Abstract
Elevated birth weight is linked to glucose intolerance and obesity health-related complications later in life. No studies have examined if infant birth weight is associated with gene expression markers of obesity and inflammation in a tissue that comes directly from the infant following birth. We evaluated the association between birth weight and gene expression on fetal programming of obesity. Foreskin samples were collected following circumcision, and gene expression analyzed comparing the 15% greatest birth weight infants (n = 7) versus the remainder of the cohort (n = 40). Multivariate linear regression models were fit to relate expression levels on differentially expressed genes to birth weight group with adjustment for variables selected from a list of maternal and infant characteristics. Glucose transporter type 4 (GLUT4), insulin receptor substrate 2 (IRS2), leptin receptor (LEPR), lipoprotein lipase (LPL), low density lipoprotein receptor-related protein 1 (LRP1), matrix metalloproteinase 2 (MMP2), plasminogen activator inhibitor-1 (PAI-1), and transcription factor 7-like 2 (TCF7L2) were significantly upregulated and histone deacetylase 1 (HDAC1) and thioredoxin (TXN) downregulated in the larger birth weight neonates versus controls. Multivariate modeling revealed that the estimated adjusted birth weight group difference exceeded one standard deviation of the expression level for eight of the 10 genes. Between 25% and 50% of variation in expression level was explained by multivariate modeling for eight of the 10 genes. Gene expression related to glycemic control, appetite/energy balance, obesity, and inflammation were altered in tissue from babies with elevated birth weight, and these genes may provide important information regarding fetal programming in macrosomic babies.
Keywords: Circumcision, fetal macrosomia, fetal programming, gestational weight gain
INTRODUCTION
Obesity has long-term, remarkable medical and public health implications.1 Obese women are more likely to deliver macrosomic infants.2, 3 Excessive weight gain in pregnancy also increases the risk for birth weight greater than the 90th percentile.4 Higher birth weights are associated with increased risk of adolescent obesity.5 An intergenerational risk of obesity and diabetes has been described, whereby maternal obesity is an independent risk factor for offspring obesity, separate from that of diabetes.6–9 The influences of fetal programming imposed by maternal obesity and diabetes may not be immediately evident at birth or early childhood, but may emerge later.10
The model of fetal programming outlined by David Barker classically describes the risk of disease among growth restricted infants during pregnancy.11 This hypothesis suggests fetal programming occurs based on maternal exposures which alters development and influences risk of future disease.11 An abnormal metabolic environment imposed by obesity or excess weight gain in pregnancy leads to fetal and neonatal overgrowth, childhood obesity and decreased insulin sensitivity.12, 13 These sequelae can lead to early onset of adult disease such as type 2 diabetes and metabolic syndrome. The cycle may continue when these women become pregnant.14 Several studies indicate that metabolic changes can be passed to subsequent generations. Responsible molecular mechanisms that contribute to offspring programming of obesity and type 2 diabetes include: hyperglycemia, impaired insulin signaling, increased circulation of adipocyte and inflammation signaling markers, abnormal adipose differentiation and metabolism, excessive placental hormone production, and alterations in the adipo-insular axis.15−17 Numerous studies have identified correlations between maternal factors and biochemical evidence of abnormal placental and fetal metabolism.18–22 In addition, animal models have tested the effects of under and over-nutrition in pregnancy and its effects on offspring.23, 24
We proposed to utilize neonatal foreskin to evaluate the effects of infant birth weight on fetal programming. Neonatal foreskin is a tissue that is readily available where circumcisions are performed and has previously been utilized to assess different cellular processes including wound healing and developmental abnormalities such as hypospadias.25–28 Importantly, the foreskin represents a terminal neonatal tissue which can be utilized in the study of developmental programming.29 We postulated that changes in gene expression involved in glucose metabolism, insulin signaling, inflammation, and oxidative stress in neonatal foreskin are associated with infant birth weight.
MATERIALS AND METHODS
Subjects
This was a birth cohort study of male neonates from 55 mother-baby couplets that was approved by the University of Kentucky Institutional Review Board. Subjects were born at the University of Kentucky from June 2012 to March 2013. Inclusion criteria were English-speaking mothers, ≥ 18 years old, and term delivery (≥ 37 weeks) of a non-anomalous, singleton male infant. Neonates admitted to the neonatal intensive-care unit were excluded. Mothers who had already consented to have a circumcision performed were approached for study enrollment. Foreskin samples were collected by study personnel after routine circumcisions were performed by the obstetric team on duty each day. The hypodermis (dartos) layer was immediately, grossly dissected from the dermis/epidermis. Samples were frozen in liquid nitrogen and stored at −80°C until processing. Eight samples were excluded because they were twins (n = 4) or preterm (n = 2), so tissues were not analyzed, or the RNA was degraded (n = 2).
Data Collection
Maternal demographic and clinical factors [pre-pregnancy body mass index (BMI), gestational weight gain, co-morbidities, and delivery data] and infant birth weight and anthropomorphic measurements (body length and head circumference) were recorded. Maternal ethnicity and smoking status were self-reported.
Sample Processing
mRNA Isolation.
Approximately 40 mg of tissue was placed in 1 mL Qiazol and homogenized using a Geno/Grinder 2010 (SPEX SamplePrep). RNA was extracted from hypodermis samples using the Qiagen RNeasy Lipid Tissue Mini Kit (Cat. No. 74804, Qiagen).30 RNA was eluted from column using 30 µl of nuclease free water. RNA integrity number (RIN) was measured using an Agilent 2100 BioAnalyzer (Agilent) and samples with RIN values lower than 6.8 were omitted (2 samples). The average RIN for the remaining 47 hypodermis samples was 8.3. cDNA was reverse transcribed using C1000 Thermal Cycler (Bio-Rad Laboratories, Inc.) and qScript cDNA SuperMix (Quanta Biosciences) for quantitative real-time PCR (qPCR).
NanoString CodeSet.
We pre-selected a panel of 120 genes involved in glucose metabolism, insulin signaling, inflammation, and oxidative stress. One hundred ng of RNA was loaded per sample for each NanoString run. NanoString results were normalized by creating scaling factors for positive controls (sum of positive controls) and pre-selected housekeeping genes (the geometric mean was calculated for 13 housekeeping genes for each sample) according to manufacturer’s suggestions. After normalization, all 13 housekeeping genes had a false discovery rate (FDR)-adjusted p-values above 0.10 in comparing the 7 largest babies to the other 40.31, 32 The FDR was defined with respect to these 13 genes. There were 17 (non-housekeeping) genes whose average corrected NanoString counts were below 15; these were excluded from the analyses described subsequently. A comprehensive list of analyzed genes is included (Supplemental Information). The NanoString nCounter system is highly reproducible and provides similar expression patterns to qPCR.33
qPCR.
Quantitative PCR was performed using a Step One Plus Real-Time PCR System (Applied Biosystems, Life Technologies). 20 ng cDNA/reaction was used in conjunction with TaqMan probes (Applied Biosystems, Life Technologies) developed using gene accession numbers associated with NanoString CodeSet above. Tubulin, beta class I (TUBB, Cat. # Hs00742828_s1) was selected as an endogenous control for normalization due to its expression levels being comparable for the two groups of babies. The top three genes from Table 2, plasminogen activator inhibitor 1 (PAI-1, Hs01126606_m1), glucose transporter type 4 (GLUT4, Hs00168966_m1), leptin receptor (LEPR, HS00174497_m1) were validated with qPCR for a subset of the samples. A subset of the control samples (n=7) were tissues collected from babies directly before or after the increased birth weight babies (n=7). Genes of interest were run in duplicate and TUBB was run in triplicate. Replicates were then averaged, and mRNA expression levels are presented as 2ΔΔCT. 34
Table 2.
Comparison of gene expression in hypodermis in the subsample with higher birth weight versus control
| Gene | Average Gene Expression Control a |
Average Gene Expression Increased Birth Weight b |
Fold Change c |
Unadjusted P value d |
FDR Adjusted P Value e |
|---|---|---|---|---|---|
| PAI-1 | 20.4 ± 1.8 | 66.6 ± 31.7 | 3.27 | 0.0011 | 0.0198 |
| GLUT4 | 27.6 ± 1.6 | 50.2 ± 7.9 | 1.82 | <0.0001 | 0.0022 |
| LEPR | 83.3 ± 3.8 | 145.6 ± 21.8 | 1.75 | <0.0001 | 0.0010 |
| LPL | 15.3 ± 0.9 | 24.6 ± 5.4 | 1.61 | 0.0038 | 0.0429 |
| MMP2 | 348.8 ± 22.3 | 558.9 ± 74.6 | 1.60 | 0.0013 | 0.0198 |
| IRS2 | 50.8 ± 2.3 | 75.9 ± 10.7 | 1.50 | 0.0007 | 0.0159 |
| LRP1 | 318.1 ± 10.0 | 443.8 ± 29.5 | 1.39 | 0.0044 | 0.0439 |
| TCF7L2 | 156.1 ± 5.9 | 201.6 ± 15.7 | 1.29 | 0.0053 | 0.0474 |
| HDAC1 | 434.5 ± 9.6 | 344.7 ± 15.4 | 0.79 | 0.0004 | 0.0120 |
| TXN | 3095.9 ± 144.8 | 2221.7 ± 178.6 | 0.72 | 0.0017 | 0.0213 |
Data are given as mean ± SEM,
Increased birth weight group is the top 15 percent from the study (n = 7) compared to the rest (n = 40),
Fold change in mean gene expression of increased birth weight divided by control,
Unadjusted p-values from t-tests are shown.
False discovery rate-adjusted p-values are displayed; since this table includes only genes with FDR-adjusted p-values below 0.05, the interpretation is that we expect 9 or 10 of these discoveries to be true.
Statistical Analysis
Statistical analyses were done using Sigma Plot 12.0 (Jandel Scientific, Chicago, IL), SAS 9.3 (SAS Institute Inc, Cary, NC), and Microsoft Excel 2013 (Microsoft Corp., Redmond WA). As this was a pilot and exploratory study, an a priori power analysis was not conducted. We targeted a sample size of 50. Greater birth weight was defined as the top 15% of the cohort (7 babies), and the control group consisted of the remainder of the samples (40 babies). In bivariate analysis (Table 1), we compared the two groups on continuous clinical factors via t-tests and on categorical clinical factors via Fisher’s exact tests. NanoString gene expression was analyzed via t-test according to birth weight stratum. If expression departed substantially from normality, a log transformation was performed before t-test; this happened once (nicotinamide phosphoribosyltransferase). If even log-transformed expression departed substantially from normality, a nonparametric rank sum test was performed in lieu of t-test; this happened once (superoxide dismutase 1). Group variances were treated as equal in the t-test unless a companion f-test yielded a contrary result (with P < 0.01), which happened three times [histone deacetylase 1 (HDAC1), low density lipoprotein receptor-related protein 1 (LRP1), and thioredoxin (TXN)]. The 90 resulting p-values (120 minus 13 [housekeeping] minus 17 [low counts]) were adjusted by FDR.31, 32 The 10 genes with FDR-adjusted P < 0.05 were ranked by fold change (Mean increased birth weight group/Mean control). In multivariate analysis on the 10 genes for which FDR-adjusted p-values were less than 0.05, the Schwarz Bayesian Criterion35 was used to select a multiple linear regression model predicting expression for each gene based on group membership (increased birth weight versus not) and a subset of variables chosen from the following list: ethnicity of the mother (Caucasian versus not), gestational weight gain category (over recommended versus not), mode of delivery (caesarean versus not), smoking during pregnancy (yes versus no), insurance status (private versus not), employment (full-time versus not), education (affirmed college degree or better versus not), feeding (complete or partial use of bottle versus not), third trimester glucose tolerance test, ponderal index, age at delivery, gravida, parity, pre-pregnancy weight, pre-pregnancy BMI, gestational weight gain, gestational age, day of life for circumcision, birth weight, birth length, and head circumference. Ten records (out of 47) had missing values on glucose tolerance test or ponderal index, which were imputed by mean value within birth weight group. Ordinary least squares was used for model fitting, unless birth weight group variances were substantially different (as judged by the aforementioned f-test), in which case weighted least squares was employed. qPCR gene expression was compared between birth weight groups by t-test and natural log transformation performed preceding t-test when normality failed (PAI-1 and GLUT4). Continuous data are summarized as mean ± SEM and categorical data by counts.
Table 1.
Maternal Demographics of Study Sample
| Maternal Variable | Total Sample (n = 47) |
Control (n = 40) |
Increased Birth Weight (n = 7) |
P a |
|---|---|---|---|---|
| Ethnicity b | 0.49 | |||
| Non-Hispanic White | 32 | 27 | 5 | |
| Non-Hispanic Black | 12 | 11 | 1 | |
| Hispanic | 1 | 1 | 0 | |
| Asian | 2 | 1 | 1 | |
| Smoker b | 15 | 13 | 2 | 1.00 |
| Parity c | 2.0 ± 0.2 | 2.1 ± 0.2 | 1.9 ± 0.3 | 0.68 |
| GA at Delivery, Weeks c | 39.3 ± 0.1 | 39.3 ± 0.2 | 39.2 ± 0.4 | 0.79 |
| Mode of Delivery b | 1.00 | |||
| SVD | 29 | 25 | 4 | |
| CS | 18 | 15 | 3 | |
| Pre-Pregnancy BMI c | 26.0 ± 1.0 | 25.5 ± 1.0 | 28.9 ± 3.6 | 0.21 |
| Gestational Weight Gain, kg c | 14.5 ± 0.8 | 13.7 ± 0.8 | 19.2 ± 1.9 | 0.01 |
| Gestational Weight Gain, Category b | 0.33 | |||
| under | 5 | 5 | 0 | |
| normal | 22 | 20 | 2 | |
| over | 20 | 15 | 5 | |
| 3rd Trimester Glucola c,d | 113.8 ± 4.0 | 111.0 ± 4.2 | 128.0 ± 10.8 | 0.12 |
Abbreviations: BMI (body mass index), CS (caesarean section), GA (gestational age), SVD (spontaneous vaginal delivery)
Continuous variables were compared with the use of Student t-test while categorical variables were compared by Fisher’s exact test;
Data given as count;
Data are given as mean ± SEM;
Glucose tolerance test (Glucola).
RESULTS
Maternal:
Table 1 outlines the demographics and obstetrical characteristics of our study sample. The mean maternal age was 27.6 years (range 20–38). Thirty-two percent of women smoked prior to pregnancy (15/47); no mother’s smoking status changed during pregnancy. The mean parity was 2.0 (range 1–5). The mean gestational age at delivery was 39.3 weeks (range from 37.2–41.3). Sixty-two percent of the study patients delivered vaginally. The mean pre-pregnancy BMI in our study cohort was 26.0 kg/m22 (range 17.5–41.4). Twenty-seven percent of women were categorized as obese with BMI > 30 and mean gestational weight gain was 14.5 kg (range 3.2–26.3). 43% (20/47) of women gained excess weight during pregnancy, 47% (22/47) gained within the recommendations, and 11% (5/47) gained less than recommended.36 Overall, gestational weight gain was similar between obese and non-obese women (P = 0.77). The mean 3rd trimester 50 g glucose challenge was 113.8 mg/dl (range 64–179) and was not significantly correlated with continuous birth weight in this cohort, (P = 0.74). Ten women underwent 3 hour glucose challenge for screening values >130 mg/dl and one was diagnosed with gestational diabetes. Six women were diagnosed with gestational hypertension, and three developed pre-eclampsia.
Offspring:
Foreskin samples from 47 neonates were used for NanoString analysis. About half of the samples were taken on day 1 of life (51%, range 0–3). The mean birth weight of the control babies was 3324 ± 60 grams compared to 4115 ± 87 grams for the top 15% of babies in the cohort (P < 0.0001).
The control and increased birth weight samples did not differ significantly according to maternal age (P = 0.84), ethnicity (P = 0.49), parity (P = 0.68), smoking (P = 1.00), or mode of delivery (P = 1.00). In this cohort, pre-pregnancy BMI was not significantly correlated with continuous birth weight overall (P = 0.36), and birth weights were not significantly different between non-obese and obese mothers (3411 ± 80 g vs. 3521 ± 120 g, P = 0.47). Gestational weight gain was significantly correlated with continuous birth weight (Pearson’s r = 0.43; P = 0.002) and women that gave birth to increased birth weight babies also had higher gestational weight gain (P = 0.01; Table 1).
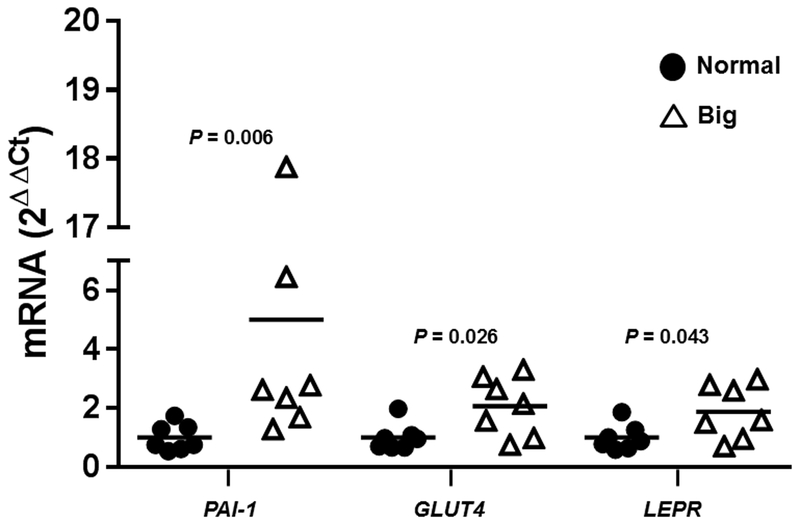
Gene expression was measured in the hypodermis of 47 neonates and expression levels of the highest 15% of birth weight babies (n = 7) were compared to those of the remaining babies (n = 40). Table 2 shows the 10 genes with an FDR adjusted P < 0.05. Both HDAC1 and TXN were significantly downregulated in the hypodermal layer in higher birth weight newborns compared to the remainder of the cohort. Eight genes were significantly upregulated with a fold change > 1.25 in the larger babies. These genes were PAI-1, GLUT4, LEPR, lipoprotein lipase (LPL), matrix metalloproteinase 2 (MMP2), insulin receptor substrate 2 (IRS2), LRP1, and transcription factor 7-like 2 (TCF7L2). We validated 3 genes with the greatest (and significantly different) fold change in mRNA differences in the NanoString CodeSet with real-time PCR (PAI-1, GLUT4, and LEPR). We found significant increases in PAI-1 (P = 0.006), GLUT4 (P = 0.026), and LEPR (P = 0.043) in babies with the 15% highest birth weights compared to a subset (n = 7) of the normal weight babies (Figure 1).
Figure 1.
qPCR validation of 3 genes with the highest (and significant) fold change in mRNA differences in the NanoString CodeSet. Horizontal line depicts the mean expression for each gene.
Multivariate analysis results are summarized in Table 3. Each column represents a different regression model, corresponding to one of the 10 genes for which the FDR-adjusted p-value was less than 0.05 in comparing birth weight groups. Eight of the 10 models contain, in addition to group, one or more maternal or infant characteristics which portend gene expression. As an example, controlling for gestational age, LEPR expression is predicted to increase by more than one-and-a-half of its standard deviations when birth weight crosses from normal to elevated; and, controlling for birth weight, each increase in gestational age by one of its standard deviations decreases predicted LEPR expression by approximately one-quarter of its standard deviation. The latter result makes sense intuitively; for instance, if a 37-week gestational age baby and a 41-week gestational age baby have the same birth weight, then the 37-week baby is larger relative to his age and would be anticipated to have greater LEPR expression consistent with being of larger size. With two exceptions, one on the high end (PAI-1, 65.9%) and one on the low end (TCF7L2, 16.1%) between 25% and 50% of each gene’s variation in expression level was accounted for by birth weight group and other maternal or infant characteristics. Besides birth weight, the predictor most often appearing in multivariate analysis was gravida, which was selected for five out of the 10 regression models. The direction of the relationship between gravida and gene expression in these five models was the same as that between birth weight and gene expression; when increased birth weight corresponded to greater gene expression, so did increased gravida, and vice versa. In addition, part or all bottle fed appeared in four out of the ten regression models with the direction of the relationship being opposite to that of birthweight and gene expression. No other predictor was selected for more than two of the regression models, though, interestingly, the model for PAI-1 contained 10 predictors; no other model contained as many as five.
Table 3.
Multivariate Analysis of Gene Expression Levels
| Predictor/ Outcome |
GLUT4 | HDAC1* | IRS2 | LEPR | LPL | LRP1* | MMP2 | PAI-1 | TCF7L2 | TXN* |
|---|---|---|---|---|---|---|---|---|---|---|
| Increased Birth Weight | 1.60 p<0.001 |
−1.41 p<0.001 |
1.16 p=0.002 |
1.62 p<0.001 |
1.22 p=0.001 |
1.59 p<0.001 |
1.31 p<0.001 |
0.90 p=0.007 |
1.11 p=0.005 |
−0.86 p<0.001 |
| Gravida | 0.29 p=0.019 |
−0.29 p=0.016 |
0.35 p=0.010 |
0.26 p=0.054 |
−0.34 p=0.008 |
|||||
| Part or All Bottle | −0.53 p=0.048 |
−0.68 p=0.018 |
−0.74 p=0.005 |
0.66 p=0.007 |
||||||
| Glucose Tolerance Test | 0.28 p=0.029 |
0.29 p=0.008 |
||||||||
| Gestational Age | −0.26 p=0.028 |
−0.27 p=0.018 |
||||||||
| Affirmed College Degree or Better | −0.62 p=0.032 |
−0.85 p=0.003 |
||||||||
| Gestational Weight Gain Over Recommended | 0.62 p=0.008 |
|||||||||
| Smoke During Pregnancy | 0.63 p=0.018 |
|||||||||
| Private Insurance | 1.22 p=0.002 |
|||||||||
| Full-Time Employment | −0.90 p=0.010 |
|||||||||
| Day of Life Circumcision | −0.21 p=0.065 |
|||||||||
| Ponderal Index | −0.26 p=0.030 |
|||||||||
| R2 | 0.391 | 0.434 | 0.403 | 0.420 | 0.299 | 0.266 | 0.272 | 0.659 | 0.161 | 0.455 |
Each column represents a separate regression model. Variables actually selected for each model are those whose cell entries are filled in. Each regression coefficient is the estimated number of standard deviations by which the outcome is expected to increase when the predictor goes from no to yes (if the predictor is binary) or when the predictor increases by one standard deviation (if the predictor is continuous), while adjusting for all other predictors in the same model; these regression coefficients turned out to be greater than 0.50 in absolute value for all binary predictors and less than 0.50 in absolute value for all continuous predictors. Accompanying each regression coefficient is a p-value. Asterisks in column headings indicate a weighted least squares analysis. The final row contains R2, the proportion of (weighted) variation in gene expression explained by the variables used to predict it.
DISCUSSION
We used human foreskin tissue to assess changes in gene expression of neonates related to obesity, weight gain in pregnancy, and birth weight. Our main finding was that birth weight was associated with the expression of genes related to metabolism and inflammation in neonatal tissue. We confirmed what others have shown37 in that birth weight was positively correlated with gestational weight gain. Understanding weight induced alterations in gene expression may be important in establishing potential mechanisms responsible for the detrimental effects of small or large birth weight and increased maternal weight gain on offspring risk of developing obesity and type 2 diabetes. While we do not anticipate that gene expression changes in the foreskin are driving whole body changes in appetite and energy balance, hyperglycemia, and inflammation, we suspect that these changes are representative of the types of alterations that are seen in other tissues. Results from this study point to the foreskin as a useful model to study developmental programming using a tissue that comes directly from the infant after birth and may not include maternal contributions like placenta38 and cord blood.39 However, given that others have shown that elevated baby birth weight impacts a number of markers related to glycemic control,40 appetite/energy balance,41, 42 obesity,43 and inflammation in placenta or cord blood,18 we assessed similar markers in the neonatal foreskin.
Upregulation of gene expression in foreskin tissue related to appetite and energy balance, hyperglycemia, and inflammation were found in babies with increased birth weight. These data support human epidemiological evidence demonstrating that high birth weight babies are at an increased risk for developing obesity44, 45 and type 2 diabetes later in life46. These data further our knowledge by providing mechanisms of dysfunction that may be predisposing high birth weight babies to an obese, insulin resistant phenotype at increased risk of developing cardiovascular disease in adulthood. 47 Several markers of obesity, insulin resistance, and cardiovascular disease had the greatest fold change in gene expression with higher birth weight (Table 2). While some markers have been measured in animal models of developmental programming or in human placenta/cord blood, this is the first study to examine them in neonatal foreskin. The skin is readily available following circumcision at birth and can be obtained later in life through skin biopsies. These repeated measures are a necessary next step in determining if the observed gene expression changes in the current study extend beyond infancy.
Results from the present study suggest that high birth weight babies have increased expression of obesity related genes (LPL and LEPR). Studies in rodents have shown that treating hyperleptinemia in offspring late in development slows neonatal weight gain and reverses prenatal adaptations resulting from stimuli that promote adulthood obesity.48 LEPR expression is increased in response to leptin insensitivity as a compensatory mechanism to defend against obesity. However, in later stages of LEPR insensitivity, there is a loss of weight homeostasis and obesity ensues 48 This provides exciting evidence of particular markers which may be targeted for therapeutic interventions in high birth weight babies to prevent adulthood obesity. Future studies in our lab will begin to investigate these mechanisms in humans.
Chronic obesity leads to whole body and skeletal muscle insulin resistance.49 A number of proteins are involved in regulating cellular insulin sensitivity. GLUT4 mRNA is increased acutely in response to hyperinsulinemia;50 however, as insulin resistance develops and progresses to type 2 diabetes, translocation of GLUT4 to the cell membrane in response to insulin is reduced.51 While infants in the present study were not obese per se, we did find that the highest 15% body weight babies had significantly elevated mRNA expression of GLUT4 and IRS2, proteins stimulated by insulin which are, in part, responsible for glucose uptake into cells. Though, it is important to note that we did not directly measure differences in protein or phosphorylation levels as part of this study.
In the area of developmental programming, elevated levels of PAI-1, an inhibitor of fibrinolysis, were found in the white adipose tissue of male rat offspring born to obese dams fed a high-fat diet during pregnancy.52 This is a phenotype that is common in obesity and is related to increased risk of developing cardiovascular disease.53 In the heart, elevated PAI-1 plays a role in the development of fibrosis.54 In the present study, PAI-1 was increased in the hypodermal layer of the foreskin in the 15% highest birth weight babies; however, whether or not this also translates into increased PAI-1 expression in the hearts of these babies which might be predisposing them to greater risk of developing cardiovascular disease is not known. Huang et al. demonstrated that fetal hearts of sheep from obese mothers had increased cardiac fibrosis,55 thus, this may be a mechanism of increased cardiovascular disease risk in babies born to obese mothers (and thus, predisposed to elevated birth weight).56 Interestingly, TXN, an antioxidant,57 was reduced in the 15% highest birth weight babies, thus providing further evidence for a phenotype which may be more predisposed to increased oxidative stress and developing cardiovascular disease later in life. In fact, previous animal studies have demonstrated that offspring born to obese dams tend to have higher rates of oxidative stress,58 potentially due to downregulation of TXN.
Although not a specific aim of the paper, it is of interest that gravida, or number of pregnancies a woman has had, appeared as a predictor in five of the regression models (Table 3), second in frequency to increased birth weight. Increased parity, or number of live births a female has had (which would also increase gravida), has been associated with weight gain and obesity in humans59 and mice.60 Given that obese mothers tend to have bigger babies,61 the fact that all of the genes whose expression levels were altered with gravida (adjusted for other variables) are also associated in the same direction with increased birth weight, is not a surprise.
There were several limitations to this study. The top 15% birth weight babies were grouped together, as opposed to using a more standard definition of macrosomia, due to our limited sample size. Further, the relatively low number of samples did not allow for analysis of gestational diabetes or hypertension as confounding factors on gene expression. While gene expression was altered in the top 15% birth weight babies compared to controls, it is significant to note that mRNA levels do not strongly correlate with protein expression.62 Finally, neonatal tissue can only be collected in males following circumcision; thus, female neonates were not included in this study and we cannot comment on a potential sex bias at this time. Despite these limitations, we have demonstrated the neonatal foreskin as a useful tissue to study developmental programming.
We found that gene expression related to glycemic control, appetite/energy balance, obesity, and inflammation was altered in tissue from babies with elevated birth weight. These genes point to potential mechanisms regarding fetal programming in macrosomic babies. Importantly, this model can be expanded in future studies to include collection of placenta, cord blood, and maternal serum for comparative analyses.
Supplementary Material
ACKNOWLEDGMENTS
We would like to acknowledge Lawrence P. Reagan from the University of South Carolina School of Medicine for his insightful discussions about GLUT4 and Wendy F. Hansen for her thoughtful comments on the manuscript.
Funding for the study was generously provided by the Graduate Center for Nutritional Sciences at the University of Kentucky. A.J.S and S.S. were supported by the National Institutes of Health (NIH) (P20GM103436–15). Additionally, A.J.S. was supported by NIH CTSA Award (UL1TR000117). R.J.C. was supported, in part, by the NIH (P20GM103527–08). L.J.R. was supported by an American Heart Association Post-Doctoral Fellowship (15POST25110002). C.S.R. was supported by NIH training grants (T32DK07778 and T32HD060556).
Footnotes
FINANCIAL SUPPORT
The authors’ views are not necessarily those of the funding agencies that supported their efforts.
CONFLICT OF INTEREST
The authors report no conflict of interest.
ETHICAL STANDARDS
The authors assert that all procedures contributing to this work comply with the Belmont Reportand with the Helinski Declaration of 1975, as revised in 2008, and has been approved by the University of Kentucky Medical Institutional Review Board.
Presented at meeting: The 61st Annual Society for Gynecological Investigation Scientific Meeting, Florence, Italy, March 2014
REFERENCES:
- 1.Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of obesity in the United States, 2009–2010. NCHS Data Brief 2012(82), 1–8. [PubMed] [Google Scholar]
- 2.American College of Obstetricians and Gynecologists Committee opinion no. 549: obesity in pregnancy. Obstet Gynecol 2013;121(1), 213–217. [DOI] [PubMed] [Google Scholar]
- 3.Ovesen P, Rasmussen S, Kesmodel U. Effect of prepregnancy maternal overweight and obesity on pregnancy outcome. Obstet Gynecol 2011;118(2 Pt 1), 305–312. [DOI] [PubMed] [Google Scholar]
- 4.Johnson J, Clifton RG, Roberts JM, et al. Pregnancy outcomes with weight gain above or below the 2009 Institute of Medicine guidelines. Obstet Gynecol 2013;121(5), 969–975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Salsberry PJ, Reagan PB. Taking the long view: the prenatal environment and early adolescent overweight. Res Nurs Health 2007;30(3), 297–307. [DOI] [PubMed] [Google Scholar]
- 6.Crume TL, Ogden L, West NA, et al. Association of exposure to diabetes in utero with adiposity and fat distribution in a multiethnic population of youth: the Exploring Perinatal Outcomes among Children (EPOCH) Study. Diabetologia. 2011;54(1), 87–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lausten-Thomsen U, Bille DS, Nasslund I, Folskov L, Larsen T, Holm JC. Neonatal anthropometrics and correlation to childhood obesity--data from the Danish Children’s Obesity Clinic. Eur J Pediatr 2013;172(6), 747–751. [DOI] [PubMed] [Google Scholar]
- 8.Oken E, Rifas-Shiman SL, Field AE, Frazier AL, Gillman MW. Maternal gestational weight gain and offspring weight in adolescence. Obstet Gynecol 2008;112(5), 999–1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sewell MF, Huston-Presley L, Super DM, Catalano P. Increased neonatal fat mass, not lean body mass, is associated with maternal obesity. Am J Obstet Gynecol 2006;195(4), 1100–1103. [DOI] [PubMed] [Google Scholar]
- 10.Dabelea D, Hanson RL, Lindsay RS, et al. Intrauterine exposure to diabetes conveys risks for type 2 diabetes and obesity: a study of discordant sibships. Diabetes. 2000;49(12), 2208–2211. [DOI] [PubMed] [Google Scholar]
- 11.Barker DJ, Hales CN, Fall CH, Osmond C, Phipps K, Clark PM. Type 2 (non-insulin-dependent) diabetes mellitus, hypertension and hyperlipidaemia (syndrome X): relation to reduced fetal growth. Diabetologia. 1993;36(1), 62–67. [DOI] [PubMed] [Google Scholar]
- 12.Guo L, Liu J, Ye R, Zhuang Z, Ren A. Gestational Weight Gain and Overweight in Children Aged 3–6 Years. J Epidemiol 2015; ; 25: 536–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marchi J, Berg M, Dencker A, Olander EK, Begley C. Risks associated with obesity in pregnancy, for the mother and baby: a systematic review of reviews. Obes Rev 2015;16(8), 621–638. [DOI] [PubMed] [Google Scholar]
- 14.Catalano PM. Obesity and pregnancy--the propagation of a viscous cycle? J Clin Endocrinol Metab 2003;88(8), 3505–3506. [DOI] [PubMed] [Google Scholar]
- 15.Garcia-Valdes L, Campoy C, Hayes H, et al. The impact of maternal obesity on iron status, placental transferrin receptor expression and hepcidin expression in human pregnancy. Int J Obes (Lond) 2015;39(4), 571–578. [DOI] [PubMed] [Google Scholar]
- 16.Brett KE, Ferraro ZM, Yockell-Lelievre J, Gruslin A, Adamo KB. Maternal-fetal nutrient transport in pregnancy pathologies: the role of the placenta. Int J Mol Sci 2014;15(9), 16153–16185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Carty D, Akehurst C, Savage R, et al. Differential gene expression in obese pregnancy. Pregnancy Hypertens. 2014;4(3), 232–233. [DOI] [PubMed] [Google Scholar]
- 18.Catalano PM, Presley L, Minium J, Hauguel-de Mouzon S. Fetuses of obese mothers develop insulin resistance in utero. Diabetes Care 2009;32(6), 1076–1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hiden U, Glitzner E, Hartmann M, Desoye G. Insulin and the IGF system in the human placenta of normal and diabetic pregnancies. J Anat 2009;215(1), 60–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McIntyre HD, Zeck W, Russell A. Placental growth hormone, fetal growth and the IGF axis in normal and diabetic pregnancy. Curr Diabetes Rev 2009;5(3), 185–189. [DOI] [PubMed] [Google Scholar]
- 21.Silverman BL, Rizzo TA, Cho NH, Metzger BE. Long-term effects of the intrauterine environment. The Northwestern University Diabetes in Pregnancy Center. Diabetes Care 1998;21 Suppl 2, B142–149. [PubMed] [Google Scholar]
- 22.Verhaeghe J, Van Bree R, Van Herck E, Laureys J, Bouillon R, Van Assche FA. C-peptide, insulin-like growth factors I and II, and insulin-like growth factor binding protein-1 in umbilical cord serum: correlations with birth weight. Am J Obstet Gynecol 1993;169(1), 89–97. [DOI] [PubMed] [Google Scholar]
- 23.Ainge H, Thompson C, Ozanne SE, Rooney KB. A systematic review on animal models of maternal high fat feeding and offspring glycaemic control. Int J Obes (Lond) 2011;35(3), 325–335. [DOI] [PubMed] [Google Scholar]
- 24.Tamashiro KL, Terrillion CE, Hyun J, Koenig JI, Moran TH. Prenatal stress or high-fat diet increases susceptibility to diet-induced obesity in rat offspring. Diabetes. 2009;58(5), 1116–1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.De Corte P, Verween G, Verbeken G, et al. Feeder layer- and animal product-free culture of neonatal foreskin keratinocytes: improved performance, usability, quality and safety. Cell Tissue Bank. 2012;13(1), 175–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mendez MV, Raffetto JD, Phillips T, Menzoian JO, Park HY. The proliferative capacity of neonatal skin fibroblasts is reduced after exposure to venous ulcer wound fluid: A potential mechanism for senescence in venous ulcers. J Vasc Surg 1999;30(4), 734–743. [DOI] [PubMed] [Google Scholar]
- 27.Qiao L, Tasian GE, Zhang H, et al. Androgen receptor is overexpressed in boys with severe hypospadias, and ZEB1 regulates androgen receptor expression in human foreskin cells. Pediatr Res 2012;71(4 Pt 1), 393–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vottero A, Minari R, Viani I, et al. Evidence for epigenetic abnormalities of the androgen receptor gene in foreskin from children with hypospadias. J Clin Endocrinol Metab 2011;96(12), E1953–1962. [DOI] [PubMed] [Google Scholar]
- 29.Reynolds LJ, Dickens BJ, Green BB, Marsit CJ, Pearson KJ. Using neonatal skin to study the developmental programming of aging. Exp Gerontol 2016; doi: 10.1016/j.exger.2016.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Berglund SR, Schwietert CW, Jones AA, Stern RL, Lehmann J, Goldberg Z. Optimized methodology for sequential extraction of RNA and protein from small human skin biopsies. J Invest Dermatol. 2007;127(2), 349–353. [DOI] [PubMed] [Google Scholar]
- 31.Benjamini Y, Hochberg, Y. Controlling the False Discovery Rate: a Practical and Powerful Approach to Multiple Testing. Journal of the Royal Statistics Society. 1995;57(1), 289–300. [Google Scholar]
- 32.Rosner B Fundamentals of Biostatistics, 6th edn, 2005. [Google Scholar]
- 33.Geiss GK, Bumgarner RE, Birditt B, et al. Direct multiplexed measurement of gene expression with color-coded probe pairs. Nat Biotechnol 2008;26(3), 317–325. [DOI] [PubMed] [Google Scholar]
- 34.Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc 2008;3(6), 1101–1108. [DOI] [PubMed] [Google Scholar]
- 35.Schwarz G Estimating the Dimension of a Model. The Annuals of Statistics. 1978;6(2), 461–464. [Google Scholar]
- 36.Rasmussen KM, Catalano PM, Yaktine AL. New guidelines for weight gain during pregnancy: what obstetrician/gynecologists should know. Curr Opin Obstet Gynecol 2009;21(6), 521–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Butte NF, Ellis KJ, Wong WW, Hopkinson JM, Smith EO. Composition of gestational weight gain impacts maternal fat retention and infant birth weight. Am J Obstet Gynecol 2003;189(5), 1423–1432. [DOI] [PubMed] [Google Scholar]
- 38.In ‘t Anker PS, Scherjon SA, Kleijburg-van der Keur C, et al. Isolation of mesenchymal stem cells of fetal or maternal origin from human placenta. Stem cells 2004;22(7), 1338–1345. [DOI] [PubMed] [Google Scholar]
- 39.Hall JM, Lingenfelter P, Adams SL, Lasser D, Hansen JA, Bean MA. Detection of maternal cells in human umbilical cord blood using fluorescence in situ hybridization. Blood. 1995;86(7), 2829–2832. [PubMed] [Google Scholar]
- 40.Kainulainen H, Jarvinen T, Heinonen PK. Placental glucose transporters in fetal intrauterine growth retardation and macrosomia. Gynecol Obstet Invest 1997;44(2), 89–92. [DOI] [PubMed] [Google Scholar]
- 41.Mazaki-Tovi S, Kanety H, Pariente C, Hemi R, Schiff E, Sivan E. Cord blood adiponectin in large-for-gestational age newborns. Am J Obstet Gynecol 2005;193(3 Pt 2), 1238–1242. [DOI] [PubMed] [Google Scholar]
- 42.Wiznitzer A, Furman B, Zuili I, Shany S, Reece EA, Mazor M. Cord leptin level and fetal macrosomia. Obstet Gynecol 2000;96(5 Pt 1), 707–713. [DOI] [PubMed] [Google Scholar]
- 43.Wang J, Shang LX, Dong X, et al. Relationship of adiponectin and resistin levels in umbilical serum, maternal serum and placenta with neonatal birth weight. Aust N Z J Obstet Gynaecol 2010;50(5), 432–438. [DOI] [PubMed] [Google Scholar]
- 44.Curhan GC, Chertow GM, Willett WC, et al. Birth weight and adult hypertension and obesity in women. Circulation. 1996;94(6), 1310–1315. [DOI] [PubMed] [Google Scholar]
- 45.Curhan GC, Willett WC, Rimm EB, Spiegelman D, Ascherio AL, Stampfer MJ. Birth weight and adult hypertension, diabetes mellitus, and obesity in US men. Circulation. 1996;94(12), 3246–3250. [DOI] [PubMed] [Google Scholar]
- 46.Wei JN, Sung FC, Li CY, et al. Low birth weight and high birth weight infants are both at an increased risk to have type 2 diabetes among schoolchildren in taiwan. Diabetes Care. 2003;26(2), 343–348. [DOI] [PubMed] [Google Scholar]
- 47.Rich-Edwards JW, Stampfer MJ, Manson JE, et al. Birth weight and risk of cardiovascular disease in a cohort of women followed up since 1976. Bmj 1997;315(7105), 396–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vickers MH, Gluckman PD, Coveny AH, et al. Neonatal leptin treatment reverses developmental programming. Endocrinology. 2005;146(10), 4211–4216. [DOI] [PubMed] [Google Scholar]
- 49.Kahn BB, Flier JS. Obesity and insulin resistance. J Clin Invest. 2000;106(4), 473–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sano H, Kane S, Sano E, et al. Insulin-stimulated phosphorylation of a Rab GTPase-activating protein regulates GLUT4 translocation. The Journal of biological chemistry. 2003;278(17), 14599–14602. [DOI] [PubMed] [Google Scholar]
- 51.Garvey WT, Maianu L, Zhu JH, Brechtel-Hook G, Wallace P, Baron AD. Evidence for defects in the trafficking and translocation of GLUT4 glucose transporters in skeletal muscle as a cause of human insulin resistance. J Clin Invest 1998;101(11), 2377–2386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pisani LP, Oller do Nascimento CM, Bueno AA, et al. Hydrogenated fat diet intake during pregnancy and lactation modifies the PAI-1 gene expression in white adipose tissue of offspring in adult life. Lipids Health Dis. 2008;7, 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Juhan-Vague I, Alessi MC. PAI-1, obesity, insulin resistance and risk of cardiovascular events. Thrombosis and haemostasis. 1997;78(1), 656–660. [PubMed] [Google Scholar]
- 54.Takeshita K, Hayashi M, Iino S, et al. Increased expression of plasminogen activator inhibitor-1 in cardiomyocytes contributes to cardiac fibrosis after myocardial infarction. Am J Pathol. 2004;164(2), 449–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Huang Y, Yan X, Zhao JX, et al. Maternal obesity induces fibrosis in fetal myocardium of sheep. American journal of physiology Endocrinology and metabolism. 2010;299(6), E968–975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Reynolds RM, Allan KM, Raja EA, et al. Maternal obesity during pregnancy and premature mortality from cardiovascular event in adult offspring: follow-up of 1 323 275 person years. Bmj. 2013;347, f4539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yoshida T, Nakamura H, Masutani H, Yodoi J. The involvement of thioredoxin and thioredoxin binding protein-2 on cellular proliferation and aging process. Ann N Y Acad Sci 2005;1055, 1–12. [DOI] [PubMed] [Google Scholar]
- 58.Bruce KD, Cagampang FR, Argenton M, et al. Maternal high-fat feeding primes steatohepatitis in adult mice offspring, involving mitochondrial dysfunction and altered lipogenesis gene expression. Hepatology. 2009;50(6), 1796–1808. [DOI] [PubMed] [Google Scholar]
- 59.Ness RB, Harris T, Cobb J, et al. Number of pregnancies and the subsequent risk of cardiovascular disease. N Engl J Med 1993;328(21), 1528–1533. [DOI] [PubMed] [Google Scholar]
- 60.Rebholz SL, Jones T, Burke KT, et al. Multiparity leads to obesity and inflammation in mothers and obesity in male offspring. American journal of physiology Endocrinology and metabolism 2012;302(4), E449–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Whitelaw AG. Influence of maternal obesity on subcutaneous fat in the newborn. Br Med J 1976;1(6016), 985–986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Vogel C, Marcotte EM. Insights into the regulation of protein abundance from proteomic and transcriptomic analyses. Nat Rev Genet 2012;13(4), 227–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.