ABSTRACT
Objective:
Functional constipation (FC) has a major impact on the health-related quality of life (HRQoL) of children. The aim of this study was to evaluate parent-child agreement on HRQoL in children (8–17 years) with FC in primary care.
Methods:
Children diagnosed with FC by their clinician were eligible. HRQoL was measured with the Defecation Disorder List (DDL, score 0–100), and the EuroQol-5-Dimension-Youth Visual Analogue Scale (EQ-5D-Y-VAS, scale 0–100). Parent-child agreement was examined with discrepancy scores, intraclass correlation coefficients and Bland-Altman plots.
Results:
Fifty-six children, median age of 10 years (IQR 8–12) and their parents were included. Parent-child agreement at a group level was good, with an intraclass correlation coefficient of 0.80 (95% confidence interval 0.67 to 0.88) for the DDL, and 0.78 (95% confidence interval 0.65 to 0.87) for the EQ-5D-Y-VAS. Mean discrepancy scores for the DDL and EQ-5D-Y-VAS were small: −2.6 and −2.9, implying that parents were slightly more positive about the HRQoL than their children. Bland-Altman plots showed considerable discordance between individual parent-child pairs. Limits of agreement were −19.7 and 14.6 for the DDL and −27.6 and 21.8 for the EQ-5D-Y-VAS.
Conclusions:
There is good parent-child agreement on HRQoL in children with FC at group level. However, a substantial number of parent-child pairs differed considerably on their rating of the HRQoL of the child. Therefore, we recommend clinicians, if they want to have an impression of the impact of the FC on the HRQoL of the child, to ask both the child and the parent(s).
Keywords: child self-report, health outcomes, parent proxy-report, quality of life
What Is Known
Functional constipation has a major impact on the health-related quality of life of children.
Children and parents may report on different aspects of the child's health-related quality of life.
What Is New
There is good parent-child agreement in children with functional constipation on a group level.
A substantial number of individual parent-child pairs differed considerably on their rating on the health-related quality of life of the child.
When clinicians want to have an impression of the impact of the functional constipation on the health-related quality of life of the child, it is recommended to assess health-related quality of life of the child to both the child and the parent.
Functional constipation (FC) is a common disorder in children, with a pooled prevalence rate of 9.5 percent (1). FC has a major impact on the health-related quality of life (HRQoL) of children and their families, with the greatest influence on the emotional and social aspects of life (2–5). Parental emotional perceptions of illness are correlated to treatment adherence in children with FC (6). Therefore, it is important for clinicians to ask for the consequences of the FC for the wellbeing of the child (7). In research, HRQoL is identified by experts as an important outcome measure in clinical trials evaluating new interventions for childhood FC (7,8).
There is substantial debate in the health outcomes literature regarding the most appropriate respondent for assessing children's HRQoL: the child self or the parent(s) (9–13). As HRQoL pertains to an individual's subjective perceptions, a child's self-report would represent the child's situation best (10,12). A parent may provide more valid information concerning more abstract health-related concepts, that is, the emotional impact of illness (10). A potential drawback of a parent‘s report may, however, be that it is affected by the impact of the child's condition on the family life (10,14). Therefore, information about the agreement between child and parent perceptions of HRQoL of the child is important to answer the question whether child self-reports and a parent proxy-reports are interchangeable.
In young children, a parent proxy-report will be the only option to assess HRQoL (10,15). In children from the age of 8 years clinicians and researchers can rely on a parent proxy-report and a child self-report when they need to be informed on the HRQoL (15,16). For practical reasons one, however, often relies on 1 of the 2 reports. Previous studies investigating the agreement between child and parents perceptions of HRQoL reported inconsistent results (10,13,17,18). In general, it seems that parents were more negative than their child on the HRQoL if their child had a chronic disease, and more positive if the child was healthy (10,13,17–19). In addition, parent-child agreement may be influenced by age and gender of the child, but the relationship between the child's age and gender and parent-child agreement is uncertain (17).
Only 1 previous study has examined parent-child agreement in children with FC. This was a population from a university hospital (20). The level of agreement in that study was low, and therefore, the authors advised to use both a parent proxy and a child self-report to measure HRQoL. In the Netherlands, children with FC are first seen in primary care. Children with diagnostic or therapeutic problems will be referred to the pediatrician or pediatric gastroenterologist. Therefore, the selection of patients with FC seen in primary care is different from that seen in a university hospital. This difference in case-mix may influence parent-child agreement. Therefore we designed a study to examine parent-child agreement on HRQoL in children (aged 8–17 years) with FC in primary care. Secondary aim was to investigate whether agreement was associated with age or gender of the child.
METHODS
Study Design and Participants
This study was designed as an agreement study. We used baseline data of an randomized controlled trial (RCT) on the effectiveness and cost-effectiveness of physiotherapy in children with FC aged 4 to 17 years (Netherlands Trial Register, number 4797). Children diagnosed with FC by their general practitioner or pediatrician were included in that trial. Exclusion criteria for the RCT were children who had already received physiotherapy or urotherapy for FC in the past 3 years, psychopathology affecting protocol adherence, and serious or terminal illness. The RCT was approved by the Medical Ethical Board of the University Medical Center Groningen (number METc 2013/331). Both parents and child (if aged ≥12 years) provided informed consent to participate in the study. For this agreement study only data of children aged 8 to 17 years were used, because children below 8 years are too young to provide a self-report of their HRQoL (10,15).
Measurements
HRQoL was measured with a disease-specific questionnaire, the Defecation Disorder List (DDL) and a health status questionnaire, the Euroqol-5-Dimensions-Youth (EQ-5D-Y) (21–23). The questionnaires were completed both by the child and by one of the parents. Children and parents were instructed to fill in the questionnaires independently.
Disease-specific Quality of Life
We used the emotional and social functioning subdomains of the DDL as these 2 subdomains of the DDL measure HRQoL (21,22). These 2 subdomains of the DDL together consist of 25 statements, answered on a 5-point Likert scale, to indicate to what extent the user agrees with that statement. This corresponds with a score of 0, 25, 50, 75, or 100 points per statement. The (subdomain) scores are computed as the sum of the items divided by the number of items answered. The lowest possible score is 0 (poorest quality of life) and the maximum score 100 (best quality of life).
Health Status
Of the EQ-5D-Y the Visual Analogue Scale (VAS) was used to measure health status (23). The lowest possible score was 0 (worst health you can imagine) and the highest score was 100 (best health you can imagine).
Demographic and Symptom-related Information
Demographic and health information, in particular age, gender, type of symptoms, duration, and onset of symptoms and (information on) the use of laxatives, were assessed based on a questionnaire completed by the parents. Symptoms related to FC were assessed using a Dutch version of the “Questionnaire on Pediatric Gastrointestinal Symptoms Rome-III” (QPGS-RIII) (24).
Statistical Analysis
Appropriate descriptive statistics were used to present patient characteristics, symptoms of FC and the quality-of-life outcomes. Less than 1% of the statements on the DDL questionnaire remained unanswered (missing). The discrepancy scores (Δ) between parent-proxy and child-self reported HRQoL were calculated for all outcomes (DDL total score, DDL emotional and social subdomain scores, and EQ-5D-Y-VAS score).
The level of parent-child agreement on HRQoL on group level was analyzed using intra-class correlation coefficients (ICC), using a 2-way random model, single measures with absolute agreement. To indicate the level of agreement the conservative criteria of Portney and Watkins were used: an ICC of ≤0.75 is then classified as poor to moderate agreement; an ICC of 0.75 to 0.90 as good agreement; and an ICC of >0.90 as “reasonable agreement for clinical measurements” (25,26).
Individual parent-child agreement was evaluated by visual inspection of the Bland-Altman plots. Perfect agreement between a child and a parent entails that the discrepancy score (Δ) is equal to zero. No systematic bias is assumed when the 95% confidence intervals (95% CI) around the mean discrepancy scores include zero. Limits of agreement were computed as follows: mean difference ± 1.96 × standard deviation of the difference. Approximately 95% of the differences between child and parent reported HRQoL will lie between the limits of agreement.
In order to determine whether age and gender of the child influenced parent-child agreement we conducted multivariate linear regression analyses. Statistical analyses were performed using SPSS Version 24.0 (IBM corp., Armonk, NY).
Sample Size
An adequate sample size is important to obtain a reliable ICC parameter with acceptable precision. When expecting an ICC of 0.8, using 2 observers per patient (child-report and parent proxy-report), and a 95% CI with a width of 0.2, a minimal sample size of 50 patients is required (27). In addition, a sample size of approximately 50 patients is required to provide a reasonable number of dots in a Bland-Altman plot to estimate the limits of agreement (28).
RESULTS
Participants
Among the 134 children participating in the RCT, 56 children fulfilled the inclusion criteria for this agreement study, that is were between 8 and 17 years of age. These were 24 boys and 32 girls, with a median age of 10 years (IQR 8–12). Patient characteristics are presented in Table 1. Disease-specific HRQoL and health status of the children reported by the children and the parents are shown in Table 2.
TABLE 1.
Baseline characteristics of the children aged 8 to 18 years with functional constipation diagnosed by their general practitioner or pediatrician (n = 56)
| Age median (IQR) in years | 10.0 (8.3–12.0) |
| Gender (% girls) | 57.1 |
| Duration of symptoms (n), months | |
| ≤3 | 8/50 |
| 3–12 | 6/50 |
| >12 | 36/50 |
| Abdominal pain/discomfort in the previous 4 weeks (n) | |
| Never | 6/56 |
| 1–3 times a month | 14/56 |
| Once a week | 7/56 |
| Multiple times a week | 21/56 |
| Every day | 8/56 |
| FC symptoms (Rome-III criteria for FC) (n) | |
| ≤2 defecations in the toilet per week | 14/56 |
| Fecal incontinence ≥1 per week | 16/56 |
| Stool withholding | 10/56 |
| Painful or hard bowel movements | 43/56 |
| Large fecal mass in the abdomen or rectum | 40/56 |
| Large stools that obstruct the toilet | 12/56 |
| Use of laxatives in the previous 4 weeks (n) | |
| Yes | 32/47 |
| No | 15/47 |
| Previous episodes of FC (n) | |
| ≥ 2 | 34/52 |
| 1 | 2/52 |
| 0 | 16/52 |
FC = functional constipation; IQR = interquartile range; n = number.
TABLE 2.
Disease-specific quality of life and health status of children with functional constipation (n = 56)
| Reported by | Results to evaluate absolute agreement | ||||
| Children | Parents | ||||
| Median (IQR) | Median (IQR) | Mean discrepancy score* (95% CI) | Limits of agreement | ICC (95% CI) | |
| DDL total score | 76 (65–84) | 78 (67–85) | −2.6 (−4.9 to −0.2) | −19.7–14.6 | 0.80 (0.67 to 0.88) |
| DDL emotional functioning | 72 (58–83) | 75 (62–83) | −2.2 (−5.2 to 0.7) | −23.9–19.5 | 0.73 (0.58 to 0.83) |
| DDL social functioning | 79 (64–89) | 82 (65–89) | −3.0 (−5.9 to 0.0) | −24.2–18.3 | 0.78 (0.65 to 0.87) |
| EQ-5D-Y-VAS | 84 (71–92) | 85 (75–94) | −2.9 (−6.3 to 0.5) | −27.6–21.8 | 0.78 (0.65 to 0.87) |
CI = confidence interval; DDL = Defecation Disorder List; EQ-5D-Y-VAS = Euroqol-5-Dimensions-Youth Visual Analogue Scale; ICC = Intraclass Correlation Coefficient; IQR = Inter Quartile Range.
*Discrepancy scores were calculated by subtracting each parent's score from their child's score. Negative differences indicate that parents evaluated the disease-specific quality of life and health status of their children better than the children did. Unlike the individual HRQoL scores reported by parents and children, the mean discrepancy scores were normally distributed in all outcome variables.
Level of Parent-child Agreement
The mean discrepancy scores and the corresponding 95% CI intervals between child self and parent proxy-reports were for the DDL total score, DDL emotional functioning subdomain, DDL social functioning subdomain, and EQ-5D-Y-VAS, −2.6 (−4.9 to −0.2), −2.2 (−5.2 to 0.7), −3.0 (−5.9 to 0.0), and −2.9 (−6.3 to 0.5), respectively. A negative score indicates that parents rated the HRQoL higher than the children did.
The level of parent-child agreement was good for the DDL total score (ICC: 0.80, 95% CI 0.67 to 0.88), the DDL social functioning subdomain (ICC: 0.78, 95% CI 0.65 to 0.87), and the EQ-5D-Y-VAS (ICC: 0.78, 95% CI 0.65 to 0.88), and poor to moderate for the DDL emotional functioning subdomain (ICC: 0.73, 95% CI 0.58 to 0.83) (Table 2).
Bland-Altman plots are shown in Figure 1. Observed limits of agreement for the DDL total score were −19.7 and 14.6, for the emotional functioning subdomain −23.9 and 19.5, for the social functioning subdomain −24.2 and 18.3, and for the EQ-5D-Y-VAS −27.6 and 21.8. With a range on the scores between 0 and 100, the intervals between the limits of agreement showed that the level of agreement varied considerably between individual parent-child pairs.
FIGURE 1.
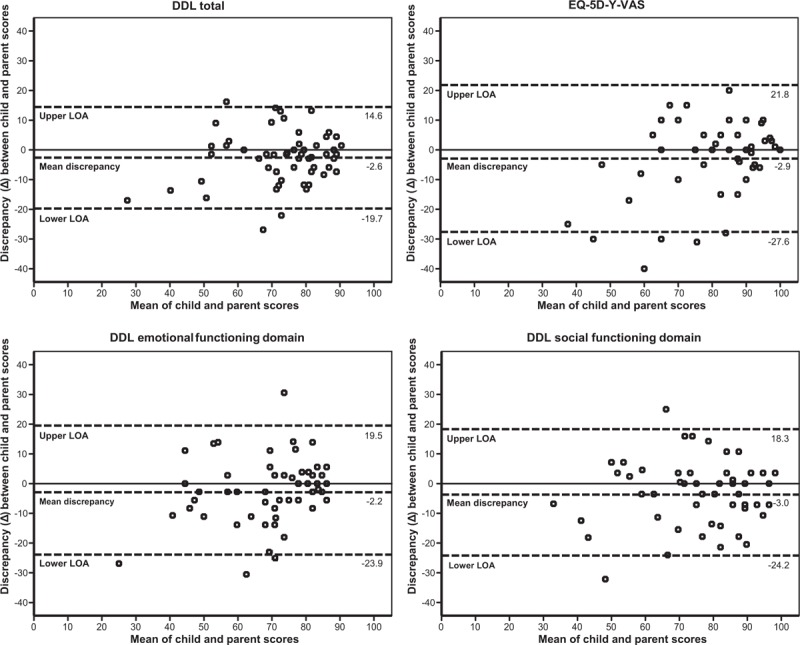
Bland-Altman plots. Top left: DDL total score, top right: EQ-5D-Y-VAS score, bottom left: DDL emotional functioning domain score, bottom right: DDL social functioning domain score. Discrepancy scores (Δ) were calculated by subtracting each parent's score from their child's score. DDL = Defecation Disorder List; EQ-5D-Y-VAS = Euroqol-5-Dimensions-Youth Visual Analogue Scale; LOA = Limits of Agreement.
Factors Associated With Parent-child Agreement
Multivariate linear regression analyses showed that age and gender of the child were not significantly associated with parent-child agreement for all outcomes (data not shown).
DISCUSSION
Main Findings
This study showed that parent-child agreement on HRQoL in children with FC was good on group level. In general, parents reported a minimally better disease-specific HRQoL and health status than the children did. For individual child-parent pairs the level of agreement, however, varied considerably. This is shown in the wide intervals between the limits of agreement, that were −19.7 and 14.6 (DDL total score), and −27.6 and 21.8 (EQ-5D-Y-VAS). Therefore, it is sufficient to use only 1 report (parent or child which is more convenient), when one is interested in the HRQoL of a group of children with FC in primary care. When one is interested in the HRQoL of an individual child, we, however, recommend to use both reports. Age and gender of a child did not affect parent-child agreement on HRQoL in children with FC in primary care.
Comparison to Literature
Consistent with our study, 3 other studies on FC, performed in a hospital setting, reported small mean discrepancy scores between child self and parent proxy-reported HRQoL (20,29,30). In our study parents were in general slightly more positive on the child's HRQoL. This overestimation of the child's HRQoL is in accordance to studies measuring parent-child agreement in healthy children (10,13,17–19). In contrast, the 3 studies on parent-child agreement in a university hospital setting showed that parents were in general slightly more negative on the HRQoL of their child than the child was. Parents of children with other chronic conditions also tend to underestimate the child's HRQoL (10,13,17–19). As stated before, on average the differences between parents and children were, however, small.
Our findings of good parent-child agreement concerning HRQoL are consistent with another study in children with FC (20). The level of parent-child agreement concerning HRQoL found in our study was better (ICCs between 0.73 and 0.80), than the parent-child agreement in the other study (ICCs between 0.55 and 0.74). Theoretically, these differences could be explained by either the other questionnaires that were used (DDL/EQ-5D-Y-VAS in this study vs PedsQL 4.0 Generic Core Scale in the other study), their clinical settings (primary care vs tertiary care) or the limitation of comparing ICCs for the level of parent-child agreement (31).
Strengths and Limitations
This is the first study examining agreement for HRQoL in children with FC for individual parent-child pairs. In addition, parent-child agreement was assessed for 2 different types of questionnaires measuring HRQoL, a disease-specific and a generic questionnaire. Disease-specific instruments are more sensitive to detect small but relevant changes in the patient's HRQoL, whereas generic instruments are more useful to compare HRQoL across different patient groups (32). By analyzing different aspects of agreement, such as by using discrepancy scores, ICCs, and Bland-Altman plots, our study attempts to comprehensively report on the nature of discrepancies between parent proxy and child self-reports concerning HRQoL in children with FC.
Our findings should be interpreted in the light of the following limitations. Parents and children have completed the questionnaires at home. The instruction was to complete the questionnaires independently. Although this study showed that the level of agreement between individual child-parent pairs varied considerably, there is a possibility that they colluded, and therefore both parties may have given more moderate responses, which would enhance agreement and minimize differences (17). Secondly, we did not collected demographic data of the parents. Thirdly, because of a limited sample size we only evaluated if age and gender of a child influenced parent-child agreement. We performed some hypothesize generating post hoc analyses but we found no indication that the number of Rome III criteria in a child, or separate Rome III criteria, influenced parent-child agreement on HRQoL (data not shown). In addition, there is limited knowledge about the psychometric properties of the DDL questionnaire. Finally, a limitation of the comparison of parent-child agreement using the ICC is that the ICC is an index of absolute agreement and consists of the ratio of between-subject variability and total variability (31). Less heterogeneity in HRQoL scores between children may generate lower ICCs for parent-child agreement. In primary care children were seen with recent onset symptoms but also children with symptoms of longer duration. Therefore, it can be expected that there is much heterogeneity in HRQoL between children, which will lead to a better ICC. Thus, for the comparison of the level of parent-child agreement between studies, it is important to use several methods to evaluate agreement, that is, discrepancy scores, ICCs, and Bland-Altman plots.
Implications for Research
On a group level parent-child agreement concerning HRQoL was good. Therefore, in research focusing on group results, one can use either a parent proxy-report or a child-self report to assess HRQoL. For research looking at the individual patient's level, it is recommended to assess both the parent's, and the child's perception of the impact of the disease. More research into factors, such as severity of disease, duration of symptoms, parent-child relationship, or mother or father's as proxy raters, that may influence parent-child agreement is needed. In addition, more research is needed into how and if a discrepancy between parent and child influence clinical decision making.
Implications for Clinical Practice
We found in our study that children and their parents may rate the impact of the FC on the quality of life of the child differently. Perceptions of the emotional impact of the FC may influence treatment adherence, as was found in a recent study (6). Therefore, we advise clinicians to pay attention as well to the parent's perception of the child's HRQoL as to that of the child. A short question, like “we would like to know how good or bad your health is today on a scale from 0 to 100” which is used in the EQ-5D-Y-VAS, will be most suitable in clinical practice. As FC influenced especially the emotional and social aspects of HRQoL, the DDL questionnaire will, however, be better in detecting relevant health issues of children with FC (5).
Footnotes
All phases of this study were supported by the Netherlands organization for Health Research and Development (ZonMw), project number 837001409.
Trial identification number: Netherlands trial register number 4797.
The funding organization had no role in the design or conduct of the study; the collection, management, analysis, or interpretation of the data; the preparation, review, or approval of the manuscript; or the decision to submit the manuscript for publication.
The authors report no conflicts of interest.
REFERENCES
- 1.Koppen IJN, Vriesman MH, Saps M, et al. Prevalence of functional defecation disorders in children: a systematic review and meta-analysis. J Pediatr 2018; 198:121–130. [DOI] [PubMed] [Google Scholar]
- 2.Kovacic K, Sood MR, Mugie S, et al. A multicenter study on childhood constipation and fecal incontinence: effects on quality of life. J Pediatr 2015; 166:1482–1487. [DOI] [PubMed] [Google Scholar]
- 3.Rajindrajith S, Devanarayana NM, Weerasooriya L, et al. Quality of life and somatic symptoms in children with constipation: a school-based study. J Pediatr 2013; 163:1069–1072. [DOI] [PubMed] [Google Scholar]
- 4.Varni JW, Bendo CB, Nurko S, et al. Health-related quality of life in pediatric patients with functional and organic gastrointestinal diseases. J Pediatr 2015; 166:85–90. [DOI] [PubMed] [Google Scholar]
- 5.Belsey J, Greenfield S, Candy D, et al. Systematic review: impact of constipation on quality of life in adults and children. Aliment Pharmacol Ther 2010; 31:938–949. [DOI] [PubMed] [Google Scholar]
- 6.Koppen IJN, van Wassenaer EA, Barendsen RW, et al. Adherence to polyethylene glycol treatment in children with functional constipation is associated with parental illness perceptions, satisfaction with treatment, and perceived treatment convenience. J Pediatr 2018; 199:132.e1–139.e1. [DOI] [PubMed] [Google Scholar]
- 7.Tabbers MM, DiLorenzo C, Berger MY, et al. Evaluation and treatment of functional constipation in infants and children: evidence-based recommendations from ESPGHAN and NASPGHAN. J Pediatr Gastroenterol Nutr 2014; 58:258–274. [DOI] [PubMed] [Google Scholar]
- 8.Koppen IJN, Saps M, Lavigne JV, et al. Recommendations for pharmacological clinical trials in children with functional constipation: The Rome foundation pediatric subcommittee on clinical trials. Neurogastroenterol Motil 2018; 30:e13294. [DOI] [PubMed] [Google Scholar]
- 9.Davis E, Nicolas C, Waters E, et al. Parent-proxy and child self-reported health-related quality of life: using qualitative methods to explain the discordance. Qual Life Res 2007; 16:863–871. [DOI] [PubMed] [Google Scholar]
- 10.Matza LS, Swensen AR, Flood EM, et al. Assessment of health-related quality of life in children: a review of conceptual, methodological, and regulatory issues. Value Health 2004; 7:79–92. [DOI] [PubMed] [Google Scholar]
- 11.Ravens-Sieberer U, Erhart M, Wille N, et al. Generic health-related quality-of-life assessment in children and adolescents. Pharmacoeconomics 2006; 24:1199–1220. [DOI] [PubMed] [Google Scholar]
- 12.Wallander JL, Koot HM. Quality of life in children: a critical examination of concepts, approaches, issues, and future directions. Clin Psychol Rev 2016; 45:131–143. [DOI] [PubMed] [Google Scholar]
- 13.Eiser C, Morse R. Can parents rate their child's health-related quality of life? Results of a systematic review. Qual Life Res 2001; 10:347–357. [DOI] [PubMed] [Google Scholar]
- 14.Silva N, Crespo C, Carona C, et al. Why the (dis)agreement? Family context and child-parent perspectives on health-related quality of life and psychological problems in paediatric asthma. Child Care Health Dev 2015; 41:112–121. [DOI] [PubMed] [Google Scholar]
- 15.Rebok G, Riley A, Forrest C, et al. Elementary school-aged children's reports of their health: a cognitive interviewing study. Qual Life Res 2001; 10:59–70. [DOI] [PubMed] [Google Scholar]
- 16.Riley AW. Evidence that school-age children can self-report on their health. Ambul Pediatr 2004; 4 (4 suppl):371–376. [DOI] [PubMed] [Google Scholar]
- 17.Upton P, Lawford J, Eiser C. Parent-child agreement across child health-related quality of life instruments: a review of the literature. Qual Life Res 2008; 17:895. [DOI] [PubMed] [Google Scholar]
- 18.Galloway H, Newman E. Is there a difference between child self-ratings and parent proxy-ratings of the quality of life of children with a diagnosis of attention-deficit hyperactivity disorder (ADHD)? A systematic review of the literature. Atten Defic Hyperact Disord 2017; 9:11–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Meyer M, Oberhoffer R, Hock J, et al. Health-related quality of life in children and adolescents: current normative data, determinants and reliability on proxy-report. J Paediatr Child Health 2016; 52:628–631. [DOI] [PubMed] [Google Scholar]
- 20.Hartman EE, Pawaskar M, Williams, et al. Psychometric properties of PedsQL generic core scales for children with functional constipation in the Netherlands. J Pediatr Gastroenterol Nutr 2014; 59:739–747. [DOI] [PubMed] [Google Scholar]
- 21.Voskuijl WP, van der Zaag-Loonen HJ, Ketel IJ, et al. Health related quality of life in disorders of defecation: the Defecation Disorder List. Arch Dis Child 2004; 89:1124–1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bongers ME, van Dijk M, Benninga MA, et al. Health related quality of life in children with constipation-associated fecal incontinence. J Pediatr 2009; 154:749–753. [DOI] [PubMed] [Google Scholar]
- 23.Wille N, Badia X, Bonsel G, et al. Development of the EQ-5D-Y: a child-friendly version of the EQ-5D. Qual Life Res 2010; 19:875–886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rasquin A, Di Lorenzo C, Forbes D, et al. Childhood functional gastrointestinal disorders: child/adolescent. Gastroenterology 2006; 130:1527–1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pearson Education, Portney LG, Watkins MP. Foundations of Clinical Research: Applications to Practice. 3rd ed.2013. [Google Scholar]
- 26.Trevethan R. Intraclass correlation coefficients: clearing the air, extending some cautions, and making some requests. Health Serv Outcomes Res 2017; 17:127–143. [Google Scholar]
- 27.Giraudeau B, Mary J. Planning a reproducibility study: how many subjects and how many replicates per subject for an expected width of the 95 per cent confidence interval of the intraclass correlation coefficient. Stat Med 2001; 20:3205–3214. [DOI] [PubMed] [Google Scholar]
- 28.De Vet HC, Terwee CB, Mokkink LB, et al. Measurement in Medicine: A Practical Guide. 1st ed.Cambridge: Cambridge University Press; 2011. [Google Scholar]
- 29.Youssef NN, Langseder AL, Verga BJ, et al. Chronic childhood constipation is associated with impaired quality of life: a case-controlled study. J Pediatr Gastroenterol Nutr 2005; 41:56–60. [DOI] [PubMed] [Google Scholar]
- 30.Clarke MC, Chow CS, Chase JW, et al. Quality of life in children with slow transit constipation. J Pediatr Surg 2008; 43:320–324. [DOI] [PubMed] [Google Scholar]
- 31.Crowley AL, Yow E, Barnhart HX, et al. Critical review of current approaches for echocardiographic reproducibility and reliability assessment in clinical research. J Am Soc Echocardiogr 2016; 29:1144.e7–1145.e7. [DOI] [PubMed] [Google Scholar]
- 32.Brouwer CN, Maillé AR, Rovers MM, et al. Health-related quality of life in children with otitis media. Int J Pediatr Otorhinolaryngol 2005; 69:1031–1041. [DOI] [PubMed] [Google Scholar]


