Abstract
MiR‐24‐3p, a broadly conserved, small, noncoding RNA, is abundantly expressed in mammary tissue. However, its regulatory role in this tissue remains poorly understood. It was predicted that miR‐24‐3p targets the 3′ untranslated region (3′‐UTR) of multiple endocrine neoplasia type 1 (MEN1), an important regulatory factor in mammary tissue. The objective of this study was to investigate the function of miR‐24‐3p in mammary cells. Using a luciferase assay in mammary epithelial cells (MAC‐T), miR‐24‐3p was confirmed to target the 3′‐UTR of MEN1. Furthermore, miR‐24‐3p negatively regulated the expression of the MEN1 gene and its encoded protein, menin. miR‐24‐3p enhanced proliferation of MAC‐T by promoting G1/S phase progression. MiR‐24‐3p also regulated the expression of key factors involved in phosphatidylinositol‐3‐kinase/protein kinase B/mammalian target of rapamycin and Janus kinase/signal transducer and activators of transcription signaling pathways, therefore controlling milk protein synthesis in epithelial cells. Thus, miR‐24‐3p appears to act on MAC‐T by targeting MEN1. The expression of miR‐24‐3p was controlled by MEN1/menin, indicating a negative feedback loop between miR‐24‐3p and MEN1/menin. The negatively inhibited expression pattern of miR‐24‐3p and MEN1 was active in mammary tissues at different lactation stages. The feedback mechanism is a new concept to further understand the lactation cycle of mammary glands and can possibly to be manipulated to improve milk yield and quality.
Keywords: cell proliferation, mammary epithelial cells (MAC‐T), milk protein synthesis, miR‐24‐3p, multiple endocrine neoplasia type 1(MEN1)/menin
1. INTRODUCTION
MicroRNAs (miRNAs) are evolutionarily conserved, small noncoding RNA approximately 22 nucleotides in length that generally regulate the expression of target messenger RNA (mRNA) by binding to the 3′ untranslated region (3′‐UTR). This regulatory mechanism exists widely in animals and plants (Bartel, 2004; Bushati & Cohen, 2007; Flynt & Lai, 2008) and participates in a wide range of biological processes including cell differentiation, proliferation, and apoptosis. Recently, miRNA has been identified to be differentially expressed in mammary tissues (Gu, Eleswarapu, & Jiang, 2007; Wang & Li, 2007; Wang et al., 2012; Wicik et al., 2016) and to control the mammary gland development by regulating the formation of mammary ducts and acini (Shimono et al., 2009), proliferation and apoptosis of mammary epithelial cells (MAC‐T; Avril‐Sassen et al., 2009; Tanaka, Haneda, Imakawa, Sakai, & Naqaoka, 2009) and synthesis of milk protein, lactose, and milk fat (Li, Wang, Li, & Gao, 2012; Li et al., 2015; Lian et al., 2016; Wang et al., 2014). In mice, miR‐138 was found to regulate mammary development and galactopoesis by targeting the prolactin receptor (Wang, Li, & Li, 2008), thus modulating the physiological role of prolactin in mammary cells. In addition, miR‐152 inhibited the activity of DNA methyltransferase 1 (DNMT1) and reduced the level of DNA methylation, therefore activating the lactation signal transduction genes AKT (serine/threonine protein kinase AKT) and peroxisome proliferator‐activated receptor gamma (Wang et al., 2014). Therefore, miRNA plays an important role in mammary gland development and milk synthesis in mammals.
MiR‐24 is abundantly expressed in the mammary tissue of Holstein cows (Gu et al., 2007), suggesting that miR‐24 may play an important role in mammary function in cattle. MiR‐24 is highly conserved across species, is broadly involved in the cell proliferation, differentiation, and apoptosis (Gao et al., 2015; Meng, Wang, & Jia, 2014) in liver and intestinal tract of patients. However, the specific function of miR‐24‐3p in mammary tissues is poorly understood.
Menin, a ubiquitous nuclear protein that interacts with a variety of transcription factors regulating cell proliferation, plays an essential role in maintaining the metabolic balance of organisms (Matkar, Thiel, & Hua, 2013; Yang & Hua, 2007). Menin is the product of the multiple endocrine tumor type 1 (MEN1) gene, named for its causative genetic disease in human, MEN1 syndrome (Falchetti et al., 2009). The absence of MEN1 causes abnormal cell proliferation or apoptosis by acting on the cell cycle regulator p27Kip1 and p18INK4C (Milne et al., 2005) and/or DNMT1 (Cheng et al., 2016). A series of studies have shown that MEN1/menin participates in the regulation of mammary gland development and lactation. First, the absence of menin not only caused increased secretion of prolactin through promotion of pituitary (anterior lobe) cell proliferation (Bertolino, Tong, Galendo, Wang & Zhang, 2003), but also affected the expression of prolactin mRNA by binding to its promoter in MAC‐T, therefore indirectly regulating mammary cell development and galactopoesis (Namihira et al., 2002; Schussheim et al., 2001). Second, menin also catalyzes the activity of estrogen receptor‐α and affects the development of breast acini (Dreijerink et al., 2006; Imachi et al., 2010). In addition, variable expression of menin in mammary tissue of dairy cows was observed in response to hormonal signals including insulin and prolactin. Changes in menin expression appear to directly regulate the milk protein synthesis through mammalian target of rapamycin (mTOR) signaling in MAC‐T (Li et al., 2017) and to modulate mammary epithelial cell proliferation through the cell cycle regulator cyclinD1 (Shi et al., 2017).
Other than the knowledge that menin expression is responsive to at least insulin and prolactin, the internal upstream factors controlling expression of menin in mammary tissue remain largely unknown. Interestingly, it was predicted that miR24 could target the 3′‐UTR of MEN1 gene in many species, and it was reported that miR‐24 interacted with menin in the parathyroid and pancreatic islets in human patients (Luzi et al., 2012; Vijayaraghavan, Maggi, & Crabtree, 2014). Therefore, in the current study, we hypothesized that miR24 regulates MEN1/menin in MAC‐T, thereby revealing the regulatory role of miR24 in mammary tissue.
2. MATERIALS AND METHODS
2.1. Cell cultures
Immortalized bovine MAC‐T were grown in Dulbecco's modified Eagle's medium‐F12 (Gibco, Waltham, MA) containing 10% fetal bovine serum (Biological Industries, Beth‐HaEmek, Israel), 100 IU/ml penicillin, and 100 μg/ml streptomycin (Sigma, St. Louis, MO) at 37°C under 5% CO2.
2.2. Transient transfections
Before the experimental treatments, cells were detached with 0.25%‐trypsin (Gibco) and transferred to six‐well plates at a density of 5 × 105 cells per well with antibiotic‐free medium. The bovine MAC‐T were transfected with 50 nM mimics and/or 100 nM inhibitor specific to bta‐miR‐24‐3p (bovine miR‐24‐3p), as well as their negative control (NC), using Lipofectamine 2000 (Invitrogen, Carlsbad, CA). The mimics and inhibitor were designed and synthesized by Ribobio (Guangzhou, China). Preliminary experiments were performed to assess the effectiveness of a range of each and those with the greatest effects on expression efficiency were chosen for use in the followed experiments (Supporting Information Figure S1). For the over‐ and/or underexpression of menin, MAC‐T cells were transfected with 1200 ng/well of pEGFP‐C2‐bMEN1 (bMEN1), the NC pEGFP‐C2‐Vector (vector), and/or 100 nM bovine MEN1 specific small interfering RNA (siRNA), the NC siRNA (control) as described by Li et al. (2017). Total RNA and protein were isolated from the transfected cells 24 hr post‐transfection for further analysis.
2.3. Quantitative RT‐PCR
Total RNA was isolated from transfected MAC‐T cells and/or bovine mammary gland tissue using the miRcute miRNA Isolation Kit (TIANGEN, Beijing, China) in accordance with the manufacturer's instructions. The quantity and purity of total RNA were assessed by UV absorption and gel electrophoresis. The first‐strand complementary DNA (cDNA) was synthesized using the Mir‐X miRNA First‐Strand Synthesis Kit or PrimeScriptTM RT reagent Kit with gDNA Eraser (TaKaRa, Dalian, China) according to the manufacturer's protocol. Target gene expression was assessed by quantitative polymerase chain reaction (PCR) using a ABI PRISM 7500 real‐time RT‐PCR System (ABI, Foster, CA) and a SYBR® Premix Ex Taq TM (Tli RNaseH Plus) real‐time PCR kit (TaKaRa, Dalian, China). The purity of all PCR products was confirmed by melting curve analysis. Primer sequences are described in Supporting Information Table S1. The expression levels of the target mRNA and miRNA were normalized to β‐actin and glyceraldehyde‐3‐phosphate dehydrogenase, and small nuclear RNA U6, respectively. The results are representative of at least three independent experiments to determine the statistical significance.
2.4. Cell proliferation assay
Equal numbers of MAC‐T cells (4 × 105 cells per well) were transfected with bta‐miR‐24‐3p mimics, inhibitor, or corresponding NC in six‐well plates. The cell number was measured using a Typan Blue staining Cell Viability Assay Kit (KeyGEN Bio TECH, Nanjing, China) at 0, 24, and 48 hr after transfection. Briefly, 10 µl of trypan blue staining solution was added to 90 µl of cell suspension followed by counting using an inverted microscope (n = 3). Only live cells without blue stainings were counted. Simultaneously, cell proliferation was assessed using a CCK‐8 Cell Counting kit (Vazyme, Nanjing, China) in a 96‐well plate (2 × 104 cells per well). 10 µl of CCK‐8 solution was added to 100 µl cell suspension and incubated for 2 hr, followed by absorbance assessment at 450 nm (n = 6). All experiments were performed in triplicates for each transfection.
2.5. Cell cycle analysis
MAC‐T cells transfected with bta‐miR‐24‐3p mimics or inhibitor were analyzed for cell cycle phases distribution (FACSCalibur; BD Biosciences) using a cell cycle detection kit (KeyGEN Bio TECH, Nanjing, China) according to the manufacturer's instructions. 2.5 × 105 cells were harvested, fixed, and analyzed in Vindelov's propidium iodide buffer. Data were processed using Mod Fit LT 3.2 software (Verity Software House). All of the experiments were performed three times for each transfection.
2.6. Target gene prediction
The candidate microRNA targets specific to the 3′‐UTR region of bovine MEN1 gene (NCBI NM_001076161) were identified using TargetScan6.2 (http://www.targetscan.org) and RNAhybrid 2.2 (http://bibiserv.techfak.uni-bielefeld.de/rnahybrid). The software predicted that bta‐miR‐24‐3p (miRBase MIMAT0003840) was one of the microRNAs that bind to the 3′‐UTR of MEN1 mRNA. Therefore, the interaction between bta‐miR‐24‐3p and bovine MEN1/menin were further investigated in MAC‐T.
2.7. Luciferase assays
A bovine MEN1–3′‐UTR was fused to a luciferase gene within the pMIR‐REPORT expression plasmid (Ambion Life Technologies, Grand Island, NY; referred to as pMIR‐REPORT‐bMEN1–3′‐UTR). The 789 bp fragment of MEN1 3′‐UTR was amplified from MEN1 cDNA using the forward primer 5′‐GCCACTAGTAGTACCGGGACTCCATATC‐3′ and the reverse primer 5′‐GCCAAGCTTACAAAATGTATTCATCTTCCT‐3′ (Sang Biotech, Shanghai, China). The bovine MAC‐T were transfected with 300 ng of pMIR‐REPORT‐bMEN1–3′‐UTR in combination with 50 nM mimics or 100 nM inhibitor specific to bta‐miR‐24‐3p, or the corresponding NCs (mimics NC or inhibitor NC) using Lipofectamine 2000 (Invitrogen) according to the manufacturer's instructions. pMIR‐REPORT β‐gal (25 ng/well; Ambion), a beta‐galactosidase reporter plasmid, was simultaneously transfected for each well to provide the internal normalization of transfection. Luciferase assays were performed at 48 hr after transfection using the Luciferase Assay System (Promega, Madison, WI) and β‐Galactosidase Enzyme Assay System with reporter lysis buffer (Promega). Each transfection was assayed in triplicate. All of the experiments were performed three times for each transfection.
2.8. Western blot analysis
Total protein was extracted in radio‐immunoprecipitation assay (RIPA) lysis buffer containing 1% phenylmethanesulfonyl fluoride (PMSF) (Beyotime, Nanjing, China) from MAC‐T cells 24 hr after transfection or from the mammary tissues that collected as described in Li et al. (2017). Approximately 25 μg of total protein was separated by polyacrylamide gel electrophoresis (10% SDS‐PAGE) and transferred onto nitrocellulose membranes at 200 mA of constant current, followed by western blot analysis. Primary antibodies against bovine menin (Bethyl Laboratories, TX) and β‐actin (Beyotime) were used at a dilution of 1:1000. A horseradish peroxidase (HRP)‐conjugated secondary antibody (Beyotime, Jiangsu, China) was diluted 1:1000 as working solution. Chemiluminescence detection was performed using BeyoECL Plus (Beyotime, Beijing, China). Luminescence data was normalized to the corresponding NCs. β‐actin was used as the total protein loading control. The results reported represent the mean of three independent experiments.
2.9. Animals and mammary gland tissue collection
Six healthy Holstein cows were biopsied for the mammary gland samples at Holstein Cattle Association Jiabao Farm in Shandong province. Tissue samples were collected on the day that corresponded with the peak milk period at +55 days in milking and the dry period before parturition (calving day as Day 1). The detailed tissue collection information was described previously (Li et al., 2017).
2.10. Statistical analysis
Data are expressed as the means ± standard deviation of at least three independent experiments or animals. Statistic differences among groups were compared with one‐way analysis of variance, and the difference between pair‐designed experiments were compared with Student's t tests by using SAS v8.2 (SAS Institute Inc. Cary, NC). Significant differences were declared when the p values were < 0.05 (*) or < 0.01 (**).
3. RESULTS
3.1. MiR‐24‐3p promotes the proliferation of mammary epithelial cells
The number of MAC‐T present in the udder is an important determinant of milk yield in dairy cows. The lactation cycle goes with mammary cell proliferation and/or apoptosis (Boutinaud, Guinard‐flamenta, & Jammes, 2004; Boutinaud, Lollivier, Finot, Bruckmaier & Lacasse, 2012). Studies have shown miR‐24 is a critical regulator of the survival of myeloid, B and primary hematopoietic cells. Its prosurvival activity contributes to the transformation of hematopoietic cells (Nguyen, Rich, & Dahl, 2013). MiR‐24 can inhibit proliferation of osteosarcoma cells by targeting on lysophosphatidic acid acyltransferase in vivo and in vitro (Song et al., 2013). To understand the impact of bta‐miR‐24‐3p on the proliferation of MAC‐T, bta‐miR‐24‐3p mimics and/or inhibitor, was transfected into MAC‐T cells and cell growth rate was assessed. The results showed that the number of viable cells in the bta‐miR‐24‐3p mimics treatment group was significantly greater than its NC group by 48 hr after transfection (supporting information Figure S1; Figure 1a; p = 0.0418), and, fewer live cells were observed for the bta‐miR‐24‐3p inhibitor transfection group (Figure 1b; p > 0.05). Proliferation measured by the CCK‐8 assay showed similar results with increased proliferation for bta‐miR‐24‐3p mimics (Figure 1c; p < 0.05), and proliferation inhibition for the bta‐miR‐24‐3p inhibitor (Figure 1d; p > 0.05).
Figure 1.
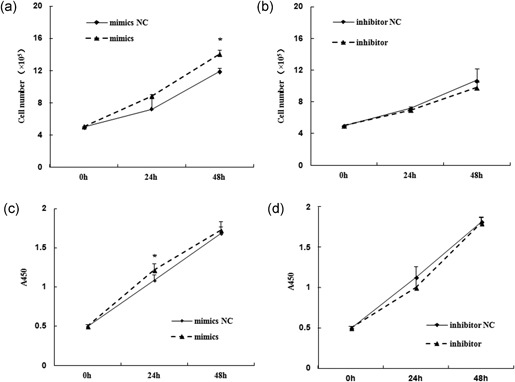
MiR‐24‐3p promotes the proliferation of mammary epithelial cells. The cell proliferation of mammary epithelial cells (MAC‐T) upon miR‐24‐3p expression modulation were assessed at 0, 24, and 48 hr after transfection of miR‐24‐3p mimics (a) and/or miR‐24‐3p inhibitor (b), as well as their corresponding negative controls (mimics NC and/or inhibitor NC), using cell counting methods after typan blue staining (a,b) and CCK‐8 assay (c,d) measuring the absorbance at 450 nm. Data are represented as the mean ± standard deviation from three independent experiments. *p < 0.05. NC: negative control
Because cell proliferation is closely related to the cell cycle regulation, we assessed whether cell cycle regulatory genes were modulated by bta‐miR‐24‐3p. Increased miR‐24‐3p was associated with decreased cells in the G0/G1 phase (Figure 2a; p = 0.04), and increased cells in the S phase (Figure 2a; p = 0.01). Conversely, downregulation of miR‐24‐3p resulted increased cells in the G0/G1 phase and decreased cells in the S phase (Figure 2b; p > 0.05). These results suggested bta‐miR‐24‐3p is involved in regulating G1/S progression of MAC‐T . Evaluation of the expression of genes controlling the cell cycle showed that increased miR‐24‐3p promoted the expression of cyclinD1 (p = 0.001) and CDK6 (p = 0.005), and markedly decreased expression of p27 (p27 Kip; p = 0.037), an important negative regulator during G1/S transition (Figure 2c). Conversely, the expression of cyclinD1 (p = 0.016) and CDK6 (p = 0.005) was significantly inhibited when bta‐miR‐24‐3p was downregulated (Figure 2d). These data indicated the regulatory role of bta‐miR‐24‐3p in the proliferation of MAC‐T through cell cycle regulator specific to G1/S transition.
Figure 2.
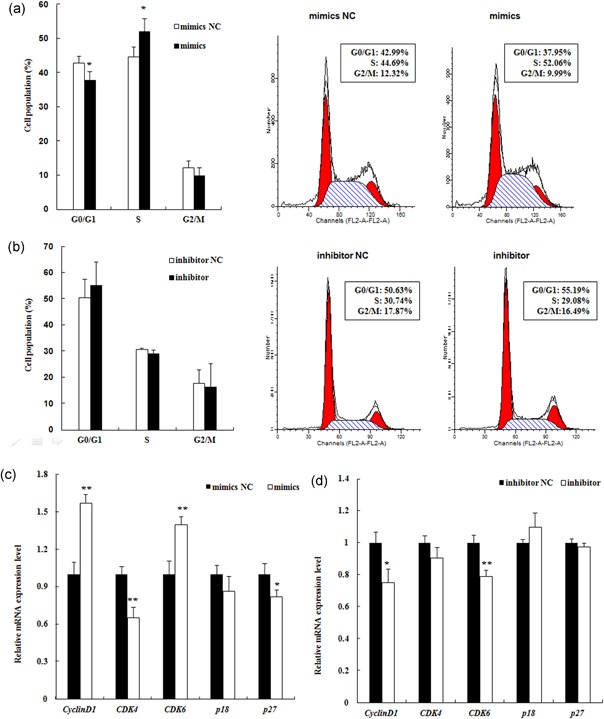
MiR‐24‐3p promotes cell cycle progression from G0/G1 to the S phase in mammary epithelial cells. MAC‐T cells were exposed to the miR‐24‐3p mimics (mimics, a) and/or miR‐24‐3p inhibitor (inhibitor, b), as well as their corresponding negative controls (mimics NC and/or inhibitor NC), for 24 hr and assessed for the distribution of cell cycle phases after propidium iodide (PI) staining. The percentages of cells in G0/G1, S, and G2/M phases are shown in (a,b), respectively. Representative FACS images of cell phase analyses illustrated changes of cell cycle in MAC‐T cells upon miR‐24‐3p overexpression (a) and/or low‐expression (b), compared with the negative controls. The cell cycle phase distribution (%) is indicated within each panel. The gene expression of cell cycle regulators specific to G1/S phase, such as cyclinD1, CDK4, CDK6, p18, and p27, were detected in the same miR‐24‐3p mimics (c) and or inhibitor (d) transfected MAC‐T cells using quantitative RT‐PCR. All of the experiments were performed three times for each transfection. **p < 0.01 and *p < 0.05. MAC‐T: mammary epithelial cell; NC: negative control; RT‐PCR: reverse transcription polymerase chain reaction [Color figure can be viewed at wileyonlinelibrary.com]
3.2. MiR‐24‐3p regulates milk protein synthesis through changes in expression of mTOR signaling pathway genes in mammary epithelial cells
Milk synthesis is the primary, important function of mammary cells in mammals. Factors involved in the phosphatidylinositol‐3‐kinase (PI3K)/protein kinase B (also known as AKT)/mTOR and the Janus kinase (JAK)/signal transducer and activators of transcription (STAT) signaling pathway have been shown to regulate in milk protein synthesis in the mammary gland (Appuhamy et al., 2014; Burgos, Dai, & Cant, 2010; Yang et al., 2008). Upon upregulation of miR‐24‐3p, the mRNA expression levels of the components involved in the PI3K/AKT/mTOR and JAK/STAT5 pathway, AKT (p = 0.01), mTOR (p = 0.003), 4E‐BP1 (eukaryotic translation initiation factor 4E binding protein 1; p = 0.029) and STAT5 (p = 0.007) were significantly decreased (Figure 3a). The expression of total and phorsphorylated protein AKT (phosphorylated at Ser473), mTOR (phosphorylated at Ser2481), and STAT5 (phosphorylated at Tyr694) were also markedly inhibited (Figures 3b,c). Simultaneously, the mRNA expression of CSNK (κ‐casein), one of the key milk proteins, was also markedly decreased (p = 0.04). By contrast, downregulation of miR‐24‐3p caused significant increased expression of AKT (p = 0.001), mTOR (p = 0.03) and 4E‐BP1 (p = 0.006; Figure 3b). The increased expression trends were also shown for phorsphorylated AKT, mTOR, and STAT5 protein (Figures 3d,e). Hence, these results suggest that miR‐24‐3p could regulate milk protein synthesis in mammary gland epithelial cells through the PI3K/AKT/mTOR and JAK/STST5 signaling pathway.
Figure 3.
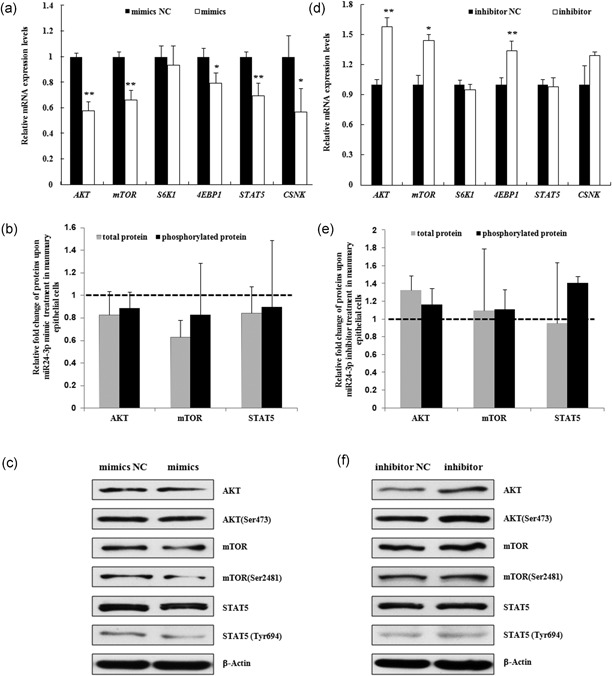
MiR‐24‐3p modulates the expression of genes that regulate milk protein synthesis in mammary epithelial cells. The expression levels of factors involved in the PI3K/AKT/mTOR (AKT, mTOR, S6K1, and 4E‐BP1) and JAK/STAT (STAT5) pathways that are associated with milk protein synthesis were assessed using quantitative real‐time PCR (qRT‐PCR) in MAC‐T cells at 24 hr after transfection with miR‐24‐3p mimics (mimics)/mimics NC (mimics NC, a) and miR‐24‐3p inhibitor (inhibitor)/inhibitor NC (inhibitor NC, b). Meanwhile, the expression of κ casein (CSNK), one of the key proteins that can be detected in MAC‐T cells, was also assessed in the cells. The data are shown as the relative expression levels normalized to the internal control, β‐actin. *p < 0.05, **p < 0.01. The phosphorylation levels of AKT at Ser473, mTOR at Ser2481, STAT5 at Tyr694 were detected simultaneously. The data are shown as the relative expression levels normalized to the loading controls, β‐actin. The horizontal dashed line represents the normalized level of their corresponding negative controls (b,d). Representative WB images of the expression of AKT, AKT phosphorylated at Ser473, mTOR, mTOR phosphorylated at Ser2481, STAT5 and STAT5 phosphorylated at Tyr694 are shown (c,e). AKT: protein kinase B; JAK: Janus kinase; MAC‐T: mammary epithelial cell; mTOR: mammalian target of rapamycin; NC: negative control; PCR: polymerase chain reaction; PI3K: phosphatidylinositol‐3‐kinase; STAT: signal transducer and activators of transcription
3.3. MiR‐24‐3p inhibits the expression of MEN1/menin through interacting with the 3′‐UTR of MEN1 gene
Interestingly, the regulatory role of miR‐24‐3p in MAC‐T was opposite to our previous observations of the effects of MEN1/menin on cell proliferation and milk protein synthesis (Li et al., 2017; Shi et al., 2017). Thus, we were curious to investigate possible interactions between miR‐24‐3p and MEN1/menin. MiR‐24‐3p, broadly conserved across species, may target the 3′‐UTR of bovine MEN1 mRNA based on predictions (Figure 4a,b). Results indicated that cotransfection of miR‐24‐3p mimics and MEN1–3′‐UTR containing plasmids significantly decreased luciferase signal (about 44%) at 48 hr after transfection (Figure 4c; p < 0.01), with no significant change in other transfection groups, suggesting that miR‐24‐3p might target the 3′‐UTR of the MEN1 gene in bovine MAC‐T.
Figure 4.
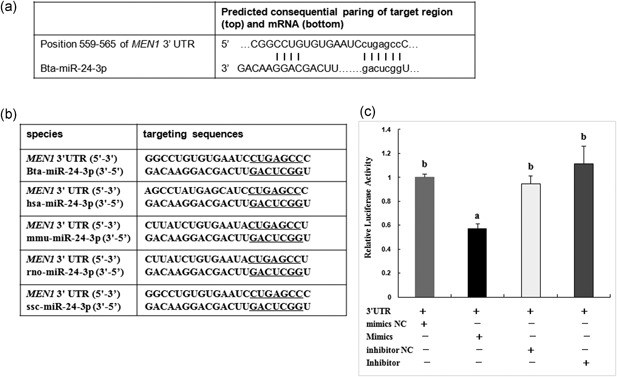
MiR‐24‐3p targets the 3′‐UTR of MEN1 mRNA. The bovine miR‐24‐3p (bta‐miR‐24‐3p) were predicted to interact with the 3′‐UTR region of MEN1 mRNA at position 559–565 using TargetScan 6.2 and RNAhybrid 2.2 software (a). MiR‐24‐3p, broadly conserved in different species, possibly also interacts with 3′‐UTR regions of MEN1 in human (has‐), mouse (mmu‐), rat (rno‐) and pig (ssc‐), with exact the same seed nucleotide sequence (underlined, b). The 3′‐UTR region (789 bp) of bovine MEN1 gene were construed into luciferase‐expressing plasmid (3′‐UTR). The plasmid was cotransfected with miR‐24‐3p mimics (mimics) and/or miR‐24‐3p inhibitor (inhibitor) into mammary epithelial cells, as well as their corresponding negative controls (mimics NC and/or inhibitor NC). The beta‐galactosidase reporter plasmid (β‐gal) was simultaneously transfected for each every transfection, serving as internal control. Significant suppressed luciferase activity was shown in the cotransfection group of 3′‐UTR plasmid and miR‐24‐3p mimics, indicating bta‐miR‐24‐3p targeted the 3′‐UTR of bovine MEN1. All of the experiments were performed three times for each transfection. Each experiment was performed in triplicates. Different letters (a,b) indicate significant difference (*p < 0.05). 3′‐UTR: 3′ untranslated region; MEN1: multiple endocrine tumor type 1; mRNA: messenger RNA; NC: negative control
3.4. MEN1/menin expression is controlled by miR‐24‐3p, but also regulates miR‐24‐3p expression, acting as a negative feedback loop in mammary tissues
As expected, the expression of MEN1 mRNA (Figure 5a; p = 0.007) and menin protein (Figure 5b; p = 0.015) were significantly inhibited upon the transfection of bta‐miR‐24‐3p mimics in MAC‐T. Conversely, inhibition of expression of bta‐miR‐24‐3p resulted in increased expression of MEN1 mRNA (Figure 5c; p = 0.001) at 24 hr after transfection, and also menin protein (Figure 5d; p = 0.002) at 48 hr after transfection. These data confirmed that miR‐24‐3p acts as an upstream factor of MEN1 gene, negatively regulating the MEN1/menin expression in MAC‐T.
Figure 5.
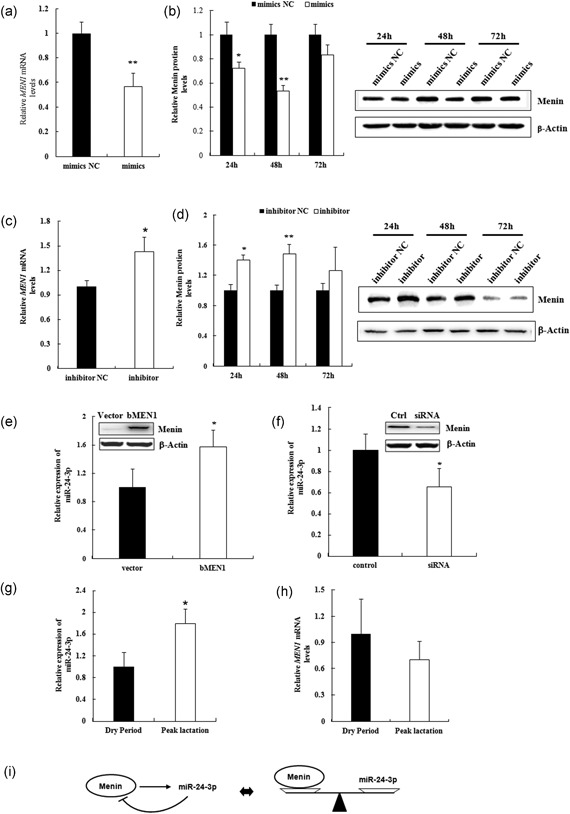
MiR‐24‐3p and MEN1 act in a negative feedback model. (a–d) Mammary epithelial cells were transfected with miR‐24‐3p mimics/mimics NC (mimics/mimics NC; a and c) and/or miR‐24‐3p inhibitor/inhibitor NC (inhibitor/ inhibitor NC; b,d), and cells were harvested for RNA and protein extraction at 24, 48, and 72 hr after transfection. The expression of bovine MEN1 mRNA at 24 hr (a,c) and protein menin in a time course (b,d) were assessed using qRT‐PCR and western blot technology, respectively. Representative WB images of the expression of protein menin at 24, 28, and 72 hr after transfection of indicated miR‐24‐3p are shown. The data are shown as the relative expression levels normalized to the internal control, β‐actin. * p < 0.05. (e,f) Mammary epithelial cells were transfected with pEGFP‐C2‐bMEN1 (bMEN1)/pEGFP‐C2‐Vector (vector) for MEN1 overexpression system (e) and/or bovine MEN1 specific siRNA/nonspecific negative control siRNA (control) for MEN1 low‐expression system (f). Cells were harvested for RNA extraction and then the expression detection of miR‐24‐3p at 24 hr after transfection. The data are shown as the relative expression levels normalized to the internal control, small nuclear (sn) RNA U6. (g,h) The expression of miR‐24‐3p (g) and MEN1 (h) were measured in mammary tissues of dairy cows at dry period stage (n = 3) and peak lactation stage (n = 3). The data are shown as the relative expression levels normalized to the internal controls, small nuclear RNA U6 for miR‐24‐3p expression (g) and β‐actin for MEN1 expression (h). *p < 0.05. (i) MiR‐24‐3p can negatively modulate the expression level of menin in mammary epithelial cells, whereas menin is a positive regulator of miR‐24‐3p expression. MiR‐24‐3p and menin were suggestive of forming a “negative feedback loop” in mammary epithelial cells, dynamically maintaining the metabolic balance in mammary glands. MEN1: multiple endocrine tumor type 1; mRNA: messenger RNA; NC: negative control; qRT‐PCR: quantitative real‐time polymerase chain reaction; siRNA: small interfering RNA
Amazingly, the expression of miR‐24‐3p was also significantly upregulated upon MEN1/menin overexpression (Figure 5e; p = 0.049) in MAC‐T, while significantly decreased upon MEN1/menin low‐expression (Figure 5f; p = 0.048). This suggested that MEN1/menin serves as an upstream controller of the expression of miR‐24‐3p in MAC‐T.
The expression profile of bta‐miR‐24‐3p in mammary gland tissues of dairy cows at different development stages was also investigated. Bta‐miR‐24‐3p expression was greater and MEN1 expression less at peak lactation (Figure 5g; p = 0.046), and the reverse for the non‐lactating dry period tissue (Figure 5h; p > 0.05). These data further support the concept of a dynamic “negative feedback loop” in MAC‐T, keeping the expression of MEN1 and bta‐miR‐24‐3p in balance. Correspondingly, it also suggests that miR‐24‐3p acts with MEN1 gene (Figure 5i) to regulate cell proliferation and milk protein synthesis in MAC‐T.
4. DISCUSSION
The milk yield of dairy cows depends on the number of MAC‐T and its secretion activity (Boutinaud et al., 2004, 2012). This novel study demonstrated that miR‐24‐3p plays an important regulatory function in mammary tissues, differentially expressing at different lactation stages (Figure 5g). First, bta‐miR‐24‐3p promotes cell cycle progression from G0/G1 phase to the S phase in bovine MAC‐T by controlling the expression of cell cycle regulator specific to the G1/S phase, such as cyclinD1, CDK6 and p27. In previous studies, a similar biological function of miR‐24 involved in cell proliferation was recently reported in different pathological conditions (Gao et al., 2015; Meng et al., 2014; Sun, Xiao, Xu, & Yuan, 2016). P18 and/or p27 were also reported to be one of the target genes of miR‐24 (Giglio et al., 2013; Karnik et al., 2005; Vijayaraghavan et al., 2014). Second, bta‐miR‐24‐3p regulates the milk protein synthesis, such as κ‐casein (Bionaz et al., 2012), through the PI3K/AKT/mTOR and/or the JAK/STAT pathway. All these data suggest an important regulatory role of miR‐24‐3p on cell number and capability of milk protein synthesis in MAC‐T. The molecular regulatory mechanism of miR‐24‐3p during the development of prenatal mammary glands would be our next interest.
The effects of bta‐miR‐24‐3p on MAC‐T proliferation were consistent with our previous discovery about MEN1/menin's role in mammary tissues (Shi et al., 2017). MEN1/menin were shown to respond to physiologic hormone insulin and prolactin (Li et al., 2017), and therefore modulate the milk protein synthesis through the PI3K/AKT/mTOR pathway (Li et al., 2017), and regulate mammary epithelial cell proliferation through cell cycle regulator cyclinD1 (Shi et al., 2017). The dual function of menin in mammary tissues was exactly the same with that of bta‐miR‐24‐3p. This indicated a possible link between menin and bta‐miR24‐3p. Bta‐miR‐24‐3p might make effects in mammary tissues through targeting MEN1.
Our data indicated that bta‐miR‐24‐3p was capable to suppress the expression of MEN1 gene at both mRNA and protein levels in bovine MAC‐T . On the other side, bta‐miR‐24‐3p expression was also regulated by MEN1/menin, forming a negative feedback action model in mammary tissues. The converse expression patterns of miR‐24‐3p and MEN1 gene were validated in the mammary tissues of dairy cows at different lactation stages. In a word, MEN1/menin played its regulatory role in mammary glands through cooperating with miR‐24‐3p (Figure 6). This negative feedback loop model between miRNAs and target genes/factors was also seen in other tissues (Keifer, Zheng, & Ambigapathy, 2015; Shi et al., 2014).
Figure 6.
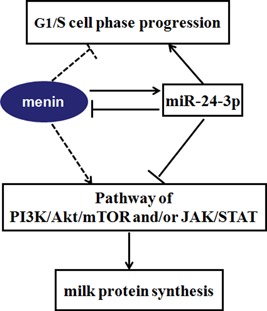
MiR‐24‐3p regulates cell proliferation and milk protein synthesis in mammary epithelial cells through cooperatively acting with MEN1/menin. MiR‐24‐3p positively regulates the proliferation of mammary epithelial cells, promoting the G1/S cell phase progression. While, MEN1/menin was found, in our previous study (Li et al., 2017), negatively control the proliferation of at G1/S phase progression, causing cell growth arrest. This is contributed to the negative feedback controlling model between miR‐24‐3p and MEN1/menin. The dynamic balance model between miR‐24‐3p and MEN1/menin also make an opposite impacts on milk protein synthesis of mammary epithelial cells through PI3K/AKT/mTOR and/or JAK/STAT signaling pathway. The solid line represents fluxes found in the current study; the dotted line represents previously found effects or fluxes (Li et al., 2017). The arrows indicate the positive regulation, and the blunt‐ended ones indicate the negative regulation. AKT: protein kinase B; JAK: Janus kinase; MEN1: multiple endocrine tumor type 1; mTOR: mammalian target of rapamycin; PI3K: phosphatidylinositol‐3‐kinase; STAT: signal transducer and activators of transcription [Color figure can be viewed at wileyonlinelibrary.com]
The functional discoveries about miR‐24‐3p in MAC‐T could further support this action model. The negatively regulated MEN1/menin by miR‐24‐3p caused enhanced cell proliferation in mammary glands. In another word, the expression reduction or elevation of cell cycle factors were likely to be the regulatory effect of miR‐24 on MEN1/Menin (Ehrlich et al., 2017; Luzi et al., 2012; Luzi, Marini, Giuffi, Galli, & Brandi, 2016; Vijayaraghavan et al., 2014). In addition, the modulation expression of MEN1/menin in MAC‐T was revealed to positively regulate miR‐24‐3p expression (Figure 5e,f). Menin possibly binds to the promoter region of primary miR‐24 and regulate its transcription (Luzi et al., 2012; Luzi et al., 2016; Vijayaraghavan et al., 2014). The detailed mechanism needs to be further investigated in mammary tissues.
However, each miRNA has the ability to regulate a large number of genes, and one single gene can be regulated by multiple miRNAs (Filipowicz, Bhattacharyya, & Sonenberg, 2008). MiR‐24‐3p might not be the only upstream factor that regulates MEN1/menin expression in mammary tissues. For example, miR‐17 promoted pancreatic β‐cell proliferation by reducing the expression of MEN1/Menin (Lu, Fei, Yang, Xu, & Li, 2015). Growing evidence indicates that other miRNAs play a regulatory role in the development and lactation processes in bovine mammary tissues (Zhou et al., 2013), such as miR‐152, miR‐138 (Wang et al., 2008, 2014). Additionally, it does not rule out the other possible routes that miR‐24‐3p might take and makes impacts on mammary glands by targeting additional genes other than MEN1.
5. CONCLUSION
MiR‐24‐3p negatively modulates the expression of MEN1/menin in MAC‐T by targeting the 3′‐UTR of MEN1. MiR‐24‐3p plays regulatory role of cell proliferation and milk protein synthesis in MAC‐T through cooperatively acting with menin.
CONFLICTS OF INTEREST
The authors declare that they have no conflicts of interest concerning this study.
Supporting information
Supporting information
Supporting information
ACKNOWLEDGMENTS
The authors would like to thank farm owner who generously allowed them to sample mammary gland tissue from their cattle.They are also thankful to Mark Hanigan (Virginia Tech) for his kindly contribution of the Immortalized bovine mammary epithelial cell line MAC‐T. We would like to thank Dr. Sunita K Agarwal (NIH/NIDDK), Dr. Robin White (Virginia Tech), Dr. Dongxiao Sun and Dr. Ying Yu (China Agricultural University), Dr. Xueyan Lin (Shandong Agricultural University), and Dr. Jinming Huang (Research Center of Dairy Cow, Shandong Academy of Agricultural Science) for their helpful discussions about the project and the manuscript. This study was financially supported by the National Natural Science Foundation of China (31402054), the Key Project of Agricultural Fine Breeding of Shandong Province (2016LZGC030), the Natural Science Foundation of Shandong (ZR2013CM013), the Modern Agricultural Industry Technology System (CARS‐36), Funds of Shandong “Double Tops” Program (SYL2017YSTD08), Tai Mountain Scholar Program (201711025).
Qiaoqiao C, Li H, Liu X, et al. MiR‐24‐3p regulates cell proliferation and milk protein synthesis of mammary epithelial cells through menin in dairy cows. J Cell Physiol. 2019;234:1522–1533. 10.1002/jcp.27017
Contributor Information
Zhonghua Wang, Email: zhwang@sdau.edu.cn.
Kerong Shi, Email: krshi@sdau.edu.cn.
References
REFERENCES
- Appuhamy, J. A. , Nayananjalie, W. A. , England, E. M. , Gerrard, D. E. , Akers, R. M. , & Hanigan, M. D. (2014). Effects of AMP‐activated protein kinase (AMPK) signaling and essential amino acids on mammalian target of rapamycin (mTOR) signaling and protein synthesis rates in mammary cells. Journal of Dairy Science, 97, 419–429. [DOI] [PubMed] [Google Scholar]
- Avril‐Sassen, S. , Goldstein, L. D. , Stingl, J. , Blenkiron, C. , Le Quesne, J. , Spiteri, I. , … Caldas, C. (2009). Characterisationof microRNA expression in post‐natal mouse mammary gland development. BMC Genom, 10, 548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartel, D. P. (2004). MicroRNAs: Genomics, biogenesis, mechanism, and function. Cell, 116, 281–297. [DOI] [PubMed] [Google Scholar]
- Bertolino, P. , Tong, W. M. , Galendo, D. , Wang, Z. Q. , & Zhang, C. X. (2003). Heterozygous MEN1 mutant mice develop a range of endocrine tumors mimicking multiple endocrine neoplasia type 1. Molecular Endocrinology, 17(9), 1880–1892. [DOI] [PubMed] [Google Scholar]
- Bionaz, M. , Periasamy, K. , Rodriguez‐Zas, S. L. , Everts, R. E. , Lewin, H. A. , Hurley, W. L. , & Loor, J. J. (2012). Old and new stories: Revelations from functional analysis of the bovine mammary transcriptome during the lactation cycle. PLoS One, 7(3), e33268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boutinaud, M. , Guinard‐Flament, J. , & Hélènejammes, H. (2004). The number and activity of mammary epithelial cells, determining factors for milk production. Reproduction, Nutrition, Development, 44, 499–508. [PubMed] [Google Scholar]
- Boutinaud, M. , Lollivier, V. , Finot, L. , Bruckmaier, R. M. , & Lacasse, P. (2012). Mammary cell activity and turnover in dairy cows treated with the prolactin‐release inhibitor quinagolide and milked once daily. Journal of Dairy Science, 95(1), 177–187. [DOI] [PubMed] [Google Scholar]
- Burgos, S. A. , Dai, M. , & Cant, J. P. (2010). Nutrient availability and lactogenic hormones regulate mammary protein synthesis through the mammalian target of rapamycin signaling pathway. Journal of Dairy Science, 93, 153–161. [DOI] [PubMed] [Google Scholar]
- Bushati, N. , & Cohen, S. M. (2007). microRNA functions. Annual Review of Cell and Developmental Biology, 23, 175–205. [DOI] [PubMed] [Google Scholar]
- Cheng, P. , Wang, Y. , Li, G. , Yang, S. , Liu, C. , Hu, H. , … Hu, X. (2016). Interplay between menin and Dnmt1 reversibly regulates pancreatic cancer cell growth downstream of the Hedgehog signaling pathway. Cancer Letters, 370(1), 136–144. [DOI] [PubMed] [Google Scholar]
- Dreijerink, K. M. A. , Mulder, K. W. , Winkler, G. S. , Höppener, J. W. M. , Lips, C. J. M. , & Timmers, H. T. M. (2006). Menin links estrogen receptor activation to histone H3K4 trimethylation. Cancer Research, 66(9), 4929–4935. [DOI] [PubMed] [Google Scholar]
- Ehrlich, L. , Hall, C. , Venter, J. , Dostal, D. , Bernuzzi, F. , Invernizzi, P. , … Glaser, S. (2017). miR‐24 inhibition increases menin expression and decreases cholangiocarcinoma proliferation. American Journal of Pathology, 187(3), 570–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falchetti, A. , Marini, F. , Luzi, E. , Giusti, F. , Cavalli, L. , Cavalli, T. , & Brandi, M. L. (2009). Multiple endocrine neoplasia type 1 (MEN1): Not only inherited endocrine tumors. Genetics in Medicine, 11, 825–835. [DOI] [PubMed] [Google Scholar]
- Filipowicz, W. , Bhattacharyya, S. N. , & Sonenberg, N. (2008). Mechanisms of post‐transcriptional regulation by microRNAs: Are the answers in sight? Nature Reviews Genetics, 9, 102–114. [DOI] [PubMed] [Google Scholar]
- Flynt, A. ‐S. , & Lai, E. ‐C. (2008). Biological principles of microRNA‐mediated regulation: Shared themes amid diversity. Nature Reviews Genetics, 9(11), 831–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao, Y. , Liu, Y. , Du, L. , Li, J. , Qu, A. , Zhang, X. , … Wang, C. (2015). Down‐regulation of miR‐24‐3p in colorectal cancer is associated with malignant behavior. Medical Oncology, 32(1), 362. [DOI] [PubMed] [Google Scholar]
- Giglio, S. , Cirombella, R. , Amodeo, R. , Portaro, L. , Lavra, L. , & Vecchione, A. (2013). MicroRNA miR‐24 promotes cell proliferation by targeting the CDKs inhibitors p27Kip1 and p16INK4a . Journal of Cellular Physiology, 228, 2015–2023. [DOI] [PubMed] [Google Scholar]
- Gu, Z. , Eleswarapu, S. , & Jiang, H. (2007). Identification and characterization of microRNAs from the bovine adipose tissue and mammary gland. FEBS Letters, 581(5), 981–988. [DOI] [PubMed] [Google Scholar]
- Imachi, H. , Murao, K. , Dobashi, H. , Bhuyan, M. M. , Cao, X. , Kontani, K. , … Yamauchi, A. (2010). Menin, a product of the MENI gene, binds to estrogen receptor to enhance its activity in breast cancer cells: Possibility of a novel predictive factor for tamoxifen resistance. Breast Cancer Research and Treatment, 122(2), 395–407. [DOI] [PubMed] [Google Scholar]
- Karnik, S. K. , Hughes, C. M. , Gu, X. , Rozenblatt‐Rosen, O. , McLean, G. W. , Xiong, Y. , … Kim, S. K. (2005). Menin regulates pancreatic islet growth by promoting histone methylation and expression of genes encoding p27Kip1 and p18INK4c . Proceedings of the National Academy of Sciences of the United States of America, 102, 14659–14664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keifer, J. , Zheng, Z. , & Ambigapathy, G. (2015). MicroRNA‐BDNF negative feedback signaling loop in brain: Implications for alzheimer's disease. Microrna, 4(2), 101–108. [DOI] [PubMed] [Google Scholar]
- Li, D. , Xie, X. , Wang, J. , Bian, Y. , Li, Q. , Gao, X. , & Wang, C. (2015). MiR‐486 regulates lactation and targets the PTEN gene in cow mammary glands. PLoS One, 10(3), e0118284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, H. , Liu, X. , Wang, Z. , Lin, X. , Yan, Z. , Cao, Q. , … Shi, K. (2017). MEN1/Menin regulates milk protein synthesis through mTOR signaling in mammary epithelial cells. Scientific Reports, 7, 5479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, H. M. , Wang, C. M. , Li, Q. Z. , & Gao, X. J. (2012). MiR‐15a decreases bovine mammary epithelial cell viability and lactation and regulates growth hormone receptor expression. Molecules, 17(10), 12037–12048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lian, S. , Guo, J. R. , Nan, X. M. , Ma, L. , Loor, J. J. , & Bu, D. P. (2016). MicroRNA Bta‐miR‐181a regulates the biosynthesis of bovine milk fat by targeting ACSL1. Journal of Dairy Science, 99(5), 3916–3924. [DOI] [PubMed] [Google Scholar]
- Lu, Y. , Fei, X. Q. , Yang, S. F. , Xu, B. K. , & Li, Y. Y. (2015). Glucose‐induced microRNA‐17 promotes pancreatic beta cell proliferation through down‐regulation of Menin. European Review for Medical and Pharmacological Sciences, 19(4), 624–629. [PubMed] [Google Scholar]
- Luzi, E. , Marini, F. , Ciuffi, S. , Galli, G. , & Brandi, M. L. (2016). An autoregulatory network between menin and pri‐miR‐24‐1 is required for the processing of its specific modulator miR2‐24‐1 in BON1 cells. Molecular Biosystems, 12, 1922–1928. [DOI] [PubMed] [Google Scholar]
- Luzi, E. , Marini, F. , Giusti, F. , Galli, G. , Cavalli, L. , & Brandi, M. L. (2012). The negative feedback‐loop between the oncomir Mir‐24‐1 and Menin modulates the MEN1 tumorigenesis by mimicking the “Knudson's second hit”. PLoS One, 7, e39767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matkar, S. , Thiel, A. , & Hua, X. (2013). Menin: A scaffold protein that controls gene expression and cell signaling. Trends in Biochemical Sciences, 38(8), 394–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng, F. L. , Wang, W. , & Jia, W. D. (2014). Diagnostic and prognostic significance of serum miR‐24‐3p in HBV‐related hepatocellular carcinoma. Medical Oncology, 31(9), 177. [DOI] [PubMed] [Google Scholar]
- Milne, T. A. , Hughes, C. M. , Lloyd, R. , Yang, Z. , Rozenblatt‐Rosen, O. , Dou, Y. , … Hess, J. L. (2005). Menin and MLL cooperatively regulate expression of cyclin‐dependent kinase inhibitors. Proceedings of the National Academy of Sciences of the USA, 102, 749–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Namihira, H. , Sato, M. , Murao, K. , Cao, W. M. , Matsubara, S. , Imachi, H. , … Ishida, T. (2002). The multiple endocrine neoplasia type 1 gene product, Menin, inhibits the human prolactin promoter activity. Journal of Molecular Endocrinology, 29(3), 297–304. [DOI] [PubMed] [Google Scholar]
- Nguyen, T. , Rich, A. , & Dahl, R. (2013). MiR‐24 promotes the survival of hematopoietic cell. PLoS One, 8(1), e55406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schussheim, D. H. , Skarulis, M. C. , Agarwal, S. K. , Simonds, W. F. , Burns, A. L. , Spiegel, A. M. , & Marx, S. J. (2001). Multiple endocrine neoplasia type 1: New clinical and basic findings. Trends in Endocrinology and Metabolism, 12(4), 173–178. [DOI] [PubMed] [Google Scholar]
- Shi, K. , Liu, X. , Li, H. , Lin, X. , Yan, Z. , Cao, Q. , … Wang, Z. (2017). Menin modulates mammary epithelial cell numbers in bovine mammary glands through Cyclin D1. Journal of Mammary Gland Biology and Neoplasia, 22, 221–233. 10.1007/s10911-017-9385-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi, L. , Jackstadt, R. , Siemens, H. , Li, H. , Kirchner, T. , & Hermeking, H. (2014). p53‐induced miR‐15a/16‐1 and AP4 form a double‐negative feedback loop to regulate epithelial‐mesenchymal transition and metastasis in colorectal cancer. Cancer Research, 74(2), 532–542. [DOI] [PubMed] [Google Scholar]
- Shimono, Y. , Zabala, M. , Cho, R. W. , Lobo, N. , Dalerba, P. , Qian, D. , … Clarke, M. F. (2009). Downregulation of miRNA‐200c links breast cancer stem cells with normal stem cells. Cell, 138(3), 592–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song, L. , Yang, J. , Duan, P. , Xu, J. , Luo, X. , Luo, F. , … Zhou, Q. (2013). MicroRNA‐24 inhibits osteosarcoma cell proliferation both in vitro and in vivo by targeting LPAATβ. Archives of Biochemistry and Biophysics, 535, 128–135. [DOI] [PubMed] [Google Scholar]
- Sun, X. , Xiao, D. , Xu, T. , & Yuan, Y. (2016). MiRNA‐24‐3p promotes cell proliferation and regulates chemosensitivity in head and neck squamous cell carcinoma by targeting CHD5. Future Oncology, 12(23), 2701–2712. [DOI] [PubMed] [Google Scholar]
- Tanaka, T. , Haneda, S. , Imakawa, K. , Sakai, S. , & Nagaoka, K. (2009). A microRNA, miR‐101a, controls mammary gland development by regulating cyclooxygenase‐2 expression. Differentiation, 77, 181–187. [DOI] [PubMed] [Google Scholar]
- Vijayaraghavan, J. , Maggi, E. C. , & Crabtree, J. S. (2014). miR‐24 regulates menin in the endocrine pancreas. American Journal of Physiology, Endocrinology and Metabolism, 307, E84–E92. [DOI] [PubMed] [Google Scholar]
- Wang, C. , & Li, Q. (2007). Identification of differentially expressed microRNAs during the development of Chinese murine mammary gland. J Genetics Genomics, 34(11), 966–973. [DOI] [PubMed] [Google Scholar]
- Wang, C. M. , Li, Q. Z. , & Li, Y. (2008). miR‐138 function and its target on mouse mammary epithelial cells. Progress in Biochemistry and Biophysics, 35, 834–838. [Google Scholar]
- Wang, J. , Bian, Y. , Wang, Z. , Li, D. , Wang, C. , Li, Q. , & Gao, X. (2014). MicroRNA‐152 regulates DNA methyltransferase 1 and is involved in the development and lactation of mammary glands in dairy cows. PLoS One, 9(7), e101358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, M. , Moisá, S. , Khan, M. J. , Wang, J. , Bu, D. , & Loor, J. J. (2012). MicroRNA expression patterns in the bovine mammary gland are affected by stage of lactation. Journal of Dairy Science, 95(11), 6529–6535. [DOI] [PubMed] [Google Scholar]
- Wicik, Z. , Gajewska, M. , Majewska, A. , Walkiewicz, D. , Osińska, E. , & Motyl, T. (2016). Characterization of microRNA profile in mammary tissue of dairy and beef breed heifers. Journal of Animal Breeding and Genetics, 133(1), 31–42. [DOI] [PubMed] [Google Scholar]
- Yang, X. , Yang, C. , Farberman, A. , Rideout, T. C. , de Lange, C. F. , France, J. , & Fan, M. Z. (2008). The mammalian target of rapamycin‐signaling pathway in regulating metabolism and growth. Journal of Animal Science, 86(Suppl. 14), E35–E350. [DOI] [PubMed] [Google Scholar]
- Yang, Y. , & Hua, X. (2007). In search of tumor suppressing functions of Menin. Molecular and Cellular Endocrinology, 265‐266, 34–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou, J. , Tian, Y. , Li, J. , Lu, B. , Sun, M. , Zou, Y. , … Ji, G. (2013). miR‐126 is downregulated in breast cancer and inhibits cell proliferation through the upregulation of CyclinD2. Biochemical and Biophysical Research Communications, 433(2), 207–212. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting information
Supporting information


