Abstract
Background
Idiopathic olfactory loss (IOL) accounts for a sizable fraction of olfactory dysfunction, but very little is known about its etiology and electrophysiological changes in the olfactory pathway.
Methods
We analyzed the physiology of IOL using chemosensory event‐related potentials (ERPs) (olfactory and trigeminal: oERP and tERP) and olfactory pathway magnetic resonance imaging (MRI) measured in adult patients with IOL and healthy controls. Subjective olfactory function was measured by Toyota and Takagi (T&T) olfactometry and Sniffin’ Sticks (SS).
Results
Olfactory function was worse in patients with IOL compared to controls (T&T, p < 0.001; SS, p < 0.001). oERPs could be evoked in 17 IOL patients. Signals in these patients showed lower amplitude in the N1 and P2 waves than controls (p < 0.05 for both), but there were no difference in latency between the 2 groups (p > 0.05). tERP were detected in all patients and controls; there were no differences in latency and nor amplitude between the 2 groups (p > 0.05). The olfactory bulb (OB) volume was significantly smaller in the IOL group than controls (p < 0.001), but there was no difference in the olfactory sulcus depth between groups (p > 0.05). Better olfactory function was associated with increasing magnitude of N1 amplitude in oERPs (p < 0.05) and increasing OB volume (p < 0.05).
Conclusion
IOL patients show neurophysiologic deficits and some anatomic differences compared to healthy controls.
Keywords: idiopathic olfactory loss, olfactory function, chemosensory event‐related potentials, MRI, clinical features
Abstract
背景
尽管特发性嗅觉下降 (IOL) 在嗅觉障碍人群中所占比例相当可观, 但是相关病因学机制及嗅觉通路相关电生理变化并不十分清楚。
方法
通过对比成人IOL及健康人群的化学感受事件相关电位 (ERPs) 及嗅觉通路核磁共振成像 (MRI) 的差异, 我们分析IOL的生理机制。同时采用Toyota和Takagi (T&T) 嗅觉测定法及Sniffin'Sticks嗅觉心理物理测试 (SS) 对患者主观嗅功能进行评定。
结果
与对照组相比, IOL患者嗅功能更差(T&T, p < 0.001; SS, p < 0.001)。17例IOL患者诱发出oERPs (嗅神经化学感受事件相关电位) , 这些患者N1、P2波振幅与对照组相比明显降低(p 均 < 0.05), 但两组间潜伏期无明显差异(p > 0.05)。所有患者及健康人群均能检测出tERP (三叉神经感受事件相关电位) ;两组间潜伏期、振幅变化无统计学差异(p > 0.05)。IOL组嗅球 (OB) 体积明显小于对照组(p < 0.001), 但组间嗅裂深度无统计学差异(p >0.05)。嗅觉功能与oERPs中N1振幅幅度、OB体积呈正相关(p 均< 0.05)。
结论
与健康对照组相比, IOL患者呈现出部分神经生理学缺陷及解剖学异常。
Olfactory loss affects approximately 5% of the healthy population and thus represents a commonly encountered medical complaint.1 Idiopathic olfactory loss (IOL) is 1 such burdensome olfactory disorder. Despite a detailed medical history that assesses common causes (negative for sinonasal disease, recent infections of the upper respiratory tract, head trauma, neurodegenerative disease, or toxic exposure) and physical examination and nasal endoscopy that determines anatomic or inflammatory causes, approximately 6% to 30% of cases (depending on setting: general practice or referral clinic) have no clear cause and are termed idiopathic.2, 3 Some clinical features are described in IOL, such as subjective olfactory dysfunction, cognitive impairment, and morphological changes involving in olfaction using imaging tests.4, 5 But very little is known about electrophysiological changes in the olfactory pathway in patients with IOL.
Although the etiology of IOL is unclear, it may be related to altered structure of brain regions involved in olfactory processing.5 IOL may also be related to neurodegenerative diseases. More than 90% of the patients with idiopathic Parkinson's disease (PD) are hyposmic or functionally anosmic at the onset of motor symptoms and poor olfaction predates the development of Alzheimer's disease (AD).6, 7 Thus, some patients with IOL may go on to develop these conditions.7, 8 Neurodegenerative pathology of olfactory bulbs (OBs) and cortex (eg, deposition of Lewy bodies, neurofibrillary tangles, or synaptic loss) may also underlie IOL, though inability to access the brain in living humans makes assessing this directly challenging. Any 1 of these abnormalities might lead to the changes in the olfactory pathway. Thus, understanding the electrophysiological and morphological changes of the olfactory pathway in IOL may have important implications for understanding the pathophysiology of IOL and related neurologic diseases.
Measurement of chemosensory event‐related potentials (ERPs), an electrophysiological test of the olfactory system, has been proposed as a more objective diagnostic tool for evaluating olfactory function.9 Chemosensory ERPs may be elicited by selective olfactory stimuli (referred to as olfactory ERPs [oERPs]) or by relatively selective trigeminal stimuli (referred to as trigeminal ERPs [tERPs]) (the trigeminal systems serves the role of detecting noxious stimuli in the environment and is separate from the olfactory chemosensory system).10 Subjects with normal olfactory function usually elicit consistent and reproducible olfactory and somatosensory ERP waves.11, 12 Few reports describe the ERP features of IOL, though they appear to change after the diagnosis of some neurodegenerative diseases.13, 14 Olfactory pathway magnetic resonance imaging (MRI) is 1 of the most widely utilized (and noninvasive) ways for assessing morphological changes of olfactory system in both clinical practice and research. It has been reported that the volume of OB decreases in some olfactory disorders (eg, posttraumatic anosmia).15 Nevertheless, in clinical practice, whether to obtain MRI scans for IOL patients to investigate intracranial abnormalities is still controversial.16
The aim of this study was to define the electrophysiological and morphological features for IOL using ERPs and MRI and to assess the use of these 2 objective tests in the clinical diagnosis of IOL.
Patients and methods
Subjects
Twenty Chinese adult IOL patients and sex‐ and age‐matched control subjects were included in this study. Standard otolaryngological evaluations and imaging examination were performed, including detailed medical history, physical examination with neurologic evaluation to exclude cognitive loss, nasal endoscopy and paranasal sinus imaging to exclude inflammatory disease. At the onset of olfactory dysfunction, all patients had no history of the following: brain trauma, acute infection of the upper respiratory airway, sinonasal or brain disease, drug or toxic exposure, or other obvious etiology for their smell loss. Chronic rhinosinusitis and other inflammatory nasal disorders were excluded by nasal endoscopy and imaging examinations (computed tomography [CT] or MRI). All patients had no other neurological or psychiatric deficits except for the loss of sense of smell. All patients were unresponsive to steroids after systemic or nasal treatment for at least 1 month. We recruited a control group from the Physical Examination Center of our institution, and all of these participates had no smell problems or brain diseases. The ethics board of Anzhen Hospital approved this study and written informed consent was obtained from all participants.
Psychophysical testing of olfactory performance
Psychophysical tests of olfactory function were performed with the Toyota and Takagi (T&T) olfactometer (Daiichi Pharmaceutical Co. Ltd., Tokyo, Japan) and Sniffin'Sticks (SS) test (Burghart GmbH, Wedel, Germany).17, 18 The T&T test was performed according to the manufacturer's instructions. The score is the average of the sum of odor threshold for 5 odorants. Standard administration was performed according to the manufacturer's instructions for the SS test.18 Olfactory function was measured by threshold (T), odor discrimination (D), and odor identification (I) tasks, and the final score was expressed as the sum of the T, D, and I values (ie, the TDI score).
Chemosensory ERP examination
The procedure was performed in a well ventilated and electrically shielded room according to published protocols. Briefly, olfactory and trigeminal stimuli (40% vol/vol phenethyl alcohol and 40% vol/vol CO2, respectively) were delivered to the olfactory area of the right nasal cavity through the olfactometer (Burghart OM6b, Wedel, Germany) at a constant temperature and humidity. A total of 60 stimulations were presented within the session corresponding to 30 stimulations of each odorant (stimulus duration 250 ms, interval 30 seconds, flow rate 8 L/minute). During the examination, white noise of approximately 60 dB was presented via a headphone to prevent the interference from noise, and a constant level of vigilance was maintained by asking subjects to avoid eye blinking.17, 19, 20 The ERP recordings took in total ∼40 minutes.
Electroencephalography (EEG) readings of 2048 ms duration, including a 512‐ms pre‐stimulus period, were recorded at Fz, Cz, and Pz on the scalp according to the international standard 10/20 method. An 8‐channel amplifier (Schubert, Rottenbach, Germany) was used for recordings. Reference electrodes were placed at the left and right earlobes (A1 and A2).19 Records contaminated by eye blinks (>50 μV in Fp2/A1 + A2) or other disturbances (eg, high‐frequency motor artifacts) were excluded during offline analysis of the recordings. Stable oERP and tERP waves were obtained after amplification and filtering. The records were amplified (20,000 times), filtered (0.2‐30 Hz band‐pass filter; an additional low‐pass filter, 15 Hz, for offline analyses), and digitized (250 Hz).21 The sampling frequency was 250 Hz. For data analysis the program EPE valuate (Kobal, Erlangen, Germany) was used. For averaging, a minimum of 6 records without artifacts for each odorant was available.22 In all averaged ERP 2 distinct peaks were measured within a defined latency range. The latency windows (all data are related to the activation of the odorous current: to obtain the real latency between stimulus onset and the components of the ERP, 512 ms have to be subtracted) were chosen using the following criteria: 320 to 450 ms for the most negative peak (Nl), 450 to 800 ms for the most positive peak (P2). The amplitudes of the N1 and P2 components were defined separately as peak‐to‐baseline amplitudes. ERPs (both oERPs and tERPs) were considered as present if the averaged waveforms demonstrated a negative‐positive complex with the amplitude value exceeding ±2 μV within the latency windows.11, 23, 24
Olfactory pathway MRI images
Patients were examined on a 3.0‐T MRI system (Siemens Trio Tim Magnetom, Germany) using a special protocol for olfactory pathway analysis. Volume of the OB and depth of the olfactory sulcus (OS) were calculated using a standardized method.4 The posterior end of the OB was determined when 2 successive slice measurements yielded the same result. OB volumes were calculated by planimetric manual contouring and all surfaces were added together to obtain the volume. The depth of the OS was measured by drawing the most perpendicular line connecting a straight line tangent to the deepest point of the sulcus. The olfactory cleft was examined to exclude problems such as obstructive lesions and structural damage.25
Statistical analysis
All analyses were performed using SPSS 17.0 (SPSS Inc., Chicago, IL). The significance level was set at 0.05. The independent sample t test was used to confirm that both groups were equivalent in term of age. Psychophysical results, ERPs, and MRI results of olfactory function were expressed as mean ± standard deviation and compared by independent sample t test. Pearson statistics were used for correlation analysis between subjective and objective olfactory function tests.
Results
Demographic and clinical information about the subjects are presented in Table 1. The age of the IOL group ranged from 26 to 70 years (mean = 44 ± 12 years) and controls ranged from 23 to 69 years (mean = 44 ± 12 years). There were no significant differences in age between the IOL and control group (p > 0.05). In both groups, there were the same number of males and females (8 males vs 12 females, p > 0.05). The mean duration of olfactory dysfunction from onset of symptoms to clinical evaluation was 2.2 years (range, 0.3‐8 years) in the IOL group.
Table 1.
Descriptive statistics of T&T olfactometer and and Sniffin' Sticks test*
| IOL patients | Controls | p | |
|---|---|---|---|
| n | 20 | 20 | |
| Age (years), mean ± SD | 44 ± 12 | 44 ± 12 | ns |
| Gender (male/female), n | M(8)/F(12) | M(8)/F(12) | ns |
| T&T scores, mean ± SD | 5.28 ± 0.74 | −1.10 ± 1.06 | 0.000 |
| Sniffin’ Sticks scores (TDIa), mean ± SD | 14.16 ± 5.42 | 32.08 ± 3.13 | 0.000 |
*Significant negative correlation between TDI scores and T&T scores in IOL patients (r = −0.90, p < 0.001).
Olfactory function measured by threshold (T), odor discrimination (D) and odor identification (I) tasks; final score expressed as the sum of the T, D, and I values (TDI score).
IOL = idiopathic olfactory loss; ns = not significant; SD = standard deviation; T&T = Toyota and Takagi.
T&T olfactometer and TDI scores were 5.28 ± 0.74 and 14.16 ± 5.42 for IOL patients vs −1.10 ± 1.06 and 32.08 ± 3.13 for controls, respectively. T&T scores were significantly negatively correlated with TDI scores (r = −0.90, p < 0.001), indicating, as expected, that greater T&T scores (worse olfactory function) generally were associated with lower TDI scores (worse olfactory function). Eight IOL patients were diagnosed with functional anosmia, and 12 patients were diagnosed with hyposmia using the standard evaluation criteria for olfactory dysfunction for each test.17, 18 Both olfactory tests were significantly different between the patients and controls groups (T&T, p < 0.001; SS, p < 0.001), indicating that olfactory function in IOL patients was worse than controls (Table 1).
oERPs
oERPs were identified in 17 of the 20 IOL patients (85%) and all 20 controls. The 3 patients with no oERPs were all functionally anosmic. The amplitude of the N1 in IOL patients range from −0.30 to −5.90 μV (mean = −2.94 ± 1.81 μV); the amplitude of controls ranged from −1.60 to −9.50 μV (mean = −4.70 ± 2.15 μV) (Fig. 1). The amplitude of the P2 in IOL patients range from +1.50 to +11.30 μV (mean = +5.82 ± 2.95 μV); the amplitude of controls ranged from +3.30 to +17.60 μV (mean = +8.54 ± 3.94 μV) (Fig. 2). Thus, IOL patients had lower amplitudes in oERPs than controls, both in N1 wave (p < 0.05) and in P2 wave (p < 0.05). Although the latencies of N1 in IOL patients was longer than in controls (490 ± 174 ms vs 439 ± 90 ms) and the latencies of P2 in IOL patients was longer than in controls (645 ± 179 vs 612 ± 103 ms), these differences did not reach statistical significance (p > 0.05 and p > 0.05, respectively) (Table 2, Fig. 3).
Figure 1.
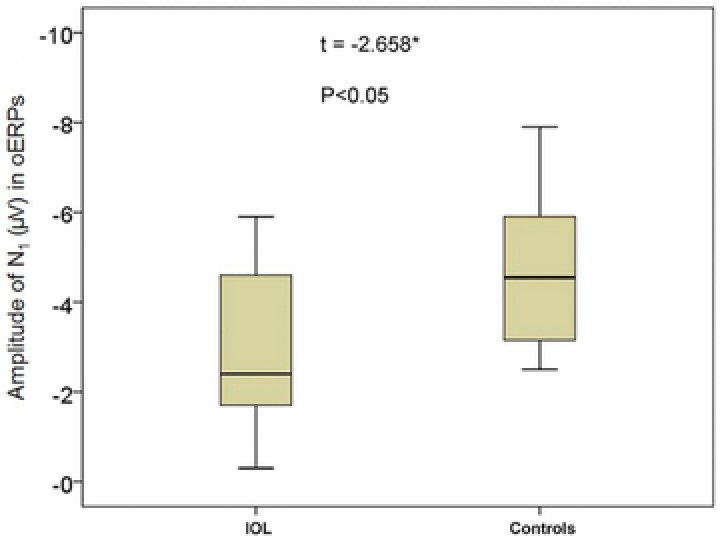
Total amplitude of N1 wave in oERPs (Cz site) from IOL patients and controls. *p < 0.05 (independent sample t test). IOL = idiopathic olfactory loss; oERP = olfactory event‐related potential.
Figure 2.
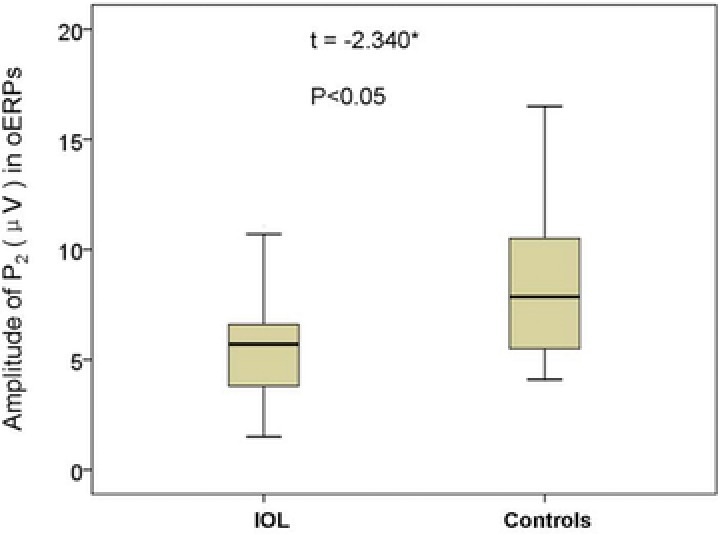
Total amplitude of P2 wave in oERPs (Cz site) from IOL patients and controls. *p < 0.05 (independent sample t test). IOL = idiopathic olfactory loss; oERP = olfactory event‐related potential.
Table 2.
ERPs
| Componentsa | IOL patients (mean ± SD) | Controls (mean ± SD) | p | |
|---|---|---|---|---|
| oERPs | Latency of N1 (ms) | 490 ± 174 | 439 ± 90 | 0.295 |
| Amplitude of N1 (μV) | −2.94 ± 1.81 | −4.70 ± 2.15 | 0.012 | |
| Latency of P2 (ms) | 645 ± 179 | 612 ± 103 | 0.513 | |
| Amplitude of P2 (μV) | +5.82 ± 2.95 | +8.54 ± 3.94 | 0.025 | |
| tERPs | Latency of N1 (ms) | 403 ± 120 | 367 ± 79 | 0.262 |
| Amplitude of N1 (μV) | −5.32 ± 2.69 | −7.16 ± 3.82 | 0.087 | |
| Latency of P2 (ms) | 560 ± 97 | 534 ± 105 | 0.435 | |
| Amplitude of P2 (μV) | +7.61 ± 3.98 | +9.07 ± 6.16 | 0.379 |
aThe results in patients and controls are recorded in Cz position and received after subtracting the 512 ms (pre‐stimulus) by the results shown in the figures.
ERP = event‐related potential; IOL = idiopathic olfactory loss; oERP = olfactory ERP; SD = standard deviation; tERP = trigeminal ERP.
Figure 3.
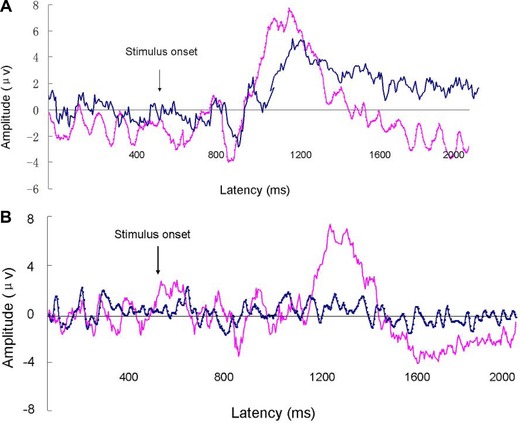
(A) Grand average for oERPs in IOL patients (blue line) and controls (red line) recording in position Cz. (B) oERPs in position Cz from a 43‐year‐old IOL patient (blue line) and an age‐matched healthy subject (red line). IOL = idiopathic olfactory loss; oERP = olfactory event‐related potential.
tERPs
All patients and controls demonstrated tERPs after stimulation with CO2. Compared with the controls, the patients had longer latencies in N1 and P2 waves (IOL 403 ± 120 ms vs controls 367 ± 79 ms and IOL 560 ± 97 ms vs controls 534 ± 105 ms, respectively), but there was no significant difference between IOL and controls (p > 0.05 and p > 0.05, respectively). The IOL patients had lower amplitudes in both N1 and P2 waves (N1 −5.32 ± 2.69 vs −7.16 ± 3.82 μV and P2 +7.61 ± 3.98 vs +9.07 ± 6.16 μV, respectively), but no difference was found between IOL and controls for N1 and P2 (p > 0.05 and p > 0.05, respectively) (Table 2, Fig. 3).
OB volume and OS depth
The average left and right OB volume of the IOL patients were 31.68 ± 3.24 mm3 and 31.94 ± 3.33 mm3, respectively, which were smaller than the control group (50.59 ± 3.68 and 49.52 ± 3.82 mm3, p < 0.001, all). No significant difference was found in OS depth between IOL patients and controls in both left and right sides (p > 0.05 for both) (Table 3, Fig. 4). There was no difference in OB volume between left and right sides in either of the 2 groups (p > 0.05 for both), nor difference in the OS depth (p > 0.05 for both).
Table 3.
OB volume and OS depth
| OB volume (mm3) | OS depth (mm) | |||||
|---|---|---|---|---|---|---|
| Left | Right | Mean | Left | Right | Mean | |
| IOL patientsa | 31.68 ± 3.24 | 31.94 ± 3.33 | 31.81 ± 3.21 | 7.79 ± 1.15 | 7.66 ± 1.00 | 7.72 ± 1.01 |
| Controlsa | 50.59 ± 3.68 | 49.52 ± 3.82 | 50.05 ± 3.53 | 7.90 ± 1.27 | 7.80 ± 1.11 | 7.85 ± 1.15 |
| p | <0.001 | <0.001 | <0.001 | 0.776 | 0.667 | 0.711 |
Values are mean ± SD.
IOL = idiopathic olfactory loss; OB = olfactory bulb; OS = olfactory sulcus; SD = standard deviation.
Figure 4.
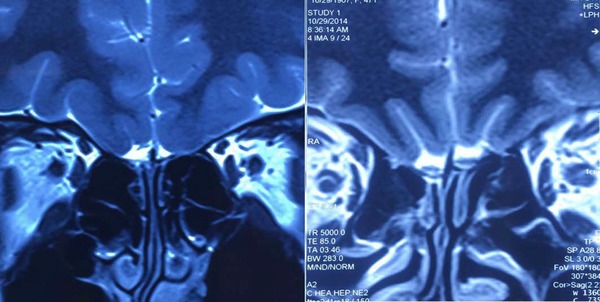
MRI of olfactory pathway: bilateral OB volume decreased in IOL patients (left); the structure of OB and OS in healthy subjects (right). IOL = idiopathic olfactory loss; OB = olfactory bulb; OS = olfactory sulcus.
Correlations between TDI score, threshold and oERPs, tERPs, OB volume, OS depth
Results from correlations between subjective and objective olfactory function tests are shown in Table 4. Better olfactory function was associated with increasing magnitude (absolute value) of N1 amplitude in oERPs (r = −0.674, p < 0.05). Furthermore, OB volume exhibited correlation with the odors threshold score (r = 0.466, p < 0.05). Sniffin’ Sticks T score ranging from 0 to 16 is determined by employing a single staircase method, and higher T score is equivalent to lower diluted concentration of the odors and greater olfactory sensitivity. Therefore, larger OB volume correlates with lower olfactory threshold and higher T score.
Table 4.
Correlations between TDI score, threshold (T) and oERPs, tERPs, OB volume, and OS depth*
| oERPs | tERPs | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| N1 Latency (ms) | N1 Amplitude (μV) | P2 Latency (ms) | P2 Amplitude (μV) | N1 Latency (ms) | N1 Amplitude (μV) | P2 Latency (ms) | P2 Amplitude (μV) | OB volume (mm3) | OS depth (mm) | |
| TDI, r a | −0.280 | −0.674 | −0.141 | −0.025 | 0.028 | 0.127 | 0.139 | 0.002 | ||
| T, r a | 0.466 | 0.411 | ||||||||
| p b | 0.276 | 0.003 | 0.591 | 0.924 | 0.908 | 0.593 | 0.560 | 0.993 | 0.044 | 0.080 |
Olfactory function measured by threshold (T), odor discrimination (D) and odor identification (I) tasks; final score expressed as the sum of the T, D, and I values (TDI score).
Bold values are significant.
ERP = event‐related potential; IOL = idiopathic olfactory loss; IOL = idiopathic olfactory loss; OB = olfactory bulb; oERP = olfactory ERP; OS = olfactory sulcus; tERP = trigeminal ERP.
Discussion
In this study, we identified electrophysiological and morphological changes of olfactory pathway associated with IOL: lower amplitude in oERPs and decreased OB volume. In addition, better olfactory function was associated with increased N1 amplitude in oERPs and larger OB volume. We report here, for the first time, on waveforms changes in oERPs in patients with IOL, and also verified the stability and validity of oERPs and MRI in the clinical diagnosis of IOL.
oERPs
Presence/absence
Here we show that IOL is associated with altered electrophysiological reactions involved in olfactory signal transduction. Patients with olfactory dysfunction usually demonstrate oERPs in approximately one‐third of cases.9 The absence of oERP observed in functional anosmia IOL patients in our study is consistent with prior work.11 However, our results demonstrate that the presence of oERPs in IOL is more common than previously found: 60% of IOL patients in our study were classified as having functional hyposmia rather than functional anosmia. Indeed, oERPs can be observed in most patients with hyposmia,24 and the probability of detecting an oERP becomes greater as hyposmia is less severe.11 In addition, a fraction of patients with the diagnosis “functional anosmia” do occasionally experience olfactory sensations or are able to identify certain odors.11 In that situation, residual olfactory function might be identified by detectable oERPs. On the other hand, the presence of oERPs may relate to specific causes of olfactory dysfunction. The presence of oERPs in previous work was lower than what we found here, which may be due to potentially mixed causes of olfactory dysfunction included. This is consistent with a previous study of subjects with functional anosmia in which the proportion of IOL subjects with detectable oERPs was higher than other olfactory disorders.11 Thus, presence of oERPs may be 1 of the characteristics of IOL that is useful in clinical diagnosis.
Amplitude and latency
To our knowledge, there are no data on the amplitude and latency of chemosensory ERP in IOL patients. In our study, patients with IOL showed lower amplitudes in N1 and P2 waves compared to the controls. The result is distinct from previous reports about the changes in oERPs that occur in other olfactory disorders such as rhinosinusitis and PD‐induced olfactory dysfunction.26, 27 We did not find a difference in latency between the 2 groups. It is generally thought that amplitude of ERPs can reflect the number of neurons involved in electrical events and that latencies can reflect the speed of information processing.17 We speculate that our results may relate to injury to and/or apoptosis of peripheral olfactory pathway neurons, leading to a decreased number of neurons and resulting in a decline in neural response amplitude centrally. Alternatively, abnormal olfactory signal pathways in the brain would also lead to a decline in amplitude due to central problems. Which is the primary deficit cannot be resolved in this pilot study; future studies will need to address this conundrum. The lack of effect on latencies may be explained by the possibility that IOL is a peripheral process that does not affect brain function. A previous study showed that patients with temporal lobe epilepsy have prolonged latencies of oERPs; these patients do indeed have central brain dysfunction.28 Taken together, these data and ours are consistent with our previous finding that IOL patients do not suffer from cognitive impairment.5
We note that oERP waves in IOL patients appear to be different from those reported in pulmonary veno‐occlusive disease (PVOD) and head trauma‐related olfactory disorders in terms of latency of N1 and P2 waves.24, 25 As the diagnosis of IOL is currently thought to depend on exclusion, these findings may provide useful in classifying olfactory loss.
Relationship with neurodegenerative diseases
We and others previously showed that patients with IOL exhibit gray matter volume loss in some regions.5, 29 Such decreases may be an early sign of neurodegenerative diseases.7, 8 The irreversible damage in neurons occurs in the early stage of neurodegenerative diseases may be reflected in the amplitudes of N1 and P2 in oERPs. Our data are consistent with other work showing that abnormalities in oERPs may presage more serious neurologic disease onset.
Indeed, oERPs have been used to investigate the olfactory deficit and the processing of odor information not only in some olfactory disorders (eg, posttraumatic anosmia) but also in idiopathic PD and AD.14, 25, 26 These studies report that oERPs are altered even earlier than when patients exhibit a decreased ability to identify odors—a phenomena that may apply broadly. Indeed, in a study focused on olfactory deficits in patients with multiple sclerosis (MS), 25% of the 45 MS patients exhibited delayed oERPs.30 Thus, patients with abnormal oERPs may be at risk for subsequent neurologic disease. This question will require additional study.
tERPs
Although some studies have demonstrated that patients with olfactory deficits (either congenital or acquired) have a reduced trigeminal sensitivity compared to controls,10, 31 we found no significant difference in this study. This might attributed to robustness of trigeminal responsiveness in IOL, which seems to be reversible and increases with the duration of the disease, and may also be due to the mechanism of sensory adaptation/compensation occuring in the interaction between olfactory and the trigeminal systems.32 We speculate that trigeminal system is more plastic in its recovery from pathologic changes in IOL. We note that our stimuli are only relatively rather than absolutely specific to each chemosensory system.
Although we found no statistical difference in tERPs between IOL patients and controls, given the small sample (N = 20) in this study, there was a trend toward a difference in lower amplitude of N1 in IOL subjects compared to controls. This result is consisted with previous reports about the change in tERPs observed in other acquired olfactory disorders, where subjects with olfactory dysfunction showed smaller central electrophysiological responses compared to controls.32 Trigeminal stimulation produces activation in the orbitofrontal cortex, superior temporal gyrus, and the caudate nucleus, which are also involved in the processing of olfactory sensations.33 Amplitude has been regarded as reflecting the orientation of neuroelectrical dipoles engaged by cortical processing of the stimulus.24 A decline in neural response centrally is showed in N1 amplitude of oERPs in this study. Thus, we speculate that olfactory loss produces central nervous changes leading to a reduced responsiveness following trigeminal stimulation, which is showed in lower N1 amplitude of tERPs. Here, the proportion of oERPs responders was higher than in some reports, and it has been demonstrated that trigeminal response amplitudes are generally larger in oERPs responders compared to nonresponders in IOL.10 This may explain the differences in our N1 amplitude findings in tERPs.
OB volume
We found decreased OB volume in IOL patients compared to controls, consistent with prior work and also data from other olfactory diseases such as postinfectious olfactory dysfunction.4, 25 OB volume typically decreases in olfactory disorders, likely related to damage of olfactory signal transduction from the periphery to the OB, leading to a decreased number of olfactory neurons on the olfactory signal pathway. This is consistent with the possibility that the category of IOL merely reflects an inability to elicit a history of antecedent trauma, upper respiratory tract infection (URTI), or inflammatory nasal disease. However, recent findings also suggest that the OB is also decreased in patients with MS, where the cause of olfactory loss is probably due to central nervous processes.34 Thus, it appears that OB volume in IOL could be due to standard etiologies which damage the olfactory system, or another way neurologic diseases present. This hypothesis is speculative and requires additional investigation.
Correlation between subjective and objective olfactory function
We found significant correlation between TDI score and N1 amplitude in oERPs, which is similar to prior work in Europeans and also data from healthy adults.24, 35 Olfactory sensitivity was found to relate to the magnitude of the oERPs component. In general, amplitudes of ERPs are particularly useful for assessing olfactory dysfunction caused by peripheral damage to the olfactory system, and correlate positively to the olfactory sensitivity in acquired olfactory dysfunction. Thus, patients with severe olfactory loss have decreased magnitude of N1 amplitude in oERPs. However, we found no correlation between P2 amplitude in oERPs and olfactory function, consistent with Murphy et al.’s report35 showing smaller N1 amplitudes but not P2 for older subjects (decreasing olfactory function). The results may demonstrate a specific association between olfactory sensitivity and magnitude of oERPs components. In generally, oERPs latencies are difficult to assess accurately in patients with olfactory dysfunction, as their oERPs responses are sometimes similar in magnitude to background noise.24 Given the small sample in the study, findings regarding P2 amplitude in oERPs will require validation.
We also found that smaller OB volume correlates with decreased olfactory sensitivity in IOL patients, consistent with prior work on IOL and other olfactory disorders.4, 36 Our data is consistent with the idea that OB volume is sensitive to subtle changes in the olfactory system.
Limitations
There are several limitations to our study. As with prior work, technical challenges can make obtaining and interpreting oERPs challenging. For example, it is difficult to classify the peaks in some recordings. This is because jitter of the oERPs from individual subjects exists and oERP responses are sometimes similar in magnitude to the background in patients with olfactory dysfunction.24 These issues have been identified in the literature and our data falls within acceptable parameters.24, 37 To address these issues, a well‐trained, independent observer with a high degree of experience is required when interpreting oERP data.
We could not analyze the effect of some relevant factors such as duration of olfactory dysfunction and age on the results of chemosensory ERPs and MRI because of the small sample size. In future studies with larger series employing chemosensory ERP and MRI comparisons between IOL and normal controls, these deficits can be overcome.
Conclusion
Reliability of oERPs is comparable to auditory and visual ERPs.38 Thus, oERPs might be a more sensitive measure of olfactory dysfunction than psychophysical tests, especially for early diagnosis of neurodegenerative diseases.13 oERPs and olfactory pathway MRI appear to provide useful information for evaluating patients with IOL. The features of oERPs and MRI in IOL found in this study, combining psychophysical testing of olfactory function, help to describe the clinical characteristic of IOL, and may help to elucidate the etiology of olfactory loss this condition. In future work, additional insights into the mechanistic deficits in IOL may be elucidated. One such direction might include longitudinal study of IOL patients to help determine if this condition is indeed a precursor to the development of neurodegenerative diseases.
How to Cite this Article: Liu J, Pinto JM, Yang L, Yao L, Miao X, Wei Y. Evaluation of idiopathic olfactory loss with chemosensory event‐related potentials and magnetic resonance imaging. Int Forum Allergy Rhinol. 2018;8:1315–1322.
Funding sources for the study: Beijing Municipal Administration of Hospitals Ascent Plan (DFL20150602); National Natural Science Foundation of China (81670903).
Potential conflict of interest: None provided.
References
- 1. Rouby C, Thomas‐Danguin T, Vigouroux M, et al. The Lyon clinical olfactory test: validation and measurement of hyposmia and anosmia in healthy and diseased populations. Int J Otolaryngol. 2011;2011:203805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Henkin RI, Levy LM, Fordyce A. Taste and smell function in chronic disease: a review of clinical and biochemical evaluations of taste and smell dysfunction in over 5000 patients at The Taste and Smell Clinic in Washington, DC. Am J Otolaryngol. 2013;34:477–489. [DOI] [PubMed] [Google Scholar]
- 3. Damm M, Temmel A, Welge‐Lüssen A, et al. [Olfactory dysfunctions. Epidemiology and therapy in Germany, Austria and Switzerland]. HNO. 2004;52:112–120. [DOI] [PubMed] [Google Scholar]
- 4. Rombaux P, Potier H, Markessis E, Duprez T, Hummel T. Olfactory bulb volume and depth of olfactory sulcus in patients with idiopathic olfactory loss. Eur Arch Otorhinolaryngol. 2010;267:1551–1556. [DOI] [PubMed] [Google Scholar]
- 5. Yao L, Pinto JM, Yi X, Li L, Peng P, Wei Y. Gray matter volume reduction of olfactory cortices in patients with idiopathic olfactory loss. Chem Senses. 2014;39:755–760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ponsen MM, Stoffers D, Twisk JW, Wolters ECh, Berendse HW. Hyposmia and executive dysfunction as predictors of future Parkinson's disease: a prospective study. Mov Disord. 2009;15;24:1060–1065. [DOI] [PubMed] [Google Scholar]
- 7. Koss E, Weiffenbach JM, Haxby JV, Friedland RP. Olfactory detection and identification performance are dissociated in early Alzheimer's disease. Neurology. 1988;38:1228–1232. [DOI] [PubMed] [Google Scholar]
- 8. Haehner A, Hummel T, Hummel C, Sommer U, Junghanns S, Reichmann H. Olfactory loss may be a first sign of idiopathic Parkinsons's disease. Mov Disord. 2007;22:839–842. [DOI] [PubMed] [Google Scholar]
- 9. Rombaux P, Mouraux A, Collet S, Eloy P, Bertrand B. Usefulness and feasibility of psychophysical and electrophysiological olfactory testing in the rhinology clinic. Rhinology. 2009;47:28–35. [PubMed] [Google Scholar]
- 10. Rombaux P, Mouraux A, Keller T, Hummel T. Trigeminal event‐related potentials in patients with olfactory dysfunction. Rhinology. 2008;46:170–174. [PubMed] [Google Scholar]
- 11. Lotsch J, Hummel T. The clinical significance of electrophysiological measures of olfactory function. Behav Brain Res. 2006;170:78–83. [DOI] [PubMed] [Google Scholar]
- 12. Han P, Schriever VA, Peters P, Olze H, Uecker FC, Hummel T. Influence of airflow rate and stimulus concentration on olfactory event‐related potentials (OERP) in humans. Chem Senses. 2018 Feb 2;43(2):89–96. [DOI] [PubMed] [Google Scholar]
- 13. Hummel T. Olfactory evoked potentials as a tool to measure progression of Parkinson's disease In: Chase T, Bedard P, eds. Focus on Medicine: New Developments in the Drug Therapy of Parkinson's Disease. Vol. 14 Oxford: Blackwell Science; 1999:47–53. [Google Scholar]
- 14. Peters JM, Hummel T, Kratzsch T, Lötsch J, Skarke C, Frölich L. Olfactory function in mild cognitive impairment and Alzheimer's disease: an investigation using psychophysical and electrophysiological techniques. Am J Psychiatry. 2003;160:1995–2002. [DOI] [PubMed] [Google Scholar]
- 15. Yousem DM, Geckle RJ, Bilker WB, Kroger H, Doty RL. Posttraumatic smell loss: relationship of psychophysical tests and volumes of the olfactory bulbs and tracts and the temporal lobes. Acad Radiol. 1999;6:264–272. [DOI] [PubMed] [Google Scholar]
- 16. Rudmik L, Smith KA, Soler ZM, Schlosser RJ, Smith TL. Routine magnetic resonance imaging for idiopathic olfactory loss: a modeling‐based economic evaluation. JAMA Otolaryngol Head Neck Surg. 2014;140:911–917. [DOI] [PubMed] [Google Scholar]
- 17. Yang L, Wei Y, Yu D, Zhang J, Liu Y. Olfactory and gustatory function in healthy adult Chinese subjects. Otolaryngol Head Neck Surg. 2010;143:554–560. [DOI] [PubMed] [Google Scholar]
- 18. Hummel T, Sekinger B, Wolf SR, Pauli E, Kobal G. ‘Sniffin’ Sticks’: olfactory performance assessed by the combined testing of odor identification, odor discrimination and olfactory threshold. Chem Senses. 1997;22:39–52. [DOI] [PubMed] [Google Scholar]
- 19. Hummel T, Bensafi M, Nikolaus J, Knecht M, Laing DG, Schaal B. Olfactory function in children assessed with psychophysical and electrophysiological techniques. Behav Brain Res. 2007;180:133–138. [DOI] [PubMed] [Google Scholar]
- 20. Hummel T, Kobal G. Olfactory event‐related potentials In: Simon SA, Nicolelis MAL, eds. Methods in Chemosensory Research. Boca Raton, FL: CRC Press; 2001:429–464. [Google Scholar]
- 21. Olofsson JK, Nordin S. Gender differences in chemosensory perception and event‐related potentials. Chem Senses. 2004;29:629–637. [DOI] [PubMed] [Google Scholar]
- 22. Gottschlich M, Hummel T. Effects of handedness on olfactory event‐related potentials in a simple olfactory task. Rhinology. 2015;53:149–153. [DOI] [PubMed] [Google Scholar]
- 23. Huart C, Rombaux P, Hummel T, Mouraux A. Clinical usefulness and feasibility of time‐frequency analysis of chemosensory event‐related potentials. Rhinology. 2013;51:210–221. [DOI] [PubMed] [Google Scholar]
- 24. Brämerson A, Millqvist E, Ydse B, Larsson C, Olofsson JK, Bende M. Event‐related potentials in patients with olfactory loss. Acta Otolaryngol. 2008;128:1126–1131. [DOI] [PubMed] [Google Scholar]
- 25. Miao X, Yang L, Gu H, et al. Evaluation of post‐traumatic anosmia with MRI and chemosensory ERPs. Eur Arch Otorhinolaryngol. 2015;272:1945–1953. [DOI] [PubMed] [Google Scholar]
- 26. Barz S, Hummel T, Pauli E, Majer M, Lang CJ, Kobal G. Chemosensory event‐related potentials in response to trigeminal and olfactory stimulation in idiopathic Parkinson's disease. Neurology. 1997;49:1424–1431. [DOI] [PubMed] [Google Scholar]
- 27. Hu B, Han D, Zhang L, et al. Olfactory event‐related potential in patients with rhinosinusitis‐induced olfactory dysfunction. Am J Rhinol Allergy. 2010;24:330–335. [DOI] [PubMed] [Google Scholar]
- 28. Hummel T, Pauli E, Schüler P, Kettenmann B, Stefan H, Kobal G. Chemosensory event‐related potentials in patients with temporal lobe epilepsy. Epilepsia. 1995;36:79–85. [DOI] [PubMed] [Google Scholar]
- 29. Bitter T, Gudziol H, Burmeister HP, Mentzel HJ, Guntinas‐Lichius O, Gaser C. Anosmia leads to a loss of gray matter in cortical brain areas. Chem Senses. 2010;35:407–415. [DOI] [PubMed] [Google Scholar]
- 30. Hawkes CH, Shephard BC, Kobal G. Assessment of olfaction in multiple sclerosis: evidence of dysfunction by olfactory evoked response and identification tests. Brain. 1997;62:436–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Frasnelli J, Hummel T. Interactions between the chemical senses: trigeminal function in patients with olfactory loss. Int J Psychophysiol. 2007;65:177–181. [DOI] [PubMed] [Google Scholar]
- 32. Frasnelli J, Schuster B, Hummel T. Interactions between olfaction and the trigeminal system: what can be learned from olfactory loss? Cereb Cortex. 2007;17:2268–2275. [DOI] [PubMed] [Google Scholar]
- 33. Hummel T, Doty RL, Yousem DM. Functional MRI of intranasal chemosensory trigeminal activation. Chem Senses. 2005;30:i205–i206. [DOI] [PubMed] [Google Scholar]
- 34. Tanik N, Serin HI, Celikbilek A, Inan LE, Gundogdu F. Olfactory bulb and olfactory sulcus depths are associated with disease duration and attack frequency in multiple sclerosis patients. J Neurol Sci. 2015;358(1‐2):304–307. [DOI] [PubMed] [Google Scholar]
- 35. Murphy C, Nordin S, de Wijk RA, Cain WS, Polich J. Olfactory‐evoked potentials: assessment of young and elderly, and comparison to psychophysical threshold. Chem Senses. 1994;19:47–56. [DOI] [PubMed] [Google Scholar]
- 36. Mueller A, Rodewald A, Reden J, Gerber J, von Kummer R, Hummel T. Reduced olfactory bulb volume in post‐traumatic and post‐infectious olfactory dysfunction. Neuroreport. 2005;16:475–478. [DOI] [PubMed] [Google Scholar]
- 37. Harada H, Shiraishi K, Kato T. Olfactory event‐related potentials in normal subjects and patients with smell disorders. Clin Electroencephalogr. 2003;34:191–196. [DOI] [PubMed] [Google Scholar]
- 38. Thesen T, Murphy C. Reliability analysis of event‐related brain potentials to olfactory stimuli. Psychophysiology. 2002;39:733–738. [DOI] [PubMed] [Google Scholar]


