Supplemental Digital Content is available in the text.
Keywords: cohort studies, intensive care, length of stay, neonatal
Abstract
Objectives:
To compare duration and changes over time in length of hospital stay for very preterm and extremely preterm infants in 10 European regions.
Design:
Two area-based cohort studies from the same regions in 2003 and 2011/2012.
Setting:
Ten regions from nine European countries.
Patients:
Infants born between 22 + 0 and 31 + 6 weeks of gestational age and surviving to discharge (Models of Organising Access to Intensive Care for Very Preterm Births cohort in 2003, n = 4,011 and Effective Perinatal Intensive Care in Europe cohort in 2011/2012, n = 4,336).
Interventions:
Observational study, no intervention.
Measurements and Main Results:
Maternal and infant characteristics were abstracted from medical records using a common protocol and length of stay until discharge was adjusted for case-mix using negative binomial regression. Mean length of stay was 63.6 days in 2003 and varied from 52.4 to 76.5 days across regions. In 2011/2012, mean length of stay was 63.1 days, with a narrower regional range (54.0–70.1). Low gestational age, small for gestational age, low 5-minute Apgar score, surfactant administration, any surgery, and severe neonatal morbidities increased length of stay. Infant characteristics explained some of the differences between regions and over time, but large variations remained after adjustment. In 2011/2012, mean adjusted length of stay ranged from less than 54 days in the Northern region of the United Kingdom and Wielkopolska, Poland to over 67 days in the Ile-de-France region of France and the Eastern region of the Netherlands. No systematic decrease in very preterm length of stay was observed over time after adjustment for patient case-mix.
Conclusions:
A better understanding of the discharge criteria and care practices that contribute to the wide differences in very preterm length of stay across European regions could inform policies to optimize discharge decisions in terms of infant outcomes and health system costs.
Very preterm infants require care in specialized neonatal units before they can be discharged home. Their length of stay (LOS) in hospital is influenced primarily by their gestational age (GA) at birth and medical conditions leading to longer stays, for example, bronchopulmonary dysplasia (BPD), persistent apnoeic spells, or need for tube feeding (1–5). The family’s socioeconomic circumstances as well as the neonatal unit’s policies and other health system factors, including the supply of beds or the availability of postdischarge home care, may also influence LOS. Although many interventions in this high-risk population are standardized, guidelines concerning when to discharge a very preterm infant are rare, reflecting the absence of evidence-based, consensual criteria (6).
Prolonged stay in hospital lengthens exposure to risks associated with the hospital environment, including nosocomial infections as well as noise and bright lights which may impact on future development (7). It may also disturb the establishment of interactions between parents and the infant (6). On the other hand, discharging a very preterm infant too early may expose the infant to the risk of life-threatening events and increase the risk of rehospitalization (8, 9). From a health system perspective, longer LOS reduces availability of beds and may limit the admission of other preterm infants to a neonatal ICU (NICU). LOS also determines healthcare costs, although other factors can offset the costs of longer hospital stays (10).
Trends toward shorter hospital stays have been observed for other hospital populations in industrialized countries, including stay in hospital after birth at term (11). For very preterm infants, however, it is possible that improvements in survival may have led to higher LOS as more infants at very low GAs or with more severe health conditions survive to discharge (12, 13). Some studies, however, show that morbidity among very preterm infants is declining, so survivors may be healthier and be discharged sooner (12–14). How these changing population characteristics affect the overall LOS for very preterm infants is not known.
The aim of this study was to compare LOS in hospital for very preterm infants across two time periods in European regions that have similar standards of living and social systems guaranteeing insurance coverage. We also sought to identify maternal, pregnancy, and newborn characteristics associated with variability in LOS. Data come from two area-based studies conducted in the same European regions in 2003 and 2011/2012 (15, 16).
MATERIALS AND METHODS
Study Design and Population
This analysis used data from the Models of Organising Access to Intensive Care for Very Preterm Births (MOSAIC) and Effective Perinatal Intensive Care in Europe (EPICE) studies, which included all stillbirths and live births from 22 + 0 to 31 + 6 weeks of GA. MOSAIC focused on comparing medical practices and the organization of care in European regions (15), whereas the primary aim of EPICE was to investigate how evidence-based medicine is introduced into the clinical care of very preterm infants (16). MOSAIC included 10 regions from nine countries, whereas EPICE included 19 European regions from 11 countries. All of the MOSAIC regions were included in the EPICE study, but several regions in France, Poland, and the United Kingdom had slightly different administrative boundaries. For this study, we included only the common geographic zones in both studies (see study flow chart, Fig. S1, Supplemental Digital Content 1, http://links.lww.com/PCC/A775; legend: study flow chart showing numbers of infants eligible, excluded, and included). In both studies, recruitment occurred over a 12-month period, except for the French region, where the recruitment period was 6 months.
Investigators abstracted data from medical records in obstetric and neonatal units using a standardized questionnaire with common definitions that was pretested in all regions. The EPICE instrument was developed from the MOSAIC instrument, although modifications were made to achieve the new study objectives. Inclusions were cross-checked against delivery ward registers in maternity units or another external data source. Infants were followed up until discharge home from hospital or into long-term care or death. The methodologies for the studies have been described in more detail previously (15, 16).
Study Population
The full MOSAIC cohort included 7,356 still and live births of which 7,125 occurred in geographic zones included in the EPICE project. Of these births, 5,133 were live births, 4,917 were admitted to a NICU and 4,176 survived to discharge, representing 81.1% of live births and 56.3% of all births. The EPICE cohort included 10,329 births of which 7,009 were in common geographic zones. Of these, 5,265 were live births, 5,079 were admitted to a NICU and 4,504 survived to discharge, 85.5% of live births and 64.2% of all births. Infants with missing location of discharge (n = 319) or missing date of discharge (n = 14) were not included in this study, but infants who were discharged into long-term institutional or foster care were included (n = 116; 1.4% of the sample). Infants with missing information on location of discharge were 4% of the total in both periods. The final study population comprised 4,011 infants surviving to discharge in 2003 and 4,336 in 2011/2012 (Fig. S1, Supplemental Digital Content 1, http://links.lww.com/PCC/A775).
Outcome and Covariables
The primary outcome measure was the length of hospital stay as calculated from the date of birth and the date of final discharge. Covariables were maternal and infant characteristics likely to influence the duration of hospitalization and included maternal and pregnancy characteristics (multiple pregnancy, prenatal corticosteroids, cesarean delivery) and infant characteristics (GA at birth, sex, birthweight, birthweight percentile for GA [< 10th, 10–24th, ≥ 25th], and the 5-min Apgar score). These covariables were selected based on the scientific literature assessing risk factors for long LOS, as well as previous studies from these cohorts identifying risk factors for adverse outcomes which could prolong stay in hospital (17–20). GA at birth was based on the best obstetric assessment, according to information on last menstrual period and/or ultrasound measures. Birthweight for GA was computed based on the observed birthweight for GA distribution in each cohort and reported in three groups previously found to be relevant for neonatal outcomes (19) Variables describing the neonatal course included any surfactant, surgery for any reason, neurologic morbidity (intraventricular hemorrhage [IVH] grade III or IV according to the classification Papile et al [21] and cystic periventricular leukomalacia [cPVL] documented on ultrasound or MRI scan), BPD (oxygen dependency or ventilation [including nasal continuous positive airway pressure] at 36 wk of GA), and any congenital malformation. Variables were collected using similar definitions with the exception of congenital malformations, for which more detail was available in the EPICE study, yielding a higher overall prevalence, which we believed to be principally linked to more thorough ascertainment of anomalies. This variable was therefore presented in descriptive tables, but not included in adjustment models.
Statistical Analysis
We first compared means and medians in LOS across periods and regions. Then we examined changes in the characteristics of women and infants between 2003 and 2011/2012. In both periods, we analyzed the impact of these population characteristics on LOS. As LOS is not a normally distributed variable, we used a negative binomial model to estimate risk ratios, as recommended (22). We also calculated the adjusted predictions in number of days for each covariable, meaning the LOS that would be observed for infants with this characteristic in a sample with the covariable profile of the overall study population. Adjusted models included all covariables. Region was entered as a fixed effect, and we adjusted for clustering of patients within region.
We then used our adjusted models to compare LOS across regions taking into consideration patient characteristics in 2003 and in 2011/2012. We computed adjusted risk ratios for each region and time period by running one combined model with interaction terms for period on all covariables and predicted adjusted mean LOS for each region in each period. The estimated mean LOS are those that would be observed if the infants born in each region in each period had the covariable profile observed in the overall study population. Using these adjusted means, we calculated the difference between the periods for each region, and we obtained CIs using the delta method (23).
Finally, we carried out a sensitivity analysis by running this final model using a more homogeneous lower risk sub-population defined as infants born at 29 weeks or over without severe congenital anomalies or severe neonatal morbidity (IVH grades III/IV, cPVL, BPD, necrotizing enterocolitis, retinopathy of prematurity) or surgery during the neonatal hospitalization.
Ethics Approval
Ethics approval was obtained for both studies in each study region from regional and/or hospital ethics committees as required by national legislation. The European studies were also approved by the French Advisory Committee on Use of Health Data in Medical Research and the French National Commission for Data Protection and Liberties.
RESULTS
In 2003, the average LOS among survivors to discharge was 63.6 days (median of 56 d), with a variation across regions from 52.4 days (median of 46 d) in Wielkopolska in Poland to 76.5 days (65 d) in Hesse in Germany (Table 1). In 2011/2012, the overall mean and median were slightly lower (63.1 and 55), but there was no overall trend toward lower LOS; regional differences remained substantial despite a narrowing of the range between regions at the extremes. The small reduction in overall LOS masked contrasting regional trends with sizable decreases in Hesse in Germany and Flanders in Belgium and increases in the Eastern-Central region of the Netherlands.
TABLE 1.
Length of Hospital Stay in the 10 European Regions in 2003 and 2011/2012
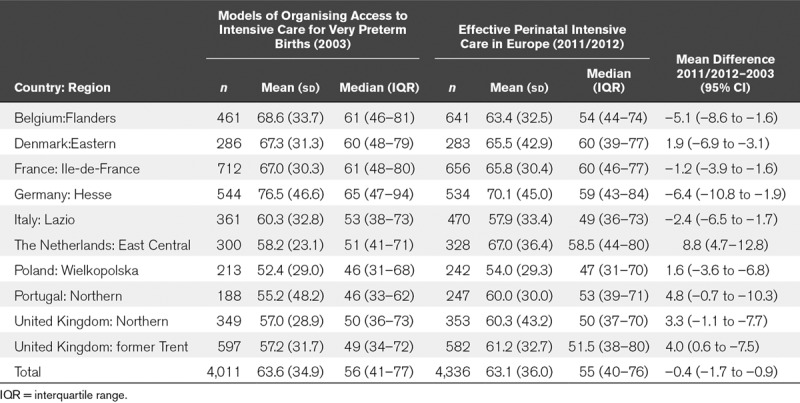
Table 2 compares the characteristics of very preterm survivors to discharge in the two time periods. The proportion of infants born to older women, as part of a multiple set and to nulliparous women increased. More active perinatal management was evidenced by changes in antenatal steroid use and a slightly higher proportion of cesarean deliveries. The GA distribution changed slightly with more survivors at lower gestations; more infants had low 5-minute Apgar scores. In contrast, the proportions of survivors with severe neurologic morbidity and BPD were significantly lower.
TABLE 2.
Maternal and Infant Characteristics in 2003 and 2011/2012
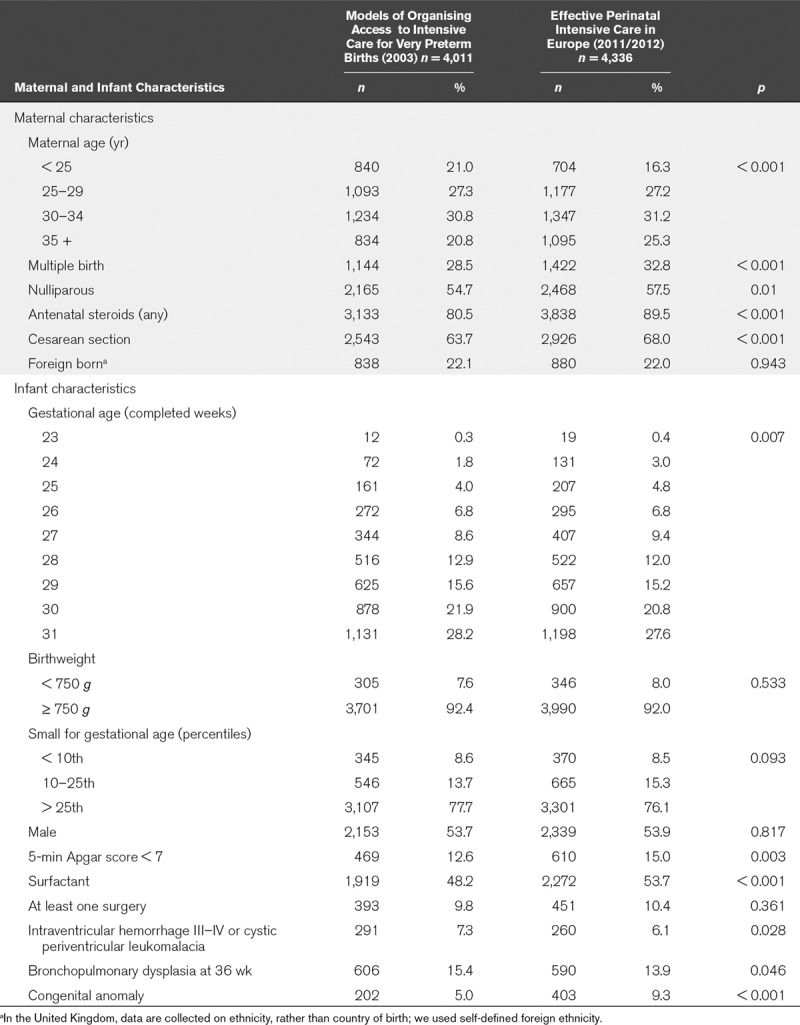
Table S1 (Supplemental Digital Content 2, http://links.lww.com/PCC/A776) illustrates how maternal and infant characteristics affected LOS in 2011/2012. GA at birth was strongly related to LOS, with an adjusted mean of 106.7 and 105.2 days for infants born at 23 and 24 weeks, respectively compared with 42.7 days at 31 weeks. Other risk factors and morbidities with a strong independent impact on LOS were small for GA (adjusted mean of 81.0 d for a birthweight percentile < 10th percentile compared with 58.2 d for a birthweight > 25th percentile), any surgery (adjusted mean of 77.5 d vs 59.4 d for no surgery), and BPD (adjusted mean of 74.5 d vs 58.8 d). Other significant predictors, but with a smaller impact, were a low 5-minute Apgar score, surfactant administration, and IVH-cPVL. The impact of these predictors on LOS was very similar in 2003 (Table S2, Supplemental Digital Content 3, http://links.lww.com/PCC/A777).
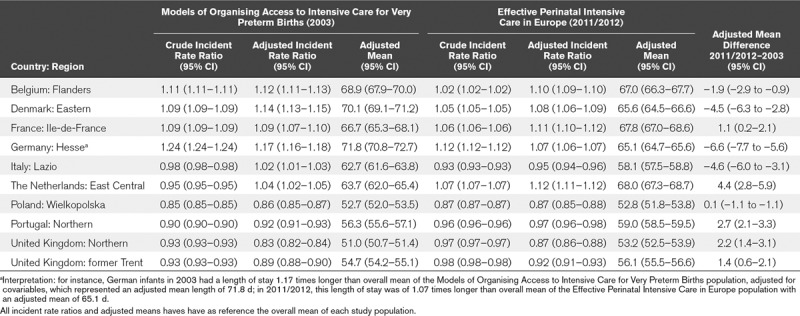
Patient characteristics contributed to the differences in LOS between regions as shown by changes in risk ratios after adjustment for population case-mix (Table 3). In particular, the extreme position in Hesse, Germany was reduced in both periods. On the other hand, the low LOS of the Netherlands in 2003 appeared to be largely explained by its patient case-mix. Nonetheless, although these differences explained some of the variation, the range of the adjusted means was still over 2 weeks: 51.0–71.8 days in MOSAIC and 52.8–68.0 days in EPICE. In general, the adjusted changes over time in mean LOS were less marked than unadjusted changes, with the exception of the Danish and Italian regions. We also observed large regional differences in LOS in sensitivity analyses using a lower risk sample, from 37.1 to 53.6 days in MOSAIC and 33.4–51.3 days in EPICE (Table S3, Supplemental Digital Content 4, http://links.lww.com/PCC/A778), with a high correlation between values in the overall and low risk population (Spearman rho of 0.95; p < 0.001 in MOSAIC and 0.92; p < 0.001 in EPICE).
TABLE 3.
Differences Between Regions in Length of Hospital Stay in 2003 and 2011/2012 and Changes Over Time After Adjustment for the Characteristics of the Patient Populations
DISCUSSION
In a large area-based study including more than 8,000 very preterm infants in two time periods, we observed a greater than 2-week difference in duration of neonatal hospitalization between regions with the lowest and highest mean LOS in both periods. Marked regional differences persisted after adjusting for maternal and infant characteristics. Although overall LOS remained largely stable, there were varying trends across time by region with decreases in Belgium, Denmark, Germany, and Italy and increases in France, the Netherlands, Portugal, and the United Kingdom. In general, regions with higher initial LOS experienced reductions, whereas those with lower initial LOS remained constant or increased which reduced some of the variation between regions over time. These regional differences raise questions about how local and regional factors determine discharge decisions, whether differences in medical practices underlie LOS variation and what consequences these decisions have for health outcomes and costs.
Our study’s strengths include its population-based design, large sample size, use of a common standardized protocol to abstract data on perinatal characteristics, and neonatal morbidity from medical records and the existence of data from the same geographical zones in two time periods. We were able to measure the entire length of hospitalization, which is not the same as the stay in the NICU (22). As organization of neonatal care differs between regions and units, it is important to include infants who are transferred to another ward or to another hospital when they do not need further intensive care (24).
Limitations are the absence of some data items relevant for the investigation of discharge decisions, including the family’s socioeconomic status, whether infants were discharged with a feeding tube or oxygen and whether postdischarge home care was provided. Although we had a wide range of variables to describe case-mix, we did not have full information on each child’s morbidity risk profile, such as sepsis, extended need for total parenteral nutrition, the number of congenital anomalies, and the complexity of surgical interventions. However, sensitivity analyses among lower risk infants with a more homogeneous risk profile found similar differences across regions, suggesting that residual confounding does not explain these findings. Other limitations were the absence of data on readmissions after discharge and on the unit practices that might contribute to variation as well as insufficient sample sizes within regions to analyze differences across units. Finally, our study questions related to infants surviving to discharge and averages for the regions based on all admissions, including in-hospital deaths, would differ.
Patient factors were strong predictors of LOS, as found in previous studies, with GA at birth having a major impact (1, 4, 5). The most immature babies born at 23, 24, and 25 weeks of gestation were hospitalized for over twice as long as those born at 30 to 31 weeks. Other predictors of a longer LOS in our sample were neonatal complications and morbidities, especially BPD and having at least one surgery, and also to a lesser extent, brain lesions, which is concordant with previous knowledge (25, 26). Being small for GA also increased LOS. In contrast, maternal sociodemographic and healthcare characteristics were not significant predictors. In this respect, our results contrast with a recent U.S. study that found that the absence of antenatal steroids prolonged LOS in very low birthweight infants (27). We also observed differences in patient case-mix over time with an increase in the proportion of the highest risk infants at very low GAs, but also a decline in some complications such as severe brain lesions and respiratory morbidity.
The variation in patient characteristics across regions had an impact on LOS, and this could be substantial in some regions. For instance, in the Netherlands, where unadjusted LOS over time increased most, the lower limit for active management of very preterm infants was lowered from 26 to 24 weeks of gestation over this time period (14, 28). In 2003, this policy meant that unadjusted LOS was lower than in the other regions because of the small proportion of extremely preterm infants. This is illustrated by the large increase in LOS once patient characteristics were taken into consideration from 58.2 to 63.7 days. Even after adjusting for case-mix, however, the LOS increased in the Netherlands over time which could reflect other residual changes in the case-mix that were not taken into consideration in our analyses. In Germany and the United Kingdom, adjusted LOS tended to be lower than unadjusted values suggesting care for a higher risk patient population, which may be related to more active management of the highest risk infants (28).
Although case-mix explained some of the variability observed in our study, wide regional differences persisted after adjustment, in line with observations from previous studies on regional and unit differences in LOS (4, 29). Multiple factors could explain these variations, including opinions among professionals about the optimal care for these infants and differences in other practices that might lengthen stay, such as respiratory management or nutritional policies, which are known to vary across units (30). For instance, local mechanical ventilation and oxygenation strategies, including specific variables used by various units to wean exogenous oxygen or ventilatory support may be a key determinant of LOS. A recent study found that implementing a collaborative quality initiative to promote evidence-based practices for feeding, discharge planning and management of apnea, bradycardia, and oxygen desaturation events made it possible to reduce postmenstrual age at discharge in California NICUs (31).
Health system factors related to financing, demand for beds or organization of postdischarge care could also play a role. In a Swedish cohort where mean postmenstrual age at discharge differed by up to 2 weeks between hospitals, it was lower with breastfeeding and domiciliary care and in hospitals without fixed discharge criteria (32). In our sample, the Hesse region provides an example of the impact of financing as hospital reimbursement was restructured from a system based on per diem charges to diagnosis-related groups, starting in 2003 on a voluntary basis and becoming obligatory in 2004 (33, 34). In this region, case-mix adjusted mean LOS declined steeply between 2003 and 2011/2012 (71.8–65.1 d).
Interventions are increasingly implemented in neonatal units to promote safe, earlier discharge of very preterm infants, including individualized developmental care, more involvement and preparation of parents for discharge, and routine discharge readiness assessment (35–39). Postdischarge organization of care, including hospital-assisted home care, can also reduce LOS in hospital (40). In contrast, gavage feeding of the infant at home has not yet been proven to successfully shorten the stay in hospital (41), while the effectiveness of prescribing monitors for use at home to shorten hospitalization is still debated (42). The usefulness of having models to predict LOS needs to be confirmed (1, 4, 25, 43). These regional differences may also make it possible to carry out research on the determinants as well as the benefits and disadvantages of short versus longer LOS in terms of rehospitalization rates, parental satisfaction, and costs. For instance, it would be possible to select regions with high versus low LOS and collect prospective data on the neonatal stay, including complications and healthcare, how the discharge decision was made for each child and the healthcare provided in the period after discharge. This could also permit use of time-varying models to understand how risk factors affect decisions across the neonatal course. A mixed method approach could complement these quantitative data with structured interviews of clinical decision-makers within the unit and with parents.
CONCLUSIONS
The large differences we found in LOS in hospital after very preterm birth among European regions could not be explained by infant characteristics. Although variation across regions narrowed from 2003 to 2011/2012, substantial differences remained. These raise questions about the diversity of current practices as well as the weak evidence-base for informing clinical and health policy decisions on the optimal hospital stays for these high-risk infants. They also raise questions about whether some care strategies may make it possible to achieve shorter LOS. Finally, understanding how differences in LOS affect readmissions, infant health, family well-being, and healthcare costs is an area for future research and could provide a basis for best practice guidelines.
ACKNOWLEDGMENTS
We acknowledge the assistance of the personnel in the maternity and neonatal units in the regions that participated in the Models of Organising Access to Intensive Care for Very Preterm Births (MOSAIC) and Effective Perinatal Intensive Care in Europe (EPICE) projects. MOSAIC and EPICE Research Groups: Belgium, Flanders (E. Martens, G. Martens); Denmark, Eastern Denmark (A. Hasselager, L. Huusom, P. Pedersen, B. Peitersen, O. Pryds, T. Weber); France, Ile-de-France (P. Y. Ancel, G. Bréart, J. L. Chabernaud, D. Delmas, E. Papiernik); Germany, Hesse (L. Gortner, W. Künzel, S. Schmidt); Italy, Lazio (R. Agostino, D. Di Lallo, R. Paesano); The Netherlands, Eastern & Central (L. den Ouden, R. de Heus, J. Gerrits, C. Hukkelhoven, M. Hulscher, C. L. Kollée, C. Koopman-Esseboom, G. Visser); Poland, Wielkopolska & Lubuskie (G. Breborowics, J. Mazela); Portugal, Northern Region (I. Campos, M. Carrapato, T. Rodrigues) United Kingdom, Trent Region (E. Boyle, D. Field, J. Konje, B. N. Manktelow); United Kingdom, Northern Region (A. Fenton, D. W. A. Milligan, S. Sturgiss); INSERM France (G. Bréart, M. Bonet).
Supplementary Material
Footnotes
*See also p. 1175.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website (http://journals.lww.com/pccmjournal).
The Models of Organising Access to Intensive Care for Very Preterm Births project was funded by a grant from the European Commission Research Directorate (QLG4-CT-2001-01907). The Effective Perinatal Intensive Care in Europe (EPICE) project was funded from the European Union’s Seventh Framework Programme (2007–2013) under grant agreement n°259882. This project has received funding from the European Union’s Horizon 2020 research and innovation programme under grant agreement No 633724. Additional funding was received for the EPICE project in the following regions: France (French Institute of Public Health Research/Institute of Public Health and its partners the French Health Ministry, the National Institute of Health and Medical Research, the National Institute of Cancer, and the National Solidarity Fund for Autonomy; grant ANR-11-EQPX-0038 from the National Research Agency through the French Equipex Program of Investments in the Future; and the PremUp Foundation); Poland (2012–2015 allocation of funds for international projects from the Polish Ministry of Science and Higher Education); and United Kingdom (funding for The Neonatal Survey from Neonatal Networks for East Midlands and Yorkshire & Humber regions.
Dr. Maier’s institution received funding from European Commission Research Directorate (QLG4-CT-2001-01907); European Union’s Seventh Framework Programme (FP7/2007–2013) under grant agreement No 259882; and European Union’s Horizon 2020 research and innovation programme under grant agreement No 633724. Dr. Van Reempts’ institution received funding from Centre of Perinatal Epidemiology in Flanders. Dr. Franco disclosed government work. Drs. Gadzinowski’s and Draper’s institutions received funding from European Union FP7, and they received support for article research from European Union FP7. Dr. Draper disclosed work for hire. Dr. Zeitlin’s institution received funding from European Commission. The remaining authors have disclosed that they do not have any potential conflicts of interest.
This work was performed at Inserm UMR 1153, Obstetrical, Perinatal and Pediatric Epidemiology Research Team, Port-Royal Maternity Unit, 53 avenue de l’Observatoire, 75014 Paris, France.
REFERENCES
- 1.Seaton SE, Barker L, Jenkins D, et al. What factors predict length of stay in a neonatal unit: A systematic review. BMJ Open 2016; 6:e010466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Eichenwald EC, Zupancic JA, Mao WY, et al. Variation in diagnosis of apnea in moderately preterm infants predicts length of stay. Pediatrics 2011; 127:e53–e58. [DOI] [PubMed] [Google Scholar]
- 3.Merritt TA, Raddish M. A review of guidelines for the discharge of premature infants: Opportunities for improving cost effectiveness. J Perinatol 1998; 18:S27–S37. [PubMed] [Google Scholar]
- 4.Manktelow B, Draper ES, Field C, et al. Estimates of length of neonatal stay for very premature babies in the UK. Arch Dis Child Fetal Neonatal Ed 2010; 95:F288–F292. [DOI] [PubMed] [Google Scholar]
- 5.Numerato D, Fattore G, Tediosi F, et al. Mortality and length of stay of very low birth weight and very preterm infants: A EuroHOPE Study. PLoS One 2015; 10:e0131685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Merritt TA, Pillers D, Prows SL. Early NICU discharge of very low birth weight infants: A critical review and analysis. Semin Neonatol 2003; 8:95–115. [DOI] [PubMed] [Google Scholar]
- 7.Santos J, Pearce SE, Stroustrup A. Impact of hospital-based environmental exposures on neurodevelopmental outcomes of preterm infants. Curr Opin Pediatr 2015; 27:254–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Doyle J, Davidson D, Katz S, et al. Apnea of prematurity and caffeine pharmacokinetics: Potential impact on hospital discharge. J Perinatol 2016; 36:141–144. [DOI] [PubMed] [Google Scholar]
- 9.Seki K, Iwasaki S, An H, et al. Early discharge from a neonatal intensive care unit and rates of readmission. Pediatr Int 2011; 53:7–12. [DOI] [PubMed] [Google Scholar]
- 10.DeRienzo C, Kohler JA, Lada E, et al. Demonstrating the relationships of length of stay, cost and clinical outcomes in a simulated NICU. J Perinatol 2016; 36:1128–1131. [DOI] [PubMed] [Google Scholar]
- 11.Organisation for Economic Co-operation and Development: Lenth of Hospital Stay (Indicator), 2017. Available at: https://data.oecd.org/healthcare/length-of-hospital-stay.htm. Accessed October 27, 2017
- 12.Ancel PY, Goffinet F, Kuhn P, et al. ; EPIPAGE-2 Writing Group: Survival and morbidity of preterm children born at 22 through 34 weeks’ gestation in France in 2011: Results of the EPIPAGE-2 cohort study. JAMA Pediatr 2015; 169:230–238. [DOI] [PubMed] [Google Scholar]
- 13.Costeloe KL, Hennessy EM, Haider S, et al. Short term outcomes after extreme preterm birth in England: Comparison of two birth cohorts in 1995 and 2006 (the EPICure studies). BMJ 2012; 345:e7976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bonet M, Cuttini M, Piedvache A, et al. Changes in management policies for extremely preterm births and neonatal outcomes from 2003 to 2012: Two population-based studies in ten European regions. BJOG 2017; 124:1595–1604. [DOI] [PubMed] [Google Scholar]
- 15.Zeitlin J, Draper ES, Kollée L, et al. ; MOSAIC Research Group: Differences in rates and short-term outcome of live births before 32 weeks of gestation in Europe in 2003: Results from the MOSAIC cohort. Pediatrics 2008; 121:e936–e944. [DOI] [PubMed] [Google Scholar]
- 16.Zeitlin J, Manktelow BN, Piedvache A, et al. ; EPICE Research Group: Use of evidence based practices to improve survival without severe morbidity for very preterm infants: Results from the EPICE population based cohort. BMJ 2016; 354:i2976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Draper ES, Zeitlin J, Fenton AC, et al. Investigating the variations in survival rates for very preterm infants in 10 European regions: The MOSAIC birth cohort. Arch Dis Child Fetal Neonatal Ed 2009; 94:F158–F163. [DOI] [PubMed] [Google Scholar]
- 18.Edstedt Bonamy AK, Zeitlin J, Piedvache A, et al. ; Epice Research Group: Wide variation in severe neonatal morbidity among very preterm infants in European regions. Arch Dis Child Fetal Neonatal Ed 2018 Jan 20. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zeitlin J, El Ayoubi M, Jarreau PH, et al. ; MOSAIC Research Group: Impact of fetal growth restriction on mortality and morbidity in a very preterm birth cohort. J Pediatr 2010; 157:733–739.e1. [DOI] [PubMed] [Google Scholar]
- 20.Draper ES, Manktelow BN, Cuttini M, et al. Variability in very preterm stillbirth and in-hospital mortality across Europe. Pediatrics 2017; 139:e20161990. [DOI] [PubMed] [Google Scholar]
- 21.Papile LA, Burstein J, Burstein R, et al. Incidence and evolution of subependymal and intraventricular hemorrhage: A study of infants with birth weights less than 1,500 gm. J Pediatr 1978; 92:529–534. [DOI] [PubMed] [Google Scholar]
- 22.Lee HC, Bennett MV, Schulman J, et al. Estimating length of stay by patient type in the neonatal intensive care unit. Am J Perinatol 2016; 33:751–757. [DOI] [PubMed] [Google Scholar]
- 23.Feiveson AH.What is the delta method and how is it used to estimate the standard error of a transformed parameter? Explanation of the Delta Method. Stata FAQ 2017. Available at: https://www.stata.com/support/faqs/statistics/delta-method/. Accessed October 24, 2017.
- 24.Attar MA, Lang SW, Gates MR, et al. Back transport of neonates: Effect on hospital length of stay. J Perinatol 2005; 25:731–736. [DOI] [PubMed] [Google Scholar]
- 25.Hintz SR, Bann CM, Ambalavanan N, et al. ; Eunice Kennedy Shriver National Institute of Child Health and Human Development Neonatal Research Network: Predicting time to hospital discharge for extremely preterm infants. Pediatrics 2010; 125:e146–e154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Klinger G, Reichman B, Sirota L, et al. ; Collaboration with the Israel Neonatal Network: Risk factors for delayed discharge home in very-low-birthweight infants:- A population-based study. Acta Paediatr 2005; 94:1674–1679. [DOI] [PubMed] [Google Scholar]
- 27.Lee HC, Bennett MV, Schulman J, et al. Accounting for variation in length of NICU stay for extremely low birth weight infants. J Perinatol 2013; 33:872–876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zegers MJ, Hukkelhoven CW, Uiterwaal CS, et al. Changing Dutch approach and trends in short-term outcome of periviable preterms. Arch Dis Child Fetal Neonatal Ed 2016; 101:F391–F396. [DOI] [PubMed] [Google Scholar]
- 29.Fatttore G, Numerato D, Peltola M, et al. ; EuroHOPE Study Group: Variations and determinants of mortality and length of stay of very low birth weight and very low for gestational age infants in seven European countries. Health Econ 2015; 24(Suppl 2):65–87. [DOI] [PubMed] [Google Scholar]
- 30.Gortner L, Misselwitz B, Milligan D, et al. ; Members of the MOSAIC Research Group: Rates of bronchopulmonary dysplasia in very preterm neonates in Europe: Results from the MOSAIC cohort. Neonatology 2011; 99:112–117. [DOI] [PubMed] [Google Scholar]
- 31.Lee HC, Bennett MV, Crockett M, et al. Comparison of collaborative versus single-site quality improvement to reduce NICU length of stay. Pediatrics 2018; 142:e20171395. [DOI] [PubMed] [Google Scholar]
- 32.Altman M, Vanpee M, Cnattingius S, et al. Moderately preterm infants and determinants of length of hospital stay. Arch Dis Child Fetal Neonatal Ed 2009; 94:F414–F418. [DOI] [PubMed] [Google Scholar]
- 33.Abler S, Verde P, Stannigel H, et al. Effect of the introduction of diagnosis related group systems on the distribution of admission weights in very low birthweight infants. Arch Dis Child Fetal Neonatal Ed 2011; 96:F186–F189. [DOI] [PubMed] [Google Scholar]
- 34.Lungen M, Lapsley I. The reform of hospital financing in Germany: An international solution? J Health Organ Manag 2003; 17:360–372. [DOI] [PubMed] [Google Scholar]
- 35.Moody C, Callahan TJ, Aldrich H, et al. Early initiation of Newborn Individualized Developmental Care and Assessment Program (NIDCAP) reduces length of stay: A quality improvement project. J Pediatr Nurs 2017; 32:59–63. [DOI] [PubMed] [Google Scholar]
- 36.Ortenstrand A, Westrup B, Broström EB, et al. The Stockholm Neonatal Family Centered Care Study: Effects on length of stay and infant morbidity. Pediatrics 2010; 125:e278–e285. [DOI] [PubMed] [Google Scholar]
- 37.Gonya J, Martin E, McClead R, et al. Empowerment programme for parents of extremely premature infants significantly reduced length of stay and readmission rates. Acta Paediatr 2014; 103:727–731. [DOI] [PubMed] [Google Scholar]
- 38.Melnyk BM, Feinstein NF, Alpert-Gillis L, et al. Reducing premature infants’ length of stay and improving parents’ mental health outcomes with the Creating Opportunities for Parent Empowerment (COPE) neonatal intensive care unit program: A randomized, controlled trial. Pediatrics 2006; 118:e1414–e1427. [DOI] [PubMed] [Google Scholar]
- 39.Hospital cuts length of stay for babies in the NICU by four days. Hosp Case Manag 2015; 23:49–50. [PubMed] [Google Scholar]
- 40.Lundberg B, Lindgren C, Palme-Kilander C, et al. Hospital-assisted home care after early discharge from a Swedish neonatal intensive care unit was safe and readmissions were rare. Acta Paediatr 2016; 105:895–901. [DOI] [PubMed] [Google Scholar]
- 41.Collins CT, Makrides M, McPhee AJ. Early discharge with home support of gavage feeding for stable preterm infants who have not established full oral feeds. Cochrane Database Syst Rev 2015; (7):CD003743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Silvestri JM. Indications for home apnea monitoring (or not). Clin Perinatol 2009; 36:87–99. [DOI] [PubMed] [Google Scholar]
- 43.Bender GJ, Koestler D, Ombao H, et al. Neonatal intensive care unit: Predictive models for length of stay. J Perinatol 2013; 33:147–153. [DOI] [PMC free article] [PubMed] [Google Scholar]


