ABSTRACT
Purpose
To determine the relationship between vitamin D status and exercise performance in a large, prospective cohort study of young men and women across seasons (study 1). Then, in a randomized, placebo-controlled trial, to investigate the effects on exercise performance of achieving vitamin D sufficiency (serum 25(OH)D ≥ 50 nmol·L−1) by a unique comparison of safe, simulated-sunlight and oral vitamin D3 supplementation in wintertime (study 2).
Methods
In study 1, we determined 25(OH)D relationship with exercise performance in 967 military recruits. In study 2, 137 men received either placebo, simulated sunlight (1.3× standard erythemal dose in T-shirt and shorts, three times per week for 4 wk and then once per week for 8 wk) or oral vitamin D3 (1000 IU·d−1 for 4 wk and then 400 IU·d−1 for 8 wk). We measured serum 25(OH)D by high-pressure liquid chromatography tandem mass spectrometry and endurance, strength and power by 1.5-mile run, maximum dynamic lift and vertical jump, respectively.
Results
In study 1, only 9% of men and 36% of women were vitamin D sufficient during wintertime. After controlling for body composition, smoking, and season, 25(OH)D was positively associated with endurance performance (P ≤ 0.01, ΔR2 = 0.03–0.06, small f2 effect sizes): 1.5-mile run time was ~half a second faster for every 1 nmol·L−1 increase in 25(OH)D. No significant effects on strength or power emerged (P > 0.05). In study 2, safe simulated sunlight and oral vitamin D3 supplementation were similarly effective in achieving vitamin D sufficiency in almost all (97%); however, this did not improve exercise performance (P > 0.05).
Conclusions
Vitamin D status was associated with endurance performance but not strength or power in a prospective cohort study. Achieving vitamin D sufficiency via safe, simulated summer sunlight, or oral vitamin D3 supplementation did not improve exercise performance in a randomized-controlled trial.
Key Words: CHOLECALCIFEROL, 25-HYDROXYVITAMIN D, UVB, ENDURANCE, STRENGTH, POWER
Vitamin D can be obtained from dietary sources but is primarily synthesized by skin exposure to sunlight ultraviolet B (UVB) radiation. Those who live at latitudes >35° or live indoors for the majority of sunlight hours and cover-up from the sun are at higher risk for vitamin D insufficiency (serum 25-hydroxyvitamin D (25(OH)D) < 50 nmol·L−1 (1,2)). Avoiding low serum 25(OH)D is essential for musculoskeletal health with current Institute of Medicine (IOM) and European Food Safety Authority (EFSA) recommendations to maintain serum 25(OH)D concentration ≥ 50 nmol·L−1 (3,4).
Vitamin D stimulates skeletal muscle protein synthesis via vitamin D receptor–mediated signaling (5) and may improve cardiac and endothelial function (6,7); as such, avoiding low serum 25(OH)D and achieving vitamin D sufficiency may be important for both strength and endurance type exercise (1,8). Positive associations between vitamin D status and physical performance have been reported in studies with elderly participants (9); for example, correcting vitamin D deficiency (serum 25(OH)D < 30 nmol·L−1) has been shown to increase strength in the elderly (10). Whether vitamin D has measurable and meaningful effects on exercise performance in young otherwise healthy adults is a matter of continued debate (11). Cross-sectional studies investigating the influence of vitamin D status on exercise performance in young healthy adults often present conflicting findings (12); likely contributing factors include small samples sizes and a lack of control over variables that may influence exercise performance (i.e., age, sex, body composition, smoking, physical activity and season) (8,13,14). Interpreting the findings from vitamin D supplementation studies is also challenging (11). The participant populations in some supplementation studies were vitamin D sufficient at baseline (15,16), and studies have used greater than currently recommended oral and ultraviolet radiation (UVR) doses; raising the risk of vitamin D toxicity (15,17,18) (tolerable upper intake 4000 IU·d−1) (3,4) and sunburn, which is a risk factor for skin cancer (19). Claims have been made of benefits to exercise performance in early UVR studies using sun lamps (8), including purported benefits of UVR for cardiovascular fitness and local muscular endurance (20). Although intriguing, these claims should be interpreted with due caution as the studies involved were not placebo-controlled and they made no assessment of serum 25(OH)D (20). As such, randomized, placebo-controlled trials investigating the influence of recommended oral vitamin D supplementation and safe simulated sunlight exposure on vitamin D status and exercise performance are required.
Here we present results from a prospective cohort study conducted during all seasons (study 1) and a randomized, placebo-controlled supplementation study that commenced in the UK winter, when vitamin D status was at its nadir (study 2). In study 1, we examined the relationship between serum 25(OH)D and endurance, strength, and power exercise performance in 967 young, healthy men and women, after adjusting for variables considered to influence exercise performance (e.g., body composition, smoking, and season). In study 2, we determined the effect of 12 wk vitamin D supplementation, by either simulated sunlight in accordance with recommendations on safe, casual sunlight exposure (21), or oral vitamin D3, on serum 25(OH)D and exercise performance. We hypothesized that wintertime vitamin D supplementation achieving vitamin D sufficiency (serum 25(OH)D ≥ 50 nmol·L−1) would improve exercise performance.
METHODS
Studies received ethics approval from the UK Ministry of Defence Research Ethics Committee and were conducted in accordance with the Declaration of Helsinki (2013). British Army recruit volunteers participated in study 1 and study 2 after providing fully informed written consent and passing a physician-screened medical assessment. Men (study 1 and study 2) were located at Infantry Training Centre Catterick, UK (latitude, 54°N) and women (study 1) were located at Army Training Centre Pirbright, UK (latitude, 51°N). All volunteers were studied during Basic Military Training that follows a generic syllabus of basic military skills including physical training, weapon handling, map reading, and field craft. The progressive, structured, physical training program included: endurance training, typically involving running in groups, with and without load carriage; circuit training, consisting of high-repetition, low force exercises using all major muscle groups; agility based gymnasium work using benches and ropes; and assault course practice. Marching with various loads while on military exercise and military drill were also undertaken.
Study 1
Participants and study design
Nine hundred sixty-seven men and women (age, 22 ± 3 yr; 95% white ethnicity; n = 621 men: body mass, 75.0 ± 10.0 kg; height, 1.78 ± 0.06 m; body mass index (BMI), 23.8 ± 2.8 kg·m−2; body fat, 19.8 ± 5.3%; current smokers, 45%; n = 346 women: body mass, 63.9 ± 7.9 kg; height, 1.65 ± 0.06 m; BMI, 23.4 ± 2.4 kg·m−2; body fat, 30.2 ± 4.9%; current smokers, 25%) participated in this prospective cohort study between January 2014 and September 2015.
Experimental procedures
We collected baseline measurements from each participant during week 1 of training; including a venous blood sample for the determination of serum 25(OH)D; body composition by dual-energy x-ray absorptiometry (DXA) (Lunar iDXA; GE Healthcare, Buckinghamshire, UK); ethnicity and smoking history by questionnaire; and exercise performance. During the DXA participants were instructed to lie motionless in the supine position for the duration of the scan, with straps fitted around their lower limbs to minimize movement. Men wore only underwear, and women wore light clothing. The DXA scanner was calibrated each morning before use. To assess endurance exercise performance, the time to complete a best effort 1.5-mile run on an outdoor course was recorded to the nearest second. The 1.5-mile run is used widely among military personnel, with performance indicative of an individual’s maximal aerobic capacity (22). Participants were highly motivated because their best effort was required for progression in their military careers. We determined maximum strength as the maximal weight lifted on a machine that simulates a power clean weightlifting movement, as described previously (23). Explosive power was assessed by counter-movement jump using a jump mat (Takei Scientific Instruments, Tokyo, Japan), as described (23), along with the following validated equation: explosive power (W) = (51.9 × maximal vertical jump height (cm)) + (48.9 × body mass (kg)) − 2007 (24). Participants were instructed to jump as high as possible three times, with their hands placed on their hips to prevent upper limb assistance. Where an increase in jump height occurred across jumps 1 to 3, indicative of a learning effect, a fourth jump was made. Maximal vertical jump height was recorded as the highest score achieved. Test–retest reliability of r ≥ 0.90 has been reported for these performance tests (23). After 12 wk of training, a cohort of 331 participants (170 men and 161 women) repeated baseline measurements; these participants were randomly selected throughout the year to provide a full seasonal spread for follow-up measurements. In addition, medical records were accessed to calculate the number of incomplete training days due to illness or injury for each participant.
Study 2
Participants and study design
Men were eligible to participate in this double-blind, randomized, placebo-controlled trial if they had sun-reactive skin types I to IV (25); were not currently consuming supplements containing vitamin D; and had not used a sun bed or travelled to a sunny climate in the 3 months before the study. The study took place in 2016 and 2017, with participants commencing in January or February when ambient UVB was negligible at UK latitudes. One hundred thirty-seven men completed the 12-wk intervention with a compliance ≥80% (100% white ethnicity; age, 22 ± 3 yr; body mass, 77.0 ± 11.5 kg; height, 1.77 ± 0.06 m; BMI, 24.4 ± 2.8 kg·m−2; body fat, 21.5% ± 5.3%; current smokers, 34%). There were no differences between treatment and control groups for demographics, anthropometrics and serum vitamin D metabolites at baseline.
Experimental procedures
Participants were block randomized within their platoons to one of four, 12-wk intervention groups: 1) solar-simulated radiation (SSR); 2) SSR placebo (SSR-P); 3) oral vitamin D3 (ORAL); or 4) oral placebo (ORAL-P). Block randomization (using randomizer.org) resulted in an equal distribution of intervention groups within each platoon, and therefore ensured any differences in training conditions between platoons did not influence the study outcomes. The intervention strategy for the SSR and ORAL groups was to restore and then maintain IOM and EFSA recommended vitamin D sufficiency (serum 25(OH)D ≥ 50 nmol·L−1). Participants completed a 4-wk restoration phase, necessary because 25(OH)D was at its winter nadir, followed by an 8-wk maintenance phase (Fig. 1). Before and after the restoration and maintenance phases, exercise performance was assessed using identical procedures to study 1 and a venous blood sample was collected for the determination of serum vitamin D metabolites. Vitamin D from the diet was estimated in week 12 using a food frequency questionnaire, and solar UVR exposure was measured in weeks 4 and 11 using polysulphone badges (26). Dietary vitamin D intake was then calculated, excluding that which participants in the ORAL group received from their intervention. On completion of the study, participants were asked to guess which intervention, that is, active or control, they thought they had been receiving.
FIGURE 1.
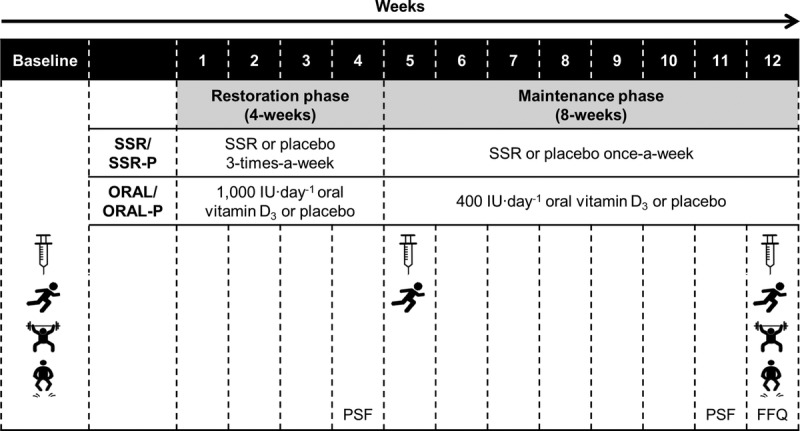
Schematic of study 2 procedures, to investigate the effect of vitamin D supplementation by SSR, oral vitamin D3 (ORAL), or placebo (SSR-P or ORAL-P) on exercise performance (1.5-mile run, maximum dynamic lift strength and explosive power), using a 4-wk restoration phase followed by an 8-wk maintenance phase. Syringe icon represents blood sample; running icon represents 1.5-mile run; weightlifting icon represents maximum dynamic lift strength; jumping icon represents explosive power; PSF, polysulphone badge; FFQ, food frequency questionnaire.
Simulated sunlight
Simulated sunlight was provided in accordance with guidelines on safe sunlight exposure for vitamin D synthesis (21); described previously to achieve serum 25(OH)D ≥ 50 nmol·L−1 in the majority of white skinned persons (19). Those assigned to the SSR intervention were exposed three times a week during the restoration phase to an experimenter-controlled constant UVR dose using a whole body irradiation cabinet (Hapro Jade, Kapelle, the Netherlands) fitted with Arimed B fluorescent tubes (Cosmedico, Stuttgart, Germany). The fluorescent tubes emitted a UVR spectrum similar to sunlight (λ, 290–400 nm; 95% UVA, 320–400 nm; 5% UVB, 290–320 nm) that was characterized by a spectroradiometer (USB2000+; Ocean Optics BV, Duiven, the Netherlands) radiometrically calibrated with traceability to UK national standards. During each exposure, participants received a 1.3× standard erythemal dose (SED), whereas wearing shorts and T-shirt to expose ~40% skin surface area. This dose is equivalent to ~15 min, midday summer sun exposure six times per week for a casually dressed individual in northern England (latitude 53.5°N) (19), and taking account of pre–vitamin D irradiance at different latitudes, can be related to exposure times at other world locations (27). For example, the equivalent exposure time in Philadelphia, Pennsylvania (40°N) would be ~12 min; and that for Oslo, Norway (60°N) would be ~18 min. During the maintenance phase, we exposed SSR participants to the same 1.3× SED dose only once a week: pilot investigations confirmed the required dose to maintain sufficiency (serum 25(OH)D ≥ 50 nmol·L−1). A constant SSR dose was maintained during the study by monitoring irradiance using a spectroradiometer (USB2000+; Ocean Optics BV) and adjusting for any decrease in measured irradiance emitted by increasing exposure time, as described (19) (mean duration of SSR exposures was 222 ± 23 s). We controlled the exposure time by using an electronic timer on the irradiation cabinet. For the SSR-P participants the number of intervention exposures each week and the exposure duration were the same as SSR except the irradiation cabinet fluorescent tubes were covered with transparent UVR blocking film (DermaGard UV film; SunGard, Woburn, MA). Spectroradiometry confirmed the UVR blocking film was effective at preventing transmission of 99.9% of UVR.
Oral vitamin D3
Participants receiving the ORAL intervention consumed a vitamin D3 capsule daily, containing a 1000 IU dose during the restoration phase and a 400 IU dose during the maintenance phase (Pure Encapsulations, Sudbury, MA). The restoration dose was based on previous predictive modeling to achieve serum 25(OH)D ≥ 50 nmol·L−1 (28), and pilot investigations that showed it achieved similar serum 25(OH)D concentrations to SSR; and was less than the tolerable upper intake recommended by the IOM and EFSA (3,4). The ORAL maintenance dose was also in accordance with recommendations (3,29). For 12 wk, ORAL-P participants consumed an identical looking cellulose placebo capsule daily (Almac Group, County Armagh, UK). Independent analysis found the vitamin D3 content of the 1000 and 400 IU capsules to be 1090 and 460 IU, respectively and confirmed the placebo did not contain vitamin D (NSF International Laboratories, Ann Arbor, MI).
Blood collection and analysis
Whole blood samples were collected by venepuncture from an antecubital vein into plain vacutainer tubes (Becton Dickinson, Oxford, UK) and left to clot for 1 h. Subsequently, samples were centrifuged at 1500g for 10 min at 4°C and the serum aliquoted into universal tubes before being immediately frozen at −80°C for later analysis. Total serum 25(OH)D was measured with high-pressure liquid chromatography tandem mass spectrometry; and serum 1,25-dihydroxyvitamin D (1,25(OH)2D; study 2) using the DiaSorin LIAISON XL 1,25(OH)2D chemiluminescent immunoassay (Stillwater, Minnesota, USA) method. Analyses were performed in a Vitamin D External Quality Assurance Scheme certified laboratory (Bioanalytical Facility, University of East Anglia, Norwich, UK).
Statistical analysis
In study 1, hierarchical multiple linear regression was used to determine the association between 25(OH)D and exercise performance at baseline and follow-up. For the 1.5-mile run, fat mass, smoking and season were included as covariates (8,13,14). For maximum dynamic lift strength and explosive power, covariates were lean body mass, smoking, height and season (8). Follow-up regression models included baseline covariates with the addition of incomplete training days to control for injury and illness (30,31). For these analyses, we estimated a minimum sample size of 155 using effect sizes from a previous study (32) and standard formula (n ≥ (8 / f2) + (number of predictors − 1)) (33). We completed final analyses on 967 participants after removing 2 men with z-scores ≥99.9th percentile for baseline 25(OH)D and 1.5-mile run time. We calculated Cohen’s f2 effect size for 25(OH)D using standard formula (34). We also compared exercise performance between baseline serum 25(OH)D quartiles using one-way ANOVA, and calculated Cohen’s d effect size (34). To correct the positive skew of variables not normally distributed, we log or square root transformed fat mass, lean body mass and serum 25(OH)D, where necessary. As required, we corrected the negative skew of 1.5-mile run time using a cube transformation. We used paired-sample t-tests to compare exercise performance between baseline and follow-up. In study 2, we used mixed model ANOVA to compare vitamin D metabolites and exercise performance between vitamin D supplementation (SSR and ORAL combined together) and placebo groups. A sample size estimation for this analysis indicated that 19 participants per group were required to produce an 80% chance of obtaining statistical significance at the 0.05 level, based on the effect size (f = 0.175) and correlation between repeated measures (r = 0.67) determined from study 1 run time data (G*Power, version 3.1.9.2). In addition, we compared individual active interventions to their respective placebos (SSR vs SSR-P; and ORAL vs ORAL-P) by mixed model ANOVA. Where statistically significant interactions were found, simple main effects were explored with one-way repeated-measures ANOVA and independent t-tests. Statistical analyses were performed using SPSS Statistics 22.0 (IBM, Armonk, NY). Statistical significance was accepted at P < 0.05.
RESULTS
Study 1
Exercise performance
For participants who completed measures at baseline and follow-up, 1.5-mile run time was faster at follow-up (men 627 ± 48 vs 578 ± 31 s; women 699 ± 54 vs 667 ± 44 s; P < 0.001). Maximum dynamic lift strength decreased in men (71 ± 12 kg vs 68 ± 11 kg; P < 0.01) but did not change in women (43 ± 9 kg vs 44 ± 9 kg; P > 0.05). From baseline to follow-up, explosive power decreased in men (3868 ± 619 W vs 3797 ± 573 W; P < 0.01) but increased in women (2766 ± 465 W vs 2840 ± 436 W; P < 0.01).
Low vitamin D status during winter
Baseline winter serum 25(OH)D was lower than all other seasons in men and lower than summer and fall in women (P < 0.001; Fig. 2A). During winter, only 9% of men and 36% of women were vitamin D sufficient (baseline 25(OH)D ≥ 50 nmol·L−1; Fig. 2B).
FIGURE 2.
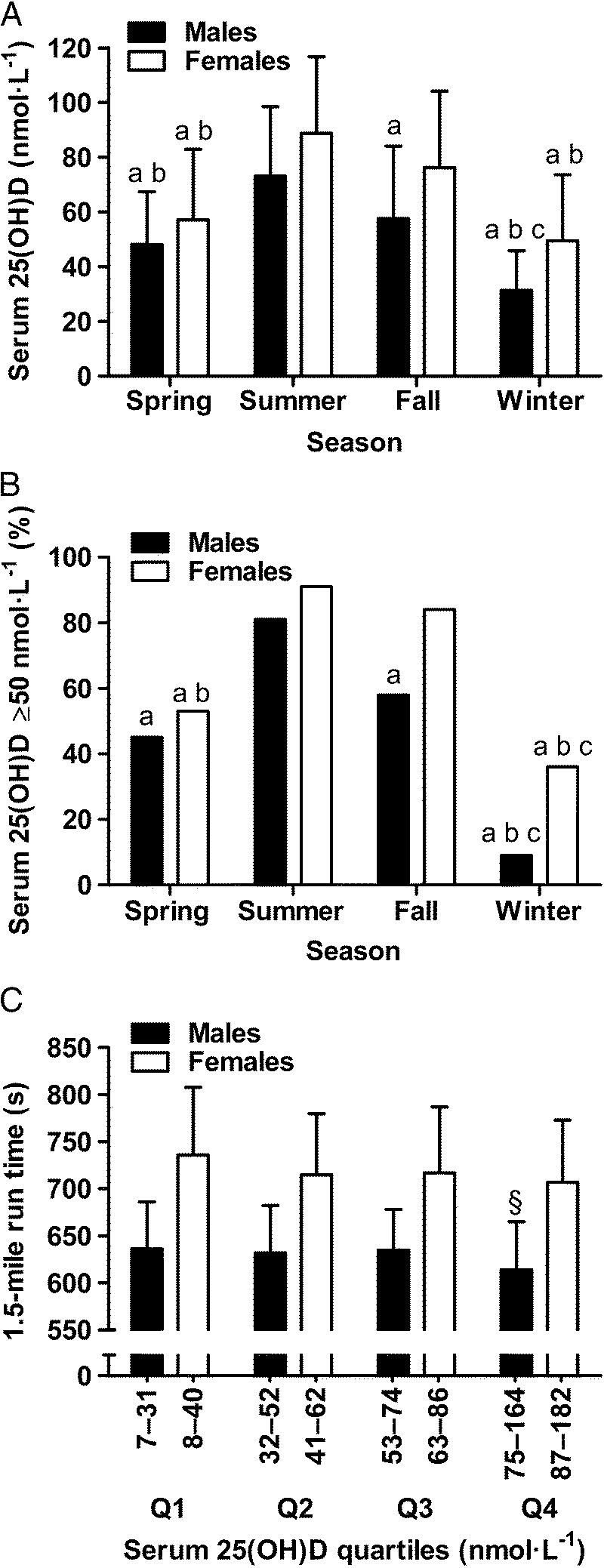
Seasonal variation in serum 25(OH)D (panel A) and percentage of participants categorized as vitamin D sufficient (serum 25(OH)D ≥ 50 nmol·L−1; panel B); and 1.5-mile run time by serum 25(OH)D quartiles (panel C) in 967 healthy, young males (n = 621) and females (n = 346) residing in the UK. a, lower than summer (P < 0.05). b, lower than fall (P < 0.05). c, lower than spring (P < 0.05). §, faster than quartiles 1, 2 and 3 (P < 0.05). Panel A and C data are mean ± SD.
Vitamin D status predicts endurance exercise performance
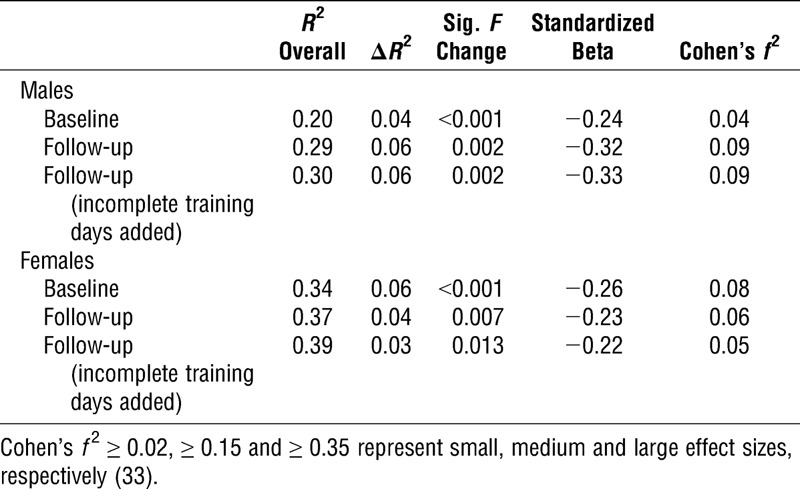
Using hierarchical multiple linear regression, serum 25(OH)D predicted endurance exercise performance after controlling for fat mass, smoking, and season; baseline serum 25(OH)D accounted for 4% and 6% of the variance in 1.5-mile run time in men and women, respectively (Table 1). Every 1 nmol·L−1 increase in 25(OH)D translated into 0.42 ± 0.16 s faster (±95% CI) 1.5-mile run time in men and 0.57 ± 0.25 s faster 1.5-mile run time in women. Although statistically significant, the small Cohen’s f2 effect sizes indicate the magnitude of additional variance explained in endurance performance by serum 25(OH)D is relatively small (Table 1). These relationships were not reliant on participants with high or low 25(OH)D concentrations because positive associations remained after removing men and women with 25(OH)D ≥ 75 or <30 nmol·L−1 (P < 0.05). At follow-up, after 12 wk of training, 25(OH)D was again positively associated with endurance exercise performance, irrespective of whether the number of incomplete training days was included in the model; 25(OH)D explained 6% and 3% of the variance in 1.5-mile run time in men and women, respectively, after controlling for fat mass, smoking, season, and the number of incomplete training days (Table 1). Using a simple one-way ANOVA, that is, without control for body composition, smoking and season, 1.5-mile run time was fastest among men with baseline serum 25(OH)D in the highest quartile (>75 nmol·L−1, P < 0.05, Cohen’s d effect size = 0.4); a similar trend was observed in women (Fig. 2C, P < 0.1; Cohen’s d effect size = 0.4).
TABLE 1.
Serum 25(OH)D predicts 1.5-mile run time after controlling for fat mass, smoking, and season, plus incomplete training days at follow-up.
Vitamin D status was not associated with strength or power exercise performance
Serum 25(OH)D was not significantly associated with maximum dynamic lift strength, or explosive power in men or women after controlling for lean mass, smoking, height and season (P > 0.05). At follow-up, once again 25(OH)D was not associated with maximum dynamic lift strength, or explosive power in men or women (P > 0.05). Analyzing quartiles of baseline serum 25(OH)D using simple one-way ANOVA, there were no differences in maximum dynamic lift strength or explosive power (P > 0.05).
Study 2
During the 12-wk intervention, daily sunlight exposure (0.22 ± 0.33 SED·d−1; P > 0.05) and daily dietary vitamin D intake were not different between groups (120 ± 88 IU·d−1, P > 0.05). Participants were sufficiently blinded to the intervention since only 35% correctly guessed their allocated group, 32% were incorrect, and 33% said they did not know whether they had received an active or placebo intervention.
Safe simulated sunlight and oral vitamin D3 restored vitamin D sufficiency in almost all
At baseline, approximately three quarters (74%) of volunteers were vitamin D insufficient (serum 25(OH)D < 50 nmol·L−1) and approximately one third (31%) were vitamin D deficient (serum 25(OH)D < 30 nmol·L−1). Both SSR and ORAL supplementation were successful strategies to achieve vitamin D sufficiency and maintain serum 25(OH)D concentrations so that at weeks 5 and 12 serum 25(OH)D concentrations in the SSR and ORAL groups were higher than their respective placebo groups (P < 0.001; Fig. 3). Indeed, by week 5, almost all SSR and ORAL participants were vitamin D sufficient (97%: serum 25(OH)D ≥ 50 nmol·L−1), and none were vitamin D deficient (serum 25(OH)D < 30 nmol·L−1); additionally, more than half (59%) had achieved the proposed optimal serum 25(OH)D ≥ 75 nmol·L−1 (1,2,35). Serum 1,25(OH)2D increased from baseline in the SSR and ORAL groups (P < 0.05; Fig. 4), and was higher than placebo groups at week 5 (P < 0.05). There was no difference between groups at week 12 (P > 0.05) because serum 1,25(OH)2D increased from weeks 5 to 12 in the placebo groups (P < 0.01).
FIGURE 3.
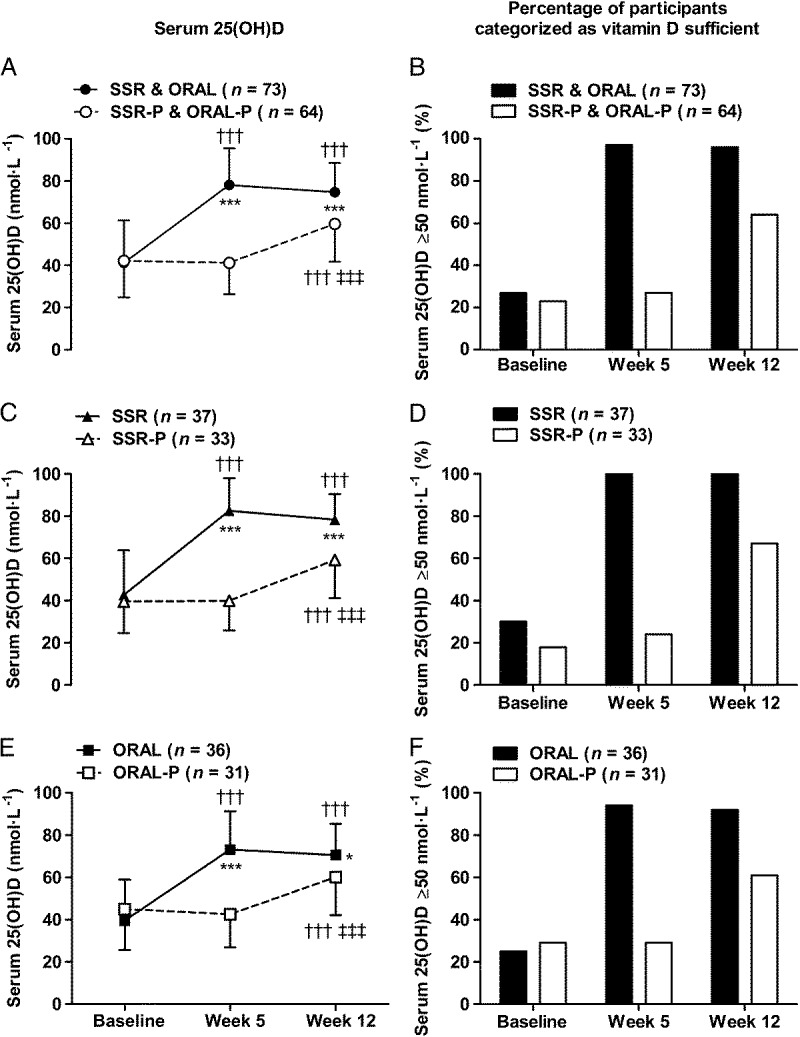
Serum 25(OH)D and percentage of participants categorized as vitamin D sufficient (serum 25(OH)D ≥ 50 nmol·L−1) in response to 12 wk of vitamin D supplementation by SSR and oral vitamin D3 (ORAL). Panels A and B show combined active interventions (SSR and ORAL) vs combined placebo (SSR-P and ORAL-P), panels C and D show SSR vs SSR-P, and panels E & F show ORAL vs ORAL-P. †††P < 0.001, greater than baseline. ‡‡‡P < 0.001, greater than week 5. *P < 0.05 and ***P < 0.001, greater than placebo. Data in panels A, C, and E are mean ± SD.
FIGURE 4.
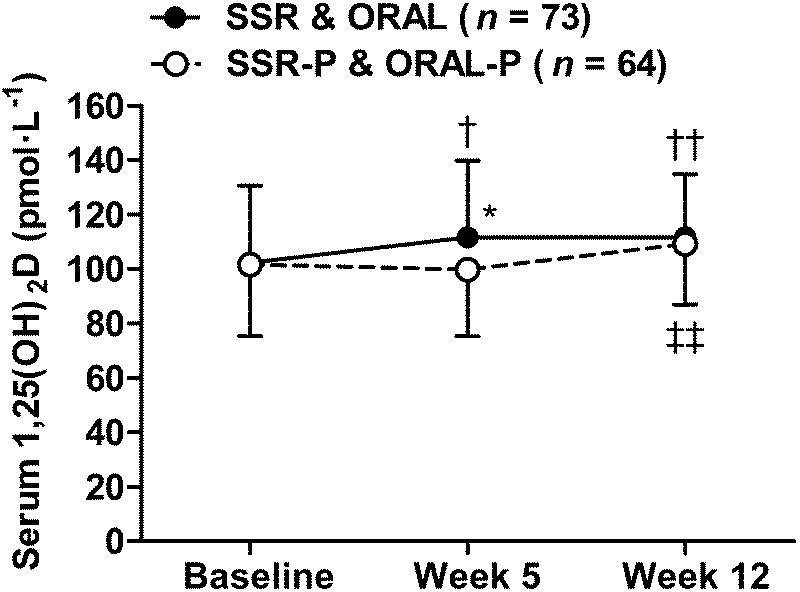
Serum 1,25(OH)2D in response to 12 wk of vitamin D supplementation by SSR and oral vitamin D3 (SSR and ORAL) vs placebo (SSR-P and ORAL-P). †P < 0.05 and ††P < 0.01, greater than baseline. ‡‡P < 0.01, greater than week 5. *P < 0.05, greater than placebo. Data are mean ± SD.
Safe simulated sunlight and oral vitamin D3 did not affect exercise performance
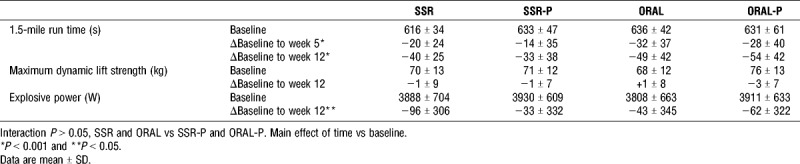
Vitamin D supplementation that achieved vitamin D sufficiency in almost all participants did not affect 1.5-mile run time, maximum dynamic lift strength or explosive power (Table 2, all interaction P values >0.05). Furthermore, participants on SSR and ORAL that achieved the proposed optimal vitamin D status (serum 25(OH)D ≥ 75 nmol·L−1) by week 5 did not improve their 1.5-mile run time more than those who received placebo and remained vitamin D insufficient (1.5-mile run time improvement by week 5: participants ≥75 nmol·L−1; −28 ± 32 s vs participants on placebo and < 50 nmol·L−1; −24 ± 34 s, P > 0.05, Cohen’s d effect size = 0.1). Additionally, those who achieved proposed optimal vitamin D status by week 12 did not improve their 1.5-mile run time, maximum dynamic lift strength or explosive power more than those who remained vitamin D insufficient (P > 0.05). As expected, 1.5-mile run time improved during training; however, explosive power decreased during training (Table 2, main effect of time; P < 0.01).
TABLE 2.
Influence of 12-weeks solar-simulated radiation (SSR), placebo solar-simulated radiation (SSR-P), oral vitamin D3 (ORAL), and oral placebo (ORAL-P) on exercise performance.
DISCUSSION
Primary Findings and Strengths
In study 1, in 967 young, healthy military recruits, we showed there was no influence of vitamin D status on muscular strength and power; however, a novel finding was that serum 25(OH)D was positively associated with endurance running performance in both men and women (Table 1). The findings of study 1 can be considered robust as they were observed in men and women; after controlling for body composition, smoking, and season; and after removing those with low or high vitamin D status (serum 25(OH)D < 30 or ≥ 75 nmol·L−1). Study 1 is the first to control for body composition in regression models investigating the relationship between vitamin D status and endurance running performance. Controlling for body composition is important because excess adipose tissue sequesters vitamin D; hence, individuals with high fat mass have lower serum 25(OH)D concentrations (36). High fat mass impairs exercise performance in young adults (13); consequently, high body fat and low availability of vitamin D may be responsible for poor performance in individuals with insufficient serum 25(OH)D concentrations. We have additional confidence in study 1 findings because the relationships were observed at the start of training and again after 12 wk of training; both before and after taking account of incomplete training days due to illness and injury (Table 1). In terms of practical significance, the magnitude of the association between serum 25(OH)D and endurance performance in study 1 can be considered small (Cohen’s f2 effect sizes <0.15). Nevertheless, in real-world terms, 1.5-mile run time was ~half-a-second faster for every 1 nmol·L−1 increase in serum 25(OH)D in men and women; equating to an ~20 s improvement in 1.5-mile run time for a 40 nmol·L−1 increase in serum 25(OH)D.
Given the low prevalence of vitamin D sufficiency during wintertime in study 1 (only 9% of men and 36% of women were vitamin D sufficient; Fig. 2B), in study 2, we explored the possibility that achieving vitamin D sufficiency from its wintertime nadir would enhance exercise performance. Study 2 involved a unique comparison of safe, simulated, casual skin sunlight exposure and oral vitamin D3 supplementation specifically designed to achieve vitamin D sufficiency. Contrary to our hypothesis, achieving and maintaining IOM and EFSA defined vitamin D sufficiency in 97% of participants, who received vitamin D supplementation (Fig. 3), did not benefit endurance, strength or power exercise performance (Table 2).
Vitamin D and Exercise Performance
Previous research relying on cross-sectional designs that did not control for important confounders (e.g., body composition) may have overestimated the influence of vitamin D on exercise performance (8,12). Consistent with this notion, in study 1, we show that endurance performance was best in those with proposed optimal serum 25(OH)D ≥ 75 nmol·L−1 (Fig. 2C) (1,2,35). Our randomized placebo-controlled trial in contrast showed no beneficial effect of achieving vitamin D sufficiency on exercise performance. Furthermore, in ~60% of our participants, vitamin D supplementation achieved the proposed optimal serum 25(OH)D ≥ 75 nmol·L−1 but this did not lead to improved exercise performance. These findings agree with other randomized, controlled trials suggesting that oral vitamin D supplementation does not directly benefit exercise performance (15,17,37). We add to this body of work by showing that exercise performance is not improved when vitamin D status is increased by oral vitamin D3 or UVB supplementation. Although supplementation restored vitamin D sufficiency, the relatively small increase in serum 1,25(OH)2D (Fig. 4) could conceivably account for the absence of a beneficial effect on exercise performance. Whether larger vitamin D doses, achieving greater than normal seasonal changes in serum 25(OH)D (e.g., > 100 nmol·L−1), would have beneficial effects on exercise performance remains unclear. Although this may appear to be the logical next step in supplementation studies, larger vitamin D doses may be ineffective because they will increase serum 24,25-dihydroxyvitamin D (24,25(OH)2D) concentrations, which may impair vitamin D receptor–1,25(OH)2D–mediated adaptations beneficial for exercise performance (11,38). Moreover, higher doses of simulated sunlight and oral vitamin D3 in excess of tolerable upper intakes (4000 IU·d−1) risk skin damage and vitamin D toxicity, respectively (3,21).
We recognize that the positive association between vitamin D status and endurance performance in study 1 may be at least partly explained by reverse causation: individuals with greater long-term physical activity are more likely to have greater aerobic fitness, spend more time outdoors exposed to sunlight and, in-turn, have higher serum 25(OH)D. A limitation of study 1 is that we did not account for long-term physical activity in our regression model. If serum 25(OH)D concentration in study 1 was reflective of an individual’s long-term vitamin D status, it may be that long-term vitamin D sufficiency is necessary for optimal endurance performance. Therefore, 12 wk of vitamin D supplementation in study 2 may have been an inadequate duration to benefit exercise performance. In accordance with this notion, an extended period of vitamin D supplementation improved skeletal muscle remodeling in untrained, young men during a progressive resistance training program (39). However, the reported benefits of longer-term vitamin D supplementation on skeletal muscle remodeling did not translate to improved muscular strength (39). A further limitation of both study 1 and study 2 was that exercise performance was assessed using relatively simple exercise tests in the field environment. Notwithstanding, the strength of these tests is that they are functionally relevant for athletic and military performance, involving multiple joints working in synergy, and have been shown to predict functional task success and injury risk (22). Participants’ performance on the tests was also typical of recruits who had passed military entry standards, and directly relevant to young, physically active adults. Further research is recommended to confirm our findings in elite athletes. A limitation of study 2 is that we only tested men; it was reasoned that vitamin D supplementation would most likely benefit exercise performance in men because vitamin D insufficiency was more prevalent in men than women in study 1. We also acknowledge that study 2 findings for SSR are only relevant to those with Fitzpatrick sun-reactive skin type I–IV (white skin) and not sun-reactive skin type V or VI (brown or black skin): serum 25(OH)D response to SSR in sun-reactive skin type V has been shown to be approximately half that achieved in those with sun-reactive skin type I–IV (40). Exposure to springtime ambient UVB in study 2 caused serum 25(OH)D to increase in the placebo groups at week 12 (Fig. 3). However, we demonstrated that exposure to ambient UVB was not different between groups, and no effect of vitamin D supplementation on exercise performance was seen when vitamin D–sufficient placebo group participants were removed from analyses.
Perspectives
The American College of Sports Medicine’s recent nutrition and athletic performance position stand called for research to investigate vitamin D’s potentially important influence on exercise performance; facilitating the determination of optimal vitamin D thresholds and supplementation recommendations (1). Our finding that vitamin D supplementation, even that which achieves the proposed optimal serum 25(OH)D ≥ 75 nmol·L−1, has no beneficial effect on exercise performance is therefore timely and has important implications for future nutrition and athletic performance recommendations. Despite evidence indicating that vitamin D supplementation does not benefit exercise performance, avoiding low serum 25(OH)D is considered important for musculoskeletal health (3,4,29). Vitamin D insufficiency is widespread in athletes and nonathletes (1,2). Correcting vitamin D insufficiency may also optimize training availability in athletes and military personnel by increasing resistance to upper respiratory tract infections (41) and reducing risk of injury (30). Our vitamin D supplementation strategies were effective in eliminating vitamin D deficiency and achieving vitamin D sufficiency in almost all. Future studies could use these methods to investigate the potential benefits of vitamin D supplementation on immune health and bone health; and the possible benefits of longer-term vitamin D supplementation.
Rather than restoring vitamin D sufficiency from its winter nadir, as in study 2, studies should investigate the effect of preventing the decline in end of summer serum 25(OH)D by commencing vitamin D supplementation in late summer or early fall and continuing until spring (~6 months). We propose the 400 IU·d−1 oral vitamin D3 dose from the maintenance phase of study 2 for the purpose of maintaining end of summer vitamin D sufficiency: this oral vitamin D3 supplementation approach corresponds with current IOM and EFSA recommendations (3,4). Oral vitamin D3 supplementation is recommended for this purpose because unlike simulated sunlight, there is no time burden for an individual; no requirement for bulky irradiation cabinets; and oral vitamin D3 supplementation is effective regardless of sun-reactive skin type (40). Studies are also required to further our understanding of how genetic variation between individuals (e.g. in vitamin D binding protein) might affect health and exercise performance outcomes to vitamin D supplementation (11). These studies, particularly those completed in diverse ethnic samples, should also consider assessing the bioavailable (free) fraction of vitamin D: health outcomes such as bone mineral density reportedly relate more closely to bioavailable vitamin D than to total serum 25(OH)D (11).
CONCLUSIONS
Vitamin D status predicted endurance exercise performance, but not strength or power, in a prospective cohort study of 967 young, healthy men and women after controlling for body composition, smoking and season. In a randomized, placebo-controlled trial, safe simulated summer sunlight or oral vitamin D3 were effective in achieving clinically important vitamin D sufficiency in almost all. However, vitamin D supplementation did not improve exercise performance, suggesting that vitamin D does not directly affect exercise performance.
Acknowledgments
The authors would like to thank Xin Hui Aw Yong, Mark Ward, Claire Potter, Anna Ferrusola-Pastrana and Dr. Thomas O’Leary for their assistance with data collection. We also thank Prof. Ann Webb and Dr. Richard Kift for providing and analyzing the polysulphone badges.
This work was funded by the Ministry of Defence (Army), UK
The authors declare no conflicts of interest. The results of study 1 and study 2 are presented clearly, honestly, and without fabrication, falsification, or inappropriate data manipulation; and, they do not constitute endorsement by ACSM.
Ethics approval for study 1 and study 2 was obtained from the UK Ministry of Defence Research Ethics Committee (protocol numbers 165/Gen/10 and 692/MoDREC/15, respectively).
REFERENCES
- 1.Thomas DT, Erdman KA, Burke LM. American College of Sports Medicine joint position statement. Nutrition and athletic performance. Med Sci Sports Exerc. 2016;48(3):543–68. [DOI] [PubMed] [Google Scholar]
- 2.Larson-Meyer DE, Willis KS. Vitamin D and athletes. Curr Sports Med Rep. 2010;9(4):220–6. [DOI] [PubMed] [Google Scholar]
- 3.Institute of Medicine. Dietary reference intakes for calcium and vitamin D. Washington. D.C.: National Academies Press; 2011. pp. 345–455. [PubMed] [Google Scholar]
- 4.European Food Safety Authority. Scientific opinion on dietary reference values for vitamin D. EFSA J. 2016;14(10):1–145. [Google Scholar]
- 5.Girgis CM, Clifton-Bligh RJ, Hamrick MW, Holick MF, Gunton JE. The roles of vitamin D in skeletal muscle: form, function, and metabolism. Endocr Rev. 2013;34(1):33–83. [DOI] [PubMed] [Google Scholar]
- 6.Allison RJ, Close GL, Farooq A, et al. Severely vitamin D-deficient athletes present smaller hearts than sufficient athletes. Eur J Prev Cardiol. 2015;22(4):535–42. [DOI] [PubMed] [Google Scholar]
- 7.Tarcin O, Yavuz DG, Ozben B, et al. Effect of vitamin D deficiency and replacement on endothelial function in asymptomatic subjects. J Clin Endocrinol Metab. 2009;94(10):4023–30. [DOI] [PubMed] [Google Scholar]
- 8.Cannell JJ, Hollis BW, Sorenson MB, Taft TN, Anderson JJ. Athletic performance and vitamin D. Med Sci Sports Exerc. 2009; 41(5):1102–10. [DOI] [PubMed] [Google Scholar]
- 9.Annweiler C, Schott AM, Berrut G, Fantino B, Beauchet O. Vitamin D-related changes in physical performance: a systematic review. J Nutr Health Aging. 2009;13(10):893–8. [DOI] [PubMed] [Google Scholar]
- 10.Stockton KA, Mengersen K, Paratz JD, Kandiah D, Bennell KL. Effect of vitamin D supplementation on muscle strength: a systematic review and meta-analysis. Osteoporos Int. 2011;22(3):859–71. [DOI] [PubMed] [Google Scholar]
- 11.Owens DJ, Allison R, Close GL. Vitamin D and the athlete: current perspectives and new challenges. Sports Med. 2018;48(1 Suppl):3–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Todd JJ, Pourshahidi LK, McSorley EM, Madigan SM, Magee PJ. Vitamin D: recent advances and implications for athletes. Sports Med. 2015;45(2):213–29. [DOI] [PubMed] [Google Scholar]
- 13.Mattila VM, Tallroth K, Marttinen M, Pihlajamaki H. Physical fitness and performance. Body composition by DEXA and its association with physical fitness in 140 conscripts. Med Sci Sports Exerc. 2007;39(12):2242–7. [DOI] [PubMed] [Google Scholar]
- 14.Song EY, Lim CL, Lim MK. A comparison of maximum oxygen consumption, aerobic performance, and endurance in young and active male smokers and nonsmokers. Mil Med. 1998;163(11):770–4. [PubMed] [Google Scholar]
- 15.Close GL, Leckey J, Patterson M, et al. The effects of vitamin D3 supplementation on serum total 25[OH]D concentration and physical performance: a randomised dose-response study. Br J Sports Med. 2013;47(11):692–6. [DOI] [PubMed] [Google Scholar]
- 16.Dubnov-Raz G, Livne N, Raz R, Cohen AH, Constantini NW. Vitamin D supplementation and physical performance in adolescent swimmers. Int J Sport Nutr Exerc Metab. 2015;25(4):317–25. [DOI] [PubMed] [Google Scholar]
- 17.Owens DJ, Webber D, Impey SG, et al. Vitamin D supplementation does not improve human skeletal muscle contractile properties in insufficient young males. Eur J Appl Physiol. 2014;114(6):1309–20. [DOI] [PubMed] [Google Scholar]
- 18.Close GL, Russell J, Cobley JN, et al. Assessment of vitamin D concentration in non-supplemented professional athletes and healthy adults during the winter months in the UK: implications for skeletal muscle function. J Sports Sci. 2013;31(4):344–53. [DOI] [PubMed] [Google Scholar]
- 19.Rhodes LE, Webb AR, Fraser HI, et al. Recommended summer sunlight exposure levels can produce sufficient (> or =20 ng ml(-1)) but not the proposed optimal (> or =32 ng ml(-1)) 25(OH)D levels at UK latitudes. J Invest Dermatol. 2010;130(5):1411–8. [DOI] [PubMed] [Google Scholar]
- 20.Allen RM, Cureton TK. Effect of ultraviolet radiation on physical fitness. Arch Phys Med Rehabil. 1945;26:641–4. [PubMed] [Google Scholar]
- 21.Advisory Group on Non-ionising Radiation. Ultraviolet radiation, vitamin D and health. London: Public Health England; 2017. pp. 7. [Google Scholar]
- 22.Friedl KE, Knapik JJ, Häkkinen K, et al. Perspectives on aerobic and strength influences on military physical readiness: report of an International Military Physiology Roundtable. J Strength Cond Res. 2015;29(11 Suppl):S10–23. [DOI] [PubMed] [Google Scholar]
- 23.Fortes MB, Diment BC, Greeves JP, Casey A, Izard R, Walsh NP. Effects of a daily mixed nutritional supplement on physical performance, body composition, and circulating anabolic hormones during 8 weeks of arduous military training. Appl Physiol Nutr Metab. 2011; 36(6):967–75. [DOI] [PubMed] [Google Scholar]
- 24.Sayers SP, Harackiewicz DV, Harman EA, Frykman PN, Rosenstein MT. Cross-validation of three jump power equations. Med Sci Sports Exerc. 1999;31(4):572–7. [DOI] [PubMed] [Google Scholar]
- 25.Fitzpatrick TB. The validity and practicality of sun-reactive skin types I through VI. Arch Dermatol. 1988;124(6):869–71. [DOI] [PubMed] [Google Scholar]
- 26.Webb AR, Kift R, Durkin MT, et al. The role of sunlight exposure in determining the vitamin D status of the U.K. white adult population. Br J Dermatol. 2010;163(5):1050–5. [DOI] [PubMed] [Google Scholar]
- 27.Webb AR, Kift R, Berry JL, Rhodes LE. The vitamin D debate: translating controlled experiments into reality for human sun exposure times. Photochem Photobiol. 2011;87(3):741–5. [DOI] [PubMed] [Google Scholar]
- 28.Cashman KD, Hill TR, Lucey AJ, et al. Estimation of the dietary requirement for vitamin D in healthy adults. Am J Clin Nutr. 2008; 88(6):1535–42. [DOI] [PubMed] [Google Scholar]
- 29.Scientific Advisory Committee on Nutrition. Vitamin D and Health. London: Public Health England; 2016. pp. 140. [Google Scholar]
- 30.Lappe J, Cullen D, Haynatzki G, Recker R, Ahlf R, Thompson K. Calcium and vitamin D supplementation decreases incidence of stress fractures in female navy recruits. J Bone Miner Res. 2008; 23(5):741–9. [DOI] [PubMed] [Google Scholar]
- 31.Halliday TM, Peterson NJ, Thomas JJ, Kleppinger K, Hollis BW, Larson-Meyer DE. Vitamin D status relative to diet, lifestyle, injury, and illness in college athletes. Med Sci Sports Exerc. 2011; 43(2):335–43. [DOI] [PubMed] [Google Scholar]
- 32.Fitzgerald JS, Peterson BJ, Warpeha JM, Johnson SC, Ingraham SJ. Association between vitamin D status and maximal-intensity exercise performance in junior and collegiate hockey players. J Strength Cond Res. 2015;29(9):2513–21. [DOI] [PubMed] [Google Scholar]
- 33.Green SB. How many subjects does it take to do a regression analysis. Multivariate Behav Res. 1991;26(3):499–510. [DOI] [PubMed] [Google Scholar]
- 34.Cohen J. Statistical Power Analysis for the Behavioral Sciences. 2nd ed Hillsdale, NJ: Lawrence Erlbaum; 1988. pp. 19–414. [Google Scholar]
- 35.Holick MF, Binkley NC, Bischoff-Ferrari HA, et al. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2011; 96(7):1911–30. [DOI] [PubMed] [Google Scholar]
- 36.Wortsman J, Matsuoka LY, Chen TC, Lu Z, Holick MF. Decreased bioavailability of vitamin D in obesity. Am J Clin Nutr. 2000;72(3): 690–3. [DOI] [PubMed] [Google Scholar]
- 37.Todd JJ, McSorley EM, Pourshahidi LK, et al. Vitamin D3 supplementation using an oral spray solution resolves deficiency but has no effect on VO2 max in Gaelic footballers: results from a randomised, double-blind, placebo-controlled trial. Eur J Nutr. 2017;56(4):1577–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Owens DJ, Tang JC, Bradley WJ, et al. Efficacy of high-dose vitamin D supplements for elite athletes. Med Sci Sports Exerc. 2017;49(2):349–56. [DOI] [PubMed] [Google Scholar]
- 39.Agergaard J, Trostrup J, Uth J, et al. Does vitamin-D intake during resistance training improve the skeletal muscle hypertrophic and strength response in young and elderly men?—a randomized controlled trial. Nutr Metab (Lond). 2015;12:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Farrar MD, Kift R, Felton SJ, et al. Recommended summer sunlight exposure amounts fail to produce sufficient vitamin D status in UK adults of South Asian origin. Am J Clin Nutr. 2011; 94(5):1219–24. [DOI] [PubMed] [Google Scholar]
- 41.Bermon S, Castell LM, Calder PC, et al. Consensus statement immunonutrition and exercise. Exerc Immunol Rev. 2017;23:8–50. [PubMed] [Google Scholar]


