Summary
The rate of identified isolated subsegmental pulmonary embolism (ssPE) has doubled with advances in computed tomography pulmonary angiography (CTPA) technology, but its clinical relevance is debated. The YEARS diagnostic algorithm was shown to safely reduce the number of required CTPAs in the diagnostic management of PE. We hypothesized that the higher threshold for performing CTPA in YEARS was associated with a lower prevalence of ssPE compared to the conventional diagnostic algorithm. We compared 2291 consecutive patients with suspected PE managed according to YEARS to 3306 consecutive control patients managed according to the Wells score for the prevalence of isolated ssPE. In the YEARS cohort, 52% were managed without CTPA, 12% had pulmonary embolism (PE) of which 10% were isolated ssPE, and the 3‐month diagnostic failure rate was 0·35%. In the control cohort, 32% were managed without CTPA, 20% had PE of which 16% were isolated ssPE, and the 3‐month failure rate was 0·73%. The isolated ssPE prevalence was significantly lower in YEARS (absolute difference 6·2% (95% confidence interval [CI] 1·4–10), Odds Ratio 0·58 (95% CI 0·37–0·90). In conclusion, YEARS is associated with a lower prevalence of isolated ssPE, due to reduction in CTPAs by the higher D‐dimer threshold. This was however not associated with a higher risk of recurrent VTE during follow‐up.
Keywords: pulmonary embolism, D‐dimer, diagnosis, computed tomography, subsegmental pulmonary embolism
Since the introduction of multi‐detector computed tomography pulmonary angiography (CTPA), the sensitivity of this diagnostic technique for visualizing smaller pulmonary embolisms (PE) has noticeably advanced. These advances have led to a more frequent detection of filling defects in subsegmental pulmonary arteries – with diameters as small as 2–3 mm – by multi‐detector computed tomography (MDCT) compared to single detector computed tomography (SDCT; Fig 1) (Carrier et al, 2010; Klok & Huisman, 2017). The rate of isolated subsegmental PE (ssPE) diagnosis has doubled since the introduction of the MDCT, from 4·7% (95% confidence interval [CI]: 2·5–7·6%) to 9·4% (95% CI: 5·5–14·2%) (Carrier et al, 2010; Konstantinides et al, 2016). Despite this increase in isolated ssPE diagnoses, the risk of fatal PE has remained unchanged, suggesting that not all isolated ssPE may be clinically relevant (Le Gal et al, 2006; Carrier et al, 2010).
Figure 1.
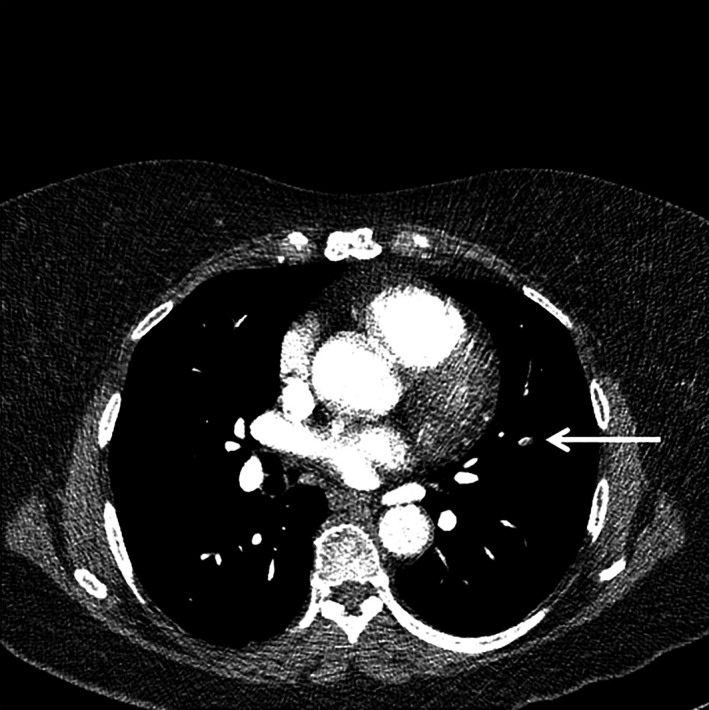
Isolated subsegmental pulmonary embolism (arrow) on computed tomography pulmonary angiography.
Recently, a number of studies have been designed to optimize diagnostic strategies for suspected acute PE. Ultimately, they aimed to lower the number of necessary CTPAs, thereby reducing the number of patients exposed to ionizing radiation and simplify diagnostic management in busy clinical practices (den Exter et al, 2013; van der Hulle et al, 2017). The YEARS algorithm involves a D‐dimer test that is combined with three clinical variables, i.e. clinical signs of deep venous thrombosis, haemoptysis and “PE most likely diagnosis”. In the absence of YEARS items, the D‐dimer threshold to rule out PE without CTPA is 1000 ng/ml and in patients with one or more YEARS items the D‐dimer threshold remains 500 ng/ml. All patients with D‐dimer levels higher than these D‐dimer thresholds require a CTPA examination (Fig 2). We have shown that this algorithm is safe with a failure rate of the overall algorithm of 0·61% (95% CI 0·36–0·96) and a resultant 14% reduction in CTPA examinations compared to the standard algorithm with the conventional Wells rule and fixed D‐dimer threshold of <500 ng/ml (van der Hulle et al, 2017).
Figure 2.
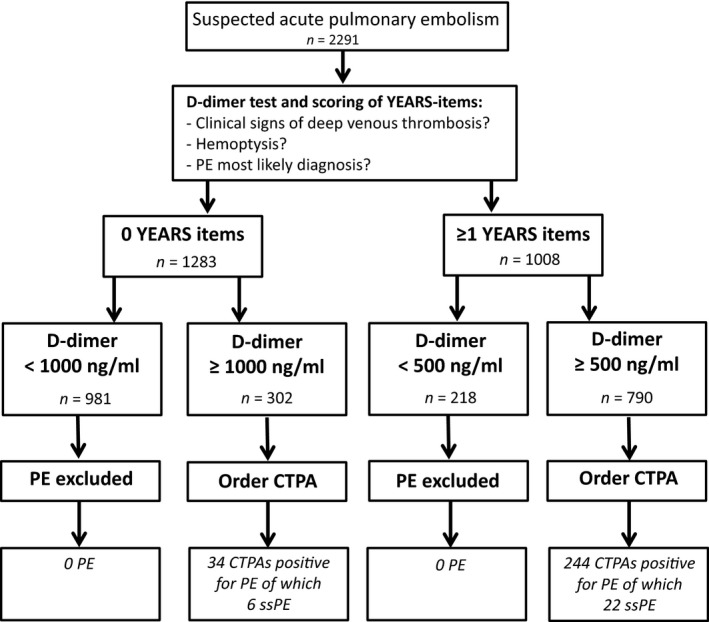
Years algorithm and study flowchart. CTPA, computed tomography pulmonary angiography; PE, pulmonary embolism; ssPE, subsegmental pulmonary embolism.
We hypothesized that this reduction in CTPA examinations has led to a decreased rate of smaller more distal emboli diagnosis without a higher incidence of recurrent VTE during the 3‐month follow‐up. To test this hypothesis, we compared the percentage of isolated ssPE diagnoses in patients managed according to the YEARS algorithm to patients managed according to the conventional algorithm that is currently recommend for use in clinical practice (Konstantinides, 2014).
Methods
Study population
This was a post‐hoc analysis with the combined data of two prospective outcome studies, i.e. the YEARS study and the Christopher study, in which consecutive, haemodynamically stable in‐ and out‐patients with clinically suspected PE had been included in the same Dutch Hospitals (van Belle et al, 2006; van der Hulle et al, 2017). We used the Christopher population as the proxy for current clinical practice.
The YEARS study evaluated the safety and efficiency of the YEARS algorithm in 3465 consecutive in‐ and out‐patients aged 18 years or older from October 2013 to July 2015 (Fig 2) (van der Hulle et al, 2017). Exclusion criteria were treatment with therapeutic doses of anticoagulants initiated ≥24 h prior to eligibility assessment, pregnancy, allergy to intravenous contrast agents, life expectancy less than 3 months or geographic inaccessibility precluding follow‐up. Patients were managed according to the YEARS algorithm (Fig 2). D‐dimer concentrations were measured with automated well validated high‐sensitive quantitative D‐dimer assays (Tinaquant, Roche Diagnostica, Mannheim, Germany; Innovance, Siemens, Malburg, Germany and STA‐LIA Diagnostica Stage, Asnieres, France) according to local practice. Patients in whom PE was ruled out were left untreated and anticoagulant treatment was started in patients with a confirmed PE diagnosis. Results were reported using an intention‐to‐diagnose approach. All patients were followed for a 3‐month period for the occurrence of (fatal) recurrent venous thromboembolism (VTE) (van der Hulle et al, 2017). The current study was restricted to 2291 patients from four study sites participating in the YEARS study, i.e. Leiden University Medical Centre (Leiden), Academic Medical Centre (Amsterdam), Flevo Hospital (Almere) and Haga Teaching Hospital (The Hague). We restricted our analysis to these four hospitals because they comprised the majority of included patients and all original CTPA examinations were readily available for analysis.
The Christopher study included 3306 normotensive consecutive in‐ and out‐patients with clinically suspected PE (van Belle et al, 2006). Patients were included from November 2002 to September 2004. The probability of PE was classified as “unlikely” with a Wells clinical decision rule score of 4 points or less, and “likely” with a Wells score of more than 4 points. In all patients with an “unlikely” score, a D‐dimer was measured and PE was considered to be ruled out if the D‐dimer was <500 ng/ml. Anticoagulant treatment was withheld in these latter patients. Patients with a D‐dimer ≥500 ng/ml and all patients with a “likely” score were directly referred for CTPA. Exclusion criteria were treatment with therapeutic doses of unfractionated or low‐molecular‐weight heparin, life expectancy <3 months, allergy to intravenous contrast agents, renal impairment (creatinine clearance <30 ml/min) or haemodynamic instability. All patients were followed for 3 months for the occurrence of symptomatic (fatal/recurrent) VTE (van Belle et al, 2006).
Radiological evaluation
An MDCT was performed in all patients from the YEARS cohort with an indication for CTPA (van der Hulle et al, 2017). In the Christopher study, CTPA was performed using either SDCT or MDCT (van Belle et al, 2006). In both studies, the pulmonary arteries were evaluated up to at least the subsegmental vessels by the attending radiologist. PE was diagnosed if a filling defect was detected or if a vessel was totally occluded by low‐attenuation material on at least 2 adjacent slices.
For all patients in this analysis, we used the previously reported frequencies of isolated ssPE by the local attending radiologist, who was either a certified radiologist, or a resident under supervision of certified radiologist. The definition of isolated ssPE was dependent on this local attending radiologist.
To assess the accuracy of the clinical ssPE diagnosis, all computed tomography (CT) scans from the YEARS study were reassessed by an independent reader who was blinded to the original CT report by the local attending radiologist, as well as to patient characteristics, clinical presentation, treatment decisions and outcome. For the patients from the Christopher study, we used previously reported frequencies of isolated ssPE (van Belle et al, 2006). These CT scans were not available for re‐evaluation.
Endpoints
This analysis was performed to determine the absolute difference in isolated ssPE prevalence between the YEARS cohort and the Christopher cohort. Other endpoints were the number of CTPA examinations required to safely confirm or rule out PE between both cohorts and the difference in failure rate (occurrence of VTE during 3‐month follow‐up).
A second analysis was performed in patients from both cohorts to evaluate if subsegmental thrombus location was associated with a lower D‐dimer level than more proximal PE. Also, we determined if isolated ssPE diagnoses would have been missed if YEARS would have been applied in the Christopher cohort.
Statistical analysis
To compare the prevalence of isolated ssPE, the required number of CTPA examinations and the failure rate in the YEARS study versus the Christopher study, absolute difference and the Odds Ratios (OR) with exact 95% CIs were calculated. To evaluate the association between D‐dimer level and location of the thrombus, we compared the median D‐dimer level in the patients with isolated ssPE versus more proximal PE from both patient cohorts. The Mann‐Whitney‐U test was performed to determine the level of significance between both groups. To evaluate if ssPE diagnoses would have been missed when applying YEARS in the Christopher cohort, the YEARS score was calculated post‐hoc for all patients of the Christopher cohort. For measurement of inter‐observer agreement between the independent reader and the local attending radiologist for isolated ssPE, Cohen's kappa was calculated in two different cohorts: (i) for the adjudication of isolated ssPE in the complete cohort, (ii) for adjudication of isolated ssPE within the group of patients with confirmed and treated PE. The kappa value for agreement was interpreted as follows: poor (<0·20), fair (0·21–0·40), moderate (0·41–0·60), good (0·61–0·80) or very good (0·81–1·00) (Cohen, 1968). P‐values <0·05 were considered statistically significant. All analysis were performed using SPSS version 23.0, Chicago, USA.
Results
YEARS cohort
The median age of the 2291 patients in the YEARS cohort was 53 years (interquartile range (IQR) 40–67), 39% of these patients were men and 88% were outpatients (van der Hulle et al, 2017). The median age of patients with confirmed isolated ssPE was 66 years (IQR 48–77), 36% of these patients were men and 89% were outpatients (Table 1).
Table 1.
Baseline characteristics
| YEARS cohort (van der Hulle et al, 2017) | Christopher cohort (van Belle et al, 2006) | |||
|---|---|---|---|---|
| All patients (n = 2291) | ssPE‐patients (n = 28) | All patients (n = 3306) | ssPE‐patients (n = 110) | |
| Age, years; median (IQR) or mean (SD) | 53 (40–67) | 66 (48–77) | 53 (18·4) | 57 (17·0) |
| Male sex, n (%) | 891 (38·9) | 10 (35·7) | 1409 (42·6) | 60 (54·5) |
| Outpatients, n (%) | 2023 (88·3) | 25 (89·3) | 2701 (81·7) | 81 (73·6) |
| Duration of complaint, days, median (IQR) | 3 (1–9) | 3 (1·0–18·5) | 2 (1–5) | 1 (0–4) |
| VTE risk factors | ||||
| Immobilization/surgery, n (%) | 255 (11·1) | 6 (21·4) | 610 (18·5) | 38 (34·5) |
| Previous VTE, n (%) | 234 (10·2) | 4 (14·3) | 480 (14·5) | 15 (13·6) |
| Active malignancy, n (%) | 212 (9·3) | 3 (10·7) | 476 (14·4) | 21 (19·1) |
| Oestrogen use, women, n (%) | 193 (8·5) | 1 (3·6) | 438 (23·1) | 16 (32·0) |
| Comorbidities | ||||
| Heart failure, n (%) | 92 (4·0) | 1 (3·6) | 243 (7·4) | 9 (8·2) |
| COPD, n (%) | 270 (11·8) | 3 (10·7) | 341 (10·3) | 11 (10·0) |
COPD, chronic obstructive pulmonary disease; IQR, interquartile range; SD, standard deviation; ssPE, subsegmental pulmonary embolism; VTE, venous thromboembolism.
According to the intention‐to‐diagnose approach, 1092 out of 2291 patients (48%) underwent CTPA and the diagnosis of PE was confirmed in 278 patients (12%). In 28 patients (10% of all PE diagnosis) PE was limited to only the subsegmental arteries, leaving 250 patients with PE localized in at least one segmental or central branch of the pulmonary artery (Table 2). The Cohen's kappa statistic comparing the assessment of the local attending radiologist and the independent blinded reviewer for isolated ssPE within the complete patient cohort (n = 2291) was 0·80 and for ssPE within the PE cohort (n = 278) it was 0·78, indicative of ‘good’ agreement. Of all patients with isolated ssPE, 22 patients had 1–3 YEARS items and a D‐dimer above 500 ng/ml and 6 patients had 0 YEARS items and a D‐dimer above 1000 ng/ml (Fig 2).
Table 2.
Prevalence and 3‐month VTE rate of both cohorts
| YEARS cohort (van der Hulle et al, 2017) (n = 2291) | Christopher cohort (van Belle et al, 2006) (n = 3306) | Absolute difference, % (95% CI) | |
|---|---|---|---|
| PE prevalence, n (%) | 278 (12) | 676 (20) | 8·3% (6·4–10) |
| Isolated ssPE prevalence, n (%) | 28 (10) | 110 (16) | 6·2% (1·4–10) |
| CTPA indicated, n (%) | 1092 (48) | 2249 (68) | 20·4% (18–23) |
| 3‐month VTE rate, % (95% CI) | 0·42 (0·21–0·82) | 0·73 (0·49–1·1) | 0·32 (−0·15–0·74) |
CI, confidence interval; CTPA, computed tomography pulmonary angiography; PE, pulmonary embolism; ssPE, subsegmental pulmonary embolism; VTE, venous thromboembolism.
Of the 1924 patients in whom PE was ruled out at baseline, who remained untreated and completed the follow‐up period of 3 months, 8 patients were diagnosed with recurrent VTE (2 fatal PE) during follow‐up for a failure rate of 0·42% (95% CI 0·21–0·82).
Christopher cohort
A total of 3306 consecutive patients were included in the Christopher study with a mean age of 53 years (standard deviation 18·4), 43% were male patients and 82% were outpatients (Table 1) (van Belle et al, 2006). A total of 1057 patients (32%) were managed without CTPA. PE was diagnosed in 676 patients for a PE‐prevalence of 20% (Table 2). MDCT was performed in 1939 patients and SDCT in 260 patients. Among all patients with confirmed PE, 110 (16%) patients were diagnosed with isolated ssPE. Of all patients who remained untreated, 23 were diagnosed with recurrent VTE (7 fatal PE) during the 3‐month follow‐up period for a failure rate of 0·73% (95% CI 0·49–1·1).
Difference between both cohorts
The prevalence of isolated ssPE was significantly lower in the YEARS cohort, with an absolute difference of isolated ssPE prevalence in patients with confirmed PE of 6·2% (95% CI 1·4–10) for an OR of 0·58 (95% CI 0·37–0·90). The absolute difference of isolated ssPE prevalence between both cohorts among all included patients was 2·1% (95% CI 1·3–2·9) for an OR of 0·36 (95% CI 0·24–0·55). CTPA was indicated in 48% of the YEARS cohort versus 68% in the Christopher cohort for an absolute difference of 20% (95% CI 18–23) in favour of the YEARS algorithm. The 0·32% difference in the 3‐month recurrent VTE rate in untreated patients between the cohorts was not statistically significant (95% CI Table 2).
Secondary endpoints
D‐dimer levels were not measured in 185 patients with confirmed PE from the Christopher cohort because they had a likely probability of PE, i.e. a Wells score of more than 4 points, and were referred for CTPA without D‐dimer measurement. In all other patients with available D‐dimer test results from both studies, the median D‐dimer level of patients with isolated ssPE was 1571 ng/ml (IQR 1010–3025) compared to 3205 ng/ml in patients with more proximal PE (IQR 1666–5000, P<0·01; Fig 3). Of all 110 patients diagnosed with isolated ssPE in the Christopher cohort, 11 patients (10% of ssPE diagnosis and 2% of all PE diagnoses) would have remained undetected if the YEARS algorithm had been applied: 7 patients with 0 YEARS items and a D‐dimer below 1000 ng/ml and 4 patients with 1–3 YEARS items and a D‐dimer below 500 ng/ml.
Figure 3.
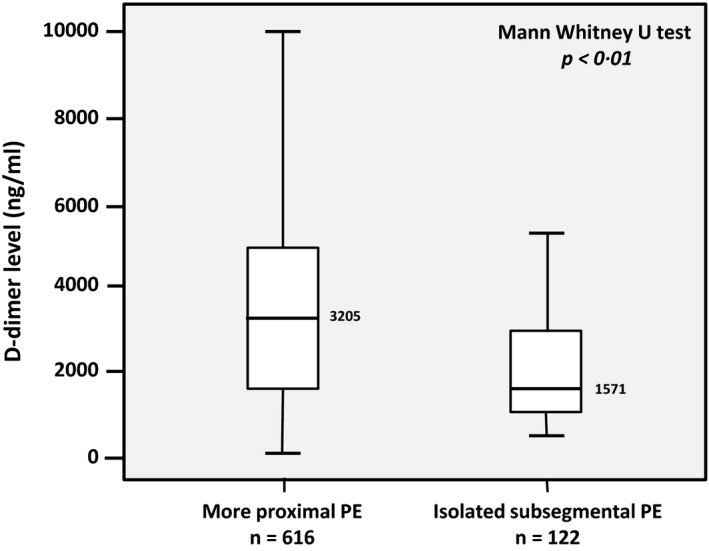
D‐dimer levels and location of pulmonary embolism (PE) in the YEARS and Christopher studies.
Discussion
This analysis demonstrates a lower prevalence of isolated ssPE in patients managed according to the YEARS algorithm compared with a traditional algorithm, without compromising the safety of the diagnostic work‐up. This difference was the consequence of a lower sensitivity of YEARS for smaller more distal emboli due to the higher threshold for performing CTPA scans because of the pre‐test probability dependent D‐dimer cut‐off. This is explained in two ways.
First, we confirmed the previously described association between D‐dimer level and location of PE, where isolated ssPE is associated with a lower median D‐dimer level than more proximal PE (De Monye et al, 2002; Klok et al, 2008; Jeebun et al, 2010). De Monye et al (2002) demonstrated the correlation between D‐dimer level and thrombus location in a prospective cohort study in patients with clinically suspected PE: D‐dimer levels were clearly lower in the group of patients with isolated ssPE (De Monye et al, 2002). Another study confirmed this observation (Jeebun et al, 2010). Second, we showed that 10% of all isolated ssPE diagnoses would have remained undetected if the YEARS algorithm would have been used instead of the standard diagnostic algorithm in the Christopher study. This notable reduction was not associated with a higher rate of symptomatic VTE events among the untreated patients of the YEARS study with an initial negative ruling by the diagnostic algorithm during the 3‐month follow‐up period. These findings support the hypothesis that some isolated ssPE cases may safely remain untreated, although our results should be regarded as hypothesis‐generating.
To date, there is limited, uncontrolled evidence in a little over 50 patients with confirmed isolated ssPE without deep venous thrombosis (DVT) who remained untreated and none suffered recurrent PE during follow‐up (Le Gal et al, 2006; Donato et al, 2010). Based on these observations, international guidelines suggest clinical surveillance over treatment with anticoagulants in patients with isolated ssPE and no proximal DVT in the legs who have a low risk for recurrent VTE, i.e. patients who are not hospitalized or have reduced mobility and those without active cancer (Class IIb, Grade 2C) (Konstantinides et al, 2014, 2016; Kearon et al, 2016). A currently active outcome study is evaluating the safety of withholding anticoagulant therapy in 300 patients without a history of VTE or cancer who have confirmed isolated ssPE without proximal DVT on bilateral lower extremity ultrasound (NCT01455818). Until the results of this study are available, the decision whether to start anticoagulant treatment in patients with isolated ssPE should be made on an individual basis, taking into account the risk of recurrent PE versus the risk of major bleeding with or without anticoagulant treatment.
We found a good inter‐observer agreement of isolated ssPE diagnosis in the cohort of all PE‐patients of 0·78. Prior studies using the same comparison have suggested that the inter‐observer agreement of the presence of PE depends on the location of the thrombus, with a fair to moderate inter observer agreement for isolated ssPE versus high agreement for more proximal PE. Miller et al (2015) demonstrated a moderate inter‐observer agreement for subsegmental pulmonary artery defects of 0·53, especially in CTPA degraded with technical artefacts, such as breathing motion artefact, artefacts due to cardiac pulsation or beam‐hardening from adjacent high‐density structures (Hutchinson et al, 2015; Miller et al, 2015). Ghanima et al (2007) found a low inter‐observer agreement of 25%, with a kappa of 0·38 (95% CI 0·0–0·89). Although our data do not allow firm conclusions on the reasons for the difference between the current and prior studies, we hypothesize that the further advancements in CT technology since 2002–2008 [inclusion period of the studies reported by Ghanima et al (2007) and Miller et al (2015)] and the very low number of technically inadequate CT scans in the YEARS study may have contributed to the good inter‐observer agreement found in the current analysis.
Strengths of our study include the prospective data collection and comparison of two large cohorts of consecutive in‐ and out‐patients with suspected PE with similar inclusion and exclusion criteria from the same Dutch hospitals. Moreover, we could clearly explain the cause of the observed lower isolated ssPE prevalence among patients managed according to YEARS, supporting the biological plausibility of our conclusion.
The fact that only half of the study population of the YEARS study was subjected to CTPA prevents accurate assessment of the actual number of patients with ssPE that were not detected and remained untreated. In contrast, it remains unclear if patients with isolated ssPE from the Christopher study who would not have been referred for CTPA according to YEARS, would have had an uneventful follow‐up without treatment. Limitations of this post‐hoc analysis are that not all patients from the YEARS study were included. We restricted our analysis to four of the participating centres, which included 66% of the total study patients and in which all original CTPA examinations were readily available for analysis. Second, the association between D‐dimer level and location of the thrombus was made with data from both study cohorts and from all four hospitals that included patients in the YEARS study. Bias could have been introduced in the analysis of the association of D‐dimer level and PE localisation because different D‐dimer assays were used in the participating hospitals and within the hospitals over time. Third, the PE prevalence in YEARS was lower than in Christopher. Although this difference can be partly explained by the lower prevalence of isolated ssPE, we cannot rule out bias towards overestimation of the primary endpoint. Further, the two studies were performed in different time periods (2002–2004 and 2013–2015), allowing for bias due to for instance differences in CT technology. Indeed, where only state‐of‐the‐art MDCT machines were used in the YEARS study, 260 patients from the Christopher cohort were managed with SDCT. Even so, because MDCT allows better visualization of segmental and subsegmental pulmonary arteries, making it easier to detect smaller more distal emboli, the prevalence of isolated ssPE was still lower in the YEARS cohort than in the Christopher cohort, supporting the validity of our findings.
In conclusion, we demonstrated a lower prevalence of isolated ssPE in patients managed according to the YEARS algorithm compared with the conventional diagnostic strategy mainly used in daily clinical practice. This lower prevalence of isolated ssPE is a consequence of the lower sensitivity of YEARS for ssPE due to the higher D‐dimer threshold. Our study provides further indirect evidence that some isolated ssPE may be left untreated in selected patients, although definite proof will only be provided by outcome studies in which patients with ssPE are left untreated. Further, these findings support the relevance of the YEARS, due to its easy applicability, the reduction in number of required CTPAs, the low failure rate and ‐last but not least‐ the associated lower prevalence of isolated ssPE. This was, however, not associated with a higher risk of recurrent VTE during follow‐up, providing indirect evidence that some ssPE may be left untreated in selected patients, although our study was underpowered to detect small differences.
Author contributions
Contribution to study concept and design: LvdP, FK. Data acquisition: LvdP, IB, TvM, TvdH, PdE, MtW, AM, SM, MH, FK. Data analysis and interpretation : LvdP, LK, FK. Assessment of imaging: LB, JvW, AM, FK. Statistical analysis: LvdP, FK. Drafting of the manuscript: LvdP, FK. Critical revision of the manuscript: LvdP, IB, TvM, TvdH, LB, LK, PdE, JvW, MtW, AM, SM, MH, FK. Final approval of the manuscript: LvdP, IB, TvM, TvdH, LB, LK, PdE, JvW, MtW, AM, SM, MH, FK.
Declaration of interests
The authors declare no competing financial interests.
Acknowledgements
This study was supported by unrestricted grants from the participating hospitals.
References
- van Belle, A. , Buller, H.R. , Huisman, M.V. , Huisman, P.M. , Kaasjager, K. , Kamphuisen, P.W. , Kramer, M.H. , Kruip, M.J. , Kwakkel‐van Erp, J.M. , Leebeek, F.W. , Nijkeuter, M. , Prins, M.H. , Sohne, M. & Tick, L.W. (2006) Effectiveness of managing suspected pulmonary embolism using an algorithm combining clinical probability, D‐dimer testing, and computed tomography. JAMA, 295, 172–179. [DOI] [PubMed] [Google Scholar]
- Carrier, M. , Righini, M. , Wells, P.S. , Perrier, A. , Anderson, D.R. , Rodger, M.A. , Pleasance, S. & Le Gal, G. (2010) Subsegmental pulmonary embolism diagnosed by computed tomography: incidence and clinical implications. A systematic review and meta‐analysis of the management outcome studies. Journal of Thrombosis and Haemostasis, 8, 1716–1722. [DOI] [PubMed] [Google Scholar]
- Cohen, J. (1968) Weighted kappa: nominal scale agreement with provision for scaled disagreement or partial credit. Psychological Bulletin, 70, 213–220. [DOI] [PubMed] [Google Scholar]
- De Monye, W. , Sanson, B.J. , Mac Gillavry, M.R. , Pattynama, P.M. , Buller, H.R. , van den Berg‐Huysmans, A.A. & Huisman, M.V. (2002) Embolus location affects the sensitivity of a rapid quantitative D‐dimer assay in the diagnosis of pulmonary embolism. American Journal of Respiratory and Critical Care Medicine, 165, 345–348. [DOI] [PubMed] [Google Scholar]
- Donato, A.A. , Khoche, S. , Santora, J. & Wagner, B. (2010) Clinical outcomes in patients with isolated subsegmental pulmonary emboli diagnosed by multidetector CT pulmonary angiography. Thrombosis Research, 126, e266–e270. [DOI] [PubMed] [Google Scholar]
- den Exter, P.L. , van Es, J. , Klok, F.A. , Kroft, L.J. , Kruip, M.J. , Kamphuisen, P.W. , Buller, H.R. & Huisman, M.V. (2013) Risk profile and clinical outcome of symptomatic subsegmental acute pulmonary embolism. Blood, 122, 1144–1149. quiz 1329. [DOI] [PubMed] [Google Scholar]
- Ghanima, W. , Nielssen, B.E. , Holmen, L.O. , Witwit, A. , Al‐Ashtari, A. & Sandset, P.M. (2007) Multidetector computed tomography (MDCT) in the diagnosis of pulmonary embolism: interobserver agreement among radiologists with varied levels of experience. Acta Radiologica, 48, 165–170. [DOI] [PubMed] [Google Scholar]
- van der Hulle, T. , Cheung, W.Y. , Kooij, S. , Beenen, L.F.M. , van Bemmel, T. , van Es, J. , Faber, L.M. , Hazelaar, G.M. , Heringhaus, C. , Hofstee, H. , Hovens, M.M.C. , Kaasjager, K.A.H. , van Klink, R.C.J. , Kruip, M. , Loeffen, R.F. , Mairuhu, A.T.A. , Middeldorp, S. , Nijkeuter, M. , van der Pol, L.M. , Schol‐Gelok, S. , Ten Wolde, M. , Klok, F.A. & Huisman, M.V. ; for the YEARS study group . (2017) Simplified diagnostic management of suspected pulmonary embolism (the YEARS study): a prospective, multicentre, cohort study. Lancet, 390, 289–297. [DOI] [PubMed] [Google Scholar]
- Hutchinson, B.D. , Navin, P. , Marom, E.M. , Truong, M.T. & Bruzzi, J.F. (2015) Overdiagnosis of pulmonary embolism by pulmonary CT angiography. AJR. American Journal of Roentgenology, 205, 271–277. [DOI] [PubMed] [Google Scholar]
- Jeebun, V. , Doe, S.J. , Singh, L. , Worthy, S.A. & Forrest, I.A. (2010) Are clinical parameters and biomarkers predictive of severity of acute pulmonary emboli on CTPA? QJM, 103, 91–97. [DOI] [PubMed] [Google Scholar]
- Kearon, C. , Akl, E.A. , Ornelas, J. , Blaivas, A. , Jimenez, D. , Bounameaux, H. , Huisman, M. , King, C.S. , Morris, T.A. , Sood, N. , Stevens, S.M. , Vintch, J.R. , Wells, P. , Woller, S.C. & Moores, L. (2016) Antithrombotic therapy for VTE Disease: CHEST guideline and expert panel report. Chest, 149, 315–352. [DOI] [PubMed] [Google Scholar]
- Klok, F.A. & Huisman, M.V. (2017) Management of incidental pulmonary embolism. European Respiratory Journal, 49, 1700275. [DOI] [PubMed] [Google Scholar]
- Klok, F.A. , Djurabi, R.K. , Nijkeuter, M. , Eikenboom, H.C. , Leebeek, F.W. , Kramer, M.H. , Kaasjager, K. , Kamphuisen, P.W. , Buller, H.R. & Huisman, M.V. (2008) High D‐dimer level is associated with increased 15‐d and 3 months mortality through a more central localization of pulmonary emboli and serious comorbidity. British Journal of Haematology, 140, 218–222. [DOI] [PubMed] [Google Scholar]
- Konstantinides, S.V. (2014) 2014 ESC guidelines on the diagnosis and management of acute pulmonary embolism. European Heart Journal, 35, 3145–3146. [DOI] [PubMed] [Google Scholar]
- Konstantinides, S.V. , Torbicki, A. , Agnelli, G. , Danchin, N. , Fitzmaurice, D. , Galie, N. , Gibbs, J.S. , Huisman, M.V. , Humbert, M. , Kucher, N. , Lang, I. , Lankeit, M. , Lekakis, J. , Maack, C. , Mayer, E. , Meneveau, N. , Perrier, A. , Pruszczyk, P. , Rasmussen, L.H. , Schindler, T.H. , Svitil, P. , Vonk Noordegraaf, A. , Zamorano, J.L. & Zompatori, M. ; Task Force for the Diagnosis and Management of Acute Pulmonary Embolism of the European Society of Cardiology . (2014) 2014 ESC guidelines on the diagnosis and management of acute pulmonary embolism. European Heart Journal, 35, 3033–3069. 3069a–3069k. [DOI] [PubMed] [Google Scholar]
- Konstantinides, S.V. , Barco, S. , Lankeit, M. & Meyer, G. (2016) Management of pulmonary embolism: an update. Journal of the American College of Cardiology, 67, 976–990. [DOI] [PubMed] [Google Scholar]
- Le Gal, G. , Righini, M. , Parent, F. , van Strijen, M. & Couturaud, F. (2006) Diagnosis and management of subsegmental pulmonary embolism. Journal of Thrombosis and Haemostasis, 4, 724–731. [DOI] [PubMed] [Google Scholar]
- Miller, W.T. Jr , Marinari, L.A. , Barbosa, E. Jr , Litt, H.I. , Schmitt, J.E. , Mahne, A. , Lee, V. & Akers, S.R. (2015) Small pulmonary artery defects are not reliable indicators of pulmonary embolism. Annals of the American Thoracic Society, 12, 1022–1029. [DOI] [PubMed] [Google Scholar]


