Abstract
Our episodic memory stores what happened when and where in life. Episodic memory requires the rapid formation and flexible retrieval of where things are located in space. Consciousness of the encoding scene is considered crucial for episodic memory formation. Here, we question the necessity of consciousness and hypothesize that humans can form unconscious episodic memories. Participants were presented with subliminal scenes, that is, scenes invisible to the conscious mind. The scenes displayed objects at certain locations for participants to form unconscious object‐in‐space memories. Later, the same scenes were presented supraliminally, that is, visibly, for retrieval testing. Scenes were presented absent the objects and rotated by 90°–270° in perspective to assess the representational flexibility of unconsciously formed memories. During the test phase, participants performed a forced‐choice task that required them to place an object in one of two highlighted scene locations and their eye movements were recorded. Evaluation of the eye tracking data revealed that participants remembered object locations unconsciously, irrespective of changes in viewing perspective. This effect of gaze was related to correct placements of objects in scenes, and an intuitive decision style was necessary for unconscious memories to influence intentional behavior to a significant degree. We conclude that conscious perception is not mandatory for spatial episodic memory formation.
Keywords: allocentric, consciousness, episodic memory, long‐term retention, unconscious
1. INTRODUCTION
Fleeting daily experiences are memorable due to episodic memory (Tulving, 1972, 2002). Episodic memory enables us to encode on the fly and to retain for the long‐term what happened where and when in life. The “what” and “where” components of episodic memory are important because they permit us to keep track of changes in the environment. If we misplace our mobile phone, we probably failed to form or reactivate the memory of putting the phone in a certain place. The neuro‐cognitive computations that underlie episodic memory formation are speed of encoding, association formation, and flexible memory representation (Cohen & Eichenbaum, 1993; Henke, 2010; McClelland, McNaughton, & O'Reilly, 1995; Moses & Ryan, 2006; Norman & O'Reilly, 2003; O'Reilly & Rudy, 2000; Treves & Rolls, 1994). A flexible memory representation can be reactivated in retrieval situations that diverge from the encoding situation, for example, when entering the space where we left the phone from another direction. According to a long‐held view, there is a further characteristic believed requisite for human episodic memory formation, namely conscious awareness of the encoding situation (Gabrieli, 1998; Graf & Schacter, 1985; Moscovitch, 1995, 2008; Schacter, 1998; Squire & Dede, 2015; Tulving, 2002). A major problem with this view is that it is exclusively based on the results from encoding protocols that use stimulus materials available to conscious perception. The only way this view can be tested is by having human subjects encode stimulus materials presented below the threshold of conscious awareness (i.e., subliminally) or by having unconscious subjects encode supraliminal stimulus material while asleep, under anesthesia, or in coma. Here, we opted for a subliminal stimulation protocol. Only few studies used subliminal stimulus presentations to test for an unconscious formation of long‐term memories. The feasibility of subliminal single word encoding and later subliminal recognition of the same words was demonstrated by (Chong, Husain, & Rosenthal, 2014). In addition, we have shown that the rapid subliminal encoding and flexible representation of face–word and word–word pairs is feasible and that the hippocampus is crucial for unconscious relational encoding and retrieval (Duss, Oggier, Reber, & Henke, 2011; Duss et al., 2014; Reber, Luechinger, Boesiger, & Henke, 2012). These earlier protocols lacked the spatial component of episodic memory. In fact, prominent theories propose that spatial processing is the most fundamental property of episodic memory (Burgess, Maguire, & O'Keefe, 2002; Maguire & Mullally, 2013; O'Keefe & Nadel, 1978). According to the cognitive map theory (O'Keefe & Nadel, 1978), the function of the hippocampus is to generate internal maps of the environment, to represent places and objects and their relations to each other in the multidimensional space in an allocentric manner. As such, cognitive maps provide the basis for spatial memory and flexible navigation, which in turn provides for the contextual details and representational flexibility of episodic memories (Burgess et al., 2002). Here, we decided to test whether consciousness is mandatory for (a) rapid spatial relational encoding of subliminal objects in scenes and (b) whether the object‐in‐space representation is invariant to perspective change (allocentric representation). This design allows us to extend previous studies on rapid unconscious learning of flexible associations that were confined to sensory and conceptual relations.
Participants were subliminally presented with a series of subliminal 3D scenes, equipped with peripheral landmarks for orientation and objects located at specific locations. Following a silent 3 min encoding‐test interval, the subliminal scenes were presented supraliminally, absent the critical objects and rotated in perspective by 0°–270°. Unconscious memory for object‐in‐space locations was evaluated with two implicit tests: (1) free viewing of a test scene for 6 s subsequent to presentation of a subliminal object cue, followed by (2) a forced‐choice location selection task for the critical (now supraliminally presented) object. During the free viewing task, the eye tracking index of successful reactivation of object‐in‐space associations was disproportionate viewing of the location formerly occupied by the subliminal object cue. Based on previous evidence of memory‐guided disproportionate viewing (Hannula & Ranganath, 2009; Hannula, Ryan, Tranel, & Cohen, 2007), we anticipated an early disproportionate viewing effect, that is, within 500–750 ms of scene onset. For the location selection task, participants were instructed to decide to which of two highlighted scene locations the object would fit better, as intuitively as possible. Here, the index of successful reactivation of object‐in‐space associations was a disproportionate selection of the object's former subliminal scene location. We encouraged participants to rely on their “gut‐feeling” when making their decisions because conscious selection strategies and intentional deliberation may affect accuracy of decision performance when memory traces are weak or implicit (Reber, Beeman, & Paller, 2013; Voss & Paller, 2010). However, data from a pilot study with the same design showed that not all participants could adopt such an intuitive decision strategy easily, and that a general preference for deliberative decision making was negatively associated with performance on the forced‐choice task (see Supporting Information). To account for this, we assessed participant's general decision making preference before experimentation. We divided participants into two groups, namely a group of intuitive decision makers and a group of deliberative decision makers. Based on the findings of the mentioned pilot experiment (Supporting Information), we expected to find in this study expressions of unconscious object‐location knowledge in the choice behavior of intuitive decision makers.
For the free viewing task, we expected that eye movements in the eye‐movement‐based implicit test would be sensitive to object‐in‐place memory for all participants. Gaze might be a more sensitive measure of unconscious memory than intentional button‐press responses because we move our eyes spontaneously in the presence of visual stimuli; consequently, eye movements may be less susceptible to conscious selection strategies and intentional deliberation, particularly when participants are simply instructed to view materials that are being presented (Hannula et al., 2010a; Hannula, Baym, Warren, & Cohen, 2012; Ryan, Althoff, Whitlow, & Cohen, 2000). In contrast, for the location selection task, we expected only the intuitive group to show above chance performance.
2. MATERIALS AND METHODS
2.1. Participants
Sixty‐four university students (23.4 (mean) ± 4.5 (SD) years old; 52 women) took part either for course credit or for 25 €. They were naïve to the purpose of the experiment and to subliminal stimulation. Therefore, only semi‐informed consent was obtained before experimentation, but a full debriefing followed experimentation. The experiment was approved by the local ethics committee. We recruited and assigned participants to one of two experimental groups after they had filled out the online PID (Preference for Intuition and Deliberation Scales; Betsch, 2004). Recruitment was continued until 32 participants were assigned to the deliberative decision maker's group and 32 to the intuitive decision maker's group. A total of six participants were excluded due to technical problems (eye tracking or experimental stimulus presentation); they were replaced.
2.2. Experimental design
The experiment started with a practice run. Four experimental runs followed, each containing a new scene and new objects. The experiment ended with the two awareness tests and debriefing.
An experimental run always started with two preparation tasks to familiarize participants with the 3D‐scene. Scenes featured large rooms with paintings, windows, doors, or plants at the walls, which acted as orientation cues, and contained a large platform with four elevated positions in the middle of the room. Then an attention task followed, where subliminal encoding of the objects in scene took place. The objects were presented at specified positions on the platform. Two objects were located at these positions in an encoding trial, but the location memory of only one object was later tested. After a short break (3 min consolidation period), the experimental run ended with the implicit retrieval tests (free view and forced choice task), where the location memory of the previously presented objects was tested (Figure 1).
Figure 1.
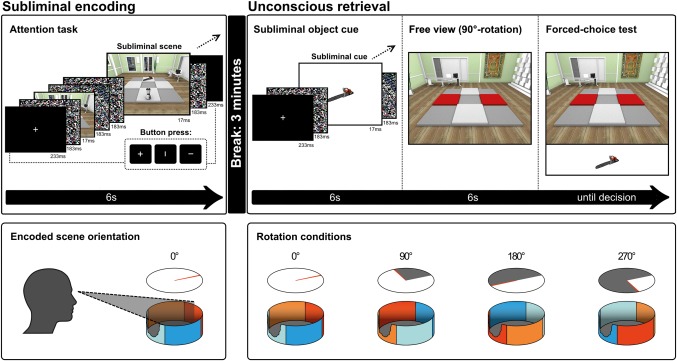
Experimental design. Upper half: Displayed is one example of a subliminal encoding trial and its corresponding retrieval trial. Each experimental run consisted of 6 consecutive encoding trials, a consolidation period of 3 min, and the corresponding 6 consecutive retrieval trials. Scenes were rotated between encoding and retrieval (0°, 90°, 180°, or 270°) with a constant rotation angle per run. Displayed is a 90°‐rotation. For subliminal encoding, a scene with two central objects was presented for 17 ms, flanked by pattern masks, in 12 repetitions in a time window of 6 s. Participants performed an attention task during subliminal encoding, which required them to fixate the center of the screen and to indicate the orientation of a line segment. There were two indirect retrieval tests of object–location associations. For the first test, a single object cue was presented subliminally (12 repetitions) to trigger the reactivation of object–location associations. Then, the scene came up visibly (“free view”), absent the objects, with the two previously occupied locations highlighted. Participants viewed the scene over 6 s for eye movement measurements. For the second (“forced‐choice”) test, the same object cue was presented visibly below the scene to trigger the reactivation of object–location associations. Participants indicated which highlighted location was a better fit for that object. Lower half: Illustration of scene rotation between subliminal encoding and implicit retrieval tasks. Scene perspective during subliminal encoding was always constant (0° rotation). For the implicit retrieval tasks, perspective on the scene could remain the same as during encoding (0°) or rotate for 90°, 180°, or 270°. This perspective was held constant throughout the six retrieval trials for a given scene [Color figure can be viewed at http://wileyonlinelibrary.com]
Each of the four experimental runs included six subliminal encoding and retrieval trials (=24 trials in total). The four runs corresponded to the four rotation conditions (i.e., perspective change, in degrees, during retrieval): 0°, 90°, 180°, and 270°. Hence, a given rotation angle was maintained throughout the six retrieval trials of a run. Scenes were also maintained throughout the trials of a run/rotation condition. At encoding, all scenes were presented at the default rotation angle of 0°. For retrieval testing, scenes were rotated by 0° (unchanged perspective), 90°, 180°, or 270° according to condition. Scenes were counterbalanced over rotation conditions across participants. Objects were trial‐unique, that is, unambiguously associated with a given location in a given scene.
2.3. Procedure
Preparation for each run: To familiarize participants with the layout of the scenes in advance of subliminal object‐in‐space encoding, participants watched a video of the upcoming scene, slowly rotating around its central axis. The video revealed all the landmarks in the periphery of the scene and participants were instructed to remember these orientation cues (landmarks), and memory for these orientation cues was tested afterward. This procedure ensured that participants had a sufficient mental representation of the scene and its landmarks from different viewing angles. The next training procedure was used to establish a task‐set—i.e., a computational routine for subliminal encoding (Kiefer & Martens, 2010; Reber & Henke, 2011). Participants needed to remember the position of two abstract figures presented at the four elevated positions at the center of the scene from different viewing angles (0°, 90°, 180°, and 270°), as in the later experimental task. These two preparatory tasks were conducted in advance of each experimental run because each run featured a distinct room, which participants needed to acquire a mental representation of.
Subliminal encoding: The masking procedure followed an established protocol (Reber et al., 2012; see Supporting Information). Participants were kept ignorant of subliminal images. During subliminal encoding, participants engaged in an attention task that required them to focus gaze on a fixation cross located at the screen center. This fixation cross was periodically presented between pattern masks and subliminal stimuli. It switched to a line segment in a subset of trials. Participants were instructed to indicate the orientation of the line segment by button press. The six subliminal encoding trials of an experimental run contained the scene, which participants had been familiarized to, including two objects that were located on demarcated positions near the center of the room. A subliminal encoding trial consisted of twelve 17 ms flashes of a particular object‐in‐scene configuration. After the six subliminal encoding trials, participants rested 3 min before retrieval testing followed.
Unconscious retrieval: The six encoding trials resulted in six retrieval trials. Each retrieval trial consisted of two implicit retrieval procedures: the first procedure was a gaze‐based unintentional retrieval test and the second was an intentional forced‐choice test requiring voluntary button‐press responses. For both implicit retrieval tests, the scene was presented supraliminally with two (of four) locations highlighted. These were the positions occupied by the two objects in a given encoding trial. Location knowledge of only one of the two encoding objects was tested. For the gaze‐based test, the object in question was flashed subliminally (12 repetitions) as an implicit retrieval cue just before the scene was presented. Participants were instructed to simply view the scene for 6 s. No additional instructions were given and no emphasis was put on viewing the highlighted locations. We hypothesized that the subliminal object cue would trigger an unconscious reactivation of object‐in‐space associations, which in turn would direct gaze to the location that had previously been occupied by the object. The forced‐choice test followed. At this point, the scene with the two highlighted locations remained on the screen with the object cue visibly displayed below the scene. Participants were instructed to spontaneously place the object where it fits better. Unconscious location memory was expected to bias choices to the location where that object was previously presented during subliminal encoding.
Awareness test: Following the experiment, we conducted two forced‐choice tests that measured stimulus awareness to validate the subliminal nature of stimulus presentations. The first test was an awareness test for scenes to validate subliminal presentations in the encoding part of the experiment. The second test was an awareness test for single objects to validate subliminal object presentations in the retrieval part of the experiment. This sequence of tests was strictly maintained. Both tests contained 24 trials each and followed a trial‐by‐trial procedure with subliminal encoding immediately followed by the respective forced‐choice test. Subliminal encoding protocols and number of stimulus presentations were exactly the same as in the main experiment. In the awareness test for scenes, participants were instructed to identify the two objects and their locations within a subliminal encoding trial. Then the scene was shown supraliminally, with the two locations highlighted, and one object was presented below the scene. Participants had to decide on which location the object had been presented subliminally. For this awareness test, four new scenes were used with six new object pairs each, and no perspective change occurred between encoding and retrieval. The awareness test for single objects consisted of single objects instead of scenes. Participants were instructed to identify the presented object within a subliminal encoding trial. Then two objects were shown supraliminally, and participants had to decide which of the two objects had been presented before. Target objects in this awareness test stemmed from the experiment; they consisted of the subliminally encoded but then not retrieval‐tested objects. The foil objects were new, that is, not used in the experiment.
2.4. Stimuli and counterbalancing
Scenes were rendered colored images of 3D‐models of nine distinct rooms (four rooms for the experiment, four rooms for the awareness test, and one room for practice trials). Scenes were limited by four sides. Each side was demarcated by at least two landmarks. Scene centers were equipped with a grey platform, on top of which four white elevated positions marked the four spots where encoding objects could be located. To equip rooms with objects for object‐in‐space encoding, we selected 136 distinct and easily recognizable images of common objects. One room and 16 objects were used for practice trials, four rooms and 48 objects were used for the experiment, another four rooms and 48 objects were used in the awareness test for scenes, and 24 objects were used as foils in the awareness test for single objects. Objects were randomly assigned to rooms, randomly paired and assigned to trials, and randomly assigned to positions in a room. Each of the four possible positions was occupied twice by an object across trials. Rendered images of scenes served as subliminal encoding stimuli and objects rendered without a background served as subliminal retrieval cues and as subliminal targets in the object awareness test. We counterbalanced the following over participants: The two sets of four rooms were counterbalanced between the experiment and the awareness test, retention testing was done for each of the two objects presented in a subliminal room an equal number of times, and positions of objects in rooms were swapped such that a specific object location was correct for only half of participants. Moreover, the order of rooms and the order of rotation conditions (runs) were distributed over participants according to a 4 × 4 Latin square. This procedure resulted in 32 experimental schemes; 32 was the number of participants in the deliberative decision makers’ group and the intuitive decision makers’ group.
2.5. Apparatus
We recorded binocular eye tracking data at 250 Hz with a head mounted video‐based eye tracker (Eyelink II system, SR Research) using a chinrest to avoid extensive head movement. Stimuli were presented on a screen at a distance of 120 cm using a DLP projector (60 Hz refresh rate) in a darkened laboratory. Subliminal stimuli had a resolution of 680 × 510 pixels and spanned a visual angle of 17° width and 12.75° height. Stimulus presentation and response logging were programmed with the Experiment Builder software (SR Research). Responses were logged using a software compatible Microsoft USB Sidewinder gamepad.
2.6. Eye movement calibration and data analysis
A nine‐point calibration procedure was performed prior to each experimental run. A short drift correction was performed after subliminal cueing at the beginning of each retrieval trial. Eye movement data were analyzed using the Data Viewer software (SR Research). Fixations were all those events that were not classified as saccades (detection thresholds: 0.15° motion, 30°/s velocity, and 8,000°/s2) or blinks (missing pupil). Regions of interest were the surface of the two highlighted locations in the test scene. A highlighted location subtended a visual angle between 2.15° and 3.5° horizontally and 0.85° and 2.6° vertically. Proportion of dwell time on the cued and uncued location was analyzed over the time course of 6 s because time course analyses reveal more detailed information about memory‐related eye movement effects (Hannula et al., 2007). Eye movement data was segmented into time bins of 250 ms. Total fixation time was computed per time bin (i.e., fixations on the stimulus display with blinks and saccades removed). Proportion of dwell time was the time spent fixating the cued or uncued location divided by total fixation time per time bin.
3. RESULTS
3.1. Early disproportionate viewing of the uncued location
To find out whether participants’ gaze would linger longer on the cued than the uncued object location during the free viewing time window of 6 s, we computed a between‐subjects repeated‐measures ANOVA. The within‐subjects factors were location (cued/uncued) and rotation angle (0°/90°/180°/270°) and the between‐subjects factor was decision style (intuitive/deliberative). The ANOVA was computed for the time window of 500–750 ms following display onset because this was the time window in which first disproportionate viewing effects due to memory have been reported (Hannula & Ranganath, 2009; Hannula et al., 2007). There was a main effect of location (F(1,62) = 7.2, p = .009, = 0.1) with a longer dwell time on the uncued than the cued location (cued location: M = 8.43%, SE = 0.62%; uncued location: M = 10.45%, SE = 0.87%). There were no significant results for rotation angle, decision style, or any interactions between factors (all p > .63). To test whether this viewing difference was maintained over the whole time window of 6 s, we computed the above ANOVA with the additional within‐subjects factor time (6 s in 250 ms levels). A main effect of time (F(8,494) = 56.2, p < .001, = 0.48) emerged but neither a main effect of location (F(1,62) = 0.08, p = .78, = 0.01) nor any other significant effect (all p > .14). We conclude that differential viewing behavior appeared following display onset and vanished thereafter (Figure 2a). Further analyses revealed that this early differential viewing pattern was caused by the first fixation made after display onset: an ANOVA comparing the proportion of trials in which the first fixation was directed to either the cued or the uncued location confirmed that there was a significantly larger proportion of trials where the fixation was directed to the uncued location: F(1,62) = 10.8, p = .002, = 0.15; M cued = 13.5%, SE cued = 1.01%, M uncued = 17.9%, SE uncued = 1.14%). There were no significant differences for the factors rotation angle (p = .98) or group (all p = .68) nor for the interactions (all p > .14; descriptive statistics are shown in Table 1). Therefore, participants generally tended to spontaneously fixate the uncued location after stimulus onset.
Figure 2.
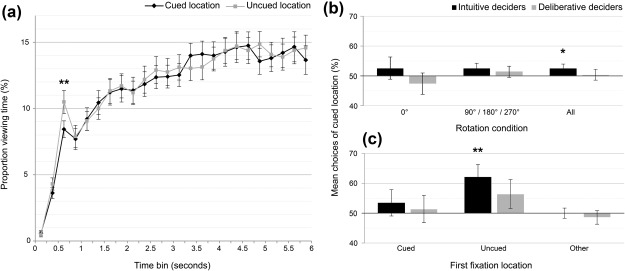
Results. (a) Proportion of viewing time directed to the cued or uncued location during the 6 s free viewing time window. More time was spent looking at the uncued location between 500 and 750 ms following scene onset. (b) Accuracy scores in the forced‐choice object placement task. Intuitive decision makers performed above chance level. (c) Accuracy scores (weighted means) plotted per first fixation condition. In retrieval trials where the first fixation during the free viewing time window went to the uncued object location, participants tended to select the correct location for the object cue later on; this effect reached statistical significance for intuitive decision makers only; this result was not influenced by rotation condition. *p < .05, one‐tailed; **p < .01, two‐tailed. Error bars represent standard errors of means
Table 1.
Descriptive statistics for target of first fixation during free view task
| Group | Location | 0° | 90° | 180° | 270° | Total |
|---|---|---|---|---|---|---|
| Intuitive | Cued | 14.1 (2.81) | 13.0 (2.77) | 12.0 (2.92) | 13.5 (2.17) | 13.2 (1.36) |
| Uncued | 17.7 (2.69) | 14.1 (2.6) | 20.3 (3.06) | 18.2 (2.74) | 17.6 (1.63) | |
| Deliberative | Cued | 12.5 (2.12) | 15.6 (2.99) | 16.1 (3.22) | 11.5 (2.3) | 13.9 (1.52) |
| Uncued | 20.3 (2.56) | 19.8 (2.3) | 13.5 (2.3) | 19.3 (2.6) | 18.2 (1.62) |
Note. Mean percentage of trials where the target of first fixation was the cued or the uncued location. Standard error in parenthesis. 0°, 90°, 180°, 270° = rotation conditions. Total = overall score.
3.2. Intuitive decision makers chose object‐cued location
Following the free viewing time window, the object cue was presented supraliminally underneath the scene for participants to spontaneously decide which of the two highlighted locations in the scene the presented object fits better to. We computed a repeated‐measures ANOVA with the within‐subjects factor rotation angle (0°, 90°, 180°, and 270°) and the between‐subjects factor decision style (intuitive or deliberative) to test the hypothesis derived from the outcome of the pilot experiment (Supporting Information) that intuitive decision makers choose the correct object location. This ANOVA yielded no significant differences (all p > .32; descriptive statistics in Table 2). Next, we tested directly whether the group of intuitive decision makers would perform above chance level given the finding of the pilot experiment indicating that intuitive decision makers tend to choose the correct object location. This pattern of results was indeed replicated: the intuitive decision makers’ performance (M = 52.6%, SE = 1.33%) exceeded chance level (t(31) = 1.95, p = .03 one‐tailed, d z = 0.35) (Figure 2b). Because the associated effect size of d z = 0.35 was smaller than the effect size obtained in the previous pilot experiment (d = 0.43), we added Bayesian statistics to evaluate the probability that the current effect size speaks for the null hypothesis. Bayes factors (i.e., the ratio of the likelihood of the data given one or the other hypothesis) are useful in determining the sensitivity with which obtained data distinguish between two hypotheses. Bayes statistics inform us whether the data support one over the other hypothesis, or are simply uninformative (Dienes, 2014). Here, we compared the relative evidence for the hypothesis that the group of intuitive decision makers yielded an effect of subliminal memory formation comparable to the effect obtained in the previous pilot experiment (4.2% correct choices above chance level) versus the hypothesis that this group performed at chance level (0%). Following the recommendations by Dienes (2014), we chose a half‐normal prior distribution with a mode of 0 and a standard deviation of 4.2% to compute the Bayes factor. The resulting Bayes factor was 3.32. According to conventions regarding the interpretation of the Bayes factor, values below 1/3 indicate substantial support for H0, while values >3 indicate substantial support for H1 (Jeffreys, 1939). Therefore, our current data clearly reject the H0 of chance performance and favor the hypothesis that the obtained effect size was comparable to the pilot study's effect size.
Table 2.
Descriptive statistics object placement task
| Group | N | 0° | 90° | 180° | 270° | Total |
|---|---|---|---|---|---|---|
| Intuitive | 32 | 52.6 (3.67) | 54.7 (3.2) | 50.5 (3.13) | 52.6 (3.67) | 52.6 (1.33) |
| Deliberative | 32 | 47.4 (3.52) | 54.7 (4.05) | 48.4 (2.93) | 51.0 (3.88) | 50.4 (1.78) |
Note. N = number of participants; 0°, 90°, 180°, 270° = rotation conditions.
Standard error in parenthesis.
Mean correct placements in percent.
There were no significant differences between rotation conditions: F(3,93) = 0.22, p = .88, = 0.01, suggesting that the overall effect was not driven solely by the 0° condition.
The group of deliberative decision makers chose the correct object location with an overall accuracy of 50.4% (1.78), which is chance level (see Table 2 for complete descriptive statistics).
3.3. Disproportionate viewing was related to choice behavior
As reported above, early eye movements during the free viewing task were disproportionately directed to the uncued (rather than the cued) scene location. If this effect indeed reflected unconscious object‐location knowledge, it should directly relate to the choices of correct object locations in the group of intuitive decision makers. We predicted that correct location choices would occur in retrieval trials where first fixations were directed to uncued locations but not in trials with first fixations to cued locations or other locations. To test this prediction, we sorted the 24 trials of the experiment into three fixation conditions: (1) first fixation to cued location (M #trials = 3.16), (2) to uncued location (M #trials = 4.22), and (3) to any other location (M #trials = 16.62). The dependent variable was percentage of correct location choices in each condition. To account for unequal numbers of trials per condition (depending on a participant's viewing behavior), we weighted (multiplied) the dependent variable “percentage of cued location choices” by (with) each participant's relative number of trials in a fixation condition (i.e., #trialsparticipant/M#trialsgroup). This procedure accounts for the fact that some participants made only few fixations and others many fixations in a fixation condition and should therefore carry less or more weight in the analysis of cued location choices. The analyses confirmed that trials with the first fixation directed to the uncued location were indeed associated with a higher percentage of correct location choices (M = 62.2%, SE = 4.06%) within the group of intuitive decision makers: t(31) = 3.01, p = .005, d z = 0.53 (Figure 2c). In contrast, in trials with the first fixation directed to the cued location, cued location choices (M = 53.5%, SE = 4.4%) did not significantly differ from chance level: t(29) = 0.79, p = .44, d z = 0.14, nor did they in trials with the first fixation directed to other locations: M = 50%, SE = 1.7%, t(31) = 0, p = 1, d z = 0. Importantly, when this analysis was computed without weighing of the means, it yielded the same results: first fixation directed to the uncued location (M = 66.3%, SE = 4.5%): t(31) = 3.59, p = .001, d z = 0.64; first fixation directed to the cued location (M = 56.8%, SE = 5.7%): t(29) = 1.2, p = .24, d z = 0.22; first fixation directed to other locations: M = 50.2%, SE = 1.8%, t(31) = 0, p = 0.9, d z = 0.02.
Because the group of deliberative decision makers did not show evidence of unconscious learning in the location selections, we did not expect this group's location selections to be better than chance in trials with first fixations to uncued locations. The corresponding analyses computed for the group of deliberative decision makers revealed nonsignificant results (all p > 0.19) (Figure 2c).
3.4. Awareness tests
Following the experiment, we conducted two directly instructed forced‐choice tests to validate objectively the subliminal nature of presentations. Accuracy in the scene test was not better than chance, neither for the group of intuitive decision makers (M = 50.0%, SE = 2.1%, t(31) = 0.0, p = 1) nor for the group of deliberative decision makers (M = 51.6%, SE = 1.58%, t(31) = 0.99, p = .33). Also, neither group performed better than chance in the object test (intuitive group: M = 47.8%, SE = 1.8%, t(31) = −1.23, p = .23; deliberative group: M = 46.9%, SE = 1.69%, t(31) = −1.85, p = .08). There were no significant performance differences between the intuitive and the deliberative decision makers in the two awareness tests (t(62) = −0.59 and t(62) = 0.37, both p > .55). Next, we tested whether the intuitive decision makers’ performance in the awareness test would predict their choices of cued locations in the experiment (Greenwald, Klinger, & Schuh, 1995). This was not the case, neither for performance in the scene awareness test (β = −.025, t(30) = −.14, p = .89) nor for performance in the object awareness test (β = −.025, t(30) = −.14, p = .89). Importantly, intercepts in both regressions were significantly larger than zero (scene test: y‐axis intercept = .026, t(30) = 1.85, p = .04; object test: y‐axis intercept = .026, t(30) = 1.92, p = .03) indicating that forced‐choice performance in the experiment remained above chance, when performance in the awareness tests was regressed to zero.
Because this regression method has been criticized recently for its lack of sensitivity (Sand & Nilsson, 2016; Shanks, 2017) and because a nonsignificant result in significance testing does not allow concluding that the null hypothesis is true, we added Bayesian statistics to help decide whether the data favor the null hypothesis (H0) of zero awareness over the alternative hypothesis (H1) of residual awareness. We compared the relative evidence for the hypothesis that the intuitive participants could not consciously perceive subliminal stimuli in the awareness tests and performed at chance level versus the evidence for the hypothesis that the intuitive participants showed a similar performance in the awareness tests as in the experiment (2.6% above chance level). We chose a half‐normal prior distribution with a mode of 0 and a standard deviation of 2.6% to calculate the Bayes factor. The resulting Bayes factor was 0.63 for the scene test and 0.29 for the object test. Hence, both Bayes factors favored the hypothesis of zero awareness over residual awareness. The Bayes factor for the data of the object test is considered substantial according to conventions regarding the interpretation of the Bayes factor. These additional analyses corroborate the hypothesis that subliminal stimuli were truly masked from conscious perception.
4. DISCUSSION
We tested the long‐held view that conscious awareness of an encoding situation is necessary for human episodic memory formation (Gabrieli, 1998; Graf & Schacter, 1985; Moscovitch, 1995, 2008; Schacter, 1998; Squire & Dede, 2015; Tulving, 2002). This view is based on the results from encoding protocols that used stimulus materials available to conscious perception. Here, we tested whether human subjects encoded objects in scenes presented below the threshold of conscious perception. Scenes were rotated between subliminal encoding and supraliminal testing to assess the representational flexibility of unconsciously formed object–location associations. The encoding test interval spanned 3 min requiring long‐term storage. To test for subliminally formed object‐location memories, we recorded eye movements as an automatic, unintentional behavior, and manual button‐presses as an intentional behavior. Data revealed that both measures reflected a successful subliminal encoding and flexible representation of object–location associations: Both the group of intuitive and the group of deliberative decision makers contributed to a disproportionate viewing effect reflecting object–location memory. Intentional forced‐choices were indicative of object–location memory in the group of intuitive decision makers only. Performance on the intentional and the unintentional memory test was correlated for intuitive decision makers only. This correlation bolsters the validity of the two procedures as tests of unconscious object‐in‐space memory. During experimentation, participants were left naive regarding the purpose of the experiment and regarding subliminal stimulus presentations. They were fully debriefed following the experiment. The validity of the applied masking technique was confirmed by the results of two objective awareness tests carried out at the end of the testing session. These tests indicated that both deliberative and intuitive decision makers were completely unable to discriminate the subliminal stimuli.
The eye movement effect unfolded early in accordance with previous findings (e.g. Hannula & Ranganath, 2009; Hannula et al., 2007) and was restricted to the first fixation after display onset. The rapid dissipation of the eye movement effect underscores past observations that unconscious memories may not affect viewing patterns beyond the first saccade (Huang, Tan, Soon, & Hsieh, 2014). We had flashed subliminal object cues to reactivate memories of where in scenes objects were located and had expected a disproportionate viewing of object‐cued locations. Yet, participants tended to direct their first fixation to uncued rather than cued locations. One might suspect that this effect was driven by a change in perspective during encoding and retrieval in the rotated conditions, that is, by an activation of an egocentric rather than an allocentric frame of reference. However, data do not support such a notion because there was no interaction with rotation condition and because uncued locations were also preferred in the 0° condition, where the viewer's perspective remained stable from encoding to test. Rather, we assume that subliminal object cues had reactivated allocentric object–location representations, which in turn elicited correct expectations about which scene location would be highlighted. We further assume that viewers’ gaze was attracted by the surprise highlighting of an additional location, namely, the uncued location. This surprise effect is reminiscent of the preference for novelty (longer viewing of new vs old item) that serves as a powerful indicator of long‐term memory in nonverbal human infants and experimental animals (Snyder, Blank, & Marsolek, 2008). Novelty preference has been found to reflect item memory and item–location associations (Hannula et al., 2010a; Hannula et al., 2010b; Ryan et al., 2000). However, other explanations are also conceivable. For example, a successful reactivation of the object–location representation during the 6 s of subliminal cueing could have directed eye movements to the correct location already prior to scene onset, so that viewing of the uncued location would reflect relative novelty or an inhibition‐of‐return effect (Posner, Rafal, Choate, & Vaughan, 1985). Within the light of the current data, these explanations remain speculative. But the interpretation of disproportionate viewing as an expression of a flexible object–location representation is supported by the intuitive decision makers’ tendency to choose the object‐cued location in the second implicit test in those trials, where they viewed the uncued location in the first implicit test. Hence, the two implicit measures, eye movements and location selections, were related: only trials where the first eye fixation was drawn to the unexpectedly highlighted uncued location resulted in an above‐chance selection of the correct object location by the intuitive group.
Retrieval success in both implicit tests seemed equal between rotated and unrotated perspectives. The obtained overall effects did not depend on a certain rotation condition. Hence, these findings speak for the hypothesis of the build‐up of flexible, view‐independent (i.e., allocentric) object‐in‐space representations from subliminal images (Bird & Burgess, 2008; King, Burgess, Hartley, Vargha‐Khadem, & O'Keefe, 2002). Yet, this result needs to be taken with caution because each rotation condition included only 6 trials. This low number of trials increased error variance in conditions such that performance in none of the rotation conditions reached significance by itself. Also, the small number of trials did not allow for reliable comparisons between the individual rotation conditions. With few trial numbers, it could happen that the easy condition, the 0° condition with no perspective change from encoding to test, drove the effects. The data (Table 2) speak against this possibility because the intuitive decision makers’ performance on the object placement task was nominally equal between the 0° and the rotated conditions (52.6%). Moreover, when the 0° condition was excluded and only the three rotated conditions were evaluated, the first‐fixation effect and the correlation between the two measures of memory still yielded significance for the group of intuitive decision makers (both p = .02).
The question arises of whether unconscious spatial processing would differ between the 0° and the rotated conditions analogous to the known susceptibility of conscious spatial processing to increasing viewpoint shifts (e.g., King et al., 2002). When spatial processing is conscious, shifts of imagined viewpoints usually show a chronometrical relationship with the size of the shifted viewpoint. Viewpoint shifts reflect an iterative mental manipulation of the shifted viewpoint towards the encoding viewpoint (Diwadkar & McNamara, 1997; King et al., 2002). The hippocampus appears critical for such viewpoint shifts as it helps translating allocentric into egocentric representations (Bird & Burgess, 2008). Reaction time data of the current experiment are suggestive of viewpoint shifts: Correct responses in the object placement task took significantly longer in rotated conditions than the 0° condition (0°: M = 1946 ms, SE = 106.1 ms; 90°: M = 2326 ms, SE = 149.6 ms; 180°: M = 2159 ms, SE = 128.1 ms; 270°: M = 2158 ms, SE = 153 ms; repeated measures ANOVA: F(2.04,124.9) = 0.22, p = .04, = 0.05; quadratic polynomial contrast p = .02). Hence, additional processing was also required with a subliminal encoding protocol and this additional processing may reflect unconscious viewpoint shifts. These results are consistent with the construction of cognitive maps (O'Keefe & Nadel, 1978) and the construction of coherent scenes (Hassabis & Maguire, 2007).
To our knowledge, this is the first evidence of unconscious object‐in‐space encoding and flexible retrieval from long‐term memory. The use of 3D‐environments, which were rotated between study and test, allowed us to bring unconscious relational learning (Duss et al., 2011; T. P. Reber & Henke, 2011) to the spatial domain. The spatial domain is a key to episodic memory according to the original definition: episodic memory is the memory of when and where things happened in the personal past (Tulving, 1972). Although brain activity was not measured in this experiment, chances are high that the hippocampus was supporting the unconscious encoding and reactivation of object‐in‐space associations because the task requires rapid spatial relational encoding and flexible retrieval—key aspects of hippocampal memory (Cohen & Eichenbaum, 1993; Henke, 2010; McClelland et al., 1995; Moses & Ryan, 2006; Norman & O'Reilly, 2003; O'Reilly & Rudy, 2000; Treves & Rolls, 1994). There is much empirical evidence that the hippocampus is necessary to store associations of items and their contexts (Diana, Yonelinas, & Ranganath, 2007; Howard, Kumaran, Ólafsdóttir, & Spiers, 2011; Libby, Hannula, & Ranganath, 2014). Theories emphasizing the role of the hippocampus in spatial processing posit that the hippocampus serves as a cognitive map of our environment (O'Keefe & Nadel, 1978) and the mental construction of spatially coherent scenes (Bird & Burgess, 2008; Hassabis & Maguire, 2007). The hippocampus appears to play a quite generic role in object location memory including short‐term memory (Crane & Milner, 2005; Esfahani‐Bayerl et al., 2016; Olson, 2006; Watson, Voss, Warren, Tranel, & Cohen, 2013; Yee, Hannula, Tranel, & Cohen, 2014; but see Allen, Vargha‐Khadem, & Baddeley, 2014; Jeneson, Mauldin, Hopkins, & Squire, 2011) and perhaps also including unconscious memory as examined here. It appears to be particularly the internal reconstruction of correct object locations—as examined in this study—that drives the hippocampus (Stepankova, Fenton, Pastalkova, Kalina, & Bohbot, 2004; Watson et al., 2013) and allocentric spatial processing in general (Hartley et al., 2007; Lee et al., 2005). Given the similarity of cognitive processes investigated in these supraliminal experiments and the subliminal experiment introduced here, it is likely that the hippocampus was also at work during unconscious object‐in‐space association formation and reactivation, as examined here.
Based on previous results (Hannula et al., 2010a, 2012; Ryan et al., 2000) and the results of the pilot experiment (Supporting Information), we had hypothesized that intentional deliberation would affect decision performance negatively in the object placement task, where implicit memory and hence intuition should guide decisions (for similar findings see Voss & Paller, 2010). Findings from both the pilot experiment and the main experiment corroborate the hypothesis that unconscious memory can guide intentional location choices, if participants habitually prefer intuitive decision making over deliberation. On the other hand, both intuitive and deliberative decision makers expressed flexible object‐location knowledge in their eye movements. We assume that decision making is not an issue when simply viewing a scene (=first implicit memory test) because eyes are usually moving spontaneously. Therefore, eye movements may be a more direct and purer measure of implicit memory than intentional choices. It should be noted that group differences between intuitive and deliberative participants were not statistically significant in the experiment. This lack of a group difference was probably not due to a lack of validity or discriminative power of the personality inventory because the inventory‐based classifications matched the impressions of participants: intuitive decision makers (vs deliberative decision makers) reported in a postexperimental survey that they felt more at ease with the decision task (10‐point Likert scale; Mdn intuitive: 3; Mdn deliberative: 3; U = 376, Z = −1.92, p = .027, one‐tailed, r = −.24) and that they had decided more intuitively than deliberatively (Mdn intuitive: 3.5; Mdn deliberative: 4; U = 378.5, Z = −1.82, p = .035, one‐tailed, r = −.23). These personal reports were not influenced by knowledge of group membership. However, neither preference for intuition according to the Betsch inventory nor self‐reported use of intuition during the object placement task correlated with placement accuracy: r(62) = .07, p = .61 and r(62) = .08, p = .55, respectively. This lack of significant correlations plus the lack of significant group differences regarding performance accuracy in the object placement task suggests that the superiority of intuition over deliberation was marginal.
In conclusion, the current findings show that completely unconscious scene perception allows for scene segmentation (background; objects) and for the formation of unconscious object‐in‐space associations that are stored flexibly in long‐term memory. The speed of association formation and the flexibility of their long‐term representation are key computational aspects of episodic memory (Cohen & Eichenbaum, 1993; Henke, 2010; McClelland et al., 1995; Moses & Ryan, 2006; Norman & O'Reilly, 2003; O'Reilly & Rudy, 2000; Treves & Rolls, 1994). Hence, according to computational definitions of episodic memory, the current findings suggest an unconscious form of spatial episodic memory. But this result challenges long‐held views assuming that conscious awareness of the encoding situation is mandatory for episodic memory formation (Gabrieli, 1998; Graf & Schacter, 1985; Moscovitch, 1995, 2008; Schacter, 1998; Squire & Dede, 2015; Tulving, 2002), while supporting the view that consciousness is not mandatory for episodic memory (Henke, 2010; Reder, Park, & Kieffaber, 2009). When considering that rapid and flexible spatial association formation is an evolutionarily old asset required for survival (Manns & Eichenbaum, 2006), it is little surprising that this cognitive processing works at various levels of consciousness.
Supporting information
Additional Supporting Information may be found online in the supporting information tab for this article.
Supporting Information 1
ACKNOWLEDGMENT
This research was supported by Grant 320000‐114012 from the Swiss National Science Foundation to K. Henke and a CCLM (Center for Cognition, Learning and Memory) PhD stipend to S. Wuethrich. The authors thank Kathrin Michel for help with data collection.
Wuethrich S, Hannula DE, Mast FW, Henke K. Subliminal encoding and flexible retrieval of objects in scenes. Hippocampus. 2018;28:633–643. 10.1002/hipo.22957
Funding information Swiss National Science Foundation, Grant/Award Number: 320000‐114012
REFERENCES
- Allen, R. J. , Vargha‐Khadem, F. , & Baddeley, A. D. (2014). Item‐location binding in working memory: Is it hippocampus‐dependent? Neuropsychologia, 59, 74–84. 10.1016/j.neuropsychologia.2014.04.013 [DOI] [PubMed] [Google Scholar]
- Betsch, C. (2004). Präferenz für Intuition und Deliberation (PID). Zeitschrift Für Differentielle Und Diagnostische Psychologie, 25(4), 179–197. [Google Scholar]
- Bird, C. M. , & Burgess, N. (2008). The hippocampus and memory: Insights from spatial processing. Nature Reviews. Neuroscience, 9(3), 182–194. [DOI] [PubMed] [Google Scholar]
- Burgess, N. , Maguire, E. A. , & O'Keefe, J. (2002). The human hippocampus and spatial and episodic memory. Neuron, 35(4), 625–641. [DOI] [PubMed] [Google Scholar]
- Chong, T. T.‐J. , Husain, M. , & Rosenthal, C. R. (2014). Recognizing the unconscious. Current Biology, 24(21), R1033–R1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen, N. J. , & Eichenbaum, H. (1993). Memory, amnesia, and the hippocampal system. Cambridge, MA: MIT Press. [Google Scholar]
- Crane, J. , & Milner, B. (2005). What went where? Impaired object‐location learning in patients with right hippocampal lesions. Hippocampus, 15(2), 216–231. [DOI] [PubMed] [Google Scholar]
- Diana, R. A. , Yonelinas, A. P. , & Ranganath, C. (2007). Imaging recollection and familiarity in the medial temporal lobe: A three‐component model. Trends in Cognitive Sciences, 11(9), 379–386. [DOI] [PubMed] [Google Scholar]
- Dienes, Z. (2014). Using Bayes to get the most out of non‐significant results. Frontiers in Psychology, 5, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diwadkar, V. A. , & McNamara, T. P. (1997). Viewpoint dependence in scene recognition. Psychological Science, 8(4), 302–307. [Google Scholar]
- Duss, S. B. , Oggier, S. , Reber, T. P. , & Henke, K. (2011). Formation of semantic associations between subliminally presented face‐word pairs. Consciousness and Cognition, 20(3), 928–935. [DOI] [PubMed] [Google Scholar]
- Duss, S. B. , Reber, T. P. , Hanggi, J. , Schwab, S. , Wiest, R. , Muri, R. M. , … Henke, K. (2014). Unconscious relational encoding depends on hippocampus. Brain, 137, 3355–3370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esfahani‐Bayerl, N. , Finke, C. , Braun, M. , Düzel, E. , Heekeren, H. R. , Holtkamp, M. , … Ploner, C. J. (2016). Visuo‐spatial memory deficits following medial temporal lobe damage: A comparison of three patient groups. Neuropsychologia, 81, 168–179. [DOI] [PubMed] [Google Scholar]
- Gabrieli, J. D. E. (1998). Cognitive neuroscience of human memory. Annual Review of Psychology, 49(1), 87–115. [DOI] [PubMed] [Google Scholar]
- Graf, P. , & Schacter, D. L. (1985). Implicit and explicit memory for new associations in normal and amnesic subjects. Journal of Experimental Psychology: Learning, Memory, and Cognition, 11(3), 501–518. [DOI] [PubMed] [Google Scholar]
- Greenwald, A. G. , Klinger, M. R. , & Schuh, E. S. (1995). Activation by marginally perceptible (“subliminal”) stimuli: Dissociation of unconscious from conscious cognition. Journal of Experimental Psychology: General, 124(1), 22–42. [DOI] [PubMed] [Google Scholar]
- Hannula, D. E. , Althoff, R. R. , Warren, D. E. , Riggs, L. , Cohen, N. J. , & Ryan, J. D. (2010a). Worth a glance: Using eye movements to investigate the cognitive neuroscience of memory. Frontiers in Human Neuroscience, 4, 166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannula, D. E. , Baym, C. L. , Warren, D. E. , & Cohen, N. J. (2012). The eyes know: Eye movements as a veridical index of memory. Psychological Science, 23(3), 278–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannula, D. E. , & Ranganath, C. (2009). The eyes have it: Hippocampal activity predicts expression of memory in eye movements. Neuron, 63(5), 592–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannula, D. E. , Ranganath, C. , Ramsay, I. S. , Solomon, M. , Yoon, J. , Niendam, T. A. , … Ragland, J. D. (2010b). Use of eye movement monitoring to examine item and relational memory in schizophrenia. Biological Psychiatry, 68(7), 610–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannula, D. E. , Ryan, J. D. , Tranel, D. , & Cohen, N. J. (2007). Rapid onset relational memory effects are evident in eye movement behavior, but not in hippocampal amnesia. Journal of Cognitive Neuroscience, 19(10), 1690–1705. [DOI] [PubMed] [Google Scholar]
- Hartley, T. , Bird, C. M. , Chan, D. , Cipolotti, L. , Husain, M. , Vargha‐Khadem, F. , & Burgess, N. (2007). The hippocampus is required for short‐term topographical memory in humans. Hippocampus, 17(1), 34–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassabis, D. , & Maguire, E. A. (2007). Deconstructing episodic memory with construction. Trends in Cognitive Sciences, 11(7), 299–306. [DOI] [PubMed] [Google Scholar]
- Henke, K. (2010). A model for memory systems based on processing modes rather than consciousness. Nature Reviews. Neuroscience, 11(7), 523–532. [DOI] [PubMed] [Google Scholar]
- Howard, L. R. , Kumaran, D. , Ólafsdóttir, H. F. , & Spiers, H. J. (2011). Double dissociation between hippocampal and parahippocampal responses to object‐background context and scene novelty. Journal of Neuroscience, 31(14), 5253–5261. 10.1523/JNEUROSCI.6055-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang, Y.‐F. , Tan, E. G. F. , Soon, C. S. , & Hsieh, P.‐J. (2014). Unconscious cues bias first saccades in a free‐saccade task. Consciousness and Cognition, 29, 48–55. [DOI] [PubMed] [Google Scholar]
- Jeffreys, H. (1939). The Theory of Probability. (1st/3rd edn). Oxford, England: Oxford University Press. [Google Scholar]
- Jeneson, A. , Mauldin, K. N. , Hopkins, R. O. , & Squire, L. R. (2011). The role of the hippocampus in retaining relational information across short delays: The importance of memory load. Learning & Memory, 18(5), 301–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiefer, M. , & Martens, U. (2010). Attentional sensitization of unconscious cognition: Task sets modulate subsequent masked semantic priming. Journal of Experimental Psychology: General, 139(3), 464–489. [DOI] [PubMed] [Google Scholar]
- King, J. A. , Burgess, N. , Hartley, T. , Vargha‐Khadem, F. , & O'keefe, J. (2002). Human hippocampus and viewpoint dependence in spatial memory. Hippocampus, 12(6), 811–820. [DOI] [PubMed] [Google Scholar]
- Libby, L. A. , Hannula, D. E. , & Ranganath, C. (2014). Medial temporal lobe coding of item and spatial information during relational binding in working memory. Journal of Neuroscience, 34(43), 14233–14242. 10.1523/JNEUROSCI.0655-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, A. C. H. , Buckley, M. J. , Pegman, S. J. , Spiers, H. , Scahill, V. L. , Gaffan, D. , … Graham, K. S. (2005). Specialization in the medial temporal lobe for processing of objects and scenes. Hippocampus, 15(6), 782–797. [DOI] [PubMed] [Google Scholar]
- Maguire, E. A. , & Mullally, S. L. (2013). The hippocampus: A manifesto for change. Journal of Experimental Psychology: General, 142(4), 1180–1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manns, J. R. , & Eichenbaum, H. (2006). Evolution of declarative memory. Hippocampus, 16(9), 795–808. [DOI] [PubMed] [Google Scholar]
- McClelland, J. L. , McNaughton, B. L. , & O'Reilly, R. C. (1995). Why there are complementary learning systems in the hippocampus and neocortex: Insights from the successes and failures of connectionist models of learning and memory. Psychological Review, 102(3), 419–457. [DOI] [PubMed] [Google Scholar]
- Moscovitch, M. (1995). Recovered consciousness: A hypothesis concerning modularity and episodic memory. Journal of Clinical and Experimental Neuropsychology, 17(2), 276–290. [DOI] [PubMed] [Google Scholar]
- Moscovitch, M. (2008). The hippocampus as a “stupid,” domain‐specific module: Implications for theories of recent and remote memory, and of imagination. Canadian Journal of Experimental Psychology/Revue Canadienne De Psychologie Expérimentale, 62(1), 62–79. [DOI] [PubMed] [Google Scholar]
- Moses, S. N. , & Ryan, J. D. (2006). A comparison and evaluation of the predictions of relational and conjunctive accounts of hippocampal function. Hippocampus, 16(1), 43–65. [DOI] [PubMed] [Google Scholar]
- Norman, K. A. , & O'Reilly, R. C. (2003). Modeling hippocampal and neocortical contributions to recognition memory: A complementary‐learning‐systems approach. Psychological Review, 110(4), 611–646. [DOI] [PubMed] [Google Scholar]
- O'Keefe, J. , & Nadel, L. (1978). The hippocampus as a cognitive map. Oxford, NY: Clarendon Press, Oxford University Press. [Google Scholar]
- Olson, I. R. (2006). Working memory for conjunctions relies on the medial temporal lobe. Journal of Neuroscience, 26(17), 4596–4601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Reilly, R. C. , & Rudy, J. W. (2000). Computational principles of learning in the neocortex and hippocampus. Hippocampus, 10(4), 389–397. [DOI] [PubMed] [Google Scholar]
- Posner, M. I. , Rafal, R. D. , Choate, L. S. , & Vaughan, J. (1985). Inhibition of return: Neural basis and function. Cognitive Neuropsychology, 2(3), 211–228. [Google Scholar]
- Reber, P. J. , Beeman, M. , & Paller, K. A. (2013). Human memory systems: A framework for understanding the neurocognitive foundations of intuition In Schmorrow D. D. & Fidopiastis C. M. (Eds.), Foundations of augmented cognition (Vol. 8027, pp. 474–483). Berlin, Heidelberg: Springer Berlin Heidelberg. [Google Scholar]
- Reber, T. P. , & Henke, K. (2011). Rapid formation and flexible expression of memories of subliminal word pairs. Frontiers in Psychology, 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reber, T. P. , Luechinger, R. , Boesiger, P. , & Henke, K. (2012). Unconscious relational inference recruits the hippocampus. Journal of Neuroscience, 32(18), 6138–6148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reder, L. M. , Park, H. , & Kieffaber, P. D. (2009). Memory systems do not divide on consciousness: Reinterpreting memory in terms of activation and binding. Psychological Bulletin, 135(1), 23–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan, J. D. , Althoff, R. R. , Whitlow, S. , & Cohen, N. J. (2000). Amnesia is a deficit in relational memory. Psychological Science, 11(6), 454–461. [DOI] [PubMed] [Google Scholar]
- Sand, A. , & Nilsson, M. E. (2016). Subliminal or not? Comparing null‐hypothesis and Bayesian methods for testing subliminal priming. Consciousness and Cognition, 44, 29–40. [DOI] [PubMed] [Google Scholar]
- Schacter, D. L. (1998). Memory and awareness. Science, 280(5360), 59–60. [DOI] [PubMed] [Google Scholar]
- Shanks, D. R. (2017). Regressive research: The pitfalls of post hoc data selection in the study of unconscious mental processes. Psychonomic Bulletin & Review, 24(3), 752–724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder, K. A. , Blank, M. P. , & Marsolek, C. J. (2008). What form of memory underlies novelty preferences? Psychonomic Bulletin & Review, 15(2), 315–321. [DOI] [PubMed] [Google Scholar]
- Squire, L. R. , & Dede, A. J. O. (2015). Conscious and unconscious memory systems. Cold Spring Harbor Perspectives in Biology, 7(3), a021667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stepankova, K. , Fenton, A. A. , Pastalkova, E. , Kalina, M. , & Bohbot, V. D. (2004). Object–location memory impairment in patients with thermal lesions to the right or left hippocampus. Neuropsychologia, 42(8), 1017–1028. [DOI] [PubMed] [Google Scholar]
- Treves, A. , & Rolls, E. T. (1994). Computational analysis of the role of the hippocampus in memory. Hippocampus, 4(3), 374–391. [DOI] [PubMed] [Google Scholar]
- Tulving, E. (1972). Episodic and semantic memory In Tulving E. & Donaldson W. (Eds.), Organization of memory (pp. 381–403). New York, NY: Academic. [Google Scholar]
- Tulving, E. (2002). Episodic memory: From mind to brain. Annual Review of Psychology, 53(1), 1–25. [DOI] [PubMed] [Google Scholar]
- Voss, J. L. , & Paller, K. A. (2010). What makes recognition without awareness appear to be elusive? Strategic factors that influence the accuracy of guesses. Learning & Memory, 17(9), 460–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson, P. D. , Voss, J. L. , Warren, D. E. , Tranel, D. , & Cohen, N. J. (2013). Spatial reconstruction by patients with hippocampal damage is dominated by relational memory errors: Relational Errors in Hippocampal Amnesia. Hippocampus, 23(7), 570–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yee, L. T. S. , Hannula, D. E. , Tranel, D. , & Cohen, N. J. (2014). Short‐term retention of relational memory in amnesia revisited: Accurate performance depends on hippocampal integrity. Frontiers in Human Neuroscience, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional Supporting Information may be found online in the supporting information tab for this article.
Supporting Information 1


