Summary
Objective
Nuclear receptors and cytochrome P450 (CYP) regulate hepatic metabolism of several drugs. Nuclear receptors are expressed at the neurovascular unit of patients with drug‐resistant epilepsy. We studied whether glucocorticoid receptor (GR) silencing or inhibition in human epileptic brain endothelial cells (EPI‐ECs) functionally impacts drug bioavailability across an in vitro model of the blood–brain barrier (BBB) by CYP‐multidrug transporter (multidrug resistance protein 1, MDR1) mechanisms.
Methods
Surgically resected brain specimens from patients with drug‐resistant epilepsy, primary EPI‐ECs, and control human brain microvascular endothelial cells (HBMECs) were used. Expression of GR, pregnane X receptor, CYP3A4, and MDR1 was analyzed pre‐ and post‐GR silencing in EPI‐ECs. Endothelial cells were co‐cultured with astrocytes and seeded in an in vitro flow‐based BBB model (DIV‐BBB). Alternatively, the GR inhibitor mifepristone was added to the EPI‐EC DIV‐BBB. Integrity of the BBB was monitored by measuring transendothelial electrical resistance. Cell viability was assessed by glucose‐lactate levels. Permeability of [3H]sucrose and [14C]phenytoin was quantified. CYP function was determined by measuring resorufin formation and oxcarbazepine (OXC) metabolism.
Results
Silencing and inhibition of GR in EPI‐ECs resulted in decreased pregnane X receptor, CYP3A4, and MDR1 expression. GR silencing or inhibition did not affect BBB properties in vitro, as transendothelial electrical resistance and Psucrose were unaltered, and glucose metabolism was maintained. GR EPI‐EC silencing or inhibition led to (1) increased Pphenytoin BBB permeability as compared to control; (2) decreased CYP function, indirectly evaluated by resorufin formation; (3) improved OXC bioavailability with increased abluminal (brain‐side) OXC levels as compared to control.
Significance
Our results suggest that modulating GR expression in EPI‐ECs at the BBB modifies drug metabolism and penetration by a mechanism encompassing P450 and efflux transporters. The latter could be exploited for future drug design and to overcome pharmacoresistance.
Keywords: bioavailability, drug‐resistant epilepsy, multidrug transporter, nuclear receptors, P450 enzymes
Key Points.
GR silencing or inhibition in EPI‐ECs decreased expression of PXR, CYP3A4, and MDR1
GR in EPI‐ECs does not control BBB structural properties such as TEER, Psucrose permeability, and physiologic glucose metabolism in vitro
Increased Pphenytoin permeability across the BBB occurred upon GR silencing or inhibition
CYP function at the BBB depended on GR expression or inhibition
GR modulation in EPI‐ECs modified OXC metabolism at the BBB in vitro.
1. INTRODUCTION
The blood–brain barrier (BBB) comprises tight‐junction endothelial cells (ECs) lining the brain capillaries, with astrocytic end‐feet in proximity. Understanding the BBB’s function in health and disease is important in epilepsy, where BBB dysfunction is reported.1, 2, 3, 4, 5 The ECs forming the BBB express variable levels of cytochrome P450 (CYP) enzymes. This variability may have relevance to drug‐resistant epilepsy (DRE),6, 7, 8 and therefore these ECs could be viewed as a regulatory interface and possible target for therapeutic interventions.
We recently reported9 that brain ECs derived from patients with intractable epilepsy possess nuclear receptors or transcription factors such as glucocorticoid receptors (GRs) and pregnane X receptor (PXR). Molecular modulation of GRs in epileptic brain endothelial cells (EPI‐ECs) resulted in changes in PXR expression and downstream CYP enzymes (eg, CYP3A4 and CYP2C9).9 Thus, we previously hypothesized a role for local drug biotransformation in the human epileptic brain, altering drug bioavailability across the BBB by CYP enzymes and multidrug efflux transporter (multidrug resistance protein 1, MDR1) mechanisms.8, 10, 11, 12, 13, 14 However, the functional relevance of GR expression at the human drug‐resistant epileptic BBB, particularly in the context of drug metabolism, remains understudied.
The purpose of this study was 2‐fold: to investigate how a mechanistic regulation of GRs in EPI‐ECs affects BBB properties and to explore the relevance of GRs to drug penetration across the BBB and to drug metabolism. To this end, we used a flow‐based in vitro BBB (DIV‐BBB) model.8, 15, 16 We performed GR silencing in EPI‐ECs and co‐culture with astrocytes for DIV‐BBB formation. We also opted for a complementary approach, pretreating EPI‐ECs with the GR inhibitor mifepristone (MF; RU486). We accomplished 3 goals: (1) We verified the pattern of GR expression and the impact on the expression of PXR and drug‐metabolizing enzymes and a drug efflux transporter; (2) we analyzed BBB integrity, cell viability, and permeability properties across the DIV‐BBB in all conditions; and (3) we monitored CYP function by measuring the enzymatic conversion of 7‐ethoxyresorufin to resorufin. Penetration and metabolism of oxcarbazepine (OXC) at the DIV‐BBB were evaluated using both GR‐silencing and GR‐inhibition approaches. These studies were designed to better understand the relevance of GRs as a possible therapeutic target in the endothelium that could influence the pharmacokinetic properties of the vasculature in the human brain with DRE.
2. METHODS
2.1. Isolation of epileptic brain microvascular endothelial cells from human surgical specimens and human brain astrocytes
Brain specimens were obtained from patients with DRE according to the principles outlined in the Declaration of Helsinki and the Cleveland Clinic Institutional Review Board–approved protocol (IRB 07‐322). Information on seizure type and frequency, duration of epilepsy, the resected brain regions, and experimental use of the specimens is provided in Table S1.
EPI‐ECs were isolated from cerebral cortex biopsies of patients who had undergone surgery due to refractory epilepsy, as described earlier.8, 16, 17, 18 Briefly, surgical specimens were incubated in collagenase type II (2 mg/mL; Worthington Biochemical Corp., Lakewood, New Jersey) to dissociate the ECs for culture (Appendix S1).9 Control human brain microvascular endothelial cells (HBMECs) were purchased from Cell Systems (Kirkland, Washington; catalog ACBRI 376). According to the company, the HBMECs were dissociated from normal human brain cortex tissue (obtained from healthy donors; see Appendix S1). The recommended culture media were used.
Normal human astrocytes (catalog 1800) were purchased from ScienCell Research Laboratories, Inc. (Carlsbad, California) and cultured in poly‐d‐lysine pre‐coated flasks (3 μg/cm2) with appropriate media.16
2.2. Small interfering RNA (siRNA) gene silencing of GR in EPI‐ECs
Gene‐specific human NR3C1 GR (catalog: E‐003424‐00‐0005) siRNA oligonucleotides were purchased from GE Dharmacon, Inc. (Lafayette, Colorado) and used at a final concentration of 1 μmol/L (Appendix S1) for EPI‐EC.9 GR silencing (human NR3C1) was performed using Accell SMARTpool siRNAs containing 4 siRNAs designed for the GR gene: (5′‐GGAGCAAAUAUAAUUGGUA‐3′, 5′‐GCAUGUACGACCAAUGUAA‐3′, 5′‐GGGUGGAGUUUCGUAAUUU‐3′, and 5′‐CUAACAUGAUUUGUGUCUA‐3′). The effects of siRNA on EPI‐EC gene expression were confirmed by immunocytochemistry (n = 6 donors in triplicate per condition).
2.3. Mifepristone treatment
Pretreatment of cells with the GR inhibitor mifepristone (MF; Sigma Aldrich, St. Louis, Missouri; catalog M8046) was selected as an alternative approach to understand the role of GR in EPI‐ECs. A 10‐mmol/L stock of MF was prepared in dimethyl sulfoxide (Fisher Scientific, Rockford, Illinois; catalog BP231‐100) and diluted to 50 μmol/L in media.19 The effects of MF on EPI‐ECs were validated by immunocytochemistry after 24 hours of incubation (n = 4 donors in triplicate per condition).
2.4. Dynamic in vitro model setup
DIV modules were purchased from FiberCell Systems Inc. (New Market, Maryland; catalog C2025). In the FiberCell Polysulfone Plus cartridge, each module contains 20 hollow‐fiber capillaries embedded inside a clear plastic chamber, which is attached to a reservoir for media circulation and connected to a pulsatile pump. Control brain ECs and EPI‐ECs (GR siRNA and non‐siRNA; 4 × 106/device) were seeded in the luminal side in different devices (n = 4 donors in triplicate per condition). Astrocytes (3 × 106/device) were co‐cultured in the abluminal side of the DIV.8, 15, 16 In a complementary experimental approach, the BBB was established using EPI‐ECs and astrocytes and exposing the EPI‐ECs to MF for 24 hours in the lumen (n = 4 donors in triplicate per condition). The experimental outline is shown in Figure S1 and Appendix S1.
2.5. DIV‐BBB TEER measurement system
The transendothelial electrical resistance (TEER) measurement provides a means of evaluating the integrity of the DIV‐BBB15, 20 (Flocel, Inc., Cleveland, Ohio) as described previously.15, 20 TEER was measured continuously throughout the course of each experiment.
2.6. Cell metabolism: glucose and lactate levels
Indicators of cell metabolism were used to monitor cell growth and the establishment of a functional BBB in vitro. Depletion of the carbohydrate component of the growth medium (glucose levels) and accumulation of metabolically produced lactic acid levels are used as indicators of cell growth.21 The lumen and extracapillary spaces (ECSs) were sampled at 0, 7, and 14 days (n = 4 donors in triplicate per condition). Data obtained show glucose and lactate levels in grams per liter.22
2.7. Drug permeability: uptake of [14C]phenytoin and [3H]sucrose
Boluses (1.0 mL each) of the radioactive tracers [14C]phenytoin (PerkinElmer, Boston, Massachusetts; catalog NEC‐246) and [3H]sucrose (Amersham Biosciences, Piscataway, New Jersey; catalog TRA‐322) were injected upstream into the lumen as described previously (Appendix S1).15, 23 The permeability across the cerebrovascular bed was calculated by graphical integration of drug concentration in the corresponding lumen and ECSs over 10 minutes (n = 4 donors in triplicate per condition).20, 22 Permeability values were obtained by integrating the area under the ECSs and the lumen according to an equation described previously.23
2.8. Immunocytochemistry
Immunocytochemistry was performed on the epileptic human brain ECs that were (1) either transfected for GR silencing (EPI‐EC GR siRNA) or not (EPI‐EC non‐siRNA) and compared with control HBMECs (n = 4 donors in triplicate per condition); (2) EPI‐ECs exposed to MF and compared with EPI‐EC (no inhibitor) (n = 4 donors in triplicate per condition). Cells were stained with antibodies against GR, PXR, CYP3A4, UGT1A4, and MDR1 and analyzed by fluorescence microscopy. Specifications on the antibodies, concentration, imaging, and quantification are provided in the Appendix S1. Images were processed using ImageJ (National Institutes of Health, Bethesda, Maryland) and Q‐Capture‐Pro software (QImaging, Inc., Surrey, Canada). All images were converted to an 8‐bit format; brightness/contrast was adjusted using constant parameters. Threshold signals were set and segmented via the watershed feature of ImageJ. Values were collected and expressed as mean ± standard error of the mean (SEM).
2.9. CYP function and resorufin formation
We performed an ethoxyresorufin‐O‐deethylase assay, determining the extent of 7‐ethoxyresorufin metabolism into resorufin by CYP enzymes.24 7‐Ethoxyresorufin (20 μg/mL, dissolved in dimethyl sulfoxide) was added to the DIV‐BBB containing EPI‐EC GR siRNA, EPI‐EC non‐siRNA, or HBMECs (n = 4 donors in triplicate per condition). A similar approach was adopted for the MF‐exposed DIV‐BBB (n = 4 donors in triplicate per condition). Samples (200 μL) were collected from the luminal and abluminal sides of the DIV‐BBB at 0, 2, 4, 6, and 24 hours (Appendix S1).24, 25, 26, 27 A standard plot for resorufin (5‐200 nmol/L) is shown in Figure S3. Samples were analyzed by a multimode reader using Gen5 software (BioTek, Winooski, Vermont; fluorescent reader) at excitation‐emission of 530/25 and 645/40, respectively.24
2.10. Oxcarbazepine treatment and HPLC‐UV analysis
To study OXC metabolism at the BBB, 25‐30 μg/mL of OXC was set as the initial concentration in the DIV‐BBB modules (EPI‐EC GR siRNA, EPI‐EC non‐siRNA, and HBMECs [n = 4 donors in triplicate per condition]).28 Similarly, EPI‐EC DIV‐BBB cells pretreated with MF were used to study OXC biotransformation at the in vitro BBB compared with MF untreated EPI‐DIV‐BBB and HBMECs (n = 4 donors in triplicate per condition). Samples were collected from the luminal and abluminal sides. Standard OXC and its metabolite licarbazepine (LiCBZ; 10, 11‐dihydro‐10‐hydroxy‐carbamazepine) were purchased from Sigma Aldrich (OXC, catalog O3764; LiCBZ, catalog 61347). OXC metabolism was assessed by measuring OXC levels at 0, 2, 6, and 24 hours by using a reversed‐phase high‐performance liquid chromatography‐ultraviolet detection (RP‐HPLC‐UV; Agilent 1200 Series) and a Zorbax Eclipse Plus C18 column (4.6 × 150 mm, 3.5 μm; see Appendix S1).14, 16
2.11. Statistical analysis
All data are expressed as mean ± standard error. Student‘s t test was used for direct comparison of 2 populations of data. One‐way analysis of variance followed by a Bonferroni test was used for comparisons. A *P value ≤0.05 was considered statistically significant (n = 4‐6 donors’ specimens used in triplicate per experimental condition). Origin 9.0 software (Origin Lab, Northampton, Massachusetts) was used for statistical analyses.
3. RESULTS
3.1. GR modulation, PXR, CYP, and MDR1 expression in EPI‐ECs and BBB properties
GR silencing and reduced expression were confirmed by immunocytochemistry (Figure 1A). A decrease was observed in PXR, CYP3A4, and MDR1 expression after GR silencing (Figure 1A).9 The latter results were comparable to results from pharmacologic GR inhibition (Figure 2A). Quantifications are provided in Figures 1A1 and 2A1. The expression of GR, PXR, and CYP in EPI‐EC GR siRNA was found comparable to the levels in control HBMECs. Together, our data indicate that GR regulates the expression of PXR, CYP3A4, and MDR1 in EPI‐ECs.
Figure 1.
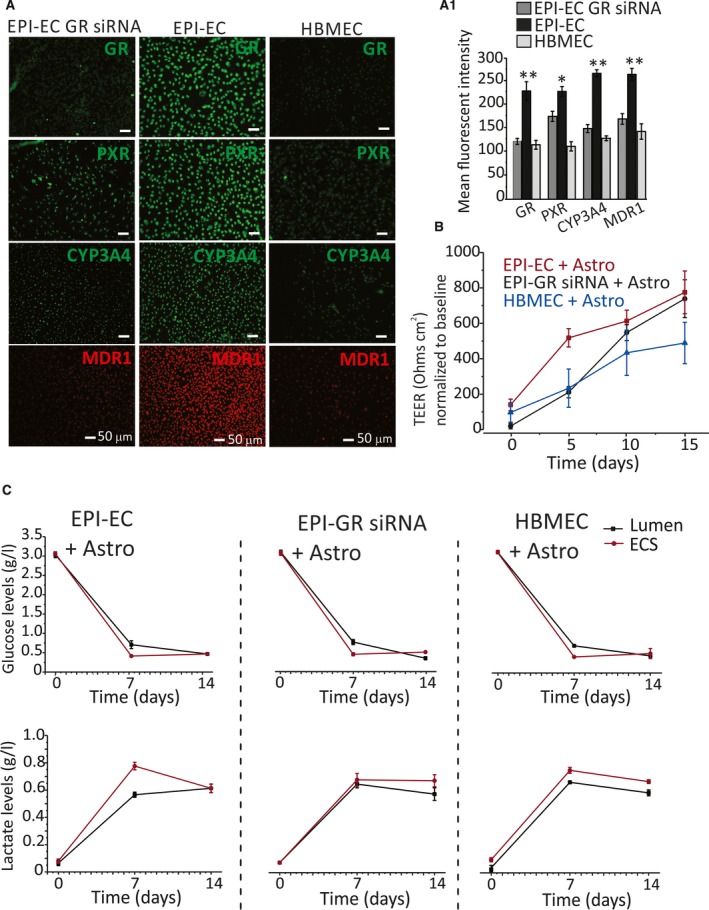
GR silencing in EPI‐EC and BBB integrity. A, ECs isolated from drug‐resistant epileptic brain resections were transfected (EPI‐EC GR siRNA; n = 6). EPI‐EC non‐siRNA and control human brain microvascular endothelial cells (HBMECs) were compared. Immunocytochemistry shows successful GR silencing by a decrease in GR levels in EPI‐ECs. Decreased PXR, CYP3A4, and MDR1 were observed in EPI‐EC GR siRNA. A1, Quantification of fluorescent signals showed a significant decrease in expression levels of GR (P ≤ 0.01), PXR (P ≤ 0.05), CYP3A4 (P ≤ 0.01), and MDR1 (P ≤ 0.01) in EPI‐EC GR siRNA (mean ± SEM). B, Transendothelial electrical resistance (TEER) measurement showed development of a barrier characterized by an increase in TEER (~600‐800 Ω/cm2) normalized with the baseline TEER reading. No statistical difference was found among TEER readings in EPI‐EC GR siRNA, EPI‐EC non‐siRNA, and HBMEC DIV‐BBB. All experiments were repeated in triplicate. C, The cellular metabolic pattern within the DIV‐BBB setup showed no significant difference in the pattern of glucose and lactate levels over time during the DIV‐BBB formation
Figure 2.
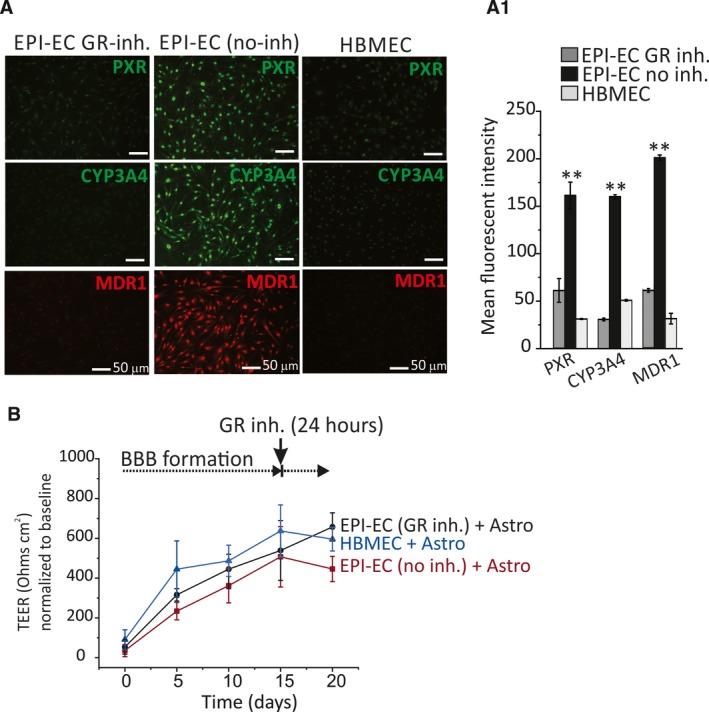
GR inhibition in EPI‐ECs and BBB integrity. A, EPI‐ECs were treated with the GR inhibitor mifepristone, MF (n = 4 donors in triplicate). Untreated EPI‐ECs (n = 4 donors in triplicate) and control HBMECs (n = 4 in triplicate) were compared. Decreased levels of PXR, CYP3A4, and MDR1 were observed in EPI‐EC GR inhibitor compared with EPI‐EC no inhibitor. A1, Quantifications are provided (mean ± SEM). B, No statistically significant difference in TEER readings was found between EPI‐EC GR inhibitor, EPI‐EC no inhibitor, and HBMEC DIV‐BBB modules
GR silencing or inhibition in EPI‐ECs did not influence the BBB TEER values and the cell viability properties of the in vitro BBB. TEER measurements reached ~600 Ω/cm2 in HBMECs and ~500‐800 Ω/cm2 in EPI‐ECs (Figures 1B and 2B). TEER values were similar in DIV‐BBBs established using EPI‐ECs, regardless of whether GR silencing or inhibition was applied. The assessment of glucose/lactate levels showed a similar trend (Figure 1C). We found no significant cell metabolic differences (glucose) between epileptic (EPI GR siRNA or non‐siRNA) and normal endothelium.
The BBB permeability of [3H]sucrose, a tracer of paracellular leakage, was comparable (Figures 3A‐A2 and 4A‐A2) among groups. Of interest, the BBB permeability of the antiepileptic drug phenytoin (Pphenytoin), a substrate of MDR transport, was 2.1‐fold higher across the EPI‐EC GR siRNA compared with EPI‐EC non‐siRNA (Figure 3B‐B1). A similar trend was found when the GR inhibitor was used (Figure 4B‐B1). These latter results suggest that permeability of phenytoin across the BBB is modified by GR silencing or inhibition that, in turn, decreases MDR1 expression, and the levels are comparable to those of controls (Figures 3B2 and 4B2).
Figure 3.
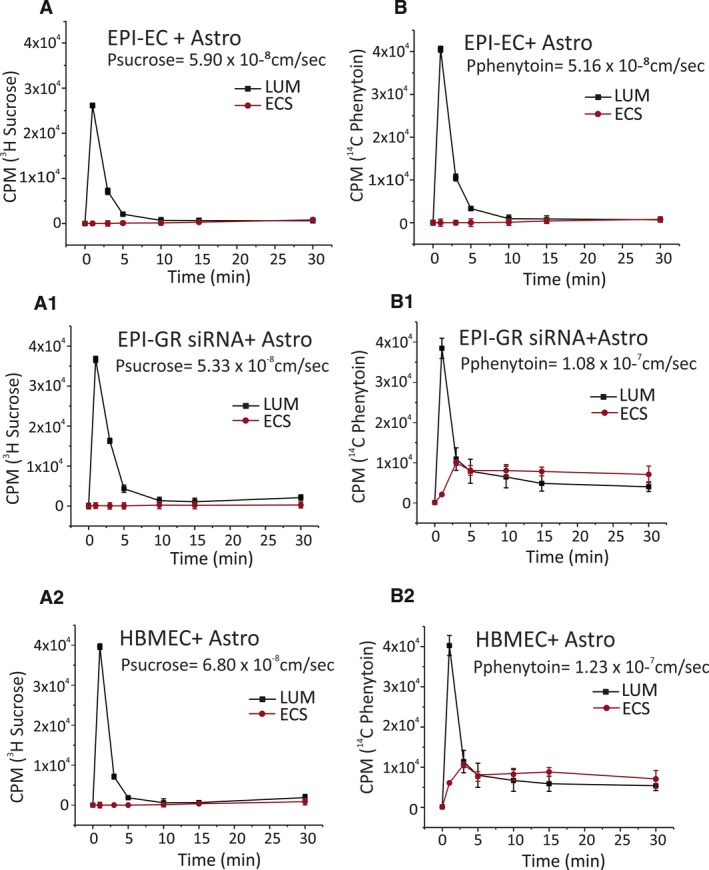
Permeability of [3H]sucrose and [14C]phenytoin in EPI‐EC GR siRNA, EPI‐EC non‐siRNA, and HBMEC DIV‐BBB. A‐A2, The tightness of the barrier evaluated by [3H]sucrose (a paracellular marker) shows no significant difference across the endothelium(s) used. B‐B2, [14C]phenytoin showed a 2.1‐fold increase in permeability levels (1.08 × 10−7 cm/s) in EPI‐EC GR siRNA DIV‐BBB (P ≤ 0.05) compared with EPI‐EC non‐siRNA BBB (5.16 × 10−8 cm/s). The permeability pattern in EPI‐EC GR siRNA at the DIV‐BBB was comparable to that of HBMEC DIV‐BBB (1.23 × 10−7 cm/s)
Figure 4.
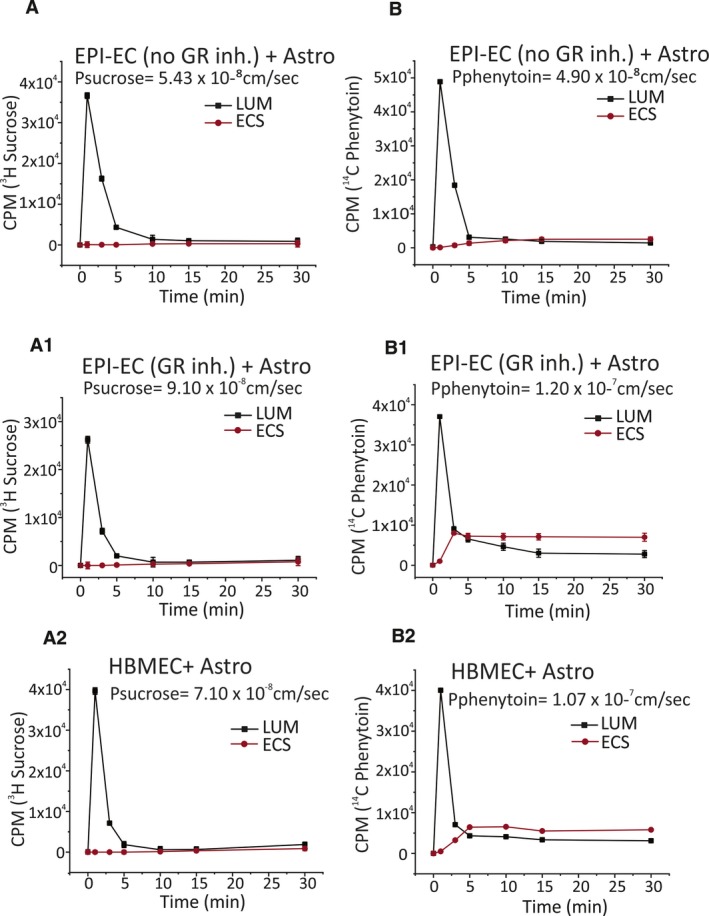
Permeability of [3H]sucrose and [14C]phenytoin evaluated in EPI‐EC GR inhibition (MF), EPI‐EC no inhibition, and HBMEC DIV‐BBB. A‐A2, [3H]sucrose permeability was comparable across experimental conditions. B‐B2, [14C]phenytoin permeability was higher (1.20 × 10−7 cm/s) in EPI‐EC GR‐inhibited DIV‐BBB (P ≤ 0.05) compared with EPI‐EC untreated BBB (4.90 × 10−8 cm/s)
3.2. CYP function is reduced upon GR silencing or inhibition in EPI‐ECs
CYP function was monitored in the DIV‐BBB by quantifying the production of fluorescent resorufin (Figures 5A,B and 6A,B). Figure S3 shows a calibration plot. Augmented resorufin levels were measured in the lumen of EPI‐EC non‐siRNA DIV‐BBB compared with EPI‐EC GR siRNA DIV‐BBB (Figure 5A). Resorufin formation in EPI‐EC GR siRNA was comparable to that seen in control HBMECs. A similar trend was found in the abluminal compartments of the DIV‐BBB (Figure 5B). Finally, pharmacologic GR inhibition produced comparable results (Figure 6). Taken together, these results support the importance of GR modulation in determining CYP function at the human BBB in vitro.
Figure 5.
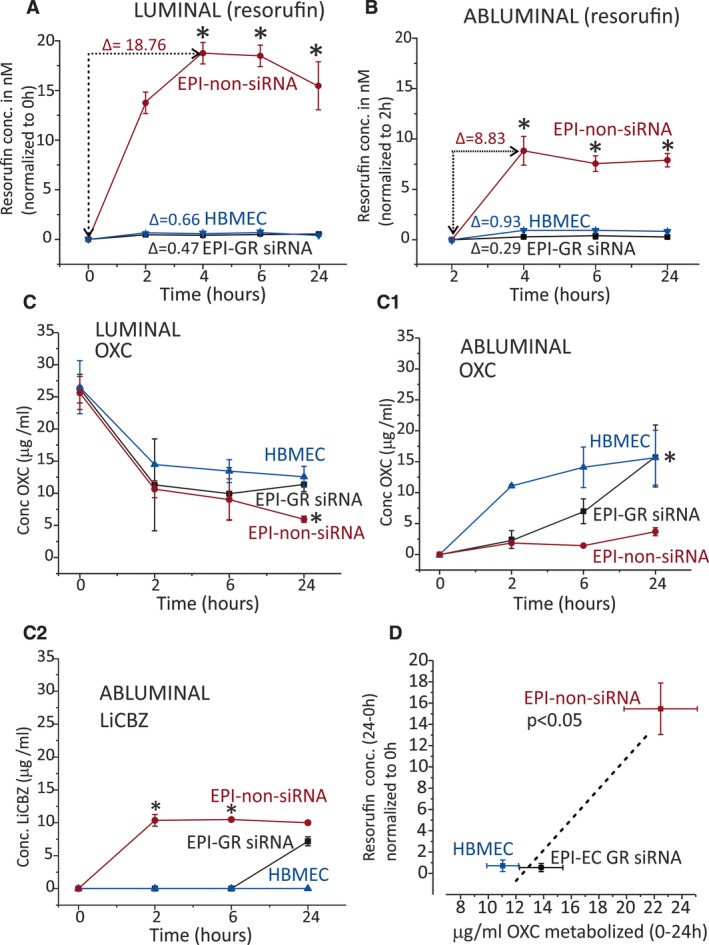
CYP drug biotransformation is supported by resorufin and OXC metabolism in EPI‐ECs after GR silencing. A, Formation of resorufin is the highest when using EPI‐EC non‐siRNA. GR silencing resulted in decreased resorufin conversion comparable to that of control DIV‐BBB (HBMECs). B, Similar results were obtained when analyzing the abluminal compartment. C, OXC metabolism is increased in EPI‐EC non‐siRNA in the luminal side compared with EPI‐EC GR siRNAs and HBMECs. C1, A relative increase in OXC penetration across the BBB was observed in EPI‐EC GR siRNA and HBMECs compared with EPI‐EC non‐siRNA DIV‐BBB. C2, Elevated LiCBZ levels in the abluminal side of EPI‐EC non‐siRNA suggest that OXC is metabolized by a GR‐CYP pathway. Such metabolism is lower in EPI‐EC GR siRNA or HBMECs. D, The levels of resorufin positively correlated with the amount (micrograms per milliliter) of OXC metabolized. Results are expressed as mean ± SEM (by analysis of variance). Asterisks indicate P ≤ 0.05
Figure 6.
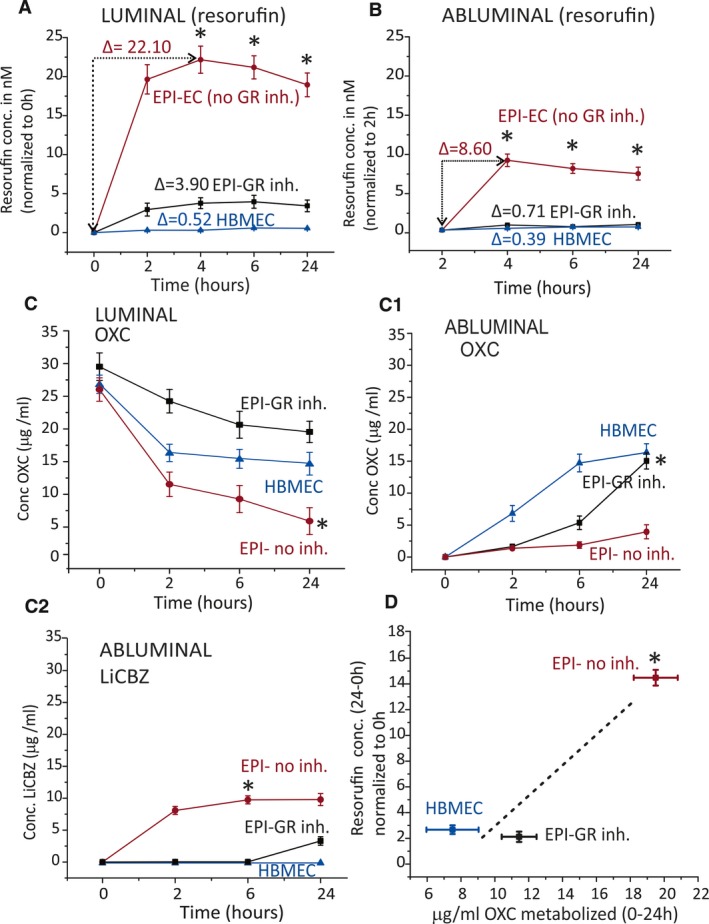
CYP drug biotransformation is supported by resorufin and OXC metabolism in EPI‐ECs after GR inhibition. A, Resorufin formation was the highest when using untreated (no MF) EPI‐ECs compared with controls. B, Similar results were obtained when analyzing the abluminal compartment of the DIV‐BBB modules. C, OXC metabolism was increased in noninhibited EPI‐EC compared with EPI‐EC GR‐inhibited and HBMECs. C1, A relative increase in OXC penetration across the BBB was observed when using EPI‐EC GR‐inhibited and HBMECs compared with EPI‐EC non‐inhibited DIV‐BBB. C2, LiCBZ formation in the abluminal side of noninhibited EPI‐ECs, indicating OXC metabolism by a GR‐CYP mechanism. OXC metabolism was lower or negligible in EPI‐ECs after GR inhibition or in HBMECs. D, Levels of resorufin positively correlated with the amount of OXC metabolized. Results are expressed as mean ± SEM. Asterisks indicate P ≤ 0.05
3.3. GR modulation at the BBB impacts OXC bioavailability
OXC metabolism and penetration were then tested. The chromatograms in Figure S3 show the standard OXC and its metabolite LiCBZ. A decrease in OXC levels occurred in the intraluminal side (0‐24 hours; Figure 5C), with EPI‐EC non‐siRNA > EPI‐EC GR siRNA > HBMECs. BBB penetration was measured in EPI‐EC GR siRNA and HBMECs (Figure 5C1), whereas only negligible OXC penetration occurred across the EPI‐EC non‐siRNA. Levels of LiCBZ (the OXC metabolite) were measured in the abluminal side of EPI‐EC non‐siRNA (Figure 5C2). We also found that there were negligible LiCBZ levels in the abluminal side of HBMEC DIV‐BBB and reduced levels in EPI‐EC GR siRNA DIV‐BBB. A correlation was calculated (Figure 5D) between OXC metabolism (intraluminally) and resorufin levels (suggesting CYP function) in EPI‐EC GR siRNA, EPI‐EC non‐siRNA, and HBMECs.
Similar patterns of OXC biotransformation were obtained when GR was pharmacologically inhibited (Figure 6C‐C1). GR noninhibited EPI‐ECs showed significantly increased OXC metabolism and LiCBZ formation (P ≤ 0.05) compared with control conditions (Figure 6C‐C2). OXC metabolism (intraluminally) in such instances was relatable to CYP function, which was indirectly evaluated by resorufin formation (Figure 6D). In the current study, we deliberately used a second‐generation antiepileptic drug (AED; phenytoin) and a third‐generation AED (OXC). [14C]Phenytoin is a suitable choice for permeation measurements across the in vitro BBB (along with the paracellular marker [3H]sucrose). OXC is a widely prescribed drug that has proven useful for drug‐metabolism studies, with particular relevance to GR in EPI‐ECs. Overall, these data support the hypothesis that metabolic enzymes and drug transporter proteins can, in concert, modify brain drug disposition and local drug metabolism, controlled by the upstream GR effector at the epileptic human BBB.
4. DISCUSSION
The purpose of this study was to explore the pharmacokinetic relevance of GR in EPI‐ECs at the BBB model and to test the hypothesis that GR is a potential target for pharmacologic modulation in DRE. The present approaches encompass the mechanistic link between GR‐P450‐MDR1 in the EPI‐ECs using a humanized in vitro BBB model associated with GR‐regulated vascular pathophysiology. The pharmacologic relevance of GR in BBB ECs derived from patients with DRE and using the in vitro platform has been shown in this study for what we believe is the first time.
The BBB is a critical site protecting the central nervous system in health and disease conditions, but it can pose a major hindrance to drug development.1, 4, 5 The flow‐based system (DIV‐BBB) has been shown previously to mimic the shear stress levels critical for tight‐junction formation.15, 16, 17, 20 This model is important for our study and to elucidate the impact that EPI‐EC molecular manipulation has at the cerebrovascular interface. At the same time, GR is an interesting target not only for EC barrier function but also for understanding the key pathophysiologic traits of brain diseases.29, 30 For instance, whether GR activation or loss mediates the immune changes and inflammation occurring in seizure disorders remains to be further studied.31, 32, 33
4.1. Genetic ablation of GR or modulation decreases PXR, CYP, and MDR1 expression
The potential of GR as a molecular target in the endothelium in brain pathologies is relevant, also considering the pattern of GR expression at the vessels in DRE regions showing reactive gliosis.9 Our recent study also showed that GR regulates CYP3A4 at the BBB.9 Other reports have shown GR regulation of BBB P‐glycoprotein.34, 35 In our current study, GR silencing (Figure 1) or GR inhibition (Figure 2) in EPI‐ECs revealed a pattern of PXR, MDR1, and CYP enzyme expression changes.9 We also found that expression of the phase II enzyme UGT1A4 is altered in EPI‐ECs by GR manipulation (Figure S2). Of interest, the multidrug efflux transporter MDR1 is significantly reduced when GR is silenced or inhibited in EPI‐ECs; this finding supports the potential link of GR‐MDR1 to pharmacoresistance (failed response to antiepileptic drugs), which still remains a critical problem for a majority of patients with DRE.36, 37 The plausible mechanism by which GR in EPI‐ECs regulates PXR‐CYP‐MDR expression is similar to the hepatic one.38, 39
4.2. GR controls drug metabolism at the BBB in drug‐resistant epileptic endothelial cells
Pharmacoresistance in epilepsy has been supported by several theories.36, 40, 41 The role of drug‐metabolizing enzymes and the formation of reactive metabolites due to enzymatic conversion by local ECs affecting drug bioavailability to the brain in DRE is one such theory.7, 10, 11, 16, 42 In the current study, CYP involvement was therefore explored in the DIV‐BBB and was found to be regulated by GR modulation in EPI‐ECs (Figures 5 and 6). The in vitro BBB model, which mimics the drug‐resistant phenotype, could be an ideal screening platform for pharmacologic compounds, similar to microfluidic liver devices used to evaluate CYP activity and function.24 Resorufin levels observed in EPI‐EC non‐siRNA, non‐GR inhibition, and controls in both luminal and abluminal sides of the DIV‐BBB suggest that ethoxyresorufin had undergone enzymatic conversion by CYP (eg, CYP1A1, CYP1B1, and CYP3A).24 Our results suggest that GR function in EPI‐ECs impacts phenytoin permeability via a contributing MDR1 mechanism.43
In the present study, OXC levels were also measured in conditions in which GR was modulated or not. Silencing GR in EPI‐ECs resulted in a reduction of OXC metabolism compared with nonsilenced conditions in EPI‐ECs. Thereby the penetration of OXC across the BBB was improved upon GR silencing or inhibition in the EPI‐ECs. Furthermore, modulation of GR further decreased OXC metabolism and LiCBZ formation (Figures 5 and 6) compared with controls. Conversely, the nonsilenced or noninhibited EPI‐EC condition demonstrated increased drug metabolic potency at the BBB, consistent with our previous reports showing a similar observation with other AEDs such as carbamazepine in the drug‐resistant epileptic brain.16 In general, GR inhibition seems to improve drug penetration and to reduce local drug biotransformation at the BBB EPI‐ECs (Figure S1). Reduction of CYP enzyme activity is a mechanism supported by the resorufin read‐out and OXC metabolism (Figures 5D and 6D), indicating GR as a controller of BBB endothelial drug biotransformation.
5. FINAL REMARKS AND PERSPECTIVE
Some evidence suggests that GR‐inhibitory and GR‐modulatory approaches could be possible and beneficial in brain disorders. For instance: (1) MF interferes with the adhesion of cancer cells to the microvasculature by downregulating cellular expression of integrins, indicating a chemopreventive effect in mouse models44; (2) Cushing‘s syndrome45; (3) maintenance of an excitation–inhibition balance to neurogenesis and psychiatric brain disorders46, 47; and (4) GR antagonism prevents the stress‐related alteration in amyloid pathology and cognitive performance in APP/PS1 mice48 (those having genetically modified amyloid precursor protein and presenilin 1) and in tau pathology.49
Our study suggests a significant role of GR in the expression of pharmacoresistance in human epilepsy through decreased drug bioavailability to epileptic brain areas. We show that the role of GR in drug resistance depends on the downstream regulation of drug‐metabolizing enzymes and efflux transporters in the ECs at the BBB. These results suggest that GR‐regulated changes in the EPI‐ECs DIV‐BBB have pharmacologic relevance pertinent to local drug delivery.
DISCLOSURE
None of the authors has any potential conflict of interest to disclose. We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
Supporting information
ACKNOWLEDGMENTS
This work was supported in part by grants R01NS078307 (to CG and NM) and R01NS095825 (to CG) from the National Institute of Neurological Disorders and Stroke/National Institutes of Health and by grants awarded from the Brain and Behavior Research Foundation (formerly NARSAD), the American Heart Association (13SDG13950015), and Alternatives Research & Development Foundation (to CG).
Ghosh C, Hossain M, Mishra S, et al. Modulation of glucocorticoid receptor in human epileptic endothelial cells impacts drug biotransformation in an in vitro blood–brain barrier model. Epilepsia. 2018;59:2049–2060. 10.1111/epi.14567
REFERENCES
- 1. Abbott NJ, Friedman A. Overview and introduction: the blood‐brain barrier in health and disease. Epilepsia. 2012;53(Suppl 6):1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Abbott NJ, Ronnback L, Hansson E. Astrocyte‐endothelial interactions at the blood‐brain barrier. Nat Rev Neurosci. 2006;7:41–53. [DOI] [PubMed] [Google Scholar]
- 3. Oby E, Janigro D. The blood‐brain barrier and epilepsy. Epilepsia. 2006;47:1761–74. [DOI] [PubMed] [Google Scholar]
- 4. Pardridge WM. Molecular biology of the blood‐brain barrier. Mol Biotechnol. 2005;30:57–70. [DOI] [PubMed] [Google Scholar]
- 5. Pardridge WM. The blood‐brain barrier: bottleneck in brain drug development. NeuroRx. 2005;2:3–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Dauchy S, Dutheil F, Weaver RJ, et al. ABC transporters, cytochromes P450 and their main transcription factors: expression at the human blood‐brain barrier. J Neurochem. 2008;107:1518–28. [DOI] [PubMed] [Google Scholar]
- 7. Ghersi‐Egea JF, Perrin R, Leininger‐Muller B, et al. Subcellular localization of cytochrome P450, and activities of several enzymes responsible for drug metabolism in the human brain. Biochem Pharmacol. 1993;45:647–58. [DOI] [PubMed] [Google Scholar]
- 8. Ghosh C, Gonzalez‐Martinez J, Hossain M, et al. Pattern of P450 expression at the human blood‐brain barrier: roles of epileptic condition and laminar flow. Epilepsia. 2010;51:1408–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ghosh C, Hossain M, Solanki J, et al. Overexpression of pregnane X and glucocorticoid receptors and the regulation of cytochrome P450 in human epileptic brain endothelial cells. Epilepsia. 2017;58:576–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ghersi‐Egea JF, Leininger‐Muller B, Cecchelli R, et al. Blood‐brain interfaces: relevance to cerebral drug metabolism. Toxicol Lett. 1995;82–83:645–53. [DOI] [PubMed] [Google Scholar]
- 11. Ghosh C, Hossain M, Puvenna V, et al. Expression and functional relevance of UGT1A4 in a cohort of human drug‐resistant epileptic brains. Epilepsia. 2013;54:1562–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ghosh C, Hossain M, Solanki J, et al. Pathophysiological implications of neurovascular P450 in brain disorders. Drug Discov Today. 2016;21:1609–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ghosh C, Hossain M, Spriggs A, et al. Sertraline‐induced potentiation of the CYP3A4‐dependent neurotoxicity of carbamazepine: An in vitro study. Epilepsia. 2015;56:439–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ghosh C, Marchi N, Desai NK, et al. Cellular localization and functional significance of CYP3A4 in the human epileptic brain. Epilepsia. 2011;52:562–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cucullo L, Hossain M, Rapp E, et al. Development of a humanized in vitro blood‐brain barrier model to screen for brain penetration of antiepileptic drugs. Epilepsia. 2007;48:505–16. [DOI] [PubMed] [Google Scholar]
- 16. Ghosh C, Marchi N, Hossain M, et al. A pro‐convulsive carbamazepine metabolite: quinolinic acid in drug resistant epileptic human brain. Neurobiol Dis. 2012;46:692–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Desai SY, Marroni M, Cucullo L, et al. Mechanisms of endothelial survival under shear stress. Endothelium. 2002;9:89–102. [DOI] [PubMed] [Google Scholar]
- 18. Dombrowski SM, Desai SY, Marroni M, et al. Overexpression of multiple drug resistance genes in endothelial cells from patients with refractory epilepsy. Epilepsia. 2001;42:1501–6. [DOI] [PubMed] [Google Scholar]
- 19. Helmestam M, Lindgren KE, Stavreus‐Evers A, et al. Mifepristone‐exposured human endometrial endothelial cells in vitro. Reprod Sci. 2014;21:408–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Santaguida S, Janigro D, Hossain M, et al. Side by side comparison between dynamic versus static models of blood‐brain barrier in vitro: a permeability study. Brain Res. 2006;1109:1–13. [DOI] [PubMed] [Google Scholar]
- 21. Stanness KA, Westrum LE, Fornaciari E, et al. Morphological and functional characterization of an in vitro blood‐brain barrier model. Brain Res. 1997;771:329–42. [DOI] [PubMed] [Google Scholar]
- 22. Goto M, Miwa H, Suganuma K, et al. Adaptation of leukemia cells to hypoxic condition through switching the energy metabolism or avoiding the oxidative stress. BMC Cancer. 2014;14:76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Cucullo L, Couraud PO, Weksler B, et al. Immortalized human brain endothelial cells and flow‐based vascular modeling: a marriage of convenience for rational neurovascular studies. J Cereb Blood Flow Metab. 2008;28:312–28. [DOI] [PubMed] [Google Scholar]
- 24. Sung JH, Choi JR, Kim D, et al. Fluorescence optical detection in situ for real‐time monitoring of cytochrome P450 enzymatic activity of liver cells in multiple microfluidic devices. Biotechnol Bioeng. 2009;104:516–25. [DOI] [PubMed] [Google Scholar]
- 25. Conaway CC, Jiao D, Chung FL. Inhibition of rat liver cytochrome P450 isozymes by isothiocyanates and their conjugates: a structure‐activity relationship study. Carcinogenesis. 1996;17:2423–7. [DOI] [PubMed] [Google Scholar]
- 26. Hagemeyer CE, Burck C, Schwab R, et al. 7‐Benzyloxyresorufin‐O‐dealkylase activity as a marker for measuring cytochrome P450 CYP3A induction in mouse liver. Anal Biochem. 2010;398:104–11. [DOI] [PubMed] [Google Scholar]
- 27. Nerurkar PV, Park SS, Thomas PE, et al. Methoxyresorufin and benzyloxyresorufin: substrates preferentially metabolized by cytochromes P4501A2 and 2B, respectively, in the rat and mouse. Biochem Pharmacol. 1993;46:933–43. [DOI] [PubMed] [Google Scholar]
- 28. Rambeck B, Jurgens UH, May TW, et al. Comparison of brain extracellular fluid, brain tissue, cerebrospinal fluid, and serum concentrations of antiepileptic drugs measured intraoperatively in patients with intractable epilepsy. Epilepsia. 2006;47:681–94. [DOI] [PubMed] [Google Scholar]
- 29. Garabedian MJ, Harris CA, Jeanneteau F. Glucocorticoid receptor action in metabolic and neuronal function. F1000Res 2017;6:1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Salvador E, Shityakov S, Forster C. Glucocorticoids and endothelial cell barrier function. Cell Tissue Res. 2014;355:597–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Maguire J, Salpekar JA. Stress, seizures, and hypothalamic‐pituitary‐adrenal axis targets for the treatment of epilepsy. Epilepsy Behav. 2013;26:352–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Oakley RH, Cidlowski JA. The biology of the glucocorticoid receptor: new signaling mechanisms in health and disease. J Allergy Clin Immunol. 2013;132:1033–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Xavier AM, Anunciato AK, Rosenstock TR, et al. Gene expression control by glucocorticoid receptors during innate immune responses. Front Endocrinol (Lausanne). 2016;7:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Bauer B, Hartz AM, Fricker G, et al. Pregnane X receptor up‐regulation of P‐glycoprotein expression and transport function at the blood‐brain barrier. Mol Pharmacol. 2004;66:413–9. [DOI] [PubMed] [Google Scholar]
- 35. Narang VS, Fraga C, Kumar N, et al. Dexamethasone increases expression and activity of multidrug resistance transporters at the rat blood‐brain barrier. Am J Physiol Cell Physiol. 2008;295:C440–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Granata T, Marchi N, Carlton E, et al. Management of the patient with medically refractory epilepsy. Expert Rev Neurother. 2009;9:1791–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Sisodiya SM, Goldstein DB. Drug resistance in epilepsy: more twists in the tale. Epilepsia. 2007;48:2369–70. [DOI] [PubMed] [Google Scholar]
- 38. Ihunnah CA, Jiang M, Xie W. Nuclear receptor PXR, transcriptional circuits and metabolic relevance. Biochim Biophys Acta. 2011;1812:956–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Pascussi JM, Gerbal‐Chaloin S, Drocourt L, et al. The expression of CYP2B6, CYP2C9 and CYP3A4 genes: a tangle of networks of nuclear and steroid receptors. Biochim Biophys Acta. 2003;1619:243–53. [DOI] [PubMed] [Google Scholar]
- 40. Hartz AM, Pekcec A, Soldner EL, et al. P‐gp protein expression and transport activity in rodent seizure models and human epilepsy. Mol Pharm. 2017;14:999–1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Tang F, Hartz AMS, Bauer B. Drug‐resistant epilepsy: multiple hypotheses, few answers. Front Neurol. 2017;8:301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Decleves X, Jacob A, Yousif S, et al. Interplay of drug metabolizing CYP450 enzymes and ABC transporters in the blood‐brain barrier. Curr Drug Metab. 2011;12:732–41. [DOI] [PubMed] [Google Scholar]
- 43. Chan GN, Hoque MT, Cummins CL, et al. Regulation of P‐glycoprotein by orphan nuclear receptors in human brain microvessel endothelial cells. J Neurochem. 2011;118:163–75. [DOI] [PubMed] [Google Scholar]
- 44. Chen W, Xiao Y, Chen J, et al. Sex‐related pharmacokinetic differences and mechanisms of metapristone (RU486 metabolite). Sci Rep. 2017;7:17190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Fleseriu M, Biller BM, Findling JW, et al. Mifepristone, a glucocorticoid receptor antagonist, produces clinical and metabolic benefits in patients with Cushing‘s syndrome. J Clin Endocrinol Metab. 2012;97:2039–49. [DOI] [PubMed] [Google Scholar]
- 46. Mayer JL, Klumpers L, Maslam S, et al. Brief treatment with the glucocorticoid receptor antagonist mifepristone normalises the corticosterone‐induced reduction of adult hippocampal neurogenesis. J Neuroendocrinol. 2006;18:629–31. [DOI] [PubMed] [Google Scholar]
- 47. Saaltink DJ, Vreugdenhil E. Stress, glucocorticoid receptors, and adult neurogenesis: a balance between excitation and inhibition? Cell Mol Life Sci. 2014;71:2499–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Lesuis SL, Weggen S, Baches S, et al. Targeting glucocorticoid receptors prevents the effects of early life stress on amyloid pathology and cognitive performance in APP/PS1 mice. Transl Psychiatry. 2018;8:53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Baglietto‐Vargas D, Medeiros R, Martinez‐Coria H, et al. Mifepristone alters amyloid precursor protein processing to preclude amyloid beta and also reduces tau pathology. Biol Psychiatry. 2013;74:357–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


