Introduction
Relapse after autologous or allogeneic hematopoietic stem cell transplantation has become the most frequent cause of treatment failure. The 3rd International Workshop on Biology, Prevention, and Treatment of Relapse after Stem Cell Transplantation held in Hamburg / Germany in November 2016 under the auspices of EBMT and ASBMT aimed to provide an update about development and achievements which have been obtained since the 1st workshop in 2009 and the 2nd in 2012.
Here, this review summarized epidemiology of relapse (M.H.), the role of microenvironment (H.S.) and tumor stem cells in the biology of relapse (A.E. / O.H. / J.V.) as well as the role of HLA loss as tumor escape mechanisms (L.V. / C.T.).
Epidemiology of posttransplant relapse: have we made any progress?
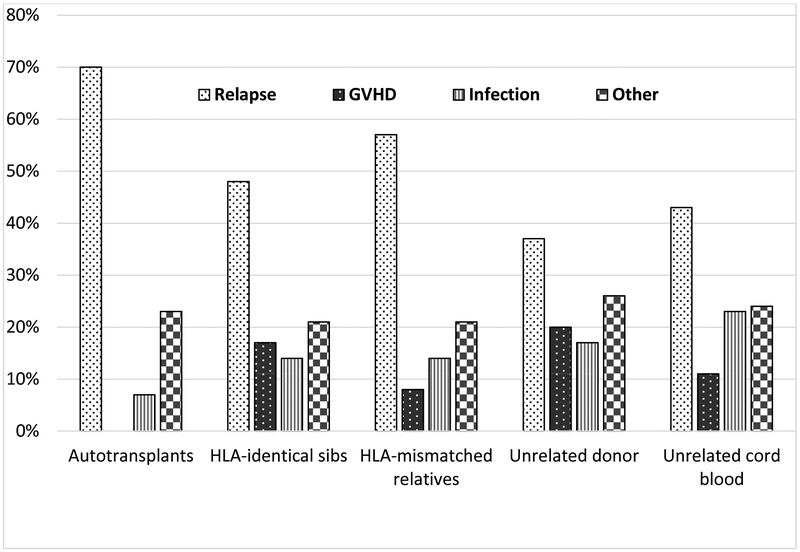
More than a million hematopoietic stem cell transplantations (HCTs) have been done since the first successful ones in 1968, with about 50,000 done annually, worldwide.1 Especially the number of transplantations is growing outside North America and Europe.2 Although initially used to treat non-malignant marrow disorders like primary immune deficiencies and aplastic anemia, most of the HCTs in the current era treat malignant disorders, primarily blood cancers. While long acknowledged to be curative, at least in some patients, in situations where conventional chemotherapy is ineffective, application of HCT for malignancy increased only slowly when first introduced in the 1970s. Recent years witnessed increased utilization, primarily due to expanded donor and graft sources, less toxic pretransplant conditioning and better supportive care leading to better outcomes, even in older and sicker patients. There is little doubt that HCT can now be done more safely, with reported rates of transplant-related mortality decreasing from about 50% in the 1970s to as low as 10% in certain patient groups in the current era.3,4,5,6,7 According to data from the Center for International Blood and Marrow Transplant Research (CIBMTR), transplant-related events such as graft-versus-host disease, Infection and organ toxicity or second cancers accounted for 30%, 52%, 43%, 63% or 57% of posttransplant deaths in patients receiving autologous, HLA-identical sibling, HLA-mismatched related, unrelated donor or unrelated cord blood HCTs, respectively, for leukemia, multiple myeloma or lymphoma in 2012–13 (Figure 1). Corresponding rates for the proportion of deaths related to relapse were 70%, 48%, 57%, 37% and 43% (Figure 1).
Figure 1.
Causes of death among patients transplanted for malignancy in 2012–2013 (Data from the Center for International Blood and Marrow Transplant Research).
The proportion of deaths due to relapse is disappointing, given that control of malignancy was the indication for all of these HCTs and raises the question of whether we have made any progress in exploiting and enhancing the anti-cancer efficacy of HCT over the past three decades. To address this question, we analyzed data for 308,745 patients reported to the CIBMTR. These patients underwent HCT in 1980–2014 for acute myelogenous leukemia (AML), acute lymphoblastic leukemia (ALL), chronic myeloid leukemia, lymphoma or multiple myeloma. Patient characteristics are shown in Table 1. We calculated the five-year rates of transplant-related mortality (TRM, defined as death occurring in the absence of relapse or progression), relapse and treatment failure (defined as occurrence of relapse or TRM or 1 - progression-free survival) by decade, for the entire cohort and for subsets defined by patient and disease characteristics. Figure 2 shows the five year rates of these outcomes according to the decade of transplantation, with Figure 2A depicting trends after allotransplantation and 2B after autotransplantation. The risk of TRM decreased over time, while the risk of relapse increased, for both auto- and alloHCTs. Overall treatment failure rates were relatively stable. Of course, the kinds of patients and diseases treated changed substantially over this 35-year period as is evident from Table 1. Additionally, reduced intensity conditioning, which decreases the toxicity of allotransplantation but may increase relapse, was used with frequency only after 2000, which might account for higher relapse risks after recent allografts. To mitigate the confounding effects of these changes in assessing trends in efficacy and toxicity, we examined outcomes in a group of patients whose eligibility for transplantation was relatively similar over the 25-year study period. For allogeneic transplantation, we chose adults, ages 20–45, transplanted for AML in first complete remission with myeloablative conditioning. For autotransplants, we chose adults, ages 20–45 years, transplanted for chemosensitive non-Hodgkin lymphoma. Figures 2 and 3 show five-years outcomes for these more restricted groups of patients. Again, we see decreasing rates of TRM but increasing rates of relapse, with lesser changes in rates of treatment failure.
Table 1.
Characteristics of 308,745 patients receiving their first allogeneic or autologous hematopoietic stem cell transplant for a hematologic malignancy in 1980-2014 and reported to the CIBMTR.
| Year of transplantation | ||||
|---|---|---|---|---|
| 1980-89 | 1990-99 | 2000-2009 | 2010-2014 | |
| Total N | 21,672 | 89,756 | 121,892 | 75,425 |
| Median age | ||||
| Allogeneic | 27 years | 32 years | 40 years | 51 years |
| Autologous | 33 years | 48 years | 57 years | 58 years |
| Disease | ||||
| Allogeneic | ||||
| Acute myeloid leukemia | 33% | 30% | 42% | 49% |
| Myelodysplastic syndromes | 2% | 5% | 10% | 15% |
| Acute lymphoblastic leukemia | 29% | 22% | 22% | 21% |
| Chronic myeloid leukemia | 30% | 32% | 13% | 4% |
| Lymphoma | 4% | 6% | 11% | 10% |
| Multiple myeloma | 1% | 3% | 2% | 1% |
| Autologous | ||||
| Acute myeloid leukemia | 30% | 14% | 6% | 1% |
| Acute lymphoblastic leukemia | 11% | 3% | <1% | <1% |
| Chronic myeloid leukemia | 4% | 2% | <1% | <1% |
| Lymphoma | 49% | 59% | 41% | 35% |
| Multiple myeloma | 5% | 22% | 51% | 63% |
| Myeloablative Conditioning (allogeneic only) |
100% | 99% | 77% | 65% |
Figure 2A-B.
Five-year outcomes after allogeneic hematopoietic stem cell transplantations for hematologic malignancy by decade (Data from the Center for International Blood and Marrow Transplant Research). (A) Five-year outcomes (in percents) after autologous hematopoietic stem cell transplantations for hematologic malignancy by decade (Data from the Center for International Blood and Marrow Transplant Research). (B)
Figure 3A-B.
Five-year outcomes (in percents) after allogeneic hematopoietic stem cell transplantations for acute myeloid leukemia in first complete remission using myeloablative conditioning in patients aged 20–45 years, by decade (Data from the Center for International Blood and Marrow Transplant Research). (A) Five-year outcomes (in percents) after autologous hematopoietic stem cell transplantations for chemosensitive non-Hodgkin lymphoma in patients aged 20–45 years, by decade (Data from the Center for International Blood and Marrow Transplant Research). (B)
What does this all mean? First of all it is a picture, even in the subset analysis, painted with broad strokes. We did not adjust for every factor that might influence either TRM or relapse risk and one can certainly find subgroups with both lower TRM and lower relapse risks than those depicted in these graphs. Additionally, the variables available for analysis across the time periods were insufficient to fully characterize the biology of the diseases transplanted. For example, as cure rates with intensive chemotherapy regimens for childhood ALL increase, the population referred for transplantation undoubtedly has disease that is biologically more aggressive and more difficult to cure, regardless of the therapy. Similarly, our ability to identify molecular abnormalities associated with prognosis means that those with good prognosis mutations (e.g., NPM1 mutations in AML) are less likely to be transplanted and those with poor prognosis mutations (e.g. FLT3 mutations) are more likely to be transplanted. But that was not the purpose of the exercise, which aimed to show, overall, in the broad population of patients undergoing transplantation, the impact of more than three decades of evolution and dedicated basic and clinical research to improve the outcomes of HCT. And there is cause for satisfaction, in that HCT has become a much safer procedure and is now available to in many more patients than was the case in the 1980s. Most of these patients have few effective alternatives. Do these data mean that increasing safety decreased the antitumor efficacy of HCT? Well, it is possible that is the case for some interventions but mostly it is a case of competing risks, defined as events where the occurrence of one precludes the occurrence of the other. Eliminating the incidence of one competing risk will inevitably increase the frequency of the other, even if the underlying risk of the other event does not change. All of the patients who now do not die early of GVHD or regimen-related toxicity remain at risk of relapse. On the other hand, the data suggest that, overall, we have not increased the anti-tumor efficacy of HCT in a meaningful way. However, given the better safety profile of HCT in general, our increasing understanding of the biology of the diseases we treat and of the mechanisms of posttransplant relapse and availability of new agents, both drugs and cellular therapies, we may now be poised to make the same kind of progress with relapse that we have seen in TRM. In assessing strategies to improve the efficacy of HCT, we must understand that success of a specific strategy will often be disease- and sub-disease specific. Even the long-recognized graft-versus-tumor effects of GVHD differ in different diseases as shown in both historic and recent studies.8,9,10,11 Evaluation of maintenance therapies that target specific mutations or tumor types must be studied in a target-specific way, which will require collaboration of many centers. Although the disease-risk index12 is a useful tool for categorizing the disease-related prognoses of patients across multiple diseases, such grouping may not be ideal to elucidate differential effects of specific agents in diseases with very different biology. And both the diseases treated and the specific drugs used may influence the impact of “dose-intensity”, which may account for the differences across studies evaluating the use myeloablative versus reduced intensity conditioning regimens. Finally, strategies may have to be different for autologous versus allogeneic HCT, or for regimens that use specific GVHD prevention strategies, e.g. post-transplant cyclophosphamide versus T-cell depletion versus standard calcineurin inhibitor based approaches. The challenges are great but no greater than the challenges of decreasing TRM and increasing donor availability, in which we have made so much progress over the past three decades.
Role of microenvironment
As stated above, relapse has become a frequent cause of failure of stem cell transplantation, and rates of failure have remained stable. Thus, novel approaches are clearly needed. We propose to target leukemia cells and their stromal microenvironment with T cells recognizing cancer-specific mutations. Key support for this powerful and exciting concept comes from (i) a much advanced understanding of leukemia and its cancer microenvironment, (ii) the affordability of defining the complete set of expressed truly cancer-specific mutations for any given patient, and (iii) mutation-specific T cell responses emerging as the unifying principle of the most effective immunotherapy not only in mice but also in humans.13
Several past studies have laid the groundwork for the new concept: (i) Niches in the bone marrow microenvironment have been defined that either promote or prevent cancer relapse after allogeneic stem cell transplantation.14 (ii) The number of minor histocompatibility antigens (mHA) as the primary targets of GVL effects15 is continuously increasing (now over 50).16 Some mHAs are preferentially expressed by the hematopoietic system, and T cells may target them as self- or allo-restricted peptide.17 Allo-MHC restricted T cells can overcome problems of self tolerance to self antigens such as mHA17 and may have higher affinity TCRs. However, neither more mHA targets nor higher avidity T cells are likely to reduce the danger of provoking GVHD. (iii) Many ongoing efforts focus on generating small molecule inhibitors that can target selectively cancer-specific mutations. So far however, even clinically effective drugs are not truly cancer-specific; for example, imatinib inhibits the normal Abelson kinase not just the BCR-ABL fusion protein.18 Therefore, no current approach targets cancer cells or their stromal microenvironment19 in a truly cancer-specific way.
By contrast, T cell receptors can recognize with an astounding and exquisite specificity single amino acid substitutions caused by a single nucleotide substitution leaving normal cells completely untouched because they express only the non-mutant peptide.20,21 There are usually 12 different MHC molecules in a patient to present somatic cancer-specific mutations that are recognizable by CD4+ or CD8+ T cells. Thus there is a large number of possible mutation-specific targets even though the absolute number of somatic mutations (10–20) in leukemias such as AML, ALL, and CLL is low compared to that observed in adult solid tumors (see below).22,23,24 Although different cancer patients may have mutations in the same genes, the sequence encoding a mutant peptides is rarely shared between patients. Therefore a truly individualized approach is required. The power of MHC class II-restricted CD4+ T cells in GVL and GVHD has been increasingly appreciated25, encouraging us to focus on targeting cancer mutation-specific MHC-Class II restricted epitopes. The bone marrow microenvironment seems to be ideal for this approach since it constitutively expresses MHC Class II on stromal cells.26 Considering the crucial role of the microenvironment for leukemic cell survival and escape14, it is obvious that targeting cancer stroma could be used to eradicate residual cancer cells. Destroying the stromal bone marrow microenvironment in which the leukemia cells reside would be restricted to the immediate vicinity of the cancer cells. Reasons for this specificity is that sufficient amounts of mutant antigen are needed for sensitizing the stromal cells for destruction. Such amounts occur only in the stromal cells directly surrounding the cancer cells. Thus, experimental evidence indicates27,28,29 that non-malignant stroma cells pick up and cross-present cancer-specific target molecules released from neighboring cancer cells. These molecules include neoantigens encoded by nsSNV or other cancer-specific mutations.20,21 Thus, by eradicating the stromal niches in which cancer cells hide, T cells can eradicate cancer cells and prevent relapse.27,28,29,30
The cancer microenvironment consists of non-malignant cells and extracellular matrix in which cancer cells are embedded (for review see31). A synonym for microenvironment is “stroma” which literally means “bed” in ancient Greek. However, cancer stroma is far more than just a physical structure holding the cancer cells. Instead, cancer stroma is essential not only for cancer cells to grow and escape but also for their eradication.32 All cancer cells depend on being surrounded by nonmalignant stromal cells. Thus, cancer cells attract the stromal cells and instruct them with very specific signals to provide factors and conditions that the cancer cells need to survive, proliferate and invade. This regulatory circuit is often being referred to as a paracrine stimulatory loop.33,34,35,36,37,38
Cancer stroma has three major cellular components, (i) neutrophils and macrophages derived from bone marrow-derived, circulating blood leukocytes27,36, (ii) fibroblasts derived from sessile (local) not circulating precursors38, and (iii) endothelial cells, also derived from sessile precursors.37 All of the three cell types neutrophils33,34,35,39,40 macrophages41,42, endothelial cells43 as well as fibroblasts38 (for review see44), play key roles in the stroma of cancers. Several lines of evidence suggest that fibroblasts differ in function dependent on their anatomic site from which they originate.44,45 This means cancer cells may disseminate to many sites but not be able to induce the fibroblast stroma conducive to survival and formation of metastasis. This may well be the basis for Paget’s 1889 seed and soil hypothesis (for review see38).
The details of the mechanisms whereby stromal destruction eradicates cancer cells are not yet fully understood. However, it is clear from chimera experiments that the BM- as well as the non-BM-derived compartments must (i) both be targeted by the T cells, (ii) express the receptors for TNF as well as IFN-γ29 and (iii) express the relevant MHC class I and/or II molecules.27,29,46,47 Surprisingly, cancer cell destruction can be very effective even when the cancer-specific antigen is exclusively being cross-presented on the stromal cell and not directly recognizable on the cancer cell.48 This is important since cancer cells can lose or lack the MHC Class I or MHC Class II molecules (see below). Thus, indirect presentation leads to highly specific eradication of cancer cells.46,48 The mechanisms involved may be related to a remarkable experimental observation: recipient CD8+ T cells recognizing alloantigen cross-presented on neighboring stromal cells eliminated allogeneic MHC Class I negative bone marrow stem cells while leaving the syngeneic, also MHC Class I negative bone marrow stem cells completely unharmed.49 Single neoepitopes50 may require the collaboration of CD4+ as well as CD8+ T cells at the effector phase46,48 for effective neoantigen-specific stromal destruction. In any case, T cell receptors exquisitely specific for mutant neoantigen can be expressed on T cells that are then adoptively transferred to eradicate advanced long established cancers.50 These T cells will destroy the niches in which residual cancer cells hide and achieve curative treatment, provided that the targeted mutant neoantigen is sufficiently cross-presented by the adjacent stromal cells. In principle, donors of the allogeneic stem cell transplants and/of the patients could also be vaccinated against mutant-antigens in an effort to prevent cancer relapse. These highly patient-individualized approaches are currently being pursued by vaccinating melanoma patients using RNA based vectors51 or long peptides52 encoding the mutant neoepitopes. Even if these active vaccinations should prove therapeutically ineffective, they could serve to generate T cells and isolate mutation-specific TCRs. These TCRs could then be used in mutation-specific TCR gene therapy.21 So-far this approach has only been tested in mice even though it had remarkable therapeutic effects. Stem cell transplantation offers excellent opportunities to pioneer such studies in patients, and these truly novel approaches may well make a significant impact reducing cancer relapse.
The role of tumor stem cells in the biology of relapse
Identification of the cells responsible for long-term tumor propagation is critical to determine which cells must be targeted to ensure disease eradication and prevent relapse. It has been established that some cancers, such as acute myeloid leukemia, follow a hierarchical stem cell model, whereby the tumor is driven by a subpopulation of rare cells with intrinsically defined stem cell properties.53,54 In contrast, the stem cell frequency in tumors such as melanoma is substantially higher.55 This points towards a stochastic model of tumor propagation, in which a high proportion of tumor cells have the capacity to drive tumor growth.
In acute lymphoblastic leukemia (ALL), the bulk of the evidence suggests that the disease follows a stochastic model. We have previously shown that blasts with the capability to reconstitute the leukemia are found in populations with mature and immature immunophenotypes.56 Limiting dilution assays also showed high leukemia-initiating cell (LIC) frequencies in ALL (1:40 to 1:2900 cells).57 More recently, we have used clonal tracking to demonstrate that xenografted ALL is driven by high numbers of functionally homogeneous founder cells which maintain the leukemia over serial transplants.58 Taken together, these data support a model where a majority of blasts can propagate the disease. This is not entirely surprising given the capacity of normal mature lymphoid cells to clonally expand. In acute myeloid leukemia (AML), the story is different and there is indeed strong evidence for a stem hierarchy with a small population of specialized leukemic stem cells that maintain and propagate the disease.59 However, there is also significant variability in respect to phenotype and frequency of the cells that can propagate the leukemia.60,61
The ability for self-renewal is intertwined with intrinsic and extrinsic factors which influence the propagating capacity of LIC.60,61 Analysis of copy number alterations has demonstrated that ALL samples consist of a pool of genetically distinct subclones, commonly related by Darwinian evolutionary trees.62,63 These subclones may differ in their leukemia repopulating capacity, leading to changes in the composition of the propagating pool.64,65 When subjected to pressures such as therapy, this process of clonal evolution can lead to the emergence of resistant clones which drive relapse.66,67,68 A complete model for the evolution of relapse must unify the elements discussed above. A typical leukemia is likely to consist of several genetically distinct subclones, with varying numbers of LIC suggesting that often a high proportion of the individual cells comprising these subclones will be capable of propagating the leukemia. This will provide an abundance of potential units for clonal selection and evolution. The evolutionary process provides a means for blasts to gain advantageous mutations in response to selective pressures, which combined with selection of fitter pre-existing clones may lead to the emergence of therapy resistant clones and relapse.69
In addition to intrinsic genetic differences, the role of extrinsic factors such as the microenvironment must be considered. Leukemic blasts are known to modulate the bone marrow environment70 which can lead to drug resistance in response to therapy.71 A recent study has demonstrated rare dormant, treatment resistant stem cells. Notably in ALL, resistance is dependent on interactions with the endosteal niche; release from this environment induced proliferation and sensitised the cells to drug treatment.72 This work demonstrates the influence of the microenvironment on the behaviour of leukemic blasts in a manner which appears to be stochastic.
It is therefore important to consider the role of the microenvironment in protecting cells from chemotherapy. Although this alone does not explain the emergence of drug resistant relapse, it will facilitate development of resistant phenotypes in cells which survive chemotherapy, which may be enhanced by epigenetic reprogramming.73 It is highly likely that these processes of niche remodelling and epigenetic plasticity are relevant in allowing cells to survive graft vs leukemia effects and immune-targeted therapies.74 Escape mutants to CAR T-cells or monoclonal antibodies such as blinatumomab have been commonly reported75,76,77 and although some may have a genetic basis, it is well established that tumor microenvironments can influence the function of immune effector cells.78
Future therapies must utilise underlying mechanistic understanding to rationally design therapeutic combinations which target both all leukemic blasts with self-renewal potential and specific pathways involved in the emergence of (intrinsically and extrinsically) treatment-resistant subclones. This will also require a thorough characterisation of interactions between leukemic cells and their niche, with the aim of disrupting pathways which facilitate the survival and selection of LIC.
Role of HLA loss
Mutation, downregulation or complete genomic loss of Human Leukocyte Antigen (HLA) molecules is commonly observed in newly-diagnosed solid tumors, suggesting that these cancers become clinically evident only after having gained a mean to evade T cell-mediated immune surveillance.79,80,81 Conversely, hematological malignancies rarely display alterations in HLA molecules at clinical presentation, with the exception of some rare subtypes.82,83 This observation suggests that either hematological tumors are more sensitive to the activity of Natural Killer cells, endowed with the ability to recognize and eliminate cells that have lost HLA Class I expression, or develop too rapidly and aggressively to be significantly edited by the patient immune system.
Nevertheless, patients suffering from hematological cancers are exposed to potent immune pressure when they undergo allogeneic hematopoietic stem cell transplantation (HSCT). Allogeneic HSCT is in fact universally recognized as a highly effective and broad-spectrum form of adoptive immunotherapy, in which donor-derived immune cells recognize not only tumor-specific antigens, but even more potently minor histocompatibility antigens or, in case of partially HLA-incompatible HSCT, the patient-specific HLA molecules.84,85 In particular, in the context of haploidentical family donor HSCT, donor T cells are preferentially unleashed against the fully mismatched HLA haplotype, and their potent primary alloreactivity, if not controlled, can rapidly lead to fatal graft-versus-host disease.86
In this highly peculiar immunogenetic context, we first described that after HSCT residual leukemic cells can undergo genomic loss of the mismatched HLA haplotype to evade the control operated by alloreactive donor T cells and outgrow into clinical relapse.87 This event, termed “HLA loss” henceforth, is the consequence of acquired uniparental disomy (also called copy-neutral loss of heterozygosity, cnLOH) of a large genomic region of chromosome 6p, often encompassing all HLA class I and class II loci. Of notice, by this mechanism not only the leukemic cells lose the main targets of donor T cell alloreactivity and render itself “invisible” to circulating T cells, but also conserve a physiological copy number of HLA class I genes, thereby limiting the activation of NK cell-mediated responses.88,89 (see figure 4)
Figure 4.
Loss of mismatched HLA in leukemic cells after haploidentical HSCT. Schematic model of the causes and consequences of genomic loss of the patient-specific HLA haplotype at relapse after transplantation. Leukemic cells, heterozygous at diagnosis for the shared (in blue) and the mismatched patient-specific (in red) haplotype, are exposed to an intense immunological pressure after transplantation, mostly mediated by donor T cells expressing alloreactive T cell receptors (in green) and targeted against the mismatched HLA haplotype. This selective environment favors the emergence of mutant variants that lack the patient-specific HLA haplotype, and are therefore no longer recognized by donor T lymphocytes.
Of interest, in all cases analyzed to date the HLA loss leukemic clone was not evident before HSCT, suggesting that it was either generated after transplant or present as a minimal fraction of the leukemic bulk in the absence of immune pressure, and providing a remarkable proof-of-concept on the ability of leukemic cells to evolve in a selective environment.
After the initial report, we and others extended the observation of HLA loss relapses to different HSCT contexts and diseases.90,91,92,93 The highest incidence has been reported in T cell-replete haploidentical HSCTs for myeloid malignancies, in which HLA loss relapses can account for up to one third of the total relapses, and have been shown to be significantly linked to the dose of donor T cells infused into the patient and to the occurrence of chronic GvHD after HSCT.94,95 Notably, HLA loss relapses have been recently reported to occur with similar frequency also after haploidentical HSCTs employing post-transplant cyclophosphamide (PT-Cy) as GvHD prophylaxis.96,97,98 This finding suggests that even though PT-Cy can dramatically decrease the risk of severe GvHD after haploidentical HSCT, it does not eliminate T cell alloreactivity against the partially HLA-mismatched leukemia.
The considerable incidence of HLA loss relapses documented in T cell-replete haploidentical transplants, together with the absence of a clear association between NK cell alloreactivity and protection from these relapse variants94, support the notion that whereas in T cell-depleted haploidentical transplants NK cell alloreactivity represents the main driver of the Graft-versus-Leukemia (GvL) effect, its role in the most recent platforms of haploidentical HSCT is blunted by competition with T cells87 or by the concomitant drugs99 and alloreactive T cells appear to be the main GvL mediators in this setting.
Cases of HLA loss have been reported also after partially HLA-mismatched unrelated donor HSCT.100,101 However, these studies (and others that failed to detect HLA loss variants in similar settings94,102) were all conducted in very small and heterogeneous cohorts. Therefore, it is to date impossible to accurately estimate the actual frequency and associated risk factors for HLA loss in unrelated donor HSCT: we might however speculate that the incidence of HLA loss relapses might be lower than in haploidentical HSCT, due to the fact that in unrelated donor HSCT donor-recipient HLA mismatches are commonly fewer than in the haploidentical setting and, if present, can involve both patient HLA haplotypes, balancing the selective pressure mediated by donor alloreactive T cells and blocking escape routes to leukemic cells. However, larger multicenter studies are warranted to confirm this hypothesis, including also the study of relapses after umbilical cord blood transplantation, to date totally unexplored.
It is important to note that the documentation of HLA loss at relapse is not merely a biological curiosity, but has relevant and direct clinical implications: by losing the main targets of donor T cell alloreactivity, HLA loss variants become in fact less susceptible to respond to donor lymphocyte infusions (DLIs).87 For patients with HLA loss relapses it is therefore advised to consider alternative salvage strategies, including re-transplantation from a different donor or cellular therapies based on unconventional target recognition modalities such as for instance chimeric antigen receptor-modified T cells.88,103 Assessment of eventual HLA loss before administering therapeutic DLIs at relapse is therefore highly recommended after haploidentical HSCT, in which the phenomenon is remarkably frequent in all reported series, and is at least worth considering for relapses after unrelated donor HSCTs.104 The recent development of “HLA-KMR”, a reliable and easy-to-implement quantitative PCR-based molecular assay for the detection of HLA loss relapses,105 will thus not only be instrumental to conduct larger retrospective and prospective studies to analyze the phenomenon in different HSCT settings, but even more importantly will facilitate rapid clinical decision-making and patient-tailored therapeutic interventions.
Footnotes
None of the authors declared any conflict of interests.
References
- 1.Gratwohl A, Pasquini MC, Aljurf M, Atsuta Y, Baldomero H, Foeken L, et al. One million haemopoietic stem-cell transplants: a retrospective observational study. The Lancet Haematol 2015; 2(3):e91–e100. doi:10.1016/S2352-3026(15)00028-9. Epub 2015 Mar 1. [DOI] [PubMed] [Google Scholar]
- 2.Passweg JR, Baldomero H, Bader P, Bonini C, Duarte RF, Dufour C et al. Use of haploidentical stem cell transplantation continues to increase: the 2015 European Societa for Blood and Marrow Transplant activity survey report. Bone Marrow Transplant 2017; 52: 811–817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.D’Souza A, Lee S, Zhu X, Pasquini M. Current use and trends in hematopoietic cell transplantation in the United States. Biol Blood Marrow Transplant 2017; 23: 1417–1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rafii H, Ruggeri A, Volt F, Brunstein CG, Carreras J, Eapen M, et al. Changing trends of unrelated umbilical cord blood transplantation for hematologic diseases in patients older than fifty years: A Eurocord-Center for International Blood and Marrow Transplant research survey. Biol Blood Marrow Transplant 2016; 22: 1717–1720. [DOI] [PubMed] [Google Scholar]
- 5.Costa LJ, Zhang M-J, Zhong X, Dispenzieri A, Lonial S, Krishnan A et al. Trends in utilization and outcomes of autologous transplantation as early therapy for multiple myeloma. Biol Blood Marrow Transplant 2013; 19: 1615–1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McCarthy PL Jr., Hahn T, Hassebroek A, Bredeson C, Gajewski J, Hale G et al. Trends in use of and survival after autologous hematopoietic cell transplantation in North America, 1995–2005: significant improvement in survival for lymphoma and myeloma during a period of increasing recipient age. Biol Blood Marrow Transplant 2013; 19: 1116–1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gooley TA, Chien JW, Pergam SA, Hingorani S, Sorror ML, Boeckh M et al. Reduced mortality after allogeneic hematopoietic-cell transplantation. N Engl J Med 2010; 363: 2091–2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Horowitz MM, Gale RP, Sondel PM, Goldman JM, Kersey JH, Kolb HJ, et al. Graft-versus-leukemia reactions after bone marrow transplantation. Blood 1990; 75: 555–562. [PubMed] [Google Scholar]
- 9.Weisdorf D, Zhang M-J, Arora M, Horowitz MM, Rizzo JD, Eapen M. Graft-versus-host disease induced graft-versus-leukemia effect: greater impact on relapse and disease-free survival after reduced intensity conditioning. Biol Blood Marrow Transplant 2012; 18: 1727–1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boyiadzis M, Arora M, Klein JP, Hassebroek A, Hemmer M, Urbano-Ispizua A et al. Impact of chronic graft-versus-host disease on late relapse and survival on 7,489 patients after myeloablative allogeneic hematopoietic cell transplantation for leukemia. Clin Cancer Res 2015; 21: 2020–2028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Urbano-Ispizua A, Pavletic SZ, Flowers ME, Klein JP, Zhang M-J, Carreras J et al. The impact of graft-versus-host disease on the relapse rate in patients with lymphoma depends on the histological subtype and the intensity of the conditioning regimen. Biol Blood Marrow Transplant 2015; 21: 1746–1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Armand P, Kim HAT, Logan BR, Wang Z, Aleya EP, Kalaycio ME, et al. Validation and refinement of the disease risk index for allogeneic stem cell transplantation. Blood 2014; 123: 3664–3671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tran E, Robbins PF, Rosenberg SA. ‘Final common pathway’ of human cancer immunotherapy: targeting random somatic mutations. Nat Immunol 2017; 18(3): 255–262. doi: 10.1038/ni.3682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fracchiolla NS, Fattizzo B, Cortelezzi A. Mesenchymal Stem Cells in Myeloid Malignancies: A Focus on Immune Escaping and Therapeutic Implications. Stem Cells Int 2017; 2017: 6720594. doi: 10.1155/2017/6720594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kolb HJ. Hematopoietic stem cell transplantation and cellular therapy. HLA 2017; 89(5): 267–277. doi: 10.1111/tan.13005 [DOI] [PubMed] [Google Scholar]
- 16.Spierings E Minor histocompatibility antigens: past, present, and future. Tissue Antigens 2014; 84(4): 374–360. doi: 10.1111/tan.12445 [DOI] [PubMed] [Google Scholar]
- 17.Stauss HJ. Immunotherapy with CTLs restricted by nonself MHC. Immunol Today 1999; 20(4): 180–183. [DOI] [PubMed] [Google Scholar]
- 18.Schreiber H, Rowley JD, Rowley DA. Targeting mutations predictably. Blood 2011; 118(4): 830–831. doi: 10.1182/blood-2011-06-357541 [DOI] [PubMed] [Google Scholar]
- 19.Shi Y, Du L, Lin L, Wang Y. Tumour-associated mesenchymal stem/stromal cells: emerging therapeutic targets. Nat Rev Drug Discov 2017; 16(1): 35–52. doi: 10.1038/nrd.2016.193 [DOI] [PubMed] [Google Scholar]
- 20.Monach PA, Meredith SC, Siegel CT, Schreiber H. A unique tumor antigen produced by a single amino acid substitution. Immunity 1995; 2: 45–59. [DOI] [PubMed] [Google Scholar]
- 21.Blankenstein T, Leisegang M, Uckert W, Schreiber H. Targeting cancer-specific mutations by T cell receptor gene therapy. Curr Opin Immunol 2015; 33: 112–119. doi: 10.1016/j.coi.2015.02.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vogelstein B, Papadopoulos N, Velculescu VE, Zhou S, Diaz LA Jr., Kinzler KW. Cancer genome landscapes. Science 2013; 339(6127): 1546–1558. doi: 10.1126/science.1235122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mullighan CG. The genomic landscape of acute lymphoblastic leukemia in children and young adults. Hematology Am Soc Hematol Educ Program 2014; 2014(1): 174–180. doi: 10.1182/asheducation-2014.1.174 [DOI] [PubMed] [Google Scholar]
- 24.Heo SG, Koh Y, Kim JK, Jung J, Kim HL, Yoon SS et al. Identification of somatic mutations using whole-exome sequencing in Korean patients with acute myeloid leukemia. BMC Med Genet 2017; 18(1): 23. doi: 10.1186/s12881-017-0382-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rutten CE, van Luxemburg-Heijs SA, Halkes CJ, van Bergen CA, Marijt EW, Oudshoorn M et al. Patient HLA-DP-specific CD4+ T cells from HLA-DPB1-mismatched donor lymphocyte infusion can induce graft-versus-leukemia reactivity in the presence or absence of graft-versus-host disease. Biol Blood Marrow Transplant 2013; 19(1): 40–48. doi: 10.1016/j.bbmt.2012.07.020 [DOI] [PubMed] [Google Scholar]
- 26.Stumpf AN, van der Meijden ED, van Bergen CA, Willemze R, Falkenburg JH, Griffioen M. Identification of 4 new HLA-DR-restricted minor histocompatibility antigens as hematopoietic targets in antitumor immunity. Blood 2009; 114(17): 3684–3692. doi: 10.1182/blood-2009-03-208017 [DOI] [PubMed] [Google Scholar]
- 27.Spiotto MT, Rowley DA, Schreiber H. Bystander elimination of antigen loss variants in established tumors. Nat Med 2004; 10(3): 294–298. [DOI] [PubMed] [Google Scholar]
- 28.Zhang B, Bowerman NA, Salama JK, Schmidt H, Spiotto MT, Schietinger A et al. Induced sensitization of tumor stroma leads to eradication of established cancer by T cells. J Exp Med 2007; 204(1): 49–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang B, Karrison T, Rowley DA, Schreiber H. IFN-gamma- and TNF-dependent bystander eradication of antigen-loss variants in established mouse cancers. J Clin Invest 2008; 118(4): 1398–1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Engels B, Engelhard VH, Sidney J, Sette A, Binder DC, Liu RB et al. Relapse or Eradication of Cancer Is Predicted by Peptide-Major Histocompatibility Complex Affinity. Cancer Cell 2013; 23(4): 516–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schreiber H Cancer Immunology In: Paul WE (ed) Fundamental Immunology, 7th edn. Lippincott-Williams & Wilkins: Philadelphia, PA, 2013, pp 1200–1234. [Google Scholar]
- 32.Singh S, Ross SR, Acena M, Rowley DA, Schreiber H. Stroma is critical for preventing or permitting immunological destruction of antigenic cancer cells. J Exp Med 1992; 175: 139–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pekarek LA, Starr BA, Toledano AY, Schreiber H. Inhibition of tumor growth by elimination of granulocytes. J Exp Med 1995; 181: 435–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Seung LP, Seung SK, Schreiber H. Antigenic cancer cells that escape immune destruction are stimulated by host cells. Cancer Res 1995; 55: 5094–5100. [PubMed] [Google Scholar]
- 35.Seung LP, Rowley DA, Dubey P, Schreiber H. Synergy between T-cell immunity and inhibition of paracrine stimulation causes tumor rejection. Proc Natl Acad Sci U S A 1995; 92: 6254–6258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cortez-Retamozo V, Etzrodt M, Newton A, Rauch PJ, Chudnovskiy A, Berger C et al. Origins of tumor-associated macrophages and neutrophils. Proc Natl Acad Sci U S A 2012; 109(7): 2491–2496. doi: 10.1073/pnas.1113744109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Purhonen S, Palm J, Rossi D, Kaskenpaa N, Rajantie I, Yla-Herttuala S et al. Bone marrow-derived circulating endothelial precursors do not contribute to vascular endothelium and are not needed for tumor growth. Proc Natl Acad Sci U S A 2008; 105(18): 6620–6625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Arina A, Idel C, Hyjek EM, Alegre ML, Wang Y, Bindokas VP et al. Tumor-associated fibroblasts predominantly come from local and not circulating precursors. Proc Natl Acad Sci U S A 2016; 113(27): 7551–7556. doi: 10.1073/pnas.1600363113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Singel KL, Segal BH. Neutrophils in the tumor microenvironment: trying to heal the wound that cannot heal. Immunol Rev 2016; 273(1): 329–343. doi: 10.1111/imr.12459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Coffelt SB, Wellenstein MD, de Visser KE. Neutrophils in cancer: neutral no more. Nat Rev Cancer 2016; 16(7): 431–446. doi: 10.1038/nrc.2016.52 [DOI] [PubMed] [Google Scholar]
- 41.Bronte V, Wang M, Overwijk WW, Surman DR, Pericle F, Rosenberg SA et al. Apoptotic death of CD8+ T lymphocytes after immunization: induction of a suppressive population of Mac-1+/Gr-1+ cells. J Immunol 1998; 161: 5313–5320.; [PMC free article] [PubMed] [Google Scholar]
- 42.Bronte V, Brandau S, Chen SH, Colombo MP, Frey AB, Greten TF et al. Recommendations for myeloid-derived suppressor cell nomenclature and characterization standards. Nat Commun 2016; 7: 12150. doi: 10.1038/ncomms12150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kammertoens T, Friese C, Arina A, Idel C, Briesemeister D, Rothe M et al. Tumour ischemia by Interferon-γ resembles physiological blood vessel regression. Nature 2017; 545(7652): 98–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ohlund D, Elyada E, Tuveson D. Fibroblast heterogeneity in the cancer wound. J Exp Med 2014; 211(8): 1503–1523. doi: 10.1084/jem.20140692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chang HY, Chi JT, Dudoit S, Bondre C, van de Rijn M, Botstein D et al. Diversity, topographic differentiation, and positional memory in human fibroblasts. Proc Natl Acad Sci U S A 2002; 99(20): 12877–12882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Arina A, Karrison T, Galka E, Schreiber K, Weichselbaum RR, Schreiber H. Transfer of allogeneic CD4+ T cells rescues CD8+ T cells in anti-PD-L1-resistant tumors leading to tumor eradication. Cancer Immunol Res 2017; 5(2): 127–136. doi: 10.1158/2326-6066.CIR-16-0293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Spiotto MT, Schreiber H. Rapid destruction of the tumor microenvironment by CTLs recognizing cancer-specific antigens cross-presented by stromal cells. Cancer Immun 2005; 5: 8. [PubMed] [Google Scholar]
- 48.Schietinger A, Philip M, Liu RB, Schreiber K, Schreiber H. Bystander killing of cancer requires the cooperation of CD4(+) and CD8(+) T cells during the effector phase. J Exp Med 2010; 207(11): 2469–2477. e-pub ahead of print 2010/10/06; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Haspot F, Li HW, Lucas CL, Fehr T, Beyaz S, Sykes M. Allospecific rejection of MHC class I-deficient bone marrow by CD8 T cells. Am J Transplant 2014; 14(1): 49–58. doi: 10.1111/ajt.12525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Leisegang M, Engels B, Schreiber K, Yew PY, Kiyotani K, Idel C et al. Eradication of Large Solid Tumors by Gene Therapy with a T-Cell Receptor Targeting a Single Cancer-Specific Point Mutation. Clin Cancer Res 2016; 22(11): 2734–2743. doi: 10.1158/1078-0432.CCR-15-2361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sahin U, Derhovanessian E, Miller M, Kloke BP, Simon P, Lower M et al. Personalized RNA mutanome vaccines mobilize poly-specific therapeutic immunity against cancer. Nature 2017; 547(7662): 222–226. doi: 10.1038/nature23003 [DOI] [PubMed] [Google Scholar]
- 52.Ott PA, Hu Z, Keskin DB, Shukla SA, Sun J, Bozym DJ et al. An immunogenic personal neoantigen vaccine for patients with melanoma. Nature 2017; 547(7662): 217–221. doi: 10.1038/nature22991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lapidot T, Sirard C, Vormoor J, Murdoch B, Hoang T, Caceres-Cortes J et al. A cell initiating human acute myeloid leukaemia after transplantation into SCID mice. Nature 1994; 367(6464): 645–648. doi: 10.1038/367645a0 [DOI] [PubMed] [Google Scholar]
- 54.Hope KJ, Jin L, Dick JE. Acute myeloid leukemia originates from a hierarchy of leukemic stem cell classes that differ in self-renewal capacity. Nat Immunol 2004; 5(7): 738–743. [DOI] [PubMed] [Google Scholar]
- 55.Quintana E, Shackleton M, Sabel MS, Fullen DR, Johnson TM, Morrison SJ. Efficient tumour formation by single human melanoma cells. Nature 2008; 456(7222): 593–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.le Viseur C, Hotfilder M, Bomken S, Wilson K, Rottgers S, Schrauder A et al. In childhood acute lymphoblastic leukemia, blasts at different stages of immunophenotypic maturation have stem cell properties. Cancer Cell 2008; 14(1): 47–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rehe K, Wilson K, Bomken S, Williamson D, Irving J, den Boer ML et al. Acute B lymphoblastic leukaemia-propagating cells are present at high frequency in diverse lymphoblast populations. EMBO molecular medicine 2013; 5(1): 38–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Elder A, Bomken S, Wilson I, Blair HJ, Cockell S, Ponthan F et al. Abundant and equipotent founder cells establish and maintain acute lymphoblastic leukaemia. Leukemia 2017; 31(12): 2577–2586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Goardon N, Marchi E, Atzberger A, Quek L, Schuh A, Soneji S, et al. Coexistence of LMPP-like and GMP-like leukemia stem cells in acute myeloid leukemia. Cancer Cell 2011; 19(1): 138–152. [DOI] [PubMed] [Google Scholar]
- 60.Quek L, Otto GW, Garnett C, Lhermitte L, Karamitros D, Stoilova B, et al. Genetically distinct leukemic stem cells in human CD34- acute myeloid leukemia are arrested at a hemopoietic precursor-like stage. J Exp Med 2016; 213(8): 1513–1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Thomas D, Majeti R. Biology and relevance of human acute myeloid leukemia stem cells. Blood 2017; 129(12): 1577–1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Anderson K, Lutz C, van Delft FW, Bateman CM, Guo Y, Colman SM et al. Genetic variegation of clonal architecture and propagating cells in leukaemia. Nature 2011; 469(7330): 356–361. doi: 10.1038/nature09650 [DOI] [PubMed] [Google Scholar]
- 63.Notta F, Mullighan CG, Wang JC, Poeppl A, Doulatov S, Phillips LA et al. Evolution of human BCR-ABL1 lymphoblastic leukaemia-initiating cells. Nature 2011; 469(7330): 362–367. e-pub ahead of print 2011/01/21; doi: 10.1038/nature09733 [DOI] [PubMed] [Google Scholar]
- 64.Bardini M, Woll PS, Corral L, Luc S, Wittmann L, Ma Z et al. Clonal variegation and dynamic competition of leukemia-initiating cells in infant acute lymphoblastic leukemia with MLL rearrangement. Leukemia 2015; 29(1): 38–50. [DOI] [PubMed] [Google Scholar]
- 65.Schmitz M, Breithaupt P, Scheidegger N, Cario G, Bonapace L, Meissner B et al. Xenografts of highly resistant leukemia recapitulate the clonal composition of the leukemogenic compartment. Blood 2011; 118(7): 1854–1864. [DOI] [PubMed] [Google Scholar]
- 66.Ma X, Edmonson M, Yergeau D, Muzny DM, Hampton OA, Rusch M et al. Rise and fall of subclones from diagnosis to relapse in pediatric B-acute lymphoblastic leukaemia. Nature Communications 2015; 6: 6604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Waanders E, Scheijen B, van der Meer LT, van Reijmersdal SV, van Emst L, Kroeze Y et al. The Origin and Nature of Tightly Clustered BTG1 Deletions in Precursor B-Cell Acute Lymphoblastic Leukemia Support a Model of Multiclonal Evolution. PLoS Genet 2012; 8(2): e1002533. e-pub ahead of print 2012/02/24; doi: 10.1371/journal.pgen.1002533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.van Delft FW, Horsley S, Colman S, Anderson K, Bateman C, Kempski H et al. Clonal origins of relapse in ETV6-RUNX1 acute lymphoblastic leukemia. Blood 2011; 117(23): 6247–6254. [DOI] [PubMed] [Google Scholar]
- 69.Mullighan CG, Phillips LA, Su X, Ma J, Miller CB, Shurtleff SA et al. Genomic analysis of the clonal origins of relapsed acute lymphoblastic leukemia. Science 2008; 322(5906): 1377–1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Polak R, de Rooij B, Pieters R, den Boer ML. B-cell precursor acute lymphoblastic leukemia cells use tunneling nanotubes to orchestrate their microenvironment. Blood 2015; 126(21): 2404–2414. [DOI] [PubMed] [Google Scholar]
- 71.Tesfai Y, Ford J, Carter KW, Firth MJ, O’Leary RA, Gottardo NG et al. Interactions between acute lymphoblastic leukemia and bone marrow stromal cells influence response to therapy. Leuk Res 2012; 36(3): 299–306. [DOI] [PubMed] [Google Scholar]
- 72.Ebinger S, Ozdemir EZ, Ziegenhain C, Tiedt S, Castro Alves C, Grunert M et al. Characterization of Rare, Dormant, and Therapy-Resistant Cells in Acute Lymphoblastic Leukemia. Cancer Cell 2016; 30(6): 849–862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Duan CW, Shi J, Chen J, Wang B, Yu YH, Qin X et al. Leukemia propagating cells rebuild an evolving niche in response to therapy. Cancer Cell 2014; 25(6): 778–793. [DOI] [PubMed] [Google Scholar]
- 74.Liu J, Zhong JF, Zhang X, Zhang C. Allogeneic CD19-CAR-T cell infusion after allogeneic hematopoietic stem cell transplantation in B cell malignancies. J Hematol Oncol 2017; 10(1): 35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sotillo E, Barrett DM, Black KL, Bagashev A, Oldridge D, Wu G et al. Convergence of Acquired Mutations and Alternative Splicing of CD19 Enables Resistance to CART-19 Immunotherapy. Cancer Discov 2015; 5(12): 1282–1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Braig F, Brandt A, Goebeler M, Tony HP, Kurze AK, Nollau P et al. Resistance to anti-CD19/CD3 BiTE in acute lymphoblastic leukemia may be mediated by disrupted CD19 membrane trafficking. Blood 2017; 129(1): 100–104. [DOI] [PubMed] [Google Scholar]
- 77.Weiland J, Pal D, Case M, Irving J, Ponthan F, Koschmieder S et al. BCP-ALL blasts are not dependent on CD19 expression for leukaemic maintenance. Leukemia 2016; 30(9): 1920–1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Whiteside TL. The tumor microenvironment and its role in promoting tumor growth. Oncogene 2008; 27(45): 5904–5912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Marincola FM, Jaffee EM, Hicklin DJ, Ferrone S. Escape of human solid tumors from T-cell recognition: molecular mechanisms and functional significance. Adv Immunol 2000; 74: 181–273. [DOI] [PubMed] [Google Scholar]
- 80.Garrido F, Ruiz-Cabello F, Cabrera T, Pérez-Villar JJ, López-Botet M, Duggan-Keen M et al. Implications for immunosurveillance of altered HLA class I phenotypes in human tumours. Immunol Today 1997; 18: 89–95. [DOI] [PubMed] [Google Scholar]
- 81.Schreiber RD, Old LJ, Smyth MJ. Cancer immunoediting: integrating immunity’s roles in cancer suppression and promotion. Science 2011; 331: 1565–1570. [DOI] [PubMed] [Google Scholar]
- 82.Booman M, Douwes J, Glas AM, Riemersma SA, Jordanova ES, Kok K et al. Mechanisms and effects of loss of human leukocyte antigen class II expression in immune-privileged site-associated B-cell lymphoma. Clin Cancer Res 2006; 12: 2698–2705. [DOI] [PubMed] [Google Scholar]
- 83.Schwindt H, Vater I, Kreuz M, Montesinos-Rongen M, Brunn A, Richter J et al. Chromosomal imbalances and partial uniparental disomies in primary central nervous system lymphoma. Leukemia 2009; 23: 1875–1884. [DOI] [PubMed] [Google Scholar]
- 84.Warren EH, Deeg HJ. Dissecting graft-versus-leukemia from graft-versus-host-disease using novel strategies. Tissue Antigens 2013; 81: 183–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Fleischhauer K, Beelen DW. HLA mismatching as a strategy to reduce relapse after alternative donor transplantation. Semin Hematol 2016; 53: 57–64. [DOI] [PubMed] [Google Scholar]
- 86.Kanakry CG, Fuchs EJ, Luznik L. Modern approaches to HLA-haploidentical blood or marrow transplantation. Nat Rev Clin Oncol 2016; 13: 10–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Vago L, Perna SK, Zanussi M, Mazzi B, Barlassina C, Stanghellini MTL et al. Loss of mismatched HLA in leukemia after stem-cell transplantation. N Engl J Med 2009; 361: 478–488. [DOI] [PubMed] [Google Scholar]
- 88.Vago L, Toffalori C, Ciceri F, Fleischhauer K. Genomic loss of mismatched human leukocyte antigen and leukemia immune escape from haploidentical graft-versus-leukemia. Semin Oncol 2012; 39: 707–715. [DOI] [PubMed] [Google Scholar]
- 89.O’Keefe C, McDevitt MA, Maciejewski JP. Copy neutral loss of heterozygosity: a novel chromosomal lesion in myeloid malignancies. Blood 2010; 115: 2731–2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Villalobos IB, Takahashi Y, Akatsuka Y, Muramatsu H, Nishio N, Hama A et al. Relapse of leukemia with loss of mismatched HLA resulting from uniparental disomy after haploidentical hematopoietic stem cell transplantation. Blood 2010; 115: 3158–3161. [DOI] [PubMed] [Google Scholar]
- 91.Hirabayashi K, Kurata T, Horiuchi K, Saito S, Shigemura T, Tanaka M et al. Loss of Mismatched HLA on the Leukemic Blasts of Patients With Relapsed Lymphoid Malignancies Following Bone Marrow Transplantation From Related Donors With HLA Class II Mismatches in the Graft Versus Host Direction. Pediatr Blood Cancer 2016; 63: 709–711. [DOI] [PubMed] [Google Scholar]
- 92.Stölzel F, Hackmann K, Kuithan F, Mohr B, Füssel M, Oelschlägel U et al. Clonal evolution including partial loss of human leukocyte antigen genes favoring extramedullary acute myeloid leukemia relapse after matched related allogeneic hematopoietic stem cell transplantation. Transplantation 2012; 93: 744–749. [DOI] [PubMed] [Google Scholar]
- 93.Park BG, Sohn YH, Oh HB, Seo EJ, Jang S, Hong SP. Loss of mismatched HLA detected in the peripheral blood of an AML patient who relapsed after haploidentical hematopoietic stem cell transplantation. Ann Lab Med 2015; 35: 551–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Crucitti L, Crocchiolo R, Toffalori C, Mazzi B, Greco R, Signori A et al. Incidence, risk factors and clinical outcome of leukemia relapses with loss of the mismatched HLA after partially incompatible hematopoietic stem cell transplantation. Leukemia 2015; 29: 1143–1152. [DOI] [PubMed] [Google Scholar]
- 95.Peccatori J, Forcina A, Clerici D, Crocchiolo R, Vago L, Stanghellini MTL et al. Sirolimus-based graft-versus-host disease prophylaxis promotes the in vivo expansion of regulatory T cells and permits peripheral blood stem cell transplantation from haploidentical donors. Leukemia 2015; 29: 396–405. [DOI] [PubMed] [Google Scholar]
- 96.Cieri N, Greco R, Crucitti L, Morelli M, Giglio F, Levati G et al. Post-transplantation Cyclophosphamide and Sirolimus after Haploidentical Hematopoietic Stem Cell Transplantation Using a Treosulfan-based Myeloablative Conditioning and Peripheral Blood Stem Cells. Biol Blood Marrow Transplant 2015; 21: 1506–1514. [DOI] [PubMed] [Google Scholar]
- 97.McCurdy SR, Iglehart BS, Batista DA, Gocke CD, Ning Y, Knaus HA et al. Loss of the mismatched human leukocyte antigen haplotype in two acute myelogenous leukemia relapses after haploidentical bone marrow transplantation with post-transplantation cyclophosphamide. Leukemia 2016; 30: 2102–2106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Grosso D, Johnson E, Colombe B, Alpdogan O, Carabasi M, Filicko-O’Hara J et al. Acquired uniparental disomy in chromosome 6p as a feature of relapse after T-cell replete haploidentical hematopoietic stem cell transplantation using cyclophosphamide tolerization. Bone Marrow Transplant 2017; 52(4): 615–619. [DOI] [PubMed] [Google Scholar]
- 99.Russo A, Oliveira G, Berglund S, Greco R, Gambacorta V, Cieri N et al. NK cell recovery after haploidentical HSCT with posttransplant cyclophosphamide: dynamics and clinical implications. Blood 2018; 131(2): 247–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Waterhouse M, Pfeifer D, Pantic M, Emmerich F, Bertz H, Finke J. Genome-wide profiling in AML patients relapsing after allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant 2011; 17: 1450–1459.e1. [DOI] [PubMed] [Google Scholar]
- 101.Toffalori C, Cavattoni I, Deola S, Mastaglio S, Giglio F, Mazzi B et al. Genomic loss of patient-specific HLA in acute myeloid leukemia relapse after well-matched unrelated donor HSCT. Blood 2012; 119: 4813–4815. [DOI] [PubMed] [Google Scholar]
- 102.Hamdi A, Cao K, Poon LM, Aung F, Kornblau S, Fernandez Vina MA et al. Are changes in HLA Ags responsible for leukemia relapse after HLA-matched allogeneic hematopoietic SCT? Bone Marrow Transplant 2015; 50: 411–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Jena B, Dotti G, Cooper LJN. Redirecting T-cell specificity by introducing a tumor-specific chimeric antigen receptor. Blood 2010; 116: 1035–1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Tsirigotis P, Byrne M, Schmid C, Baron F, Ciceri F, Esteve J et al. Relapse of AML after hematopoietic stem cell transplantation: methods of monitoring and preventive strategies. A review from the ALWP of the EBMT. Bone Marrow Transplant 2016; 51: 1431–1438. [DOI] [PubMed] [Google Scholar]
- 105.Ahci M, Toffalori C, Bouwmans E, Crivello P, Brambati C, Pultrone C et al. A new tool for rapid and reliable diagnosis of HLA loss relapses after HSCT. Blood 2017; 130(10): 1270–1273. [DOI] [PubMed] [Google Scholar]