Abstract
Isovaleric acidemia (IVA) is an organic acid disease caused by a deficiency of isovaleryl-CoA dehydrogenase. Deficiency of this enzyme leads to accumulation of organic acids, such as isovalerylcarnitine and isovalerylglycine. The proposed IVA treatments include leucine restriction and l-carnitine and/or glycine supplementation, which convert isovaleric acid into non-toxic isovalerylcarnitine and isovalerylglycine, respectively. We examined the therapeutic response using the leucine load test and performed a 10-year follow-up in the patient.
Methods
We evaluated the patient with IVA beginning at 5 years of age, when he presented with a mild to intermediate metabolic phenotype. Ammonia, free carnitine, isovalerylcarnitine, and isovalerylglycine were analyzed in the urine and blood after a meal consisting of 1600 mg leucine with glycine alone (250 mg/kg/day), l-carnitine alone (100 mg/kg/day), or both glycine and l-carnitine for four days each.
Results
(Leucine load test) Three hours after the meal, serum ammonia levels increased most dramatically with glycine treatment alone, then with both in combination, and least with l-carnitine alone. Urinary isovalerylglycine levels increased 2-fold more with glycine supplementation than those following supplementation with both agents or with l-carnitine alone. Treatment with both agents resulted in a gradual increase in urinary acylcarnitine levels during the 6-h period following the leucine load, reaching concentrations comparable to those observed with l-carnitine alone. (Clinical course) After initiation of both glycine (200 mg/kg/day) and l-carnitine (100 mg/kg/day) supplementation at 5 years of age, doses were gradually reduced to 111.7 mg/kg/day and 55.8 mg/kg/day, respectively, at 15 years of age. His mind and body had developed without any sequelae.
Discussion
We concluded that l-carnitine conjugated isovaleric acid earlier than glycine. Additionally, during the 10-year follow-up period, the patient displayed no clinical deterioration.
Keywords: Isovaleric acidemia, Glycine, l-carnitine, Isovalerylcarnitine, Isovalerylglycine
1. Introduction
Isovaleric acidemia (IVA; MIM 243500) is an autosomal recessive disorder of organic acid metabolism caused by a deficiency of isovaleryl-CoA dehydrogenase (IVD). IVD catalyzes the conversion of isovaleryl-CoA to 3-methylcrotonyl-CoA during leucine catabolism [1]. IVD deficiency results in an accumulation of derivative organic acids, including isovaleric acid, 3-hydroxyisovaleric acid, isovaleryl (C5)-carnitine (IVC), and isovalerylglycine (IVG) [2], [3]. Clinical and laboratory findings in patients with IVA include episodic vomiting, metabolic acidosis, ketosis, hyperammonemia, dehydration, lethargy, distinctive “odor of sweaty feet” because of isovaleric acid buildup, and mental retardation. IVA manifestation varies widely. The severe neonatal onset form presents with early onset of metabolic decompensation. The chronic intermittent form with onset in infancy or childhood presents with developmental delays and/or failure to thrive. Finally, the asymptomatic form is identified through newborn screening of blood spots by tandem mass spectrometry [4], [5].
The proposed IVA treatments include dietary leucine restriction and dietary supplementation with l-carnitine and/or glycine to conjugate isovaleric acid, resulting in its conversion into non-toxic IVC and IVG that are subsequently excreted in urine [6], [7]. As the glycine dose increases in patients with IVA, IVG excretion in the urine concomitantly increases. However, one study reported that an increase in the glycine dose from 300 to 600 mg/kg/day led to a decrease in IVG excretion [8]. Thus, they suggested that glycine overdose might inhibit glycine conjugation by glycine N-acyltransferase (E.C. 2.3.1.13). Fries et al. [9] determined that combined glycine (250 mg/kg/day) and l-carnitine (100 mg/kg/day) therapy maximally increased IVA and IVG excretion in 12-h urine specimens collected overnight after a 2000 mg leucine load in an 8-year-old patient with IVA. Previous studies have indicated that combined therapy with carnitine and glycine maximized the total excretion of isovaleryl-CoA conjugates, but the clinical benefits of combined versus single therapy have not been established in controlled studies [7], [10], [11]. The relative merits of the two therapies either singly or in combination in patients with IVA remain unclear. Concern has been raised about potential glycine toxicity, though no reports of such an occurrence have been published.
We examined the acute biochemical response, including serum ammonia levels, to a leucine load test following supplementation with glycine, l-carnitine, or both glycine and l-carnitine in combination. Additionally, we followed the patient with IVA for ten years under stable conditions.
2. Methods and clinical course
2.1. Clinical course (until IVA diagnosis)
The patient had an uneventful delivery after a 39 week-gestation period with a birth weight of 3088 g. Ten days after birth, he presented with anemia and thrombocytopenia. At 3 years of age, mild mental retardation was identified. As he exhibited symptoms, such as repetitive vomiting with hyperammonemia, he was diagnosed with IVA at 5 years and 6 months of age. His height was 119 cm (+ 1.8 SD), and weight was 19.2 kg (mean). Tsumori's Mental Development Test identified mild mental retardation (DQ = 69). Molecular analysis of the IVD gene revealed a compound heterozygosity of missense mutations consisting of p.Y403C and p.E411K [12]. The biochemical phenotype of the patient was metabolically mild or intermediate type, based on a C5 acylcarnitine concentration of 0.8–6 mmol/L and a urine isovalerylglycine concentration of 15–195 mmol/mol creatinine [3], [4].
2.2. Leucine load test
We examined the initial biochemical response for 6 h following a leucine load in the patient with IVA. We used a meal containing 1600 mg of leucine. At diagnosis, the patient typically consumed meals containing 1800 mg of leucine. He received a supplement of glycine (250 mg/kg/day), l-carnitine (100 mg/kg/day), or both glycine and l-carnitine for four days each. Following the 1600 mg leucine meal, we measured ammonia, free carnitine, acylcarnitine, IVC, and IVG levels in dry blood spots and urine samples for 6 h. The study was performed in accordance with the standards of the Ethics Committee in the Ryukyus Graduate School of Medicine (Okinawa, Japan).
2.3. Clinical course (following IVA diagnosis)
Following the leucine load test, the patient received both glycine (200 mg/kg/day) and l-carnitine (100 mg/kg/day) supplementation and had been followed carefully with an almost constant dose of glycine and l-carnitine for ten years. Magnetic resonance imaging (MRI) of the brain and echocardiography were examined. We measured serum ammonia, serum glycine, free carnitine, and IVG levels from dry blood spots 3 h after lunch during an outpatient visit. We analyzed the relationship between ammonia and glycine, ammonia and free carnitine, and ammonia and IVC levels using Pearson's correlation coefficient in Microsoft Excel to determine the effective quantity of glycine and l-carnitine.
3. Results
3.1. Leucine load test
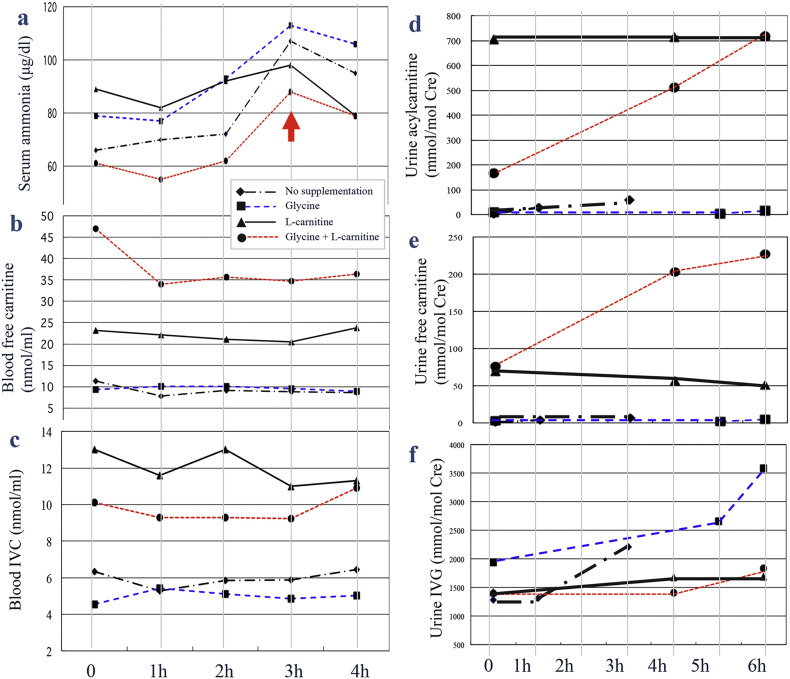
Three hours after the test meal, serum ammonia levels displayed the smallest increase with l-carnitine supplementation only, followed by both agents in combination, and the largest increase with glycine supplementation only (Fig. 1a and Supplementary Fig. 1). Blood free carnitine levels were highest following supplementation with both agents (Fig. 1b). Initially, blood IVC levels were the highest following supplementation with l-carnitine only; however, 4 h after the leucine load, IVC levels following supplementation with l-carnitine only or both agents were comparable (Fig. 1c). The increase in urinary acylcarnitine was the highest following l-carnitine supplementation. Supplementation with both agents, however, resulted in a gradual increase over 6 h post-leucine load to concentrations comparable to those observed with l-carnitine alone (Fig. 1d). Treatment with a combination of both agents was associated with a dramatic increase in urinary free carnitine levels (Fig. 1e). The increase in urinary IVG levels following glycine supplementation was twice that of both agents in combination and of l-carnitine alone (Fig. 1f).
Fig. 1.
Biochemical data following a leucine load.
For 6 h post-leucine load, we analyzed levels of (a) serum ammonia (normal range: 36–86 μg/dL), (b) blood free carnitine, (c) blood isovalerylcarnitine (IVC), (d) urine acylcarnitine, (e) urine free carnitine, and (f) urine isovalerylglycine (IVG). The arrow indicates the most dramatic increase in serum ammonia following the leucine load.
3.2. Clinical course (following IVA diagnosis)
Following initiation of treatment with both glycine (200 mg/kg/day) and l-carnitine (100 mg/kg/day) in combination with protein restriction to 50 g per day (1.6 g/kg) at five years and 7 months of age, daytime sleepiness was reduced. He had been infected with the influenza virus three times notwithstanding vaccination. During each hospitalization, he was administered l-carnitine (100 mg/kg/day) intravenously and recovered without metabolic acidosis, hyperammonemia, or any sequelae. An MRI of the brain presented no abnormal findings at 6, 10, or 14 years of age. An echocardiogram exhibited normal findings. At 13 years of age, an examination of the Wechsler Intelligence Scale for Children (WISC) III revealed mild mental retardation (IQ = 70). The growth curve indicated that the patient was in the normal range for both height and weight (Supplementary Fig. 2). At 15 years and 7 months of age, his height was 179.6 cm (+ 1.85 SD), and his weight was 58.2 kg (− 0.2 SD). A WISC IV examination revealed mild mental retardation (Full Scale IQ, 66; Verbal Comprehension Index, 66; Perceptual Reasoning Index, 74; Working Memory Index, 73; and Processing Speed Index, 76). He was a student of a technical high school. During the ten-year follow-up period, the serum ammonia concentrations 3 h after a meal at the time of outpatient consultation were within an almost normal range, except when he forgot to take both agents following a meal. There were no significant correlations (p > 0.05) between ammonia and glycine, ammonia and free carnitine, or ammonia and IVC levels (Supplementary Fig. 3).
4. Discussion
We described clinical observations following an initial leucine load test and during a 10-year follow-up period in a patient with IVA that was treated with both glycine and l-carnitine. We estimated the patient's ammonia levels for 6 h following a leucine load and during the basal state before the leucine load following supplementation with glycine, l-carnitine, or both glycine and l-carnitine for four days each. The patient's baseline ammonia levels might vary as a result of the amount of isovaleric excretion caused by these supplements over the 4-day treatment period. During the 6 h following a leucine load, changes in ammonia levels might result from an early reaction to these supplements.
Glycine conjugation of toxic acyl-CoAs derived from organic acids, such as IVA, by glycine N-acyltransferase in mitochondria is an important metabolic pathway responsible for maintaining mitochondrial energy metabolism [13]. Carnitine acyltransferases are important enzymes for energy homeostasis and fat metabolism. When acyl-CoA accumulates, it may become a substrate for carnitine acyltransferases, resulting in the formation of acyl-carnitine that can be excreted in the urine [13], [14], [15]. Secondary hyperammonemia is presumed to be due to inhibition of N-acetylglutamate synthetase by isovaleryl-CoA and/or intracellular depletion of acetyl-CoA, leading to reduced N-acetylglutamate synthesis and impairment of the urea cycle [16], [17]. At a concentration of 3 mM, propionyl-CoA, methylmalonyl-CoA, and isovaleryl-CoA inhibit N-acetylglutamate synthetase in rat liver by 73%, 28%, and 70%, respectively [16]. We suggest that serum ammonia is a key parameter, in addition to IVG and IVC, during a leucine load test. In previous studies, leucine load tests have been performed using 2 g of leucine in patients with IVA between 4 and 5 years of age. In our study, we used 1600 mg of leucine, which was likely safe for the patient, as his typical intake at IVA diagnosis was 1800 mg. We performed a leucine load test following glycine (250 mg/kg/day) only, l-carnitine (100 mg/kg/day) only, or both glycine and l-carnitine supplementation. We observed that before the leucine load, serum ammonia levels were lowest following supplementation with both agents. The lowest baseline ammonia level might result from the highest isovaleric excretion following treatment with both agents for four days. In contrast, after the leucine load, serum ammonia levels were lowest following l-carnitine supplementation alone. It is unclear whether our patient response is out of proportion. However, the amount of isovaleric excretion and the speed of conjugation of IVA and l-carnitine or glycine might be one possible explanation for our results. We hypothesized that l-carnitine would conjugate earlier than glycine in patients with IVA, because serum ammonia levels displayed the greatest reduction following l-carnitine supplementation alone. During acute metabolic decompensation l-carnitine treatment might reduce free isovaleric acid levels faster, thereby more efficiently decreasing serum ammonia levels. During the stable condition before a leucine load test, supplementation with both agents in combination resulted in the lowest serum ammonia levels. Leucine would conjugate slowly and might gradually reduce the accumulation of IVA in the body.
During routine follow-up examinations, there is no established laboratory marker for monitoring therapeutic control or disease state [3]. Body measurements are key parameters to monitor on a routine basis, and the growth curve of the patient was age-appropriate. Metabolic decompensation was not observed in our patient either acutely or chronically over ten years. During the initial medical examination of our patient with IVA, serum glycine levels were not deficient. Glycine is not an essential amino acid and is produced from biological pathways, including production from threonine by threonine aldolase. We considered an effective dose of glycine and l-carnitine might be lower than the initial doses of both agents, but we could not determine the most appropriate glycine and l-carnitine doses based on blood concentrations from our sampling dates over 10 years. Our information might be useful for determining glycine and l-carnitine doses depending on age in patients with IVA. The heterogeneous presentation in individuals with IVA and the appropriate clinical management is still a matter of debate [3], [18], [19].
There are currently insufficient data to determine the appropriate doses of glycine and l-carnitine individually, and further investigation is warranted.
Compliance with ethics
The study (reception number: H18.3-8) was performed in accordance with the standards of the Ethics Committee at the Ryukyus Graduate School of Medicine (Okinawa, Japan).
Conflict of interest
All authors contributed to the original article have no conflicts of interest with any other party. Yasutsugu Chinen, Sadao Nakamura, Kunihito Tamashiro, Osamu Sakamoto, Kyoko Tashiro, and Takahiro Inokuchi declare that they have no conflicts of interest.
Informed consent
Informed consent for human subjects was obtained by Dr. Yasutsugu Chinen.
Animal rights
Not applicable.
Contributions to the project
Yasutsugu Chinen was the Principal Investigator of this project and contributed to the conception, analysis of data, and reporting of the work described in the article. He and his team performed patient follow-up.
Sadao Nakamura contributed to clinical management and neurological assessment.
Kunihito Tamashiro contributed to clinical management and neurological assessment.
Osamu Sakamoto contributed to DNA analysis of the enzyme isovaleryl-CoA dehydrogenase.
Kyoko Tashiro contributed to the laboratory analysis of gas chromatography tandem mass spectrometry.
Takahiro Inokuchi provided advice on data analysis and contributed to the laboratory analysis of gas chromatography tandem mass spectrometry.
Funding
This research did not receive any specific grants from funding agencies in the public, commercial, or not-for-profit sectors.
Acknowledgements
We thank Ryuji Tasaki of the Chemo-Sero-therapeutic Research Institute (Kumamoto, Japan) for analyses of tandem mass spectrometry. We are indebted to the patient, his parents, nurses, and physicians who supported this study.
Footnotes
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.ymgmr.2017.03.002.
Appendix A. Supplementary data
Supplementary Fig. 1 Serum ammonia levels following a leucine load.
Supplementary Fig. 2 The growth chart of the patient. The arrows indicate initiation of supplementation with both l-carnitine and glycine in combination.
Supplementary Fig. 3 Correlations between ammonia levels and glycine, free carnitine, or IVC levels. The relationships between (a) ammonia and glycine, (b) ammonia and free carnitine (C0), and (c) ammonia and IVC (C5) levels were not significant (p > 0.05).
References
- 1.Tanaka K., Budd M.A., Efron M.L., Isselbacher K.J. Isovaleric acidemia; a new genetic defect of leucine metabolism. Proc. Natl. Acad. Sci. U. S. A. 1966;56:236–242. doi: 10.1073/pnas.56.1.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tanaka K., Isselbacher K.J. The isolation and identification of N-isovalerylglycine from urine of patients with isovaleric acidemia. J. Biol. Chem. 1967;242:2966–2972. [PubMed] [Google Scholar]
- 3.Vockley J., Ensenauer R. Isovaleric acidemia: new aspects of genetic and phenotypic heterogeneity. Am. J. Med. Genet. C: Semin. Med. Genet. 2006;142:95–103. doi: 10.1002/ajmg.c.30089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ensenauer R., Vockley J., Willard J.M., Huey J.C., Sass J.O., Edland S.D., Burton B.K., Berry S.A., Santer R., Grünert S., Koch H.G., Marquardt I., Rinaldo P., Hahn S., Matern D. A common mutation is associated with a mild, potentially asymptomatic phenotype in patients with isovaleric acidemia diagnosed by newborn screening. Am. J. Hum. Genet. 2004;75:1136–1142. doi: 10.1086/426318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grünert S.C., Wendel U., Lindner M., Leichsenring M., Schwab K.O., Vockley J., Lehnert W., Ensenauer R. Clinical and neurocognitive outcome in symptomatic isovaleric acidemia. Orphanet J. Rare Dis. 2012;7:1–9. doi: 10.1186/1750-1172-7-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yudkoff M., Cohn R.M., Puschak R., Rothman R., Segal S. Glycine therapy in isovaleric acidemia. J. Pediatr. 1978;92:813–817. doi: 10.1016/s0022-3476(78)80164-4. [DOI] [PubMed] [Google Scholar]
- 7.Roe C.R., Millington D.S., Maltby D.A., Kahler S.G., Bohan T.P. L-carnitine therapy in isovaleric acidemia. J. Clin. Invest. 1984;74:2290–2295. doi: 10.1172/JCI111657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Naglak M., Salvo R., Madsen K., Dembure P., Elsas L. The treatment of isovaleric acidemia with glycine supplement. Pediatr. Res. 1988;24:9–13. doi: 10.1203/00006450-198807000-00004. [DOI] [PubMed] [Google Scholar]
- 9.Fries M.H., Rinaldo P., Schmidt-Sommerfeld E., Jurecki E., Packman S. Isovaleric acidemia: response to a leucine load after three weeks of supplementation with glycine, l-carnitine, and combined glycine-carnitine therapy. J. Pediatr. 1996;129:449–452. doi: 10.1016/s0022-3476(96)70081-1. [DOI] [PubMed] [Google Scholar]
- 10.Itoh T., Ito T., Ohba S., Sugiyama N., Mizuguchi K., Yamaguchi S., Kidouchi K. Effect of carnitine administration on glycine metabolism in patients with isovaleric acidemia: significance of acetylcarnitine determination to estimate the proper carnitine dose. Tohoku J. Exp. Med. 1996;179:101–109. doi: 10.1620/tjem.179.101. [DOI] [PubMed] [Google Scholar]
- 11.Mayatepek E., Kurczynski T.W., Hoppel C.L. Long-term l-carnitine treatment in isovaleric acidemia. Pediatr. Neurol. 1991;7:137–140. doi: 10.1016/0887-8994(91)90011-9. [DOI] [PubMed] [Google Scholar]
- 12.Sakamoto O., Arai-Ichinoi N., Mitsubuchi H., Chinen Y., Haruna H., Maruyama H., Sugawara H., Kure S. Phenotypic variability and newly identified mutations of the IVD Gene in Japanese patients with isovaleric acidemia. Tohoku J. Exp. Med. 2015;236:103–106. doi: 10.1620/tjem.236.103. [DOI] [PubMed] [Google Scholar]
- 13.Badenhorst C.P., van der Sluis R., Erasmus E., van Dijk A.A. Glycine conjugation: importance in metabolism, the role of glycine N-acyltransferase, and factors that influence interindividual variation. Expert Opin. Drug Metab. Toxicol. 2013;9:1139–1153. doi: 10.1517/17425255.2013.796929. [DOI] [PubMed] [Google Scholar]
- 14.Sakuma T. Alteration of urinary carnitine profile induced by benzoate administration. Arch. Dis. Child. 1991;66:873–875. doi: 10.1136/adc.66.7.873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Feoli-Fonseca J.C., Lambert M., Mitchell G., Melançon S.B., Dallaire L., Millington D.S., Qureshi I.A. Chronic sodium benzoate therapy in children with inborn errors of urea synthesis: effect on carnitine metabolism and ammonia nitrogen removal. Biochem. Mol. Med. 1996;57:31–36. doi: 10.1006/bmme.1996.0006. [DOI] [PubMed] [Google Scholar]
- 16.Coude F.X., Sweetman L., Nyhan W.L. Inhibition by propionyl-coenzyme A of N-acetylglutamate synthetase in rat liver mitochondria. A possible explanation for hyperammonemia in propionic and methylmalonic acidemia. J. Clin. Invest. 1979;64:1544–1551. doi: 10.1172/JCI109614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stewart P.M., Walser M. Failure of the normal ureagenic response to amino acids in organic acid-loaded rats. Proposed mechanism for the hyperammonemia of propionic and methylmalonic acidemia. J. Clin. Invest. 1980;66:484–492. doi: 10.1172/JCI109879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Castorina M., Rigante D., Antuzzi D., Sciascia Cannizzaro G., Ricci R. Different outcome in isovaleric acidemia might be related to unsatisfactory diet compliance. Scand. J. Gastroenterol. 2008;43:767–768. doi: 10.1080/00365520801912128. [DOI] [PubMed] [Google Scholar]
- 19.Knerr I., Weinhold N., Vockley J., Gibson K.M. Advances and challenges in the treatment of branched-chain amino/keto acid metabolic defects. J. Inherit. Metab. Dis. 2012;35:29–40. doi: 10.1007/s10545-010-9269-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Fig. 1 Serum ammonia levels following a leucine load.
Supplementary Fig. 2 The growth chart of the patient. The arrows indicate initiation of supplementation with both l-carnitine and glycine in combination.
Supplementary Fig. 3 Correlations between ammonia levels and glycine, free carnitine, or IVC levels. The relationships between (a) ammonia and glycine, (b) ammonia and free carnitine (C0), and (c) ammonia and IVC (C5) levels were not significant (p > 0.05).