Abstract
Purpose
The intensity of prostate-specific membrane antigen (PSMA) expression increases as the tumor grade increases and the uptake of Ga-68-PSMA is higher in high-grade tumors. The aim of the present study was to evaluate the correlation of preoperative tracer uptake of primary tumor to Gleason Score in patients who underwent prostatectomy.
Patients and methods
We retrospectively evaluated 141 patients who had Ga-68-PSMA positron emission tomography/computed tomography (PET/CT) imaging and who underwent prostatectomy. All patients had a diagnosis of prostate cancer on the basis of 10–24 cores transrectal ultrasound-guided biopsy (TRUS-Bx). Histological assessment was performed according to the New Contemporary Prostate Cancer Grading System. All patients had a prostate-specific antigen (PSA) level measurement within maximum of 28 days before Ga-68-PSMA PET/CT. Region of interests were drawn manually around the prostate gland, avoiding the bladder activity, to calculate the maximum standardized uptake values (SUVmax) values.
Results
The median PSA values for all patients were 10.0 ng/ml. PSA values for low-risk patients were significantly lower than those of high-risk patients (P<0.001). There were 41.1% upgrades and 7.8% downgrades following prostatectomy in terms of Grade Groups. According to the final pathology reports, 21% (n=16) of patients moved from a low-risk level (grade groups 1+2) to a high-risk level (grade groups 3+4+5). The median SUVmax value was 8.8, ranging from 2.1 to 62.4. There was a strong correlation between SUVmax values and grade groups (Pearson ρ=0.66) (P<0.001). The mean SUVmax values of high-risk patients were significantly higher than those of low-risk patients (18.9±12.1 vs. 7.16±6.2, respectively) (P<0.001). Receiver operation characteristic curve analysis of SUVmax at the cut-off value of 9.1 showed a high sensitivity (78%) and specificity (81%) for detection of high risk disease.
Conclusion
SUVmax values correlate significantly with the grade groups of the primary tumor. The intraprostatic accumulation sites may predict clinically significant cancer and potentially serve as a target for biopsy sampling in conjunction with mpMRI in selected patients.
Keywords: Gallium-68, Gleason scoring system, guided prostate biopsy, prostate cancer, Prostate Cancer Grading System, prostate-specific membrane antigen, Positron emission tomography/computed tomography
Introduction
To plan the optimum treatment, it is mandatory to assess the precise local and systemic staging of newly diagnosed prostate cancer (PCa). Risk stratification is performed on the basis of a number of pre-treatment parameters, including digital rectal examination (DRE) findings, prostate-specific antigen (PSA) level, and Gleason Score (GS) grading of biopsy findings. Current guidelines recommend MRI, computed tomography (CT), and bone scintigraphy for the staging of PCa to guide treatment options. However, the limitations of these tools may result in inaccurate information, which may lead to under-staging and under-treatment. According to the European Association of Urology guidelines 10–12 core systematic transrectal ultrasonography-guided prostate biopsy (TRUSBx) is the standard of care for the primary diagnosis and risk stratification of prostate cancer 1. However, it has been reported that 20–30% of clinically important cancers are missed because of anterior or apical localization, leading to inaccurate grading and staging 2–4. Although the use of pre-biopsy multiparametric MRI (mpMRI) increased the accuracy, the benefit was limited to the re-biopsy subgroups 5–7.
Molecular imaging with Ga-68-labeled prostate-specific antigen inhibitors (PSMA) positron emission tomography (PET/CT) has been one of the critical steps forward in practicing individualized medicine in prostate cancer management 8–10. 68Ga HBED-CC PSMA has been suggested as a novel tracer that can detect prostate cancer relapses and metastases with high contrast by targeting PSMA 11,12. PSMA is a type II transmembrane glycoprotein with high expression by prostatic cancer cells 13,14. The expression of PSMA was found to be low or moderate in normal or hyperplastic prostatic tissue and high in primary adenocarcinomas 15. Furthermore, it has also been shown that the intensity of PSMA expression is increasing as the tumor grade increases and the uptake of 68Ga-PSMA, defined as maximum standardized uptake value (SUVmax), may differentiate the malignant from the benign lesions with a high accuracy, which may guide the site of biopsy decrease false-negative biopsy results 16. In a recent immunohistochemical study, which evaluated the correlation between the SUVmax values and PSMA expression in tissue samples, it was shown that the tracer uptake directly correlates with the intensity of PSMA expression, but not with the GS 17. However, Uprimny et al. 18 have reported that the intensity of tracer uptake within the primary tumor correlated with the GS and PSA level. Moreover, they have analyzed the tracer uptake and SUVmax values with GS obtained from biopsy specimens, but not from the final whole gland pathology from radical prostatectomy.
The aim of the present study was to evaluate the correlation of preoperative tracer uptake to GS obtained from a definitive pathology report in patients who underwent radical prostatectomy and had a 68Ga-PSMA PET/CT performed for primary staging.
Patients and methods
Patients
We retrospectively evaluated the files of 141 patient who had 68Ga-PSMA PET/CT imaging for the purpose of primary staging and who underwent radical prostatectomy. All patients had a diagnosis of prostate cancer on the basis of 10–24 cores TRUS-Bx. As a tertiary center, many patients had been referred from other institutions; thus, biopsy reports did not have uniformity. However, the final pathology reports after radical prostatectomy were obtained from 4 different referral centers and had a consistent documentation. All patients were subjected to a histopathology analysis of biopsy and final postoperative tissue specimens. In addition to the Gleason scoring system, grade groups are reported according to The 2014 International Society of Urological Pathology 19 Consensus, which was later on adopted by the WHO for the 2016 edition of Pathology and Genetics: grade group 1 (GS ≤6), grade group 2 (GS 3+4=7), grade group 3 (GS 4+3=7), grade group 4 (GS 4+4=8, 3+5=8, 5+3=8), and grade group 5 (GS 9–10). For analysis, GRADE GROUPS are used to underline the clinical significance of GS 3+4 and 4+3. All patients had a PSA level measurement within maximum of 28 days before a 68Ga-PSMA scan. The study was carried out in accordance with the Helsinki Declaration and its later amendments. Written consent was obtained from all patients in accordance with our institution rules.
Image acquisition
After the preparation and quality control of the radiotracer, all patients received 113–384 MBq (mean: 215.3±67.2 MBq, <2 nmol PSMA ligand) of 68Ga-PSMA-11 according to the yield of the radiolabeling. Whole-body images were acquired 45–60 min p.i. of radiotracer using either of two integrated PET/CT scanners: Biograph 6 (Siemens, Knoxville, Tennessee, USA) or Discovery PET-CT 710 (GE Healthcare, Milwaukee, Wisconsin, USA). The patients were placed on the scanner table in a supine position and a CT transmission scan without intravenous contrast enhancement was acquired with a low tube current (130 kVp, 48–76 mAs), a slice thickness of 4.0 mm, 0.6 s gantry rotation, and a collimator width of 6×3 mm. Then, PET emission scanning with a duration of 3 min per bed position was performed with the identical transverse field of view in the caudocranial direction. For attenuation correction, CT transmission images were used and an iterative method was used for image reconstruction.
Image analysis
PET images were reviewed using Advantage Workstation (Version: 4.6; GE Healthcare). Region of interests were drawn manually around the prostate gland, avoiding the bladder activity, to calculate the maximum SUV (SUVmax) values. A segmental analysis of the prostate gland was not carried out. The highest SUVmax value from the whole prostate gland was assumed to be the highest site of PSMA expression and recorded for further evaluation. The scope of the study was restricted to primary tumor uptake and its correlation to GS in pathology reports; thus, we did not evaluate other 68Ga-PSMA PET/CT findings.
Statistical analysis
All results were expressed as median and mean±sd. For statistical analysis, dedicated statistical software was used (SPSS, version 21; SPSS Inc., Chicago, Illinois, USA). For comparison of the results, a nonparametric Mann–Whitney U-test was used. For the correlation of different parameters, a Pearson correlation test was used. Grade group results were compared using the chi square test. Receiver operation characteristic curve analysis was carried out to calculate sensitivity and specificity. All tests on statistical significance were performed as two tailed and a P value lower than 0.05 was considered significant.
Results
There were 141 patients, mean age 64.6±6.9 years, and ranged in age from 54 to 86 years (median: 65 years) (Table 1). The mean PSA value for all patients was 18.1±20.9 ng/ml, ranging from 1.8 to 128 ng/ml, with a median value of 10.0 ng/ml. PSA values for low-risk patients were significantly lower than those of high-risk patients, which were 12.8±14.3 and 23.0±24.5 ng/ml, respectively (P<0.001). There was no correlation between PSA values and Grade groups of the tumor (Pearson’s ρ=0.26). The median PSA values are presented in Table 1.
Table 1.
Patient data of age, prostate-specific antigen level, and SUVmax values according to grade groups obtained from biopsy results and definitive pathology results after prostatectomy
There were 37 (26%) patients in grade group 1, 41 (29%) patients in grade group 2, 31 (22%) patients in grade group 3, 19 (13%) patients in grade group 4 and 13 (9%) patients in grade group 5 according to pre-operative biopsy reports. In terms of Gleason sum, there were 37% upgrade and 2% downgrade following radical prostatectomy. When we compared the pathology reports of prostatectomy specimens with the biopsy results, we observed 49% changes in grade groups, which was significant (P<0.001). There were 1 level upgrade of grade groups in 43 patients, 2 level upgrade in 12 patients, 3 level upgrade in two patients, and 4 level upgrade in one patient [total 58 (41.1%) patients] (Table 1). We also observed 1 level downgrade in 10 patients and 3 level downgrade in one patient [total 11 (%7.8) patients]. Following radical prostatectomy, 21% (n=16) of patients moved from a low-risk level (grade groups 1+2) to a high-risk level (grade groups 3+4+5) and 10% (n=6) of patients moved from a high-risk level to a low-risk level. If we look closely at the PSMA PET images of subgroup of patients who were moved from low-risk to high risk group, the SUVmax cut-off of 9.1 would have predicted upstage in 10 out of those 16 (62.5%) patients.
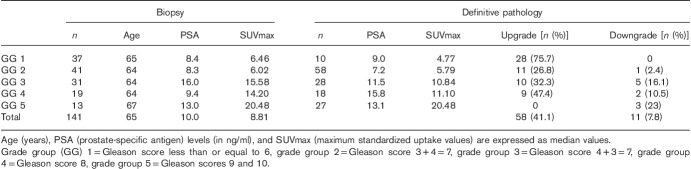
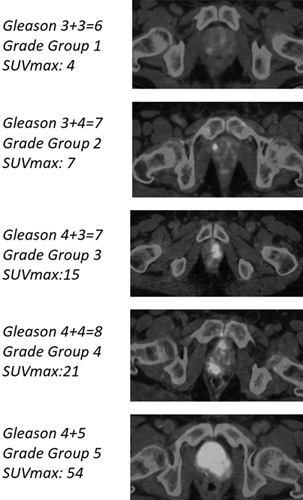
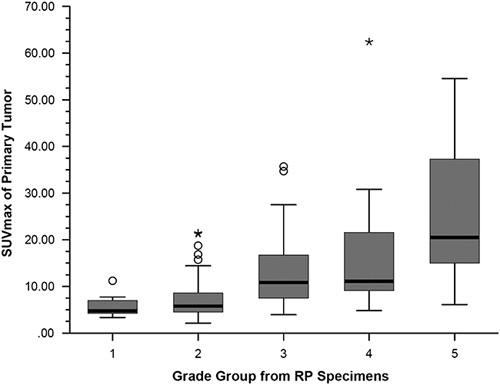
The mean SUVmax value was 13.2±11.7, with a median of 8.8, ranging from 2.1 to 62.4. The SUVmax values according to grade groups are presented in Table 1 and representative 68Ga-PSMA PET/CT fusion images for each grade group are presented in Fig. 1. There was a moderate correlation between the SUVmax values and grade groups (Pearson’s ρ=0.50) (P<0.001) obtained from biopsy reports while a strong correlation observed between SUVmax values and grade groups obtained from the final pathology reports (Pearson’s ρ=0.66) (P<0.001) (Fig. 2). The mean SUVmax value for grade group 3 tumors was 13.3±8.5 and it was significantly higher than grade group 2 tumors, which was 7.4±4.6 (P<0.001). The mean SUVmax values of high-risk patients according to the final pathology reports were significantly higher than those of low-risk patients, which were 18.9±12.1 and 7.16±6.2, respectively (P<0.001) (Fig. 3). Receiver operation characteristic curve analysis of SUVmax (area under curve=0.85, 95% CI: 0.79–0.91) showed a cut-off value of 9.1, yielding a sensitivity and a specificity of 78 and 81%, respectively.
Fig. 1.
Representative Ga-68-PSMA PET/CT fusion images for each grade group. Maximum standardized uptake values (SUVmax). PSMA PET/CT, prostate-specific membrane antigen positron emission tomography/computed tomography.
Fig. 2.
Box plot display of SUVmax values according to grade groups obtained from pathology reports after radical prostatectomy (RP). Pearson Pearson ρ 0.66 (P<0.001).
Fig. 3.
Box plot display of SUVmax values of patients with low-risk (grade group 1 and 2) and high-risk (grade groups 3, 4 and 5) prostate cancer according to pathology reports after radical prostatectomy (P<0.001). GG, grade group.
Discussion
After the introduction of PSA screening, the number of patients who were diagnosed with low-grade indolent prostate cancer increased significantly 20. Many of these patients are considered to be candidates for close follow-up rather than a radical treatment, which may have critical consequences like urinary, sexual and bowel dysfunction 21–23. Unfortunately, the inconsistency between the biopsy Gleason scores and the pathology Gleason scores is well known 2–7. In agreement with the previous reports, we observed a 21% upgrade to grade group 3 or higher after radical prostatectomy that was initially reported to be grade group 1 or 2 in biopsy reports. Using a cut-off value of 9.1, PSMA PET predicted those patients with a rate of 62.5% (Fig. 4). Estimation of clinically important upstaging at this rate may have a significant value for initial treatment planning, and may enable prediction of outcome.
Fig. 4.
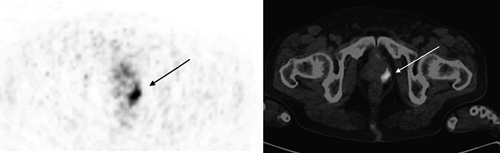
Ga-68 PSMA PET/CT scan of a 72-year-old patient who had a grade group 1 PCa. The PSA level was 12.5 ng/ml and SUVmax was 9.22. After prostatectomy, his grade group was found to be 3. PSA, prostatespecific antigen; PSMA PET/CT, prostate-specific membrane antigen positron emission tomography/computed tomography.
The expression of PSMA was found to be low or moderate in normal or hyperplastic prostatic tissue and high in primary adenocarcinomas 24,25. The radiotracer uptake was found to be high within the primary tumor, yielding high SUVmax values, and could be delineated easily from the normal prostatic tissue with high lesion contrast compared with normal tissue uptake. In a recent article, Woythal et al. 17 have shown that SUVmax of primary cancer was significantly higher than normal prostate tissue and SUVmax significantly correlated with PSMA expression in the primary tumor, which enables the detection of prostate cancer with very high sensitivity and specificity. Similarly, Rahbar et al. 16 have shown that the uptake of the tracer strongly correlates with the primary tumor, and may predict intraprostatic tumor location even in low-grade tumors. Rhee et al. 26, in a prospective study with 20 patients, compared mpMRI findings and 68Ga-PSMA PET/CT and found that use of PSMA PET/CT imaging in the pre-biopsy setting would improve the local staging of the patients who are undergoing radical prostatectomy. These findings may have an important implication and the site of increased uptake may potentially serve as a target for biopsy in conjunction with mpMRI.
Moreover, it has also been shown that the intensity of PSMA expression increases as the tumor grade increases 14. In agreement with this finding, initial reports have shown higher SUVmax values in higher GS 8 and 9. In a preliminary report of 6 cases, the authors have suggested that uptake of 68Ga-PSMA, defined as SUVmax, may differentiate the malignant from the benign lesions with high accuracy 16. Moreover, they suggested that a pre-biopsy 68Ga-PSMA PET/CT scan might guide the site of biopsy to decrease false-negative biopsy results. Uprimny et al. 18 have reported that SUVmax values of primary tumor correlated with the GS and SUVmax values of grade groups 1, 2, and 3 were significantly lower than grade groups 4 and 5. They did not find any difference between grade groups 2 and 3. However, their study was carried out according to the biopsy results, which may not reflect the actual grade group as in our series, 12 of 42 (29%) patients upgraded from grade group 2 to grade group 3. In our patient cohort, SUVmax values were significantly higher in grade group 3 compared with those of grade group 2, which were confirmed with the final pathology report. This finding suggests that the level of the SUVmax value may potentially predict clinically significant cancer.
This study has limitations because of its retrospective nature. Furthermore, we did not carry out a segmental analysis of the pathology specimens and the accumulation sites of the tracer in the 68Ga-PSMA PET/CT scan. Instead, we used the highest SUVmax value throughout the prostatic lesions and compared with the grade group. Although we are aware that some tumors may not express PSMA and may not show tracer accumulation, it is well documented that uptake of the tracer is significantly correlated with PSMA expression and grade groups of the tumor 16–18. However, a prospective study is essential to understand the value of tracer uptake and prediction of clinically significant cancer.
Conclusion
In conclusion, SUVmax values correlate significantly with the Grade Group of the primary tumor. The intraprostatic accumulation sites of the radiotracer may predict clinically significant cancer, and may potentially serve as a target for biopsy sampling in conjunction with mpMRI in selected patients. However, prospective studies are needed to determine the value of tracer uptake sites as a guide to sampling.
Acknowledgements
Conflicts of interest
There are no conflicts of interest.
References
- 1.Mottet N, Bellmunt J, Bolla M, Briers E, Cumberbatch MG, de Santis M, et al. EAU-ESTRO-SIOG guidelines on prostate cancer. Part1: screening, diagnosis and local treatment with curative intent. Eur Urol 2017; 71:618–629. [DOI] [PubMed] [Google Scholar]
- 2.Barzell WE, Melamed MR, Cathcart P, Moore CM, Ahmed HU, Emberton M. Identifying candidates for active surveillance: an evaluation of the repeat biopsy strategy for men with favorable risk prostate cancer. J Urol 2012; 188:762–767. [DOI] [PubMed] [Google Scholar]
- 3.Berglund RK, Masterson TA, Vora KC, Eggener SE, Eastham JA, Guillonneau BD. Pathological upgrading and up stagingwith immediate repeat biopsy in patients eligible for active surveillance. J Urol 2008; 180:1964–1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.King CR, McNeal JE, Gill H, Presti JC Jr. Extended prostate biopsy scheme improves reliability of Gleason grading: implications for radiotherapy patients. Int J Radiat Oncol Biol Phys 2004; 59:386–391. [DOI] [PubMed] [Google Scholar]
- 5.Tontilla PP, Lantto J, Paakko E, Piippo U, Kauppila S, Lammentausta E, et al. Prebiopsy multiparametric magnetic resonance imaging for prostate cancer diagnosis in biopsy-naive men with suspected prostate cancer based on elevated prostate-specific antigen values: results from a randomized prospective blinded controlled trial. Eur Urol 2016; 69:419–425. [DOI] [PubMed] [Google Scholar]
- 6.Siddiqui MM, Rais-Bahrami S, Turkbey B, George AK, Rothwax J, Shakir N, et al. Comparison of MR/ultrasound fusion-guided biopsy with ultrasound-guided biopsy fort he diagnosis of prostate cancer. JAMA 2015; 27:313390–313397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Füttere JJ, Briganti A, Visschere PD, de Visschere P, Emberton M, Giannarini G, et al. Can clinically significant cancer be detected with multiparamatric magnetic resonance imaging? A systematic review of the literature. Eur Urol 2015; 68:1045–1053. [DOI] [PubMed] [Google Scholar]
- 8.Eder M, Schäfer M, Bauder-Wüst U, Haberkorn U, Eisenhut M, Kopka K. Preclinical evaluation of a bispecific low-molecular heterodimer targeting both PSMA and GRPR for improved PET imaging and therapy of prostate cancer. Prostate 2014; 74:659–668. [DOI] [PubMed] [Google Scholar]
- 9.Afshar-Oromieh A, Malcher A, Eder M, Eisenhut M, Linhart HG, Hadaschik BA, et al. PET imaging with a [68Ga]gallium-labelled PSMA ligand for the diagnosis of prostate cancer: biodistribution in humans and first evaluation of tumour lesions. Eur J Nucl Med Mol Imaging 2013; 40:486–495. [DOI] [PubMed] [Google Scholar]
- 10.Öbek C, Doğanca T, Demirci E, Ocak M, Kural AR, Yildirim A, et al. The accuracy of 68Ga-PSMA PET/CT in primary lymph node staging in high-risk prostate cancer. Eur J Nucl Med Mol Imaging 2017; 44:1806–1812. [DOI] [PubMed] [Google Scholar]
- 11.Afshar-Oromieh A, Holland-Letz T, Giesel FL, Kratochwil C, Mier W, Haufe S, et al. Erratum to: diagnostic performance of 68Ga-PSMA-11 (HBED-CC) PET/CT in patients with recurrent prostate cancer: evaluation in 1007 patients. Eur J Nucl Med Mol Imaging 2017; 44:1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kabasakal L, Demirci E, Nematyazar J, Akyel R, Razavi B, Ocak M, et al. The role of PSMA PET/CT imaging in restaging of prostate cancer patients with low prostate-specific antigen levels. Nucl Med commun 2017; 38:149–155. [DOI] [PubMed] [Google Scholar]
- 13.Bařinka C, Rojas C, Slusher B, Pomper M. Glutamate Carboxypeptidase II in diagnosis and treatment of neurologic disorders and prostate cancer. Curr Med Chem 2012; 19:856–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wright GL Jr, Haley C, Beckett ML, Schellhammer PF. Expression of prostate-specific membrane antigen in normal, benign and malignant prostate tissues. Urol Oncol 1995; 1:18–28. [DOI] [PubMed] [Google Scholar]
- 15.Perner S, Hofer MD, Kim R, Shah RB, Li H, Möller P, et al. Prostate-specific membrane antigen expression as a predictor of prostate cancer progression. Human Pathol 2007; 38:696–701. [DOI] [PubMed] [Google Scholar]
- 16.Rahbar K, Weckesser M, Huss S, Semjonow A, Breyholz HJ, Schrader AJ, et al. Correlation of intraprostatic tumor extent with 68Ga-PSMA distribution in patients with prostate cancer. J Nucl Med 2016; 57:563–567. [DOI] [PubMed] [Google Scholar]
- 17.Woythal N, Arsenic R, Kempkensteffen C, Miller K, Janssen JC, Huang K, et al. Immunohistochemical validation of PSMA-expression measured by Ga-68-PSMA PET/CT in primary prostate cancer. J Nucl Med 2017; 59:238–243. [DOI] [PubMed] [Google Scholar]
- 18.Uprimny C, Kroiss AS, Decristoforo C, Fritz J, von Guggenberg E, Kendler D, et al. 68Ga-PSMA-11 PET/CT in primary staging of prostate cancer: PSA and Gleason score predict the intensity of tracer accumulation in the primary tumour. Eur J Nucl Med Mol Imaging 2017; 44:941–949. [DOI] [PubMed] [Google Scholar]
- 19.Epstein JI, Egevad L, Amin MB, Delahunt B, Srigley JR, Humphrey PA, et al. the Grading Committee. The 2014 International Society of Urological Pathology (ISUP) Consensus Conference on Gleason Grading of Prostatic Carcinoma: Definition of Grading Patterns and Proposal for a New Grading System. Am J Surg Pathol 2016; 40:244–252. [DOI] [PubMed] [Google Scholar]
- 20.Hayes JH, Barry MJ. Screening for prostate cancer with the prostate-specific antigen test: a review of current evidence. JAMA 2014; 311:1143–1149. [DOI] [PubMed] [Google Scholar]
- 21.Corcoran NM, Casey RG, Hong MK, Pedersen J, Connolly S, Peters J, et al. The ability of prostate-specific antigen (PSA) density to predict an upgrade in Gleason score between initial prostate biopsy and prostatectomy diminishes with increasing tumour grade due to reduced PSA secretion per unit tumour volume. BJU Int 2012; 110:36–42. [DOI] [PubMed] [Google Scholar]
- 22.Gearman DJ, Morlacco A, Cheville JC, Rangel LJ, Karnes RJ. Comparison of pathological and oncological outcomes in “favorable risk” GS 3+4 and low risk GS6 prostate cancer:considerations for active surveillance. J Urol 2017; 199:1188. [DOI] [PubMed] [Google Scholar]
- 23.Loeb S, Bruinsma SM, Nicholson J, Briganti A, Pickles T, Kakehi Y, et al. Active surveillance for prostate cancer: a systematic review of clinicopathologic variables and biomarkers for risk stratification. Eur Urol 2015; 67:619–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Demirci E, Sahin OE, Ocak M, Akovali B, Nematyazar J, Kabasakal L. Normal biodistribution and physiological variants of Ga-68-PSMA-11 PET/CT imaging. Nucl Med Commun 2015; 37:1169–1179. [DOI] [PubMed] [Google Scholar]
- 25.Kabasakal L, Demirci E, Ocak M, Akyel R, Nematyazar J, Aygun A, et al. Evaluation of PSMA PET/CT imaging using a 68Ga-HBED-CC ligand in patients with prostate cancer and the value of early pelvic imaging. Nucl Med Commun 2015; 36:582–587. [DOI] [PubMed] [Google Scholar]
- 26.Rhee H, Thomas P, Shepherd B, Gustafson S, Vela I, Russell PJ, et al. Prostate specific membrane antigen positron emission tomography may improve the diagnostic accuracy of multiparametric magnetic resonance imaging in localized prostate cancer. J Urol 2016; 196:1261–1267. [DOI] [PubMed] [Google Scholar]