Abstract
Background:
Glioblastoma multiform (GBM) is the most common and most malignant of the glial tumors that begins primarily in brain tissue. Genetic background could be considered as an important predisposing factor in GBM. Autocrine motility factor receptor (AMFR) is a cytokine receptor that participates in a lot of physiologic and pathologic processes like: Cellular motility and metastasis. So, it seems that this protein has an essential role in pathophysiology of several cancers and could be a potential diagnostic and or therapeutic target in GBM. The aim of this study is to investigate the association of AMFR (rs2440472, rs373191257) gene polymorphism and GBM in a representative Iranian population.
Materials and Methods:
This study includes 81 cases of GBM and 117 control subjects. After DNA extraction, polymerase chain reaction - high resolution melting reaction was performed. For each single nucleotide polymorphisms, 12 samples were selected for sequencing. Data was analyzed using Chi-square test and Logistic regression.
Results:
For rs2440472, frequency of GG genotype in the case group was increased compared to the control group (51.9% vs. 34.2% respectively, P = 0.013). After adjusting for sex and age by logistic regression our results were the same (P = 0.017, odds ratio = 2.056). Allelic frequencies for rs2440472 among cases and controls were not significantly different (P = 0.058). For rs373191257, genotypic and allelic frequencies were not significantly different between two groups.
Conclusion:
Our results showed the possible association between the AMFR rs2440472 gene polymorphism with susceptibility to GBM.
Keywords: Autocrine motility factor receptor, cancer, glioblastoma multiform, polymorphism, single nucleotide polymorphisms
INTRODUCTION
Gliomas are among the most prevalent primary tumors of central nervous system (CNS). Glioblastoma multiform (GBM) as the most fatal malignant primary tumor of CNS, consists 54% of all Gliomas.[1] This tumor can originate from normal brain cells or develop from an existing low-grade astrocytoma.[2] There are several possible risk factors such as prior exposure to radiotherapy for GBM; however genetic background could be considered as an important predisposing factor in GBM.[3] The current treatments of GBM only provides a 12–18 month survival period postdiagnosis.[1] In spite of surgical treatment and chemotherapy, almost all GBM patients undergo tumor recurrence.[4] This necessitates more investigation to define molecular pathogenesis of this malignancy.
One of the most important molecular changes that occur during carcinogenesis is reprogramming of cell's metabolic pathways. In this process expression of some genes that control key metabolic pathways like glycogenesis, lipogenesis and nucleic acid synthesis is changed. These changes are thought to be adaptive changes to cope with new situations and cause more aggressive phenotype.[5]
Autocrine motility factor/phosphoglucose isomeras (AMF/PGI) gene shows increased expression in cancerous cells. This gene codes glucose-6-phosphate isomerase; an enzyme which catalyzes the conversion of glucose-6-phosphate to fructose-6-phosphate in glycolysis and gluconeogenesis pathways. The product of this gene in nervous system acts as a neurotropic factor.[6] Also it acts as maturation factor (MF) that causes differentiation and maturation of human myeloid leukemia cells.[7] It was shown that P53, a tumor suppressor gene, is regulated downstream to AMF/PGI. Also P21, a cyclin dependent kinase, exhibited increased expression in AMF/PGI knock downed cells.[8] These findings are consistent with a role of this gene in cell-cycle regulation.
AMF receptor (AMFR/gp78) is a cytokine cell membrane receptor for AMF/PGI that plays a possible role in various physiologic and pathologic processes like: Signal transduction, cellular motility, and metastasis.[7] AMFR also expressed in the surface of endoplasmic reticulum as a part of E3 ubiquitin ligase.[9] Here by making a connection to KAI1 (CD28), a tumor suppressor protein, causes its degeneration. This is the possible mechanism for the roll of AMFR in metastasis.[10]
Regarding to the possible role of AMFR gene in carcinogenesis and the importance of determining molecular pathogenesis in GBM patients, the aim of this study is to investigate the association of 2 single nucleotide polymorphisms (SNPs) (rs2440472, rs373191257) of AMFR gene and GBM in a representative sample of Iranian population.
MATERIALS AND METHODS
Ethical approval of this study
The protocol of this study was confirmed by regional ethics committee of Isfahan University of Medical Sciences (293404). Before enrolling the subjects to this study, they were informed about the goal of this study and the process of collecting samples. Informed consent was taken from subjects.
Subjects
This case-control study includes 81 cases and 117 control subjects.
47 paraffin embedded brain tissue samples were taken from pathology Department of Alzahra University Hospital (the major referral hospital of Isfahan University of Medical Sciences). Pathologic diagnosis for these tissue samples was GBM. These samples were taken from 2013 to 2014. Blood samples were taken from another 34 GBM patients, who were under chemotherapy and radiotherapy regimens from Milad Hospital, Isfahan. These samples were taken from 2013 to 2015. Two mentioned hospitals are 2 major regional referral hospitals in center of Iran. One-hundred seventeen blood samples of coronary artery disease negative individuals (their angiographic reports don’t show any coronary arterial disease) who had a negative history of cancer and neurological symptoms, were used in this study as control group. These samples were taken from Selenegene study.[11]
Genomic DNA extraction and genotyping
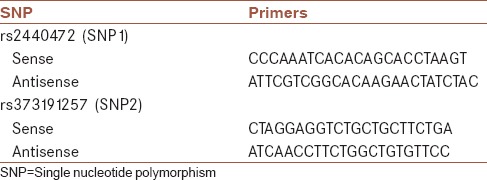
DNA extraction from brain tissue samples was performed using paraffin embedded tissue - DNA extraction kit (Yektatajhiz Inc., Tehran, Iran) and for whole blood samples, blood - DNA extraction kit (Yektatajhiz Inc, Tehran, Iran) was used according to the manufacturer's protocol. NCBI Gene and Ensembl databases were used to detect SNP1 (rs2440472). SNP2 (rs373191257) was selected according to a previous study. In this study, rs373191257 was identified as a mutation in cancerous patients.[12] Primers were designed by Beacon Designer v. 8 (Premier Biosoft International, Palo Alto, California, USA). Primers’ sequences are shown in Table 1. The amplicon length for SNP1 (rs2440472) and SNP2 (rs373191257) were 228 bps and 221 bps respectively. Polymerase chain reaction - high resolution melting (PCR-HRM) reaction, as an SNP detecting method,[13] was performed on 198 samples (81 cases and 117 controls) by Rotor-gene 6000 (Corbett Life Sciences, Australia). Type-it HRM PCR kit (QIAGEN Inc., Germany) was used in this study. In this reaction final volume was set to 10 μL (2 μL of genomic DNA containing 20 ng of extracted DNA, 2 μL of RNase free water, 1 μL of primer, 5 μL of HRM master mix). Temperature program for PCR stage of this reaction was as following: 15 min at 95°C (Holding stage); 40 PCR thermal cycles (10 s at 95°C, 30 s at 65°C, 20 s at 72°C). At the end, HRM stage of this reaction was performed from 70°C to 90°C with increasing 0.1°C at each stage. PCR-HRM reaction results analyzed by Rotor-Gene Q Series (Corbett Life Sciences, Australia) Software program. For each SNP, 12 samples selected for sequencing. DNA sequencing was performed by Bioneer Inc. Korea.
Table 1.
Primers’ sequences
Data analysis
All the data analysis was performed with SPSS software version 18.0 (IBM Inc., New York, United States). Hardy-Weinberg equation was tested to compare the observed genotype frequencies to the expected ones by Chi-square analysis. Chi-square test was used to find any difference between allelic and genotypic frequencies among cases and controls. Logistic regression analysis was used to analyze the effect of AMFR gene polymorphism (rs2440472, rs373191257) on the susceptibility to GBM after adjustment for sex and age. P < 0.05 considered statistically significant.
RESULTS
In the present study, AMFR rs2440472 and rs373191257 gene polymorphisms were investigated in 81 patients and 117 healthy controls. Demographic characteristic of all participants is shown in Table 2. Genotypic frequencies of cases (P < 0.01) and controls (P < 0.01) were not consistent with Hardy-Weinberg equation. Difference in sex between two groups of this study was not statistically significant (P = 0.258).
Table 2.
Demographic features of participants
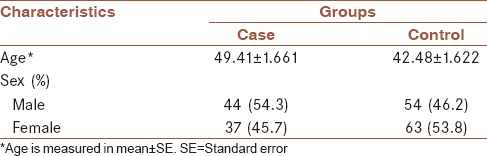
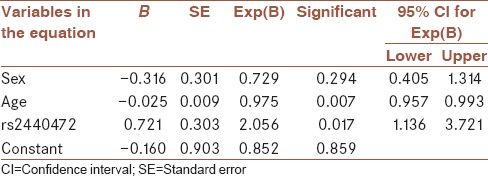
Table 3 demonstrates the allele frequencies and genotype profile of cases and controls. For SNP1 (rs2440472), 82 individuals were observed with GG genotype and 116 individuals were observed with GA genotype. For rs2440472 frequency of GG genotype in the case group was increased compared to the control group by using a Chi-square test (51.9% vs. 34.2% respectively, P = 0.013). Actually in this investigation, increased occurrence of the disease was observed with GG genotype (odds ratio [OR] = 2.073, 95% confidence interval = 1.161–3.701). After adjusting for sex and age by logistic regression our results were the same (P = 0.017, OR = 2.056). Complementary analysis, by adding age and sex to our model in logistic regression analysis are shown in Table 4. Allelic frequencies for rs2440472 among cases and controls were not significantly different using a Chi-square test (75.9% vs. 67.1% respectively, P = 0.058). For SNP2 (rs373191257) all individuals in case and control groups showed homozygote wild type genotype (TT).
Table 3.
Allelic and genotypic distributions in case and control groups
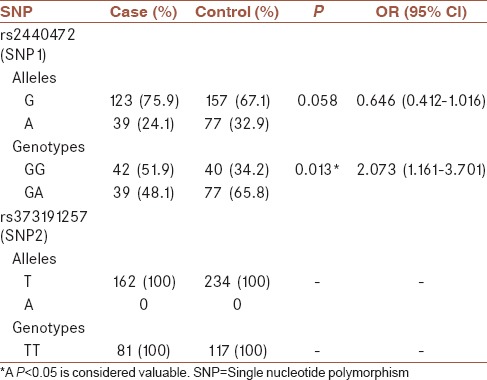
Table 4.
Logistic regression for genotype analysis
DISCUSSION
After analysis, our data showed a possible relationship between AMFR gene polymorphism and GBM. This relationship was shown in genotypic frequencies of SNP1 (rs2440472). The mutant heterozygote genotype (GA) was significantly higher in GBM patients. About SNP2 (rs373191257), our study didn’t show any difference between allelic and genotypic frequencies, because all the individuals showed the same genotype (TT). This is possibly due to very low Frequency of mutant genotype in selected population. We believe that this is the first paper that investigated AMFR gene polymorphism in GBM patients. Earlier studies investigated role of this gene in pathogenesis of other cancers.
AMF participates in different molecular pathways during tumorigenesis. AMF, by binding to its cell surface receptor (AMFR) enhances metastasis.[7] Via a mitogen activated protein kinase pathway, AMF induces Matrix metalloproteinase 3 expression. This protease destructs basement membrane and extracellular matrix and possibly promotes invasion of cancerous cells.[14] AMFR is also defined as a dowstream target of miR-139-5p in colorectal cancer. In this cancer, MiR-139-5p suppresses cellular invasion and metastasis by down regulating AMFR and NOTCH1.[15] AMFR regulate ROCK2 expression. ROCK2 via its downstream target cofilin manipulates cell motility. Also, AMFR controls cell adhesive properties by upregulating E-cadherin and intercellular adhesion molecule-1.[16] AMF overexpression mediates epithelial to mesenchymal transition[17] by upregulating ZEB1/ZEB2 as a downstream target of miR-200.[18]
A study by Kara et al. showed an association between AMFR and vascular endothelial growth factor (VEGF) expression levels.[19] AMF is upregulated in hypoxic conditions as a downstream target of hypoxia inducible factor-1 and VEGF. By this mechanism hypoxia enhances invasiveness of cancerous cells.[20] AMF induces fms like tyrosine kinase (Flt-1) expression by activating phosphatidylinositol 3 kinase and protein kinase C. Flt-1 is known as VEGF receptor.[21]
AMF also mediates cell cycle regulation. In a study, reduced p27Kip1 expression was observed in cells with induced AMF expression.[22] By activating ERK, AMF promotes G1/S phase transition.[23] AMF protects cells from apoptosis via PI3K/PKB pathway.[24] Apaf-1 and caspase-9 are two proteins of the “apoptosome” complex. AMF by downregulating these proteins, could protect cells from apoptosis. This mechanism is suggested as a novel route of chemotherapy resistance.[25] Also, anti-apoptotic properties of AMF plays a pathogenic role in other disease like rheumatoid arthritis.[23]
AMF and/or AMFR showed increased expression in breast cancer,[26] gastric cancer,[27] nonsmall cell lung cancer,[19] and many other cancers. In these studies, patients’ clinical outcomes, survival rates, and metastatic ability of tumors were in negative relation with the degree of expression. In a study, metastatic ability of sarcomas was related to AMF expression level.[28] In another study, Kho et al. showed that AMF overexpression causes resistance to trastuzumab in breast cancer patients.[29] Ascites accumulation as a complication of some solid tumors was observed in higher AMF expression levels.[30]
So, it seems that AMF and AMFR has an essential role in molecular pathways of carcinogenesis, patient outcomes, and treatment complications. AMF and AMFR could be a potential diagnostic and/or therapeutic target in cancers especially malignant ones like GBM.
Previous studies mentioned, the occurrence of GBM is considerably high in different populations.[1] But because this tumor is the most fatal primary tumor of brain tissue, nearly all the patients die in the 1st year after diagnosis. Because of this matter, the prevalence of GBM is low. In the 2 years that we collect our sample size we cannot find much patients alive to complete our sample size. Then we decided to use not only blood samples of alive patients, but also the samples from died patients. The only sample that we could find from these patients were brain tissue samples that were used to confirm the pathologic diagnosis of the patients. After data analysis, for rs2440472 the difference between genotypic (47.1% vs. 55.3% respectively, P = 0.505) and allelic (71.6% vs. 79.5% respectively, P = 0.271) frequencies were not statistically significant between blood and brain samples. Because of this matter we decided to compare control samples with all of the GBM samples (without separating analysis of blood and brain samples). Another limitation of our study is that our data was not consistent with Hardy-Weinberg equation. Small sample size and limited participated ethnic groups were other limitations of this study. It is notable to say that, due to mentioned limitations, our results are preliminary. Studies with larger sample sizes and more diverse ethnic groups are needed to confirm our results.
CONCLUSION
In conclusion our data for the first time, demonstrated an association between AMFR rs2440472 gene polymorphism and susceptibility to GBM in a representative Iranian population after adjustment for age and sex. Studies with larger sample sizes and with participants from more diverse ethnical groups are needed to confirm our results.
Financial support and sponsorship
This work was supported by Isfahan University of Medical Sciences, Isfahan, Iran (Grant # 293404).
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
Authors would like to appreciate collaborations of Dr. Alireza Amuheidari for diagnosing GBM patients and also Mohamadhasan Tajaddini for his guidance in PCR-HRM reaction setup.
REFERENCES
- 1.Renault IZ, Golgher D. Molecular genetics of glioblastomas: Defining subtypes and understanding the biology. Neuroimaging Clin N Am. 2015;25:97–103. doi: 10.1016/j.nic.2014.09.007. [DOI] [PubMed] [Google Scholar]
- 2.Wesseling P, Kros JM, Jeuken JWM. The pathological diagnosis of diffuse gliomas: Towards a smart synthesis of microscopic and molecular information in a multidisciplinary context. Diagn Histopathol. 2011;17:486–94. [Google Scholar]
- 3.Gallego O. Nonsurgical treatment of recurrent glioblastoma. Curr Oncol. 2015;22:e273–81. doi: 10.3747/co.22.2436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Osuka S, Van Meir EG. Overcoming therapeutic resistance in glioblastoma: The way forward. J Clin Invest. 2017;127:415–26. doi: 10.1172/JCI89587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Furuta E, Okuda H, Kobayashi A, Watabe K. Metabolic genes in cancer: Their roles in tumor progression and clinical implications. Biochim Biophys Acta. 2010;1805:141–52. doi: 10.1016/j.bbcan.2010.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tian K, Zhong W, Zheng X, Zhang J, Liu P, Zhang W, et al. Neuroleukin/Autocrine motility factor receptor pathway promotes proliferation of articular chondrocytes through activation of AKT and smad2/3. Sci Rep. 2015;5:15101. doi: 10.1038/srep15101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shimizu K, Tani M, Watanabe H, Nagamachi Y, Niinaka Y, Shiroishi T, et al. The autocrine motility factor receptor gene encodes a novel type of seven transmembrane protein. FEBS Lett. 1999;456:295–300. doi: 10.1016/s0014-5793(99)00966-7. [DOI] [PubMed] [Google Scholar]
- 8.Funasaka T, Hu H, Hogan V, Raz A. Down-regulation of phosphoglucose isomerase/autocrine motility factor expression sensitizes human fibrosarcoma cells to oxidative stress leading to cellular senescence. J Biol Chem. 2007;282:36362–9. doi: 10.1074/jbc.M706301200. [DOI] [PubMed] [Google Scholar]
- 9.Fairbank M, St-Pierre P, Nabi IR. The complex biology of autocrine motility factor/phosphoglucose isomerase (AMF/PGI) and its receptor, the gp78/AMFR E3 ubiquitin ligase. Mol Biosyst. 2009;5:793–801. doi: 10.1039/b820820b. [DOI] [PubMed] [Google Scholar]
- 10.Tsai YC, Mendoza A, Mariano JM, Zhou M, Kostova Z, Chen B, et al. The ubiquitin ligase gp78 promotes sarcoma metastasis by targeting KAI1 for degradation. Nat Med. 2007;13:1504–9. doi: 10.1038/nm1686. [DOI] [PubMed] [Google Scholar]
- 11.Gharipour M, Sadeghi M, Salehi M, Behmanesh M, Khosravi E, Dianatkhah M, et al. Association of expression of selenoprotein P in mRNA and protein levels with metabolic syndrome in subjects with cardiovascular disease: Results of the selenegene study. J Gene Med. 2017;19 doi: 10.1002/jgm.2945. ??? [DOI] [PubMed] [Google Scholar]
- 12.Sjoblom T, Jones S, Wood LD, Parsons DW, Lin J, Barber TD, et al. The consensus coding sequences of human breast and colorectal cancers. Science (N Y) 2006;314:268–74. doi: 10.1126/science.1133427. [DOI] [PubMed] [Google Scholar]
- 13.Zandifar A, Soleimani S, Iraji N, Haghdoost F, Tajaddini M, Javanmard SH, et al. Association between promoter region of the uPAR (rs344781) gene polymorphism in genetic susceptibility to migraine without aura in three iranian hospitals. Clin Neurol Neurosurg. 2014;120:45–8. doi: 10.1016/j.clineuro.2014.02.003. [DOI] [PubMed] [Google Scholar]
- 14.Haga A, Funasaka T, Deyashiki Y, Raz A. Autocrine motility factor stimulates the invasiveness of malignant cells as well as up-regulation of matrix metalloproteinase-3 expression via a MAPK pathway. FEBS Lett. 2008;582:1877–82. doi: 10.1016/j.febslet.2008.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Song M, Yin Y, Zhang J, Zhang B, Bian Z, Quan C, et al. MiR-139-5p inhibits migration and invasion of colorectal cancer by downregulating AMFR and NOTCH1. Protein Cell. 2014;5:851–61. doi: 10.1007/s13238-014-0093-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang L, Hou G, Xue L, Li J, Wei P, Xu P, et al. Autocrine motility factor receptor signaling pathway promotes cell invasion via activation of ROCK-2 in esophageal squamous cell cancer cells. Cancer Invest. 2010;28:993–1003. doi: 10.3109/07357907.2010.483503. [DOI] [PubMed] [Google Scholar]
- 17.Funasaka T, Hogan V, Raz A. Phosphoglucose isomerase/autocrine motility factor mediates epithelial and mesenchymal phenotype conversions in breast cancer. Cancer Res. 2009;69:5349–56. doi: 10.1158/0008-5472.CAN-09-0488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ahmad A, Aboukameel A, Kong D, Wang Z, Sethi S, Chen W, et al. Phosphoglucose isomerase/autocrine motility factor mediates epithelial-mesenchymal transition regulated by miR-200 in breast cancer cells. Cancer Res. 2011;71:3400–9. doi: 10.1158/0008-5472.CAN-10-0965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kara M, Ohta Y, Tanaka Y, Oda M, Watanabe Y. Autocrine motility factor receptor expression in patients with stage I non-small cell lung cancer. Ann Thorac Surg. 2001;71:944–8. doi: 10.1016/s0003-4975(00)02135-4. [DOI] [PubMed] [Google Scholar]
- 20.Funasaka T, Yanagawa T, Hogan V, Raz A. Regulation of phosphoglucose isomerase/autocrine motility factor expression by hypoxia. FASEB J. 2005;19:1422–30. doi: 10.1096/fj.05-3699com. [DOI] [PubMed] [Google Scholar]
- 21.Yanagawa T, Funasaka T, Tsutsumi S, Watanabe H, Raz A. Novel roles of the autocrine motility factor/phosphoglucose isomerase in tumor malignancy. Endocr Relat Cancer. 2004;11:749–59. doi: 10.1677/erc.1.00811. [DOI] [PubMed] [Google Scholar]
- 22.Tsutsumi S, Yanagawa T, Shimura T, Fukumori T, Hogan V, Kuwano H, et al. Regulation of cell proliferation by autocrine motility factor/phosphoglucose isomerase signaling. J Biol Chem. 2003;278:32165–72. doi: 10.1074/jbc.M304537200. [DOI] [PubMed] [Google Scholar]
- 23.Zong M, Lu T, Fan S, Zhang H, Gong R, Sun L, et al. Glucose-6-phosphate isomerase promotes the proliferation and inhibits the apoptosis in fibroblast-like synoviocytes in rheumatoid arthritis. Arthritis Res Ther. 2015;17:100. doi: 10.1186/s13075-015-0619-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tsutsumi S, Hogan V, Nabi IR, Raz A. Overexpression of the autocrine motility factor/phosphoglucose isomerase induces transformation and survival of NIH-3T3 fibroblasts. Cancer Res. 2003;63:242–9. [PubMed] [Google Scholar]
- 25.Haga A, Funasaka T, Niinaka Y, Raz A, Nagase H. Autocrine motility factor signaling induces tumor apoptotic resistance by regulations apaf-1 and caspase-9 apoptosome expression. Int J Cancer. 2003;107:707–14. doi: 10.1002/ijc.11449. [DOI] [PubMed] [Google Scholar]
- 26.Jiang WG, Raz A, Douglas-Jones A, Mansel RE. Expression of autocrine motility factor (AMF) and its receptor, AMFR, in human breast cancer. J Histochem Cytochem. 2006;54:231–41. doi: 10.1369/jhc.5A6785.2005. [DOI] [PubMed] [Google Scholar]
- 27.Huang Z, Zhang N, Zha L, Mao HC, Chen X, Xiang JF, et al. Aberrant expression of the autocrine motility factor receptor correlates with poor prognosis and promotes metastasis in gastric carcinoma. Asian Pac J Cancer Prev. 2014;15:989–97. doi: 10.7314/apjcp.2014.15.2.989. [DOI] [PubMed] [Google Scholar]
- 28.Dobashi Y, Watanabe H, Matsubara M, Yanagawa T, Raz A, Shimamiya T, et al. Autocrine motility factor/glucose-6-phosphate isomerase is a possible predictor of metastasis in bone and soft tissue tumours. J Pathol. 2006;208:44–53. doi: 10.1002/path.1878. [DOI] [PubMed] [Google Scholar]
- 29.Kho DH, Nangia-Makker P, Balan V, Hogan V, Tait L, Wang Y, et al. Autocrine motility factor promotes HER2 cleavage and signaling in breast cancer cells. Cancer Res. 2013;73:1411–9. doi: 10.1158/0008-5472.CAN-12-2149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Funasaka T, Haga A, Raz A, Nagase H. Tumor autocrine motility factor induces hyperpermeability of endothelial and mesothelial cells leading to accumulation of ascites fluid. Biochem Biophys Res Commun. 2002;293:192–200. doi: 10.1016/S0006-291X(02)00202-4. [DOI] [PubMed] [Google Scholar]


