Summary
There has been increased interest in the prophylactic and therapeutic use of high‐flow nasal oxygen in patients with, or at risk of, non‐hypercapnic respiratory failure. There are no randomised trials examining the efficacy of high‐flow nasal oxygen in high‐risk cardiac surgical patients. We sought to determine whether routine administration of high‐flow nasal oxygen, compared with standard oxygen therapy, leads to reduced hospital length of stay after cardiac surgery in patients with pre‐existing respiratory disease at high risk for postoperative pulmonary complications. Adult patients with pre‐existing respiratory disease undergoing elective cardiac surgery were randomly allocated to receive high‐flow nasal oxygen (n = 51) or standard oxygen therapy (n = 49). The primary outcome was hospital length of stay and all analyses were carried out on an intention‐to‐treat basis. Median (IQR [range]) hospital length of stay was 7 (6–9 [4–30]) days in the high‐flow nasal oxygen group and 9 (7–16 [4–120]) days in the standard oxygen group (p=0.012). Geometric mean hospital length of stay was 29% lower in the high‐flow nasal group (95%CI 11–44%, p = 0.004). High‐flow nasal oxygen was also associated with fewer intensive care unit re‐admissions (1/49 vs. 7/45; p = 0.026). When compared with standard care, prophylactic postoperative high‐flow nasal oxygen reduced hospital length of stay and intensive care unit re‐admission. This is the first randomised controlled trial examining the effect of prophylactic high‐flow nasal oxygen use on patient‐centred outcomes in cardiac surgical patients at high risk for postoperative respiratory complications.
Keywords: cardiothoracic surgery, length of stay, morbidity, oxygen therapy
Introduction
Patients undergoing cardiac surgery are at significant risk of postoperative pulmonary complications, and these complications may increase morbidity and mortality and lead to prolonged intensive care unit (ICU) and hospital length of stay (LOS) 1. The reported incidence of postoperative pulmonary complications following cardiac surgery ranges from 8% to 79% 2. Postoperative pulmonary complications manifest early as hypoxaemia, later pneumonia, and in rare cases also as acute respiratory distress syndrome 3. The incidence of postoperative pulmonary complications is increased in patients with intrinsic respiratory disease, asthma, chronic obstructive pulmonary disease (COPD) and heavy smokers 4. These patients often stay longer in the ICU after surgery due to lower respiratory tract infections, impaired ventilation and the need for prolonged ventilatory support. They are also more likely to require re‐admission to ICU for unplanned continuous positive airways pressure (CPAP), non‐invasive or invasive mechanical ventilation. Therefore, hospital stay is prolonged compared with low‐risk patients after cardiac surgery 5, 6, 7. A contributing mechanism of postoperative pulmonary complications is atelectasis, which has been shown to affect up to 90% of patients undergoing cardiac surgery. Atelectasis has been shown to be resistant to simple techniques such as patient positioning and incentive spirometry 8. Lung recruitment manoeuvres and positive airways pressure may reduce atelectasis development, but this effect is lost after tracheal extubation 9.
Prophylactic nasal CPAP reduces postoperative pulmonary complications after cardiac surgery 10. However, CPAP is costly and requires more intensive involvement by hospital staff. In many hospitals, its use requires admission to at least a high dependency area or even ICU, thus further increasing costs. Apart from the expense and extra healthcare provision costs, common potential side‐effects from CPAP include mask discomfort, skin abrasions, inability to communicate effectively, inability to eat or drink while the device is in use, inability to mobilise and irritation from device noise 11, 12.
High‐flow nasal oxygen therapy delivers warmed humidified oxygen and low level, flow‐dependent positive airways pressure, and may be better tolerated than CPAP or non‐invasive ventilation; moreover, high‐flow nasal oxygen enhances washout of nasopharyngeal dead space, thus improving oxygenation 13, 14, 15, 16. It has been shown that high‐flow nasal oxygen is both safe and non‐inferior to conventional CPAP in providing prophylactic support to very preterm neonates after extubation while the incidence of nasal trauma was significantly lower than in the CPAP group 17. No study has assessed the effect of prophylactic use of high‐flow nasal oxygen on hospital stay in adult cardiac surgical patients with significant risk factors for postoperative pulmonary complications. We therefore, decided to study high‐risk patients with pre‐existing lung disease (COPD, asthma, recent lower respiratory tract infection), heavy smokers or morbidly obese patients (body mass index (BMI) ≥ 35 kg.m−2), who were expected to stay longer in ICU and hospital due to increased respiratory complications. We tested the hypothesis that routine administration of high‐flow nasal oxygen leads to reduced hospital length of stay after cardiac surgery compared with standard oxygen therapy.
Methods
Following national research ethics service (East Midlands Research Ethics Committee UK) and local Research and Development approval, written informed consent was obtained from all patients. Patients scheduled for elective cardiac surgery (coronary artery bypass grafting (CABG), valve surgery or both) were screened for eligibility. Patients were included in the trial if they were aged > 18 years, had one or more patient‐related risk factors for postoperative pulmonary complications (COPD, asthma, lower respiratory tract infection in preceding four weeks, BMI ≥ 35 kg.m−2, current (within last six weeks) heavy smokers (> 10 pack years)) and were capable of performing a 6‐minute walk test (6MWT). Only patients with a formal COPD or asthma diagnosis (as defined by British Thoracic Society and National Institute for Health and Care Excellence) on inhaled therapy were enrolled 18, 19. Lower respiratory tract infection was defined according to National Institute for Health and Care Excellence as an acute illness, usually with cough as the main symptom, and with at least one other lower respiratory tract symptom (such as fever, sputum production, breathlessness, wheeze or chest discomfort/pain) and no alternative explanation 20. According to the trial protocol, patients in whom high‐flow nasal oxygen was contraindicated (presence of a nasal septal defect), those who needed CPAP pre‐operatively or those who did not meet tracheal extubation criteria by 10.00 the day after surgery (as they did not follow the local protocolised peri‐operative care pathway), were not studied. Randomisation was performed using a computer‐generated assignment sequence and a centralised online system before the induction of anaesthesia while the patients were in the operating theatre. Participating patients were randomly allocated in a 1:1 ratio (block randomisation procedure with randomly selected block sizes keeping the investigators blinded to the size of each block) to either the high‐flow nasal oxygen or standard oxygen therapy group. A research nurse or clinical investigator not involved in the clinical care of the patient obtained the treatment allocation and informed the ICU nursing staff in order that the allocated therapy was prepared. We were, therefore, unable to blind patients or nursing staff in the ICU. Surgical and nursing teams responsible for deciding when patients were discharged (and therefore hospital stay) and other aspects of their postoperative care on the surgical ward were blinded as to group allocation.
Study participants underwent a 6MWT before the operation, conducted in a standard manner according to the guidelines of the American Thoracic Society 21. Additionally, patients underwent pre‐operative spirometry testing. This was repeated three times, and average values for forced expiratory volume in one second (FEV1) and forced vital capacity (FVC), were calculated.
The anaesthetic technique and surgical procedure were not affected by patients’ participation in this study. Anaesthesia was induced with midazolam, fentanyl and propofol, neuromuscular blockade achieved with pancuronium and anaesthesia maintained with a continuous infusion of propofol and/or inhalational anaesthetic agent at the discretion of the attending anaesthetist. All patients’ lungs were ventilated using volume control ventilation and the same intra‐operative ventilatory settings, in accordance with local practice: tidal volume 6–8 ml.kg−1 predicted body weight; positive end‐expiratory pressure (PEEP) level of 5 cmH2O; cessation of mechanical ventilation and zero PEEP during cardiopulmonary bypass.
At the end of surgery, patients were transferred to the ICU where they received standard post‐cardiac surgery monitoring and treatment. Patients’ tracheas were extubated once they were normothermic and not bleeding, had established a regular respiratory pattern, had no significant residual neuromuscular blockade and did not complain of anything other than mild pain. Postoperative pain relief was provided by regular paracetamol and opioid analgesia for all patients, unless they had a specific contraindication (e.g. known drug allergy).
Following tracheal extubation, patients received either high‐flow nasal oxygen or standard oxygen therapy, that is, low‐flow oxygen via nasal prongs or a soft facemask, according to the randomisation performed before surgery. The high‐flow nasal oxygen was set up and connected to the patients by the ICU nurses who had been trained before the start of the study. The fraction of inspired oxygen (FIO2) delivered was titrated to that which resulted in a pulse oximeter saturation of at least 95% (93% for those at risk of hypercapnic respiratory failure, such as confirmed COPD patients and morbidly obese patients) according to the British Thoracic Society guidelines for oxygen use in adult patients 22. The FIO2 was actively reduced to the minimum level which achieved this goal. Gas flow for the high‐flow nasal oxygen was calculated for each patient, based on their body characteristics and comfort level. The standard starting flow rate was 30 l.min−1, and this was adjusted up or down between a range of 20–50 l.min−1 with the aim of achieving a respiratory rate of less than 16 breaths per minute and patient comfort. Patients randomised to receive standard oxygen therapy were fitted with nasal prongs or a soft facemask and the oxygen flow titrated to provide oximetry saturations of at least 95% (93% for those at risk of hypercapnic respiratory failure, such as confirmed COPD patients and morbidly obese patients). Both groups of patients had oxygen therapy prescribed for the first 24 h postoperatively and were transferred to surgical wards once they fulfilled pre‐specified physiological criteria. Oxygen therapy was discontinued after 24 h unless there was evidence of respiratory deterioration (dyspnoea, oxygen saturation < 95% (or < 93% for COPD and morbidly obese patients), respiratory rate > 20 breaths per minute). Patients randomly allocated to receive high‐flow nasal oxygen could have high‐flow nasal oxygen continued for more than 24 h (in the ICU or surgical ward) if it was deemed necessary. Patients who continued to be in respiratory distress (respiratory rate > 20 breaths per minute, oxygen saturation < 95% (or < 93% for COPD and morbidly obese patients)) were treated initially by increasing the FIO2. Failing these measures, CPAP, non‐invasive ventilation or, if necessary, invasive mechanical ventilation were considered, as is standard practice at our institution. Participation in the study did not preclude any measures which the clinical team caring for the patient felt were necessary. As part of their standard care, all patients were instructed on appropriate respiratory exercises postoperatively. On postoperative day 5 or 6, patients had both the 6MWT and spirometry testing repeated. Removal of chest drains was decided upon by the surgical team, who also decided when patients would be discharged from the hospital. The surgical team and the physiotherapists were unaware of the study group allocations unless oxygen therapy was continued after discharge from ICU.
The primary outcome of the study was hospital stay. Pre‐specified secondary outcomes were: ICU stay; ICU re‐admission rate; in‐hospital mortality rate; pulmonary function tests (postoperative FEV1 and FVC); 6MWT (pre‐ and postoperatively) and postoperative quality of recovery assessed using the postoperative quality of recovery scale (PQRS) questionnaire 23. The PQRS was completed on the day of admission (baseline) and before discharge (approximately 1 week after surgery), and again 1 month following surgery by telephone contact. The PQRS data were reported as the proportion of patients recovered at each time‐point (baseline, first postoperative week, first postoperative month). We focused on non‐physiological recovery domains (nociceptive, cognitive, activities of daily living (ADL), emotive) and overall patient satisfaction. The PQRS questionnaires were conducted face‐to‐face during hospital stay, and by telephone after hospital discharge. Detailed information around how to use the test and how different domains are being assessed is provided on the PQRS website (http://www.postopqrs.com/).
When planning our study, we analysed our hospital database for more than 2000 cardiac surgical procedures carried out in the year 2014, and found that mean (SD) hospital stay was 10 (3) days in patients with the same risk factors. We expected high‐flow nasal oxygen to reduce mean length of stay by 2 days (a 20% relative reduction) to 8 days; data from our trial in thoracic surgery patients showed a 35% reduction in length of stay, so we proposed that 20% was both feasible and clinically significant. Sample size calculation demonstrated that 92 patients (46 per group) would provide 90% power to detect a mean difference of 2 days in hospital length of stay. The required sample size was increased to 100 patients (50 per group) to allow for a 5% loss to follow‐up and drop‐outs. Baseline data were described using frequencies and percentages for categorical variables and either mean and SD or median, IQR and range for continuous variables. Total hospital stay was calculated for all patients who were discharged alive. As total hopsital length of stay was positively skewed, comparisons between groups were made using the Wilcoxon Rank Sum test and the ratio of geometric means. A risk ratio (RR) was used to compare prolonged stay (defined as total hospital stay > 10 days) in the two groups. Intensive care re‐admission and death were compared using Fisher's exact test, and 6MWT and pulmonary function tests were compared using linear regression. Recovery profiles were compared using Chi‐squared tests and multi‐level mixed‐effect logistic regression. All analyses were by intention‐to‐treat and were performed using Stata version 15.1 (StataCorp. 2017. Stata Statistical Software: Release 15. College Station, TX: StataCorp LLC) software and a p value < 0.05 was considered statistically significant.
Results
One hundred patients were enrolled in the study, 51 patients were allocated to receive high‐flow nasal oxygen and 49 to receive standard oxygen therapy. Of these, six patients were not included in the final analysis due to protocol violation (two patients in each group were not extubated by 10:00 on the morning after surgery), surgery being cancelled (one patient in the standard oxygen group) and withdrawal of consent (one patient in the standard oxygen group) (Fig. 1). All other randomly allocated patients were included in the intention‐to‐treat analysis. Baseline and clinical characteristics (including logistic EuroScore) are shown in Table 1. Study end‐points are reported in Table 2. Median (IQR [range]) hospital length of stay was 7 (6–9 [4–30]) and 9 (7–16 [4–120]) days in the high‐flow nasal oxygen group and the standard oxygen group, respectively, p = 0.012. The geometric mean hospital length of stay was 29% lower in the high‐flow nasal oxygen group (95%CI: 11–44%), p = 0.004. A sensitivity analysis removing the single outlying value (120 days) from the standard oxygen group did not materially alter the conclusions (Figure 2). The risk of prolonged stay was 18.7% in the high‐flow nasal oxygen group compared with 38.6% in the standard oxygen group (RR 0.49, 95%CI: 0.24–0.97), p = 0.0343. There was no difference in ICU length of stay (p = 0.949) with the median (IQR [range]) being 1 (1–2 [1–15]) and 1 (1–2 [1–23]) in the high‐flow nasal oxygen group and standard oxygen group, respectively. Patients in the high‐flow nasal oxygen group had fewer ICU re‐admissions (1/49 vs. 7/45; p = 0.026). There was one death in each group. Less than half of the patients enrolled performed a postoperative 6MWT. The mean (SD) distance was 207.3 (98.9) m in the high‐flow nasal oxygen group compared with 186.1 (114.9) m in the standard oxygen group; mean difference was 21.3 m (95%CI: −44.0 to 86.6), p = 0.510. Postoperative pulmonary function tests were performed in two‐thirds of the patients. There was no significant difference between the two groups in terms of FEV1 and FVC. The number of patients requiring escalation of respiratory support (unplanned CPAP, non‐invasive or invasive ventilation) was three (6.1%) in the high‐flow nasal oxygen group compared with six (13.3%) in the standard oxygen group (p = 0.190). One patient (in the standard oxygen group) required a tracheostomy to aid weaning from mechanical ventilation. Four patients in the standard oxygen group crossed over and received high‐flow nasal oxygen after 24 h (two patients required high‐flow nasal oxygen for 48 h and the other two for 72 h). There were no significant between‐group differences in extra‐pulmonary postoperative complications (Table 3). The recovery profiles for each PQRS domain are summarised in Table 4. The differences in patient‐reported outcomes between the two groups were not statistically significant.
Figure 1.
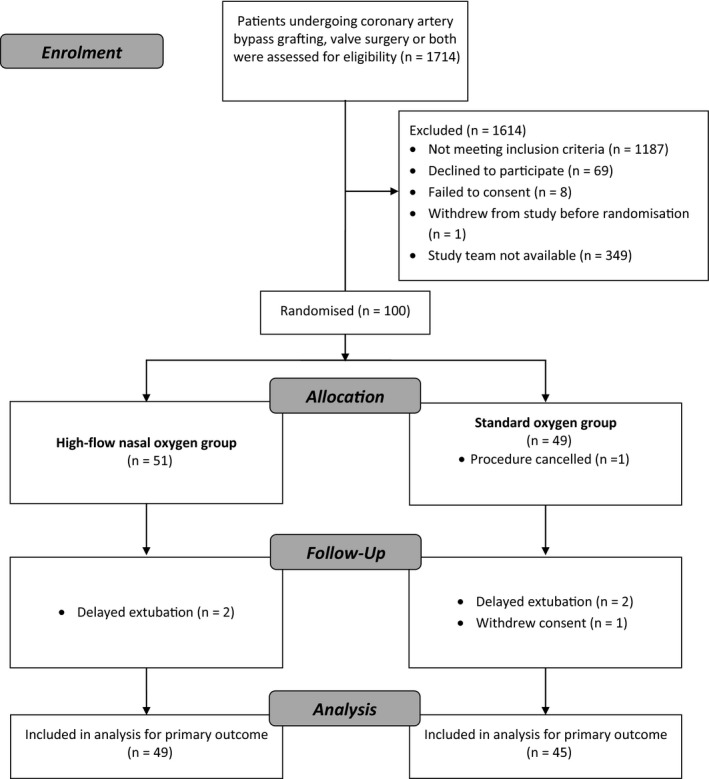
Study flow (CONSORT) chart showing patients allocated to either high‐flow nasal oxygen or standard oxygen therapy.
Table 1.
Baseline characteristics of patients receiving high‐flow nasal oxygen or standard oxygen therapy. Values are mean (SD), number (proportion) or median (IQR [range])
| High‐flow nasal oxygen group | Standard oxygen group | |
|---|---|---|
| n = 49 | n = 45 | |
| Age; y | 67.3 (9.3) | 69.1 (11.1) |
| Sex; female | 19 (38.8%) | 17 (37.8%) |
| Comorbiditiesa | ||
| COPDb | 14 (28.6%) | 15 (33.3%) |
| Asthmab | 18 (36.7%) | 19 (42.2%) |
| Smoker | 10 (20.4%) | 10 (22.2%) |
| BMI ≥ 35 kg.m−2 | 13 (26.5%) | 12 (26.7%) |
| Recent LRTIc | 0 | 0 |
| BMI; kg.m−2 | 32 (5.5) | 30.2 (6.6) |
| Pre‐operative Hb; g.l−1 | 137 (129–145 [111–156]) | 131 (127–141 [85–166]) |
| Pre‐operative creatinine; μmol.l−1 | 80 (71–90 [52–141]) | 83 (74–96 [38–180]) |
| Logistic EUROScored | 4 (3–7 [1–30]) | 4 (3–10 [1–26]) |
| Six‐minute walk test; m | 334.4 (283.0–397.1 [108.0–481.0]) | 348.2 (275.0–392.5 [130.0–596.0]) |
| FEV1; l | 2.3 (1.8–2.7 [0.8–3.8]) | 2.0 (1.6–2.4 [1.0–3.9]) |
| FEV1; % of predicted value | 87 (72–101 [41–127]) | 81 (68–89 [42–147]) |
| FVC; l | 3.3 (2.4–3.7 [1.5–5.2]) | 3.0 (2.4–3.5 [1.5–5.4]) |
| FVC; % of predicted value | 89 (81–107 [49–138]) | 92 (77–104 [41–169]) |
| Procedure | ||
| CABG | 17 (34.7%) | 14 (31.1%) |
| Valve(s) | 24 (49.0%) | 18 (40.0%) |
| CABG + Valve(s) | 8 (16.3%) | 13 (28.9%) |
| Surgery time; min | 197 (176–225 [100–327]) | 202 (169–274 [55–470]) |
BMI, body mass index; CABG, coronary artery bypass graft; COPD, chronic obstructive pulmonary disease; EuroScore, European System for Cardiac Operative Risk Evaluation; Hb, haemoglobin; FEV1, forced expiratory volume in one second; FVC, forced vital capacity.
More than one pathology may be present in each patient.
Only patients with a formal COPD or asthma diagnosis (as defined by British Thoracic Society and National Institute for Health and Care Excellence) on inhaled therapy were enrolled 18, 19.
LRTI was defined according to National Institute for Health and Care Excellence as an acute illness, usually with cough as the main symptom, and with at least one other lower respiratory tract symptom (such as fever, sputum production, breathlessness, wheeze or chest discomfort or pain) and no alternative explanation 20.
Logistic EUROSCORE is a risk model which allows prediction of mortality after cardiac surgery. It includes 17 factors (patient‐, cardiac‐ and operation related) and uses logistic regression to calculate mortality risk.
Table 2.
Study end‐points for patients randomly allocated to either the high‐flow nasal oxygen group or standard oxygen group. Values are median (IQR [range]) or number (proportion)
| High‐flow nasal oxygen group | Standard oxygen group | p value | |
|---|---|---|---|
| n = 49 | n = 45 | ||
| Total length of stay; days | 7 (6–9 [4–30]) | 9 (7–16 [4–120]) | 0.012 |
| ICU length of stay; days | 1 (1–2 [1–15]) | 1 (1–2 [1–23]) | 0.949 |
| Re‐admission to ICU | 1 (2.0%) | 7 (15.6%) | 0.026 |
| Six‐minute walk test; m | 214 (116–280 [40–380]) | 165 (98–251 [60–510]) | 0.330 |
| Lung function | |||
| FVC; l | 1.9 (1.6–2.3 [0.8–3.8]) | 1.9 (1.5–2.3 [0.9–3.5]) | 0.480 |
| FVC; % of predicted value | 57 (45–66 [31–102]) | 57 (47–69 [34–123]) | 0.990 |
| FEV1; l | 1.5 (1.1–1.7 [0.7–2.5]) | 1.2 (1.1–1.5 [0.5–2.7]) | 0.180 |
| FEV1; % of predicted value | 54 (42–64 [29–81]) | 53 (39–65 [18–83]) | 0.690 |
ICU, intensive care unit; FEV1, forced expiratory volume in one second; FVC, forced vital capacity.
Figure 2.
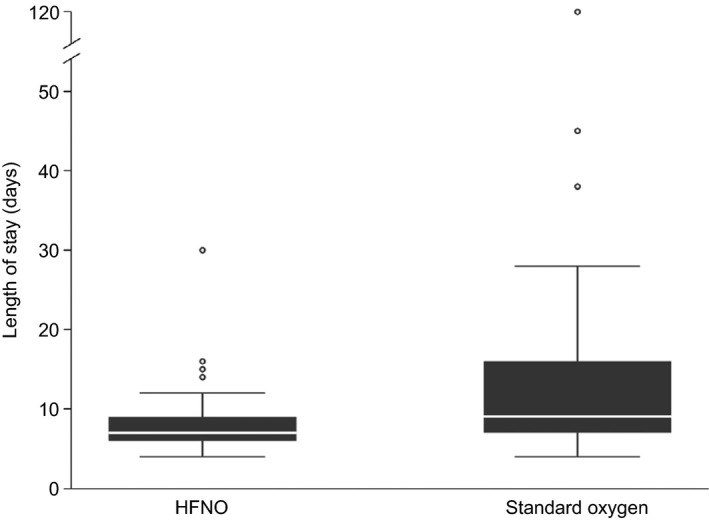
Box plot showing hospital length of stay in cardiac surgical patients allocated to either high‐flow nasal oxygen (HFNO) or standard oxygen therapy. The horizontal line is the median value, the box is the interquartile range and the whiskers extend out to the adjacent values. Outliers are plotted individually as open circles.
Table 3.
Extra‐pulmonary complications in patients allocated to either high‐flow nasal oxygen or standard oxygen therapy
| High‐flow nasal oxygen group | Standard oxygen group | p value | |
|---|---|---|---|
| n = 51 | n = 49 | ||
| Return to operating theatre | 2 | 3 | 0.628 |
| Atrial fibrillation | 1 | 5 | 0.101 |
| Pacemaker insertion | 1 | 4 | 0.190 |
| Renal replacement therapy | 3 | 3 | 1.000 |
| Sternal wound infection | 0 | 1 | 0.479 |
| Delirium | 2 | 4 | 0.421 |
Table 4.
Patient‐reported outcomes from patients randomly allocated to high‐flow nasal oxygen or standard oxygen therapy. Values are proportion recovery from baseline
| Domain | Time | High‐flow nasal oxygen (%) | Standard oxygen (%) | p valuee | p valuef |
|---|---|---|---|---|---|
| Nociceptivea | 5 days | 40.5 | 35.9 | 0.677 | 0.535 |
| 1 month | 40.5 | 37.9 | 0.830 | ||
| Emotionalb | 5 days | 78.4 | 71.8 | 0.508 | 0.773 |
| 1 month | 65.8 | 75.9 | 0.372 | ||
| ADLc | 5 days | 86.5 | 83.8 | 0.744 | 0.576 |
| 1 month | 89.5 | 86.2 | 0.683 | ||
| Cognitive | 5 days | 65.7 | 69.2 | 0.772 | 0.483 |
| 1 month | 72.2 | 87.5 | 0.227 | ||
| Satisfactiond | 5 days | 97.4 | 97.4 | 1.000 | 0.239 |
| 1 month | 87.8 | 96.6 | 0.198 |
Nociception, pain and nausea.
Emotional, anxiety and depression.
ADL, activities of daily living (eat, walk, stand, dress).
Satisfaction, overall satisfaction with anaesthetic care, reported as satisfied or very satisfied.
p value for comparison at each visit.
p value for comparison over both follow‐up visits.
Discussion
In this study, we randomly assigned patients undergoing cardiac surgery and at high risk for postoperative pulmonary complications to receive either high‐flow nasal oxygen or standard oxygen therapy following tracheal extubation. This is the first randomised controlled trial examining the effect of prophylactic use of high‐flow nasal oxygen on clinical outcomes in cardiac surgical patients with significant risk factors for peri‐operative pulmonary complications. We demonstrated that high‐flow nasal oxygen use resulted in a statistically significant reduction in hospital length of stay and fewer re‐admissions to ICU. There were no significant between‐group differences in other secondary outcomes.
Airflow limitation strongly predicts increased hospital stay and in‐hospital mortality after cardiac surgery 24. The beneficial effect of high‐flow nasal oxygen after cardiac surgery and reduction in hospital stay in our study cohort could potentially be explained by the following mechanisms: washout of nasopharyngeal dead space; reduced work of breathing; improved respiratory mechanics; and generation of low‐level PEEP 25, 26, 27. The warmed and humidified oxygen facilitates optimum function of the airway mucosa and mucociliary clearance, and inhibits bronchomotor response, thus preventing bronchospasm and increases in airway resistance 28, 29. It has been shown that high‐flow nasal oxygen reduces dead space ventilation in a flow‐ and time‐dependent manner, leading to a reduction in rebreathing, more effective alveolar ventilation and decreased work of breathing 26. Furthermore, high‐flow nasal oxygen provides PEEP of 3–5 cmH2O at flows of 30–50 l.min−1 which could potentially reduce postoperative atelectasis 15. Prevention of postoperative hypoxaemia and hypercapnia, together with improved pulmonary mechanics, not only reduces pulmonary morbidity but may also improve cardiac function by reducing myocardial oxygen demand and the adverse effects of impaired gas exchange on pulmonary vasculature. These mechanisms could explain the beneficial effect of prophylactic high‐flow nasal oxygen use in the immediate postoperative period. We would, therefore, expect an improvement in postoperative pulmonary function and 6MWT which we were unable to demonstrate. A possible explanation for these results could be that the study was not powered to compare those secondary outcomes, or that patients had limited physiological reserve due to chronic lung disease (COPD, asthma), recent sternotomy and major surgery.
In the non‐cardiac surgery setting, high‐flow nasal oxygen is increasingly being used as a first‐line therapy in acute respiratory failure. This strategy is supported by data from well‐designed and adequately powered trials showing that high‐flow nasal oxygen application reduces tracheal re‐intubation rate in low‐risk patients 30 when compared with conventional oxygen therapy, confers survival benefit 31 and results in a lower tracheal re‐intubation rate 32 in patients with, or at risk of, non‐hypercapnic hypoxaemic respiratory failure. However, a recent systematic review of 11 randomised trials (n = 1972) examining the safety and efficacy of high‐flow nasal oxygen in general ICU patients requiring respiratory support found insufficient evidence to determine superiority of high‐flow nasal oxygen in the ICU setting 33. In a pragmatic randomised controlled trial, Parke et al. demonstrated that high‐flow nasal oxygen did not improve outcomes as assessed by oxygen saturation to fraction of inspired oxygen (SpO2/FIO2) ratio after cardiac surgery, but did reduce the requirement for escalation of respiratory support 34. In a recent randomised controlled trial, Corley et al. compared prophylactic tracheal extubation followed by high‐flow nasal oxygen for 8 h with standard care post‐cardiac surgery in obese patients (BMI ≥ 30 kg.m−2) 35. Primary outcome was atelectasis on postoperative chest radiograph. Prophylactic tracheal extubation followed by high‐flow nasal oxygen did not lead to improvements in respiratory function or a statistically significant difference in ICU stay. The limited high‐flow nasal oxygen exposure time (8 h) in the Corley trial and use of surrogate (SpO2/FIO2 ratio and atelectasis score) markers, rather than patient‐oriented primary outcomes (which would have required a much larger sample size), were the main limitations of these studies. Furthermore, reporting ‘non‐validated’ surrogate primary outcomes is more likely to result in overestimation of treatment effect and uncertainty in predicting treatment benefit 36.
We selected hospital stay as the primary outcome because it is more relevant to patients and healthcare providers than physiological parameters such as oxygenation or haemodynamic data. Length of stay integrates postoperative pulmonary and extra‐pulmonary complications, and speed of postoperative recovery. It is, therefore, a non‐mortality patient‐centred outcome which reflects quality and value‐based healthcare delivery 37, 38.
A recent meta‐analysis which included the aforementioned two randomised trials only, examined the efficacy and safety of high‐flow nasal oxygen after cardiac surgery compared with conventional oxygen therapy and found that post‐extubation application of high‐flow nasal oxygen was associated with a significant reduction in escalation of respiratory support (RR, 0.61; 95%CI, 0.46–0.82; z = 3.32, p < 0.001) 39. However, due to the small number of studies analysed, the methods utilised to detect publication bias were underpowered. In addition, the definitions, strategies and criteria for escalation of respiratory support in the studies included in the meta‐analysis were non‐specific, making the validity of the results questionable. The differences in escalation of respiratory support (unplanned CPAP, non‐invasive or invasive ventilation) between the high‐flow nasal oxygen group and the standard oxygen group in our study cohort were non‐significant.
In a large (n = 830) ‘non‐inferiority’ multi‐centre randomised trial, high‐flow nasal oxygen was compared with BiPAP in patients with, or at risk of, respiratory failure after cardiothoracic surgery 40. The primary outcome in this study was treatment failure, which was defined as a composite of tracheal re‐intubation, switch to the other study therapy or early discontinuation of the assigned therapy, and the authors concluded that high‐flow nasal oxygen was not inferior to BiPAP. The control group in this study does not necessarily represent the standard of care or best practice because there are no robust data indicating that BiPAP improves outcomes in the cardiac surgery setting. In addition, by using a composite primary outcome, the effect may be small for important individual components (e.g. tracheal re‐intubation rate) and large for less important clinical components (e.g. crossover to another treatment group), limiting the generalisability of the results 41. A recent post‐hoc analysis in the subset of obese (BMI > 30 kg.m−2) patients from the same randomised controlled trial showed that continuous application of high‐flow nasal oxygen compared with intermittent BiPAP in patients with or without respiratory failure did not lead to a higher rate of treatment failure 42. The use of BiPAP was associated with improved oxygenation indices; however this did not translate into improved clinical outcomes.
None of the high‐flow nasal oxygen studies previously undertaken in the cardiac surgery setting were powered to detect differences in clinically important primary outcomes such as mortality or length of stay. No study exclusively investigated the effect of high‐flow nasal oxygen on outcomes in patients at significantly higher risk of postoperative pulmonary dysfunction, such as patients with pre‐existing pulmonary disease. Two studies focused on obese patients who are at higher risk of postoperative pulmonary complications, but methodological flaws relating to their ‘non‐inferiority’ design and post‐hoc analysis diminish the validity of their results 40, 42.
Our trial has several limitations. Firstly, there is potential bias due to a lack of blinding of the patients and healthcare providers in the immediate postoperative period due to the obvious differences between the study interventions. Secondly, our study was underpowered for some of the secondary outcomes, for example, 6MWT and patient‐reported outcomes, and the composite of postoperative complications. Although we did not discern significant between‐group differences in patient‐reported outcomes, we highlight the importance of reporting recovery profiles as they reflect value‐based care and provide potentially useful information in order to adequately power future studies. Thirdly, the single centre status of our study and potentially associated large intervention effect may not be directly transferable to other settings 43.
In conclusion, when compared with standard care, prophylactic postoperative use of high‐flow nasal oxygen in cardiac surgical patients at high risk for postoperative respiratory complications reduced hospital length of stay and re‐admissions to ICU. This has implications for reduced healthcare costs and potentially morbidity. We recommend routine use of high‐flow nasal oxygen after tracheal extubation in this cohort of patients and further testing of our hypothesis in large multi‐centre randomised trials.
Acknowledgements
The authors thank F.E. Botrill HNC (clinical trials co‐ordinator) for her contribution in helping with study design, patient recruitment and site training, W. Lawson‐Brown and T. Kriz (research fellow) who assisted with patient recruitment and data collection, and J. Hernandez‐Sanchez (statistician) for his advice and guidance on sample size calculation. This trial was registered at clinicaltrials.gov (NCT02496923).
The trial was funded by the Association of Anaesthetists of Great Britain and Ireland via a grant awarded by the National Institute of Academic Anaesthesia, and was approved by the UK National Institute of Health Research (NIHR) for portfolio status, and Royal Papworth Hospital acknowledges the support of the NIHR Clinical Research Network. Fisher and Paykel provided equipment and disposables, but were not involved with data collection or analysis, writing the manuscript or the decision to submit the manuscript for publication. AK has received educational grant funding, honoraria or assistance with travel from CSL Behring, Massimo, Pharmacosmos, Fisher and Paykel, Brightwake Ltd and Vifor Pharma. AK is the Editor‐in‐Chief of Anaesthesia. No other external funding or competing interests declared.
Presented in part at the Association for Cardiothoracic Anaesthesia and Critical Care (ACTACC) meeting, Birmingham, UK, June 2017 and at the Association of Anaesthetists Annual Congress, Liverpool, UK, September 2017
You can respond to this article at http://www.anaesthesiacorrespondence.com
References
- 1. Pasquina P, Merlani P, Granier JM, Ricou B. Continuous positive airway pressure versus noninvasive pressure support ventilation to treat atelectasis after cardiac surgery. Anesthesia and Analgesia 2004; 99: 1001–8. [DOI] [PubMed] [Google Scholar]
- 2. Wynne R, Botti M. Postoperative pulmonary dysfunction in adults after cardiac surgery with cardiopulmonary bypass: clinical significance and implications for practice. American Journal of Critical Care 2004; 13: 384–93. [PubMed] [Google Scholar]
- 3. Taggart DP, El‐Fiky M, Carter R, Bowman A, Wheatley DJ. Respiratory dysfunction after uncomplicated cardio‐pulmonary bypass. Annals of Thoracic Surgery 1993; 56: 1123–8. [DOI] [PubMed] [Google Scholar]
- 4. Weissman C. Pulmonary complications after cardiac surgery. Seminars in Cardiothoracic and Vascular Anesthesia 2004; 8: 185–211. [DOI] [PubMed] [Google Scholar]
- 5. Adabag AS, Wassif HS, Rice K, et al. Preoperative pulmonary function and mortality after cardiac surgery. American Heart Journal 2010; 159: 691–7. [DOI] [PubMed] [Google Scholar]
- 6. Leavitt BJ, Ross CS, Spence B, et al. Long‐term survival of patients with chronic obstructive pulmonary disease undergoing coronary artery bypass surgery. Circulation 2006; 114: I430–4. [DOI] [PubMed] [Google Scholar]
- 7. Manganas H, Lacasse Y, Bourgeois S, Perron J, Dagenais F, Maltais F. Postoperative outcome after coronary artery bypass grafting in chronic obstructive pulmonary disease. Canadian Respiratory Journal 2007; 14: 19–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Duggan M, Kavanagh BP. Pulmonary atelectasis: a pathogenic perioperative entity. Anesthesiology 2005; 102: 838–54. [DOI] [PubMed] [Google Scholar]
- 9. Lumb AB, Greenhill SJ, Simpson MP, Stewart J. Lung recruitment and positive airway pressure before extubation does not improve oxygenation in the post‐anaesthesia care unit: a randomized clinical trial. British Journal of Anaesthesia 2010; 104: 643–7. [DOI] [PubMed] [Google Scholar]
- 10. Zarbock A, Mueller E, Netzer S, Gabriel A, Feindt P, Kindgen‐Milles D. Prophylactic nasal continuous positive airway pressure following cardiac surgery protects from postoperative pulmonary complications: a prospective, randomized, controlled trial in 500 patients. Chest 2009; 135: 1252–9. [DOI] [PubMed] [Google Scholar]
- 11. Kindgen‐Milles D, Müller E, Buhl R, et al. Nasal‐continuous positive airway pressure reduces pulmonary morbidity and length of hospital stay following thoracoabdominal aortic surgery. Chest 2005; 128: 821–8. [DOI] [PubMed] [Google Scholar]
- 12. Westerlind A, Nilsson F, Ricksten SE. The use of continuous positive airway pressure by face mask and thoracic epidural analgesia after lung transplantation. Gothenburg Lung Transplant Group. Journal of Cardiothoracic and Vascular Anesthesia 1999; 13: 249–52. [DOI] [PubMed] [Google Scholar]
- 13. Cuquemelle E, Lellouche F. Assessment of humidification performance: still no easy method!. Respiratory Care 2013; 58: 1559–61. [DOI] [PubMed] [Google Scholar]
- 14. Frizzola M, Miller TL, Rodriguez ME, et al. High‐flow nasal cannula: impact on oxygenation and ventilation in an acute lung injury model. Pediatric Pulmonology 2011; 46: 67–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Parke R, McGuinness S, Eccleston M. Nasal high‐flow therapy delivers low level positive airway pressure. British Journal of Anaesthesia 2009; 103: 886–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Parke RL, Eccleston ML, McGuinness SP. The effects of flow on airway pressure during nasal high‐flow oxygen therapy. Respiratory Care 2011; 56: 1151–5. [DOI] [PubMed] [Google Scholar]
- 17. Manley BJ, Owen LS, Doyle LW, et al. High‐flow nasal cannulae in very preterm infants after extubation. New England Journal of Medicine 2013; 369: 1425–33. [DOI] [PubMed] [Google Scholar]
- 18. National Institute for Health and Care Excellence . Chronic obstructive pulmonary disease in over 16s: diagnosis and management. NICE guideline [CG101]. 2010. https://www.nice.org.uk/guidance/cg101/chapter/1-Guidance#diagnosing-copd (accessed 12/12/2017). [PubMed]
- 19. British Thoracic Society and Scottish Intercollegiate Guidelines Network . British guideline on the management of asthma. A national clinical guideline. London: British Thoracic Society, 2014. p22 https://www.brit-thoracic.org.uk/document-library/clinical-information/asthma/btssign-asthma-guideline-2014/ (accessed 25/07/2015). [Google Scholar]
- 20. National Institute for Health and Care Excellence . Pneumonia in adults: diagnosis and management. NICE guideline [CG191]. 2014. https://www.nice.org.uk/guidance/cg191 (accessed 25/07/2015). [PubMed]
- 21. Copsfcpfl ATS. ATS statement: guidelines for the six‐minute walk test. American Journal of Respiratory and Critical Care Medicine 2002; 166: 111–17. [DOI] [PubMed] [Google Scholar]
- 22. O'Driscoll BR, Howard LS, Davison AG. BTS guideline for emergency oxygen use in adult patients. Thorax 2008;63 Suppl 6: vi1–68. [DOI] [PubMed] [Google Scholar]
- 23. Royse CF, Newman S, Chung F, et al. Development and feasibility of a scale to assess postoperative recovery: the post‐operative quality recovery scale. Anesthesiology 2010; 113: 892–905. [DOI] [PubMed] [Google Scholar]
- 24. McAllister DA, Wild SH, MacLay JD, et al. Forced expiratory volume in one second predicts length of stay and in‐hospital mortality in patients undergoing cardiac surgery: a retrospective cohort study. PLoS ONE 2013; 8: e64565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Dysart K, Miller TL, Wolfson MR, Shaffer TH. Research in high flow therapy: mechanisms of action. Respiratory Medicine 2009; 103: 1400–5. [DOI] [PubMed] [Google Scholar]
- 26. Möller W, Feng S, Domanski U, et al. Nasal high flow reduces dead space. Journal of Applied Physiology 2017; 122: 191–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Delorme M, Bouchard PA, Simon M, Simard S, Lellouche F. Effects of high‐flow nasal cannula on the work of breathing in patients recovering from acute respiratory failure. Critical Care Medicine 2017; 45: 1981–8. [DOI] [PubMed] [Google Scholar]
- 28. Williams R, Rankin N, Smith T, Galler D, Seakins P. Relationship between the humidity and temperature of inspired gas and the function of the airway mucosa. Critical Care Medicine 1996; 24: 1920–9. [DOI] [PubMed] [Google Scholar]
- 29. Fontanari P, Zattara‐Hartmann MC, Burnet H, Jammes Y. Nasal eupnoeic inhalation of cold, dry air increases airway resistance in asthmatic patients. European Respiratory Journal 1997; 10: 2250–4. [DOI] [PubMed] [Google Scholar]
- 30. Hernández G, Vaquero C, González P, et al. Effect of postextubation high‐flow nasal cannula vs conventional oxygen therapy on reintubation in low‐risk patients: a randomized clinical trial. Journal of the American Medical Association 2016; 315: 1354–61. [DOI] [PubMed] [Google Scholar]
- 31. Frat JP, Thille AW, Mercat A, et al. High‐flow oxygen through nasal cannula in acute hypoxemic respiratory failure. New England Journal of Medicine 2015; 372: 2185–96. [DOI] [PubMed] [Google Scholar]
- 32. Maggiore SM, Idone FA, Vaschetto R, et al. Nasal high‐flow versus Venturi mask oxygen therapy after extubation. Effects on oxygenation, comfort, and clinical outcome. American Journal of Respiratory and Critical Care Medicine 2014; 190: 282–8. [DOI] [PubMed] [Google Scholar]
- 33. Corley A, Rickard CM, Aitken LM, et al. High‐flow nasal cannulae for respiratory support in adult intensive care patients. Cochrane Database of Systematic Reviews 2017; 5: CD010172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Parke R, McGuinness S, Dixon R, Jull A. Open‐label, phase II study of routine high‐flow nasal oxygen therapy in cardiac surgical patients. British Journal of Anaesthesia 2013; 111: 925–31. [DOI] [PubMed] [Google Scholar]
- 35. Corley A, Bull T, Spooner AJ, Barnett AG, Fraser JF. Direct extubation onto high‐flow nasal cannulae post‐cardiac surgery versus standard treatment in patients with a BMI ≥30: a randomised controlled trial. Intensive Care Medicine 2015; 41: 887–94. [DOI] [PubMed] [Google Scholar]
- 36. Ciani O, Buyse M, Garside R, et al. Comparison of treatment effect sizes associated with surrogate and final patient relevant outcomes in randomised controlled trials: meta‐epidemiological study. British Medical Journal 2013; 346: f457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Myles PS, Shulman MA, Heritier S, et al. Validation of days at home as an outcome measure after surgery: a prospective cohort study in Australia. British Medical Journal Open 2017; 7: e015828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Hyder JA, Hirschberg RE, Nguyen LL. Home discharge as a performance metric for surgery. The Journal of the American Medical Association Surgery 2015; 150: 96–7. [DOI] [PubMed] [Google Scholar]
- 39. Zhu Y, Yin H, Zhang R, Wei J. High‐flow nasal cannula oxygen therapy vs conventional oxygen therapy in cardiac surgical patients: a meta‐analysis. Journal of Critical Care 2017; 38: 123–8. [DOI] [PubMed] [Google Scholar]
- 40. Stéphan F, Barrucand B, Petit P, et al. High‐flow nasal oxygen vs noninvasive positive airway pressure in hypoxemic patients after cardiothoracic surgery: a randomized clinical trial. Journal of the American Medical Association 2015; 313: 2331–9. [DOI] [PubMed] [Google Scholar]
- 41. Del Sorbo L, Ferguson ND. High‐flow nasal cannulae or noninvasive ventilation for management of postoperative respiratory failure. The Journal of the American Medical Association 2015; 313: 2325–6. [DOI] [PubMed] [Google Scholar]
- 42. Stéphan F, Bérard L, Rézaiguia‐Delclaux S, Amaru P, BiPOP SG. High‐flow nasal cannula therapy versus intermittent noninvasive ventilation in obese subjects after cardiothoracic surgery. Respiratory Care 2017; 62: 1193–202. [DOI] [PubMed] [Google Scholar]
- 43. Bafeta A, Dechartres A, Trinquart L, Yavchitz A, Boutron I, Ravaud P. Impact of single centre status on estimates of intervention effects in trials with continuous outcomes: meta‐epidemiological study. British Medical Journal 2012; 344: e813. [DOI] [PMC free article] [PubMed] [Google Scholar]


