Abstract
Background
The composition of the intestinal microbiota and its effect on septic shock patients in the intensive care unit (ICU) is unknown. In the present study we explored the hypothesis that bacterial diversity is decreased in septic shock patients and that this diversity may be improved by use of probiotics or enteral nutrition.
Material/Methods
A total of 15 stool samples were collected prospectively from septic shock patients in the ICU, while 15 samples from healthy subjects served as controls. Bacterial DNA was submitted for 16S rDNA gene sequencing. The relationship between intestinal microbiota and prognosis was evaluated.
Results
Significantly lower bacterial diversity was found in septic shock patients compared with healthy subjects (p<0.05). However, there was no difference in bacterial diversity in the presence or absence of probiotics (p=0.59), enteral nutrition (p=0.59), or in-hospital death (p=0.93) in septic shock patients. A high abundance of Proteobacteria and Fusobacteria was observed in most septic shock patients, whereas low abundance was observed in healthy subjects (mean relative proportion: 23.71% vs. 3.53%, p<0.05; 1.27% vs. 0.12%, p=0.59).
Conclusions
Bacterial diversity was decreased, and 1 or 2 rare bacterial species were overgrown in septic shock patients. Bacterial diversity was not improved by use of probiotics or enteral nutrition. The small sample size of our study limits the interpretation of results.
MeSH Keywords: Critical Illness; RNA, Ribosomal, 16S; Sepsis
Background
Sepsis is a complex syndrome caused by an uncontrolled systemic inflammatory response to infection. Septic shock is a subset of sepsis, with circulatory and cellular/metabolic dysfunction associated with a high risk of mortality [1]. Several interventions have been reported to improve the prognosis of sepsis; however, the clinical trials of various therapies, such as activated protein C [2], heparin [3], or carnitine [4], have failed to demonstrate consistent benefits; thus, a more personalized approach towards sepsis care is urgently needed. Until now, efforts to stratify septic patients have been hampered by the lack of more specific biomarkers. Genetic diversity defined as the carriage of single-nucleotide polymorphisms of genes accounts for some of the individual variability. Cytokines, soluble receptors, cell-surface molecules, and vasoactive hormones have all been evaluated for their diagnostic and prognostic utility in sepsis [5]. Evidence shows a single biomarker cannot successfully reflect the complex pathophysiologic processes that occur in sepsis, and more biomarkers are needed to achieve the goal of individualized management of sepsis [6]. The gut microbiota is a highly metabolically active human ‘organ’ that may be a novel focus of sepsis research.
The changes in the diversity and structure of the gut microbiota have been implicated in the pathogenesis of several metabolic and inflammatory conditions, including diabetes, obesity, and atherosclerosis [7]. In the case of intensive care unit (ICU) patients, Bacteroides and Firmicutes are the main categories of healthy intestinal flora, accounting for 89–98% of all involved bacteria [8]. The gut microbiome of patients with sepsis is often characterized by low diversity, low abundance of key commensal genera (such as Faecalibacterium, Blautia, and Ruminococcus), and is sometimes overgrowth by a single genus [9]. Gut microbiome dysbiosis can be attributed to the use of parenteral feeding, gastric acid inhibitory drugs, antibiotics, and mechanical ventilation [9]. However, previous studies have not focused on specific diseases [10,11].
Septic shock is an extreme case of infection, whereby alteration in the gut microbiota might be dramatically different from that in common illnesses. This phenomenon can seriously affect the prognosis of septic shock. Thus, studying the change in gut microbiota may provide a new and effective direction of treatment. Herein, we assume bacterial diversity is decreased in septic shock patients, and it may be improved by probiotics or enteral nutrition.
Material and Methods
Subjects
The present observational study of clinical trials prospectively and consecutively enrolled patients with septic shock from a Chinese teaching hospital from June 2016 through February 2017. The study protocol was approved by the local ethics committees, and written informed consent was obtained from all participants or their authorized personnel; the registered Chinese Clinical Trial Registry Platform identification number was ChiCTR-RPC-17013870 (chictr.org). This study strictly complies with the Helsinki Declaration. Patients were eligible if they fulfilled the following criteria: (1) Age ≥18 years and (2) Sepsis was defined as life-threatening organ dysfunction caused by a dysregulated host response to infection. Organ dysfunction can be assessed by an increase in the Sequential [Sepsis-related] Organ Failure Assessment (SOFA) score of 2 points or more [1]. Septic shock was defined as a subset of sepsis, which was identified by a vasopressor requirement to maintain a mean arterial pressure of 65 mmHg or greater and serum lactate level greater than 2 mmol/L (>18 mg/dL) in the absence of hypovolemia. APACHE II scores were recorded at the time of admission to the ICU. The first fecal sample was collected immediately after admission to the ICU. Fifteen non-smoking, healthy volunteers formed a control group
Intestinal microbiota analysis
We collected fresh stools on the first day after ICU admission and immediately froze them to −80°C. Enemas were used to obtain samples from patients with no normal bowel movement on the day of sampling. Samples were analyzed with 16S rDNA gene sequencing according to the method used by Lankelma et al. [12]. We used a bead-beating protocol to extract DNA according to the manufacturer’s instructions [13]. Libraries were prepared according to a previous standardized protocol [14]. Briefly, the libraries were constructed from the PCR amplicons of the V3–V4 region of the 16S rDNA gene generated using unique dual-index primers for each sample and TransGen AP221-02 (TransGen Biotech, Beijing, China), followed by sequencing on the Illumina MiSeq sequencing platform (Illumina, San Diego, CA, USA). Data were analyzed using QIIME and Mothur software.
Statistical analysis
The categorical variables were compared using the chi-square test or Fisher’s exact test, as appropriate. The Mann-Whitney U test was applied to continuous variables. The correlation analyses were conducted by Spearman’s rank correlation test. The rarefied alpha diversity (Shannon diversity index) and beta diversity (Nonmetric Multidimensional Scaling) were calculated in QIIME. The fold-change in relative abundance was calculated by dividing the mean relative abundance in each category. All data analyses were performed using SPSS (V.22; IBM, NY, USA). A p-value <0.05 was considered to be statistically significant.
Results
Patient characteristics
Fecal samples were collected from 15 septic shock patients (mean age 59 years; 80% males) on the first day after admission to the ICU, and 15 healthy volunteers (mean age 57 years; 73% males) served as controls. The characteristics of the patients are listed in Table 1. The age and sex ratios were matched (p=0.59 and 0.39). The primary diagnosis of patients across the group differed with respect to severe acute pancreatitis, acute exacerbation of chronic obstructive pulmonary disease, severe pneumonia, gastrointestinal perforation, and severe hepatitis. A major source of infection was lung (10/15) and enterocoelia (4/15). Enteral nutrition and probiotics were used in 11/15 and 8/15 subjects. Most patients were administered more than 3 types of antimicrobial drugs. Blood culture was positive in only 2 patients: Escherichia coli and Parabacteroides distasonis, respectively. On the other hand, sputum culture mainly consisted of Acinetobacter baumannii, Pseudomonas aeruginosa, Klebsiella pneumoniae, and Candida albicans. About one-third of the patients had died. Only 2/15 pathogens were identified in blood cultures in our study, which was quite low. It may due to high rate of antibiotics used before the patients were transferred from general ward. The antibiotics used for a long time before blood cultures was conducted would result in a low positive rate of blood culture.
Table 1.
Characteristics of all included patients.
| No. of patients | Age (y)/gender | Main diagnosis | APACHE II score | Source of infection | Enteral nutrition | Probiotics | Antibiotics use prior to enrollment (days) | Antibacterial | Anti-fungal | Blood culture | Sputum culture | Diarrhea | Death in hospital |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 75/M | Severe acute pancreatitis, septic shock | 27 | Lung | Yes | Yes | 5 | Biapenem | No | Escherichia coli | None | Yes | Yes |
| 2 | 35/M | Intestinal infection, septic shock | 25 | Enterocoelia | No | No | 7 | Biapenem; Teicoplanin | No | None | NA | No | No |
| 3 | 47/M | Serious hepatitis, septic shock | 23 | Lung | Yes | No | 3 | Vancomycin; Tigecycline | Fluconazole | None | Acinetobacter baumannii | Yes | Yes |
| 4 | 56/F | Immune thrombocytopenic purpura, septic shock | 27 | Lung | Yes | Yes | 3 | Meropein, Levofloxacin; Linazolamide | Voriconazole; Caspofungin | None | None | No | Yes |
| 5 | 51/M | Craniocerebral tumor; septic shock | 19 | Lung | Yes | No | 1 | Cefoperazone Sulbatan, Biapernan | No | None | NA | No | No |
| 6 | 69/M | Acute Exacerbation Chronic obstructive pulmonary disease, septic shock | 28 | Lung | Yes | Yes | 0 | Meropenem | Voriconazole | NA | NA | No | No |
| 7 | 89/M | Submandibular abscess, septic shock | 19 | Skin tissue | Yes | No | 5 | Imipenem and Cilastatin sodium, ornidazole, Linezolid | No | NA | None | No | Yes |
| 8 | 63/M | Silicosis, severe pneumonia, septic shock | 21 | Lung | No | No | 4 | Moxifloxacin, Penticillin, Etimicin | No | NA | Pseudomonas aeruginosa | No | No |
| 9 | 68/M | Severe pneumonia, septic shock | 22 | Lung | Yes | Yes | 7 | Minocycline, Levofloxacin, Linezolid | No | None | Klebsiella pneumoniae, Acinetobacter baumannii | No | No |
| 10 | 61/M | Esophagus cancer, Severe pneumonia, septic shock | 23 | Lung | Yes | Yes | 3 | Piperacillin | Voriconazole | NA | Klebsiella pneumoniae Acinetobacter baumannii, Candida albicans | No | No |
| 11 | 53/M | Gastrointestinal perforation, septic shock | 24 | Enterocoelia | No | Yes | 2 | Mezlocillin, Vancomycin, Levofloxacin, Ornidazole | No | Parabacteroides distasonis | NA | No | No |
| 12 | 51/F | Multiple organ failure, septic shock | 25 | Lung | Yes | No | 1 | Cotrimoxazole | Voriconazole | None | Pseudomonas aeruginosa, Candida albicans | No | No |
| 13 | 46/M | Aortic dissection, septic shock | 29 | Lung | Yes | Yes | 0 | Meropenem, Levofloxacin, Mezlocillin | No | NA | Klebsiella pneumoniae, Acinetobacter baumannii | No | No |
| 14 | 64/F | Serious hepatitis, septic shock | 29 | Enterocoelia | Yes | Yes | 1 | Vancomycin, Biapenem | No | None | Acinetobacter baumannii | No | Yes |
| 15 | 64/F | Gastrointestinal perforation, septic shock | 20 | Enterocoelia | No | No | 2 | Biapenem, Teicoplanin | Fluconazole, Caspofungin | None | Klebsiella pneumoniae | No | No |
M – Male; F – Female; NA – not available.
Characteristics of bacterial composition in the stool
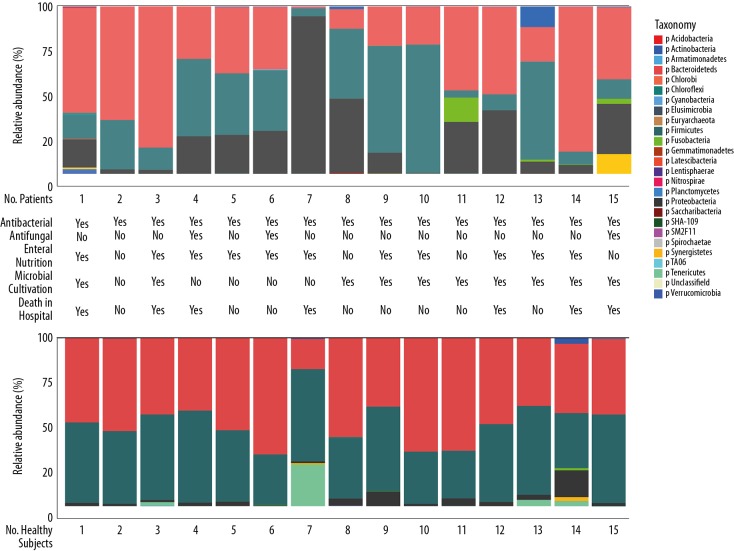
In healthy subjects, the microbial composition of fecal samples was Bacteroidetes and Firmicutes (Figure 1) at the phylum level (mean relative proportion: 49.85% vs. 43.54%). The abundance of Proteobacteria in these samples was <2% in most healthy subjects (mean 3.52%), while Tenericutes was found in 4 healthy subjects (mean 2.30%). Compared to healthy subjects, the stool microbiota composition of septic shock patients showed a remarkable individual variation. Although Bacteroidetes and Firmicutes were the primary bacteria, the mean proportion of both decreased (42.38% vs. 30.27%). A significantly higher proportion of Proteobacteria was found in sepsis patients as compared to healthy subjects (mean relative proportion: 23.71% vs. 3.53%, p<0.05); similarly, a higher proportion of Fusobacteria (mean relative proportion: 1.27% vs. 0.12%, p=0.59) did not differ significantly. Furthermore, patient no. 7 demonstrated a high proportion of Proteobacteria (94%) in the stool sample and was eventually died in the hospital. Another patient, no. 13, presented 12.23% Actinobacteria. This phylum of Gram-positive bacteria, especially Streptomyces spp., was identified as the producer of several bioactive metabolites that are beneficial to humans. Actinobacteria-derived antibiotics included aminoglycosides, anthracyclines, chloramphenicol, macrolide, and tetracyclines.
Figure 1.
Microbial composition of fecal samples at the phylum level in septic shock patients and healthy subjects. Fecal samples were collected at the same time from patients and healthy subjects. Total bacterial 16S rDNA was isolated and sequenced to investigate the bacterial composition of these samples. Each bar represents the microbiota composition of a patient or healthy subject (number indicated at the bottom of each bar) at the phylum level. Data are presented as the percentage of total 16S rDNA reads in each sample and colors indicate different phyla. Table 1 summarizes the characteristics of each included patient.
At the genus level, the individual variation in the stool composition was enhanced. A total of 29 types of bacteria were classified, while a majority remained unclassified (Figure 2). Bacteroides (30%) were found in healthy subjects, and other types of bacteria were found in different persons. A single bacterial genus was overgrown in 3 septic shock patients (no. 2, 6, and 9), making up >80% of the gut microbiota in these 3 patients.
Figure 2.
Microbial composition of fecal samples at the genus level in septic shock patients and healthy subjects. Each bar represents the microbiota composition of an individual patient or healthy subject (number indicated at the bottom of each bar) at the phylum level. Data are presented as the percentage of total 16S rDNA reads in each sample and colors indicate different genus. “H” represents “healthy subjects,” “P” represents “patients.”
Decreased intra-individual bacterial diversity in septic patients
A significant lower bacterial diversity was found in septic shock patients compared with healthy subjects (p<0.05, Figure 3). Herein, we explored the potential effect of using probiotics and enteral nutrition on stool bacterial diversity, as well as the association between bacterial diversity and mortality. We found no difference between septic shock patients with or without the usage of probiotics (p=0.59) and enteral nutrition (p=0.59, Figure 3). Similarly, no difference was observed in bacterial diversity between the dead or alive patients in the hospital (p=0.93, Figure 3). The overall microbial composition of patients with septic shock showed a clear shift as compared to the healthy subjects in measures of beta diversity (a nonmetric multidimensional scaling method, Figure 4).
Figure 3.
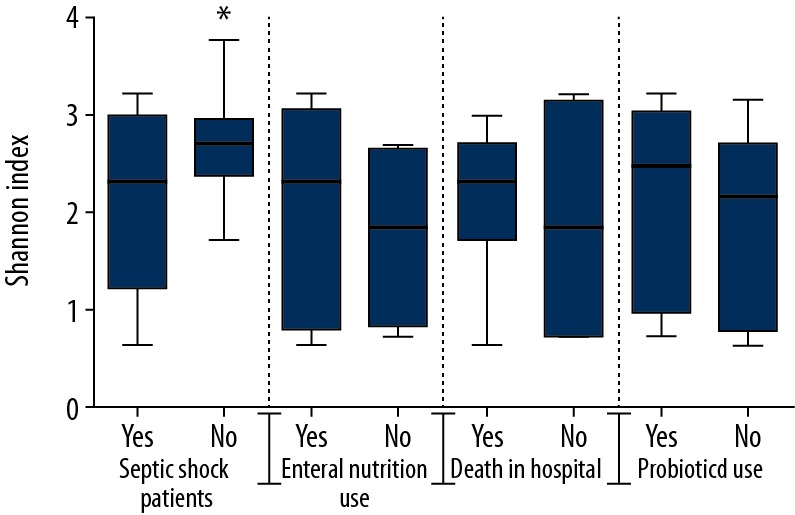
Intestinal microbiota diversity was decreased in septic shock patients. Microbiota diversity, presented as the Shannon index, was calculated from 15 healthy subjects and 15 septic shock patients. Data are presented as box-whisker plot. * p<0.05. Bacterial diversity was decreased in septic shock patients compared with healthy subjects. No difference in bacterial diversity was found in septic shock patients with respect to probiotics, enteral nutrition, or in-hospital death.
Figure 4.
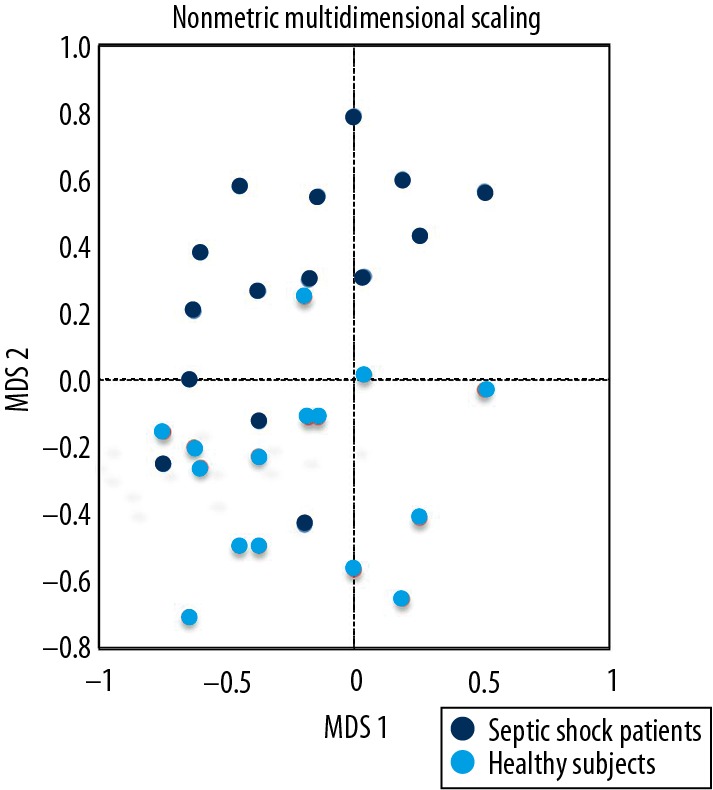
The overall microbial composition of patients with septic shock, showing a definite shift as compared to healthy subjects (nonmetric multidimensional scaling method). Each point represents a sample, with the same color representing the same sample group. The horizontal axis represents the first dimension and the vertical axis represents the second dimension. A shorter distance between dots indicates similar composition of samples.
Discussion
In this observational clinical trial, we identified large differences in the gut microbiota of patients with septic shock as compared to the controls. In septic shock patients, bacterial diversity was decreased; however, Proteobacteria and Fusobacteria showed an overgrowth. We found no effect of probiotics and enteral nutrition on gut microbiota.
The microbiome plays a major role in health and disease, including diabetes, obesity, atherosclerosis, and inflammatory bowel disease. However, little is known about the composition of the intestinal microbiota and its effect on septic shock patients. An observational study by Lankelma et al. [10] collected stool samples from 34 ICU patients and found that more than half of the patients had significantly reduced intestinal diversity. They also found no correlation between microbiota diversity and prognosis. Moreover, the Shannon index in all healthy subjects was > 4, while that in the current study was <4. This phenomenon might be due to the difference in race and dietary habits between the Eastern and Western hemispheres. However, that study started in 2012, so sepsis was not defined according to the most recently proposed criteria, including organ failure. The present study, which included patients meeting the Third International Consensus definitions for sepsis and septic shock, demonstrated similar results, including reduced microbiota diversity and overgrowth of 1 or 2 bacterial species. A study by Ojima et al. [11] evaluated the mechanism underlying the gut microbiota change in ICU patients. They collected fecal samples on days 1–2, 2–4, 5–8, and 7–10 after admission. Subsequent dynamic changes in the percentages of Bacteroidetes and Firmicutes were significant; however, the result was limited by the small sample size (12 patients), and the analysis of gut microbiota was limited to the phylum level. In another small-sample study by Zaborin et al. [15], 14 selected patients, during their prolonged stay in the ICU, were analyzed and local environmental conditions in the gut were found to direct the pathogen communities to adapt to either a commensal or pathogenic style. McDonald et al. [16] collected fecal, oral, and skin samples from 115 mixed ICU patients, and found that critical illness was associated with the loss of normal, “health promoting” bacteria, allowing overgrowth of disease-promoting pathogenic bacteria (dysbiosis), which in turn makes patients susceptible to hospital-acquired infections, sepsis, and organ failure. The limitation was that the study included ICU patients with varying diagnosis types and ages, which contributed to increased variability in the data.
Sepsis is the major cause of mortality from any infectious disease worldwide. Precision medicine provides an opportunity to improve the outcomes of patients with sepsis [17]. A key to the precision medicine approach is the accuracy of biomarkers to stratify patients into subgroups based on specific pathophysiological conditions [18]. Hence, biomarkers can be the key to personalized medicine. The complex pathophysiology of sepsis makes it unlikely that a single biomarker can provide sufficient information about the derailment of the host response [19]. Biomarkers have included Procalcitonin, C-reactive protein (CRP), lipopolysaccharide binding protein, and IL-6 [20], but none of these is specific enough to be used alone in the management of critically ill patients. Some biomarkers were designed, based on the gut microbiota, to predict the clinical outcomes, such as bacterial diversity and abundance of bacteria [10]. Consistent results were not found in these studies. However, research on new biomarkers based on the gut microbiota is still in the early stages and more studies are needed.
In the present study, using probiotics and enteral nutrition was found to have no effect on bacterial diversity; however, research on stool community structure is challenging. Furthermore, the present study was a non-randomized, controlled study, and the effect of probiotics or enteral nutrition on septic shock needs further verification.
To the best of our knowledge, this is the first clinical study evaluating the gut microbiota composition in septic shock patients and its association with clinical outcome parameters based on 16S rRNA gene sequencing technology. Most previous studies were focused on ICU patients, but not on specific diseases [10,11,15].
An important limitation of this study is its small sample size, as it was conducted in a single center and the small sample size limits the interpretation of results. A fecal sampling was collected once for each patient, and the following dynamic changes could not be studied. Additionally, stool samples cannot provide information about the upper gastrointestinal tract and cannot satisfactorily reflect the condition of the surface of the intestinal mucosa.
Conclusions
This study showed that bacterial diversity was decreased, and 1 or 2 rare bacterial species were overgrown in septic shock patients. Bacterial diversity was not be improved by use of probiotics or enteral nutrition. The small sample size of our study limits interpretation of results. A larger-sample study is needed to verify the relationship between intestinal microbiota, probiotics, enteral nutrition, and mortality.
Footnotes
Source of support: This study was supported by the China Postdoctoral Science Foundation (No. 2015M582058)
Conflict of interests
None.
References
- 1.Singer M, Deutschman CS, Seymour CW, et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3) JAMA. 2016;315(8):801–10. doi: 10.1001/jama.2016.0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ranieri VM, Thompson BT, Barie PS, et al. Drotrecogin alfa (activated) in adults with septic shock. N Engl J Med. 2012;366(22):2055–64. doi: 10.1056/NEJMoa1202290. [DOI] [PubMed] [Google Scholar]
- 3.Zarychanski R, Abou-Setta AM, Kanji S, et al. The efficacy and safety of heparin in patients with sepsis: a systematic review and metaanalysis. Crit Care Med. 2015;43(3):511–18. doi: 10.1097/CCM.0000000000000763. [DOI] [PubMed] [Google Scholar]
- 4.Puskarich MA, Kline JA, Krabill V, et al. Preliminary safety and efficacy of L-carnitine infusion for the treatment of vasopressor-dependent septic shock: A randomized control trial. J Parenter Enteral Nutr. 2014;38(6):736–43. doi: 10.1177/0148607113495414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Christaki E, Giamarellos-Bourboulis EJ. The beginning of personalized medicine in sepsis: Small steps to a bright future. Clin Genet. 2014;86(1):56–61. doi: 10.1111/cge.12368. [DOI] [PubMed] [Google Scholar]
- 6.Gibot S, Bene MC, Noel R, et al. Combination biomarkers to diagnose sepsis in the critically ill patient. Am J Respir Crit Care Med. 2012;186(1):65–71. doi: 10.1164/rccm.201201-0037OC. [DOI] [PubMed] [Google Scholar]
- 7.Giongo A, Gano KA, Crabb DB, et al. Toward defining the autoimmune microbiome for type 1 diabetes. ISME J. 2011;5(1):82–91. doi: 10.1038/ismej.2010.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jacobs MC, Haak BW, Hugenholtz F, Wiersinga WJ. Gut microbiota and host defense in critical illness. Curr Opin Cirt Care. 2017;23(4):257–63. doi: 10.1097/MCC.0000000000000424. [DOI] [PubMed] [Google Scholar]
- 9.Haak BW, Wiersinga WJ. The role of the gut microbiota in sepsis. Lancet Gastroenterol Hepatol. 2017;2(2):135–43. doi: 10.1016/S2468-1253(16)30119-4. [DOI] [PubMed] [Google Scholar]
- 10.Lankelma JM, van Vught LA, Belzer C, et al. Critically ill patients demonstrate large interpersonal variation in intestinal microbiota dysregulation: a pilot study. Intensive Care Med. 2017;43(1):59–68. doi: 10.1007/s00134-016-4613-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ojima M, Motooka D, Shimizu K, et al. Metagenomic analysis reveals dynamic changes of whole gut microbiota in the acute phase of Intensive Care Unit patients. Dig Dis Sci. 2016;61(6):1628–34. doi: 10.1007/s10620-015-4011-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lankelma JM, Cranendonk DR, Belzer C, et al. Antibiotic-induced gut microbiota disruption during human endotoxemia: A randomised controlled study. Gut. 2017;66(9):1623–30. doi: 10.1136/gutjnl-2016-312132. [DOI] [PubMed] [Google Scholar]
- 13.Salonen A, Nikkila J, Jalanka-Tuovinen J, et al. Comparative analysis of fecal DNA extraction methods with phylogenetic microarray: Effective recovery of bacterial and archaeal DNA using mechanical cell lysis. J Microbiol Methods. 2010;81(2):127–34. doi: 10.1016/j.mimet.2010.02.007. [DOI] [PubMed] [Google Scholar]
- 14.Kozich JJ, Westcott SL, Baxter NT, et al. Development of a dual-index sequencing strategy and curation pipeline for analyzing amplicon sequence data on the MiSeq Illumina sequencing platform. Appl Environ Microbiol. 2013;79(17):5112–20. doi: 10.1128/AEM.01043-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zaborin A, Smith D, Garfield K, et al. Membership and behavior of ultra-low-diversity pathogen communities present in the gut of humans during prolonged critical illness. MBio. 2014;5(5):e1314–61. doi: 10.1128/mBio.01361-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McDonald D, Ackermann G, Khailova L, et al. Extreme dysbiosis of the microbiome in critical illness. mSphere. 2016;1(4) doi: 10.1128/mSphere.00199-16. pii: e00199-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Calfee CS. Opening the debate on the new sepsis definition. Precision medicine: An opportunity to improve outcomes of patients with sepsis. Am J Respir Crit Care Med. 2016;194(2):137–39. doi: 10.1164/rccm.201604-0697ED. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.van der Poll T, van de Veerdonk FL, Scicluna BP, et al. The immunopathology of sepsis and potential therapeutic targets. Nat Rev Immunol. 2017;17(7):407–20. doi: 10.1038/nri.2017.36. [DOI] [PubMed] [Google Scholar]
- 19.Gibot S, Bene MC, Noel R, et al. Combination biomarkers to diagnose sepsis in the critically ill patient. Am J Respir Crit Care Med. 2012;186(1):65–71. doi: 10.1164/rccm.201201-0037OC. [DOI] [PubMed] [Google Scholar]
- 20.Pierrakos C, Vincent JL. Sepsis biomarkers: A review. Crit Care. 2010;14(1):R15. doi: 10.1186/cc8872. [DOI] [PMC free article] [PubMed] [Google Scholar]