Abstract
Objective
The aim of this study was to assess the efficacy and safety of intravenous ferric carboxymaltose (FCM) following hospitalization for acute gastrointestinal bleeding (AGIB) in the context of a restrictive transfusion strategy.
Patients and methods
A retrospective single-center study analyzed patients with AGIB (excluding AGIB secondary to portal hypertension) administered a single FCM dose with or without blood transfusion.
Results
Eighty-six episodes in 84 patients were analyzed. Seventy-nine patients had upper AGIB. Nineteen episodes were associated with hemodynamic instability. FCM was administered during hospitalization as a single dose of 1000 mg iron in 84/86 episodes and as a single dose of 500 mg iron in two episodes, with blood transfusion in 60/86 (69.8%) episodes. The mean hemoglobin (Hb) was 9.0 g/dl at admission, 7.6 g/dl at the lowest in-hospital value, 9.4 g/dl at discharge, and 12.7 g/dl at follow-up (mean: 55 days postdischarge) (P<0.001 for follow-up vs. all other timepoints). The lowest mean in-hospital Hb value was 7.2 and 8.8 g/dl, respectively, in patients with transfusion+FCM versus FCM alone; the mean Hb was 12.4 versus 13.7 g/dl at follow-up. In patients administered FCM alone, the mean Hb at follow-up in the subpopulations aged older than or equal to 75 years (n=33), Charlson comorbidity index of at least 3 (n=48), and Hb of up to 10 g/dl at admission (n=47) were 12.6, 13.1, and 13.3 g/dl, respectively. No adverse effects were detected.
Conclusion
Treatment with FCM for AGIB is associated with a good erythropoietic response and anemia correction after hospitalization, even in severe episodes or when transfusion is needed. FCM is safe and well tolerated, and may support a restrictive transfusion policy.
Keywords: acute gastrointestinal bleeding, anemia, ferric carboxymaltose, hemoglobin, intravenous iron
Introduction
Acute gastrointestinal bleeding (AGIB) is a common cause of hospitalization. Recent prospective studies have estimated the incidence to be 87 cases per 100 000 population for acute upper or lower gastrointestinal (GI) bleeding 1,2, and up to 76 patients are hospitalized per 100 000 population as a result 3,4. Mortality is high, with estimates typically in the range 3–14% 5–7.
The immediate clinical priority is to stabilize the patient, stop the hemorrhage, and where necessary use blood transfusion to correct severe anemia 8. Blood transfusion is widely used in cases of AGIB, but it is increasingly being recognized that overtransfusion should be avoided to improve survival rates 9. Transfusion increases the risk of nosocomial infection and is associated with increased mortality in high-risk hospitalized patients 10, as well as incurring substantial financial costs. Moreover, it is associated with an increased risk for rebleeding and higher mortality 9,11. Restricted use of blood transfusion, whereby it is administered only to patients with very low hemoglobin (Hb) levels, has become the standard of care in many types of AGIB 12. European and US guidelines recommend a restrictive transfusion strategy that targets a Hb level between 7 and 9 g/dl or higher Hb target levels if the patient has intravascular volume depletion or significant comorbidity such as ischemic cardiovascular disease 13,14. A recent meta-analysis of five randomized-controlled trials showed that compared with a liberal transfusion strategy, restrictive transfusion is associated with a significant reduction in all-cause mortality 15.
Longer-term restoration of normal Hb levels after AGIB, however, is often neglected. Current recommendations do not address the use of iron supplementation 13,14 despite the fact that anemia affects 50–80% of patients discharged after AGIB 16–18 and iron deficiency has been documented in ~50% of patients at discharge 19. Management of anemia is clearly important in this population, but a survey conducted in Canada in 2016 found that fewer than 15% of clinicians prescribe iron therapy in patients who are anemic after AGIB, and that oral iron is the selected mode of administration in 80% of treated patients 20. Another survey, in Denmark, reported that only 13% of discharged anemic patients are administered oral iron supplementation after AGIB, with none administered intravenous (i.v.) iron 16,21.
Some experts suggest that high-dose i.v. iron formulations would be logical after AGIB because iron replenishment is rapid and treatment is well tolerated, and i.v. therapy avoids the risk of poor compliance that can occur with oral iron treatment 22. In recent years, new i.v. iron preparations have been developed that allow larger doses of iron to be administered in a single dose, reducing the cumulative risk for infusion reactions as well as improving convenience for physicians and patients 23. One of these is ferric carboxymaltose (FCM), an i.v. iron preparation in which a nondextran, stable carbohydrate shell facilitates the release of iron in a controlled manner 24. FCM can be infused in a single dose of up to 1000 mg iron over 15 min. A network meta-analysis of six randomized-controlled trials across various indications of chronic kidney disease 25, inflammatory bowel disease 26, postpartum anemia 27,28, heavy uterine bleeding 29, and fatigue 30 has shown that FCM improves levels of serum ferritin and transferrin saturation, and corrects Hb levels, more rapidly than oral iron, and it is well tolerated 31. It has a low risk of hypersensitivity reactions 24.
There are no studies that assess the efficacy and safety of i.v. iron treatment in patients with AGIB. A retrospective analysis was carried out to assess the efficacy and safety of i.v. FCM following hospital admission for AGIB at a center where a restrictive blood transfusion policy is followed.
Patients and methods
This was a retrospective analysis of patients admitted with AGIB to the Gastroenterology Department of the Hospital Universitari Arnau de Vilanova, Lleida, Spain, in whom treatment included FCM therapy. The center routinely treats anemia in hospitalized patients with FCM (Ferinject, Vitor Pharma España S.L., Barcelona, Spain). The study included all eligible patients admitted from October 2012 to December 2015. Patients for inclusion in the current analysis were identified by searching the pharmacy records for all patients who received FCM during the study period, and then each clinical history was checked manually. Patients with AGIB because of portal hypertension were excluded. No other exclusion criteria were applied.
AGIB was defined as externalized bleeding (hematemesis, melena, or rectal bleeding) with/without hemodynamic instability [systolic blood pressure<100 mmHg with/without tachycardia (>100 bpm)].
Blood transfusion was administered on admission or during hospitalization if the Hb level was lower than 7 g/dl, or at higher levels of Hb if comorbidities or hemodynamic instability were present (at the physician’s discretion). The decision on whether or not to administer blood transfusion was not influenced by the patient’s iron status on the basis of mean corpuscular hemoglobin or mean corpuscular volume measurements.
FCM was administered as a single dose of 1000 mg iron over 15 min during hospitalization after the patient had been stabilized. A follow-up visit took place ~2 months after discharge, and included blood sampling for the determination of Hb level 32.
The following information was obtained from patients’ medical records: demographic and clinical data, presentation and severity of AGIB (symptoms, hemodynamic instability, Charlson comorbidity index 33, Glasgow–Blatchford index score 34), endoscopic features (cause of hemorrhage, endoscopic treatment, and Rockall index score 32), medical treatment (number of blood transfusion units, total dose of FCM), Hb level (at hospital admission, the lowest value during hospital stay, at discharge, and at follow-up), and the duration of hospital stay. The mean change in Hb from the lowest value during hospitalization to (a) the value at hospital discharge and (b) the follow-up visit was calculated. Changes in Hb to both timepoints were also analyzed in the following subgroups: (i) patients aged younger than 75 or older than or equal to 75 years at admission (ii) patients with a Charlson comorbidity index less than 3 or at least 3 and (iii) patients with Hb above 10 g/dl or up to 10 g/dl at admission to hospital. These subgroups were considered to be high-risk populations: older age is associated with a higher risk of mortality; comorbidity as measured by the Charlson comorbidity index incurs a higher risk of mortality; and the Hb level indicates the severity of the GI bleeding (Hb≤10 g/dl at hospital admission predicts iron deficiency anemia at 30 days after AGIB 18).
Values are reported as n (%) for categorical variables and as mean±SD values for continuous variables. Values were compared between subpopulations using the χ2-test, Student’s t-test, or paired t-test, as appropriate.
Study conduct
The study was carried out in accordance with the Declaration of Helsinki and Good Clinical Practice Guidelines. The study protocol was approved by the Hospital Ethics Committee and Health Authorities (protocol number: RBC-HE-2018-01). All authors approved the procedures for data analysis and approved the final version of the manuscript for publication.
Results
Study population
In total, 84 patients were admitted with AGIB and treated with FCM during the study period. Seventy-nine (94.0%) patients had upper GI bleeding and five patients had lower GI bleeding (6%). The majority of patients (58/84) were male. The population had a high rate of comorbidities, with 51 (60.7%) patients having a Charlson comorbidity index score of at least 3. Thirteen (15.5%) patients had experienced previous AGIB (Table 1). Overall, only four patients had previously received antiaGGreGant medication, and Hb levels at admission, discharge, and follow-up in these individuals were similar to the rest of the study population. No other patient had been treated with antiaGGreGant therapy or nonsteroidal anti-inflammatory drugs. Two patients were admitted twice for AGIB bleeding and received FCM and blood transfusion on both occasions, such that a total of 86 episodes were analyzed.
Table 1.
Demographic and clinical characteristics of patients admitted for acute gastrointestinal bleeding and treated with ferric carboxymaltose
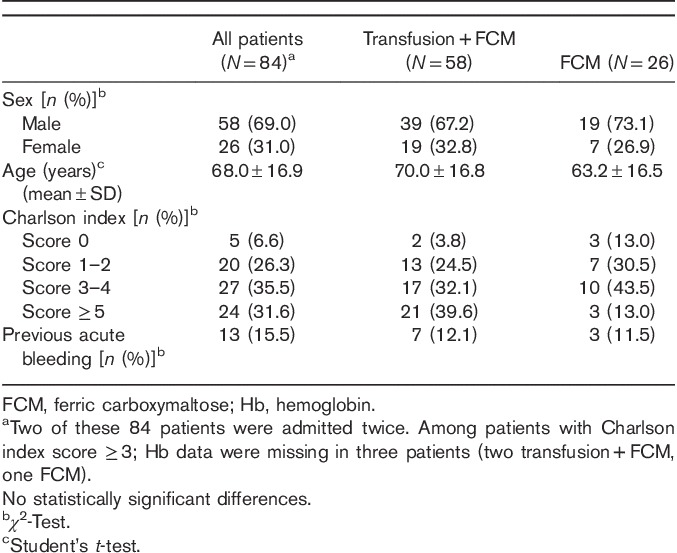
On admission, the majority presented with melenas [66/86 (76.7%)] and 21 (23.5%) patients presented with hematemesis. Approximately one in five [19/86 (22.1%)] had hemodynamic instability confirmed at hospital admission. Multiple presentations were common, with 17 patients having melena and symptoms of hemodynamic instability and nine having melena and hematemesis. In six (7%) patients, the initial symptom was weakness, presenting GI bleeding at admission. The most common causes of hemorrhage were duodenal and gastric ulceration (Table 2).
Table 2.
Characteristics of acute gastrointestinal bleeding episodes in patients treated with ferric carboxymaltose
The mean±SD levels of mean corpuscular hemoglobin (29.8±3.38 pg) and mean corpuscular volume (90.1±7.59 fl) across the study population were within the normal range at admission.
Treatment and hospital stay
The total dose of FCM was 1000 mg iron in 84/86 (96.5%) episodes, administered as a single infusion. In two further patients, who weighed less than 50 kg, a total dose of only 500 mg iron was calculated to be necessary. One patient was administered a dose of 1000 mg iron and also received an additional FCM dose of 500 mg iron after a low Hb value was recorded at the follow-up visit.
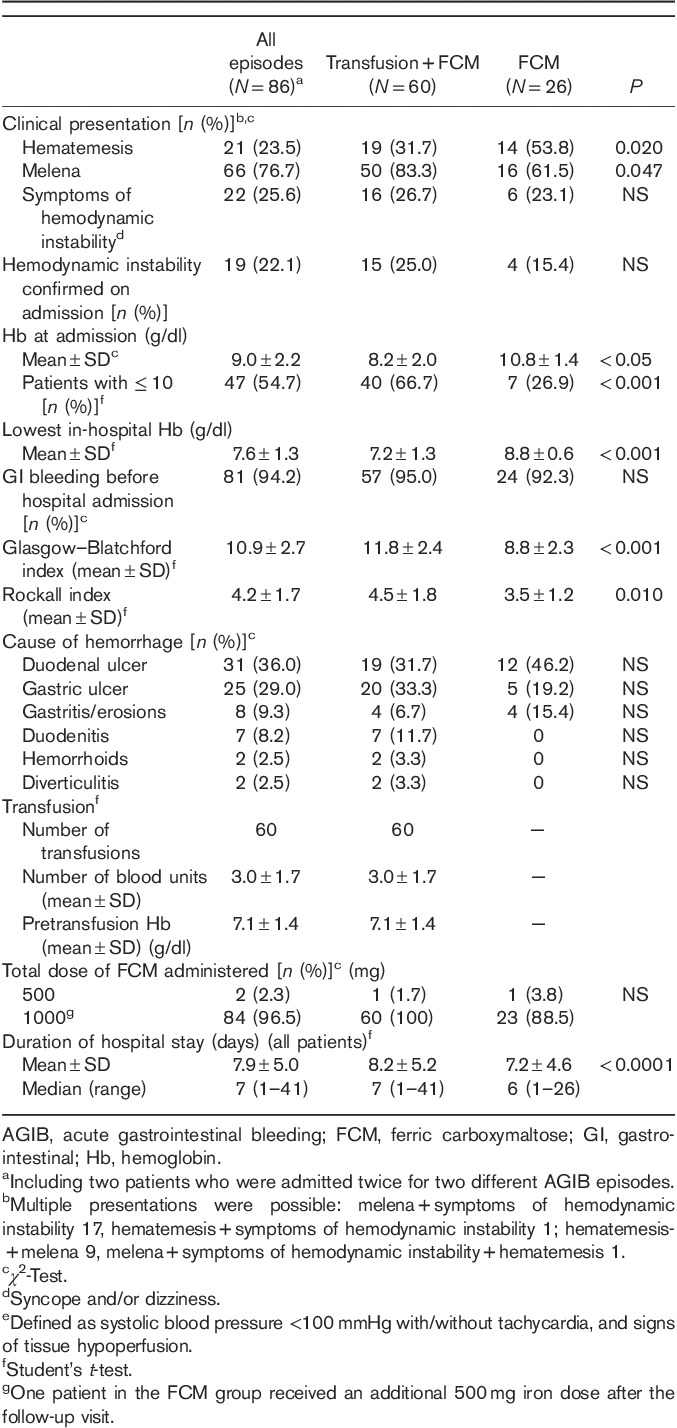
One or more blood transfusion was administered for 60/86 (69.8%) episodes using a mean of 3.0±1.7 units of blood (Table 2). No oral iron or vitamin supplements were administered to any patient.
The mean hospital stay associated with AGIB was 7.9±5.0 days. Patients requiring transfusion remained in hospital a mean of 8.2±5.2 days compared with 7.2±4.6 for those without transfusion (P<0.0001).
The postdischarge follow-up visit at the hospital outpatient clinic took place at a mean of 60.9±39.6 days after leaving hospital.
Safety events
There were no severe adverse events associated with FCM administration in any patient during hospitalization, and none were reported at the follow-up visit.
One patient experienced a rebleeding episode in the upper GI tract because of cameron ulcers at 3.5 months after hospital discharge. Two patients died because of underlying pathologies: one death occurred at 1 month after hospital discharge as a result of previously diagnosed pancreatic neoplasia and one death occurred 3 months after discharge because of complications related to GI bleeding in a patient with multiple comorbidities.
Hemoglobin response
In the total study cohort, the mean Hb at the time of hospital admission was 9.0±2.2 g/dl, decreasing to 7.6±1.3 g/dl at the lowest point during the hospital stay and before transfusion or administration of FCM. By the time of discharge, the mean Hb had increased to 9.4±1.1 g/dl (P<0.001 vs. the lowest in-hospital Hb value). The increase in the mean Hb during the hospital stay was largely accounted for by the group who received one or more blood transfusion (Fig. 1). The lowest mean Hb level during hospitalization was lower in the group administered blood transfusion plus FCM versus FCM alone (7.2±1.3 vs. 8.8±0.6 g/dl, P<0.001).
Fig. 1.
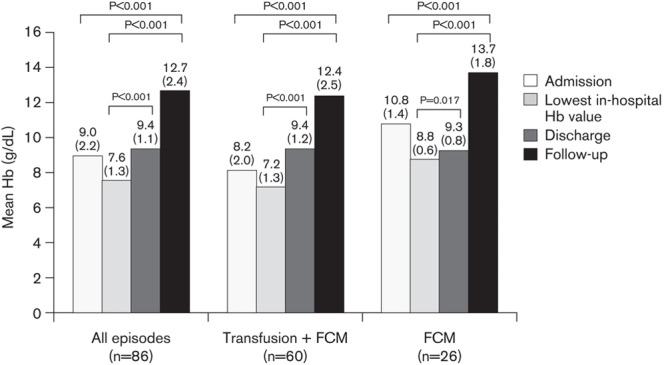
Mean hemoglobin (Hb) level during all episodes of acute gastrointestinal bleeding and in episodes treated with FCM with or without blood transfusion. Values are shown at the time of hospital admission, the lowest Hb value recorded during hospitalization (before treatment administration), at the time of hospital discharge, and at follow-up. Numbers in brackets indicate SD. P values are based on the paired t-test. FCM, ferric carboxymaltose.
By the time of discharge, both groups had a similar mean Hb level (9.4±1.2 and 9.3±0.8 g/dl, respectively) (Fig. 1). At the postdischarge follow-up visit, the mean Hb was 12.7±2.4 g/dl (P<0.001 vs. Hb at admission and vs. the lowest in-hospital Hb value).
The mean Hb was in the normal range both in the patients administered transfusion and FCM (12.4±2.5 g/dl) or FCM alone (13.7±1.8 g/dl) (Fig. 1). The Hb level was up to 10 g/dl at follow-up in 5/40 patients administered FCM plus transfusion FCM (13.9%) compared with 0/7 patients administered FCM alone. The increase in Hb from admission and from the lowest in-hospital Hb level to follow-up was significant with FCM with or without transfusion (P<0.001 in all cases) (Fig. 1). At follow-up, Hb levels normalized (Hb≥12 g/dl in women and ≥13 g/dl in men) in 60.0% of patients administered FCM plus transfusion versus 75.0% given FCM alone.
Hemoglobin response in at-risk patient subpopulations
Patients older than or equal to 75 years who received FCM plus transfusion [28/33 (84.8%)] had a mean Hb at admission of 8.0 versus 11.1 g/dl for those administered FCM alone (P=0.001); Hb was 9.7 vs. 9.6 g/dl at discharge (NS) and 12.7 vs. 12.6 g/dl at follow-up (NS). Patients treated with FCM and transfusion who had a Charlson comorbidity index of at least 3 [36/46 (78.3%) among patients for whom Hb data were available] had a mean Hb at admission of 7.9 versus 11.2 g/dl for FCM plus transfusion versus FCM alone (P<0.001); the mean Hb was 9.6 versus 9.7 g/dl at discharge (NS) and 12.0 versus 13.1 g/dl at follow-up (NS). Finally, for patients with Hb of up to 10 g/dl at admission, the mean Hb at admission in the group administered FCM plus transfusion [40/47 (85.1%)] was 7.3 versus 9.1 g/dl in the FCM group (P=0.004), 9.5 versus 8.9 g/dl at discharge (NS), and 12.6 versus 13.3 g/dl at follow-up (NS).
The mean increase in Hb from the lowest in-hospital Hb value to the point of discharge and to the follow-up visit was analyzed overall (Fig. 2a), in older patients [≥75 years (n=33)] (Fig. 2b), in patients with the highest morbidity scores [Charlson index≥3 (n=48)] (Fig. 2c), and in patients with moderate or severe anemia [Hb≤10 g/dl (n=47)] at admission (Fig. 2d). These subgroups each showed a larger mean increase in Hb from the lowest in-hospital Hb value to the value at discharge than in their lower-risk counterparts: 2.1±1.7 versus 1.4±1.5 g/dl for patients aged older than or equal to 75 years versus younger than 75 years (P<0.001); 1.9±1.6 versus 1.3±1.5 g/dl for patients with Charlson index of at least 3 versus less than 3 (P<0.001); and 2.0±1.7 versus 1.0±1.3 g/dl for patients with Hb of up to 10 g/dl at admission versus more than 10 g/dl (P<0.001).
Fig. 2.
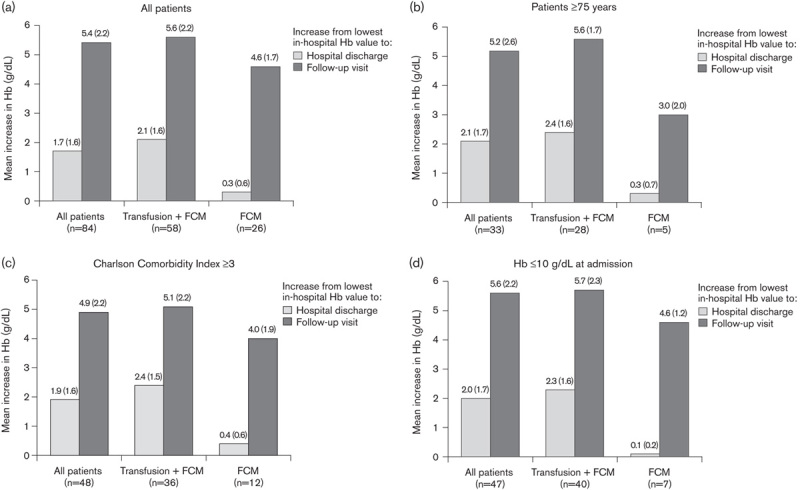
Mean increase in hemoglobin (Hb) from the lowest in-hospital Hb value to hospital discharge and to the follow-up visit in (a) all FCM-treated patients, (b) patients aged older than or equal to 75 years, (c) patients with Charlson Comorbidity Index of at least 3, and (d) patients with Hb of up to 10 g/dl at hospital admission. Values are shown at the time of hospital admission, the lowest Hb value recorded during hospitalization (before treatment administration), at the time of hospital discharge, and at follow-up. Numbers in brackets indicate SD. P values are based on the paired t-test. FCM, ferric carboxymaltose.
In each of these subpopulations, the change in the mean Hb level to the follow-up visit was higher in the patients administered blood transfusion in addition to FCM versus FCM alone (Fig. 2b–d). However, even in patients administered only FCM, the mean Hb level was within the normal range or close to normal by the follow-up visit: 12.6±2.3 g/dl in patients aged older than or equal to 75 years, 13.1±2.0 g/dl in patients with Charlson index of at least 3, and 13.3±1.3 g/dl in patients with Hb of up to 10 g/dl at admission.
Discussion
International recommendations advise that transfusion should be restricted to cases of severe anemia (<7 g/dl) in patients with AGIB, except under specific circumstances 13,14. At our center, use of blood transfusion was restricted to patients with the lowest Hb levels (mean 7.2 g/dl at the lowest in-hospital measurement in the current cohort), a strategy shown to improve survival rates 9.
The rapid rate of erythropoiesis that is required after acute blood loss demands an adequate supply of iron. Well-designed trials of i.v. iron therapy in this setting are rare. A randomized comparative trial by Ferrer-Barceló et al. 35, which compared FCM versus oral iron in 55 patients with anemia after AGIB, showed a faster Hb response and normalization of transferrin saturation by day 7 with FCM versus oral iron, with fewer adverse events and improved tolerance in the FCM cohort. A double-blind, placebo-controlled study has been carried out in which 97 patients with nonvariceal upper GI hemorrhage were randomized to i.v. FCM, oral iron, or placebo 36. The erythropoietic response was similar with either FCM or oral iron, but ferritin levels increased more rapidly with FCM (mean: 874 vs 149 μg/l at week 4). In our study, a single FCM dose was adequate to manage anemia without blood transfusion in 30% of patients. The mean Hb level at follow-up was either normal or close to normal both in patients administered transfusion and FCM and in those managed with FCM alone, with 60% of transfused patients and 75% of those receiving only FCM achieving normal Hb levels.
Three patient subpopulations considered to be at risk for poor outcomes were examined separately. The first group included older patients (≥75 years). Older age is a known risk factor for anemia, often associated with iron deficiency or renal insufficiency 37, and has been reported to increase the risk of anemia after AGIB 18. Older patients may also be more likely to experience rebleeding and in-hospital mortality than younger patients 6,38,39. The second group included patients with multiple comorbidities (Charlson comorbidity index≥3). Highly comorbid patients frequently have primary or secondary renal dysfunction, reducing erythropoietin production and restricting erythropoiesis, with an associated increase in predicted health costs 40 and are at greater risk for death following AGIB 41. The last group was patients with moderate or severe anemia at admission (≤10 g/dl). Low Hb (≤10 g/dl) at baseline is, as one would expect, predictive of subsequent anemia 18. Hb less than 10 g/dl has been shown to be an independent predictor for rebleeding, need for intervention, or death following AGIB 42. In these three groups, FCM alone achieved a mean Hb increase in the range 3.0–4.6 g/dl, and the mean Hb at follow-up was normal or close to normal.
According to our results, 15% of patients older than or equal to 75 years, 25% of patients with Charlson comorbidity index of at least 3, and 15% of patients with Hb of up to 10 g/dl at admission were managed only with FCM, achieving a significant increase in Hb and normalizing or almost normalizing Hb values at follow-up with a mean Hb of 12.6 g/dl in older than or equal to 75 years; 13.1 g/dl in Charlson index of at least 3; and 13.2 g/dl in those with Hb of up to 10 g/dl on admission.
These are encouraging findings, given the importance of achieving a sustained hematopoietic response and avoiding transfusion in these vulnerable patient groups.
In our series of 84 patients (86 AGIB episodes), no clinical adverse events were observed following FCM treatment. A meta-analysis of 11 randomized trials and three cohort studies of FCM at a dose of up to 1000 mg iron, in a range of clinical settings, has concluded that FCM is associated with fewer GI adverse events compared with oral iron, and that there are no FCM-related safety concerns 43. Another recent meta-analysis, which compared different i.v. iron preparations, observed that FCM tends to be better tolerated than iron sucrose or iron isomaltoside 44.
This analysis reflects routine clinical practice, including the typical range of patients who present with AGIB. The only exclusion was patients with bleeding secondary to portal hypertension, which represents a different condition and requires different management. The major limitation of the study is the absence of a control group receiving only transfusions. In this group of patients with a high rate of comorbidities and low baseline Hb, routine practice at our center mandates i.v. iron to ensure rapid repletion of iron stores after acute hemorrhage. Second, as a retrospective analysis, transfusion decisions were made by the responsible clinician on the basis of the center’s standard restrictive transfusion policy. Third, data on Hb levels after hospital discharge were obtained at routine outpatient follow-up visits and the timing varied between patients. However, as production of new red cells takes ~25 days 45 and the mean interval between hospital discharge and the follow-up visit was 60 days, follow-up data are likely to have reflected the peak erythropoietic response to iron repletion by FCM. Finally, markers of iron status were only available infrequently in the patient records, reflecting the retrospective nature of the study. Thus, although the high rate of Hb normalization points to effective iron replenishment, this cannot be confirmed directly. However, the randomized study by Ferrer-Barceló et al. 35, which compared FCM versus oral iron in AGIB patients, reported significantly greater repletion of iron deposits under FCM versus oral iron at the end of the 6-week study (75 vs. 26% P=0.0007).
Conclusion
Treatment with a single dose of FCM in patients with AGIB as part of a restrictive transfusion policy is associated with a good erythropoietic response and recovery from anemia after the immediate period of hospitalization. Although it is not the recommended dose to replenish iron deposits, a good erythropoietic response to a single infusion of FCM 1000 mg was observed in at-risk subgroups including patients aged older than or equal to 75 years, those with high comorbidity, and patients with moderate to severe anemia. Almost normal mean Hb values were achieved in these three groups, in whom improving Hb levels may be particularly important. FCM therapy was safe and well tolerated in this population, which included a high proportion of elderly patients and patients with multiple comorbidities, with no severe adverse events. Providing effective iron repletion during hospitalization through i.v. FCM therapy can help to avoid transfusion even in high-risk patients and also avoid the longer-term anemia so frequently observed after AGIB.
Acknowledgements
These data were presented as posters at the Asociación Española de Gastroenterología 20th Annual Congress, Madrid, 8–10 March 2017 and at United European Gastroenterology Week Congress, 25th Annual Congress, Barcelona, 28 October 2017 to 1 November 2017.
Data analysis and medical writing support from a freelance medical writer (C. Dunstall) were funded by Vifor Pharma, Glattbrugg, Switzerland.
Authors’ contributions: Raquel Ballester-Clau, Gisela Torres Vicente, Tania Voltà-Pardo, Laura López-Barroso, Josep Maria Reñé-Espinet, and Montse Planella de Rubinat, managed the patients and collected the data. Mercedes Cucala-Ramos provided medical support. All authors critically analyzed the data, reviewed the manuscript, and approved the final version for publication.
Conflicts of interest
Mercedes Cucala-Ramos is an employee of Vifor Pharma España S.L. For the remaining authors, there are no conflicts of interest.
References
- 1.Hreinsson JP, Kalaitzakis E, Gudmundsson S, Björnsson ES. Upper gastrointestinal bleeding: incidence, etiology and outcomes in a population-based setting. Scand J Gastroenterol 2013; 48:439–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hreinsson JP, Gumundsson S, Kalaitzakis E, Björnsson ES. Lower gastrointestinal bleeding: incidence, etiology and outcomes in a population-based setting. Eur J Gastro Hepatol 2013; 25:37–43. [DOI] [PubMed] [Google Scholar]
- 3.Paspatis GA, Konstantinidis K, Chalkiadakis I, Tribonias G, Chlouverakis G, Roussomoustakaki M. Changing trends in acute upper gastrointestinal bleeding in Crete, Greece: a population-based study. Eur J Gastroenterol Hepatology 2012; 24:102–103. [DOI] [PubMed] [Google Scholar]
- 4.Loperfido S, Baldo V, Piovesana E, Bellina L, Rossi K, Groppo M, et al. changing trends in acute upper-GI bleeding: a population-based study. Gastrointest Endosc 2009; 70:212–224. [DOI] [PubMed] [Google Scholar]
- 5.van Leerdam ME. Epidemiology of acute upper gastrointestinal bleeding. Best Pract Res Clin Gastroenterol 2008; 22:209–224. [DOI] [PubMed] [Google Scholar]
- 6.Aoki T, Nagata N, Niikura R, Shimbo T, Tanaka S, Sekine K, et al. Recurrence and mortality among patients hospitalized for acute lower gastrointestinal bleeding. Clin Gastroenterol Hepatol 2015; 13:488–494. [DOI] [PubMed] [Google Scholar]
- 7.Wysocki JD, Srivastav S, Winstead NS. A nationwide analysis of risk factors for mortality and time to endoscopy in upper gastrointestinal haemorrhage. Aliment Pharmacol Ther 2012; 36:30–36. [DOI] [PubMed] [Google Scholar]
- 8.PRODIGGEST Project. Healthcare PROtocols to improve inter Disciplinary management of gastrointestinal diseases in hospital settings. Asociación Española de Gastroenterología Protocolos Asistenciasles (Prodiggest). 2017. Available at: http://www.aegastro.es/publicaciones/publicaciones-aeg/protocolos-asistenciasles-prodiggest/anemia-y-ferropenia.
- 9.Villanueva C, Colomo A, Bosch A, Concepción M, Hernandez-Gea V, Aracil C, et al. Transfusion strategies for acute upper gastrointestinal bleeding. N Engl J Med 2013; 368:11–21. [DOI] [PubMed] [Google Scholar]
- 10.Marik PE, Corwin HL. Efficacy of red blood cell transfusion in the critically ill: a systematic review of the literature. Crit Care Med 2008; 36:2667–2674. [DOI] [PubMed] [Google Scholar]
- 11.Jairath V, Hearnshaw S, Brunskill SJ, Doree C, Hopewell S, Hyde C, et al. Red cell transfusion for the management of upper gastrointestinal haemorrhage. Cochrane Database Syst Rev 2010; 8:006613. [DOI] [PubMed] [Google Scholar]
- 12.Oakland K, Jairath V, Murphy MF. Advances in transfusion medicine: gastrointestinal bleeding. Transfus Med 2018; 28:132–139. [DOI] [PubMed] [Google Scholar]
- 13.Gralnek IM, Dumonceau JM, Kuipers EJ, Lanas A, Sanders DS, Kurien M, et al. Diagnosis and management of nonvariceal upper gastrointestinal hemorrhage: European Society of Gastrointestinal Endoscopy (ESGE) Guideline. Endoscopy 2015; 47:1–46. [DOI] [PubMed] [Google Scholar]
- 14.Laine L, Jensen DM. Management of patients with ulcer bleeding. Am J Gastroenterol 2012; 107:345–360. [DOI] [PubMed] [Google Scholar]
- 15.Odutayo A, Desborough MJ, Trivella M, Stanley AJ, Dorée C, Collins GS, et al. Restrictive versus liberal blood transfusion for gastrointestinal bleeding: a systematic review and meta-analysis of randomised controlled trials. Lancet Gastroenterol Hepatol 2017; 2:354–360. [DOI] [PubMed] [Google Scholar]
- 16.Bager P, Dahlerup JF. Lack of follow-up of anaemia after discharge from an upper gastrointestinal bleeding centre. Dan Med J 2013; 60:4583. [PubMed] [Google Scholar]
- 17.Lee JM, Kim ES, Chun HJ, Hwang YJ, Lee JH, Kang SH, et al. Discharge hemoglobin and outcome in patients with acute nonvariceal upper gastrointestinal bleeding. Endosc Int Open 2016; 4:865–869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Planella de Rubinat M, Teixidó Amorós M, Ballester Clau R, Trujillano Cabello J, Ibarz Escuer M, Reñé Espinet JM. Incidence and predictive factors of iron deficiency anemia after acute non-variceal upper gastrointestinal bleeding without portal hypertension. Gastroenterol Hepatol 2015; 38:525–533. [DOI] [PubMed] [Google Scholar]
- 19.El-Halabi MM, Green MS, Jones C, Salyers WJ., Jr Under-diagnosing and under-treating iron deficiency in hospitalized patients with gastrointestinal bleeding. World J Gastrointest Pharmacol Ther 2016; 7:139–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fortinsky KJ, Martel M, Razik R, Spiegle G, Gallinger ZR, Grover SC, et al. Red blood cell transfusions and iron therapy for patients presenting with acute upper gastrointestinal bleeding: a survey of Canadian gastroenterologists and hepatologists. Can J Gastroenterol Hepatol 2016; 2016:5610838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mearin F, Lanas Á, Bujanda L, Canelles P, Cotter J, Hervás A, et al. Open questions and misconceptions in the diagnosis and management of anemia in patients with gastrointestinal bleeding. Gastroenterol Hepatol 2018; 41:63–76. [DOI] [PubMed] [Google Scholar]
- 22.Schiefke I, Stein J, Wehkamp J, Hankey C, Wendt R, Clarke P, et al. Delphi study on the management of iron deficiency anaemia in patients with gastrointestinal bleeding. United European Gastroenterol J 2016; 4 (Suppl 5):A157–A720. [Google Scholar]
- 23.Auerbach M, Macdougall I. The available intravenous iron formulations: history, efficacy, and toxicology. Hemodial Int 2017; 21 (Suppl 1): 83–S92. [DOI] [PubMed] [Google Scholar]
- 24.Keating GM. Ferric carboxymaltose: a review of its use in iron deficiency. Drugs 2015; 75:101–127. [DOI] [PubMed] [Google Scholar]
- 25.Agarwal R, Rizkala AR, Bastani B, Kaskas MO, Leehey DJ, Besarab A. A randomized controlled trial of oral versus intravenous iron in chronic kidney disease. Am J Nephrol 2006; 26:445–454. [DOI] [PubMed] [Google Scholar]
- 26.Kulnigg-Dabsch S, Schmid W, Howaldt S, Stein J, Mickisch O, Waldhör T, et al. Iron deficiency generates secondary thrombocytosis and platelet activation in IBD: the randomized, controlled thromboVIT trial. Inflamm Bowel Dis 2013; 19:1609–1616. [DOI] [PubMed] [Google Scholar]
- 27.Seid MH, Derman RJ, Baker JB, Banach W, Goldberg C, Rogers R. Ferric carboxymaltose injection in the treatment of postpartum iron deficiency anemia: a randomized controlled clinical trial. Am J Obstet Gynecol 2008; 199:435–437. [DOI] [PubMed] [Google Scholar]
- 28.Van Wyck DB, Martens MG, Seid MH, Baker JB, Mangione A. Intravenous ferric carboxymaltose compared with oral iron in the treatment of postpartum anemia: a randomized controlled trial. Obstet Gynecol 2007; 110:267–278. [DOI] [PubMed] [Google Scholar]
- 29.Van Wyck DB, Mangione A, Morrison J, Hadley PE, Jehle JA, Goodnough LT. Large-dose intravenous ferric carboxymaltose injection for iron deficiency anemia in heavy uterine bleeding: a randomized, controlled trial. Transfusion 2009; 49:2719–2728. [DOI] [PubMed] [Google Scholar]
- 30.Krayenbuehl PA, Battegay E, Breymann C, Furrer J, Schulthess G. Intravenous iron for the treatment of fatigue in nonanemic, premenopausal women with low serum ferritin concentration. Blood 2011; 118:3222–3227. [DOI] [PubMed] [Google Scholar]
- 31.Rognoni C, Venturini S, Meregaglia M, Marmifero M, Tarricone R. Efficacy and safety of ferric carboxymaltose and other formulations in iron-deficient patients: a systematic review and network meta-analysis of randomised controlled trials. Clin Drug Investig 2016; 36:177–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rockall TA, Logan RF, Devlin HB, Northfield TC. Risk assessment after acute upper gastrointestinal haemorrhage. Gut 1996; 38:316–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987; 40:373–383. [DOI] [PubMed] [Google Scholar]
- 34.Blatchford O, Murray WR, Blatchford M. A risk score to predict need for treatment for upper-gastrointestinal haemorrhage. Lancet 2000; 356:1318–1321. [DOI] [PubMed] [Google Scholar]
- 35.Ferrer-Barceló L, Sanchis Artero L, Sempere García-Argüelles J, Canelles Gamir P, Pérez Gisbert J, Monzó Gallego A, et al. Tratamiento de la anemia ferropénica posthemorragia digestive aguda: ferroterapia oral frente a intravenosa [Spanish]. Gastroenterol Hepatol 2016; 39:156. [Google Scholar]
- 36.Bager P, Dahlerup JF. Randomised clinical trial: oral vs. intravenous iron after upper gastrointestinal haemorrhage: a placebo-controlled study. Aliment Pharmacol Ther 2014; 39:176–187. [DOI] [PubMed] [Google Scholar]
- 37.Patel KV. Epidemiology of anemia in older adults. Semin Hematol 2008; 45:210–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.González-González JA, Monreal-Robles R, García-Compean D, Paz-Delgadillo J, Wah-Suárez M, Maldonado-Garza HJ. Nonvariceal upper gastrointestinal bleeding in elderly people: Clinical outcomes and prognostic factors. J Dig Dis 2017; 18:212–221. [DOI] [PubMed] [Google Scholar]
- 39.Saltzman JR, Tabak YP, Hyett BH, Sun X, Travis AC, Johannes RS. A simple risk score accurately predicts in-hospital mortality, length of stay, and cost in acute upper GI bleeding. Gastrointest Endosc 2011; 74:1215–1224. [DOI] [PubMed] [Google Scholar]
- 40.Charlson M, Wells MT, Ullman R, King F, Shmukler C. The Charlson comorbidity index can be used prospectively to identify patients who will incur high future costs. PLoS ONE 2014; 9:112479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Marmo R, Koch M, Cipolletta L, Bianco MA, Grossi E, Rotondano G, et al. PNED 1 and PNED 2 Investigators. Predicting mortality in patients with in-hospital nonvariceal upper GI bleeding: a prospective, multicenter database study. Gastrointest Endosc 2014; 79:741–749. [DOI] [PubMed] [Google Scholar]
- 42.Hoffmann V, Neubauer H, Heinzler J, Smarczyk A, Hellmich M, Bowe A, et al. A novel easy-to-use prediction scheme for upper gastrointestinal bleeding: Cologne-WATCH (C-WATCH) Risk Score. Medicine (Baltimore) 2015; 94:1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Moor RA, Gaskell H, Rose P, Allan J. Meta-analysis of efficacy and safety of intravenous ferric carboxymaltose (Ferinject) from clinical trial reports and published trial data. BMC Blood Disord 2011; 11:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Aksan A, Işik H, Radeke HH, Dignass A, Stein J. Systematic review with network meta-analysis: comparative efficacy and tolerability of different intravenous iron formulations for the treatment of iron deficiency anaemia in patients with inflammatory bowel disease. Aliment Pharmacol Ther 2017; 45:1303–1318. [DOI] [PubMed] [Google Scholar]
- 45.Kalantar-Zadeh K, Streja E, Miller JE, Nissenson AR. Intravenous iron versus erythropoiesis-stimulating agents: friends or foes in treating chronic kidney disease anemia? Adv Chronic Kidney Dis 2009; 16:143–151. [DOI] [PubMed] [Google Scholar]


