Abstract
Background:
Ameloblastic carcinoma (ACA) is a malignant neoplasm with overlapping histopathological features of benign aggressive solid multicystic ameloblastoma (SMA). This often leads to misdiagnosis with direct implication on the management protocol. The need of the hour is to adopt reliable tissue biomarkers to differentiate these lesions accurately that will help to implement an appropriate treatment modality. Few studies to differentiate ACA and SMA in literature with a limitation of a single marker and lack of availability of cases have prompted us to undertake this study. Thereby, this study is aimed at resolving the diagnostic dilemma in differentiating ACA and aggressive SMA using SOX-2, OCT-4 and CD44.
Materials and Methods:
Tissue samples involved 40 archival cases of histopathologically confirmed cases of ACA (n = 20) and SMA (n = 20). The sections were subjected to immunohistochemical staining using antibodies to SOX-2, OCT-4 and CD44. Nuclear staining for SOX-2 and OCT-4 and membranous reactivity for CD44 was considered positive.
Results:
The expression of SOX-2 and OCT-4 in ACA was statistically significant when compared to SMA (P < 0.001). CD44 showed an insignificant statistical value of <0.077 in differentiating ACA and SMA. SOX-2 and OCT-4 expression in ACA showed a significant correlation coefficient of 0.616 at P < 0.004.
Conclusions:
SOX-2 and OCT-4 could serve as independent novel markers in resolving the diagnostic dilemma between ACA and aggressive SMA.
Keywords: Ameloblastic carcinoma, CD44, OCT-4, SOX-2, solid multicystic ameloblastoma
Introduction
Solid multicystic ameloblastoma (SMA) is the most common benign odontogenic tumor of the jaws that constitutes about 1% of all cysts and tumors of the jaws.[1] It is generally painless, slow-growing and locally aggressive. SMA is treated by enucleation or surgical excision depending on size and type of the lesion.
Ameloblastic carcinoma (ACA) is a rare malignant odontogenic tumor exhibiting not only features of ameloblastoma but also that of carcinoma. It is usually painless or symptomatic, rapidly growing and invasive. Management dictates a more aggressive surgical approach than that of a simple ameloblastoma and has to be customized for each individual patient.[2] Histologically, it has overlapping features with aggressive SMA that makes it challenging to diagnose and differentiate from a benign SMA. In addition to this, there are no standardized histologic criteria to distinguish it objectively leading to misdiagnosis and direct implication on management and treatment outcome. Furthermore, ACA has a high rate of recurrence than conventional SMA. Benlyazid et al., 2007 retrospectively reviewed 66 patients with ACA and the majority exhibited lung metastasis, which indicated the requirement for aggressive systemic therapy.[3] Yoon et al., 2009 reported a recurrence rate of 92.3% following curettage alone and 28.3% following partial resection.[4] Whereas for SMA, the treatment regimens encompass enucleation, curettage, marsupialization, and radical surgery which includes resection with or without continuity defect. The rate of recurrence for SMA ranges from 17.7% for en bloc resection to 34.7% for conservative therapy.[3]
Clinical and therapeutic implications of cancer stem cells (CSCs) have attracted growing attention, including early detection and prognostication of cancer.[5] Previous studies have shown the role of stem cell markers in predicting malignant transformation of oral potentially malignant disorders as they correlate well with the biologic behavior. In addition, CSCs can be identified and isolated by expression of distinctive markers. The dilemma in differentiating ACA from SMA can be overcome with the aid of stem cell markers. This would navigate the surgeon to effectively treat ACA resulting in better treatment outcome and patient survival. This study would also be a preliminary step to undertake genetic studies that will facilitate to help pinpoint tumor-specific genes specific for ACAs. Thus, the current study aims to resolve the diagnostic dilemma in differentiating ACA and aggressive SMA using SOX-2, OCT-4, and CD44.
Materials and Methods
Tissue specimens
Formalin fixed paraffin-embedded tissue blocks were retrieved from archives of the Oral Pathology and microbiology Department, Faculty of Dental Sciences, M. S. Ramaiah University of Applied Sciences, Bengaluru. A total of 40 histopathologically diagnosed cases (20 ACA and 20 SMA) were selected for the study. Unicystic ameloblastomas were excluded since they are not aggressive lesions and recurrent cases of ACA or SMA were also excluded since only primary tumors were included. Oral squamous cell carcinoma (OSCC, n = 5) being a malignant tumor of the oral cavity is known to be strongly positive for CSC markers. Hence, it was taken as control tissue for ACA while odontogenic epithelium from dental follicle (OEDF, n = 5) was considered as control tissue for SMA as it relates to the origin of SMA. Negative controls were performed by omission of the primary antibody.
Tissue processing and immunohistochemistry
Tissue sections of the cases and controls were initially prepared for routine hematoxylin and eosin staining. Serial sections of 4 μm thickness were obtained on silane-coated slides followed by deparafinization and rehydration. Endogenous peroxidases in tissue sections were blocked by immersing and gently agitating the slides in phosphate-buffered saline (PBS) containing 0.3% hydrogen peroxide (to eliminate false-positive reactions). Antigen retrieval was performed in three cycles using the microwave method. The staining protocol was optimized for each of the antibodies by titration of antibodies to select the optimum concentration in which the positive control tissue gave the best positive staining with minimal background staining. The tissue sections were stained with anti-rabbit monoclonal primary antibody in a humidifying chamber for 45 min using SOX-2 (Sigma-Aldrich, USA at 1:300 dilution), OCT-4 (Sigma-Aldrich, USA at 1:300 dilution) and CD44 (Sigma-Aldrich, USA at 1:200 dilution). The slides were washed in PBS for 5 min at room temperature (to avoid tissue drying leading to nonspecific binding and resultant background staining) followed by incubation with rabbit monoclonal secondary antibody labeled with streptavidin-biotin peroxidase. The slides were reimmersed in PBS for 5 min at room temperature followed by treatment with chromogen 3, 3’-diaminobenzidine. The tissue sections were counterstained with Harris’ hematoxylin for 2 min and washed in running tap water for 5 min. Dehydration was performed in increasing concentrations of alcohol (50%, 60%, 70%, 80% and 100%). The slides were immersed in a xylene bath for 6 min at room temperature. The sections were mounted in distrene dibutylphthalate xylene and interpreted under a compound light microscope (Olympus Optical Company, India). In each staining batch of slides, positive and negative controls were compared to assure consistent interpretation. A negative control slide was taken where the addition of primary antibody was omitted that was maintained for all antibodies used.
Interpretation and scoring
Two investigators (WK and DA) independently scored the stained slides. Dark brown staining of tumor cell nucleus for SOX-2 and OCT-4 was considered positive. Dark brown membrane staining of the tumor cells was considered positive for CD44. The distribution pattern in ACA and SMA was also analyzed. SOX-2, OCT-4, and CD44 immunoreactivity was evaluated by scanning the slides at ×100 and ×400 magnification. Five areas were randomly selected which were rich in lesional cells of ACA and SMA. The immunoreactivity for SOX-2 and OCT-4 was semi quantitatively assessed using a scoring criterion by Ge et al., 2010.[6] Scores were recorded based on the proportion of cells stained: 0 = negative, 1 = <1/4, 2 = 1/4–1/2, 3 = 1/2–3/4, and 4 ≥ 3/4 and intensity of staining was interpreted as 0 = negative, 1 = weak, 2 = intermediate and 3 = strong. The proportion and intensity scores were then added to obtain a total score, which ranged from 0 to 7. The cases were categorized into one of two groups according to their overall scores: (1) Low expression, <4 points and (2) high expression, 4–7 points. The scoring criteria of Srinath et al., 2014 was adopted for CD44 based on proportion of cells stained as follows: 0 = negative; 1 = low, 1%–10% positive cells; 2 = intermediate, 11%–50% positive cells; and 3 = high, >50% positive cells.[7]
Data analysis
The statistical analysis was performed using Statistical Package for the Social Sciences Version 22, IBM, NY, United States of America. Mann–Whitney U-test was used to evaluate significance in expression between the groups. Spearman's correlation coefficient was determined between the markers in ACA and SMA.
Results
A total of 40 cases comprising of 20 ACA and 20 SMA were evaluated for immunohistochemical expression of stem cell markers SOX-2, OCT-4, and CD44.
SOX-2 expression in ameloblastic carcinoma and solid multicystic ameloblastoma
Among the 20 ACA, 18 cases (90%) showed a positive nuclear immunoreactivity for SOX-2 and 2 cases (10%) were negative [Figure 1a–c]. Among the 20 SMA, positive immunoreactivity was observed in 3 cases (15%) for SOX-2 while 17 cases (85%) were negative [Figure 2a–c]. Among the 18 SOX-2-positive cases of ACA, 16 cases (88.88%) showed high expression (>4) and 2 cases (11.12%) showed weak expression (<4). The 3 SOX-2 positive cases of SMA showed a weak expression (<4) [Table 1]. The expression of SOX-2 in ACA was statistically significant when compared to SMA (P < 0.001) [Table 2]. Pattern of SOX-2 expression in ACA was diffuse. Nuclear staining was observed in the tumor islands with predominantly weak staining in central areas. In the 3 SOX-2 positive cases of SMA, weak positive expression was seen in the basal and parabasal cells of the follicles [Table 3].
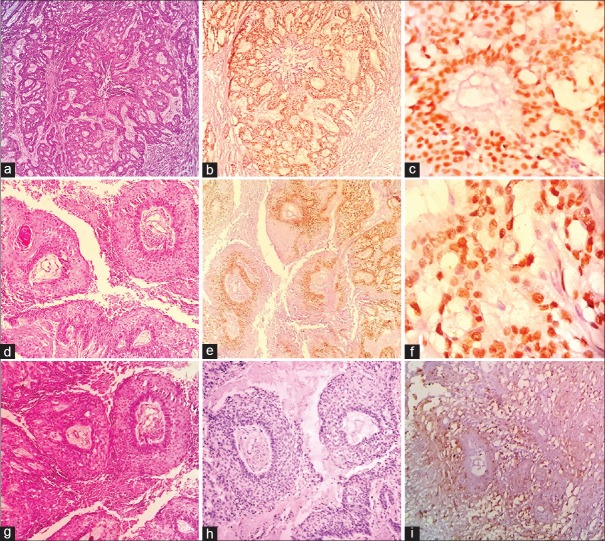
Figure 1.
Photomicrographs of ameloblastic carcinoma (a) Proliferating odontogenic islands (H and E, ×100). (b) Diffuse and high SOX-2 nuclear staining of basal and parabasal layers (IHC ×100). (c) High SOX-2 nuclear staining of tumor cells (IHC ×200). (d) Odontogenic island with central necrosis (H and E, ×100). (e) Diffuse and high OCT-4 nuclear staining of basal and parabasal layers (IHC ×100). (f) High OCT-4 nuclear staining of tumor cells (IHC ×400). (g) Odontogenic islands with central necrosis and retraction artifact (H and E, ×100). (h) Negative staining for OCT-4 in tumor islands (IHC ×100). (i) Low membrane expression for CD44 in the tumor islands (IHC ×400)
Figure 2.
Photomicrographs of solid multicystic ameloblastoma (a) Odontogenic follicle (H and E, ×400). (b) Diffuse SOX-2 nuclear staining of the basal layer (IHC ×200). (c) Negative SOX-2 staining in an odontogenic follicle (IHC ×400). (d) Ameloblast like cells with central stellate reticulum (H and E, ×400). (e) Negative OCT-4 staining of odontogenic epithelium (IHC ×100). (f) Negative OCT-4 staining of odontogenic epithelium and stellate reticulum (IHC ×400). (g) Odontogenic follicle with central stellate reticulum (H and E, ×100). (h) High membrane staining for CD44 with majority of central areas being negative (IHC ×100). (i) Negative membrane expression for CD44 (IHC ×400)
Table 1.
SOX-2 and OCT-4 scoring of ameloblastic carcinoma and solid multicystic ameloblastoma
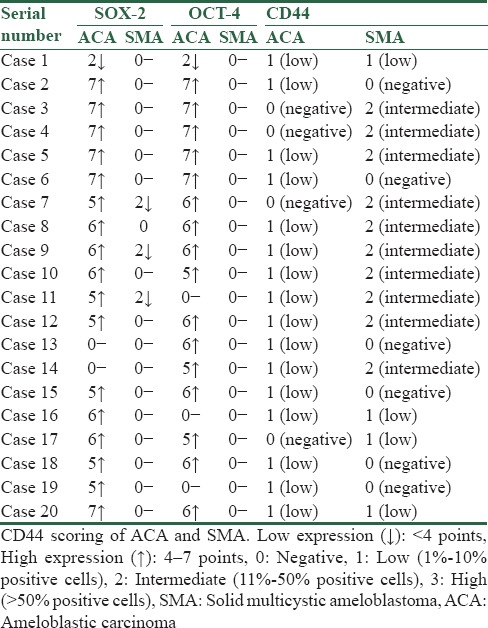
Table 2.
Expression of SOX-2, OCT-4 and CD44 in 20 cases of ameloblastic carcinoma and solid multicystic ameloblastoma analyzed by Mann-Whitney U-test
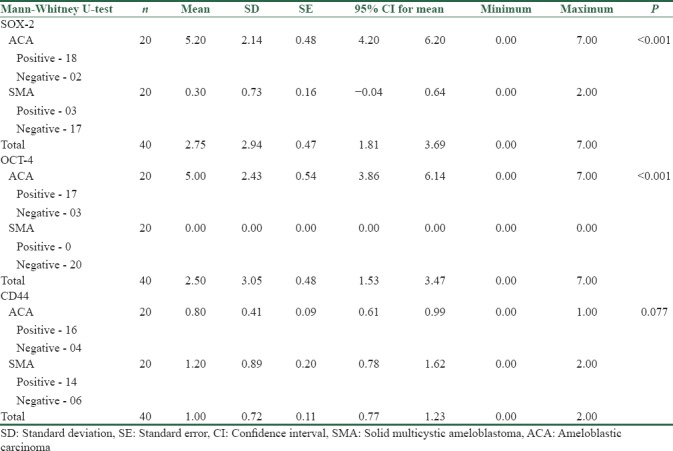
Table 3.
Pattern of expression of SOX-2, OCT-4, and CD44 in 20 cases of ameloblastic carcinoma and solid multicystic ameloblastoma

OCT-4 expression in ameloblastic carcinoma and solid multicystic ameloblastoma
Among the 20 ACA, 17 cases (85%) showed a positive nuclear immunoreactivity for OCT-4 and 3 cases (15%) were negative [Figure 1d–f]. All 20 SMAs were immunonegative for OCT-4 [Figure 2d–f]. Among the 17 OCT-4-positive cases of ACA, 16 cases (94.11%) showed strong expression (>4) and 1 case (5.89%) showed weak expression (<4) [Table 1]. The expression of OCT-4 in ACA was statistically significant when compared to SMA (P < 0.001) [Table 2]. Pattern of OCT-4 expression in ACA showed nuclear staining in the tumor islands with predominantly weak staining in central areas [Table 3].
CD44 expression in ameloblastic carcinoma and solid multicystic ameloblastoma
Among the 20 ACA, 16 cases (80%) showed positive membrane immunoreactivity for CD44 and 4 cases (20%) were negative [Figure 1g–i]. Among the 20 SMA, positive immunoreactivity was observed in 14 cases (70%) for CD44 while 6 cases (30%) were negative [Figure 2g–i]. Among the 16 CD44-positive cases of ACA, all showed low expression (1%–10% positive cells). In 14 CD44-positive cases of SMA, 6 cases were negative, 4 cases showed low expression (1%–10% positive cells), and 10 cases showed intermediate expression (11%–50% positive cells) [Table 1]. The expression of CD44 in ACA was statistically non-significant when compared to SMA (P < 0.077) [Table 2]. Pattern of CD44 expression in ACA was variable either in intensity or localization, with some cases being positive in focal areas while others were considered negative. In SMA, most central stellate reticulum-like cells were negative with higher membrane expression in basal and suprabasal layers [Table 3].
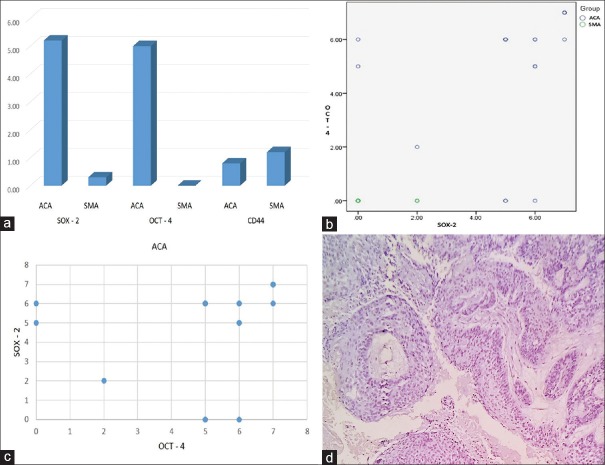
SOX-2 and OCT-4 immunoexpression was significant between ACA and SMA as analyzed by Mann–Whitney U-test at statistical significance (P < 0.001) [Figure 3a]. The scoring of SOX-2 and OCT-4 in ACA is shown in Figure 3b. SOX-2 and OCT-4 expression in ACA showed a significant correlation coefficient of 0.616 at (P < 0.004) [Figure 3c]. In the control tissues stained [Figure 4a–h], high expression was observed in all 5 cases for SOX-2 and OCT-4 in OSCC (control for ACA) while 3 cases showed an intermediate expression for CD44 staining and 2 were negative. OEDF (control for SMA) showed weak SOX-2 expression limited to basal layer in 2 controls, while 3 were negative. OCT-4 was negative in all 5 OEDF controls. High expression to CD44 was observed in 3 controls of OEDF, while 2 were negative. The negative control slide maintained for all antibodies used showed no staining in all cases of IHC staining [Figure 3d].
Figure 3.
(a) Bar graph comparing SOX-2, OCT-4, and CD44 expression in ameloblastic carcinoma and solid multicystic ameloblastoma. (b) Scatterplot comparing SOX-2 and OCT-4 scoring in ameloblastic carcinoma. (c) Scatterplot for Spearman coefficient of correlation between SOX-2 and OCT-4 in ameloblastic carcinoma. (d) Negative control slide with the absence of antibody immunoexpression (IHC ×100)
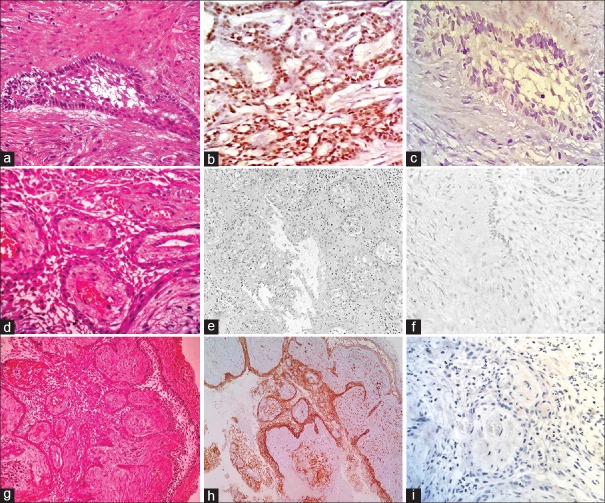
Figure 4.
Photomicrographs of stained control tissue sections for solid multicystic ameloblastoma (odontogenic epithelium from dental follicle, a-d). (a) Odontogenic epithelium overlying a dense fibrous stroma (H and E, ×400). (b) Low SOX-2 focal nuclear staining of basal layer (IHC ×400). (c) Negative staining for OCT-4 (IHC ×400). (d) High membrane positivity for CD44 (IHC ×400). Photomicrographs of stained control tissue sections for ameloblastic carcinoma (oral squamous cell carcinoma, e-h). (e) Malignant epithelial island with anaplasia (H and E, ×400). (f) High nuclear staining for SOX-2 in tumor cells (IHC ×400). (g) High nuclear staining for OCT-4 in tumor cells (IHC ×400). (h) Intermediate membrane positivity for CD44 in tumor cells (IHC ×400)
Discussion
Odontogenic tumors are a set of heterogeneous group of lesions present with different routes of origin. Some originate from the remnants of odontogenic epithelium that are laid down after the completion of tooth development while others develop from the malignant transformation of a cyst or tumor.[8] ACA is a rare entity that is challenging to diagnose, especially when spindle cells are present in a conventional SMA. In addition, there are no standardized microscopic criteria to distinguish ACA histopathologically from aggressive SMA.
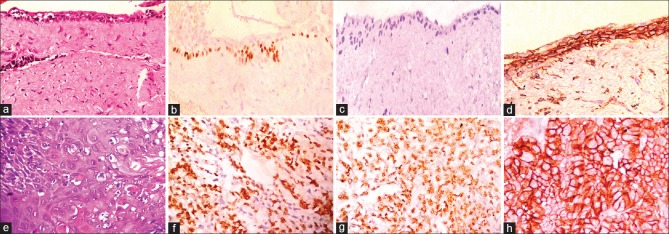
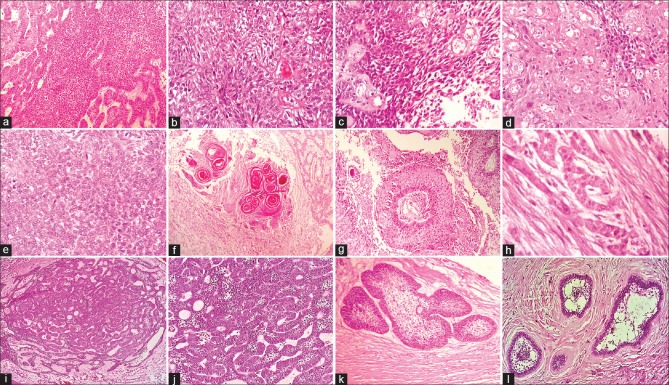
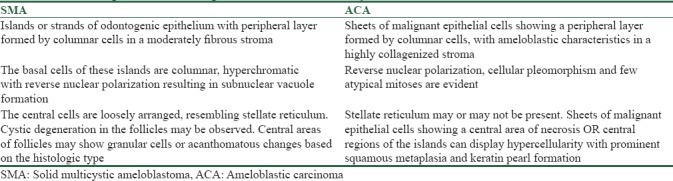
Distinguishing between ACA and aggressive SMA is challenging, as a single conclusive microscopic criterion for ACA is indefinable and the distinction from aggressive SMA is subjective. ACA has features of the conventional ameloblastoma in addition to the carcinomatous component. In ACA dysplastic features such as hypercellularity or basaloid differentiation of the stellate reticulum, loss of ameloblastic differentiation, nuclear pleomorphism, increased cytoplasmic ratio, nuclear hyperchromatism, and increased mitotic rate have been described.[9] In addition, infiltrative growth patterns, nuclear and vascular invasion and necrosis have also been employed to categorize ACA [Figure 5a–h]. SMA consists of islands or strands of odontogenic epithelium in a fibrous stroma. The basal cells of these islands are columnar, hyperchromatic with subnuclear vacuole formation, and central cells are loosely arranged, resembling stellate reticulum.[9] [Figure 5i–l]. Other features seen reflecting the malignant process are tumor necrosis, vascular, and neural invasion. Ghost cells, clear cells, calcifications, individual cell keratinization, keratin pearl formation, and melanin have also been reported in ACAs. The stroma is usually fibrous, inflamed with areas of hemorrhage and hemosiderin.[10] The histopathological features of SMA and ACA are summarized in Table 4. Conventional SMA sometimes presents with increased proliferation of cells with occasional budding of the follicles with basilar hyperplasia. Added to this, the presence of mild pleomorphism in a SMA adds to the diagnostic dilemma when differentiating it from ACA.
Figure 5.
Photomicrographs of ameloblastic carcinoma and solid multicystic ameloblastoma (H and E stain). (a) Sheet of proliferating cells (×100). (b) Odontogenic follicle with few atypical cells (×400). (c) Dysplastic features (×400). (d) Malignant cells and areas of dyskeratosis (×100). (e) Round cells and ovoid cells (×400). (f) Keratin pearl with ameloblastic differentiation (×100). (g) Odontogenic follicle with central necrosis and retraction artifact (×100). (h) Perineural invasion (×400). (i) Plexiform pattern with central follicular areas (×100). (j) Columnar cells with reversal of polarity (×400). (k) Odontogenic follicle with central areas of stellate reticulum (×100). (l) Odontogenic follicle with stellate reticulum and small areas of squamous metaplasia (×100)
Table 4.
Comparison of histologic features of solid multicystic ameloblastoma and ameloblastic carcinoma
The present study was performed to resolve the diagnostic dilemma in differentiating ACA and SMA by determining a novel immunohistochemistry (IHC) marker that reliably stains ACA. IHC aids in the analysis of abnormal cells such as those frequently found in cancerous tumors. The assessment of cellular activity by IHC analysis has become an essential tool to provide useful information about the biologic behavior of several tumors.[11] IHC is relatively economical and the procedure is easy to perform with reproducible results. The need of the hour is to adopt reliable biomarkers that can differentiate ACA from aggressive SMA.
CSC markers SOX-2 and OCT-4 are two major transcription factors, which are required to maintain the pluripotent and self-renewal capacity. SOX-2 is a cancer stem marker that promotes ectodermal development, which gives rise to odontogenic epithelium.[12] In squamous cell carcinoma, gene amplifications frequently target the 3q26.3 region. The gene for SOX-2 lies within this region, which effectively characterizes SOX-2 as an oncogene. Its overexpression also activates cellular migration and anchorage-independent growth.[13] Two recent studies have found that SOX-2 is expressed in dental lamina and is involved in the renewal of all epithelial cell lineages of tooth development, including ameloblasts in mouse models.[14,15] OCT-4 (also known as POU5F1) an octamer-binding transcription factor 4 is a key regulator of self-renewal in embryonic stem cells. It is frequently used as a marker for undifferentiated cells.[16,17] OCT-4 has been implicated in tumorigenesis of adult germ cells. Dysplastic lesions of the skin and intestine in adult mice have been strongly related to ectopic OCT-4 expression.[18] The CD44 gene is unique for all the various isoforms of the protein. CD44 is expressed in a wide variety of cell types, including hematopoietic cells, fibroblasts, macrophages, epithelial cells, muscle cells, and glial cells. CD44 functions as a principal receptor for hyaluronic acid, a major glycosaminoglycan of the extracellular matrix.[19] Altered CD44 expression has been detected in various neoplasms and is thought to play a part in tumor cell differentiation, progression, and metastasis.[20,21,22,23]
In the present study, we employed CSC markers SOX-2, OCT-4 and CD44 in 20 cases each of ACA and aggressive SMA respectively. ACA showed strong positive expression for both SOX-2 and OCT-4 markers in its tumor component in comparison with SMA with a statistically significant result (P < 0.001) as tested by Mann-Whitney U-test. Only 3 cases of SMA were positive for SOX-2 while 17 cases were negative. Lack of OCT-4 immunoexpression was observed in all 20 cases of SMA. The expression of CD44 in ACA was statistically nonsignificant when compared to SMA (P < 0.077). SOX-2 and OCT-4 were found to be independent and highly accurate markers for ACA. SOX-2 and OCT-4 expression in ACA showed a significant correlation coefficient of 0.616 at (P < 0.004).
In the past proliferative markers in a limited number of ACA's have been used to determine differences between ACA and SMA. Martínez-Martínez et al., 2017 conducted a study on 15 cases of SMA and 7 cases of ACA and stated that Ki-67, p53, and p63 expression was higher in ACA as compared to SMA, suggesting that these markers can be useful when considering diagnosis of malignancy.[24] In another study, Loyola et al., 2016 studied the IHC features of 17 ACA and found that ACA showed increased immunoexpression of CK18, CK19, p16, p53, and Ki-67 as compared to benign cases.[25] Yoon et al., 2011 assessed the expression of CK7, CK14, CK18, CK19, MMP-2, and Ki-67 in 10 cases of ameloblastoma and 7 cases ACA. A statistically significant expression of CK18, parenchymal MMP-2, stromal MMP-9, and Ki-67 was found between ACA and ameloblastoma.[26]
The current study has employed 3 CSC markers to characterize the differences between ACA and SMA. Few studies have been reported in literature evaluating CSC markers in odontogenic lesions. Banerjee et al., 2016 reported the immunoexpression of stem cell markers OCT-4 and SOX-2 in the odontogenic lesions and stated that SOX-2 and OCT-4 serve as reliable markers for identifying stem cell population.[27] The results of SOX-2 expression of ACA and SMA in the present study was in accordance with Lei et al., 2014 who assessed the immunoexpression of SOX-2 in ameloblastoma, aggressive ameloblastoma, and ACA and stated that diffuse nuclear staining pattern of SOX-2 is suggestive of a high-grade process in ameloblastic neoplasms. SOX-2 was diffusely positive in ACA tumor aggregates.[28]
A negative SOX-2 expression of 17 cases (85%) of SMA and lack of OCT-4 expression was observed in the present study. This was in accordance with the findings of Bandyopadhyay et al., 2017 who evaluated SOX-2 and OCT-4 in SMA and keratocystic odontogenic tumor (KCOT). The results reported that no OCT-4 positivity was found in SMA or KCOT. However, SMA showed SOX-2 negativity while KCOT showed high SOX-2 expression.[29]
OCT-4 is a rarely explored marker in odontogenic lesions that has been recently employed in other head and neck squamous cell carcinomas (HNSCCs). Reers et al., 2014 stated that Oct-4A isoform is a marker of stemness in HNSCC and plays a pivotal role in the detection of cancer cells with enhanced chemoresistance.[30] OCT-4A provides evidence for the existence of cancer stem-like cells in HNSCC. Ge et al., 2010 studied the expression of OCT-4 and SOX-2 in hypopharyngeal squamous cell carcinoma and reported that OCT-4 and SOX-2 might play an important role in carcinogenesis and tumor progression and may be used as an indicator of the patient prognosis.[6] In the current study, 17 cases (85%) out of 20 showed a positive nuclear immunoreactivity for OCT-4 and only 3 cases (15%) were negative. Results of the present study showed that SOX-2 and OCT-4 are independent, accurate markers for ACA with high coefficient of correlation.
Among the 20 cases of SMA considered in the current study, positive immunoreactivity was observed in 14 cases (70%) for CD44 while 6 cases (30%) were negative. These results were similar to the study conducted by Sathi et al., 2012 who analyzed the expression of CSC markers CD133, CD44, and ABCG2 along with proliferation marker Ki-67 in 23 ameloblastomas and found that all three candidate CSC markers were expressed in ameloblastoma and are possibly involved in cell proliferation, tumor progression, and recurrence.[31]
Srinath et al., 2014 analyzed the expression of CD44 in ameloblastoma, dentigerous cyst and radicular cyst and found that CD44 was widely expressed in tumor cells of ameloblastoma. The authors concluded that IHC staining of odontogenic lesions for CD44s may be useful in detecting the active cells in the odontogenic lesions to predict the tumor progression.[7] There are no reported studies in literature that have analyzed CD44 in ACA using a large sample size. The 20 ACA cases considered in the present study showed a positive membrane immunoreactivity for CD44 in 16 cases (80%) while 4 cases (20%) were negative. No significant difference between ACA and SMA was found involving CD44.
Pattern of expression of SOX-2 and OCT-4 was in accordance with its biologic function as a transcription factor. Diffuse and strong nuclear staining in the tumor islands of ACA for SOX-2 and OCT-4 were observed, staining was predominantly weak in the central areas. Only 3 cases of SMA showed low positivity for SOX-2 in the basal and parabasal cells of the follicles, this pattern was in accordance with Juuri et al., 2013 where immunostaining showed that SOX-2 protein was expressed in the majority of the preameloblast-like cells and central cells of SMA. In control tissues (OSCC), high nuclear positivity was observed for SOX-2 and OCT-4. Focal nuclear positivity of the basal layer of 2 OEDF control tissues showed low SOX-2 expression. SOX-2 has been previously localized in the dental lamina of developing human primary molars.[32] Low membrane expression of CD44 was observed in basal, suprabasal layers and small tumor follicles of ACA, whereas SMA showed intermediate to high membrane expression in several cases. Intermediate CD44 membrane expression was seen in control tissue of ACA (OSCC) whereas a high and uniform CD44 membrane expression was observed in control tissue of SMA (OEDF). Usually, it is expressed strongly in dental epithelial derivatives including dental lamina, inner dental epithelium and ameloblasts.[7]
The results of the present study confirm that SOX-2 and OCT-4 are useful markers to distinguish ACA and aggressive SMA. The present study has contributed in resolving the diagnostic dilemma between ACA and aggressive SMA which is significant in the current global scenario where such dilemmas are often encountered. Evaluation of these markers to differentiate other ambiguous benign and malignant tumors of the head and neck is an interesting prospect to investigate.
Conclusions
Few studies in literature have attempted to resolve the diagnostic dilemma between ACA and SMA, either analyzing only few cases of ACA or with a limitation of a single marker. The highlight of the present study is that 20 cases each of ACA and SMA were analyzed immunohistochemically with control tissue (OSCC and OEDF) in addition to the usage of 3 CSC markers. SOX-2 and OCT-4 can be employed as independent markers to help differentiate aggressive SMA and ACA with high accuracy. This study advocates the use of IHC markers that may help in early diagnosis of malignancies, resulting in better prognosis and survival rate of patients.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgement
The authors thank Dr. Puneet Gupta for statistical analysis of data.
References
- 1.Slater LJ. Odontogenic malignancies. Oral Maxillofac Surg Clin North Am. 2004;16:409–24. doi: 10.1016/j.coms.2004.03.009. [DOI] [PubMed] [Google Scholar]
- 2.Jiang C, Xu Q, Zhang Q, Wang S, Carrasco LR, Le AD. Benign odontogenic tumors: Origins, immunophenotypic features, and genetic alterations. Curr Oral Health Rep. 2016;3:93–101. [Google Scholar]
- 3.Benlyazid A, Lacroix-Triki M, Aziza R, Gomez-Brouchet A, Guichard M, Sarini J. Ameloblastic carcinoma of the maxilla: Case report and review of the literature. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2007;104:e17–24. doi: 10.1016/j.tripleo.2007.05.026. [DOI] [PubMed] [Google Scholar]
- 4.Yoon HJ, Hong SP, Lee JI, Lee SS, Hong SD. Ameloblastic carcinoma: An analysis of 6 cases with review of the literature. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2009;108:904–13. doi: 10.1016/j.tripleo.2009.06.045. [DOI] [PubMed] [Google Scholar]
- 5.Cheng L, Alexander R, Zhang S, Pan CX, MacLennan GT, Lopez-Beltran A, et al. The clinical and therapeutic implications of cancer stem cell biology. Expert Rev Anticancer Ther. 2011;11:1131–43. doi: 10.1586/era.11.82. [DOI] [PubMed] [Google Scholar]
- 6.Ge N, Lin HX, Xiao XS, Guo L, Xu HM, Wang X, et al. Prognostic significance of Oct4 and So×2 expression in hypopharyngeal squamous cell carcinoma. J Transl Med. 2010;8:94. doi: 10.1186/1479-5876-8-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Srinath S, Iyengar A, Mysorekar V. CD 44 expression in dentigerous cyst, radicular cyst and ameloblastoma, by immunohistochemical analysis. IOSR J Dent Med Sci. 2014;13:80–3. [Google Scholar]
- 8.Imran A, Jayanthi P, Tanveer S, Gobu SC. Classification of odontogenic cysts and tumors – Antecedents. J Oral Maxillofac Pathol. 2016;20:269–71. doi: 10.4103/0973-029X.185935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kumaran PS, Anuradha V, Gokkulakrishnan S, Thambiah L, Jagadish AK, Satheesh G. Ameloblastic carcinoma: A case series. J Pharm Bioallied Sci. 2014;6:S208–11. doi: 10.4103/0975-7406.137473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reichart PA, Philipsen HP. Odontogenic Tumors and Allied Lesions. Hanover, Germany: Quintessence Publishing; 2004. [Google Scholar]
- 11.Hunter KD, Speight PM. The diagnostic usefulness of immunohistochemistry for odontogenic lesions. Head Neck Pathol. 2014;8:392–9. doi: 10.1007/s12105-014-0582-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Luo W, Li S, Peng B, Ye Y, Deng X, Yao K. Embryonic stem cells markers SOX2, OCT4 and Nanog expression and their correlations with epithelial-mesenchymal transition in nasopharyngeal carcinoma. PLoS One. 2013;8:e56324. doi: 10.1371/journal.pone.0056324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hussenet T, Dali S, Exinger J, Monga B, Jost B, Dembelé D, et al. SOX2 is an oncogene activated by recurrent 3q26.3 amplifications in human lung squamous cell carcinomas. PLoS One. 2010;5:e8960. doi: 10.1371/journal.pone.0008960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Juuri E, Saito K, Ahtiainen L, Seidel K, Tummers M, Hochedlinger K, et al. Sox2+ stem cells contribute to all epithelial lineages of the tooth via Sfrp5+ progenitors. Dev Cell. 2012;23:317–28. doi: 10.1016/j.devcel.2012.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Juuri E, Jussila M, Seidel K, Holmes S, Wu P, Richman J, et al. Sox2 marks epithelial competence to generate teeth in mammals and reptiles. Development. 2013;140:1424–32. doi: 10.1242/dev.089599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zeineddine D, Hammoud AA, Mortada M, Boeuf H. The Oct4 protein: More than a magic stemness marker. Am J Stem Cells. 2014;3:74–82. [PMC free article] [PubMed] [Google Scholar]
- 17.Niwa H, Miyazaki J, Smith AG. Quantitative expression of Oct-3/4 defines differentiation, dedifferentiation or self-renewal of ES cells. Nat Genet. 2000;24:372–6. doi: 10.1038/74199. [DOI] [PubMed] [Google Scholar]
- 18.Hochedlinger K, Yamada Y, Beard C, Jaenisch R. Ectopic expression of Oct-4 blocks progenitor-cell differentiation and causes dysplasia in epithelial tissues. Cell. 2005;121:465–77. doi: 10.1016/j.cell.2005.02.018. [DOI] [PubMed] [Google Scholar]
- 19.Jiang WG, Sanders AJ, Katoh M, Ungefroren H, Gieseler F, Prince M, et al. Tissue invasion and metastasis: Molecular, biological and clinical perspectives Semin Cancer Biol? 2015 Dec;35(Suppl):S244–S275. doi: 10.1016/j.semcancer.2015.03.008. doi: 10.1016/j.semcancer.2015.03.008. Epub 2015 Apr 10. [DOI] [PubMed] [Google Scholar]
- 20.Mao M, Zheng X, Jin B, Zhang F, Zhu L, Cui L. Effects of CD44 and E-cadherin overexpression on the proliferation, adhesion and invasion of ovarian cancer cells. Exp Ther Med. 2017;14:5557–63. doi: 10.3892/etm.2017.5259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Basakran NS. CD44 as a potential diagnostic tumor marker. Saudi Med J. 2015;36:273–9. doi: 10.15537/smj.2015.3.9622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Olsson E, Honeth G, Bendahl PO, Saal LH, Gruvberger-Saal S, Ringnér M, et al. CD44 isoforms are heterogeneously expressed in breast cancer and correlate with tumor subtypes and cancer stem cell markers. BMC Cancer. 2011;11:418. doi: 10.1186/1471-2407-11-418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fan Z, Li M, Chen X, Wang J, Liang X, Wang H, et al. Prognostic value of cancer stem cell markers in head and neck squamous cell carcinoma: A meta-analysis. Sci Rep. 2017;7:43008. doi: 10.1038/srep43008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Martínez-Martínez M, Mosqueda-Taylor A, Carlos-Bregni R, Pires FR, Delgado-Azanero W, Neves-Silva R, et al. Comparative histological and immunohistochemical study of ameloblastomas and ameloblastic carcinomas. Med Oral Patol Oral Cir Bucal. 2017;22:e324–32. doi: 10.4317/medoral.21901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Loyola AM, Cardoso SV, de Faria PR, Servato JP, Eisenberg AL, Dias FL, et al. Ameloblastic carcinoma: A Brazilian collaborative study of 17 cases. Histopathology. 2016;69:687–701. doi: 10.1111/his.12995. [DOI] [PubMed] [Google Scholar]
- 26.Yoon HJ, Jo BC, Shin WJ, Cho YA, Lee JI, Hong SP, et al. Comparative immunohistochemical study of ameloblastoma and ameloblastic carcinoma. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2011;112:767–76. doi: 10.1016/j.tripleo.2011.06.036. [DOI] [PubMed] [Google Scholar]
- 27.Banerjee A, Kamath VV, Sundaram L, Krishnamurthy SS. OCT4 and SOX2 are reliable markers in detecting stem cells in odontogenic lesions. J Orofac Sci. 2016;8:16–21. [Google Scholar]
- 28.Lei Y, Jaradat JM, Owosho A, Adebiyi KE, Lybrand KS, Neville BW, et al. Evaluation of SOX2 as a potential marker for ameloblastic carcinoma. Oral Surg Oral Med Oral Pathol Oral Radiol. 2014;117:608–160. doi: 10.1016/j.oooo.2014.01.017. [DOI] [PubMed] [Google Scholar]
- 29.Bandyopadhyay A, Nishat R, Behura SS, Panda A, Ramachandra S, Mohiddin G. Cancer stem cell markers, SO×2 and OCT 4 in ameloblastoma and keratocystic odontogenic tumor: An immunohistochemical study. J Int Oral Health. 2017;9:28–32. [Google Scholar]
- 30.Reers S, Pfannerstill AC, Maushagen R, Pries R, Wollenberg B. Stem cell profiling in head and neck cancer reveals an Oct-4 expressing subpopulation with properties of chemoresistance. Oral Oncol. 2014;50:155–62. doi: 10.1016/j.oraloncology.2013.12.006. [DOI] [PubMed] [Google Scholar]
- 31.Sathi GA, Tamamura R, Tsujigiwa H, Katase N, Lefeuvre M, Siar CH, et al. Analysis of immunoexpression of common cancer stem cell markers in ameloblastoma. Exp Ther Med. 2012;3:397–402. doi: 10.3892/etm.2011.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Juuri E, Isaksson S, Jussila M, Heikinheimo K, Thesleff I. Expression of the stem cell marker, SOX2, in ameloblastoma and dental epithelium. Eur J Oral Sci. 2013;121:509–16. doi: 10.1111/eos.12095. [DOI] [PubMed] [Google Scholar]