Abstract
The objective of this study was to assess the clinical utility of the AUSDRISK tool for determining risk of Type 2 diabetes mellitus (T2DM). In this secondary analysis from the HealthTrack study, the AUSDRISK tool was applied to data from overweight/obese volunteers completing a lifestyle intervention trial. Participants were volunteer residents of the Illawarra region recruited in 2014–2015. From 377 trial participants (BMI 25–40 kg/m2, 25–54 yr), 161 provided data required for measurement of AUSDRISK, collected at 0 and 12 months. They had been randomised to one of two lifestyle interventions (±a healthy food sample, 30 g walnuts/day, I and IW) delivered by dietitians, or a control intervention (C) delivered by nurse practitioners. HbA1c measures were considered for comparison. At baseline the AUSDRISK score indicated n = 83 (51.5%) were at high risk of T2DM within 5 years (≥12 points). After 12 months the proportion scored as high risk significantly decreased in the IW group (51.5% vs 33.3%; p = 0.005), but not I (51.2% vs 39.0%; p = 0.063) or C group (51.9% vs 38.9%; p = 0.065). By comparison, HbA1c measures indicated high risk in n = 24 (17%) of 139 participants at baseline and borderline non-significant changes over time in the randomised groups. In conclusion, the AUSDRISK tool has reasonable clinical utility in identifying T2DM risk in clinical samples of overweight/obese individuals.
Keywords: Obesity, Diabetes risk, Lifestyle intervention, AUSDRISK, HbA1c
1. Introduction
The global economic burden of type 1 and type 2 diabetes in 2015 was US$1·31 trillion (95% CI 1·28–1·36) or 1·8% (95% CI 1·8–1·9) of global gross domestic product (Bommer et al., 2017). Obesity is a significant aetiological cause of Type 2 Diabetes Mellitus (T2DM); being overweight with substantial abdominal fat, or obese account for approximately 80–90% of all T2DM (Astrup and Finer, 2000). The relative risk for T2DM among women who gain 5.0 to 7.9 kg was 1.9 and those that gained 8.0 to 10.9 kg was 2.7 (Colditz et al., 1995). Clinical trials have demonstrated that as little as 5% weight loss is sufficient to prevent most obese subjects with impaired glucose tolerance developing T2DM (Colditz et al., 1995; Hamman et al., 2006; Delahanty et al., 2014). The rapid increase in obesity prevalence indicates an epidemic and reversing the scale would reduce the prevalence of T2DM. The obesity rate in Australia has tripled in the last three decades with one in four Australians considered obese (Body Mass Index (BMI) > 30 kg/m2) (Buchmueller and Johar, 2015). The impact on healthcare expenditure is substantial. It has been estimated that, compared to a person of normal weight, a person with a BMI between 30 and 35 kg/m2 has 19% higher healthcare costs, and a person with BMI > 35 kg/m2 has a 51% higher healthcare expenditure (Buchmueller and Johar, 2015). Early intervention is key to the prevention of chronic disease. It has been well documented that multi-disciplinary lifestyle interventions integrating nutrition, exercise and psychological support produces the most significant and sustained results for weight loss (NHMRC, 2013).
Determining which patients are at greatest risk to target for lifestyle intervention is challenging for clinicians. Some overweight and obese people may be “metabolically healthy”, in that they do not present with dyslipidemia, glucose perturbations and lack cardiovascular disease symptoms (Phillips, 2016). General Practitioners (GPs) need a simple tool to screen overweight/obese patients to identify those at greatest risk of T2DM and refer them for lifestyle intervention before medical intervention is required.
There are a number of methods to assess T2DM risk, and they differ in sensitivity and specificity within various populations. The gold standard to assess insulin sensitivity is the Euglycaemic clamp, which is invasive and not appropriate at a population level. The oral glucose tolerance test lacks specificity as 40% of incident diabetes arises in people who had a normal glucose tolerance 3–5 years earlier (Unwin et al., 2002). The Homeostatic Model Assessment (HOMA) requires the measurement of fasting glucose and insulin, but insulin is an expensive and non-routine test. HbA1c reflects average glucose levels over the preceding weeks and is used to routinely diagnose T2DM and detect individuals at risk (American Diabetes Association, 2013). The Australian Type 2 Diabetes Risk Assessment Tool (AUSDRISK) (Chen et al., 2010) has been developed for use within an Australian population and incorporates ten different medical, lifestyle and demographic variables to determine 5 year T2DM risk. The AUSDRISK tool was introduced in July 2008 by the Australian Government Department of Health and Ageing and attracts a Medicare rebate for eligible patients (Australian Government of Health and Ageing, 2008).
The utility of the AUSDRISK tool in clinical practice has not been fully explored and clinical trials for obesity management provide a useful opportunity. For example, the HealthTrack study incorporated the services of all three allied health disciplines (dietetics, exercise physiology, psychology) into a streamlined lifestyle intervention for an overweight/obese clinical cohort and compared effects with usual care. One experimental group received the interdisciplinary intervention, another received the interdisciplinary intervention plus a healthy food sample (30 g walnuts/day), and both reported a significant reduction in weight over 12 months compared to control participants (Tapsell et al., 2017).
In this secondary analysis of data from HealthTrack we measured T2DM risk by the AUSDRISK tool to investigate its clinical utility to measure prevalence and change in an “at risk” population. The aim of this exploratory study was to assess the change in T2DM risk in an overweight/obese clinical sample undergoing a lifestyle intervention.
2. Subjects
The subjects of this analysis were HealthTrack study participants described previously (Tapsell et al., 2015). In summary they were volunteer residents of the Illawarra region, aged 25–54 years, with a BMI 25–40 kg/m2, and randomly assigned to standard care (C), interdisciplinary intervention (I), or interdisciplinary intervention + healthy food sample (30 g walnuts/day) (IW) for 12 months. C participants received general advice on diet and exercise from a nurse practitioner and a follow up phone call. I and IW participants received this advice from an Accredited Practising Dietitian (APD) discussing individualised changes in specific food choices and providing advice on physical activity with support from an Exercise Physiologist. They also received scripted motivational telephone calls from trained health coaches.
3. Materials and methods
This study involved the application of the AUSDRISK tool (Australian Government of Health and Ageing, 2008) to data made available from the HealthTrack study. Information required was age, sex, ethnicity, parental history of diabetes, history of high blood glucose, antihypertensive use, smoking, physical inactivity, waist circumference and fruit/vegetable consumption. The tool is available on line (www.health.gov.au/internet/main/publishing.nsf/Content/diabetesRiskAssessmentTool). The sensitivity and specificity of the AUSDRISK tool has been reported as 74.0% and 67.7%, respectively for a score of >21%, with a positive predictive value (PPV) of 12.7% (Australian Government of Health and Ageing, 2008).
Full details of the HealthTrack study protocol and results are reported elsewhere (Tapsell et al., 2017; Tapsell et al., 2015). Briefly, a population-based survey collected self-reported familial, medical and pharmacological history (completed at screening and repeated at the 12 month follow-up visit). Dietary intake was assessed using a diet history interview (Martin et al., 2003) and physical activity using the International Physical Activity Questionnaire (IPAQ) (Craig et al., 2003) and via a pedometer (Yamax Digiwalker SW200, Pedometers Australia) worn for a four day period every quarter. Waist circumference was measured at baseline and 12 month visits by trained personnel using standard protocols.
3.1. Statistical analysis
Data was available for this analysis from the HealthTrack study at baseline and 12 months. After applying the AUSDRISK tool participants were categorised according to the following criteria: <12 points = not at high risk, and 12+ points = high risk of developing T2DM.
Statistical analysis was conducted using SPSS (version 21.0, IBM Corp, Chicago IL, 2012). The categorised scores were compared between treatment groups at baseline and 12 months using McNemar's test for nominal data. The AUSDRISK scores for the three randomised groups over time was log transformed and compared using mixed measures repeated ANOVA.
For comparative purposes, data on HbA1c levels were also assessed as a proxy for standard care. A HbA1c level of >5.7% or taking glycaemic medication was considered at risk of T2DM. The HbA1c (%) was also compared between treatment groups over time (baseline and 12 months) using a mixed measures repeated ANOVA. The HbA1c categorised data was compared between treatment groups at baseline and 12 months using McNemar's test for nominal data.
4. Results
Characteristics of study participants at baseline for both the full study sample (n = 377) and participants included in this analysis (n = 161) are presented in Table 1. There were no significant differences in baseline characteristics between treatment groups (data not shown).
Table 1.
Baseline demographics for original study sample (n = 377), and AUSDRISK analysis sample (n = 161).
| Intervention + walnut | Intervention | Control | |
|---|---|---|---|
| Original study sample (n = 377) | |||
| N | 126 | 125 | 126 |
| Gender (% female) | 74.6 | 73.6 | 73 |
| Age (years), mean ± standard deviation | 42.1 ± 8.7 | 43.9 ± 7.9 | 43.8 ± 7.5 |
| BMI (kg/m2), mean ± standard deviation | 32.6 ± 4.3 | 32.6 ± 4.3 | 32.5 ± 4.1 |
| HbA1c (%), median (interquartile range) | 5.1 (4.9–5.4) | 5.2 (4.9–5.4) | 5.2 (5.0–5.5) |
| AUSDRISK analysis sample (n = 161) | |||
| N | 66 | 41 | 54 |
| Gender (% female) | 65.2 | 65.9 | 70.4 |
| Age (years), mean ± standard deviation | 42.3 ± 8.8 | 45.1 ± 7.9 | 44.4 ± 7.6 |
| BMI (kg/m2), mean ± standard deviation | 32.2 ± 4.0 | 31.5 ± 4.1 | 31.6 ± 4.2 |
| HbA1c (%), median (interquartile range) | 5.2 (5.0–5.4) | 5.1 (4.9–5.4) | 5.2 (4.9–5.5) |
The AUSDRISK scores were calculated at baseline and 12 months for the HealthTrack cohort and the data for each treatment arm are outlined in Table 2. AUSDRISK data was available at both time points on 161 participants. Across all randomised groups, 83 out of the 161 completers (51.5%) were considered at high risk of developing T2DM within the next 5 years at baseline. When comparing change in AUSDRISK continuous data between randomised groups there was a significant reduction in scores over time (F(1,158) = 65.2, p < 0.005) but no difference between treatment groups (F(2,158) = 0.048, p = 0.95). The change in the continuous measures of HbA1c (%) over time are summarised in Table 1. HbA1c data was only available at both time points on 139 participants. Across all randomised groups, 24 out of the 139 completers (17%) were considered at high risk of developing T2DM based on HbA1c criteria at baseline. There were no statistically significant differences between baseline and 12 months or between treatment groups in HbA1c levels (F(3.2,221) = 0.67, p = 0.58).
Table 2.
AUSDRISK and HbA1c (%) at baseline and 12 months in the 3 randomised arms of the HealthTrack study conducted on Illawarra Residents recruited in 2014 and 2015.
| Measure | Time point | Intervention + walnut |
Intervention |
Control |
|||
|---|---|---|---|---|---|---|---|
| Mean n = 66 |
SD | Mean n = 41 |
SD | Mean n = 54 |
SD | ||
| AUSDRISK score | Baseline | 11.85 | 4.74 | 12.10 | 5.86 | 11.52 | 4.46 |
| 12 months⁎ | 9.61 | 5.11 | 9.95 | 8.00 | 10.11 | 4.67 | |
| Measure | Time point | Intervention + walnut |
Intervention |
Control |
|||
|---|---|---|---|---|---|---|---|
| Median n = 59 |
IQR | Median n = 35 |
IQR | Median n = 45 |
IQR | ||
| HbA1c % | Baseline | 5.2 | 4.9–5.5 | 5.1 | 4.9–5.4 | 5.1 | 4.9–5.5 |
| 12 months | 5.1 | 4.9–5.4 | 5.1 | 4.9–5.4 | 5.2 | 5.0–5.4 | |
AUSDRISK score significantly reduced over time (p < 0.005) but not between treatment groups (p = 0.95). There was no significant change in HbA1c over time or between groups.
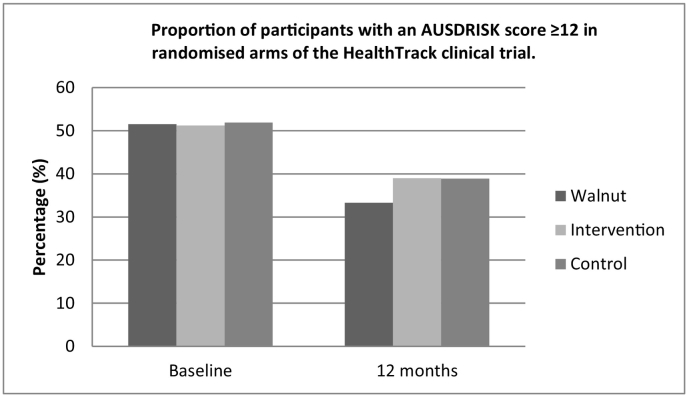
When the data was analysed by categorising the data as “high risk” or “not at high risk”, the proportion of participants in the IW group with an AUSDRISK score ≥ 12 was 51.5% (n = 34) at baseline and 33.3% (n = 22) at 12 months, which was a significant reduction over time (p = 0.005) (Fig. 1). The proportion of I and C groups with an AUSDRISK score ≥ 12 points was lower after the 12 month intervention, but this was borderline non-significant (I 51.2 (n = 21) vs 39.0% (n = 16); p = 0.063 and C 51.9 (n = 28) vs 38.9% (n = 21); p = 0.065) (Fig. 2).
Fig. 1.
Proportion of participants with an AUSDRISK score ≥ 12 in randomised arms of the HealthTrack clinical trial.
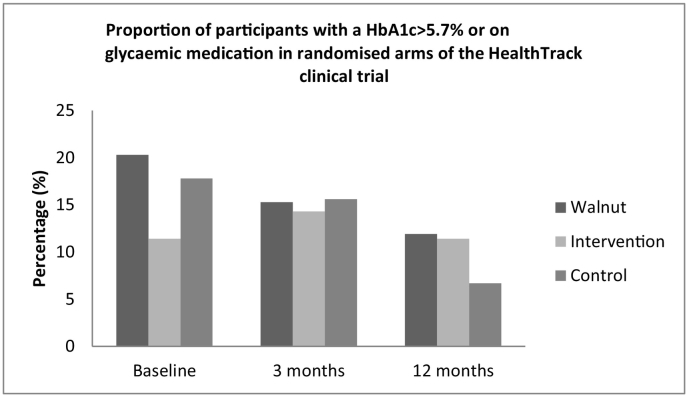
Fig. 2.
Proportion of participants with a HbA1c > 5.7% or on glycaemic medication in randomised arms of the HealthTrack clinical trial.
The proportion of participants categorised as “at risk” of T2DM using the HbA1c > 5.7% criteria are summarised in Fig. 2. The proportion of HbA1c considered “at risk” of T2DM was non-significantly reduced between baseline and 12 months (IW 20 (n = 12) vs 12% (n = 7), p = 0.06; I 11 (n = 4) vs 11% (n = 4), p = 0.99; C 18 (n = 8) vs 7% (n = 3), p = 0.06) in the randomised arms.
5. Discussion
This secondary analysis of data from the HealthTrack trial examined the utility of the AUSDRISK tool (and by comparison the routinely measured HbA1c) in an overweight/obese population undertaking lifestyle intervention. The AUSDRISK tool identified participants at high risk of developing T2DM within 5 years. The clinical utility of the tool was evident as it showed a significant reduction in the proportion at high risk of T2DM after 12 months intervention in the IW group, consistent with the greater weight loss observed in this group reported in the primary analysis of the trial (Tapsell et al., 2017).
Identification of individuals at increased risk of T2DM is vital in ensuring targeted treatment and management to reduce the global disease burden of diabetes. Overweight and obesity is associated with insulin resistance, and reducing weight and increasing exercise can improve insulin sensitivity (Espeland, 2007; Esposito et al., 2003). There is a need to identify which overweight/obese patients are at greatest risk of developing T2DM and which tool general practitioners could best use to identify these patients. Not all overweight or obese people will go on to develop chronic disease in the immediate future. In fact “metabolically healthy obesity” is a recent phenotype described in the literature and may account for between 10 and 40% of obese people (Phillips, 2016).
The problem that arises is there are multiple tools to assess risk of T2DM in an individual. The important aspects for screening tools at a population level are low cost, non-invasive and easy to access. HbA1c is considered the standard method to assess insulin resistance and is routinely measured, non-fasting, and inexpensive. It has been suggested that HbA1c can predict future onset of diabetes better then fasting blood glucose (Bonora and Tuomilehto, 2011). However, others suggest HbA1c is less sensitive, stating that HbA1c 6–6.4% missed 90% of individuals at risk of diabetes and 5.7–6.4% missed 75% (Lorenzo et al., 2010). In the HealthTrack cohort only 17.3% of participants had HbA1c > 5.7% or taking glycaemic medication at baseline. The proportion of participants identified as “at risk” using HbA1c was substantially lower than those identified using the AUSDRISK tool (51.6%). This may infer that the AUSDRISK score is a better tool to predict 5 year risk, and HbA1c may not be associated with a long-term forecast, but more importantly an assessment of current T2DM status.
The AUSDRISK tool was developed in 2008 by the Australian Government Department of Health and Ageing, and was validated in 2010 using the AusDiab study population (Chen et al., 2010). It was translated into a “patient-friendly” version by the Baker IDI Heart and Diabetes Institute (http://www.health.gov.au/internet/main/publishing.nsf/Content/diabetesRiskAssessmentTool). A systematic literature review identified 65 T2DM risk assessment tools worldwide, with only 10 reporting on their use as a screening tool (Dhippayom et al., 2014). Since AUSDRISK was validated there has only been two further studies assessing the use of this tool in practice. Pasco et al. (2010) applied AUSDRISK to a cohort of women >25 years old (n = 1494), and reported that while 6.6% had ≥12 points but only n = 28 (1.9%) developed diabetes during the 3 year follow-up (Pasco et al., 2010). This proportion at risk of T2DM is much lower than in our study however our cohort were all overweight or obese and included males and females. The publication by Pasco (2010) was a letter to the editor so no other data were provided on lifestyle or weight, making it difficult to compare results. Aguiar et al. (2015) applied AUSDRISK to 101 Australian men aged 18–65 years, with BMI 25–40 kg/m2 and no diabetes (Aguiar et al., 2015); 40% scored 12-15points, 24% scored 16–19 points, 37% scored ≥20 points (i.e. 100% considered “at risk”). These results are higher than assessed for our clinical cohort (51% scored >12 points). This difference may be due to our population being a mixed population of men (26%) and women (74%), as men have a greater incidence of insulin resistance than women. According to the AUSDRISK report an individual with an AUSDRISK score between 12 and 15 points has a 7% risk of developing T2DM, and between 16 and 19 points has a 14% risk, whereas an individual with an AUSDRISK score ≥ 20 points has a 33% chance of developing T2DM (Australian Government Department of Health and Ageing, 2010).
In Australia, the AUSDRISK tool attracts a Medicare rebate in patients aged 40–49 years who are at high risk of developing T2DM. However, only about 23% of general practitioners are aware of AUSDRISK and only 14% have actually used AUSDRISK (Wong et al., 2011). The utility of the AUSDRISK tool has now been demonstrated in overweight/obese participants in the HealthTrack study. There is value in referring “at risk” patients for multi-disciplinary lifestyle intervention before the onset of chronic disease. This analysis also reflected the primary weight loss finding of HealthTrack (Tapsell et al., 2017), where lifestyle intervention, whether nurse-led, or Accredited Practising Dietitian (APD) delivered with health coaching, can lead to reduced risk of T2DM over 12 months. However, an inter-disciplinary approach lead by an APD along with the provision of a healthy food (30 g daily walnuts) may reduce T2DM risk significantly more than both a healthy lifestyle intervention alone or usual care.
The improvement in diabetes risk observed in the walnut lifestyle intervention arm may simply reflect the greater weight loss in this group, but the types of foods consumed may be implicated. The addition of a healthy supplement into a lifestyle intervention providing additional beneficial affects has been demonstrated previously (Salas-Salvadó et al., 2008). Regular consumption of nuts has consistently shown to be associated with a reduced risk of cardiovascular disease (Kris-Etherton, 2014), however evidence of the relationship between nuts and T2DM has been mixed (Zhou et al., 2014). Compared with most nuts, which contain monounsaturated fatty acids, walnuts are a rich source of polyunsaturated fatty acids, particularly Ω3 fatty acid, α linolenic acid and Ω6 linoleic acid. Walnuts also provide several bioactive constituents that may exert anti-inflammatory effects, such as antioxidants (Kris-Etherton, 2014). Therefore this finding that the walnut supplemented group reduced T2DM risk significantly is not surprising, and it is supported by a recent large scale intervention involving the Mediterranean diet (Salas-Salvadó et al., 2008), which included greater consumption of nuts (50% of which were walnuts), fish and oils.
This study was limited primarily because it was a secondary analysis of trial data and so was not powered to detect significant differences in these endpoints. Additionally, the withdrawal rate over the 12 months of the lifestyle clinical trial was high, with only 161 participants providing the data required to calculate AUSDRISK at 12 months from an original 377 randomised. Power statistics could not be calculated as it was a secondary analysis of all available data. In addition, the use of different sample sizes between groups and between measurements reduces the sensitivity of these analyses. Even with those limitations we were able to observe a statistically significant reduction in T2DM risk in the walnut intervention. The lower number of participants in the HbA1c analyses may account for the non-significant findings. Additionally, the statistically significant change in AUSRISK in the IW group may be an artefact of the higher weight loss and greater vegetable consumption, factors included in the AUSDRISK model. However, improving these factors is also known clinically to reduce the incidence of T2DM, so the results are to be expected. Further prospective studies measuring AUSDRISK and HbA1c that include a 5 year follow-up would be required to confirm our findings.
It is important to recognise that this exploratory research considered the use of the AUSDRISK tool in a particular trial context, given that the trial reflected a clinical setting. Nonetheless we are unable to generalise beyond this context. Rather we are mobilising knowledge that suggests value in the AUSDRISK tool as a resource that goes beyond observations of HbA1c and draws attention to important lifestyle factors amenable to change. Translation of these findings to changes in clinical practice requires further exploration, which should consider strategies to translate this knowledge within the confines of the current health system, while also acknowledging the inherent complexity of the system (Holmes et al., 2017).
The AUSDRISK tool was able to identify a reduction in T2DM risk and differentiate between treatment groups in overweight/obese participants attending a lifestyle intervention over 12 months. Applying the AUSDRISK tool to calculate T2DM 5 year risk in overweight and obese people and implementing lifestyle change, may reduce the chronic disease burden of the obesity epidemic.
Acknowledgments
Acknowledgments
The authors would like to acknowledge Prof Marijka Batterham and Ms. Rhoda Ndanuko for the contribution to the statistics conducted in these analyses and the participants in the HealthTrack study for their time and commitment. Funding was provided by the Illawarra Health and Medical Research Institute and supplementary funding and walnuts samples from the California Walnut Commission. The funding bodies had no involvement in the study design, collection, analysis or interpretation of the data.
Conflict of interest statement
All authors declare the following competing interest. Linda Tapsell declares she has served as an unpaid member of the Californian Walnut Commission Steering Advisory Committee and has received grant funds for projects and consultancies relating to walnuts. Both Linda Tapsell and Elizabeth Neale have received a competitive grant from the International Nut and Dried Fruit Council, and have received funds for a consultancy with Nuts for Life. Allison Humphries has no competing interests.
References
- Aguiar E.J., Morgan P.J., Collins C.E. Characteristics of men classified at high-risk for type 2 diabetes mellitus using the AUSDRISK screening tool. Diabetes Res. Clin. Pract. 2015;108(1):45–54. doi: 10.1016/j.diabres.2015.01.017. [DOI] [PubMed] [Google Scholar]
- American Diabetes Association Standards of medical care in diabetes—2013. Diabetes Care. 2013;36(Supplement 1):S11–S66. doi: 10.2337/dc13-S011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Astrup A., Finer N. Redefining type 2 diabetes: ‘diabesity’ or ‘obesity dependent diabetes mellitus’? Obes. Rev. 2000;1(2):57–59. doi: 10.1046/j.1467-789x.2000.00013.x. [DOI] [PubMed] [Google Scholar]
- Australian Government Department of Health and Ageing . Australian Government Department of Health and Ageing; Canberra: 2010. The Australian Type 2 Diabetes Risk Assessment Tool (AUSDRISK) [Google Scholar]
- Australian Government of Health and Ageing . DOHA; Canberra: 2008. The Australian Type 2 Diabetes Risk Assessment Tool.https://www.health.gov.au/internet/main/publishing.nsf/Content/diabetesRiskAssessmentTool [Google Scholar]
- Bommer C., Heesemann E., Sagalova V. The global economic burden of diabetes in adults aged 20–79 years: a cost-of-illness study. Lancet Diabetes Endocrinol. 2017 doi: 10.1016/S2213-8587(17)30097-9. [DOI] [PubMed] [Google Scholar]
- Bonora E., Tuomilehto J. The pros and cons of diagnosing diabetes with A1C. Diabetes Care. 2011;34(Supplement 2):S184–S190. doi: 10.2337/dc11-s216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchmueller T.C., Johar M. Obesity and health expenditures: evidence from Australia. Econ. Hum. Biol. 2015;17:42–58. doi: 10.1016/j.ehb.2015.01.001. [DOI] [PubMed] [Google Scholar]
- Chen L., Magliano D.J., Balkau B. AUSDRISK: an Australian type 2 diabetes risk assessment tool based on demographic, lifestyle and simple anthropometric measures. Med. J. Aust. 2010;192(4):197. doi: 10.5694/j.1326-5377.2010.tb03507.x. [DOI] [PubMed] [Google Scholar]
- Colditz G.A., Willett W.C., Rotnitzky A. Weight gain as a risk factor for clinical diabetes mellitus in women. Ann. Intern. Med. 1995;122(7):481–486. doi: 10.7326/0003-4819-122-7-199504010-00001. [DOI] [PubMed] [Google Scholar]
- Craig C.L., Marshall A.L., Sjöström M. International physical activity questionnaire: 12-country reliability and validity. Med. Sci. Sports Exerc. 2003;35:1381–1395. doi: 10.1249/01.MSS.0000078924.61453.FB. [DOI] [PubMed] [Google Scholar]
- Delahanty L.M., Pan Q., Jablonski K.A. Effects of weight loss, weight cycling, and weight loss maintenance on diabetes incidence and change in cardiometabolic traits in the Diabetes Prevention Program. Diabetes Care. 2014;37(10):2738–2745. doi: 10.2337/dc14-0018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhippayom T., Chaiyakunapruk N., Krass I. How diabetes risk assessment tools are implemented in practice: a systematic review. Diabetes Res. Clin. Pract. 2014;104(3):329–342. doi: 10.1016/j.diabres.2014.01.008. [DOI] [PubMed] [Google Scholar]
- Espeland M. Reduction in weight and cardiovascular disease risk factors in individuals with type 2 diabetes: one-year results of the look AHEAD trial. Diabetes Care. 2007;30(6):1374–1383. doi: 10.2337/dc07-0048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esposito K., Pontillo A., Di Palo C. Effect of weight loss and lifestyle changes on vascular inflammatory markers in obese women: a randomized trial. JAMA. 2003;289(14):1799–1804. doi: 10.1001/jama.289.14.1799. [DOI] [PubMed] [Google Scholar]
- Hamman R.F., Wing R.R., Edelstein S.L. Effect of weight loss with lifestyle intervention on risk of diabetes. Diabetes Care. 2006;29(9):2102–2107. doi: 10.2337/dc06-0560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes B.J., Best A., Davies H. Mobilising knowledge in complex health systems: a call to action. Evid. Policy J. Res. Debate Pract. 2017;13(3):539–560. [Google Scholar]
- Kris-Etherton P.M. Walnuts decrease risk of cardiovascular disease: a summary of efficacy and biologic mechanisms. J. Nutr. 2014;144(4):547S–554S. doi: 10.3945/jn.113.182907. [DOI] [PubMed] [Google Scholar]
- Lorenzo C., Wagenknecht L.E., Hanley A.J. A1C between 5.7 and 6.4% as a marker for identifying pre-diabetes, insulin sensitivity and secretion, and cardiovascular risk factors. Diabetes Care. 2010;33(9):2104–2109. doi: 10.2337/dc10-0679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin G.S., Tapsell L.C., Denmeade S. Relative validity of a diet history interview in an intervention trial manipulating dietary fat in the management of type II diabetes mellitus. Prev. Med. 2003;36:420–428. doi: 10.1016/s0091-7435(02)00054-3. [DOI] [PubMed] [Google Scholar]
- NHMRC Department of Health and Ageing; 2013. Clinical practice guidelines for the management of overweight and obesity in adults, adolescents and children in Australia. http://www.health.gov.au/internet/main/publishing.nsf/Content/obesityguidelines-index.htm
- Pasco J.A., Henry M.J., Nicholson G.C. Evaluating AUSDRISK for predicting incident diabetes in an independent sample of women. Med. J. Aust. 2010;193(6):374. doi: 10.5694/j.1326-5377.2010.tb03955.x. [DOI] [PubMed] [Google Scholar]
- Phillips C.M. Metabolically healthy obesity: personalised and public health implications. Trends Endocrinol. Metab. 2016;27(4):189–191. doi: 10.1016/j.tem.2016.02.001. [DOI] [PubMed] [Google Scholar]
- Salas-Salvadó J., Fernández-Ballart J., Ros E. Effect of a Mediterranean diet supplemented with nuts on metabolic syndrome status: one-year results of the PREDIMED randomized trial. Arch. Intern. Med. 2008;168(22):2449–2458. doi: 10.1001/archinte.168.22.2449. [DOI] [PubMed] [Google Scholar]
- Tapsell L.C., Lonergan M., Martin A. Interdisciplinary lifestyle intervention for weight management in a community population (HealthTrack study): study design and baseline sample characteristics. Contemp. Clin. Trials. 2015;45:394–403. doi: 10.1016/j.cct.2015.10.008. [DOI] [PubMed] [Google Scholar]
- Tapsell L.C.L.M., Batterham M.J., Neale E.P. Effect of interdisciplinary care on weight loss: a randomised controlled trial. BMJ Open. 2017;7 doi: 10.1136/bmjopen-2016-014533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unwin N., Shaw J., Shorteed S.M. Impaired glucose tolerance and impaired fasting glycaemia: the current status on definition and intervention. Diabet. Med. 2002;19:708–723. doi: 10.1046/j.1464-5491.2002.00835.x. [DOI] [PubMed] [Google Scholar]
- Wong K.C., Brown A.M., Li S.C. AUSDRISK: application in general practice. Aust. Fam. Physician. 2011;40(7):524. [PubMed] [Google Scholar]
- Zhou D., Yu H., He F. Nut consumption in relation to cardiovascular disease risk and type 2 diabetes: a systematic review and meta-analysis of prospective studies. Am. J. Clin. Nutr. 2014;100(1):270–277. doi: 10.3945/ajcn.113.079152. [DOI] [PubMed] [Google Scholar]