Abstract
Aim
To investigate the impact of renal function on the safety and efficacy of insulin glargine 300 U/mL (Gla‐300) and insulin glargine 100 U/mL (Gla‐100).
Materials and Methods
A meta‐analysis was performed using pooled 6‐month data from the EDITION 1, 2 and 3 trials (N = 2496). Eligible participants, aged ≥18 years with a diagnosis of type 2 diabetes (T2DM), were randomized to receive once‐daily evening injections of Gla‐300 or Gla‐100. Pooled results were assessed by two renal function subgroups: estimated glomerular filtration rate (eGFR) <60 and ≥60 mL/min/1.73 m2.
Results
The decrease in glycated haemoglobin (HbA1c) after 6 months and the proportion of individuals with T2DM achieving HbA1c targets were similar in the Gla‐300 and Gla‐100 groups, for both renal function subgroups. There was a reduced risk of nocturnal (12:00‐5:59 am) confirmed (≤3.9 mmol/L [≤70 mg/dL]) or severe hypoglycaemia with Gla‐300 in both renal function subgroups (eGFR <60 mL/min/1.73 m2: relative risk [RR] 0.76 [95% confidence interval {CI} 0.62‐0.94] and eGFR ≥60 mL/min/1.73 m2: RR 0.75 [95% CI 0.67‐0.85]). For confirmed (≤70 mg/dL [≤3.9 mmol/L]) or severe hypoglycaemia at any time of day (24 hours) the hypoglycaemia risk was lower with Gla‐300 vs Gla‐100 in both the lower (RR 0.94 [95% CI 0.86‐1.03]) and higher (RR 0.90 [95% CI 0.85‐0.95]) eGFR subgroups.
Conclusions
Gla‐300 provided similar glycaemic control to Gla‐100, while indicating a reduced overall risk of confirmed (≤3.9 and <3.0 mmol/L [≤70 and <54 mg/dL]) or severe hypoglycaemia, with no significant difference between renal function subgroups.
Keywords: basal insulin, glycaemic control, hypoglycaemia, insulin analogues, meta‐analysis, type 2 diabetes
1. INTRODUCTION
Diabetes and chronic kidney disease (CKD) are common chronic comorbid conditions, with diabetic kidney disease accounting for approximately half of all end‐stage renal disease cases in developed countries.1 The US National Health and Nutrition Examination Survey (NHANES) reported that ~40% of survey participants with diabetes had CKD.2 Similarly, in the UK, CKD stage 3 was reported in ~29% of individuals with diabetes, vs ~7% of those without diabetes3, while CKD stages 4 and 5 were reported in 2.1% vs 0.2%, and 0.3% vs 0.03% of individuals with and without diabetes, respectively.3 Additionally, the incidence of CKD in diabetes may be under‐reported, with one US primary care study indicating that CKD stages 1 to 5 affected 54% of individuals with type 2 diabetes (T2DM), although it had only been diagnosed in 12% of cases.4 Rates of CKD are higher in elderly populations, and as the proportion of people aged >60 years increases so will the rates of CKD.5
Renal impairment complicates the management of diabetes because it increases the risk of hypoglycaemia, is associated with increased risk of cardiovascular morbidity and mortality, and limits the options for glucose‐lowering therapy.6, 7, 8, 9 For example, cardiovascular disease has been reported in 53% of people with CKD,10 and as many as 59% of people who also have T2DM.4 The rate of hypoglycaemia in people with diabetes and CKD can be double that of individuals without CKD,11 therefore, the choice of glucose‐lowering therapy should account for this increased risk.6, 8 Additionally, dose adjustments, or even drug withdrawal, may be necessary, as the clearance of some therapies may be affected.8 However, there is a lack of evidence available, particularly from randomized clinical trials, to inform the choice of therapy and treatment goals in the management of diabetes in people with renal impairment,8 or regarding optimal glycaemic control in individuals with diabetes and more advanced CKD, for whom glycated haemoglobin (HbA1c) targets 53 mmol/mol (<7.0%) may be appropriate.8
For people with T2DM and CKD, insulin remains an appropriate option when other agents such as metformin may be contraindicated or are used at lower than standard doses.12 While insulin requirements are generally lower in people with impaired renal function, there are no specific guidelines regarding insulin dose adjustment in this population other than general recommendations that glycaemic targets should be individualized.8 Insulin glargine 300 U/mL (Gla‐300), a second‐generation basal insulin, provides a more stable and prolonged pharmacokinetic and pharmacodynamic profile compared with insulin glargine 100 U/mL (Gla‐100).13 Data from the phase III EDITION 1, 2 and 3 clinical trials showed that over 6 months, Gla‐300 provided similar glycaemic control to Gla‐100 in participants with T2DM, with less hypoglycaemia.14, 15, 16 Given the reduced hypoglycaemia risk with Gla‐300 vs Gla‐100, we were interested to see if this benefit persisted in individuals with impaired renal function. The objective of the present post hoc patient‐level meta‐analysis of EDITION 1, 2 and 3 was to investigate the impact of renal function on the safety and efficacy of Gla‐300, with a focus on hypoglycaemia risk.
2. MATERIALS AND METHODS
2.1. Study design and participants
EDITION 1, 2 and 3 (NCT01499082, NCT01499095, NCT01676220) were multicentre, randomized (1:1), open‐label, two‐arm, parallel‐group, phase IIIa studies.14, 15, 16 Briefly, eligible participants were aged ≥18 years with T2DM and receiving the following: EDITION 1, basal (≥42 U/d) and prandial insulin therapy ± metformin for ≥1 year; EDITION 2, basal insulin therapy (≥42 U/d) in combination with oral antidiabetic drugs; EDITION 3, oral antidiabetic drugs received for ≥6 months before screening and insulin‐naïve. People with severe, unstable or end‐stage renal disease (CKD stage 5, estimated glomerular filtration rate [eGFR] <15 mL/min/1.73 m2) were excluded. Participants were randomized (1:1) to once‐daily evening injections of Gla‐300 (Toujeo; Sanofi, Paris, France) or Gla‐100 (Lantus, Sanofi), titrated to a fasting self‐monitored plasma glucose target of 4.4 to 5.6 mmol/L (80‐100 mg/dL); sulphonylurea use was not allowed.
In the present post hoc analysis, study populations were pooled and results assessed across two renal function subgroups, according to baseline eGFR (calculated using the Modification of Diet in Renal Disease study equation): ≥15 to <60 mL/min/1.73 m2 (CKD stage 3‐4, indicating mild‐to‐moderate renal impairment) and ≥ 60 mL/min/1.73 m2 (indicating preserved renal function).
2.2. Outcomes
The following endpoints were examined: HbA1c and fasting plasma glucose (FPG) change from baseline to month 6, percentage of participants achieving HbA1c 53 mmol/mol (<7.0%) and 58.5 mmol/mol (<7.5%) at month 6, percentage of participants attaining FPG <5.6 mmol/L and <6.7 mmol/L, change in insulin dose and body weight from baseline to month 6, percentage of participants with ≥1 hypoglycaemic event, hypoglycaemic event rates per participant‐year and cumulative mean number of hypoglycaemic events per participant (during the night [12:00‐5:59 am] or at any time of day [24 hours]), and adverse events during the 6‐month study period. Hypoglycaemia endpoints were based on American Diabetes Association (ADA) definitions17; confirmed or severe hypoglycaemia was defined as any event that was documented symptomatic or asymptomatic with a plasma glucose measurement of ≤3.9 mmol/L (≤70 mg/dL) or <3.0 mmol/L (<54 mg/dL), or severe (requiring third‐party assistance).
2.3. Data analysis and statistics
Efficacy outcomes were analysed using the modified intention‐to‐treat (mITT) population, defined as all randomized participants who received at least 1 dose of study insulin and had both a baseline and at least 1 post‐baseline efficacy assessment. The safety population comprised all participants who had received at least 1 dose of study insulin.
Change in HbA1c was analysed using a mixed model for repeated measurements (MMRM) approach. Relative risk (RR) of hypoglycaemia was analysed using the Cochran–Mantel–Haenzel method and annualized rates of hypoglycaemia were analysed using an overdispersed Poisson regression model. For HbA1c, and hypoglycaemia, the homogeneity of the treatment effect among subgroups was assessed using subgroup‐by‐treatment interaction. Differences in treatment effect across subgroups were only considered relevant if significant heterogeneity was observed (P < 0.05). Body weight, insulin dose, FPG, adverse events and patient satisfaction (evaluated using the Diabetes Treatment Satisfaction Questionnaire [DTSQ]) were assessed descriptively.
3. RESULTS
3.1. Baseline characteristics
The mITT and safety populations included 2474 and 2488 individuals, respectively. Of the 2496 participants randomized to treatment in EDITION 1, 2 and 3, 2075 (83.1%) had a baseline eGFR ≥60 mL/min/1.73 m2 (Gla‐300: n = 1039; Gla‐100: 1036) and 421 (16.9%) had a baseline eGFR <60 mL/min/1.73 m2 (Gla‐300: n = 208; Gla‐100: 213). Baseline characteristics are summarized in Table 1. Participants were, on average, older in the lower vs higher eGFR subgroup (~65 years vs 57 years). Most participants in the study were white. The mean duration of diabetes was longer in the lower vs higher eGFR subgroup (~16 years vs 12 years). Those in the lower eGFR subgroup had received ~1 year more of insulin therapy than those in the higher eGFR subgroup.
Table 1.
Baseline demographics and participant characteristics, by renal function subgroup (pooled randomized population)
| Renal function subgroup, baseline eGFR | ||||
|---|---|---|---|---|
| <60 mL/min/1.73 m2 | ≥60 mL/min/1.73 m2 | |||
| Gla‐300 (N = 208) | Gla‐100 (N = 213) | Gla‐300 (N = 1039) | Gla‐100 (N = 1036) | |
| Mean age, years | 65.0 (7.9) | 65.0 (7.5) | 57.4 (9.0) | 57.2 (9.3) |
| Gender: male, n (%) | 109 (52.4) | 101 (47.4) | 548 (52.7) | 548 (52.9) |
| Race, n (%) | ||||
| White | 188 (90.4) | 199 (93.4) | 908 (87.4) | 896 (86.5) |
| Black | 13 (6.3) | 5 (2.3) | 77 (7.4) | 89 (8.6) |
| Asian/Oriental | 4 (1.9) | 7 (3.3) | 44 (4.2) | 42 (4.1) |
| Other | 3 (1.4) | 2 (0.9) | 10 (1.0) | 9 (0.9) |
| Body weight, kg | 101.2 (21.3) | 99.5 (20.5) | 99.6 (23.2) | 99.9 (22.0) |
| BMI, kg/m2 | 35.5 (7.0) | 35.4 (6.3) | 34.5 (6.9) | 34.7 (6.4) |
| eGFR, mL/min/1.73 m2 | 49.0 (8.5) | 48.2 (9.6) | 85.1 (16.6) | 85.0 (16.6) |
| HbA1c, mmol/mol | 66.3 (9.2) | 65.2 (8.3) | 67.5 (10.2) | 67.9 (10.1) |
| HbA1c, % | 8.2 (0.8) | 8.1 (0.8) | 8.3 (0.9) | 8.4 (0.9) |
| Haemoglobin: males, g/L | 138.4 (14.3) | 140.3 (15.8) | 146.0 (11.6) | 145.6 (12.1) |
| Number of males below gender‐ specific haemoglobin range, n (%) | 19 (17.4) | 17 (16.8) | 22 (4.0) | 26 (4.7) |
| Haemoglobin: females, g/L | 128.2 (13.4) | 128.3 (12.32) | 132.6 (11.3) | 132.3 (12.0) |
| Number of females below gender‐specific haemoglobin range, n (%) | 15 (15.2) | 18 (16.1) | 33 (6.7) | 35 (7.2) |
| Albumin creatinine ratio assessed, n | 201 | 210 | 1018 | 1013 |
| Categories, n (%) | ||||
| <30 mg/g | 120 (59.7) | 136 (64.8) | 777 (76.3) | 784 (77.4) |
| 30 to 300 mg/g | 54 (26.9) | 50 (23.8) | 204 (20.0) | 198 (19.5) |
| >300 mg/g | 27 (13.4) | 24 (13.4) | 37 (3.6) | 31 (3.1) |
| Duration of diabetes, years | 15.7 (7.8) | 15.9 (7.9) | 12.1 (7.0) | 12.0 (7.3) |
| Duration of basal insulin, yearsa | 5.7 (4.9) | 6.1 (4.5) | 5.1 (4.4) | 4.9 (4.3) |
| Basal daily insulin dose, U/kga | 0.67 (0.23) | 0.69 (0.26) | 0.67 (0.25) | 0.67 (0.25) |
| Total daily insulin dose, U/kgb | 1.22 (0.54) | 1.25 (0.45) | 1.19 (0.48) | 1.19 (0.45) |
| Previous antihyperglycaemic medication excluding insulin, n (%) | 154 (74.0) | 161 (75.6) | 912 (87.8) | 919 (88.7) |
| Biguanides | 134 (64.4) | 145 (68.1) | 876 (84.3) | 877 (84.7) |
| Metformin | 115 (55.3) | 118 (55.4) | 702 (67.6) | 715 (69.0) |
| Metformin hydrochloride | 17 (8.2) | 28 (13.1) | 173 (16.7) | 164 (15.8) |
| Sulphonylureas | 47 (22.6) | 39 (18.3) | 229 (22.0) | 230 (22.2) |
| Dipeptidyl peptidase‐4 inhibitors | 26 (12.5) | 31 (14.6) | 97 (9.3) | 118 (11.4) |
| Previous insulins and analogues, n (%) | 152 (73.1) | 150 (70.4) | 656 (63.1) | 661 (63.8) |
| Insulin glargine | 134 (64.4) | 128 (60.1) | 543 (52.3) | 579 (55.9) |
| Insulin detemir | 1 (0.5) | 2 (0.9) | 7 (0.7) | 3 (0.3) |
| Insulin degludec | 0 (0) | 0 (0) | 0 (0) | 1 (<0.1) |
| Any statins, n (%) | 149 (71.6) | 150 (70.4) | 570 (54.9) | 591 (57.0) |
| Antihypertensive agents, n (%) | 185 (88.9) | 177 (83.1) | 760 (73.1) | 774 (74.7) |
| Any ACE inhibitors | 108 (51.9) | 107 (50.2) | 463 (44.6) | 477 (46.0) |
| Any other antihypertensive agents | 24 (11.5) | 26 (12.2) | 66 (6.4) | 74 (7.0) |
Abbreviations: ACE, angiotensin converting enzyme; BMI, body mass index; eGFR, estimated glomerular filtration rate; Gla‐100, insulin glargine 100 U/mL; Gla‐300, insulin glargine 300 U/mL; HbA1c, glycated haemoglobin.
Data are pooled from EDITION 1, 2 and 3, and are presented as mean (SD), unless otherwise indicated.
eGFR derived using the Modification of Diet in Renal Disease study equation.
Only EDITION 1 and 2.
Only EDITION 1.
The proportions of participants with comorbidities reported at baseline were similar in the two renal function subgroups (Table 1), although there was a general trend for more participants reporting complications in the lower eGFR subgroup. The most common overall complication was diabetic sensory and motor neuropathy, reported in ~41% and 32% of participants in the lower and higher eGFR subgroups, respectively. Diabetic macroangiopathy was reported in an average of 9.1% and 6.1% of participants in the lower and higher eGFR subgroups, respectively (Table S1, Supporting Information). Cardiac disorders were documented in 39.2% of participants in the lower eGFR subgroup, compared with 25.4% in the higher eGFR subgroup.
3.2. Renal function
The EDITION 1 study contributed the largest proportion of participants to the <60 mL/min/1.73 m2 subgroup (n = 188; Table S2, Supporting Information). At baseline, the pooled average eGFR for the lower and higher renal function subgroups were 48.6 mL/min/1.73 m2 and 85.0 mL/min/1.73 m2, respectively. These values did not change markedly after 6 months' treatment, regardless of eGFR subgroup or treatment arm (50.5 and 85.0 mL/min/1.73 m2).
3.3. Previous and concomitant medications
At baseline, most participants were using non‐insulin antihyperglycaemic medications (~75% and 88% in the lower and higher eGFR subgroups, respectively), of which metformin was the most common (Table 1). The proportion of participants using metformin in the lower eGFR subgroup (55%) was lower than in the higher eGFR subgroup (68%). By comparison, more participants in the lower eGFR subgroup than in the higher eGFR subgroup had previously been using insulins (72% and 63%, respectively) or antihypertensive agents (86% and 74%, respectively). Most participants were using statins, irrespective of renal function (Table 1). At month 6, there was very little change in medication usage patterns from baseline in either treatment arm or renal function subgroup (Table S3, Supporting Information).
3.4. Glycaemic control
The HbA1c reductions from baseline were similar in the Gla‐300 and the Gla‐100 treatment group, regardless of renal function (least squares [LS] mean difference 0.14 [95% confidence interval {CI} −0.04 to 0.32 and −0.03 [95% CI −0.11 to 0.05]) in the eGFR <60 mL/min/1.73 m2 and ≥60 mL/min/1.73 m2 subgroups, respectively. There was no evidence of heterogeneity of treatment effect across subgroups (P = 0.097; Figure 1A). Similar proportions of participants achieved HbA1c target <53 mmol/mol (<7.0%) between treatment arms in both renal function subgroups. In the lower eGFR subgroup, 36.4% and 39.2% of participants achieved target with Gla‐300 and Gla‐100, respectively. In the higher eGFR subgroup, 36.2% and 34.7% of participants achieved target with Gla‐300 and Gla‐100 (Figure 1B). As observed for the HbA1c 53 mmol/mol (<7.0%) target, the proportion of participants achieving HbA1c target 58.5 mmol/mol (<7.5%) was similar for the Gla‐300 and Gla‐100 treatment arms in both renal function subgroups. In the Gla‐300 and Gla‐100 treatment arms, respectively, 54.9% and 58.4% of participants achieved target in the lower eGFR subgroup, and 54.0% and 51.8% of participants achieved target in the higher eGFR subgroup. The mean change in FPG from baseline to month 6 was similar for both treatment arms and renal function subgroups (Table S4, Supporting Information). The proportion of participants attaining FPG <5.6 mmol/L was 24.8% and 32.5% in the lower eGFR subgroup, and 25.2% and 25.1% in the higher eGFR subgroup, with Gla‐300 and Gla‐100, respectively; for attainment of FPG <6.7 mmol/L, the proportions were 45.1% and 50.2% in the lower eGFR subgroup, and 44.5% and 46.6% in the higher eGFR subgroup, with Gla‐300 and Gla‐100, respectively.
Figure 1.
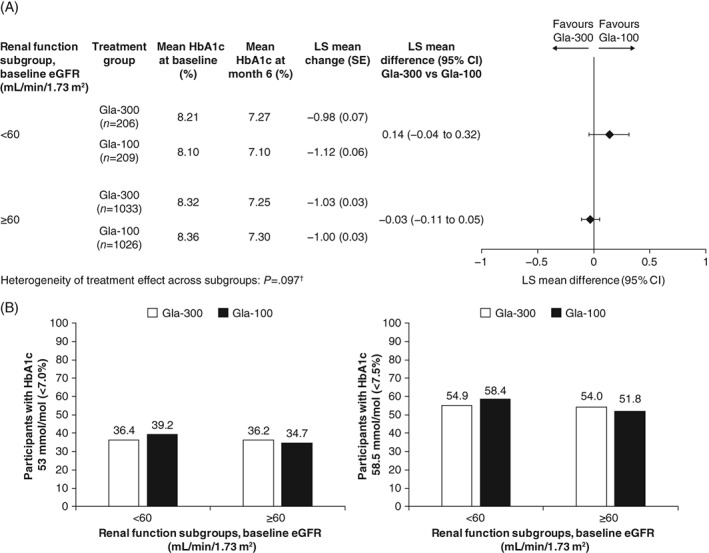
A, Mean change in glycated haemoglobin (HbA1c) from baseline to month 6 according to renal function subgroup (modified intention‐to‐treat [mITT] population). †Logistic method; P < 0.05 corresponds to significant heterogeneity of treatment effect. B, Percentage of participants achieving HbA1c targets (53 mmol/mol [<7.0%] and 58.5 mmol/mol [<7.5%]) at month 6 (mITT population). CI, confidence interval; eGFR, estimated glomerular filtration rate; Gla‐100, insulin glargine 100 U/mL; Gla‐300, insulin glargine 300 U/mL; LS, least squares; MMRM, mixed model for repeated measurements
3.5. Hypoglycaemia
More participants in the lower vs the higher eGFR subgroup experienced ≥1 confirmed (≤3.9 mmol/L [≤70 mg/dL] and <3.0 mmol/L [<54 mg/dL]) or severe hypoglycaemic event at night (12:00‐05:59 am) or at any time of day (24 hours), regardless of the insulin used (Figure 2A). The risk of confirmed (≤3.9 mmol/L [≤70 mg/dL]) or severe hypoglycaemic events at night (12:00‐05:59 am) was lower with Gla‐300 than with Gla‐100 (RR 0.76 [95% CI 0.62‐0.94] and 0.75 [95% CI 0.67‐0.85] in the lower and higher eGFR subgroups, respectively). Similarly, the risk of any‐time‐of‐day (24‐hour) confirmed (≤3.9 mmol/L [≤70 mg/dL]) or severe hypoglycaemic events was lower with Gla‐300 than with Gla‐100 in the lower and higher eGFR subgroups (RR 0.94 [95% CI 0.86‐1.03 and 0.90 [95% CI 0.85‐0.95], respectively). There was no significant difference between renal function subgroups (no evidence of heterogeneity of treatment effect across subgroups, P = 0.662 for nocturnal events; P = 0.794 for events at any time of day). Consistent results were observed when using the more stringent glycaemic threshold of <3.0 mmol/L (<54 mg/dL; Figure 2B).
Figure 2.
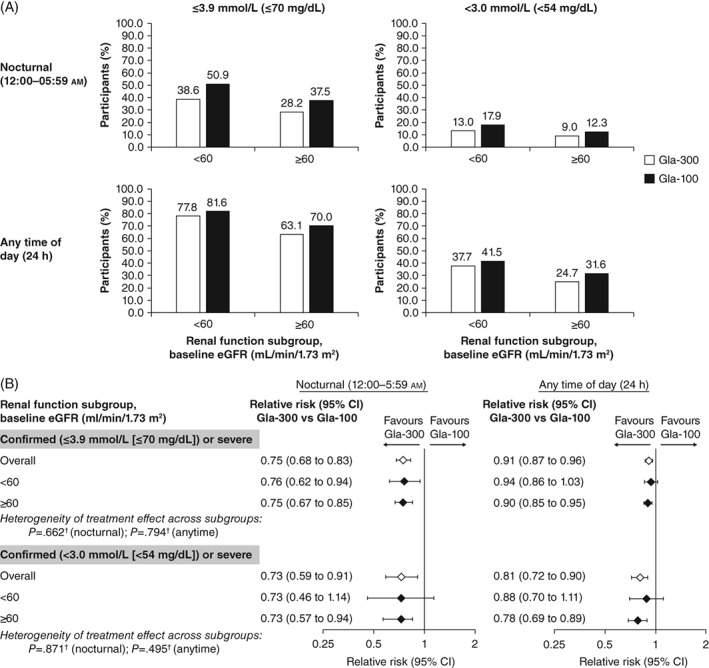
A, Percentage of participants experiencing ≥1 confirmed or severe hypoglycaemic event (safety population). B, Relative risk of experiencing ≥1 hypoglycaemic event with insulin glargine 300 U/mL (Gla‐300) vs insulin glargine 100 U/mL (Gla‐100) by renal function subgroup (safety population). †Logistic method. P < 0.05 corresponds to significant heterogeneity of treatment effect. CI, confidence interval; eGFR, estimated glomerular filtration rate
The rates of confirmed or severe nocturnal (12:00‐05:59 am) or any‐time (24‐hour) hypoglycaemic events per participant‐year, at both glycaemic thresholds, were higher in the lower vs the higher eGFR subgroup, irrespective of the insulin used (Figure 3A). The annualized rate of confirmed (≤3.9 mmol/L [≤70 mg/dL]) or severe hypoglycaemic events at night (12:00‐05:59 am) and at any time of day (24 hours) in the overall population was lower with Gla‐300 than with Gla‐100 (Figure 3B), and there was no significant difference between renal function subgroups (no evidence of heterogeneity of treatment effect across subgroups, P = 0.986 for nocturnal events; P = 0.604 for events at any time of day). The rate ratio of nocturnal confirmed (≤3.9 mmol/L [≤70 mg/dL]) or severe hypoglycaemic events was 0.69 (95% CI 0.44‐1.08) in the lower and 0.69 (95% CI 0.56‐0.85) in the higher eGFR subgroup. Similarly, for any‐time‐of‐day (24‐hour) confirmed (≤3.9 mmol/L [≤70 mg/dL]) or severe hypoglycaemia the rate ratios for Gla‐300 vs Gla‐100 were 0.81 (95% CI 0.64‐1.04) in the lower eGFR subgroup and 0.88 (95% CI 0.77‐1.00) in the higher eGFR subgroup. Generally, these results were consistent at the lower glucose threshold, with an overall lower annualized rate of nocturnal events for Gla‐300 vs Gla‐100, but not for hypoglycaemic events that occurred at any time of day (Figure 3B), and no heterogeneity of treatment effect across the subgroups (P = 0.707 for nocturnal events; P = 0.792 for events at any time of day).
Figure 3.
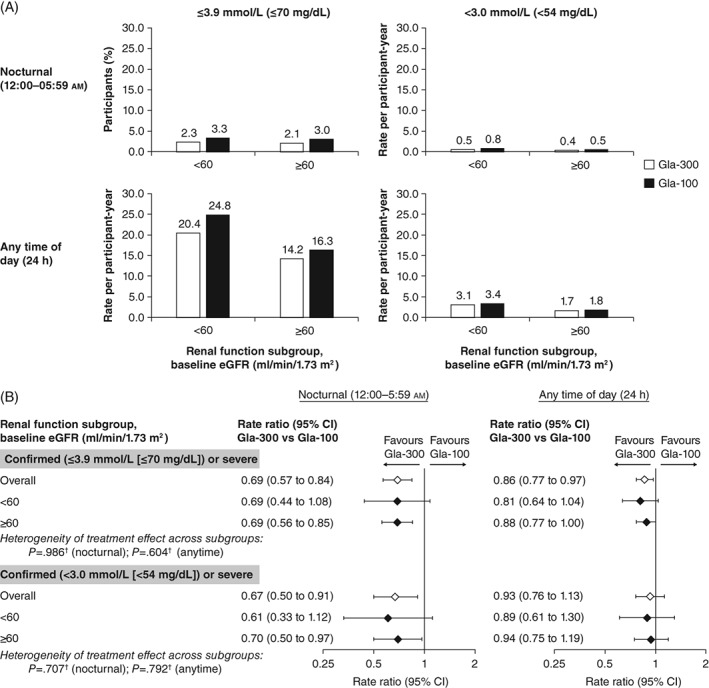
A, Rate of confirmed or severe hypoglycaemia by renal function subgroup (safety population). B, Ratio of annualized hypoglycaemia event rates by renal function subgroup (safety population). †Logistic method. P < 0.05 corresponds to significant heterogeneity of treatment effect. CI, confidence interval; eGFR, estimated glomerular filtration rate
Severe hypoglycaemia was experienced by 28/1242 participants (2.3%) in the Gla‐300 group (lower eGFR subgroup: 11/207 [5.3%]; higher eGFR subgroup: 17/1035 [1.6%]) and 33/1236 (2.6%) in the Gla‐100 group (lower eGFR subgroup: 9/212 [4.2%]; higher eGFR subgroup: 24/1034 [2.3%]). Annualized rates of severe hypoglycaemic events at any time of day (24 hours) were 0.35 and 0.26 events per participant‐year in the Gla‐300 and Gla‐100 treatment groups, respectively, for participants in the lower eGFR subgroup. For the higher eGFR subgroup, annualized rates were 0.06 and 0.08 events per participant‐year, respectively.
The proportion of participants who achieved HbA1c targets (53 mmol/mol [<7.0%] or 58.5 mmol/mol [<7.5%]) without experiencing confirmed or severe hypoglycaemia were similar across renal function subgroups and between treatment arms, for both hypoglycaemia thresholds evaluated (Table S5, Supporting Information).
3.6. Body weight and insulin dose
The mean (SD) change in body weight from baseline to month 6 was small in both treatment groups across both renal function subgroups: 0.14 (4.06) kg and 0.42 (3.68) kg for participants in the lower eGFR subgroup; 0.59 (3.48) kg and 0.92 (3.20) kg for participants in the higher eGFR subgroup, in the Gla‐300 and Gla‐100 groups, respectively. At month 6, insulin dose in the lower eGFR subgroup increased from baseline by 0.30 and 0.21 U/kg (an average increase of 89.3% and 72.4%) for Gla‐300 and Gla‐100, respectively. In the higher eGFR subgroup, the insulin dose increase from baseline was 0.35 and 0.26 U/kg (an average increase of 117.0% and 89.2%) for Gla‐300 and Gla‐100, respectively (Table S4, Supporting Information). This reflected a −23.7% difference in dose increase for Gla‐300 and a −18.8% difference in dose increase for Gla‐100 between the lower vs higher eGFR subgroups.
3.7. Adverse events
Treatment‐emergent adverse events (TEAEs) were observed more commonly in participants in the eGFR <60 mL/min/1.73 m2 vs the ≥60 mL/min/1.73 m2 subgroup. In the lower eGFR subgroup, TEAEs were reported in 64.7% and 59.4% of participants in the Gla‐300 and Gla‐100 treatment groups, respectively. In the higher eGFR subgroup, TEAEs were reported in 55.8% and 52.5% of participants in the Gla‐300 and Gla‐100 treatment groups, respectively. The proportions of participants who experienced at least one TEAE relating to injection site reaction, hypersensitivity, cardiovascular events, and acute kidney injury, are shown in Table S6 (Supporting Information).
3.8. Participant satisfaction
There were no differences in patient satisfaction either by eGFR subgroup or treatment arm. The mean (SD) change in total DTSQ score from baseline to month 6 in the higher eGFR subgroup was 3.53 (6.50) and 3.93 (6.84) with Gla‐300 and Gla‐100, respectively. In the lower eGFR subgroup, mean change was 3.01 (6.43) and 3.30 (6.44) with Gla‐300 and Gla‐100, respectively. The LS mean difference (SE) between Gla‐300 vs Gla‐100 in total DTSQ scores after 6 months of treatment was −0.22 (0.21) in the higher eGFR subgroup, compared with −0.33 (0.46) in the lower eGFR subgroup.
4. DISCUSSION
This patient‐level meta‐analysis from the EDITION 1, 2 and 3 trials of participants with T2DM by baseline eGFR subgroup demonstrated consistent and similar reductions in FPG and HbA1c levels for the Gla‐300 and Gla‐100 groups, regardless of renal function subgroup. This finding was accompanied by a lower risk of nocturnal confirmed (≤3.9 mmol/L [≤70 mg/dL]) or severe hypoglycaemia with Gla‐300 than Gla‐100, which was not influenced by renal function. Hence, overall, the pooled results presented here are consistent with the individual EDITION studies,14, 15, 16 which also reported similar glycaemic control and reduced risk of hypoglycaemia with Gla‐300 compared with Gla‐100. Importantly, however, these data show that this advantage of Gla‐300 over Gla‐100 was maintained in people with T2DM and impaired renal function. While the results of the present patient‐level meta‐analysis are encouraging and suggest that Gla‐300 therapy may also be used in people with T2DM and renal impairment, the scarcity of literature on insulin use in this population, and the post hoc nature of this analysis, highlight the need for randomized clinical trials to more fully evaluate the impact of renal function and CKD on diabetes therapy.
As expected, participants with lower eGFR experienced higher incidence and rates of hypoglycaemia. While the mean insulin doses increased in both treatment arms, there was a tendency for a larger increase with Gla‐300, consistent with findings reported for the overall EDITION population.14, 15, 16 Although speculative, one possible reason underlying this greater increase in dose with Gla‐300 than Gla‐100 may be related to the lower bioavailability of Gla‐300, owing to the greater stability of its subcutaneous depot that might make it more prone to enzymatic inactivation compared with Gla‐100.18 It is also important to consider that the dose changes observed may be a consequence of the treat‐to‐target design of the EDITION trials; dose increases were to be expected given that participants switched to, or initiated, a new basal insulin in this study because they were uncontrolled on their previous treatment. In a meta‐analysis of the EDITION studies, Gla‐300 was associated with lower weight gain despite higher doses compared with Gla‐100,18 and the present analysis demonstrates a similar trend in participants with renal impairment.
Notably, the higher insulin doses observed in the EDITION studies with Gla‐300 were not associated with an increased risk of hypoglycaemic events, and the mean insulin doses were similar between renal function subgroups in this analysis. Differences between renal function subgroups in the relative increases of insulin dose are unlikely to account for the higher incidence of hypoglycaemia in the lower eGFR subgroup as, if anything, dose increases were lower in the <60 mL/min/1.73 m2 than in the ≥60 mL/min/1.73 m2 subgroup. Furthermore, the pooled data show that renal function did not change after treatment with either Gla‐300 or Gla‐100 for 6 months. These findings suggest that the observed differences between the renal subgroups in the rate and incidence of hypoglycaemia were unlikely to be related to dose or dose increase, but are consistent with the higher risk for hypoglycaemia reported in CKD.11 In terms of safety, as expected, TEAE incidence was generally higher in the lower vs the higher eGFR subgroup, but there were no major differences in TEAEs between Gla‐300 and Gla‐100 within the two subgroups, nor between either renal function or treatment groups for injection site reactions, hypersensitivity, cardiovascular events or acute kidney injury.
The 2016 ADA guidelines do not include specific HbA1c targets for individuals with T2DM and renal impairment, although goals that are less stringent than the general target of 53 mmol/mol (7.0%) (e.g. 63.9 mmol/mol (8.0%)) are suggested for people with comorbidities.19 Indeed, the present analysis shows that HbA1c targets of 58.5 mmol/mol (<7.5%) are achievable for many people with T2DM. Furthermore, the reduced risk of hypoglycaemia with Gla‐300 is particularly important in this patient group, where there is a high risk of cardiovascular disease, as the experience of hypoglycaemia may be associated with cardiovascular events and mortality.20
This post hoc analysis provides data supporting the use of Gla‐300 in individuals with T2DM and mild renal impairment, which will help inform and guide management decisions. The comparison with Gla‐100 is important, as this is the “gold standard” of treatment in many countries. The lower risk of hypoglycaemia with Gla‐300 than Gla‐100 is of particular interest as the risk of hypoglycaemia is increased in people with renal impairment,11 therefore, therapy options with lower risk of hypoglycaemia may be particularly beneficial in this population. Basal insulin therapy requires a balance between achieving appropriate individualized glycaemic targets and minimizing or avoiding hypoglycaemia. Consistent with the data in the present study in participants with impaired renal function, a meta‐analysis of EDITION 1, 2 and 3 data indicated that treatment with Gla‐300 could allow people with T2DM to achieve equivalent glycaemic control vs Gla‐100 but with less hypoglycaemia.21
The limitations of the present analysis include its post hoc nature and the exclusion of participants with severe renal impairment (CKD stage 5) from the EDITION programme. In addition, the lower number of participants with eGFR <60 mL/min/1.73 m2 compared with the ≥60 mL/min/1.73 m2 subgroup lead to wider CIs for the hypoglycaemia results for this subgroup compared with the ≥60 mL/min/1.73 m2 subgroup, although the absolute point estimates were similar between each subgroup (Figures 2B and 3B); however, the present analysis provided hypothesis‐generating data that may be explored further by stratifying participants according to renal function in future randomized controlled clinical studies, dedicated to evaluating insulin treatment in participants with T2DM.
In summary, as previously demonstrated overall in the EDITION programme, Gla‐300 had similar effectiveness to Gla‐100 in improving glycaemic control in a group of challenging‐to‐treat people with T2DM and renal impairment; this clinical goal was achieved in tandem with a consistent overall reduction in the risk of confirmed (≤3.9 and <3.0 mmol/L [≤70 and <54 mg/dL]) or severe hypoglycaemia, with no significant difference between renal function subgroups.
Supporting information
Table S1. Comorbidities at baseline (randomized population).
Table S2. eGFR by study and in pooled population.
Table S3. Concomitant medications at month 6 (pooled randomized population).
Table S4. Change in FPG and insulin dose from baseline, by renal function subgroup (pooled mITT population).
Table S5. Percentage of participants achieving target HbA1c without hypoglycemia.
Table S6. Participants with reports of at least one injection site reaction, hypersensitivity, cardiovascular and renal TEAEs.
ACKNOWLEDGMENTS
The authors thank the study participants, trial staff and investigators for their participation. Editorial and writing assistance was provided by Arthur Holland (PhD) of Fishawack Communications Ltd and was funded by Sanofi. Sanofi sponsored, designed and coordinated the clinical trials presented in this meta‐analysis.
Data from this manuscript have been published in abstract form (Escalada J, et al. Abstract 69‐OR Diabetes. 2016;65(suppl. 1):A18; Escalada J, et al. Diabetes Technology & Therapeutics. February 19, 2017(S1): A116) at the ADA 2016 and Advanced Technologies & Treatments for Diabetes (ATTD) 2017 congresses, and presented as an oral presentation at ADA 2016 and poster presentation at ATTD 2017.
Conflicts of interest
J.E. has participated in advisory panels for MSD, Novo Nordisk and Sanofi, and has participated in speaker's bureau for AstraZeneca, Boehringer Ingelheim, Eli Lilly, MSD, Novo Nordisk, and Sanofi. S.H. has participated in advisory boards, conferences and acted as a consultant for Ascensia, AstraZeneca, Bayer, Becton‐Dickinson, Boehringer Ingelheim, Janssen, Lifescan, Eli Lilly, MSD, Novartis, Novo Nordisk, and Sanofi. P.S. has participated in advisory panels, advisory boards, and acted as a consultant for Abbott, AstraZeneca, Eli Lilly, Genzyme, GlaxoSmithKline, Janssen, Medtronic, Novo Nordisk, Sanofi and Servier, received research support from AstraZeneca, Boehringer Ingelheim, Prometic, Novo Nordisk, Sanofi, Servier and Viacyte, and participated in speaker's bureaus for AstraZeneca, Boehringer Ingelheim, Eli Lilly, Janssen, Lifescan, Novartis, Novo Nordisk, Sanofi and Valeant. M.B. and A.C. are employees of Sanofi. L.M.‐M is an employee of IVIDATA Group, providing consultancy to Sanofi. J.K. has received research support from AstraZeneca and Sanofi, and participated in speaker's bureaus for AstraZeneca, Boehringer Ingelheim, Eli Lilly, and Janssen. R.R. has acted as a consultant for AstraZeneca, MSD, Novo Nordisk, Sanofi and Servier, and participated in speaker's bureaus for AstraZeneca, Bristol‐Myers Squibb, Eli Lilly, MSD, Novartis, Novo Nordisk and Sanofi.
Author contributions
M.B. and A.C. were involved in the design of the meta‐analysis and acquisition of the data. L.M.‐M performed the statistical analysis of the data. All authors had access to the relevant study data and interpreted data, reviewed and commented on several drafts of the manuscript, and had final responsibility to submit the article for publication. J.E. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Javier Escalada F, Halimi S, Senior PA, et al. Glycaemic control and hypoglycaemia benefits with insulin glargine 300 U/mL extend to people with type 2 diabetes and mild‐to‐moderate renal impairment. Diabetes Obes Metab. 2018;20:2860–2868. 10.1111/dom.13470
Funding information Sanofi sponsored, designed and coordinated the clinical trials presented in this meta‐analysis.
REFERENCES
- 1. Tuttle KR, Bakris GL, Bilous RW, et al. Diabetic kidney disease: a report from an ADA Consensus Conference. Am J Kidney Dis. 2014;64:510‐533. [DOI] [PubMed] [Google Scholar]
- 2. Saran R, Li Y, Robinson B, et al. US Renal Data System 2015 Annual Data Report: Epidemiology of Kidney Disease in the United States. Am J Kidney Dis. 2016;67:A7‐A8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. New JP, Middleton RJ, Klebe B, et al. Assessing the prevalence, monitoring and management of chronic kidney disease in patients with diabetes compared with those without diabetes in general practice. Diabet Med. 2007;24:364‐369. [DOI] [PubMed] [Google Scholar]
- 4. Szczech LA, Stewart RC, Su HL, et al. Primary care detection of chronic kidney disease in adults with type‐2 diabetes: the ADD‐CKD Study (awareness, detection and drug therapy in type 2 diabetes and chronic kidney disease). PLoS One. 2014;9:e110535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Nitta K, Okada K, Yanai M, Takahashi S. Aging and chronic kidney disease. Kidney Blood Press Res. 2013;38:109‐120. [DOI] [PubMed] [Google Scholar]
- 6. Alsahli M, Gerich JE. Hypoglycemia in patients with diabetes and renal disease. J Clin Med. 2015;4:948‐964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Pathak RD, Schroeder EB, Seaquist ER, et al. Severe hypoglycemia requiring medical intervention in a large cohort of adults with diabetes receiving care in U.S. Integrated Health Care Delivery Systems: 2005‐2011. Diabetes Care. 2016;39:363‐370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. National Kidney Foundation . KDOQI Clinical Practice Guideline for Diabetes and CKD: 2012 update. Am J Kidney Dis. 2012;60:850‐886. [DOI] [PubMed] [Google Scholar]
- 9. Inzucchi SE, Bergenstal RM, Buse JB, et al. Management of hyperglycemia in type 2 diabetes, 2015: a patient‐centered approach: update to a position statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2015;38:140‐149. [DOI] [PubMed] [Google Scholar]
- 10. Hyre AD, Fox CS, Astor BC, Cohen AJ, Muntner P. The impact of reclassifying moderate CKD as a coronary heart disease risk equivalent on the number of US adults recommended lipid‐lowering treatment. Am J Kidney Dis. 2007;49:37‐45. [DOI] [PubMed] [Google Scholar]
- 11. Moen MF, Zhan M, Hsu VD, et al. Frequency of hypoglycemia and its significance in chronic kidney disease. Clin J Am Soc Nephrol. 2009;4:1121‐1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Busch M, Nadal J, Schmid M, et al. Glycaemic control and antidiabetic therapy in patients with diabetes mellitus and chronic kidney disease ‐ cross‐sectional data from the German Chronic Kidney Disease (GCKD) cohort. BMC Nephrol. 2016;17:59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Becker RH, Dahmen R, Bergmann K, Lehmann A, Jax T, Heise T. New insulin glargine 300 Units . mL‐1 provides a more even activity profile and prolonged glycemic control at steady state compared with insulin glargine 100 Units . mL‐1. Diabetes Care. 2015;38:637‐643. [DOI] [PubMed] [Google Scholar]
- 14. Riddle MC, Bolli GB, Ziemen M, et al. New insulin glargine 300 units/mL versus glargine 100 units/mL in people with type 2 diabetes using basal and mealtime insulin: glucose control and hypoglycemia in a 6‐month randomized controlled trial (EDITION 1). Diabetes Care. 2014;37:2755‐2762. [DOI] [PubMed] [Google Scholar]
- 15. Yki‐Jarvinen H, Bergenstal R, Ziemen M, et al. New insulin glargine 300 units/mL versus glargine 100 units/mL in people with type 2 diabetes using oral agents and basal insulin: glucose control and hypoglycemia in a 6‐month randomized controlled trial (EDITION 2). Diabetes Care. 2014;37:3235‐3243. [DOI] [PubMed] [Google Scholar]
- 16. Bolli GB, Riddle MC, Bergenstal RM, et al. New insulin glargine 300 U/ml compared with glargine 100 U/ml in insulin‐naive people with type 2 diabetes on oral glucose‐lowering drugs: a randomized controlled trial (EDITION 3). Diabetes Obes Metab. 2015;17:386‐394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Workgroup on Hypoglycemia, American Diabetes Association . Defining and reporting hypoglycemia in diabetes: a report from the American Diabetes Association Workgroup on Hypoglycemia. Diabetes Care. 2005;28:1245‐1249. [DOI] [PubMed] [Google Scholar]
- 18. Ritzel R, Roussel R, Bolli GB, et al. Patient‐level meta‐analysis of the EDITION 1, 2 and 3 studies: glycaemic control and hypoglycaemia with new insulin glargine 300 U/ml versus glargine 100 U/ml in people with type 2 diabetes. Diabetes Obes Metab. 2015;17:859‐867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. American Diabetes Association. Standards of medical care in diabetes‐2016: summary of revisions. Diabetes Care. 2016;39(suppl 1):S4‐S5. [DOI] [PubMed] [Google Scholar]
- 20. Zoungas S, Patel A, Chalmers J, et al. Severe hypoglycemia and risks of vascular events and death. N Engl J Med. 2010;363:1410‐1418. [DOI] [PubMed] [Google Scholar]
- 21. Bonadonna RC, Yale J‐F, Brulle‐Wohlhueter C, Boëlle‐Le Corfec E, Choudhary P, Bailey TS. Hypoglycemia as a Function of HbA1c in Type 2 Diabetes (T2DM): Insulin Glargine 300 U/mL in a Patient‐Level Meta‐analysis of EDITION 1, 2, and 3. Diabetes. 2016; 65(suppl 1):A239. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Comorbidities at baseline (randomized population).
Table S2. eGFR by study and in pooled population.
Table S3. Concomitant medications at month 6 (pooled randomized population).
Table S4. Change in FPG and insulin dose from baseline, by renal function subgroup (pooled mITT population).
Table S5. Percentage of participants achieving target HbA1c without hypoglycemia.
Table S6. Participants with reports of at least one injection site reaction, hypersensitivity, cardiovascular and renal TEAEs.


