Abstract
Background and aims
Studies examining the next‐day cognitive effects of heavy alcohol consumption have produced mixed findings, which may reflect inconsistencies in definitions of ‘hangover’. Recent consensus has defined hangover as ‘mental and physical symptoms, experienced the day after a single episode of heavy drinking, starting when blood alcohol concentration (BAC) approaches zero’. In light of this, we aimed to review the literature systematically to evaluate and estimate mean effect sizes of the next‐day effects of heavy alcohol consumption on cognition.
Methods
Embase, PubMed and PsycNET databases were searched between December 2016 and May 2018 using terms based on ‘alcohol’ and ‘hangover’. Studies of experimental designs which reported the next‐day cognitive effects of heavy alcohol consumption in a ‘hangover’ group with BAC < 0.02% were reviewed. A total of 805 articles were identified. Thirty‐nine full‐text articles were screened by two independent reviewers and 19 included in the systematic review; 11 articles provided sufficient data to be included in the meta‐analysis; 1163 participants across 19 studies conducted since 1970 were included in the analysis. Data for study design, hangover severity, BAC at testing and cognitive performance were extracted and effect estimates calculated.
Results
The systematic review suggested that sustained attention and driving abilities were impaired during hangover. Mixed results were observed for: psychomotor skills, short‐ (STM) and long‐term memory (LTM) and divided attention. The meta‐analysis revealed evidence of impairments in STM [g = 0.64, 95% confidence interval (CI) = 0.15–1.13], LTM (Hedges’ g = 0.59, 95% CI = 0.01–1.17) sustained attention (g = 0.47, 95% CI = 0.07–0.87) and psychomotor speed (Hedges’ g = 0.66, 95% CI = 0.31–1.00) during alcohol hangover.
Conclusion
The research literature suggests that alcohol hangovers may involve impaired cognitive functions and performance of everyday tasks such as driving.
Keywords: Alcohol, cognition, driving, hangover, memory, psychomotor, sustained attention
Introduction
Research examining the cognitive effects of alcohol hangover have produced conflicting findings. While several studies report impairment in spatial and visual abilities 1, 2, attention 3, 4, 5, 6, 7, memory 6, 8, 9, 10, 11, information processing speed 3, 12, reaction times 8, 9, 12 and intellectual processes 1, 2, others reveal no clear evidence that hangover affects cognition 1, 5, 6, 11, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22. Tasks reflecting work‐place performance have also produced mixed results, with impairments in driving 23, 24, 25, 26, flying 27, 28, 29 and surgical performance 30, 31, 32, but not managerial decisions 33 or problem‐solving in a ship engine 34.
Disagreements in the definition of alcohol hangover may contribute to inconstancies with study designs and measures 35, 36. Some researchers argue that hangover constitutes any next‐day effects following a night of heavy alcohol consumption, and often do not measure blood alcohol concentration (BAC) or hangover at the time of testing. However, some individuals may be hangover‐resistant 37, 38, 39, experiencing no symptoms despite sufficient alcohol to induce hangover. Indeed, the importance of measuring hangover symptoms is highlighted in a recent definition, which received consensus from academics in the field. It states that hangover is a ‘combination of mental and physical symptoms, experienced the day after a single episode of heavy drinking, starting when BAC approaches zero’ 40.
Peak BAC during a night of ‘heavy’ drinking may also contribute to conflicting results 35, 41. To induce a hangover, high amounts of alcohol (> 1 g/kg) are consumed 42, and the higher the amount the more severe are the cognitive impairments 5. Hangovers are studied using either an experimental approach, where an alcohol challenge is administered, or using the naturalistic approach, where participants consume alcohol at a time and place typical for the individual. In experimental studies, hangover may not be induced reliably, as practical and ethical issues could prevent doses > 1 g/kg being administered—again highlighting the need to include measures of hangover in order to validate the hangover condition. Conversely, naturalistic studies have reported alcohol consumption at approximately 1.6 g/kg 9 yet, unlike experimental studies, do not allow for the control of extraneous variables (e.g. food). Although naturalistic and experimental methods may reveal different impairments, it is important to assess convergence of findings across these different methodologies 43, 44.
Previous reviews have highlighted other methodological limitations which contribute to conflicting findings, preventing firm conclusions 36, 41, 45, 46, 47. These include; no BAC measurement at testing, no counterbalance to avoid order effects and poor controls of potentially confounding factors. These reviews excluded studies with BAC > 0 at testing 36, 41, 46. However, alcohol hangover starts when BAC is approaching zero 40, indicating that these reviews may have excluded potentially informative studies. As acute intoxication can produce cognitive effects at BAC > 0.02%, studies which include participants above this threshold cannot disassociate hangover from acute intoxication effects.
The perspective taken here is that BAC should be < 0.02% at testing and hangover symptoms should be measured to validate the hangover condition. However, we acknowledge that, despite mean scores indicating higher hangover severity in hangover conditions, individuals within these groups may not experience hangover symptoms. As separate analysis is not typically reported for those with and without hangover following heavy alcohol consumption, this review should be regarded as examining next‐day effects of heavy alcohol consumption. We acknowledge that hangover has also been explored in animal models; however, the translational value of this work is currently unclear, and so only human studies are included in this review.
To our knowledge, there have been no previous systematic reviews that have estimated mean effect sizes in a meta‐analysis. This review aims to critically evaluate and estimate mean effect sizes to explore the next‐day cognitive effects of heavy alcohol consumption.
Methods
Search strategy and inclusion criteria
A literature search was conducted from December 2016–May 2018 to identify studies examining the cognitive effects of alcohol hangover. PubMed, Embase and PsycNET were searched using the strategy ‘alcohol’ OR ‘ethanol’ OR ‘alcohol intoxication’ OR ‘alcohol drinking patterns’ AND ‘hangover’ OR ‘next day effects’. Search terms were adapted for each database and references searched for additional articles. Articles were screened by two independent reviewers and disagreements resolved by discussion in the first instance. If consensus was not reached, a third reviewer was consulted. The inclusion criteria for studies were developed based upon the consensus on hangover research report 48. Only studies that examined healthy human adults (18+ years of age) and contained a no‐hangover control condition were included in the review. Studies had to include a measure which validated the presence of hangover, such as a questionnaire assessing symptoms, and were required to report a BAC < 0.02% at testing. The inclusion criteria were based on a stringent set of criteria for hangover; however it is acknowledged that other approaches may be more inclusive of studies (e.g. including studies which do not include a measure of hangover or BAC at testing).
Data extraction
Data were extracted from included studies for study design, cognitive tasks, hangover measurement and BAC during hangover. Where possible, quantitative data were extracted and effect estimates calculated 49, 50. Tasks were coded into their corresponding cognitive components 51. Components and their subcategories comprised: attention/vigilance (selective, sustained, divided and vigilance attention), memory [working memory (WM), short‐term memory (STM) and long‐term memory (LTM)] and psychomotor (speed and accuracy).
Data analysis
All meta‐analyses were performed using RevMan 52. Hedges’ g effect size estimates were calculated 18, 19 for each outcome. For those studies with multiple outcomes in each category of cognition, effect sizes were averaged so that no study carried undue weight in determining overall effect. The weight given to each study was the inverse of the variance of the effect size, thus larger studies with smaller standard errors were given more weight.
Results
Identification of studies
Agreement between reviewers was 95% with two ‘disagreements’ which were resolved through discussion, without the need to consult a third reviewer. In one case, upon both reviewers revisiting the paper, it was clear that the paper did not measure hangover. In the other case, inclusion criteria for one study were reported across two papers. The reviewers agreed that the inclusion criteria were met by collating data from both papers.
The literature search identified 19 studies that could be included in the systematic review 1, 2, 4, 5, 6, 7, 8, 9, 11, 12, 17, 18, 19, 23, 24, 25, 33, 34, 53, and 11 with sufficient data to be included in the meta‐analysis 1, 5, 6, 7, 8, 9, 11, 12. Of the 20 articles excluded during full text screening, 12 studies failed to measure hangover at testing, two of which 30 were reported in the same article 21, 27, 28, 29, 31, 32, 54, 55, 56, 57. Two studies, which included measures on subjective feelings during hangover, only found increases in fatigue or arousal 14, 20, therefore it was unclear if participants were experiencing a hangover. Seven studies failed to measure BAC at testing 3, 10, 27, 29, 30, 54, 58, and two studies which did measure BAC showed that participants achieved BAC > 0.02% 21, 26. Two studies included other treatments in their research design 20, 59. To avoid interference from either the substance or the placebo effect, these studies were excluded. Finally, a further study was excluded 60, as the data analysed were already included in this review via another article from the same authors 9. Figure 1 represents a PRISMA (Preferred Reporting Items for Systematic Reviews and Meta‐Analyses) diagram of study exclusion. Assuming studies which did not report participant attrition did not experience any, total participants recruited across all included studies was 1163. The total number of participants for which data were reported was 846, an attrition rate of 27.3%.
Figure 1.
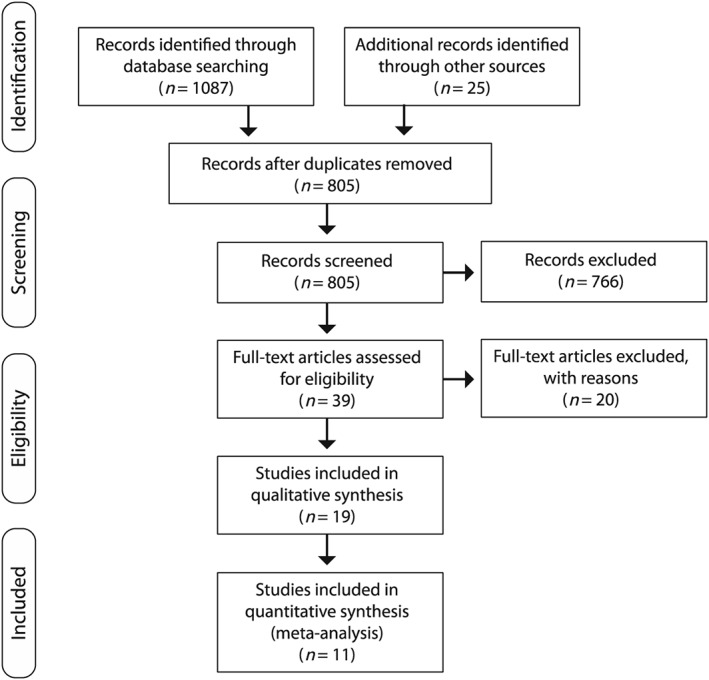
PRISMA (Preferred Reporting Items for Systematic Reviews and Meta‐Analyses) flow diagram; 805 articles were screened by two independent reviewers, and 39 had full‐text assessed. Nineteen articles were included in the review and 11 provided sufficient data to be included in meta‐analysis
Included studies
A total of 19 studies were included in the qualitative synthesis, as illustrated in Table 1. The 11 laboratory studies 1, 2, 4, 5, 6, 11, 17, 18, 33, 34, 53 typically administered lower doses of alcohol than were consumed during the eight naturalistic drinking studies 7, 8, 9, 11, 12, 19, 23, 24, 25. Ten studies explored multiple aspects of cognition 1, 2, 5, 7, 8, 9, 11, 18, 19. Risk of bias was assessed using RevMan (56; see Fig. 2). One study did not randomize sufficiently to condition 2, and for all studies it was unclear whether there was bias for selective reporting due to a lack of study pre‐registration. Fifty per cent of studies were at risk of other biases, including non‐randomization of task administration and sampling biases. Blinding was not considered a risk of bias as participants readily guess conditions during experimental hangover research, despite blinding 5, 6.
Table 1.
Description of included studies.
| Study | n | Design | Alcohol | BAC at testing | Hangover measure | Tests used | Cognitive domain | Main finding | Comments |
|---|---|---|---|---|---|---|---|---|---|
| Collins & Chiles, 1979 | 11 | Within‐subjects, laboratory | 13 g/kg | < 0.01% | 20‐item hangover questionnaire |
Choice RT Meter Monitoring Pattern Identification Compensatory Tracking Problem solving |
P SA STM DA PS |
Non‐significant results | |
| Collins 1980 | 8 | Within‐subjects, laboratory | 1.3 g/kg | 0.012% | 20‐item hangover questionnaire | Tracking task with RT | DA | Non‐significant results | |
| Finnigan et al. 2005 | 71 | 2 × 3 mixed design, naturalistic | 1.77 g/kg | 0% | Subjective feelings questionnaire |
Psychomotor vigilance Dual task Probe memory recall |
SA DA STM |
Non‐significant results | Group impaired in V, post‐hoc significant for ‘acute and hangover’ only |
| Grange et al. 2016 | 31 | Within‐subjects, naturalistic | 1.55 g/kg | 0% | AHS | Choice RT | P | Impaired RT | Anecdotal evidence for impaired accuracy |
| Howland et al. 2010 | 184–193 | Within‐subjects, laboratory | 0.99 g/kg | 0% | AHS |
PVT CPT ADST‐B APASAT VST‐B PMT |
SA SA WM WM WM STM |
Impaired Non‐significant Non‐significant Non‐significant Impaired Female only impairment |
|
| Kim et al. 2003 | 13 | Within‐subjects, naturalistic | 1.5 g/kg | < 0.01% | Subjective Hangover Scale | LNNB | Various | Impairments in ‘memory’, ‘Visual’ and ‘intellectual’ components | Excluded from meta‐analysis as components cannot be subcategorized |
| Kruisselbrink et al. 2006 | 12 | Within‐subjects, laboratory | 1.36 g/kg | 0% | Rated common symptoms | Choice RT | P |
Non‐significant RT Impaired accuracy |
Female participants Alcohol g/kg maximum dose |
| Laurell & Törnros, 1983 | 22 | Within‐subjects, naturalistic | 1.25 g/kg | 0 | Rated severity | Driving ability | RL | Impaired | |
| McKinney et al. 2004 | 48 | Within‐subjects, naturalistic | 1.54 g/kg | < 0.01% | Questionnaire on signs & symptoms |
Free recall Delayed recognition Simple RT Choice RT |
STM LTM P P |
Impaired | STM impaired at 9:00 a.m. only, alcohol g/kg averaged male & female |
| McKinney et al. 2007 | 78 | Mixed design, naturalistic | 1.67 g/kg | < 0.01% | Questionnaire on signs & symptoms |
Free recall Delayed recognition Simple RT Choice RT |
STM LTM P P |
Impaired | Stressor between‐subject condition. ES calculated for group effect (hangover/no‐hangover). Alcohol g/kg averaged male & female |
| McKinney et al. 2012 | 48 | Within‐subjects, naturalistic | 1.54 g/kg | < 0.01% | Questionnaire on signs & symptoms |
Sustained attention Divided attention Erikson Flanker Stroop Spatial attention |
SA DA SelA SelA SpaA |
Impaired Non‐significant Impaired Impaired Non‐significant |
Alcohol g/kg averaged male & female |
| Mrysten et al. 1970 | 15 | Within‐subjects, laboratory | 1.43 g/kg | < 0.01% | Rated severity |
Simple RT Choice RT F‐test Correction test |
P P EF SA |
All non‐significant except ‘spatial’ factor of F‐test | |
| Rohers et al. 1991 | 5 | Within‐subjects, laboratory | 0.8 g/kg | 0% | Rated hangover | Divided attention | DA | Impaired tracking, but not RT | |
| Rohsenow et al. 2006 | 61 | 2 × 2 mixed, laboratory | 1.1 g/kg | < 0.02% | AHS | Simulated ship performance | PS | Non‐significant | Outcome overall time. Alcohol g/kg averaged male & female |
| Rohsenow et al. 2010 | 89–95 | 2 × 2 × 2 mixed, laboratory | 1.15 g/kg | 0 | AHS |
PVT CPT ADST‐B APASAT VST‐B PMT |
SA SA WM WM WM STM |
Impaired Impaired Non‐significant Non‐significant Non‐significant Non‐significant |
Alcohol g/kg averaged male & female |
| Streufert et al. 1995 | 21 | Within‐subjects, laboratory | 1 g/kg | 0 | Drug effects questionnaire | Managerial simulations | EF | Non‐significant | Involved decision making and planning |
| Törnros & Laurell, 1991 | 24 | Within‐subjects, naturalistic | 1.42 g/kg | < 0.02%a | Rated severity | Driving speed | RL | Non‐significant | overall impaired, post‐hoc BAC < 0.02% non‐significant |
| Verster et al. 2003 | 48 | Within‐subjects, naturalistic | 1.4 g/kg | 0 | Severity scored |
Immediate recall Delayed recall Delayed recognition Macworth clock |
STM LTM LTM VA |
Non‐significant Impaired Non‐significant Non‐significant |
46 participants completed memory tasks |
| Verster et al. 2014 | 42 | Within‐subjects, naturalistic | 1.55 g/kg | < 0.01b | Severity scored | Driving ability | RL |
Ability impaired Speed non‐significant |
Alcohol g/kg averaged male & female |
P = psychomotor; SA = sustained attention; DA = divided attention; SelA = selective attention; SpaA = spatial attention; VA = vigilance attention; STM = short‐term memory; LTM = long‐term memory; WM = working memory; PS = problem solving; EF = executive function (non‐specified); RL = ‘real‐life’; AHS = acute hangover scale.
BAC > 0.02% at 9 a.m. session;
BAC > 0.02% for four participants; however, inclusion did not impact results (correspondence with authors).
Figure 2.
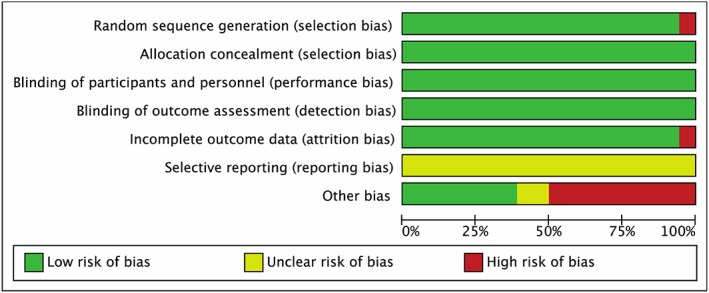
Risk of bias graph. One study was at risk of insufficient randomization procedures; all studies were at risk of reporting bias as there were no pre‐registered study protocols, and 50% of studies were at risk of biases such as non‐randomized task order and sampling bias. [Colour figure can be viewed at http://wileyonlinelibrary.com]
Attention
Sustained attention
Five studies explored sustained attention, three laboratory 1, 5, 6 and two naturalistic 7, 19. Howland et al. 6, McKinney et al. 7 and Rohsenow et al. 5 reported impairments, whereas Finnigan et al. 19 and Mrysten et al. 1 showed no evidence of next‐day effects on sustained attention. Two studies used tasks from a tool validated for assessing cognitive impairments: the neurobehavioural evaluation system‐3 5, 6, 61, two used a sustained attention task which presented stimuli at a consistent rate and participants responded to consecutive stimuli 7, 19, and one used a ‘correction test’ where participants marked identical rows in a list of two columns 1. Four studies provided sufficient information to be included in the meta‐analysis 1, 7, which revealed an overall impairment in sustained attention during hangover [Hedges’ g = 0.47, 95% confidence interval (CI) = 0.07–0.87, I 2 = 50%]. This is shown graphically in Fig. 3.
Figure 3.
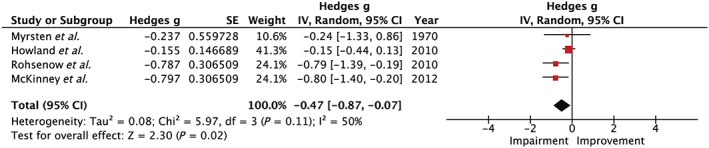
Forest plot for sustained attention. Testing for an overall effect revealed a significant impairment (P = 0.02) with a small to medium effect estimate of 0.47, 95% confidence interval = 0.07–0.87. [Colour figure can be viewed at http://wileyonlinelibrary.com]
Divided attention
Five studies included measures of divided attention 4, 7, 17, 18, 19. Of these, one (with a small sample size; n = 5) reported impairments in divided attention 4. The four other studies showed no evidence of a next‐day effect on divided attention. Four studies were included in a meta‐analysis 4, 7, 17, 18 which showed no evidence of a next‐day effect on divided attention.
Other attention
Verster et al. 11 analysed vigilance using the Macworth clock test and found no evidence of next‐day effects. McKinney et al. 7 found slowed reaction times (RT) for both near and far distractors in a selective attention task, and increased interference the day after heavy alcohol consumption in the Stroop test. As only one study explored vigilance and only one explored selective attention, a meta‐analysis was not performed for these categories of attention.
Memory
Short‐term memory
Short‐term memory (STM) was assessed in seven studies 5, 6, 8, 9, 11, 18, 19, three naturalistic 8, 9, 19 and four laboratory 5, 6, 11, 18. McKinney & Coyle 8, 9 and Howland et al. 6 reported impairments, with Howland et al. showing a female only impairment, whereas Collins & Chiles 18, Finnigan et al. 19, Rohsenow et al. 5 and Verster et al. 11 reported no evidence of a next‐day effect. Three studies used a word recall task 8, 9, 11, one used a similar task which measured probed recall 19, two used a pattern memory test 5, 6 and one used a ‘pattern identification task’ 18. Five studies provided sufficient information to be included in the meta‐analysis 6, 8, 9, 11, 18 which, as indicated in Fig. 4, revealed an overall impairment for STM during hangover (Hedges’ g = 0.64, 95% CI = 0.15–1.13, I 2 = 73%).
Figure 4.
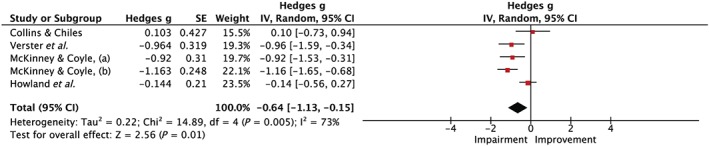
Forest plot for short‐term memory. Testing for an overall effect revealed a significant impairment (P = 0.01) with a medium effect estimate of 0.64, 95% confidence interval = 0.15–1.13. [Colour figure can be viewed at http://wileyonlinelibrary.com]
Long‐term memory
Four studies, two naturalistic 8, 9 and two laboratory 6, 11, assessed LTM. Verster et al. 11 and McKinney & Coyle 8, 9 used a word recall task and reported impairments in LTM. However, in Howland et al. 6, where participants were required to learn lecture materials pre‐intoxication, there was no evidence of a next‐day effect on LTM. Figure 5 shows that when all four studies were included in a meta‐analysis there was an overall impairment in LTM during hangover (Hedges’ g = 0.59, 95% CI = 0.01–1.17, I 2 = 84%).
Figure 5.
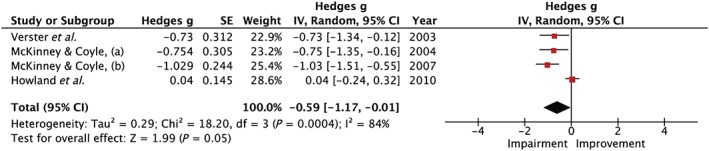
Forest plot for long‐term memory. Testing for an overall effect revealed a significant impairment (P = 0.05) with a medium effect estimate of 0.59, 95% confidence interval = 0.01–1.17. [Colour figure can be viewed at http://wileyonlinelibrary.com]
Other memory
Howland et al. 6 and Rohsenow et al. 5 investigated working memory using the adaptive paced auditory serial addition test (APASAT), the visual span‐backwards (VST‐B) and the auditory digit span‐backwards (ADS‐B). They found no evidence of a next‐day effect in the APASAT or the ADS‐B; however, Howland et al. 6 reported impairments in the VST‐B during hangover. Kim et al. 2 also reported impairments in the memory domain of the Luria‐Nebraska Neurobehavioural Battery (LNNB), although as this domain encompasses STM, LTM and WM 62, it is unclear which aspects of memory were impaired.
Psychomotor performance
Speed
Psychomotor speed was measured using RT in six studies 1, 8, 9, 12, 18, 53. Three naturalistic studies 8, 9, 12 found slower RT the day after an evening of heavy alcohol consumption, whereas three laboratory studies found no evidence to support this 1, 18, 53. Five studies 1, 8, 9, 12, 18 were included in the meta‐analysis which, as shown in Fig. 6, indicated that psychomotor speed was slowed the following day (Hedges’ g = 0.66, 95% CI = 0.31–1.00, I 2 = 36%).
Figure 6.
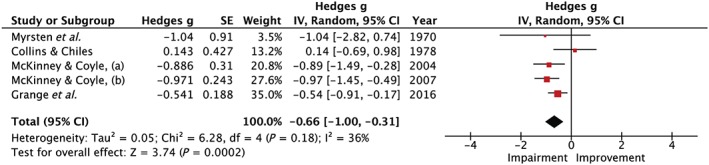
Forest plot for psychomotor speed. Testing for an overall effect revealed a significant impairment (P < 0.001) with a medium effect estimate of 0.66, 95% confidence interval = 0.31–1.00. [Colour figure can be viewed at http://wileyonlinelibrary.com]
Accuracy
Two studies reported psychomotor accuracy 12, 53. Kruisselbrink et al. 53 found a decrease in psychomotor accuracy following an evening of heavy alcohol consumption, whereas Grange et al. 12 reported no evidence of an effect on accuracy.
‘Real‐life’ simulations
Six studies included a ‘real‐life’ simulation that required cognitive performance. Rohsenow et al. 34 reported no evidence of an effect for solving a mechanical failure during a simulated ship scenario. Streufert et al. 33 reported no clear evidence of an effect on performance in scenarios which require managerial skills, and Howland et al. 6 reported no evidence of a next‐day effect for General Record Examination scores on two factors; verbal and quantitative. For studies that analysed driving following an evening of heavy alcohol consumption 23, 24, 25, the ability to control a vehicle, as measured by deviation from a set course, was impaired 23, 25, whereas there was no clear evidence to suggest a next‐day effect on driving speed 24. Due to considerable differences in research methodology it was not possible to conduct a meta‐analysis for ‘real‐life’ simulations.
Discussion
The systematic review and meta‐analyses indicate that STM, LTM sustained attention and psychomotor speed are impaired the day after an evening of heavy alcohol consumption. Results were mixed for the impact of next‐day effects on WM, and there was no clear evidence of an effect on divided attention or vigilance, suggesting that specific components of cognition are influenced the next day. The meta‐analysis showed that psychomotor speed, STM and LTM had medium overall effect estimates (Hedges’ g = 0.66, 0.64 and 0.59, respectively), and sustained attention had a small effect estimate (Hedges’ g = 0.47).
Our systematic review indicated that sustained attention was impaired in studies using naturalistic and laboratory methodologies, with meta‐analysis revealing an overall impairment with a small effect size (Hedges’ g = 0.47). For divided attention, only Rohers et al. reported an impairment 4; however, the reliability of this study is potentially limited by the small sample size (n = 5). Meta‐analysis data revealed no evidence of a next‐day effect on divided attention. Next‐day impairments in sustained attention may reflect accumulating mental fatigue, induced by prolonged attentional demands 63. Fatigue is a common symptom of hangover 64 and involves reward‐cost trade‐offs 65, 66. Therefore, hangover‐induced fatigue may contribute to impairments observed in sustained attention. The lack of clear evidence for an effect in some studies of sustained attention may reflect insensitivity of the cognitive task used. Studies which used tasks that have not previously demonstrated sensitivity to state changes in drug use 1, 17, 18 tended to report no evidence of a next‐day effect, whereas studies 7 that used cognitive tasks that have previously detected state changes 67 were more likely to report next‐day related impairments. Next‐day effects on sustained and divided attention may also have been masked by low statistical power. For example, Finnigan et al. 19 had small, unequal group sizes in their between‐subjects design (n = 13, n = 25, n = 33 for ‘acute and hangover’, hangover and control groups, respectively).
Our review highlights converging evidence from both methodologies (experimental and naturalistic) that STM and LTM may be influenced the morning following a night of heavy alcohol consumption, with the meta‐analysis revealing impairments in both. It is possible that memory formation, rather than retrieval, may be affected, as indicated by the differential next‐day effects on studies in which learning took place following heavy alcohol consumption versus sober state. An important process for memory formation in the hippocampus is long‐term potentiation (LTP)—the strengthening of signals between neurones 68. Given the detrimental effect of elevated interleukin (IL)‐6 69, 70, 71 and cortisol 72 on LTP 73 and the increase of these in the morning following heavy alcohol consumption 74, 75, 76, this could be a possible mechanism underlying next‐day related impairment of memory formation. Three studies examined memory processes using a naturalistic methodology, two of which reported impairments in STM and LTM, whereas Finnigan et al. 19 reported no evidence of impairments in STM. However, as mentioned above, this study may have lacked the statistical power to identify next‐day effects. Conversely, experimental studies have largely reported no evidence of next‐day impairment of memory, although studies where participants reached higher BACs tended to report impairments 6, 11. As with studies of attention, some studies that reported no clear evidence of an effect on memory may have used tasks that are insensitive to the acute next‐day effects.
Systematic review revealed conflicting results for next‐day influences on psychomotor speed. However, when effect estimates were combined in the meta‐analysis, there was an overall impairment with a medium effect estimate (Hedges’ g = 0.66). It is important to consider the suitability of RT as an outcome measure when assessing the next‐day effects on cognition. For example, Howland et al. 6 and Rohsenow et al. 5 use RT as an outcome measure in tasks of sustained attention. Both reveal impairments; however, it is unclear whether the impairment is related to sustained attention or psychomotor speed. Some cognitive tasks of sustained attention, which do not use RT as an outcome measure, revealed no clear evidence of next‐day effects on attention 1. Three naturalistic studies reported slower RTs, whereas three laboratory studies reported no evidence of an effect, although Kruisselbrink et al. 53 reported decreased accuracy. Studies using experimental manipulation of ‘hangover’ typically administered lower doses of alcohol than studies where ‘hangover’ occurred ‘naturally’ (1.3–1.43 and ~1.54–1.67 g/kg, respectively), and had smaller sample sizes (n = 8–12), which may impact reliability 77. It should be noted that, due to insufficient information, one laboratory study 53 could not be included in the meta‐analysis, which may over‐inflate the effect estimate reported.
Three naturalistic studies identified in this review assessed driving the morning following a night of heavy alcohol consumption. Verster et al. and Laurell & Törnros 23, 25 reported impairments in ability to control the vehicle. However, Törnros & Laurell 24 reported no effect on speed the next day. These studies have important implications for road safety, especially given that hangover may contribute to road‐traffic accidents 78. The impairments observed in ability to drive may be driven by next‐day effects on underlying cognitive components. Driving uses psychomotor speed and sustained attention 79, both of which appear to be impaired in this review. Studies using experimental manipulation of hangover, which assessed task performance using measures of executive function (problem‐solving and decision‐making), as well as academic performance, all found no clear evidence of a next‐day effect. However, an outcome measure of overall completion time, as in Rohsenow et al. 34, and the managerial task used in Streufert et al. 33, may not be sufficient to detect next‐day effects. Together, these findings echo the recommendations of previous reviews 36, 41, indicating that further research is needed to determine hangover effects on executive functions. We also suggest that future studies of executive function should use validated measures known to be sensitive to state changes in drug use, such as the Iowa Gambling Task 80.
In line with previous reviews 35, 36, 41, 46, this systematic review and meta‐analysis revealed several methodological issues which limit the interpretation of evidence from studies of alcohol hangover on cognition. Although the studies included in this review met rigorous criteria there was a high degree of variability in the design of individual studies, possibly reflected by the high level of heterogeneity observed. Our review highlights that low sensitivity of tasks to detect next‐day impairments may underlie null next‐day effects on cognition. The use of cognitive tasks sensitive to state changes in substance use is essential for studies exploring cognitive effects the day after a night of heavy alcohol consumption 81. Thought should also be given to the sensitivity of visual stimuli to next‐day effects, as opposed to auditory stimuli. Studies using cognitive tasks with auditory stimuli revealed no evidence of a next‐day effect on cognition, in contrast to effects observed when using visual stimuli. This discrepancy is supported by evidence of impairments of the ‘visual’ component of the LNNB task battery 2. Another factor that may influence the next‐day effect on cognition is study design. Our review suggests a greater likelihood of next‐day impairment in studies of naturalistic design. In studies where hangover is induced ‘naturally’, alcohol consumption was higher (mean alcohol dose = 1.54 g/kg) than in experimental studies (mean alcohol dose = 1.21 g/kg). This finding suggests that higher alcohol doses are associated with greater next‐day performance impairments 82.
Finally, several other limitations should be considered. One study 2 did not randomize condition order, while others did not randomize task administration order 7, 8, 9. Randomization to condition is important to prevent practice effects, and randomizing task order limits confounding variables such as fatigue. Several studies did not control for nicotine use 1, 17, 18, which is known to influence cognitive performance 83. Our review also highlighted variability between study design in the amount of time between alcohol consumption and cognitive testing, possibly depriving participants of sleep. Sleep time is an important consideration when researching cognition, as cognitive components are affected differentially by sleep loss 84. Although in real‐life drinking some individuals may reduce sleep time for drinking time 85, variability between studies for the time allowed for sleep make it difficult to draw firm conclusions regarding cognitive effects.
Based on these shortcomings, we make the following recommendations for future research. First, to address the shortcoming of low statistical power, studies should conduct a priori power analysis to determine an estimate of required sample sizes. Secondly, studies should adopt tasks that have been validated and shown to be sensitive to state changes in drug use. Thirdly, consideration should be given for the use of RT as an outcome measure in tasks, and interpretation should acknowledge the potential impact of next‐day psychomotor impairments. Fourthly, future research should seek to address the paucity of robust research examining executive functions the morning following a night of heavy alcohol consumption.
Conclusion
To our knowledge, this study is the first to systematically review the literature exploring next‐day effects on cognitive performance and to estimate mean effect sizes. Our review reveals next‐day impairments in STM, LTM, psychomotor speed and sustained attention, with mixed findings for next‐day effects on working memory, and no clear evidence of an effect on divided attention. Results from our meta‐analysis indicate medium effect sizes for psychomotor speed, STM and LTM, and a small effect size for sustained attention. These findings suggest that specific cognitive functions may be impaired the morning following a night of heavy alcohol consumption, with implications for everyday task performance (e.g. driving).
Declaration of interests
S.A. and M.R.M. are members of the UK Centre for Tobacco and Alcohol Studies. The other authors have no relevant conflicts of interest to disclose.
Acknowledgements
This work was funded by a University of Bath research studentship. Funding from British Heart Foundation, Cancer Research UK, Economic and Social Research Council, Medical Research Council, and the National Institute for Health Research, under the auspices of the UK Clinical Research Collaboration, is gratefully acknowledged. We also thank Professor Richard Stephens and Dr Jim Grange for their support in providing information for this review.
Pre‐registered hypothesis
http://www.crd.york.ac.uk/PROSPERO/display_record.php?ID=CRD42017054617
Gunn, C. , Mackus, M. , Griffin, C. , Munafò, M. R. , and Adams, S. (2018) A systematic review of the next‐day effects of heavy alcohol consumption on cognitive performance. Addiction, 113: 2182–2193. 10.1111/add.14404.
References
- 1. Myrsten A. L., Neri A., Kelly M., Rydberg U. Acute effects and after‐effects of alcohol on psychological and physiological function. Report no. 314. Stockholm: Psychological Laboratory of the University of Stockholm; 1970.
- 2. Kim D.‐J., Yoon S.‐J., Lee H.‐P., Choi B.‐M., Go H. J. The effects of alcohol hangover on cognitive functions in healthy subjects. Int J Neurosci 2003; 113: 581–594. [DOI] [PubMed] [Google Scholar]
- 3. Anderson S., Dawson J. Neuropsychological correlates of alcoholic hangover. S Afr J Sci 1999; 95: 145–147. [Google Scholar]
- 4. Roehrs T., Yoon J., Roth T. Nocturnal and next‐day effects of ethanol and basal level of sleepiness. Hum Psychopharmacol Clin Exp 1991; 6: 307–311. [Google Scholar]
- 5. Rohsenow D. J., Howland J., Arnedt J. T., Almeida A. B., Greece J., Minsky S. et al Intoxication with bourbon versus vodka: effects on hangover sleep and next‐day neurocognitive performance in young adults. Alcohol Clin Exp Res 2010; 34: 509–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Howland J., Rohsenow D. J., Greece J. A., Littlefield C. A., Almeida A., Heeren T. et al The effects of binge drinking on college students’ next‐day academic test‐taking performance and mood state. Addiction 2010; 105: 655–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. McKinney A., Coyle K., Penning R., Verster J. C. Next day effects of naturalistic alcohol consumption on tasks of attention. Hum Psychopharmacol 2012; 27: 587–594. [DOI] [PubMed] [Google Scholar]
- 8. McKinney A., Coyle K. Next‐day effects of alcohol and an additional stressor on memory and psychomotor performance. J Stud Alcohol Drugs 2007; 68: 446–454. [DOI] [PubMed] [Google Scholar]
- 9. McKinney A., Coyle K. Next day effects of a normal night's drinking on memory and psychomotor performance. Alcohol Alcohol 2004; 39: 509–513. [DOI] [PubMed] [Google Scholar]
- 10. McCaul M. E., Turkkan J. S., Svikis D. S., Bigelow G. E. Alcohol and secobarbital effects as a function of familial alcoholism: extended intoxication and increased withdrawal effects. Alcohol Clin Exp Res 1991; 15: 94–101. [DOI] [PubMed] [Google Scholar]
- 11. Verster J. C., van Duin D., Volkerts E., Schreuder A., Verbaten M. Alcohol hangover effects on memory functioning and vigilance performance after an evening of binge drinking. Neuropsychopharmacology 2003; 28: 740–746. [DOI] [PubMed] [Google Scholar]
- 12. Grange J. A., Stephens R., Jones K., Owen L. The effect of alcohol hangover on choice response time. J Psychopharmacol 2016; 30: 654–661. [DOI] [PubMed] [Google Scholar]
- 13. Carroll J. R., Ashe W. F., Roberts L. B. Influence of the aftereffects of alcohol combined with hypoxia on psychomotor performance. Aerosp Med 1964; 35: 990–993. [PubMed] [Google Scholar]
- 14. Finnigan F., Hammersley R., Cooper T. An examination of next‐day hangover effects after a 100 mg/100 ml dose of alcohol in heavy social drinkers. Addiction 1998; 93: 1829–1838. [DOI] [PubMed] [Google Scholar]
- 15. Ideström C. M., Cadenius B. Time relations of the effects of alcohol compared to placebo. Psychopharmacologia 1968; 13: 189–200. [DOI] [PubMed] [Google Scholar]
- 16. Morrow D., Leirer V., Yesavage J. The influence of alcohol and aging on radio communication during flight. Aviat Sp Environ Med 1990; 61: 12–20. [PubMed] [Google Scholar]
- 17. Collins W. E. Performance effects of alcohol intoxication and hangover at ground level and at simulated altitude. Aviat Sp Environ Med 1980; 51: 327–335. [PubMed] [Google Scholar]
- 18. Collins W. E., Chiles W. D. Laboratory performance during acute alcohol intoxication and hangover. Hum Factors 1980; 22: 445–462. [DOI] [PubMed] [Google Scholar]
- 19. Finnigan F., Schulze D., Smallwood J., Helander A. The effects of self‐administered alcohol‐induced ‘hangover’ in a naturalistic setting on psychomotor and cognitive performance and subjective state. Addiction 2005; 100: 1680–1689. [DOI] [PubMed] [Google Scholar]
- 20. Chait L. D., Perry J. L. Acute and residual effects of alcohol and marijuana, alone and in combination, on mood and performance. Psychopharmacology (Berl) 1994; 115: 340–349. [DOI] [PubMed] [Google Scholar]
- 21. Lemon J., Chesher G., Fox A., Greeley J., Nabke C. Investigation of the ‘hangover’ effects of an acute dose of alcohol on psychomotor performance. Alcohol Clin Exp Res 1993; 17: 665–668. [DOI] [PubMed] [Google Scholar]
- 22. Dowd P. J., Wolfe J. W., Cramer R. L. Aftereffects of alcohol on the perception and control pitch altitude during centripetal acceleration. Aerosp Med 1973; 44: 928–930. [PubMed] [Google Scholar]
- 23. Verster J. C., Bervoets A. C., de Klerk S., Vreman R. A., Olivier B., Roth T. et al Effects of alcohol hangover on simulated highway driving performance. Psychopharmacology (Berl) 2014; 231: 2999–3008. [DOI] [PubMed] [Google Scholar]
- 24. Tornros J., Laurell H. Acute and hangover effects of alcohol on simulated driving performance. Blutalkohol 1991; 28: 24–30. [PubMed] [Google Scholar]
- 25. Laurell H., Tornros J. Investigation of alcoholic hangover effects on driving performance. Natl Swedish Road Traffic Res Inst 1983; 581: 489–499. [Google Scholar]
- 26. Seppålå T., Leino T., Linnoila M., Huttunen M., YIikahri R. Effects of hangover on psychomotor skills related to driving: modification by fructose and glucose. Acta Pharmacol Toxicol (Copenh) 1976; 38: 209–218. [DOI] [PubMed] [Google Scholar]
- 27. Petros T., Bridewell J., Jensen W., Ferraro F. R., Bates J., Moulton P. et al Postintoxication effects of alcohol on flight performance after moderate and high blood alcohol levels. Int J Aviat Psychol 2003; 13: 287–300. [Google Scholar]
- 28. Yesavage J. A., Leirer V. O. Hangover effects on aircraft pilots 14 hours after alcohol ingestion: a preliminary report. Am J Psychiatry 1986; 143: 1546–1550. [DOI] [PubMed] [Google Scholar]
- 29. Yesavage J. A., Dolhert N., Taylor J. L. Flight simulator performance of younger and older aircraft pilots: effects of age and alcohol. J Am Geriatr Soc 1994; 42: 577–582. [DOI] [PubMed] [Google Scholar]
- 30. Gallagher A. G., Boyle E., Toner P., Neary P. C., Andersen D. K., Satava R. M. et al Persistent next‐day effects of excessive alcohol consumption on laparoscopic surgical performance. Arch Surg 2011; 146: 419–426. [DOI] [PubMed] [Google Scholar]
- 31. Kocher H. M., Warwick J., Al‐Ghnaniem R., Patel A. G. Surgical dexterity after a ‘night out on the town’. Aust NZ J Surg 2006; 76: 110–112. [DOI] [PubMed] [Google Scholar]
- 32. Van Dyken I., Szlabick R. E., Sticca R. P. Effect of alcohol on surgical dexterity after a night of moderate alcohol intake. Am J Surg 2013; 206: 964–969. [DOI] [PubMed] [Google Scholar]
- 33. Streufert S., Pogash R., Braig D., Gingrich D., Kantner A., Landis R. et al Alcohol hangover and managerial effectiveness. Alcohol Clin Exp Res 1995; 19: 1141–1146. [DOI] [PubMed] [Google Scholar]
- 34. Rohsenow D. J., Howland J., Minsky S. J., Arnedt J. T. Effects of heavy drinking by maritime academy cadets on hangover, perceived sleep, and next‐day ship power plant operation. J Stud Alcohol 2006; 67: 406–415. [DOI] [PubMed] [Google Scholar]
- 35. Stephens R., Grange J. A., Jones K., Owen L. A critical analysis of alcohol hangover research methodology for surveys or studies of effects on cognition. Psychopharmacology (Berl) 2014; 231: 2223–2236. [DOI] [PubMed] [Google Scholar]
- 36. Stephens R., Ling J., Heffernan T. M., Heather N., Jones K. A review of the literature on the cognitive effects of alcohol hangover. Alcohol Alcohol 2008; 43: 163–170. [DOI] [PubMed] [Google Scholar]
- 37. Howland J., Rohsenow D. J., Allensworth‐Davies D., Greece J., Almeida A., Minsky S. J. et al The incidence and severity of hangover the morning after moderate alcohol intoxication. Addiction 2008; 103: 758–765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Verster J. C., de Klerk S., Bervoets A. C., Kruisselbrink L. D. Editorial: Can hangover immunity be really claimed? Curr Drug Abuse Rev 2014; 6: 253–254. [DOI] [PubMed] [Google Scholar]
- 39. Howland J., Rohsenow D. J., Edwards E. M. Are some drinkers resistant to hangover? A literature review. Curr Drug Abuse Rev 2008; 1: 42–46. [DOI] [PubMed] [Google Scholar]
- 40. Van Schrojenstein Lantman M., van de Loo A. J. A. E., Mackus M., Verster J. C. Development of a definition for the alcohol hangover: consumer descriptions and expert consensus. Curr Drug Abuse Rev 2017; 9: 148–154. [DOI] [PubMed] [Google Scholar]
- 41. Prat G., Adan A., Perez‐Pamies M., Sanchez‐Turet M. Neurocognitive effects of alcohol hangover. Addict Behav 2008; 33: 15–23. [DOI] [PubMed] [Google Scholar]
- 42. Chapman L. F. Experimental induction of hangover. Q J Stud Alcohol 1970; 5: 67–86. [PubMed] [Google Scholar]
- 43. Finnigan F., Hammersley R. The effects of alcohol on performance. Handb Hum Perform 1992; 2: 73–126. [Google Scholar]
- 44. Howitt D., Cramer D. Introduction to Research Methods in Psychology. London: Pearson Education; 2007. [Google Scholar]
- 45. Gauvin D. V., Cheng E. Y., Holloway F. A. Biobehavioral correlates In: Galanter M., editor. Recent Developments in Alcoholism: Ten Years of Progress, Social and Cultural Perspectives Physiology and Biochemistry Clinical Pathology Trends in Treatment. Boston, MA: Springer US; 1993, pp. 281–304. [PubMed] [Google Scholar]
- 46. Ling J., Stephens R., Heffernan T. M. Cognitive and psychomotor performance during alcohol hangover. Curr Drug Abuse Rev 2010; 3: 80–87. [DOI] [PubMed] [Google Scholar]
- 47. Frone M., Verster J. Editorial: Alcohol hangover and the workplace: a need for research. Curr Drug Abuse Rev 2013; 6: 177–179. [DOI] [PubMed] [Google Scholar]
- 48. Verster J. C., Stephens R., Penning R., Rohsenow D., McGeary J., Levy D. et al The alcohol hangover research group consensus statement on best practice in alcohol hangover research. Curr Drug Abuse Rev 2010; 3: 116–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Hunter J. E., Schmidt F. L. Methods of Meta‐Analysis: Correcting Error and Bias in Research Findings, 2nd edn. Newbury Park, CA: Sage Publications; 2004, pp. 1–581. [Google Scholar]
- 50. Lakens D. Calculating and reporting effect sizes to facilitate cumulative science: a practical primer for t‐tests and ANOVAs. Front Psychol 2013; 4: 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Selnes O. A., Grega M. A., Bailey M. M., Pham L., Zeger S., Baumgartner W. A. et al Neurocognitive outcomes 3 years after coronary artery bypass graft surgery: a controlled study. Ann Thorac Surg 2007; 84: 1885–1896. [DOI] [PubMed] [Google Scholar]
- 52. RevMan . Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration; 2014. [Google Scholar]
- 53. Kruisselbrink L. D., Martin K. L., Megeney M., Fowles J. R., Murphy R. J. L. Physical and psychomotor functioning of females the morning after consuming low to moderate quantities of beer. J Stud Alcohol 2006; 67: 416–420. [DOI] [PubMed] [Google Scholar]
- 54. Hartung B., Schwender H., Roth E. H., Hellen F., Mindiashvili N., Rickert A. et al The effect of alcohol hangover on the ability to ride a bicycle. Int J Leg Med 2015; 129: 751–758. [DOI] [PubMed] [Google Scholar]
- 55. Roehrs T., Beare D., Zorick F., Roth T. Sleepiness and ethanol effects on simulated driving. Alcohol Clin Exp Res 1994; 18: 154–158. [DOI] [PubMed] [Google Scholar]
- 56. Stock A. K., Hoffmann S., Beste C. Effects of binge drinking and hangover on response selection sub‐processes—a study using EEG and drift diffusion modeling. Addict Biol 2016; 22: 1355–1365. [DOI] [PubMed] [Google Scholar]
- 57. Wolff N., Gussek P., Stock A.‐K., Beste C. Effects of high‐dose ethanol intoxication and hangover on cognitive flexibility. Addict Biol 2016; 23: 503–514. [DOI] [PubMed] [Google Scholar]
- 58. Howse A. D., Hassall C. D., Williams C. C., Hajcak G., Krigolson O. E. Alcohol hangover impacts learning and reward processing within the medial‐frontal cortex. Psychophysiology 2018; 10.1111/psyp.13081. [DOI] [PubMed] [Google Scholar]
- 59. Myrsten A., Rydberg U., Lamble R. Alcohol intoxication and hangover: modification of hangover by chlormethiazole. Psychopharmacology (Berl) 1980; 125: 117–125. [DOI] [PubMed] [Google Scholar]
- 60. McKinney A., Coyle K., Verster J. C. Direct comparison of the cognitive effects of acute alcohol with the morning after a normal night's drinking. Hum Psychopharmacol Clin Exp 2012; 27: 295–304. [DOI] [PubMed] [Google Scholar]
- 61. White R. F., James K. E., Vasterling J. J., Letz R., Marans K., Delaney R. et al Neuropsychological screening for cognitive impairment using computer‐assisted tasks. Assessment 2003; 10: 86–101. [DOI] [PubMed] [Google Scholar]
- 62. Golden C. J., Hammeke T. A., Purisch A. D. Diagnostic validity of a standardized neuropsychological battery derived from Luria's neuropsychological tests. J Consult Clin Psychol 1978; 46: 1258–1265. [PubMed] [Google Scholar]
- 63. Langner R., Willmes K., Chatterjee A., Eickhoff S. B., Sturm W. Energetic effects of stimulus intensity on prolonged simple reaction‐time performance. Psychol Res 2010; 74: 499–512. [DOI] [PubMed] [Google Scholar]
- 64. Penning R., McKinney A., Verster J. C. Alcohol hangover symptoms and their contribution to the overall hangover severity. Alcohol Alcohol 2012; 47: 248–252. [DOI] [PubMed] [Google Scholar]
- 65. Boksem M. A. S., Meijman T. F., Lorist M. M. Effects of mental fatigue on attention: an ERP study. Cogn Brain Res 2005; 25: 107–116. [DOI] [PubMed] [Google Scholar]
- 66. Boksem M. A. S., Tops M. Mental fatigue: costs and benefits. Brain Res Rev 2008; 59: 125–139. [DOI] [PubMed] [Google Scholar]
- 67. Tiplady B., Drummond G. B., Cameron E., Gray E., Hendry J., Sinclair W. et al Ethanol, errors, and the speed–accuracy trade‐off. Pharmacol Biochem Behav 2001; 69: 635–641. [DOI] [PubMed] [Google Scholar]
- 68. Carter D., Murphy D. Molecular Neuroscience, 1st edn. London, UK: Pearson Education; 1999, pp. 117–145. [Google Scholar]
- 69. Tancredi V., D'Antuono M. Inhibitory effects of interleukin‐6 on synaptic plasticity in the rat hippocampus are associated with an inhibition of mitogen‐activated protein kinase ERK. J Neurochem 2000; 75: 634–643. [DOI] [PubMed] [Google Scholar]
- 70. Li A.‐J., Katafuchi T., Oda S., Hori T., Oomura Y. Interleukin‐6 inhibits long‐term potentiation in rat hippocampal slices. Brain Res 1997; 748: 30–38. [DOI] [PubMed] [Google Scholar]
- 71. Bellinger F. P., Madamba S. G., Campbell I. L., Siggins G. R. Reduced long‐term potentiation in the dentate gyrus of transgenic mice with cerebral overexpression of interleukin‐6. Neurosci Lett 1995; 198: 95–98. [DOI] [PubMed] [Google Scholar]
- 72. Monk C. S., Nelson C. A. The effects of hydrocortisone on cognitive and neural function: a behavioral and event‐related potential investigation. Neuropsychopharmacology 2002; 26: 505–519. [DOI] [PubMed] [Google Scholar]
- 73. Balschun D., Wetzel W., Del Rey A., Pitossi F., Schneider H., Zuschratter W. et al Interleukin‐6: a cytokine to forget. FASEB J 2004; 18: 1788–1790. [DOI] [PubMed] [Google Scholar]
- 74. Van de Loo A. J. A. E., Hogewoning A., Raasveld S. J., De Zeeuw R., Bosma E. R., Bouwmeester N. H. et al Saliva cytokine concentrations the day after heavy alcohol consumption in drinkers suffering from a hangover versus those who claim to be hangover resistant. Alcohol Alcohol 2015; 50; i1–67 FOC7–2. [Google Scholar]
- 75. Kim D.‐J., Kim W., Yoon S.‐J., Choi B.‐M., Kim J.‐S., Go H. J. et al Effects of alcohol hangover on cytokine production in healthy subjects. Alcohol 2003; 31: 167–170. [DOI] [PubMed] [Google Scholar]
- 76. Linkola J., Fyhrquist F., Ylikahri R. Renin, aldosterone and cortisol during ethanol intoxication and hangover. Acta Physiol Scand 1979; 106: 75–82. [DOI] [PubMed] [Google Scholar]
- 77. Button K. S., Ioannidis J. P. A., Mokrysz C., Nosek B. A., Flint J., Robinson E. S. J. et al Power failure: why small sample size undermines the reliability of neuroscience. Nat Rev Neurosci 2013; 14: 365–376. [DOI] [PubMed] [Google Scholar]
- 78. Hoiseth G., Fosen J. T., Liane V., Bogstrand S. T., Morland J. Alcohol hangover as a cause of impairment in apprehended drivers. Traffic Inj Prev 2015; 16: 323–328. [DOI] [PubMed] [Google Scholar]
- 79. Allen A. J., Meda S. A., Skudlarski P., Calhoun V., Astur R., Ruopp K. C. et al Effects of alcohol on performance on a distraction task during simulated driving. Alcohol Clin Exp Res. 2009; 33: 617–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Bechara A., Tranel D., Damasio H. Characterization of the decision‐making deficit of patients with ventromedial prefrontal cortex lesions. Brain 2000; 123: 2189–2202. [DOI] [PubMed] [Google Scholar]
- 81. Parrott A. C. Performance tests in human psychopharmacology (1): test reliability and standardization. Hum Psychopharmacol Clin Exp 1991; 6: 1–9. [Google Scholar]
- 82. Liu Y., Ho C. H. Effects of different blood alcohol concentrations and post‐alcohol impairment on driving behavior and task performance. Traffic Inj Prev 2016; 11: 334–341. [DOI] [PubMed] [Google Scholar]
- 83. Swan G. E., Lessov‐Schlaggar C. N. The effects of tobacco smoke and nicotine on cognition and the brain. Neuropsychol Rev 2007; 17: 259–273. [DOI] [PubMed] [Google Scholar]
- 84. Jackson M. L., Gunzelmann G., Whitney P., Hinson J. M., Belenky G., Rabat A. et al Deconstructing and reconstructing cognitive performance in sleep deprivation. Sleep Med Rev 2013; 17: 215–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Verster J. C. The alcohol hangover—a puzzling phenomenon. Alcohol Alcohol 2008; 43: 124–126. [DOI] [PubMed] [Google Scholar]


