Abstract
Background
With the well established shift to neoadjuvant treatment for locally advanced rectal cancer, there is increasing focus on the use of radiosensitizers to improve the efficacy and tolerability of radiotherapy. There currently exist few randomized data exploring novel radiosensitizers to improve response and it is unclear what the clinical endpoints of such trials should be.
Methods
A qualitative systematic review was performed according to the PRISMA guidelines using preset search criteria across the PubMed, Cochrane and Scopus databases from 1990 to 2017. Additional results were generated from the reference lists of included papers.
Results
A total of 123 papers were identified, of which 37 were included; a further 60 articles were obtained from additional referencing to give a total of 97 articles. Neoadjuvant radiosensitization for locally advanced rectal cancer using fluoropyrimidine‐based chemotherapy remains the standard of treatment. The oral derivative capecitabine has practical advantages over 5‐fluorouracil, with equal efficacy, but the addition of a second chemotherapeutic agent has yet to show a consistent significant efficacy benefit in randomized clinical assessment. Preclinical and early‐phase trials are progressing with promising novel agents, such as small molecular inhibitors and nanoparticles.
Conclusion
Despite extensive research and promising preclinical studies, a definite further agent in addition to fluoropyrimidines that consistently improves response rate has yet to be found.
Short abstract
Promising agents coming
Introduction
Rectal cancer treatment has continued to improve in recent years as a result of optimized surgical technique, advances in staging, pathological quality control and multidisciplinary management. Neoadjuvant chemoradiotherapy (CRT) is considered the standard of care for locally advanced rectal cancer (LARC). It is well recognized that the response to neoadjuvant CRT is both variable and unpredictable for the individual patient, and techniques to risk‐stratify patients and predict response are an expanding area of research. Favourable responses to CRT are independently associated with conferring a long‐term survival advantage to patients who undergo resection, and in more recent years the possibility of deferral of surgery and organ preservation has also been raised1.
A complete response to CRT may be classified as either a clinical complete response (cCR) or a pathological complete response (pCR). Although the two terms are often used interchangeably, these responses are assessed differently, and one does not necessarily imply the other. A pCR is based on pathological findings after resection, commonly using the Dworak or Mandard tumour regression grading systems. A cCR is defined according to a combination of clinical examination (including digital rectal examination), radiological (in particular diffusion‐weighted MRI) and endoscopic appearances.
Following the initial description by Habr‐Gama and colleagues2, there are now a growing number of series reporting the use of neoadjuvant CRT as the sole treatment for rectal cancer that undergoes a cCR, resulting in further interest in the role of organ preservation in rectal cancer3. It is, however, important to be able to differentiate which tumours are more susceptible to undergoing a cCR. At present, the most reliable predictor of an increased response is tumour stage, with early tumours more likely to display a cCR. The use of CRT in combination with local excision is perhaps becoming better defined in early T1 rectal cancers, but its value in more advanced cancer is less clear4. The STAR‐TReC trial (ISRCTN14240288)5 will compare three different strategies for more advanced tumours up to T3b N0, and assess the feasibility of randomizing to a trial with organ preservation arms. However, the role of neoadjuvant CRT as sole treatment for even more locally advanced tumours that perhaps threaten the circumferential resection margin (CRM) is unknown, and it is likely that studies examining such tumours will need to incorporate the development of intensified CRT regimens.
Patients who have an apparent cCR may be offered entry into a watch‐and‐wait surveillance policy after a full and complete discussion. If patients are fit for intervention, salvage surgery is recommended for those who display tumour regrowth, which is most often luminal rather than nodal1. There is clearly an interest in both predicting patients who may undergo a cCR or pCR and/or improving cCR and/or pCR rates as there are currently no reliable clinical (apart from earlier stage), biochemical or molecular predictive biomarkers in clinical practice.
Radiotherapy (RT) is typically delivered via either a short‐ or long‐course strategy, the latter being employed to downstage tumours. A recent short study by the UK National Bowel Cancer Audit6 revealed that the median time from completion of CRT to surgical resection is currently 11 weeks in the UK, suggesting that the concept of delayed resection is gaining traction in clinical practice. A recent study7 suggested that increasing the interval between the end of CRT and surgical resection improves the response rate. Similarly, short‐course RT may be combined with a delayed interval to surgery; the recent Stockholm III trial8 has demonstrated improved tumour regression over traditional short‐course treatment.
Radiosensitizers are employed routinely to improve the radiosensitivity of rectal cancer to RT; the standard of care is a concurrent single‐agent fluoropyrimidine. A number of studies have analysed novel agents or combination therapies that aim to improve radiosensitivity and cCR and/or pCR rates. The critical target for RT is DNA and the accumulation of DNA damage, particularly DNA double‐strand breaks, and the ability of tumour cells to repair this damage, contributes significantly to the therapeutic effect. Some agents and combination therapies (such as oxaliplatin, irinotecan and poly(ADP‐ribose) polymerase (PARP) inhibitors) might typically take advantage of this by creating additional DNA damage or inhibiting DNA damage repair, exacerbating the effects of RT. The aim of this review was to summarize the current and novel agents that have been employed in the treatment of LARC, and to consider their role in the context of cCR and organ preservation. A summary of radiosensitizing agents is provided in Fig. 1.
Figure 1.
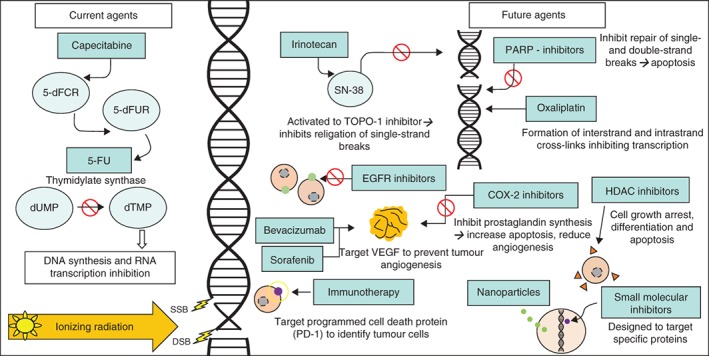
Summary of current and potential radiosensitizing agents. 5‐dFCR, 5′‐deoxy‐5‐fluorocytidine; 5‐dFUR, 5′‐deoxy‐5‐fluorouridine; 5‐FU, 5‐fluorouracil; dUMP, deoxyuridine monophosphate; dTMP, deoxythymidine monophosphate; SSB, single‐strand break; DSB, double‐strand break; TOPO, topoisomerase; EGFR, epidermal growth factor receptor; VEGF, vascular endothelial growth factor; PARP, poly(ADP‐ribose)polymerase; COX, cyclo‐oxygenase; HDAC, histone deacetylase
Methods
A literature search was performed for published full‐text articles using PubMed, Cochrane and Scopus databases using the search criteria string (‘radiosensitiser’ OR ‘radiosensitizer’) AND (‘rectal’ OR ‘rectum’) AND ‘cancer’ in November 2017. Additional papers were detected by scanning the references of relevant papers. Search results were initially included based on a relevant title, and these papers were then read in full. Inclusion criteria were: papers published in the English language, those with a focus on rectal cancer, all study types, and articles published between 1990 and 2017. Studies focusing on a primary malignancy other than rectal cancer were excluded. Two reviewers were involved at each stage, with search results being loaded into the Covidence system to enable joint reviews to take place methodically. Once eligible articles had been identified, a search was undertaken to exclude duplicated results or duplicated data sets to produce the final list of papers included (Fig. 2).
Figure 2.
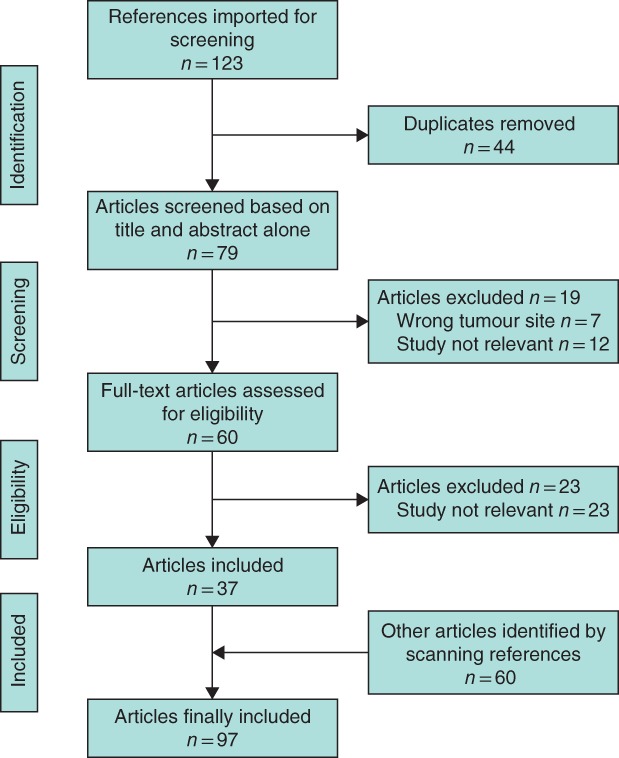
PRISMA diagram showing selection of articles for review
Standard chemotherapy regimens
5‐Fluorouracil
5‐Fluorouracil (5‐FU) is an antimetabolite fluoropyrimidine. It is one of the most common chemotherapeutic agents used in cancer treatment, in particular breast and colorectal cancer. It was the first agent to be used as a radiosensitizer in conjunction with RT, predominantly in colorectal cancer. It works by inhibiting essential biosynthetic processes, and also by affecting cellular DNA and RNA functions. The mechanism of cytotoxicity of 5‐FU has been ascribed to the misincorporation of fluoronucleotides into RNA and DNA, and to inhibition of the nucleotide synthetic enzyme thymidylate synthase9.
There are a number of mechanisms by which 5‐FU could increase radiation sensitivity at the cellular level. One is thought to involve the killing of S‐phase cells, which are relatively radioresistant10, 11. This does not account for all of the increased radiation sensitivity produced by the drug because non‐cytotoxic concentrations can also increase sensitivity. Radiosensitization under non‐cytotoxic conditions occurs only when cells are incubated with the drug before and during radiation treatment. Several studies have suggested that 5‐FU should be given continuously during a course of fractionated radiation to achieve radiosensitization of most fractions12, 13. UK National Institute for Health and Care Excellence guidance14 focusing on stage III tumours, examining randomized comparisons of bolus versus infusional regimens, suggests that infusional therapy is equivalent to bolus treatment in terms of effectiveness, but has relatively reduced toxicity. Owing to concerns regarding the increased cost of infusional treatment and patient inconvenience, there remains geographical variation across the UK in the technique employed14, although 5‐FU has largely been superseded by oral capecitabine. Trials of 5‐FU are summarized in Table 1.
Table 1.
Summary of fluoropyrimidine agents
| Results (%)* | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Reference | Phase | Disease stage | Test drug | Single/combination regimen | Cohort size | pCR rate | cCR rate | Other endpoints | Toxicity |
| 15 | III | T3–4 N–/+ | 5‐FU | Adjuvant RT versus RT + 5‐FU | 204 | n.a. | – | 5‐year recurrence 41·5 (62·7) | Increased risk of GI or haematological problems with 5‐FU use; only 1 severe |
| 16 | III | T3–4 N+/– | 5‐FU | Neoadjuvant RT versus RT + 5‐FU | 773 | 11·4 (3·6) | – |
5‐year LR 8·1 (16·5) 5‐year OS 67 (67) |
Grade 3–4 toxicity 14·6 (3·6) |
| 17 | III | T3–4 N–/+ | 5‐FU | Neoadjuvant versus adjuvant RT + 5‐FU | 267 | 15·0 | 0·8 |
5‐year DFS 64·7 (53·4) 5‐year OS 74·5 (65·6) |
Grade 4 GI disturbance 24 (13) |
| 18 | III | T3–4 N–/+ | 5‐FU | Neoadjuvant RT versus RT + 5‐FU | 1011 | 13·7 (5·3) | – | – | – |
| 19 | III | T3–4 N–/+ | 5‐FU | Neoadjuvant versus adjuvant RT + 5‐FU | 823 | 8 | – |
5‐year LRR 6 (13) 5‐year DFS 68 (65) 5‐year OS 76 (74) |
Grade 3–4 toxicity 27 versus 40 |
| 20 | II | T3–4 N–/+ | Capecitabine | Single | 95 | 12 | – | Downstaging 76 | Grade 3 toxicity 3 |
| 21 | II | T3–4 N–/+ | Capecitabine | Single | 54 | 18 | – |
Downstaging 51 Sphincter salvage 67 |
Grade 3–4 GI toxicity 2 |
| 22 | II | T3–4 N–/+ | Capecitabine | Single | 31 | 7 | – |
Downstaging 54 3‐year DFS 60 3‐year OS 77 |
Grade 3–4 GI toxicity 36 Proctitis 32 |
| 23 | III | T3–4 N–/+ | Capecitabine | Capecitabine + oxaliplatin versus 5‐FU | 42 | 24 (14) | – | Downstaging 81 (67) |
Grade 3 GI toxicity 19 (14) Haematological 19 (14) |
| 24 | III | T3–4 N–/+ | Capecitabine | Capecitabine versus 5‐FU | 392 | 14 (5) | – |
LR 6 (7) 5‐year OS 76 (67) 3‐year DFS 75 (67) |
Grade 3–4 GI toxicity 9 (2) |
| 25 | III | T3–4 N–/+ | Capecitabine | Capecitabine +/– oxaliplatin versus 5‐FU +/– oxaliplatin | 1608 | 20·7 (17·8) | – |
Downstaging 21·1 (21·3) Sphincter salvage 59·3 (59·4) |
Grade 3–5 GI toxicity 11·7 (11·7) Addition of oxaliplatin increased GI disturbance (P < 0·001) |
Results for control group are shown in parentheses. pCR, pathological complete response; cCR, clinical complete response; 5‐FU, 5‐fluorouracil; RT, radiotherapy; n.a., not applicable; GI, gastrointestinal; LR, local recurrence; OS, overall survival; DFS, disease‐free survival; LRR, locoregional recurrence.
Krook and colleagues15 first conducted an RCT to assess adjuvant RT with or without systemic bolus 5‐FU chemotherapy, and confirmed an improvement in local relapse rates with a survival benefit in favour of 5‐FU‐based RT in comparison with RT alone. The phase III French Federation Francophone de Cancerologie Digestive (FFCD) 9203 study16 randomized patients with stage II–III rectal cancer to receive RT alone or with infusional 5‐FU/leucovorin. Patients in both arms subsequently underwent surgery and four cycles of 5‐FU/leucovorin. The preoperative chemoradiation arm showed a significant improvement in pCR rate (11·4 versus 3·6 per cent; P < 0·050) and local relapse rate (8·1 versus 16·5 per cent; P < 0·050). The 5‐year survival in both arms was 67 per cent.
The National Surgical Adjuvant Breast and Bowel Project (NSABP) R‐03 phase III study17 compared the use of neoadjuvant and adjuvant CRT in patients with T3–T4 or node‐positive rectal cancer using 5‐FU and leucovorin. Those receiving neoadjuvant therapy had a pCR rate of 15·0 per cent and 5‐year disease‐free survival (DFS) rate of 64·7 per cent. Among those undergoing adjuvant CRT, 39·2 per cent had sphincter‐saving surgery (versus 47·8 per cent in the neoadjuvant cohort) and a DFS rate of 53·4 per cent. Five‐year overall survival rates were 74·5 and 65·6 per cent in the neoadjuvant and adjuvant treatment groups respectively, supporting the use of CRT before rather than after operation. In a 2012 Cochrane review, Petersen and colleagues26 considered the use of 5‐FU for additional adjuvant chemotherapy. The pooled data from 21 RCTs, including almost 10 000 patients, found improved DFS and overall survival with use of adjuvant chemotherapy. Owing to lack of tumour stage‐specific data, however, it was not possible to draw a link of benefit to specific locally advanced tumours, potentially indicating an area for further work.
The phase III European Organisation for Research and Treatment of Cancer (EORTC) 22921 study18 randomized patients with stage II–III rectal cancer to neoadjuvant RT alone versus RT with concurrent bolus 5‐FU/leucovorin, with subsequent randomization to postoperative chemotherapy or not. The authors concluded that adding 5‐FU‐based chemotherapy, either before (as part of CRT) or after operation, conferred a significant advantage in terms of local control.
The German Rectal Cancer Study Group19 randomly assigned 823 patients with clinical stage T3 or T4 or node‐positive disease to receive either preoperative or postoperative CRT. The results showed a significantly lower 5‐year cumulative incidence of local relapse in favour of the preoperative treatment group (6 versus 13 per cent; P = 0·006). Five‐year DFS (68 versus 65 per cent) and overall survival (76 versus 74 per cent) rates were similar. Significant tumour downstaging was seen after preoperative combined treatment, with a pCR rate of 8 per cent.
In a pooled analysis of 5‐FU phase II–III trials27 including 3157 patients, the pCR rate was 13·5 per cent. On multivariable analysis, statistically significant factors for a higher pCR rate were the addition of a second chemotherapy agent and the method of continuous infusion.
Capecitabine
The development of an oral 5‐FU drug was driven by the desire to overcome the perceived limitations associated with intravenous infusion of 5‐FU, such as extended hospital stay, the need for intravenous lines and associated healthcare costs. Capecitabine (Xeloda®; Roche, Basle, Switzerland) is an oral prodrug of 5‐FU; it is a fluoropyrimidine carbamate that undergoes a three‐step in vivo enzymatic conversion to 5‐FU. The final step is mediated by the enzyme thymidine phosphorylase, which is upregulated in tumour tissue compared with adjacent healthy tissue. This theoretically allows selective activation of the drug and low systemic toxicity28. After oral administration, capecitabine passes rapidly and extensively through the intestinal membrane as an intact molecule. Capecitabine is not cytotoxic itself; the only cytotoxic moiety is 5‐FU, which is generated preferentially in human cancer cells. Preferential activation of capecitabine to 5‐FU in malignant tumour was demonstrated in animal models bearing human xenografts29. Table 1 provides an overview of clinical trials of capecitabine.
The first phase II trials20, 21 showed that RT plus capecitabine is well tolerated and easier to administer than protracted intravenous infusion of 5‐FU, with a pCR rate comparable to intravenous infusion of 5‐FU for LARC. In 2008, another phase II trial22 of 31 patients with LARC showed that capecitabine was well tolerated orally and had radiosensitizing effects comparable to those of neoadjuvant 5‐FU therapy. In 2011, Swellengrebel et al.30 again showed that oral capecitabine had an acceptable acute toxicity profile in a cohort of 147 patients with LARC. In 2015, Saha and co‐workers23 conducted a randomized control pilot study comparing capecitabine–oxaliplatin (CAPOX) as a radiosensitizer with 5‐FU–leucovorin; the two arms were comparable in terms of objective response rate, pCR rate, R0 resection and toxicity profile23. Noh and colleagues31, 32 investigated different timings for administration of capecitabine among 171 patients undergoing RT followed by total mesorectal excision (TME) 4–6 weeks after neoadjuvant therapy and assessed the radiosensitization response by pCR. The optimal radiosensitizing effects of capecitabine were achieved if it was administered 1 h before RT.
A phase III RCT24 between 2002 and 2007 recruited nearly 400 patients with stage II and III LARC, comparing CRT with capecitabine versus 5‐FU. The primary endpoint was 5‐year overall survival, which in the capecitabine group was non‐inferior to that in the 5‐FU group (76 versus 67 per cent; P < 0·001). The local recurrence rate was similar in the two groups (6 versus 7 per cent); however, the rate of distant metastasis was 9 per cent lower in the capecitabine group, with increased 3‐year DFS.
The phase III NSABP R‐04 trial25 aimed to ascertain the optimal neoadjuvant chemotherapy regimen alongside RT for stage II–III rectal cancer. Infusion of 5‐FU and oral capecitabine with or without intravenous oxaliplatin were compared in 1608 patients. The pCR rate was 17·8 per cent for those receiving 5‐FU and 20·7 per cent among those receiving capecitabine. Sphincter salvage rates were largely comparable between the groups at 59·4 and 59·3 per cent respectively, as were rates of tumour downstaging (21·3 versus 21·1 per cent). The addition of oxaliplatin led to a small increase in pCR rate, but a reduction in sphincter salvage and downstaging, and a significant increase in toxicity.
Additional chemotherapy agents to enhance radiosensitivity
Oxaliplatin
Oxaliplatin is a third‐generation platinum‐based drug that enhances radiation‐induced cytotoxicity via irreparable DNA damage through formation of interstrand and intrastrand crosslinks, induction of G2/M cell‐cycle arrest, blockage of DNA replication and inhibition of transcription33, 34. Preclinical data indicated potent radiosensitizing properties of the drug, with synergism between oxaliplatin and RT35, 36; these findings have been applied to several clinical trials for patients with LARC.
Phase I–II studies focusing on the addition of oxaliplatin to 5‐FU‐based neoadjuvant CRT reported promising results33. pCR rates varied between 7 and 28 per cent, compared with 8–15 per cent in the 5‐FU‐alone group34, 37. Single weekly dosing was the most effective regimen, with diarrhoea and neuropathy the most commonly reported adverse effects.
Six large phase III trials to date have compared fluoropyrimidine CRT with or without oxaliplatin. The results from these trials are summarized in Table 2. The ACCORD 12/prodige 2 trial38 randomized 598 patients to standard capecitabine‐based neoadjuvant CRT or additional weekly dosing with oxaliplatin together with an increased radiation dose. The difference in pCR rate of 13·9 versus 19·2 per cent was not significant (P = 0·09), although ACCORD had been powered to detect an increase from 11 to 20 per cent with CAPOX. There was an increase in grade 3–4 toxicity with oxaliplatin.
Table 2.
Summary of other chemotherapy agents
| Results (%)* | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Reference | Phase | Disease stage | Test drug | Single/combination | Cohort size | pCR rate | cCR rate | Other endpoints | Toxicity |
| 38 | III | T3–4 N–/+ | Oxaliplatin | Capecitabine + oxaliplatin versus capecitabine alone | 598 | 19·2 (13·9) | 0·7 (0) | Positive CRM 9·9 (19·3) | Grade 3–4 toxicity 25 (1) |
| 39 | III | T3–4 N–/+ | Oxaliplatin | 5‐FU + oxaliplatin versus 5‐FU alone | 1236 | 17 (13) | – | 3‐year DFS 75·9 (71·2) | Grade 3–4 toxicity 23 (20) |
| 40 | III | T3–4 N–/+ | Oxaliplatin | 5‐FU +/– oxaliplatin versus capecitabine +/– oxaliplatin | 1608 | – | – |
LR 11·2 (11·8) 5‐year DFS 66·4 (67·7) 5‐year OS 81·3 (79) |
Addition of oxaliplatin significantly increased toxicity (P < 0·001) |
| 41 | III | T3–4 N–/+ | Oxaliplatin | Oxaliplatin +5‐FU versus 5‐FU alone | 1094 | – | – | 3‐year DFS 74·5 (73·9) | ‐ |
| 42 | III | T3–4 N–/+ | Oxaliplatin | 5‐FU + oxaliplatin versus 5‐FU alone | 747 | 16 (16) | – | Positive CRM 7 (4) |
Grade 3–4 toxicity 24 (8) Discontinued owing to toxicity 17 (4) |
| 43 | III | T3–4 N–/+ | Oxaliplatin | 5‐FU + oxaliplatin versus 5‐FU alone | 495 | 27·5 (14·0) | – | Negative nodes 87·4 (80·1) | Grade 3–4 haematological toxicity 19 (12·9) |
| 44 | I–II | T3–4 N–/+ | Irinotecan | Irinotecan +5‐FU | 59 | 25 | – |
Downstaging 41 3‐year DFS 40 |
Grade 3–4 toxicity 28·8 |
| 45 | II | T3–4 N–/+ | Irinotecan | Irinotecan + capecitabine | 36 | 15 | – | 3‐year OS 80 | Grade 3–4 haematological toxicity 25 |
| 46 | II | T3–4 N–/+ | Irinotecan | Irinotecan + capecitabine | 48 | 25 | – |
5‐year DFS 75 5‐year OS 94 |
Grade 3 toxicity 10·5 No grade 4 toxicity |
| 47 | II | T3–4 N–/+ | Irinotecan | Irinotecan + 5‐FU versus 5‐FU alone | 106 | 26 (30) |
Downstaging 75 (74) 5‐year OS 75 (61) 5‐year DFS 85 (78) |
Grade 3 toxicity 13 (8) | |
| 48 | II | T3–4 N–/+ | Irinotecan | Irinotecan + capecitabine | 110 | 21·8 | – |
Negative CRM 89·1 3‐year DFS 96·9 3‐year OS 88·2 |
Grade 3 GI toxicity 22 No grade 4 toxicity |
Results for control group are shown in parentheses. pCR, pathological complete response; cCR, clinical complete response; CRM, circumferential resection margin; 5‐FU, 5‐fluorouracil; DFS, disease‐free survival; LR, local recurrence; OS, overall survival; GI, gastrointestinal.
The CAO/ARO/AIO‐04 study39, which included over 1000 participants, was the only trial to find a significantly improved pCR rate with oxaliplatin (from 13 to 17 per cent; P = 0·038). This was also the only trial to report an advantage for oxaliplatin in terms of 3‐year DFS (71·2 to 75·9 per cent). There were no significant differences in grade 3–4 toxicities or postoperative complications. However, the infusional 5‐FU regimen was changed between the control and experimental arms, with oxaliplatin being added to 16 weeks of postoperative adjuvant chemotherapy compared with 16 weeks of bolus 5‐FU alone in the control arm; this means that the relative contributions of oxaliplatin in CRT compared with adjuvant chemotherapy are difficult to define.
The NSABP R‐0440 and PETACC‐641 trials, published only in abstract form, found that addition of oxaliplatin to 5‐FU‐based neoadjuvant therapy led to decreased treatment compliance and increased toxicity, with no associated improvement in pathological tumour downstaging. The STAR‐01 trial42 randomized 747 patients to standard 5‐FU chemotherapy or additional oxaliplatin. Interim analysis detected no difference in pCR and toxicity problems with the addition of oxaliplatin42.
Interestingly, initial results from the Chinese FOWARC trial43 showed that use of a modified FOLFOX (oxaliplatin, leucovorin, 5‐FU) 6 regimen in addition to 5‐FU + RT gave significantly improved rates of pCR compared with single‐agent 5‐FU + RT (27·5 versus 14·0 per cent respectively). The trial also showed comparable downstaging and acceptable toxicity in patients with stage II–III disease, and good compliance. Long‐term data are awaited and may still be important for future practice.
The evidence at present, including subsequent meta‐analyses49, 50, still supports the use of a single‐agent fluoropyrimidine as the standard of care because of a lack of consistent improvement in pCR and 3‐year DFS rates51 with the combined regimen, and the greater toxicity due to oxaliplatin52.
Irinotecan
Irinotecan, a topoisomerase (TOPO) 1 inhibitor, inhibits religation of single‐strand DNA breaks through the formation of camptothecin 11–TOPO‐1–DNA complexes53. A preclinical study54 has demonstrated irinotecan to be not only a feasible addition to 5‐FU chemotherapy, but also a potent radiosensitizing agent in colorectal cancer, even under hypoxic conditions.
In a small phase I trial in 2008, Choi et al.55 examined the addition of weekly irinotecan to traditional 5‐FU neoadjuvant CRT in 16 patients with locally advanced T3–T4 rectal cancers. Some 94 per cent of patients were eligible to progress with surgical resection at the time of restaging, with 93 per cent achieving a R0 resection. In eight patients the disease was downstaged based on the TMN classification, with a pCR rate of 25 per cent. Although the numbers were too small to draw any firm conclusions, the evidence was promising in terms of combination potential.
Mehta and colleagues56 conducted a phase II trial with the same dosing strategy in a cohort of 32 patients, of whom 38 per cent achieved a pCR and 71 per cent TNM downstaging. However, 56 per cent experienced acute toxicity with an initial dose of 50 mg/m2, requiring dose alteration or delay in administration. Overall, small phase II studies44, 45, 46, 56 focusing on this approach have achieved pCR rates of 14–37 per cent and tumour downstaging in 24–71 per cent. A summary of these trials is provided in Table 2.
Mohiuddin et al.47 reported 5‐year outcomes on 106 patients randomized to either basic 5‐FU CRT or additional 4‐week doses of 50 mg/m2 irinotecan. They reported an increase in overall survival of 14 per cent with the addition of irinotecan; however, the locoregional recurrence rate was 17 per cent, compared with 16 per cent without irinotecan, and respective distal recurrence rates were 21 and 16 per cent. There was no significant difference between the treatment arms in terms of pCR or downstaging, but an increased rate of acute toxicity was reported in the irinotecan group. Gollins and colleagues48 reported on 110 patients with MRI‐defined locally advanced rectal cancer threatening or involving the surgical CRM treated with a regimen of irinotecan 60 mg/m2 weekly for the first 4 weeks of a 5‐week course of capecitabine CRT. In total, 24 patients (21·8 per cent) had a pCR and 98 (89·1 per cent) a negative CRM. A further study focusing on long‐term outcome in 115 patients57 found no significant difference between the two treatment arms in terms of pCR, with a higher overall survival rate of 87 per cent and DFS rate of 79 per cent in the irinotecan group (median follow‐up 60 months).
Despite the promise of the above studies, no phase III trial of concurrent irinotecan has yet been reported58. This will be rectified in the future by the ongoing UK ARISTOTLE trial, which will complete accrual (target 600 patients) in mid‐2018. In MRI‐defined high‐risk rectal cancer, ARISTOTLE will compare CRT with concurrent capecitabine with or without irinotecan.
Epidermal growth factor receptor inhibitors
Epidermal growth factor receptor (EGFR), a member of the ErbB family of receptors, is relevant in colorectal cancer because overexpression or upregulation of the EGFR gene occurs in 60–80 per cent of cases59, 60, 61. Expression of the gene is also associated with poor survival62, 63, 64. The anti‐EGFR monoclonal antibodies cetuximab and panitumumab are already approved for the treatment of RAS wild‐type metastatic colorectal cancer65, but their role in LARC remains unclear.
There have been several clinical trials of EGFR‐targeting monoclonal antibodies as radiosensitizers in neoadjuvant therapy for LARC. These trials are summarized in Table 3. Early efficacy results in terms of pCR rate were around 5–10 per cent74, 75, 76. However, these studies did not investigate tumour RAS status, which is used as a predictive biomarker for anti‐EGFR monoclonal antibody response in metastatic colorectal cancer77, 78. Potentially, optimal ordering of chemotherapy, RT and the EGFR inhibitor might unlock the full radiosensitizing potential of anti‐EGFR monoclonal antibodies79.
Table 3.
Summary of phase II trials of epidermal growth factor receptor inhibitors
| Results (%)* | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Reference | Phase | Disease stage | Test drug | Single/combination | Cohort size | pCR rate | cCR rate | Other endpoints | Toxicity |
| 66 | II | T3–4 N–/+ | Panitumumab | Single | 19 | 0 | – |
Downstaging 41 Negative CRM 76 LRC 90 DFS 79 |
GI disturbance 89 Grade 4 toxicity 21 |
| 67 | II | T3–4 N–/+ | Cetuximab | Cetuximab + capecitabine | 31 | 0 | – | Downstaging 42 |
GI disturbance 13 Grade 4 toxicity 3 |
| 68 | II | T3–4 N–/+ | Panitumumab | Panitumumab + oxaliplatin +5‐FU | 60 | 21 | – | Downstaging 58 |
GI disturbance 39 1 death |
| 69 | II | T3–4 N–/+ | Panitumumab | Panitumumab + capecitabine versus capecitabine alone | 68 | 10 (18) | – |
R0 resection 85 (93) Sphincter salvage 69 (70) Downstaging 87 (85) |
GI disturbance 10 (4) |
| 70 | II | T3–4 | Cetuximab | Cetuximab + capecitabine + oxaliplatin versus capecitabine + oxaliplatin | 165 | 11 (9) | 11 (7) | Radiological response 71 (51) | GI disturbance 8 (9) |
| 71 | II | T2–4 N–/+ | Cetuximab | Cetuximab + capecitabine + irinotecin | 82 | 17 | 5 | R0 resection 82 |
GI disturbance 25 Grade 4 toxicity 10 |
| 72 | II | T3–4 N–/+ | Cetuximab | Cetuximab + capecitabine | 47 | 8 | – |
3‐year DFS 72 3‐year RFS 74 3‐year OS 68 |
2 of 32 unable to complete treatment owing to GI disturbance and leucopenia |
| 73 | I–II | T3–4 N–/+ | Cetuximab | Cetuximab + capecitabine + oxaliplatin | 60 | 8 | – |
5‐year OS 76 3‐year DFS 88 5 ‐year CSS 78 |
Grade 2 toxicity 5 |
Results for control group are shown in parentheses. pCR, pathological complete response; cCR, clinical complete response; CRM, circumferential resection margin; LRC, locoregional control; DFS, disease‐free survival; GI, gastrointestinal; 5‐FU, 5‐fluorouracil; RFS, relapse‐free survival; OS, overall survival; CSS, cancer‐specific survival.
A phase II trial66 was designed to assess the pCR (primary endpoint) following neoadjuvant therapy with panitumumab and RT. Of 19 enrolled patients, 17 were evaluable for pathology assessment. Although no pCR was observed, seven patients (41 per cent) had grade 3 Dworak pathological tumour regression. As the primary endpoint was not achieved, the authors were unable to make any recommendation for the use of panitumumab in treatment of LARC. Similar findings were reported in other phase II trials where the primary endpoint of pCR was not achieved67, 68, and toxicity was high69.
EXPERT‐C70 was a randomized phase II trial of neoadjuvant CAPOX with or without cetuximab, followed by capecitabine‐based CRT with or without cetuximab, followed by surgery and then adjuvant CAPOX with or without cetuximab in 165 high‐risk patients with rectal cancer. Cetuximab did not improve the primary outcome (pCR), so it was not felt to have contributed significantly to increased radiation‐induced cytotoxicity. The EXPERT‐C trial did, however, find that TP53 tumour suppressor protein wild‐type status was a predictive biomarker in favour of cetuximab‐based therapy.
The prospective phase II EXCITE trial71, published in 2017, focused on the addition of cetuximab to an irinotecan–capecitabine‐based neoadjuvant CRT regimen in 82 patients. Fourteen patients (17 per cent) had a pCR. As a side point of interest, contrary to the planned protocol, four patients achieved an endoscopically and MRI‐confirmed cCR, and were managed using the emerging watch‐and‐wait approach. Overall 24 patients (29 per cent) had an excellent clinical or pathological response. Using next‐generation sequencing, 46 per cent of matched biopsy–resection specimens were discrepant for EGFR pathway mutations. Intratumoral heterogeneity was suggested as a possible explanation, manifesting as a geographical biopsy miss or chemoradiation‐driven emergence of new mutations.
Phase II studies so far have failed to suggest a benefit in terms of pCR rate and DFS, and have shown no consistent correlation with KRAS status72, 73, 80. There is currently no role for the addition of EGFR‐targeted therapy as a radiosensitizer in the treatment of LARC81. However, a pilot study82 of RT with personalized chemotherapy and biological therapy, based on molecular markers among 16 patients with T3 or N1 rectal cancers, showed a pCR rate of 50 per cent, which may be the basis for future molecular guided studies.
Antiangiogenesis therapy
Bevacizumab
Bevacizumab is a monoclonal antibody that targets vascular epithelial growth factor (VEGF). In combination with cytotoxic chemotherapy, it has shown potential for rectal cancer treatment; the evidence is, however, currently limited to phase I–II trials83. Salazar et al.84 undertook a multicentre randomized phase II trial in 90 patients with LARC, of capecitabine with or without bevacizumab. The pCR rate was 16 per cent in the bevacizumab arm, compared with 11 per cent in the control arm, and an additional 20 per cent of tumours were downstaged. However, the predefined efficacy endpoint of a difference in treatment arms of 10 per cent was not met, despite these encouraging results.
Landry et al.85 performed a phase II trial of the addition of bevacizumab therapy with 5‐year follow‐up. Of 57 patients included in the data analysis, 17 per cent achieved a pCR, with an overall 5‐year survival rate of 80 per cent and relapse‐free survival rate of 81 per cent. The pCR endpoint of 30 per cent was not reached and, owing to substantial side‐effects (1 death was attributed to study therapy), the regimen was not considered worthy of further work by the authors.
Sorafenib
Sorafenib is a multikinase inhibitor that blocks the receptor tyrosine kinase of VEGF, platelet‐derived growth factor and the RAF serine–threonine kinases along the RAF–mitogen‐activated protein kinase kinase–extracellular signal‐related kinase pathway. Jeong and colleagues86 assessed its potential as a radiosensitizer using three colorectal cell lines, and a xenograft animal model. They were able to demonstrate a scientific rationale for combination therapy, with enhanced radiosensitivity being shown in all three cell lines and the xenograft model, and delayed DNA damage repair caused by the radiation treatment. Van Moos et al.87 evaluated its effect in a cohort of 54 patients with KRAS‐mutated rectal tumours in combination with capecitabine‐based CRT. The pCR rate was 60 per cent, with downstaging in 82 per cent. A second phase I study88 also produced encouraging results, with a pCR of 36 per cent.
Poly(ADP‐ribose) polymerase inhibition
PARPs, particularly PARP‐1, play a critical role in the recognition and repair of DNA single‐ and double‐strand breaks. Higher PARP activity has been noted in cancer cells with increased proliferation and chemoradioresistance, and this has led to the development of PARP inhibitors, which reduce the cancer cell's ability to repair single‐ and double‐strand breaks generated by RT and lead to cell death. Preclinical trials have demonstrated radiosensitizing effects in multiple colorectal cell lines89, although this effect is potentially largely dependent on the status of oncogenes such as BRCA1/BRCA2 90 generating defective DNA double‐strand break repair (termed synthetic lethality), and dysregulation of P5391.
Veliparib (ABT‐888), a potent orally bioavailable PARP‐1/2 inhibitor, has been shown to enhance the antitumor activity of chemotherapy and RT in preclinical colorectal cancer models92. In in vitro and in vivo experiments in colorectal cancer, veliparib had independent radiosensitization effects and was synergistic with chemotherapy, especially with irinotecan. Final results from a phase Ib dose‐escalation study of veliparib plus capecitabine‐based CRT and surgery were published in 201793, demonstrating a pCR rate of 28 per cent. As with the EGFR monoclonal antibodies, this class of potential radiosensitizer remains an area of interest and future studies are needed to elucidate its role in rectal cancer. Potential predictive biomarkers have not been identified. A recent report94 has described pharmacodynamic assays that are able to measure the low levels at which PARP inhibitors are active.
Immunotherapy
The immune system plays an intricate and complex role in all aspects of cancer from carcinogenesis to treatment95. Over the past 10 years, a great deal of work has been done to better understand that role, with the development of therapies such as programmed cell death protein 1 (PD‐1)/programmed death ligand 1 (PD‐L1) inhibitors, cancer vaccines and adoptive cell therapy. In a phase I–II trial96 of adjuvant immunotherapy involving sentinel lymph node T lymphocytes in 55 patients with metastatic colorectal cancer, there was no treatment‐related toxicity, and 24‐month survival rates were 56 and 18 per cent in the treatment and control groups respectively.
Use of cytokine therapy, although very early in terms of research into colorectal cancer, has been approved by the US Food and Drug Administration for melanoma and renal cell carcinoma (interleukin 2). Although much of the work focusing on colorectal malignancy is in its early phases (I–II), there is evidence to suggest the potential use of these therapies in a combination role for a correctly selected cohort97. Specifically, the PD‐1 immune checkpoint inhibitors pemrolizumab and nivolumab have shown promising activity in DNA mismatch repair‐deficient (dMMR)/microsatellite instability – high colorectal cancers, which carry a high mutation load and an active immune microenvironment98, 99. A small proportion of rectal cancers are dMMR, but one possible area of research is to determine whether the proinflammatory properties of RT might enhance the response of microsatellite‐stable tumours to PD‐1 blockade. The R‐IMMUNE phase II study100 is currently recruiting to compare the use of atezolizumab as a radiosensitizer with 5‐FU‐based neoadjuvant CRT. More studies are in the pipeline, with a recent UK proposal aiming to assess the effect of the PD‐L1 inhibitor durvalumab in combination with RT.
Novel agents
With a clear focus of research on optimizing neoadjuvant therapy, several novel agents ranging from cyclo‐oxygenase 2 inhibitors to nanoparticles have been investigated in the preclinical setting (Table 4). Further phase I studies are in preparation to examine both prostaglandin E2 receptor inhibitors (PRAER 1 trial) and Ad3/Ad11p chimeric adenoviruses (CEDAR trial).
Table 4.
Summary of novel radiosensitizing agents
| Reference | Study design | Findings |
|---|---|---|
| COX‐2 inhibitors | Cox‐2 is an inducible enzyme that regulates prostaglandin synthesis and is overexpressed at sites of inflammation and in epithelial malignancy tumours101. It is involved in the regulation of apoptosis, angiogenesis and tumour cell invasiveness. Preclinical studies suggest the potential of COX‐2 inhibitors as selective radiosensitizers102 | |
| Debucquoy et al.103 | Double‐blind randomized phase II; in addition to 5‐FU; 35patients |
Improved downstaging No increased toxicity |
| Nanoparticles | Aim to improve the therapeutic index of chemoradiotherapy and overcome potential systemic excess toxicity. Focus on particle size sub‐50 nm | |
| Caster et al.104 | Particles 50, 100 and 150 nm in size loaded with 2 DNA repair inhibitor model drugs in colorectal cancer cell lines |
All sizes potent radiosensitizers Good toxicity tolerance |
| Tian et al.105 | CRLX101 in combination with oxaliplatin and 5‐FU |
Increased efficacy of chemoradiotherapy Early stage; needs expansion |
| Histone deacetylase inhibitors | Emerging therapeutic concept attempting to target epigenetic regulatory mechanisms and act as a radiosensitizer in combination therapy. SAHA approved as a single agent for refractory cutaneous T‐cell lymphoma | |
| Folkvord et al.106 | Preclinical study of SAHA using 2 xenograft models |
In vitro: improved radiosensitivity (P ≤ 0·050) across cell lines at all radiation doses less than 6 h after exposure In vivo: pCR achieved in 1 model |
| Saelen et al.107 | Vorinostat assessed under hypoxic conditions in vitro |
Enhanced radiosensitivity across cell lines Warrants further research |
| Small molecular inhibitors | Low molecular weight; able to target both extracellular and intracellular proteins | |
| Kleiman et al 108 |
Preclinical Focus on radiosensitizers for KRAS mutant tumours 28 known radiosensitizers assessed |
6 effective; AZD7762 most highly potent Suggested investigation into role of CHK2 inhibitors |
| Nelfinavir | HIV protease inhibitor; inhibits Akt at standard clinical doses and results in radiosensitivity | |
| Hill et al.109 | Non‐randomized SONATINA clinical trial focusing on safety in 10 patients with T3–4 N0–2 M1 rectal cancers recruited over 2 years 14 days total oral treatment (7 days preoperative) |
2 discontinued owing to toxicity 5 grade 3 toxicity Warrants further research |
| Buijsen et al.110 |
Phase I trial including 12 patients Escalating doses with capecitabine Primary endpoint: dose‐limiting toxicity |
4 of 6 experienced toxicity, precluding further dose escalation pCR 27% Further toxicity concerns |
| Zerumbone | Cyclic sesquiterpene from rhizomes of edible ginger plant; emerging evidence of potential for inhibition of proliferation of human colonic adenocarcinoma cells, with minimal toxicity111 | |
| Deorukhkar et al.112 |
3 colorectal cancer cell lines Inhibition of proliferation identified in dose‐dependentmanner |
Marked radiosensitizer in clonogenic survival curves Little effect on normal fibroblasts Warrants further research |
| Bortezomib | Modified dipeptidyl boronic acid derived from leucine and phenylalanine that acts as a 26S proteasome inhibitor. The ubiquitin–proteasome pathway is involved in intracellular protein degradation in eukaryotic cells | |
| O'Neil et al.113 | 10 patients with stage II or III rectal cancer received 5‐FU‐based chemoradiotherapy plus bortezomib twiceper week |
pCR 10% High toxicity – diarrhoea Study not progressed |
COX, cyclo‐oxygenase; 5‐FU, 5‐fluorouracil; SAHA, suberoylanilide hydroxamic acid; pCR, pathological complete response; CHK2, serine–threonine kinase 2; HIV, human immunodeficiency virus.
Alternatives to standard radiotherapy strategies
Dose escalation
An alternative potential method of enhancing the effectiveness of CRT is by increasing the radiation dose, via an increased external‐beam dose or endocavitary brachytherapy. There is evidence to suggest that a dose–response relationship with pCR exists114.
A prospective single‐centre study115 from Denmark in patients with T2–3 cancers within 6 cm of the anal verge used radiation dose intensification to the primary tumour delivered with intensity‐modulated external‐beam RT to 60 Gy in 30 fractions over 6 weeks, with 50 Gy to the pelvic nodes, combined with an endorectal brachytherapy tumour boost to 5 Gy and tegafur/uracil on treatment days. Of the 51 patients treated, 78 per cent achieved a cCR and organ preservation; the local recurrence rate was 26 per cent at 2 years. Grade 3 diarrhoea occurred in 8 per cent, and long‐term rectal bleeding was of concern during follow‐up.
Gerard and colleagues116 demonstrated improved clinical (24 versus 2 per cent) and pathological (57 versus 34 per cent) responses using the 50‐Kv Papillon technique for contact X‐ray brachytherapy (CXB). Patients with a clinical incomplete response to external‐beam CRT have been shown to achieve a cCR after a CXB boost, with only 11 per cent developing recurrence117.
With both approaches, there is a lack of randomized data. The recently funded UK APHRODITE study will randomize patients with T1–T3b rectal adenocarcinomas with a maximum diameter of 4 cm, considered unsuitable for radical TME surgery, to standard CRT versus RT dose‐escalated CRT. The OPERA trial will randomize patients with early cT2–T3a–b tumours smaller than 5 cm in diameter, treated with external‐beam CRT, to either an external‐beam CRT boost or a CXB boost.
Delivery modification
An alternative strategy to dose escalation is the development of novel delivery methods that reduce toxicity, particularly to the small bowel. Intensity‐modulated RT is one such technique that has been proposed owing to its highly conformal dose distribution. There are currently few published prospective data to support its routine use; however, a recent meta‐analysis118 of retrospective studies has suggested that it has a significantly lower toxicity profile than routine three‐dimensional CRT. Future developments may ultimately lead to traditional photon irradiation being replaced with charged particles such as protons or carbon ions, which may have even greater biological effectiveness while maintaining a favourable toxicity profile. At the present time, further clinical studies and access to treatment facilities are required to assess the applicability of these techniques fully119, 120.
Preoperative chemotherapy given sequentially with (chemo)radiotherapy
The twofold rationale for giving neoadjuvant chemotherapy sequentially, either before or after (C)RT, followed by surgery, is to improve the response of the primary tumour and to reduce the distant metastasis rate. Owing to morbidity from RT and pelvic surgery, individuals who have undergone preoperative CRT then surgery may fail to start adjuvant chemotherapy or tolerate it poorly, resulting in dose reductions121. A meta‐analysis122 of four trials including preoperative RT, however, has questioned the benefit of postoperative chemotherapy (hazard ratio for DFS 0·91, 95 per cent c.i. 0·77 to 1·07; P = 0·230), possibly for this reason. Giving chemotherapy before surgery allows an increased dose intensity to be delivered, potentially increasing the response rate.
However, although the concept of ‘total neoadjuvant therapy’ is gaining traction123, there is currently very little randomized phase II (and no phase III) evidence specifically examining the benefit of neoadjuvant chemotherapy. Grupo Cancer de Recto (GCR) 3124 was a randomized phase II study of preoperative CAPOX followed by CRT then surgery versus CRT then surgery then postoperative CAPOX in 108 patients. Less toxicity (P < 0·001) and better compliance (P < 0·001) were demonstrated for the same regimen used as neoadjuvant chemotherapy compared with adjuvant chemotherapy, although the pCR rate was no different (13 versus 14 per cent respectively).
A non‐randomized US–Canadian trial125 examined four sequential study groups of patients with LARC, examining CRT followed by chemotherapy then surgery. Group 1 had CRT followed by TME 6–8 weeks later. Groups 2, 3 and 4 had two, four and six 2‐weekly cycles of modified FOLFOX delivered between CRT and TME. The pCR rate was 18, 25, 30 and 38 per cent for groups 1–4 respectively. Although promising, it is not clear whether the increased downstaging occurred because of a greater gap between CRT and surgery (6, 8, 12 and 16 weeks for groups 1–4 respectively).
Randomized studies are urgently needed to examine the efficacy of intensified neoadjuvant CRT regimens for rectal cancer, including the sequential addition of preoperative chemotherapy, in comparison to standard neoadjuvant CRT alone.
Neoadjuvant chemotherapy alone
In the modern TME era, local recurrence rates have fallen to as low as 5 per cent. However, CRT has not affected distant metastatic relapse, which affects up to 30 per cent of patients. Although surgery is associated with long‐term sexual, bowel and bladder dysfunction, preoperative RT can exacerbate this morbidity126. Consideration should be given to whether chemotherapy alone can be as effective as CRT in terms of DFS, thereby avoiding some acute and long‐term toxicity.
Several small single‐arm studies using mainly oxaliplatin‐based chemotherapy have reported promising DFS rates. In addition, studies127, 128 examining neoadjuvant CAPOX followed by CRT have clearly shown the substantial downstaging efficacy of chemotherapy, using MRI after chemotherapy but before CRT. The FOWARC Chinese phase III study43 randomized 495 patients with LARC to either standard neoadjuvant CRT using concurrent 5‐FU, CRT with concurrent 5‐FU and oxaliplatin, or FOLFOX chemotherapy alone. Although tumour downstaging was comparable between the standard CRT and chemotherapy‐alone arms (37·1 and 35·5 per cent respectively), the pCR rate was inferior with chemotherapy alone (14·0 versus 6·6 per cent). It was reported recently that there was no difference in DFS or overall survival between the three arms129. At present, there is more evidence to support the replacement of neoadjuvant CRT with chemotherapy using DFS as the primary endpoint, than for a cCR/organ preservation endpoint.
Discussion
The ideal radiosensitizing agent would be one that could target cancer cells selectively130, 131, enhancing the efficacy of treatment with minimal local and systemic toxicity. Exploiting the benefits of neoadjuvant therapy, accurately staging and assessing cCR could open up the era of increasingly personalized medicine and the avoidance of resection altogether132. It is an area of research that could bring significant patient benefits including improvements in health‐related quality of life. However, future clinical trials of radiosensitizers, with the aim of organ preservation, need carefully to consider the endpoints that are used to assess efficacy132.
Although there is much emerging evidence with regard to potential new radiosensitizing agents, the current standard treatment alongside RT remains 5‐FU or capecitabine chemotherapy. The addition of any second systemic agent has yet to show a consistent increase in efficacy in randomized studies. Many promising radiosensitizers have failed to progress beyond the preclinical and early clinical phases (I–II) owing to systemic toxicity and varying rates of pCR. Unfortunately, the quality of phase II studies of potential intensifying agents has been poor. A systematic review133 of 92 phase II trials showed that only eight were randomized.
There remains the fundamental question of the optimal primary endpoint. In virtually all studies in this review, the pCR rate was employed as the determinant of success. At present, there is no predetermined set definition of what constitutes a pCR. It may be defined as the absence of neoplastic cells in the surgical resection specimen as a result of neoadjuvant treatment (ypT0 and ypN0)134 and indeed may still occur even in the presence of mucosal abnormalities following treatment135. Published rates of pCR range widely from 15 to 40 per cent136, 137. However, despite small cohort sizes being accounted for, very few trials have noted the potential introduction of bias due to lack of standardization of pathologist reporting. The Royal College of Pathologists138 specifies that pathologists should embed all of the tumour‐associated scar and examine three deeper levels on each block before calling a pCR. The lack of reliable lymph node involvement status, dependence on pathologist block and level sampling intensity, and varying time points between the end of RT and surgery affecting tumour regression, could potentially lead to inflated pCR results. The associated benefits of a true pCR include a reduced recurrence rate and enhanced overall survival136, 139, 140.
Tumour regression grading is a semiquantitative assessment of residual tumour cells versus fibroinflammatory tissue in the rectal wall, and has been shown to be able to stratify tumour response to CRT and predict prognosis on an individual‐patient level in two large prospective phase III trials141, 142. Identifying patients who have achieved a cCR following CRT, and who could be followed prospectively with an active surveillance or watch‐and‐wait strategy, is gathering increasing interest. Of 183 patients with T2–T4 N0–2 M0 distal rectal cancers receiving neoadjuvant CRT in a trial published in 2014 by Habr‐Gama and colleagues143, 49 per cent were deemed to have achieved a cCR; 31 per cent of these patients went on to develop local recurrence and the salvage rate was 93 per cent. The rate of local disease control was 94 per cent, with 78 per cent organ preservation. The Habr‐Gama protocol involved clinical, endoscopic, radiological and serological reassessment of patients 8 weeks after completion of neoadjuvant therapy. A cCR was defined as the absence of residual ulceration, stenosis or mass lesion within the rectum on digital palpation and endoscopic imaging. MRI was performed, and the carcinoembryonic antigen level was measured.
The International Watch & Wait Database Consortium144 recently published the long‐term outcomes of the largest series of patients managed by this strategy, reporting a 2‐year cumulative regrowth rate of 25·2 per cent among 1009 patients. Surgical treatment data were available for only 148 of the 213 patients who experienced regrowth; 115 proceeded to TME resection, with histologically clear margins in 88 per cent. Overall 5‐year survival rates of 84·7 per cent in this group are comparable to those of major resection. However, before this approach can be established as a standard of care, standardized definitions of cCR and surveillance protocols need to be developed. Criteria for shared decision‐making with the patient for this approach also need to be addressed. An increasing consensus views a two‐stage assessment as optimum for identifying a cCR, at 3 and then 6 months following CRT, allowing enough time for a cCR to develop in initially good responders145. MRI tumour regression grade following CRT has been shown to be predictive of DFS in a cohort of 66 patients from the MERCURY study, suggesting the value of MRI assessment after CRT as part of the protocol for selecting patients for a non‐operative approach146. Although there are still many questions surrounding watch and wait147, it clearly has an increasingly important place in modern rectal cancer management and strategies to intensify CRT need further exploration.
For patients in whom there has been a good response but not an apparent cCR, local transanal excision is an alternative to major resection148, 149. MRI can be useful in guiding patient selection for such treatments150. However, the recently published GRECCAR 2 study151, which used a composite endpoint of surgical complications and recurrence, failed to show a difference between the two approaches in this setting, suggesting that more prospective studies are needed in this area.
It is imperative that studies employ standardized pathological reporting to ensure that the pCR rates quoted are both realistic and comparable. In view of this, use of the pCR as a primary endpoint for research studies and/or clinical trials should perhaps be questioned. If the ultimate goal is organ preservation regardless of whether the patient has undergone a cCR or pCR, perhaps organ preservation should be the primary endpoint. Against this is the morbidity associated with rectal surgery in terms of bowel, urinary and sexual dysfunction. However, there are few data on long‐term toxicity and health‐related quality of life for an active surveillance approach, which clearly needs to be addressed in future prospective studies. A recent patient consultation exercise revealed that, even in the context of cancer care, patients regarded quality of life and presence of a stoma as more important than overall survival152. Future trials of neoadjuvant therapy for rectal cancer need to ensure that patient experience and reported endpoints are addressed.
Although not available at the present time, it is hoped that the development of biomarker‐based stratified treatment will be used to guide neoadjuvant therapy on a personalized basis in the future153. Such biomarkers may be purely molecular (DNA alterations, gene expression, protein expression, epigenetic or circulating) or a combination of molecular markers and imaging findings154. Reliable pretreatment biomarkers do not currently exist, although ongoing research is attempting to identify pretreatment markers that are predictive of response155, 156. It is essential that future neoadjuvant trials incorporate a translational element to further develop biomarker‐guided therapy. As such, it is critical that such translational arms adhere to a robust biopsy protocol to ensure that enough appropriate biological material is available for downstream analysis in addition to the routine histopathological biopsies taken for diagnostic purposes. Factors that need to be considered include the person taking the biopsies, the quantity of material and timing. Patients who undergo a cCR or pCR will have little or no tumour to access at either clinical follow‐up or at the time of surgical resection; this must be considered in the translational design, which may need to include liquid biopsies157.
Acknowledgements
The authors acknowledge Yorkshire Cancer Research for funding the academic programmes at North Wales Cancer Treatment Centre and the University of Leeds.
Disclosure: The authors declare no conflict of interest.
References
- 1. Renehan AG, Malcomson L, Emsley R, Gollins S, Maw A, Myint AS et al Watch‐and‐wait approach versus surgical resection after chemoradiotherapy for patients with rectal cancer (the OnCoRe project): a propensity‐score matched cohort analysis. Lancet Oncol 2016; 17: 174–183. [DOI] [PubMed] [Google Scholar]
- 2. Habr‐Gama A, Perez RO, Nadalin W, Sabbaga J, Ribeiro U Jr, Silva e Sousa AH Jr et al Operative versus nonoperative treatment for stage 0 distal rectal cancer following chemoradiation therapy: long‐term results. Ann Surg 2004; 240: 711–717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Dossa F, Chesney TR, Acuna SA, Baxter NN. A watch‐and‐wait approach for locally advanced rectal cancer after a clinical complete response following neoadjuvant chemoradiation: a systematic review and meta‐analysis. Lancet Gastroenterol Hepatol 2017; 2: 501–513. [DOI] [PubMed] [Google Scholar]
- 4. Borstlap WAA, Van Oostendorp SE, Klaver CEL, Hahnloser D, Cunningham C, Rullier E et al; Research committee of the European Society of Coloproctology. Organ preservation in rectal cancer: a synopsis of current guidelines. Colorectal Dis 2017; [Epub ahead of print]. [DOI] [PubMed]
- 5. ISRCTNregistry . Can We Save the Rectum by Watchful Waiting or Transanal Surgery Following (Chemo)Radiotherapy Versus Total Mesorectal Excision for Early Rectal Cancer? ISRCTN14240288. http://www.isrctn.com/ISRCTN14240288?q=&filters=conditionCategory:Cancer&sort=&offset=4&totalResults=1864&page=1&pageSize=10&searchType=basic-search [accessed 15 August 2018].
- 6. National Bowel Cancer Audit (NBCA) . Optimal Time Interval Between Neoadjuvant Long‐Course Radiotherapy and Major Resection in English Rectal Cancer Patients Diagnosed between 2011 and 2014 Short report 3; 2017. https://www.nboca.org.uk/content/uploads/2017/07/NBOCA-short-report-timing-RT-surgery2017.pdf [accessed 16 August 2018].
- 7. Sloothaak DA, Geijsen DE, van Leersum NJ, Punt CJ, Buskens CJ, Bemelman WA et al Dutch Surgical Colorectal Audit. Optimal time interval between neoadjuvant chemoradiotherapy and surgery for rectal cancer. Br J Surg 2013; 100: 933–939. [DOI] [PubMed] [Google Scholar]
- 8. Pettersson D, Lörinc E, Holm T, Iversen H, Cedermark B, Glimelius B et al Tumour regression in the randomized Stockholm III Trial of radiotherapy regimens for rectal cancer. Br J Surg 2015; 102: 972–978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Longley DB, Harkin DP, Johnston PG. 5‐Fluorouracil: mechanisms of action and clinical strategies. Nat Rev Cancer 2003; 3: 330–338. [DOI] [PubMed] [Google Scholar]
- 10. Byfield JE. Useful interactions between 5‐fluorouracil and radiation in man: 5‐fluorouracil as a radiosensitizer In Antitumor Drug–Radiation Interactions, Hill BT, Bellamy AS. (eds). CRC Press: Boca Raton, 1990; 87–105. [Google Scholar]
- 11. Ojima E, Inoue Y, Watanabe H, Hiro J, Toiyama Y, Miki C et al The optimal schedule for 5‐fluorouracil radiosensitization in colon cancer cell lines. Oncol Rep 2006; 16: 1085–1091. [PubMed] [Google Scholar]
- 12. Byfield JE, Calabro‐Jones P, Klisak I, Kulhanian F. Pharmacologic requirements for obtaining sensitization of human tumor cells in vitro to combined 5‐fluorouracil or ftorafur and X rays. Int J Radiat Oncol Biol Phys 1982; 8: 1923–1933. [DOI] [PubMed] [Google Scholar]
- 13. Ishikawa T, Tanaka Y, Ishitsuka H, Ohkawa T. Comparative antitumor activity of 5‐fluorouracil and 5′‐deoxy‐5‐fluorouridine in combination with radiation therapy in mice bearing colon 26 adenocarcinoma. Jpn J Cancer Res 1989; 80: 583–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. National Institute for Health and Care Excellence . Capecitabine and Oxaliplatin in the Adjuvant Treatment of Stage III (Dukes' C) Colon Cancer; 2006. https://www.nice.org.uk/guidance/ta100 [accessed 16 August 2018].
- 15. Krook JE, Moertel CG, Gunderson LL, Wieand HS, Collins RT, Beart RW et al Effective surgical adjuvant therapy for high‐risk rectal carcinoma. N Engl J Med 1991; 325: 519–520. [DOI] [PubMed] [Google Scholar]
- 16. Gérard JP, Conroy T, Bonnetain F, Bouché O, Chapet O, Closon‐Dejardin MT et al Preoperative radiotherapy with or without concurrent fluorouracil and leucovorin in T3–4 rectal cancers: results of FFCD 9203. J Clin Oncol 2006; 24: 4620–4625. [DOI] [PubMed] [Google Scholar]
- 17. Roh MS, Colangelo LH, O'Connell MJ, Yothers G, Deutsch M, Allegra CJ et al Preoperative multimodality therapy improves disease‐free survival in patients with carcinoma of the rectum: NSABP R‐03. J Clin Oncol 2009; 27: 5124–5130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bosset JF, Calais G, Mineur L, Maingon P, Radosevic‐Jelic L, Daban A et al Enhanced tumorocidal effect of chemotherapy with preoperative radiotherapy for rectal cancer: preliminary results – EORTC 22921. J Clin Oncol 2005; 23: 5620–5627. [DOI] [PubMed] [Google Scholar]
- 19. Sauer R, Becker H, Hohenberger W, Rödel C, Wittekind C, Fietkau R et al; German Rectal Cancer Study Group. Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med 2004; 351: 1731–1740. [DOI] [PubMed] [Google Scholar]
- 20. Kim JC, Kim TW, Kim JH, Yu CS, Kim HC, Chang HM et al Preoperative concurrent radiotherapy with capecitabine before total mesorectal excision in locally advanced rectal cancer. Int J Radiat Oncol Biol Phys 2005; 63: 346–353. [DOI] [PubMed] [Google Scholar]
- 21. Krishnan S, Janjan NA, Skibber JM, Rodriguez‐Bigas MA, Wolff RA, Das P et al Phase II study of capecitabine (Xeloda) and concomitant boost radiotherapy in patients with locally advanced rectal cancer. Int J Radiat Oncol Biol Phys 2006; 66: 762–771. [DOI] [PubMed] [Google Scholar]
- 22. Bazarbashi S, El‐Bassiouni M, Abdelsalam M, Soudy H, Sanea NA, Jabbar AA et al A modern regimen of pre‐operative concurrent chemo‐radiation therapy in locally advanced rectal cancer. J Surg Oncol 2008; 98: 167–174. [DOI] [PubMed] [Google Scholar]
- 23. Saha A, Ghosh SK, Roy C, Saha ML, Choudhury KB, Chatterjee K. A randomized controlled pilot study to compare capecitabine–oxaliplatin with 5‐FU–leucovorin as neoadjuvant concurrent chemoradiation in locally advanced adenocarcinoma of rectum. J Cancer Res Ther 2015; 11: 88–93. [DOI] [PubMed] [Google Scholar]
- 24. Hofheinz RD, Wenz F, Post S, Matzdorff A, Laechelt S, Hartmann JT et al Chemoradiotherapy with capecitabine versus fluorouracil for locally advanced rectal cancer: a randomised, multicentre, non‐inferiority, phase 3 trial. Lancet Oncol 2012; 13: 579–588. [DOI] [PubMed] [Google Scholar]
- 25. O'Connell MJ, Colangelo LH, Beart RW, Petrelli NJ, Allegra CJ, Sharif S et al Capecitabine and oxaliplatin in the preoperative multimodality treatment of rectal cancer: surgical end points from National Surgical Adjuvant Breast and Bowel Project Trial R‐04. J Clin Oncol 2014; 32: 1927–1934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Petersen SH, Harling H, Kirkeby LT, Wille‐Jørgensen P, Mocellin S. Postoperative adjuvant chemotherapy in rectal cancer operated for cure. Cochrane Database Syst Rev 2012; (3)CD004078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hartley A, Ho KF, McConkey C, Geh JI. Pathological complete response following pre‐operative chemoradiotherapy in rectal cancer: analysis of phase II/III trials. Br J Radiol 2005; 78: 934–938. [DOI] [PubMed] [Google Scholar]
- 28. Schüller J, Cassidy J, Dumont E, Roos B, Durston S, Banken L et al Preferential activation of capecitabine in tumor following oral administration to colorectal cancer patients. Cancer Chemother Pharmacol 2000; 45: 291–297. [DOI] [PubMed] [Google Scholar]
- 29. Ishikawa T, Utoh M, Sawada N, Sekiguchi F, Fukase Y, Ishitsuka H. Xeloda (capecitabine): an orally available tumor‐selective puoro‐pyrimidine carbamate. Proc Am Soc Clin Oncol 1997; 16: 208a. [Google Scholar]
- 30. Swellengrebel HA, Marijnen CA, Verwaal VJ, Vincent A, Heuff G, Gerhards MF et al Toxicity and complications of preoperative chemoradiotherapy for locally advanced rectal cancer. Br J Surg 2011; 98: 418–426. [DOI] [PubMed] [Google Scholar]
- 31. Noh YJ, Choi WS, Kim JH, Kim JC, Yu CS, Kim HC et al Optimal timing for the administration of capecitabine with preoperative chemoradiation for locally advanced rectal cancer. Cancer Res Treat 2006; 38: 30–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Yu CS, Kim TW, Kim JH, Choi WS, Kim HC, Chang HM et al Optimal time interval between capecitabine intake and radiotherapy in preoperative chemoradiation for locally advanced rectal cancer. Int J Radiat Oncol Biol Phys 2007; 67: 1020–1026. [DOI] [PubMed] [Google Scholar]
- 33. Martin LK, Bekaii‐Saab T. Optimizing neoadjuvant therapy for rectal cancer with oxaliplatin. J Natl Compr Canc Netw 2013; 11: 298–307. [DOI] [PubMed] [Google Scholar]
- 34. Hermann RM, Rave‐Fränk M, Pradier O. Combining radiation with oxaliplatin: a review of experimental results. Cancer Radiother 2008; 12: 61–67. [DOI] [PubMed] [Google Scholar]
- 35. Magné N, Fischel JL, Formento P, Etienne MC, Dubreuil A, Marcié S et al Oxaliplatin–5‐fluorouracil and ionizing radiation. Importance of the sequence and influence of p53 status. Oncology 2003; 64: 280–287. [DOI] [PubMed] [Google Scholar]
- 36. Folkvord S, Flatmark K, Seierstad T, Røe K, Rasmussen H, Ree AH. Inhibitory effects of oxaliplatin in experimental radiation treatment of colorectal carcinoma: does oxaliplatin improve 5‐fluorouracil‐dependent radiosensitivity? Radiother Oncol 2008; 86: 428–434. [DOI] [PubMed] [Google Scholar]
- 37. Rosenthal DI, Catalano PJ, Haller DG, Landry JC, Sigurdson ER, Spitz FR et al Phase I study of preoperative radiation therapy with concurrent infusional 5‐fluorouracil and oxaliplatin followed by surgery and postoperative 5‐fluorouracil plus leucovorin for T3/T4 rectal adenocarcinoma: ECOG E1297. Int J Radiat Oncol Biol Phys 2008; 72: 108–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Gérard JP, Azria D, Gourgou‐Bourgade S, Martel‐Laffay I, Hennequin C, Etienne PL et al Comparison of two neoadjuvant chemoradiotherapy regimens for locally advanced rectal cancer: results of the phase III trial ACCORD 12/0405‐prodige 2. J Clin Oncol 2010; 28: 1638–1644. [DOI] [PubMed] [Google Scholar]
- 39. Rödel C, Liersch T, Becker H, Fietkau R, Hohenberger W, Hothorn T et al; German Rectal Cancer Study Group. Preoperative chemoradiotherapy and postoperative chemotherapy with fluorouracil and oxaliplatin versus fluorouracil alone in locally advanced rectal cancer: initial results of the German CAO/ARO/AIO‐04 randomised phase 3 trial. Lancet Oncol 2012; 13: 679–687. [DOI] [PubMed] [Google Scholar]
- 40. Allegra C, Yothers G, O'Connell M, Roh M, Lopa S, Petrellia N et al Final results from NSABP protocol R‐04: neoadjuvant chemoradiation (RT) comparing continuous infusion (CIV) 5‐FU with capecitabine (Cape) with or without oxaliplatin (Ox) in patients with stage II and III rectal cancer. J Clin Oncol 2014; 32(Suppl): Abstract 3603. [Google Scholar]
- 41. Schmoll H, Haustermans K, Price T, Nordlinger B, Hoftheinz R, Daisne J et al Preoperative chemoradiotherapy and postoperative chemotherapy with capecitabine +/− oxaliplatin in locally advanced rectal cancer: interim analysis for disease‐free survival of PETACC‐6. Ann Oncol 2014; 25(Suppl 4): iv167–iv209. [Google Scholar]
- 42. Aschele C, Cionini L, Lonardi S, Pinto C, Cordio S, Rosati G et al Primary tumor response to preoperative chemoradiation with or without oxaliplatin in locally advanced rectal cancer: pathologic results of the STAR‐01 randomized phase III trial. J Clin Oncol 2011; 29: 2773–2780. [DOI] [PubMed] [Google Scholar]
- 43. Deng Y, Chi P, Lan P, Wang L, Chen W, Cui L et al Modified FOLFOX6 with or without radiation versus fluorouracil and leucovorin with radiation in neoadjuvant treatment of locally advanced rectal cancer: initial results of the Chinese FOWARC multicenter, open‐label, randomized three‐arm phase III Trial. J Clin Oncol 2016; 34: 3300–3307. [DOI] [PubMed] [Google Scholar]
- 44. Glynne‐Jones R, Falk S, Maughan TS, Meadows HM, Sebag‐Montefiore D. A phase I/II study of irinotecan when added to 5‐fluorouracil and leucovorin and pelvic radiation in locally advanced rectal cancer: a Colorectal Clinical Oncology Group Study. Br J Cancer 2007; 96: 551–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Willeke F, Horisberger K, Kraus‐Tiefenbacher U, Wenz F, Leitner A, Hochhaus A et al A phase II study of capecitabine and irinotecan in combination with concurrent pelvic radiotherapy (CapIri‐RT) as neoadjuvant treatment of locally advanced rectal cancer. Br J Cancer 2007; 96: 912–917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Hong YS, Kim DY, Lim SB, Choi HS, Jeong SY, Jeong JY et al Preoperative chemoradiation with irinotecan and capecitabine in patients with locally advanced resectable rectal cancer: long‐term results of a phase II study. Int J Radiat Oncol Biol Phys 2011; 79: 1171–1178. [DOI] [PubMed] [Google Scholar]
- 47. Mohiuddin M, Paulus R, Mitchell E, Hanna N, Yuen A, Nichols R et al Neoadjuvant chemoradiation for distal rectal cancer: 5‐year updated results of a randomized phase 2 study of neoadjuvant combined modality chemoradiation for distal rectal cancer. Int J Radiat Oncol Biol Phys 2013; 86: 523–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Gollins S, Sun Myint A, Haylock B, Wise M, Saunders M, Neupane R et al Preoperative chemoradiotherapy using concurrent capecitabine and irinotecan in magnetic resonance imaging‐defined locally advanced rectal cancer: impact on long‐term clinical outcomes. J Clin Oncol 2011; 29: 1042–1049. [DOI] [PubMed] [Google Scholar]
- 49. An X, Lin X, Wang FH, Goodman K, Cai PQ, Kong LH et al Short term results of neoadjuvant chemoradiotherapy with fluoropyrimidine alone or in combination with oxaliplatin in locally advanced rectal cancer: a meta analysis. Eur J Cancer 2013; 49: 843–851. [DOI] [PubMed] [Google Scholar]
- 50. Yang YJ, Cao L, Li ZW, Zhao L, Wu HF, Yue D et al Fluorouracil‐based neoadjuvant chemoradiotherapy with or without oxaliplatin for treatment of locally advanced rectal cancer: an updated systematic review and meta‐analysis. Oncotarget 2016; 7: 45513–45524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Jansen RL, Beets GL. Which way is forward in the treatment of rectal cancer? Nat Rev Clin Oncol 2013; 10: 12–13. [DOI] [PubMed] [Google Scholar]
- 52. Hill EJ, Nicolay NH, Middleton MR, Sharma RA. Oxaliplatin as a radiosensitiser for upper and lower gastrointestinal tract malignancies: what have we learned from a decade of translational research? Crit Rev Oncol Hematol 2012; 83: 353–387. [DOI] [PubMed] [Google Scholar]
- 53. Coveler AL, Richard P, Apisarnthanarax S, Gabriela Chiorean E. Is there a best radiosensitizing agent in the treatment of locally advanced rectal cancer? Curr Colorectal Cancer Rep 2016; 12: 189–200. [Google Scholar]
- 54. Boscia RE, Korbut T, Holden SA, Ara G, Teicher BA. Interaction of topoisomerase I inhibitors with radiation in cis‐diamminedichloroplatinum(II)‐sensitive and ‐resistant cells in vitro and in the FSAIIC fibrosarcoma in vivo . Int J Cancer 1993; 53: 118–123. [DOI] [PubMed] [Google Scholar]
- 55. Choi HJ, Kim NK, Keum KC, Cheon SH, Shin SJ, Baik SH et al Phase I trial of neoadjuvant concurrent chemoradiotherapy with S‐1 and weekly irinotecan in locally advanced rectal cancer. Radiother Oncol 2008; 87: 361–366. [DOI] [PubMed] [Google Scholar]
- 56. Mehta VK, Cho C, Ford JM, Jambalos C, Poen J, Koong A et al Phase II trial of preoperative 3D conformal radiotherapy, protracted venous infusion 5‐fluorouracil, and weekly CPT‐11, followed by surgery for ultrasound‐staged T3 rectal cancer. Int J Radiat Oncol Biol Phys 2003; 55: 132–137. [DOI] [PubMed] [Google Scholar]
- 57. Nakamura T, Yamashita K, Sato T, Ema A, Naito M, Watanabe M. Neoadjuvant chemoradiation therapy using concurrent S‐1 and irinotecan in rectal cancer: impact on long‐term clinical outcomes and prognostic factors. Int J Radiat Oncol Biol Phys 2014; 89: 547–555. [DOI] [PubMed] [Google Scholar]
- 58. Illum H. Irinotecan and radiosensitization in rectal cancer. Anticancer Drugs 2011; 22: 324–329. [DOI] [PubMed] [Google Scholar]
- 59. Messa C, Russo F, Caruso MG, Di Leo A. EGF, TGF‐alpha, and EGF‐R in human colorectal adenocarcinoma. Acta Oncol 1998; 37: 285–289. [DOI] [PubMed] [Google Scholar]
- 60. Porebska I, Harlozińska A, Bojarowski T. Expression of the tyrosine kinase activity growth factor receptors (EGFR, ERB B2, ERB B3) in colorectal adenocarcinomas and adenomas. Tumour Biol 2000; 21: 105–115. [DOI] [PubMed] [Google Scholar]
- 61. Salomon DS, Brandt R, Ciardiello F, Normanno N. Epidermal growth factor‐related peptides and their receptors in human malignancies. Crit Rev Oncol Hematol 1995; 19: 183–232. [DOI] [PubMed] [Google Scholar]
- 62. Goldstein NS, Armin M. Epidermal growth factor receptor immunohistochemical reactivity in patients with American Joint Committee on Cancer Stage IV colon adenocarcinoma: implications for a standardized scoring system. Cancer 2001; 92: 1331–1346. [DOI] [PubMed] [Google Scholar]
- 63. Mayer A, Takimoto M, Fritz E, Schellander G, Kofler K, Ludwig H. The prognostic significance of proliferating cell nuclear antigen, epidermal growth factor receptor, and mdr gene expression in colorectal cancer. Cancer 1993; 71: 2454–2460. [DOI] [PubMed] [Google Scholar]
- 64. Cunningham D, Humblet Y, Siena S, Khayat D, Bleiberg H, Santoro A et al Cetuximab monotherapy and cetuximab plus irinotecan in irinotecan‐refractory metastatic colorectal cancer. N Engl J Med 2004; 351: 337–345. [DOI] [PubMed] [Google Scholar]
- 65. Machiels JP, Sempoux C, Scalliet P, Coche JC, Humblet Y, Van Cutsem E et al Phase I/II study of preoperative cetuximab, capecitabine, and external beam radiotherapy in patients with rectal cancer. Ann Oncol 2007; 18: 738–744. [DOI] [PubMed] [Google Scholar]
- 66. Mardjuadi FI, Carrasco J, Coche JC, Sempoux C, Jouret‐Mourin A, Scalliet P et al Panitumumab as a radiosensitizing agent in KRAS wild‐type locally advanced rectal cancer. Target Oncol 2015; 10: 375–383. [DOI] [PubMed] [Google Scholar]
- 67. Eisterer W, De Vries A, Öfner D, Rabl H, Koplmüller R, Greil R et al; Austrian Breast and Colorectal Cancer Study Group (ABCSG). Preoperative treatment with capecitabine, cetuximab and radiotherapy for primary locally advanced rectal cancer – a phase II clinical trial. Anticancer Res 2014; 34: 6767–6773. [PubMed] [Google Scholar]
- 68. Pinto C, Di Fabio F, Maiello E, Pini S, Latiano T, Aschele C et al Phase II study of panitumumab, oxaliplatin, 5‐fluorouracil, and concurrent radiotherapy as preoperative treatment in high‐risk locally advanced rectal cancer patients (StarPan/STAR‐02 Study). Ann Oncol 2011; 22: 2424–2430. [DOI] [PubMed] [Google Scholar]
- 69. Helbling D, Bodoky G, Gautschi O, Sun H, Bosman F, Gloor B et al Neoadjuvant chemoradiotherapy with or without panitumumab in patients with wild‐type KRAS, locally advanced rectal cancer (LARC): a randomized, multicenter, phase II trial SAKK 41/07. Ann Oncol 2013; 24: 718–725. [DOI] [PubMed] [Google Scholar]
- 70. Dewdney A, Cunningham D, Tabernero J, Capdevila J, Glimelius B, Cervantes A et al Multicenter randomized phase II clinical trial comparing neoadjuvant oxaliplatin, capecitabine, and preoperative radiotherapy with or without cetuximab followed by total mesorectal excision in patients with high‐risk rectal cancer (EXPERT‐C). J Clin Oncol 2012; 30: 1620–1627. [DOI] [PubMed] [Google Scholar]
- 71. Gollins S, West N, Sebag‐Montefiore D, Myint AS, Saunders M, Susnerwala S et al Preoperative chemoradiation with capecitabine, irinotecan and cetuximab in rectal cancer: significance of pre‐treatment and post‐resection RAS mutations. Br J Cancer 2017; 117: 1286–1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Velenik V, Ocvirk J, Oblak I, Anderluh F. Cetuximab in preoperative treatment of rectal cancer – term outcome of the XERT trial. Radiol Oncol 2012; 46: 252–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Fokas E, Conradi L, Weiss C, Sprenger T, Middel P, Rau T et al Preoperative chemoradiation therapy with capecitabine/oxaliplatin and cetuximab in rectal cancer: long‐term results of a prospective phase 1/2 study. Int J Radiat Oncol Biol Phys 2013; 87: 992–999. [DOI] [PubMed] [Google Scholar]
- 74. Rödel C, Arnold D, Hipp M, Liersch T, Dellas K, Iesalnieks I et al Phase I–II trial of cetuximab, capecitabine, oxaliplatin, and radiotherapy as preoperative treatment in rectal cancer. Int J Radiat Oncol Biol Phys 2008; 70: 1081–1086. [DOI] [PubMed] [Google Scholar]
- 75. Bertolini F, Chiara S, Bengala C, Antognoni P, Dealis C, Zironi S et al Neoadjuvant treatment with single‐agent cetuximab followed by 5‐FU, cetuximab, and pelvic radiotherapy: a phase II study in locally advanced rectal cancer. Int J Radiat Oncol Biol Phys 2009; 73: 466–472. [DOI] [PubMed] [Google Scholar]
- 76. Horisberger K, Treschl A, Mai S, Barreto‐Miranda M, Kienle P, Ströbel P et al; MARGIT (Mannheimer Arbeitsgruppe för Gastrointestinale Tumoren). Cetuximab in combination with capecitabine, irinotecan, and radiotherapy for patients with locally advanced rectal cancer: results of a phase II MARGIT trial. Int J Radiat Oncol Biol Phys 2009; 74: 1487–1493. [DOI] [PubMed] [Google Scholar]
- 77. Peeters M, Douillard JY, Van Cutsem E, Siena S, Zhang K, Williams R et al Mutant KRAS codon 12 and 13 alleles in patients with metastatic colorectal cancer: assessment as prognostic and predictive biomarkers of response to panitumumab. J Clin Oncol 2013; 31: 759–765. [DOI] [PubMed] [Google Scholar]
- 78. Douillard JY, Oliner KS, Siena S, Tabernero J, Burkes R, Barugel M et al Panitumumab–FOLFOX4 treatment and RAS mutation in colorectal cancer. N Engl J Med 2013; 369: 1023–1034. [DOI] [PubMed] [Google Scholar]
- 79. Ahsan A, Hiniker SM, Davis MA, Lawrence TS, Nyati MK. Role of cell cycle in epidermal growth factor receptor inhibitor‐mediated radiosensitization. Cancer Res 2009; 69: 5108–5114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Gollins S, Sebag‐Montefiore D. Neoadjuvant treatment strategies for locally advanced rectal cancer. Clin Oncol (R Coll Radiol) 2016; 28: 146–151. [DOI] [PubMed] [Google Scholar]
- 81. Glynne‐Jones R, Mawdsley S, Harrison M. Antiepidermal growth factor receptor radiosensitizers in rectal cancer. Anticancer Drugs 2011; 22: 330–340. [DOI] [PubMed] [Google Scholar]
- 82. Cubillo A, Hernando‐Requejo O, García‐García E, Rodriguez‐Pascual J, De Vicente E, Morelli P et al A prospective pilot study of target‐guided personalized chemotherapy with intensity‐modulated radiotherapy in patients with early rectal cancer. Am J Clin Oncol 2014; 37: 117–121. [DOI] [PubMed] [Google Scholar]
- 83. Nieder C, Wiedenmann N, Andratschke NH, Astner ST, Molls M. Radiation therapy plus angiogenesis inhibition with bevacizumab: rationale and initial experience. Rev Recent Clin Trials 2007; 2: 163–168. [DOI] [PubMed] [Google Scholar]
- 84. Salazar R, Capdevila J, Laquente B, Manzano JL, Pericay C, Villacampa MM et al A randomized phase II study of capecitabine‐based chemoradiation with or without bevacizumab in resectable locally advanced rectal cancer: clinical and biological features. BMC Cancer 2015; 15: 60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Landry JC, Feng Y, Prabhu RS, Cohen SJ, Staley CA, Whittington R et al Phase II trial of preoperative radiation with concurrent capecitabine, oxaliplatin, and bevacizumab followed by surgery and postoperative 5‐fluorouracil, leucovorin, oxaliplatin (FOLFOX), and bevacizumab in patients with locally advanced rectal cancer: 5‐year clinical outcomes ECOG‐ACRIN Cancer Research Group E3204. Oncologist 2015; 20: 615–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Jeong YK, Kim MS, Lee JY, Kim EH, Kim W, Ha H et al Sorafenib acts synergistically in combination with radiotherapy without causing intestinal damage in colorectal cancer. Tumori 2013; 99: 176–182. [DOI] [PubMed] [Google Scholar]
- 87. Von Moos R, Koeberle D, Schacher S, Hayoz S, Winterhalder RC, Roth A et al; Swiss Group for Clinical Cancer Research (SAKK). Neoadjuvant radiotherapy combined with capecitabine and sorafenib in patients with advanced KRAS‐mutated rectal cancer: a phase I/II trial (SAKK 41/08). Eur J Cancer 2018; 89: 82–89. [DOI] [PubMed] [Google Scholar]
- 88. Kim R, Prithviraj GK, Shridhar R, Hoffe SE, Jiang K, Zhao X et al Phase I study of pre‐operative continuous 5‐FU and sorafenib with external radiation therapy in locally advanced rectal adenocarcinoma. Radiother Oncol 2016; 118: 382–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Page P, Yang LX. Novel chemoradiosensitizers for cancer therapy. Anticancer Res 2010; 30: 3675–3682. [PubMed] [Google Scholar]
- 90. Verhagen CV, de Haan R, Hageman F, Oostendorp TP, Carli AL, O'Connor MJ et al Extent of radiosensitization by the PARP inhibitor olaparib depends on its dose, the radiation dose and the integrity of the homologous recombination pathway of tumor cells. Radiother Oncol 2015; 116: 358–365. [DOI] [PubMed] [Google Scholar]
- 91. Sabbatino F, Fusciello C, Somma D, Pacelli R, Poudel R, Pepin D et al Effect of p53 activity on the sensitivity of human glioblastoma cells to PARP‐1 inhibitor in combination with topoisomerase I inhibitor or radiation. Cytometry A 2014; 85: 953–961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Shelton JW, Waxweiler TV, Landry J, Gao H, Xu Y, Wang L et al In vitro and in vivo enhancement of chemoradiation using the oral PARP inhibitor ABT‐888 in colorectal cancer cells. Int J Radiat Oncol Biol Phys 2013; 86: 469–476. [DOI] [PubMed] [Google Scholar]
- 93. Czito BG, Deming DA, Jameson GS, Mulcahy MF, Vaghefi H, Dudley MW et al Safety and tolerability of veliparib combined with capecitabine plus radiotherapy in patients with locally advanced rectal cancer: a phase 1b study. Lancet Gastroenterol Hepatol 2017; 2: 418–426. [DOI] [PubMed] [Google Scholar]
- 94. de Haan R, Pluim D, Van Triest B, Van den Heuvel M, Peulen H, Van Berlo D et al Improved pharmacodynamic (PD) assessment of low dose PARP inhibitor PD activity for radiotherapy and chemotherapy combination trials. Radiother Oncol 2018; 126: 443–449. [DOI] [PubMed] [Google Scholar]
- 95. Markman JL, Shiao SL. Impact of the immune system and immunotherapy in colorectal cancer. J Gastrointest Oncol 2015; 6: 208–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Zhen YH, Liu XH, Yang Y, Li B, Tang JL, Zeng QX et al Phase I/II study of adjuvant immunotherapy with sentinel lymph node T lymphocytes in patients with colorectal cancer. Cancer Immunol Immunother 2015; 64: 1083–1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Lynch D, Murphy A. The emerging role of immunotherapy in colorectal cancer. Ann Transl Med 2016; 4: 305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Le DT, Durham JN, Smith KN, Wang H, Bartlett BR, Aulakh LK et al Mismatch repair deficiency predicts response of solid tumors to PD‐1 blockade. Science 2017; 357: 409–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Overman MJ, McDermott R, Leach JL, Lonardi S, Lenz HJ, Morse MA et al Nivolumab in patients with metastatic DNA mismatch repair‐deficient or microsatellite instability‐high colorectal cancer (CheckMate 142): an open‐label, multicentre, phase 2 study. Lancet Oncol 2017; 18: 1182–1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. http://ClinicalTrials.gov. Safety and Efficacy of Atezolizumab Combined to Preoperative Radio‐chemotherapy in Localized Rectal Cancer (R‐IMMUNE) NCT03127007. https://clinicaltrials.gov/ct2/show/NCT03127007 [accessed 15 August 2018].
- 101. Sinicrope FA, Gill S. Role of cyclooxygenase‐2 in colorectal cancer. Cancer Metastasis Rev 2004; 23: 63–75. [DOI] [PubMed] [Google Scholar]
- 102. Zhu AX, Willett CG. Chemotherapeutic and biologic agents as radiosensitizers in rectal cancer. Semin Radiat Oncol 2003; 13: 454–468. [DOI] [PubMed] [Google Scholar]
- 103. Debucquoy A, Roels S, Goethals L, Libbrecht L, Van Cutsem E, Geboes K et al Double blind randomized phase II study with radiation + 5‐fluorouracil +/– celecoxib for resectable rectal cancer. Radiother Oncol 2009; 93: 273–278. [DOI] [PubMed] [Google Scholar]
- 104. Caster JM, Yu SK, Patel AN, Newman NJ, Lee ZJ, Warner SB et al Effect of particle size on the biodistribution, toxicity, and efficacy of drug‐loaded polymeric nanoparticles in chemoradiotherapy. Nanomedicine 2017; 13: 1673–1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Tian X, Nguyen M, Foote HP, Caster JM, Roche KC, Peters CG et al CRLX101, a nanoparticle–drug conjugate containing camptothecin, improves rectal cancer chemoradiotherapy by inhibiting DNA repair and HIF1α. Cancer Res 2017; 77: 112–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Folkvord S, Ree AH, Furre T, Halvorsen T, Flatmark K. Radiosensitization by SAHA in experimental colorectal carcinoma models – in vivo effects and relevance of histone acetylation status. Int J Radiat Oncol Biol Phys 2009; 74: 546–552. [DOI] [PubMed] [Google Scholar]
- 107. Saelen MG, Ree AH, Kristian A, Fleten KG, Furre T, Hektoen HH et al Radiosensitization by the histone deacetylase inhibitor vorinostat under hypoxia and with capecitabine in experimental colorectal carcinoma. Radiat Oncol 2012; 7: 165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Kleiman LB, Krebs AM, Kim SY, Hong TS, Haigis KM. Comparative analysis of radiosensitizers for K‐RAS mutant rectal cancers. PLoS One 2013; 8: e82982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Hill EJ, Roberts C, Franklin JM, Enescu M, West N, MacGregor TP et al Clinical trial of oral nelfinavir before and during radiation therapy for advanced rectal cancer. Clin Cancer Res 2016; 22: 1922–1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Buijsen J, Lammering G, Jansen RL, Beets GL, Wals J, Sosef M et al Phase I trial of the combination of the Akt inhibitor nelfinavir and chemoradiation for locally advanced rectal cancer. Radiother Oncol 2013; 107: 184–188. [DOI] [PubMed] [Google Scholar]
- 111. Spalding AC, Lawrence TS. New and emerging radiosensitizers and radioprotectors. Cancer Invest 2006; 24: 444–456. [DOI] [PubMed] [Google Scholar]
- 112. Deorukhkar A, Ahuja N, Mercado AL, Diagaradjane P, Raju U, Patel N et al Zerumbone increases oxidative stress in a thiol‐dependent ROS‐independent manner to increase DNA damage and sensitize colorectal cancer cells to radiation. Cancer Med 2015; 4: 278–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. O'Neil BH, Raftery L, Calvo BF, Chakravarthy AB, Ivanova A, Myers MO et al A phase I study of bortezomib in combination with standard 5‐fluorouracil and external‐beam radiation therapy for the treatment of locally advanced or metastatic rectal cancer. Clin Colorectal Cancer 2010; 9: 119–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Appelt AL, Pløen J, Vogelius IR, Bentzen SM, Jakobsen A. Radiation dose–response model for locally advanced rectal cancer after preoperative chemoradiation therapy. Int J Radiat Oncol Biol Phys 2013; 85: 74–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Appelt AL, Pløen J, Harling H, Jensen FS, Jensen LH, Jørgensen JC et al High‐dose chemoradiotherapy and watchful waiting for distal rectal cancer: a prospective observational study. Lancet Oncol 2015; 16: 919–927. [DOI] [PubMed] [Google Scholar]
- 116. Gerard JP, Chapet O, Nemoz C, Hartweig J, Romestaing P, Coquard R et al Improved sphincter preservation in low rectal cancer with high‐dose preoperative radiotherapy: the Lyon R96‐02 randomized trial. J Clin Oncol 2004; 22: 2404–2409. [DOI] [PubMed] [Google Scholar]
- 117. Sun Myint A, Smith FM, Gollins S, Wong H, Rao C, Whitmarsh K et al Dose escalation using contact X‐ray brachytherapy after external beam radiotherapy as nonsurgical treatment option for rectal cancer: outcomes from a single‐center experience. Int J Radiat Oncol Biol Phys 2018; 100: 565–573. [DOI] [PubMed] [Google Scholar]
- 118. Wee CW, Kang HC, Wu HG, Chie EK, Choi N, Park JM et al Intensity‐modulated radiotherapy versus three‐dimensional conformal radiotherapy in rectal cancer treated with neoadjuvant concurrent chemoradiation: a meta‐analysis and pooled‐analysis of acute toxicity. Jpn J Clin Oncol 2018; 48: 458–466. [DOI] [PubMed] [Google Scholar]
- 119. Hathout L, Williams TM, Jabbour SK. The impact of novel radiation treatment techniques on toxicity and clinical outcomes in rectal cancer. Curr Colorectal Cancer Rep 2017; 13: 61–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Kamada T, Tsujii H, Blakely EA, Debus J, De Neve W, Durante M et al Carbon ion radiotherapy in Japan: an assessment of 20 years of clinical experience. Lancet Oncol 2015; 16: e93–e100. [DOI] [PubMed] [Google Scholar]
- 121. Bosset JF, Calais G, Mineur L, Maingon P, Stojanovic‐Rundic S, Bensadoun RJ et al; EORTC Radiation Oncology Group. Fluorouracil‐based adjuvant chemotherapy after preoperative chemoradiotherapy in rectal cancer: long‐term results of the EORTC 22921 randomised study. Lancet Oncol 2014; 15: 184–190. [DOI] [PubMed] [Google Scholar]
- 122. Breugom AJ, Swets M, Bosset JF, Collette L, Sainato A, Cionini L et al Adjuvant chemotherapy after preoperative (chemo)radiotherapy and surgery for patients with rectal cancer: a systematic review and meta‐analysis of individual patient data. Lancet Oncol 2015; 16: 200–207. [DOI] [PubMed] [Google Scholar]
- 123. Franke AJ, Parekh H, Starr JS, Tan SA, Iqbal A, George TJ Jr. Total neoadjuvant therapy: a shifting paradigm in locally advanced rectal cancer management. Clin Colorectal Cancer 2018; 17: 1–12. [DOI] [PubMed] [Google Scholar]
- 124. Fernández‐Martos C, Pericay C, Aparicio J, Salud A, Safont M et al Phase II, randomized study of concomitant chemoradiotherapy followed by surgery and adjuvant capecitabine plus oxaliplatin (CAPOX) compared with induction CAPOX followed by concomitant chemoradiotherapy and surgery in magnetic resonance imaging‐defined, locally advanced rectal cancer: Grupo Cancer de Recto 3 Study. J Clin Oncol 2010; 28: 859–865. [DOI] [PubMed] [Google Scholar]
- 125. Garcia‐Aguilar J, Chow OS, Smith DD, Marcet JE, Cataldo PA, Varma MG et al; Timing of Rectal Cancer Response to Chemoradiation Consortium. Effect of adding mFOLFOX6 after neoadjuvant chemoradiation in locally advanced rectal cancer: a multicentre, phase 2 trial. Lancet Oncol 2015; 16: 957–966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Gilbert A, Ziegler L, Martland M, Davidson S, Efficace F, Sebag‐Montefiore D et al Systematic review of radiation therapy toxicity reporting in randomized controlled trials of rectal cancer: a comparison of patient‐reported outcomes and clinician toxicity reporting. Int J Radiat Oncol Biol Phys 2015; 92: 555–567. [DOI] [PubMed] [Google Scholar]
- 127. Chau I, Brown G, Cunningham D, Tait D, Wotherspoon A, Norman AR et al Neoadjuvant capecitabine and oxaliplatin followed by synchronous chemoradiation and total mesorectal excision in magnetic resonance imaging‐defined poor‐risk rectal cancer. J Clin Oncol 2006; 24: 668–674. [DOI] [PubMed] [Google Scholar]
- 128. Gollins S, West N, Sebag‐Montefiore D, Susnerwala S, Falk S, Brown et al A prospective phase II study of pre‐operative chemotherapy then short‐course radiotherapy for high risk rectal cancer: COPERNICUS. Br J Cancer 2018; (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Deng Y, Chi P, Lan P, Wang L, Chen W, Cui L et al Modified FOLFOX6 with or without radiation in neoadjuvant treatment of locally advanced rectal cancer: final results of the Chinese FOWARC randomized trial. J Clin Oncol 2018; 36(Suppl): Abstract 3502. [Google Scholar]
- 130. Weiser MR, Zhang Z, Schrag D. Locally advanced rectal cancer: time for precision therapeutics. Am Soc Clin Oncol Educ Book 2015; 35: e192–e196. [DOI] [PubMed] [Google Scholar]
- 131. Koyama FC, Lopes Ramos CM, Ledesma F, Alves VAF, Fernandes JM, Vailati BB et al Effect of Akt activation and experimental pharmacological inhibition on responses to neoadjuvant chemoradiotherapy in rectal cancer. Br J Surg 2018; 105: e192–e203. [DOI] [PubMed] [Google Scholar]
- 132. Sammour T, Price BA, Krause KJ, Chang GJ. Nonoperative management or ‘watch and wait’ for rectal cancer with complete clinical response after neoadjuvant chemoradiotherapy: a critical appraisal. Ann Surg Oncol 2017; 24: 1904–1915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Teo MTW, McParland L, Appelt AL, Sebag‐Montefiore D. Phase 2 neoadjuvant treatment intensification trials in rectal cancer: a systematic review. Int J Radiat Oncol Biol Phys 2018; 100: 146–158. [DOI] [PubMed] [Google Scholar]
- 134. Pozo ME, Fang SH. Watch and wait approach to rectal cancer: a review. World J Gastrointest Surg 2015; 7: 306–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Smith FM, Chang KH, Sheahan K, Hyland J, O'Connell PR, Winter DC. The surgical significance of residual mucosal abnormalities in rectal cancer following neoadjuvant chemoradiotherapy. Br J Surg 2012; 99: 993–1001. [DOI] [PubMed] [Google Scholar]
- 136. Maas M, Nelemans PJ, Valentini V, Das P, Rödel C, Kuo LJ et al Long‐term outcome in patients with a pathological complete response after chemoradiation for rectal cancer: a pooled analysis of individual patient data. Lancet Oncol 2010; 11: 835–844. [DOI] [PubMed] [Google Scholar]
- 137. Habr‐Gama A, Perez RO, Proscurshim I, Campos FG, Nadalin W, Kiss D et al Patterns of failure and survival for nonoperative treatment of stage c0 distal rectal cancer following neoadjuvant chemoradiation therapy. J Gastrointest Surg 2006; 10: 1319–1328. [DOI] [PubMed] [Google Scholar]
- 138. Loughrey MB, Quirke P, Shepherd NA. Standards and Datasets for Reporting Cancers. Dataset for Colorectal Cancer Histopathology Reports. Royal College of Pathologists: London, 2014. [Google Scholar]
- 139. Martin ST, Heneghan HM, Winter DC. Systematic review and meta‐analysis of outcomes following pathological complete response to neoadjuvant chemoradiotherapy for rectal cancer. Br J Surg 2012; 99: 918–928. [DOI] [PubMed] [Google Scholar]
- 140. Smith FM, Waldron D, Winter DC. Rectum‐conserving surgery in the era of chemoradiotherapy. Br J Surg 2010; 97: 1752–1764. [DOI] [PubMed] [Google Scholar]
- 141. Fokas E, Liersch T, Fietkau R, Hohenberger W, Beissbarth T, Hess C et al Tumor regression grading after preoperative chemoradiotherapy for locally advanced rectal carcinoma revisited: updated results of the CAO/ARO/AIO‐94 trial. J Clin Oncol 2014; 32: 1554–1562. [DOI] [PubMed] [Google Scholar]
- 142. Fokas E, Ströbel P, Fietkau R, Ghadimi M, Liersch T, Grabenbauer GG et al; German Rectal Cancer Study Group. Tumor regression grading after preoperative chemoradiotherapy as a prognostic factor and individual‐level surrogate for disease‐free survival in rectal cancer. J Natl Cancer Inst 2017; 109. doi: http://onlinelibrary.wiley.com/doi/10.1093/jnci/djx095. [DOI] [PubMed] [Google Scholar]
- 143. Habr‐Gama A, Gama‐Rodrigues J, São Julião GP, Proscurshim I, Sabbagh C, Lynn PB et al Local recurrence after complete clinical response and watch and wait in rectal cancer after neoadjuvant chemoradiation: impact of salvage therapy on local disease control. Int J Radiat Oncol Biol Phys 2014; 88: 822–828. [DOI] [PubMed] [Google Scholar]
- 144. van der Valk MJM, Hilling DE, Bastiaannet E, Meershoek‐Klein Kranenbarg E, Beets GL, Figueiredo NL et al; IWWD Consortium. Long‐term outcomes of clinical complete responders after neoadjuvant treatment for rectal cancer in the International Watch & Wait Database (IWWD): an international multicentre registry study. Lancet 2018; 391: 2537–2545. [DOI] [PubMed] [Google Scholar]
- 145. Martens MH, Maas M, Heijnen LA, Lambregts DM, Leijtens JW, Stassen LP et al Long‐term outcome of an organ preservation program after neoadjuvant treatment for rectal cancer. J Natl Cancer Inst 2016; 108: pii: djw171. [DOI] [PubMed] [Google Scholar]
- 146. Patel UB, Taylor F, Blomqvist L, George C, Evans H, Tekkis P et al Magnetic resonance imaging‐detected tumor response for locally advanced rectal cancer predicts survival outcomes: MERCURY experience. J Clin Oncol 2011; 29: 3753–3760. [DOI] [PubMed] [Google Scholar]
- 147. Glynne‐Jones R, Hughes R. Critical appraisal of the ‘wait and see’ approach in rectal cancer for clinical complete responders after chemoradiation. Br J Surg 2012; 99: 897–909. [DOI] [PubMed] [Google Scholar]
- 148. Verseveld M, de Graaf EJ, Verhoef C, van Meerten E, Punt CJ, de Hingh IH et al; CARTS Study Group. Chemoradiation therapy for rectal cancer in the distal rectum followed by organ‐sparing transanal endoscopic microsurgery (CARTS study). Br J Surg 2015; 102: 853–860. [DOI] [PubMed] [Google Scholar]
- 149. Creavin B, Ryan E, Martin ST, Hanly A, O'Connell PR, Sheahan K et al Organ preservation with local excision or active surveillance following chemoradiotherapy for rectal cancer. Br J Cancer 2017; 116: 169–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Jang JK, Lee JL, Park SH, Park HJ, Park IJ, Kim JH et al Magnetic resonance tumour regression grade and pathological correlates in patients with rectal cancer. Br J Surg 2018. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 151. Rullier E, Rouanet P, Tuech JJ, Valverde A, Lelong B, Rivoire M et al Organ preservation for rectal cancer (GRECCAR 2): a prospective, randomised, open‐label, multicentre, phase 3 trial. Lancet 2017; 390: 469–479. [DOI] [PubMed] [Google Scholar]
- 152. McNair AG, Heywood N, Tiernan J, Verjee A, Bach SP, Fearnhead NS; ORACLE Collaboration . A national patient and public colorectal research agenda: integration of consumer perspectives in bowel disease through early consultation. Colorectal Dis 2017; 19: O75–O85. [DOI] [PubMed] [Google Scholar]
- 153. Leong KJ, Beggs A, James J, Morton DG, Matthews GM, Bach SP. Biomarker‐based treatment selection in early‐stage rectal cancer to promote organ preservation. Br J Surg 2014; 101: 1299–1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Molinari C, Matteucci F, Caroli P, Passardi A. Biomarkers and molecular imaging as predictors of response to neoadjuvant chemoradiotherapy in patients with locally advanced rectal cancer. Clin Colorectal Cancer 2015; 14: 227–238. [DOI] [PubMed] [Google Scholar]
- 155. Rogers AC, Gibbons D, Hanly AM, Hyland JM, O'Connell PR, Winter DC et al Prognostic significance of tumor budding in rectal cancer biopsies before neoadjuvant therapy. Mod Pathol 2014; 27: 156–162. [DOI] [PubMed] [Google Scholar]
- 156. Bowden DL, Sutton PA, Wall MA, Jithesh PV, Jenkins RE, Palmer DH et al Proteomic profiling of rectal cancer reveals acid ceramidase is implicated in radiation response. J Proteomics 2018; 179: 53–60. [DOI] [PubMed] [Google Scholar]
- 157. Nordgård O, Tjensvoll K, Gilje B, Søreide K. Circulating tumour cells and DNA as liquid biopsies in gastrointestinal cancer. Br J Surg 2018; 105: e110–e120. [DOI] [PubMed] [Google Scholar]


