorders. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32:1362–74. doi: 10.1016/j.pnpbp.2008.01.008. [DOI] [PubMed] [Google Scholar]
- 31.Sani G, Serra G, Kotzalidis GD, Romano S, Tamorri SM, Manfredi G, et al. The role of memantine in the treatment of psychiatric disorders other than the dementias: A review of current preclinical and clinical evidence. CNS Drugs. 2012;26:663–90. doi: 10.2165/11634390-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 32.Serra G, De Chiara L, Koukopoulos A, Serra G. Antimanic and long-lasting mood stabilizing effect of memantine in bipolar I mood disorder: Two case reports. J Clin Psychopharmacol. 2013;33:715–7. doi: 10.1097/JCP.0b013e31829b62ba. [DOI] [PubMed] [Google Scholar]
- 33.Serra G, De Chiara L, Manfredi G, Koukopoulos AE, Sani G, Girardi P, et al. Memantine in the management of affective recurrences of bipolar disorders after the discontinuation of long-term lithium treatment: Three case histories. Ther Adv Psychopharmacol. 2014;4:53–5. doi: 10.1177/2045125313507737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.De Chiara L, Serra G, Koukopoulos AE, Koukopoulos A, Serra G. Memantine in the treatment and prophylaxis of bipolar type II mood disorder and co-morbid eating disorder: A case report. Riv Psichiatr. 2014;49:192–4. doi: 10.1708/1600.17462. [DOI] [PubMed] [Google Scholar]
- 35.Anand A, Gunn AD, Barkay G, Karne HS, Nurnberger JI, Mathew SJ, et al. Early antidepressant effect of memantine during augmentation of lamotrigine inadequate response in bipolar depression: A double-blind, randomized, placebo-controlled trial. Bipolar Disord. 2012;14:64–70. doi: 10.1111/j.1399-5618.2011.00971.x. [DOI] [PubMed] [Google Scholar]
- 36.Lee SY, Chen SL, Chang YH, Chen PS, Huang SY, Tzeng NS, et al. Add-on memantine to valproate treatment increased HDL-C in bipolar II disorder. J Psychiatr Res. 2013;47:1343–8. doi: 10.1016/j.jpsychires.2013.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Depp CA, Davis CE, Mittal D, Patterson TL, Jeste DV. Health-related quality of life and functioning of middle-aged and elderly adults with bipolar disorder. J Clin Psychiatry. 2006;67:215–21. doi: 10.4088/jcp.v67n0207. [DOI] [PubMed] [Google Scholar]
- 38.Dean BB, Gerner D, Gerner RH. A systematic review evaluating health-related quality of life, work impairment, and healthcare costs and utilization in bipolar disorder. Curr Med Res Opin. 2004;20:139–54. doi: 10.1185/030079903125002801. [DOI] [PubMed] [Google Scholar]
- 39.Keck PE, Jr, McElroy SL, Arnold LM. Bipolar disorder. Med Clin North Am. 2001;85:645–61. doi: 10.1016/s0025-7125(05)70334-5. ix. [DOI] [PubMed] [Google Scholar]