Abstract
Purpose
Baclofen is widely used off‐label for alcohol use disorders (AUD) in France, despite its uncertain efficacy and safety, particularly at high doses. This study was designed to evaluate the safety of this off‐label use compared to the main approved drugs for AUD (acamprosate, naltrexone, nalmefene).
Methods
This cohort study from the French Health Insurance claims database included patients, aged 18 to 70 years, with no serious comorbidity (assessed by the Charlson score) initiating baclofen or approved drugs for AUD between 2009 and 2015. The risk of hospitalisation or death associated with baclofen, at variable doses over time (from low doses <30 mg/day to high doses ≥180 mg/day), compared to approved drugs, was evaluated by a Cox model adjusted to sociodemographic and medical characteristics.
Results
The cohort included 165 334 patients, 47 614 of whom were exposed to baclofen. Patients exposed to baclofen differed from those treated with approved drugs in terms of sociodemographic and medical characteristics (more females, higher socioeconomic status, fewer hospitalisations for alcohol‐related problems), but these differences tended to fade at higher doses of baclofen. Baclofen exposure was significantly associated with hospitalisation (hazard ratio [HR] = 1.13 [95%CI: 1.09‐1.17]) and death (HR = 1.31 [95%CI: 1.08‐1.60]). The risk increased with dose, reaching 1.46 [1.28‐1.65] for hospitalisation and 2.27 [1.27‐4.07] for death at high doses. Similar results were in patients with a history of hospitalisation for alcohol‐related problems.
Conclusions
This study raises concerns about the safety of baclofen for AUD, particularly at high doses, with higher risks of hospitalisation and mortality than approved drugs.
Keywords: adverse effects, alcohol use disorders, baclofen, claims database, cohort study, pharmacoepidemiology
KEY POINTS.
Baclofen is widely used off‐label for alcohol use disorders in France, despite its uncertain efficacy and safety, particularly at high doses.
Exposure to baclofen vs approved drugs for alcohol use disorders was associated with an increased risk of hospitalisation and death in a large nationwide observational patient cohort.
High‐dose baclofen were associated with an increased risk of hospitalisation and death.
These associations particularly concerned drug intoxication (hospitalisation and death) and death from an unknown cause by the physician reporting the death.
1. INTRODUCTION
Baclofen has been indicated for the treatment of painful involuntary muscle spasms since 1972. It is a GABAB receptor agonist that crosses the blood‐brain barrier. In the 2000s, case reports and case series and several clinical trials suggested a possible dose‐related efficacy of baclofen to treat alcohol use disorders (AUD), including alcohol dependence and reduction of alcohol consumption or craving.1, 2, 3, 4, 5, 6, 7
In France, this use of baclofen has become very popular in the medical community and the general press since the publication of “The End of my Addiction”8, 9 in 2008, by O. Ameisen, an alcoholic physician popularising his self‐case report.2 French physicians have subsequently prescribed off‐label baclofen to several thousands of patients.10, 11, 12
About 120 000 people initiated drug for AUD each year between 2009 and 2015 in France, with an annual prevalence of 500 000 treated persons.13
In 2014, the French National Agency for Medicines and Health Products Safety (ANSM) enacted a temporary authorization for use14 to monitor this use while awaiting more convincing studies on the efficacy and safety of baclofen for AUD.
Several clinical trials have been conducted in various countries,15, 16, 17 but no consensus has been reached concerning efficacy, as some trials reported reduced craving and higher abstinence rates,1, 3, 5, 15 whereas others failed to show any effect.4, 16, 17, 18 In terms of safety, the adverse effects reported in these trials concerned neurological effects with confusion, seizures, psychiatric disorders, etc. and several serious adverse reactions have been reported by the French pharmacovigilance network and case reports.19, 20, 21 These studies were not designed to assess the safety of baclofen in terms of all‐cause hospitalisation and death, particularly at doses much higher than those recommended for the approved indication.22, 23
This study was conducted to evaluate the risk of hospitalisation and death (specific and all‐cause) of real‐life use of baclofen compared to the main approved drugs for AUD.
2. MATERIAL AND METHODS
2.1. Data sources
The study was conducted on medical administrative data from the French national health insurance information system database (SNIIRAM) linked to the hospital discharge database (PMSI) and crossed with the Epidemiology Centre on Medical Causes of Death (CépiDc) database. SNIIRAM and PMSI have already been described24, 25, 26 (see Supporting Information S1) and successfully used in epidemiological and pharmacoepidemiological studies.27, 28, 29, 30, 31 Data on causes of death are generated in France by CépiDc32 (see also Supporting Information S1).
2.2. Study population
This study included patients, aged 18 to 70 years, who initiated one of the main approved drugs for AUD (acamprosate, naltrexone, nalmefene) or baclofen between 1 January 2009 and 31 December 2015, with no other reimbursement for these drugs or disulfiram (another drug for AUD, which induces an Antabuse reaction in the case of alcohol intake, and which can only be used in abstinent patients) during the previous 3 years.
Baclofen had to be prescribed for an off‐label use. During the 3 years preceding initiation of baclofen, patients with hospitalisation or ongoing severe or costly long‐term disease for a neurological condition possibly responsible for painful involuntary muscle spasms (ICD‐10 codes: C70‐C71, C793‐C794, D32‐D33, D42‐D43, G04‐G06, G09, G114, G12‐G13, G24‐G26, G31‐G32, G35‐G37, G46, G80‐G83, G91, G93, G95, I60‐I64), reimbursement for wheelchairs or related devices or dantrolene,10, 11 were therefore identified and excluded from the cohort. The same selection criteria were applied to patients initiating approved drugs.
Patients reimbursed for opioid substitution therapy or hospitalised for a serious alcohol‐related disease (eg, alcohol liver disease, chronic pancreatitis, polyneuropathy; codes: E244, G312, G621, G721, I426, K70, K860) during the 3 years preceding inclusion were excluded. Patients with a long‐term disease or hospitalised for a disease during the year preceding inclusion, defined as increasing the risk of 1‐year mortality according to the Charlson score33 matched to SNIIRAM data,34 were also excluded. Approved drugs or baclofen had to be initiated by a physician managing patients for AUD, namely general practitioners, hospital physicians, or psychiatrists. Patients had to have received at least a second reimbursement for the same drug within 60 days after the first reimbursement.
2.3. Exposure and follow‐up
Patients were exposed to only the first drug reimbursed. Patients were assumed to be exposed to fixed doses of approved drugs based on their indications. Patients exposed to baclofen were either considered globally, regardless of whether or not the baclofen dose varied over time, or categorised according to their mean daily dose, calculated at each dispensing d: quantity (mg) of baclofen dispensed at d − 1, expressed in relation to the duration (days) between dispensings d − 1 and d. Mean daily dose classes considered in this study corresponded to doses <30 mg/day (low dose), 30 to 75 mg/day, 75 to 180 mg/day, and ≥180 mg/day (high dose).
Patients were followed for a maximum of 1 year. Follow‐up started at the second dispensing and ended at the first of the following events: hospitalisation, death, switch from the initial drug to another drug, end of study (31 December 2015, or 31 December 2014 for the causes of death study), or 60 days after the last dispensing of the same drug.
2.4. Outcomes
Primary outcomes were death from any cause (or hospitalisation followed by death during the following 30 days, considered to be a death at the date of hospitalisation. Deaths occurring more than 30 days after hospitalisation were ignored), and all‐cause hospitalisation, except for obstetric or elective day‐only hospitalisation.
Secondary outcomes were death due to specific causes (before 31 December 2014, when the cause of death was available) described by main groups of diseases and according to the most frequent underlying cause, deaths by suicide (ICD‐10: X60‐X84), and hospitalisations for specific causes described by the main groups of diseases and according to the most frequent primary diagnoses.
2.5. Statistical analysis
Patients were compared in terms of the following sociodemographic and medical characteristics: age, gender, deprivation index of the patient's area of residence,35 speciality of the physician who initiated drug for AUD, history of hospitalisation for alcohol‐related problems (eg, acute intoxication, harmful use, withdrawal, or dependence syndrome; ICD‐10: F10, K292, R780, T51, X45, X65, Y15, Y573, Y90, Y91, Z502, Z714, Z721) during the 3 years preceding inclusion, exposure to antipsychotics, anxiolytics, hypnotics, or antidepressants during the year preceding inclusion, history of comorbidity, and year of inclusion. Patients exposed to baclofen were also compared according to the dose reached at the end of follow‐up.
Risks of specific and all‐cause hospitalisation and death were assessed for patients exposed to baclofen vs approved drugs. Patients exposed to baclofen were considered globally, regardless of the mean daily dose reached during follow‐up, and were then categorised according to classes of mean daily dose varying over time. The following indicators were used to measure these risks: incidence per 1000 person‐years standardised for gender and age structured in 10‐year age‐groups of the entire cohort, and a hazard ratio (HR) calculated from a Cox model adjusted for gender and age, or fully adjusted (HRf) for gender, age, deprivation index, speciality of the physician who initiated an approved drug or baclofen, exposure to psychiatric drugs, history of hospitalisation for alcohol‐related problems, history of comorbidity, and year of inclusion, for classes of doses varying over time or regardless of the dose for baclofen exposure vs approved drugs.
We repeated our analyses by stratifying our Cox model on one or several adjustment covariates.
Complementary analyses were performed on the subset of patients with a history of hospitalisation for alcohol‐related problems, to assess the robustness of the results by studying a more homogeneous subgroup of patients.
3. RESULTS
3.1. Study population
The study included 165 334 patients: 47 614 (28.8%) exposed to baclofen and 117 720 (71.2%) exposed to approved drugs (Figure 1). Median follow‐up of patients exposed to baclofen or approved drugs was 87 and 81 days, respectively. At the end of follow‐up, 45.1% of patients taking baclofen were exposed to a mean daily dose <30 mg/day, 35.9% to 30 to 75 mg/day, 15.3% to 75 to 180 mg/day, and 3.6% to ≥180 mg/day (Table 1).
Figure 1.
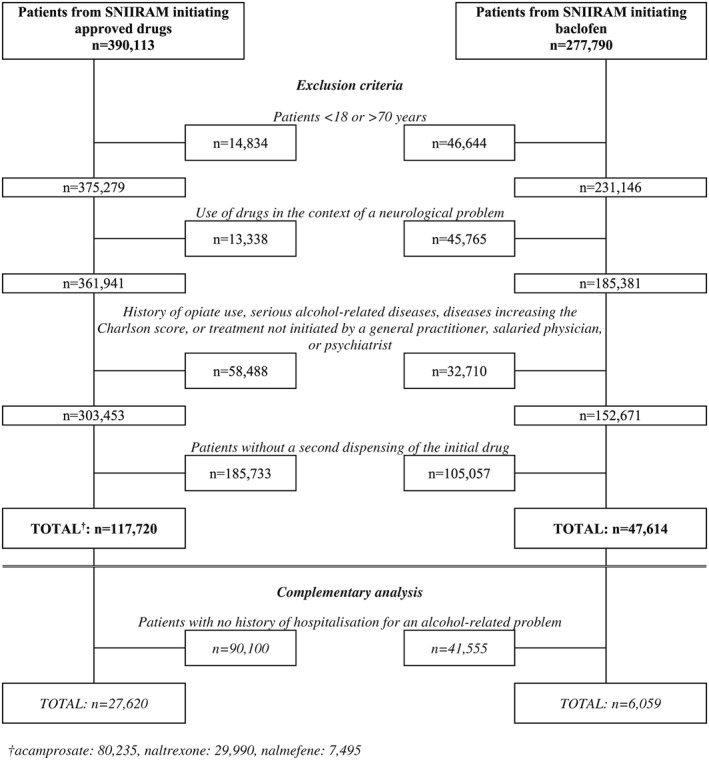
Flow chart
Table 1.
Sociodemographic, medical, and follow‐up characteristics of patients initiating approved drugs (acamprosate, naltrexone, nalmefene) or baclofen for alcohol use disorders
| Approved drugs | Baclofen | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| All doses | Doses (mg/day) at the end of follow‐upa | |||||||||||
| Characteristics | < 30 | 30‐75 | 75‐180 | ≥ 180 | ||||||||
| Number of incident patients, n | 117 720 | 47 614 | 21 495 | 17 103 | 7274 | 1742 | ||||||
| Age, median (IQR) | 47 (39‐55) | 48 (40‐56) | 50 (41‐58) | 48 (40‐56) | 46 (38‐54) | 44 (36‐53) | ||||||
| Age groups, n (%) | ||||||||||||
| <30 | 7028 | (6.0) | 2843 | (6.0) | 1188 | (5.5) | 997 | (5.8) | 521 | (7.2) | 137 | (7.9) |
| 30‐40 | 22 492 | (19.1) | 8440 | (17.7) | 3351 | (15.6) | 3023 | (17.7) | 1610 | (22.1) | 456 | (26.2) |
| 40‐50 | 38 834 | (33.0) | 14 462 | (30.4) | 6170 | (28.7) | 5322 | (31.1) | 2394 | (32.9) | 576 | (33.1) |
| 50‐60 | 33 620 | (28.6) | 13 723 | (28.8) | 6507 | (30.3) | 4969 | (29.1) | 1862 | (25.6) | 385 | (22.1) |
| 60‐70 | 15 746 | (13.4) | 8146 | (17.1) | 4279 | (19.9) | 2792 | (16.3) | 887 | (12.2) | 188 | (10.8) |
| Gender: Male, n (%) | 81 519 | (69.2) | 26 736 | (56.2) | 10 890 | (50.7) | 10 138 | (59.3) | 4596 | (63.2) | 1112 | (63.8) |
| Deprivation index, n (%) | ||||||||||||
| 1 (least deprived) | 16 387 | (13.9) | 8543 | (17.9) | 3240 | (15.1) | 3223 | (18.8) | 1641 | (22.6) | 439 | (25.2) |
| 2 | 20 867 | (17.7) | 8856 | (18.6) | 3740 | (17.4) | 3215 | (18.8) | 1506 | (20.7) | 395 | (22.7) |
| 3 | 23 723 | (20.2) | 9576 | (20.1) | 4308 | (20.0) | 3455 | (20.2) | 1469 | (20.2) | 344 | (19.7) |
| 4 | 25 726 | (21.9) | 9841 | (20.7) | 4662 | (21.7) | 3479 | (20.3) | 1408 | (19.4) | 292 | (16.8) |
| 5 (more deprived) | 28 862 | (24.5) | 9802 | (20.6) | 5005 | (23.3) | 3386 | (19.8) | 1165 | (16.0) | 246 | (14.1) |
| DOM (index not available.) | 2155 | (1.8) | 996 | (2.1) | 540 | (2.5) | 345 | (2.0) | 85 | (1.2) | 26 | (1.5) |
| Patients according to the speciality of the physician initiating treatment, n (%) | ||||||||||||
| General practitioner | 77 997 | (66.3) | 33 652 | (70.7) | 16 642 | (77.4) | 11 778 | (68.9) | 4256 | (58.5) | 976 | (56.0) |
| Salaried physician | 30 707 | (26.1) | 9498 | (19.9) | 3686 | (17.1) | 3467 | (20.3) | 1863 | (25.6) | 482 | (27.7) |
| Psychiatrist | 9016 | (7.7) | 4464 | (9.4) | 1167 | (5.4) | 1858 | (10.9) | 1155 | (15.9) | 284 | (16.3) |
| Hospitalisation for an alcohol‐related problem, n (%) | 27 620 | (23.5) | 6059 | (12.7) | 1717 | (8.0) | 2580 | (15.1) | 1411 | (19.4) | 352 | (20.2) |
| Psychiatric drugs, n (%) | ||||||||||||
| Antipsychotic | 16 279 | (13.8) | 5778 | (12.1) | 1817 | (8.5) | 2367 | (13.8) | 1277 | (17.6) | 317 | (18.2) |
| Anxiolytic | 84 241 | (71.6) | 21 259 | (44.6) | 8142 | (37.9) | 8453 | (49.4) | 3787 | (52.1) | 877 | (50.3) |
| Antidepressant | 51 494 | (43.7) | 18 555 | (39.0) | 7473 | (34.8) | 7106 | (41.5) | 3227 | (44.4) | 749 | (43.0) |
| Hypnotic | 28 520 | (24.2) | 9820 | (20.6) | 3827 | (17.8) | 3837 | (22.4) | 1748 | (24.0) | 408 | (23.4) |
| Comorbidities according to cumulative Charlson score, n (%) | ||||||||||||
| 0 (no comorbidity) | 98 298 | (83.5) | 38 480 | (80.8) | 17 029 | (79.2) | 13 917 | (81.4) | 6051 | (83.2) | 1483 | (85.1) |
| 0 (score 0 with comorbidities) | 6386 | (5.4) | 3080 | (6.5) | 1585 | (7.4) | 1052 | (6.2) | 377 | (5.2) | 66 | (3.8) |
| 1 (score 1 with comorbidities) | 13 036 | (11.1) | 6054 | (12.7) | 2881 | (13.4) | 2134 | (12.5) | 846 | (11.6) | 193 | (11.1) |
| Number of dispensings during treatment, n (%) | ||||||||||||
| 2 dispensings | 49 247 | (41.8) | 18 353 | (38.5) | 10 565 | (49.2) | 6116 | (35.8) | 1421 | (19.5) | 251 | (14.4) |
| 3 dispensings | 24 657 | (20.9) | 8584 | (18.0) | 4096 | (19.1) | 3179 | (18.6) | 1082 | (14.9) | 227 | (13.0) |
| 4 to 6 dispensings | 25 894 | (22.0) | 10 392 | (21.8) | 3886 | (18.1) | 4016 | (23.5) | 2069 | (28.4) | 421 | (24.2) |
| More than 6 dispensings | 17 922 | (15.2) | 10 285 | (21.6) | 2948 | (13.7) | 3792 | (22.2) | 2702 | (37.1) | 843 | (48.4) |
| Patients on treatment at 6 months, n (%) | 19 698 | (16.7) | 10 355 | (21.7) | 3311 | (15.4) | 3908 | (22.8) | 2475 | (34.0) | 661 | (37.9) |
| Duration of treatment in days, median (IQR) | 81 (60‐138) | 87 (60‐162) | 60 (60‐127) | 88 (60‐169) | 120 (63‐237) | 129 (64‐278) | ||||||
| Reason for end of follow‐upa, n (%) | ||||||||||||
| Events | 11 918 | (10.1) | 4864 | (10.2) | 1797 | (8.4) | 1876 | (11.0) | 923 | (12.7) | 268 | (15.4) |
| End of exposure | 87 374 | (74.2) | 34 067 | (71.5) | 16 809 | (78.2) | 12 050 | (70.5) | 4293 | (59.0) | 915 | (52.5) |
| Switch | 6593 | (5.6) | 1364 | (2.9) | 357 | (1.7) | 580 | (3.4) | 327 | (4.5) | 100 | (5.7) |
| One year after initiation | 6445 | (5.5) | 4414 | (9.3) | 1323 | (6.2) | 1600 | (9.4) | 1157 | (15.9) | 334 | (19.2) |
| Other | 5390 | (4.6) | 2905 | (6.1) | 1209 | (5.6) | 997 | (5.8) | 574 | (7.9) | 125 | (7.2) |
Abbreviations: DOM, French overseas department (Departement d'Outre Mer); IQR, interquartile range.
mean daily dose of baclofen reached at the end of follow‐up.
Events: hospitalisation or death. End of exposure: 60 days after the last dispensing of the same approved drug or baclofen. Switch: switch from the initial approved drug or baclofen to another drug. Other: end of the study (31 December 2015), change of health insurance scheme, obstetric hospitalisation.
Patients exposed to baclofen differed from those exposed to approved drugs in terms of sociodemographic and medical characteristics: more likely to be female (43.8% vs 30.8%, respectively), higher socioeconomic status (17.9% vs 13.9% for the least deprived area of residence), lower frequency of history of hospitalisation for alcohol‐related problems (12.7% vs 23.5%), and lower frequency of exposure to anxiolytics (44.6% vs 71.6%). With increasing doses, from <30 to ≥180 mg/day, patients exposed to baclofen tended to resemble those receiving approved drugs, particularly in terms of age, gender, history of hospitalisation for alcohol‐related problems, and exposure to anxiolytics (Table 1).
Among patients with a history of hospitalisation for alcohol‐related problems (33 679), those exposed to baclofen were relatively similar to those exposed to approved drugs in terms of the various characteristics evaluated (Table 2).
Table 2.
Sociodemographic, medical, and follow‐up characteristics of patients with a history of hospitalisation for alcohol‐related problems initiating approved drugs (acamprosate, naltrexone, nalmefene) or baclofen for alcohol use disorders
| Approved drugs | Baclofen | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| All doses | Doses (mg/day) at the end of follow‐upa | |||||||||||
| Characteristics | < 30 | 30‐75 | 75‐180 | ≥ 180 | ||||||||
| Number of incident patients, n | 27 620 | 6059 | 1717 | 2580 | 1410 | 352 | ||||||
| Age, median (IQR) | 47 (40‐54) | 48 (40‐55) | 49 (42‐57) | 48 (41‐55) | 46 (38‐53) | 45 (36‐53) | ||||||
| Age groups, n (%) | ||||||||||||
| <30 | 1687 | (6.1) | 329 | (5.4) | 87 | (5.1) | 124 | (4.8) | 89 | (6.3) | 29 | (8.2) |
| 30‐40 | 4968 | (18.0) | 1087 | (17.9) | 240 | (14.0) | 443 | (17.2) | 321 | (22.8) | 83 | (23.6) |
| 40‐50 | 9342 | (33.8) | 2048 | (33.8) | 535 | (31.2) | 887 | (34.4) | 502 | (35.6) | 124 | (35.2) |
| 50‐60 | 8320 | (30.1) | 1779 | (29.4) | 561 | (32.7) | 771 | (29.9) | 363 | (25.7) | 84 | (23.9) |
| 60‐70 | 3303 | (12.0) | 8160 | (13.5) | 294 | (17.1) | 355 | (13.8) | 135 | (9.6) | 32 | (9.1) |
| Gender: Male, n (%) | 19 314 | (69.9) | 4001 | (66.0) | 1144 | (66.6) | 1693 | (65.6) | 937 | (66.5) | 227 | (64.5) |
| Deprivation index, n (%) | ||||||||||||
| 1 (least deprived) | 3004 | (10.9) | 885 | (14.6) | 217 | (12.6) | 352 | (13.6) | 253 | (17.9) | 63 | (17.9) |
| 2 | 4583 | (16.6) | 1161 | (19.2) | 294 | (17.1) | 503 | (19.5) | 292 | (20.7) | 72 | (20.5) |
| 3 | 5812 | (21.0) | 1355 | (22.4) | 386 | (22.5) | 576 | (22.3) | 306 | (21.7) | 87 | (24.7) |
| 4 | 6234 | (22.6) | 1343 | (22.2) | 393 | (22.9) | 582 | (22.6) | 306 | (21.7) | 62 | (17.6) |
| 5 (more deprived) | 7469 | (27.0) | 1196 | (19.7) | 369 | (21.5) | 526 | (20.4) | 239 | (17.0) | 62 | (17.6) |
| DOM (index not available.) | 518 | (1.9) | 119 | (2.0) | 58 | (3.4) | 41 | (1.6) | 14 | (1.0) | 6 | (1.7) |
| Patients according to the speciality of the physician initiating treatment, n (%) | ||||||||||||
| General practitioner | 12 960 | (46.9) | 3313 | (54.7) | 1125 | (65.5) | 1396 | (54.1) | 643 | (45.6) | 149 | (42.3) |
| Salaried physician | 12 962 | (46.9) | 1984 | (32.7) | 433 | (25.2) | 855 | (33.1) | 547 | (38.8) | 149 | (42.3) |
| Psychiatrist | 1698 | (6.1) | 762 | (12.6) | 159 | (9.3) | 329 | (12.8) | 220 | (15.6) | 54 | (15.3) |
| Psychiatric drugs, n (%) | ||||||||||||
| Antipsychotic | 5846 | (21.2) | 1528 | (25.2) | 361 | (21.0) | 651 | (25.2) | 416 | (29.5) | 100 | (28.4) |
| Anxiolytic | 21 871 | (79.2) | 4160 | (68.7) | 1146 | (66.7) | 1782 | (69.1) | 984 | (69.8) | 248 | (70.5) |
| Antidepressant | 13 232 | (47.9) | 3084 | (50.9) | 787 | (45.8) | 1332 | (51.6) | 767 | (54.4) | 198 | (56.3) |
| Hypnotic | 9415 | (34.1) | 2139 | (35.3) | 533 | (31.0) | 929 | (36.0) | 547 | (38.8) | 130 | (36.9) |
| Comorbidities according to cumulative Charlson score, n (%) | ||||||||||||
| 0 (no comorbidity) | 21 959 | (79.5) | 4662 | (76.9) | 1286 | (74.9) | 1978 | (76.7) | 1119 | (79.4) | 279 | (79.3) |
| 0 (score 0 with comorbidities) | 1830 | (6.6) | 438 | (7.2) | 138 | (8.0) | 199 | (7.7) | 86 | (6.1) | 15 | (4.3) |
| 1 (score 1 with comorbidities) | 3831 | (13.9) | 959 | (15.8) | 293 | (17.1) | 403 | (15.6) | 205 | (14.5) | 58 | (16.5) |
| Number of dispensings during treatment, n (%) | ||||||||||||
| 2 dispensings | 10 931 | (39.6) | 1835 | (30.3) | 686 | (40.0) | 822 | (31.9) | 271 | (19.2) | 56 | (15.9) |
| 3 dispensings | 5493 | (19.9) | 1075 | (17.7) | 352 | (20.5) | 470 | (18.2) | 199 | (14.1) | 54 | (15.3) |
| 4 to 6 dispensings | 6269 | (22.7) | 1517 | (25.0) | 371 | (21.6) | 674 | (26.1) | 402 | (28.5) | 70 | (19.9) |
| More than 6 dispensings | 4927 | (17.8) | 1632 | (26.9) | 308 | (17.9) | 614 | (23.8) | 538 | (38.2) | 172 | (48.9) |
| Patients on treatment at 6 months, n (%) | 5126 | (18.6) | 1550 | (25.6) | 323 | (18.8) | 616 | (23.9) | 478 | (33.9) | 133 | (37.8) |
| Duration of treatment in days, median (IQR) | 82 (60‐146) | 95 (60‐184) | 87 (60‐148) | 91 (60‐175) | 116 (60‐237) | 117 (60‐267) | ||||||
| Reason for end of follow‐upb, n (%) | ||||||||||||
| Events | 4835 | (17.5) | 1314 | (21.7) | 318 | (18.5) | 556 | (21.6) | 334 | (23.7) | 106 | (30.1) |
| End of exposure | 18 216 | (66.0) | 3489 | (57.6) | 1162 | (67.7) | 1519 | (58.9) | 666 | (47.2) | 142 | (40.3) |
| Switch | 1589 | (5.8) | 242 | (4.0) | 65 | (3.8) | 106 | (4.1) | 54 | (3.8) | 17 | (4.8) |
| One year after initiation | 1761 | (6.4) | 665 | (11.0) | 112 | (6.5) | 250 | (9.7) | 243 | (17.2) | 60 | (17.0) |
| Other | 1219 | (4.4) | 349 | (5.8) | 60 | (3.5) | 149 | (5.8) | 113 | (8.0) | 27 | (7.7) |
Abbreviations: DOM, French overseas department (Departement d'Outre Mer); IQR, interquartile range.
Mean daily dose of baclofen reached at the end of follow‐up.
Events: hospitalisation or death. End of exposure: 60 days after the last dispensing of the same approved drug or baclofen. Switch: switch from the initial approved drug or baclofen to another drug. Other: end of the study (31 December 2015), change of health insurance scheme, obstetric hospitalisation.
3.2. Risks of all‐cause hospitalisation or death
A total of 16 226 patients (9.8% of the population) were hospitalised and 556 patients (0.34%) died during follow‐up. In “gender‐age” analysis, standardised incidences per 1000 person‐years for patients exposed to baclofen vs approved drugs were lower for all‐cause hospitalisation (288 vs 321) and higher for all‐cause death (10.8 vs 10.6), with similar trends for the risk of hospitalisation and death (HR = 0.91 [95%CI: 0.88‐0.94] and HR = 1.03 [95%CI: 0.86‐1.24], respectively). In multivariate analysis, the risk of hospitalisation and death were higher for patients treated with baclofen vs approved drugs (HRf = 1.13 [1.09‐1.17] and HRf = 1.31 [1.08‐1.60], respectively). For patients exposed to baclofen vs approved drugs, the risk of hospitalisation and death increased from doses <30 mg/day (HRf = 1.09 [1.03‐1.15] and HRf = 1.00 [0.74‐1.36], respectively) to doses ≥180 mg/day (HRf = 1.46 [1.28‐1.65] and HRf = 2.27 [1.27‐4.07], respectively) (Table 3). Associations of the various covariates with hospitalisation and death are presented in Table S2.
Table 3.
Risk of all‐cause hospitalisation or death of patients initiating approved drugs (acamprosate, naltrexone, nalmefene) or baclofen for alcohol use disorders
| Approved drugs | Baclofen | |||||
|---|---|---|---|---|---|---|
| All doses | Doses (mg/day) over timea | |||||
| Outcomes | < 30 | 30‐75 | 75‐180 | ≥ 180 | ||
| All‐cause hospitalisation | ||||||
| N | 11 533 | 4693 | 1747 | 1803 | 887 | 256 |
| Gender‐age standardised incidence per 1000 p.y. | 321 | 288 | 272 | 295 | 299 | 367 |
| Gender‐age adjusted HR (95%CI) | 1 | 0.91 (0.88‐0.94) | 0.83 (0.79‐0.87) | 0.94 (0.89‐0.99) | 0.99 (0.92‐1.06) | 1.22 (1.08‐1.38) |
| HRf b (95%CI) | 1 | 1.13 (1.09‐1.17) | 1.09 (1.03‐1.15) | 1.12 (1.06‐1.18) | 1.15 (1.07‐1.23) | 1.46 (1.28‐1.65) |
| All‐cause mortality | ||||||
| N | 385 | 171 | 50 | 73 | 36 | 12 |
| Gender‐age standardised incidence per 1000 p.y. | 10.6 | 10.8 | 7.5 | 12.2 | 12.5 | 18.1 |
| Gender‐age adjusted HR (95%CI) | 1 | 1.03 (0.86‐1.24) | 0.74 (0.55‐0.99) | 1.16 (0.91‐1.50) | 1.25 (0.89‐1.76) | 1.82 (1.02‐3.25) |
| HRf b (95%CI) | 1 | 1.31 (1.08‐1.60) | 1.00 (0.74‐1.36) | 1.41 (1.09‐1.84) | 1.50 (1.06‐2.14) | 2.27 (1.27‐4.07) |
Abbreviations: CI, confidence interval; HR, hazard ratio; p.y., person‐year.
Time‐varying mean daily dose of baclofen during follow‐up.
HR fully adjusted for age, gender, deprivation index, speciality of the physician who initiated treatment, psychiatric drugs, history of hospitalisation for an alcohol‐related problem, history of comorbidity, and year of inclusion in the study.
The subpopulation of patients with a history of hospitalisation for alcohol‐related problems treated with baclofen had a significantly higher risk of all‐cause hospitalisation than those receiving an approved drug, both regardless of the dose (HRf = 1.15 [1.08‐1.23]) and for all dose classes greater than 30 mg/day, and a higher risk of all‐cause death for doses ≥180 mg/day (HRf = 2.72 [1.10‐6.72]) (Table 4).
Table 4.
Risk of all‐cause hospitalisation or death of patients with a history of hospitalisation for alcohol‐related problems initiating approved drugs (acamprosate, naltrexone, nalmefene) or baclofen for alcohol use disorders
| Approved drugs | Baclofen | |||||
|---|---|---|---|---|---|---|
| All doses | Doses (mg/day) over timea | |||||
| Outcomes | < 30 | 30‐75 | 75‐180 | ≥ 180 | ||
| All‐cause hospitalisation | ||||||
| N | 4679 | 1268 | 307 | 533 | 327 | 101 |
| Gender‐age standardised incidence per 1000 p.y. | 540 | 574 | 576 | 563 | 567 | 843 |
| Gender‐age adjusted HR (95%CI) | 1 | 1.10 (1.03‐1.17) | 1.03 (0.92‐1.16) | 1.06 (0.97‐1.16) | 1.14 (1.02‐1.27) | 1.53 (1.26‐1.87) |
| HRf b (95%CI) | 1 | 1.15 (1.08‐1.23) | 1.10 (0.97‐1.23) | 1.11 (1.01‐1.21) | 1.19 (1.06‐1.34) | 1.60 (1.31‐1.95) |
| All‐cause mortality | ||||||
| N | 156 | 46 | 11 | 23 | 7 | 5 |
| Gender‐age standardised incidence per 1000 p.y. | 18.0 | 21.1 | 18.9 | 24.4 | 11.3 | 34.2 |
| Gender‐age adjusted HR (95%CI), | 1 | 1.20 (0.86‐1.67) | 1.09 (0.59‐2.00) | 1.37 (0.88‐2.12) | 0.75 (0.35‐1.60) | 2.40 (0.98‐5.88) |
| HRf b (95%CI) | 1 | 1.37 (0.96‐1.95) | 1.28 (0.69‐2.40) | 1.55 (0.98‐2.45) | 0.84 (0.39‐1.82) | 2.72 (1.10‐6.72) |
HR: Hazard Ratio. CI: confidence interval. p.y.: person‐year.
Time‐varying mean daily dose of baclofen during follow‐up
HR fully adjusted for age, gender, deprivation index, speciality of the physician who initiated treatment, psychiatric drugs, history of comorbidity, and year of inclusion in the study.
Similar results were obtained whether covariates were taken into account by stratification or by adjustment. In particular, models adjusted or stratified for all covariates were similar for both the risks of all‐cause hospitalisation and death (Tables S3 and S4).
3.3. Primary hospital discharge diagnoses and specific causes of death
Primary hospital discharge diagnoses varied greatly. Several risks were significantly associated with patients treated with baclofen vs approved drugs, particularly, (1) at doses ≥180 mg/day, “injury, poisoning, and certain other consequences of external causes” (codes: S‐T; 17% of hospitalisations) with a HRf = 2.14 [95%CI: 1.68‐2.72], epilepsy (code: G40, 1.2% of hospitalisations) with a HRf = 4.42 [2.22‐8.82], and (2) regardless of dose class, “diseases of the musculoskeletal system” (code: M; 8% of hospitalisations) with a HRf = 1.80 [1.60‐2.04], and “symptoms, signs” (code: R; 6% of hospitalisations) with a HRf = 1.76 [1.53‐2.02] (Table S5).
The risk of death for “other ill‐defined and unspecified causes of mortality” (code: R99, ie, unknown cause by the physician reporting the death) was significantly higher for patients exposed to baclofen (HRf = 1.90 [1.00‐3.64]). The risk of “intentional self‐poisoning by and exposure to other and unspecified drugs, medicaments, and biological substances” (code: X64) was also associated with baclofen (HRf = 2.49 [1.04‐5.98]), although the risk of suicide (codes: X64‐X80) was not (HRf = 1.04 [0.65‐1.65]) (Table S6).
Hospitalisation for “injury, poisoning and certain other consequences of external causes” (codes S‐T; 20% of hospitalisations) in patients with a history of hospitalisation for alcohol‐related problems was associated with exposure to baclofen for all dose classes (HRf = 1.43 [1.12‐1.82], HRf = 1.24 [1.01‐1.51], HRf = 1.29 [1.01‐1.65], HRf = 2.04 [1.38‐3.01], for dose classes from <30 to ≥180 mg/day). No clear associations for causes of death were observed for patients in this subgroup exposed to baclofen vs approved drugs, because of the very small number of deaths (Tables S7 and S8).
4. DISCUSSION
4.1. Main results
In this study based on a cohort of more than 165 000 patients, exposure to baclofen for AUD was associated with an increased risk of hospitalisation (+13%) and death (+31%) compared to exposure to approved drugs. This association was particularly marked at high doses ≥180 mg/day of baclofen (+46% for hospitalisation and + 117% for death), was also observed for the subset of patients with a history of hospitalisation for alcohol‐related problems, and particularly concerned drug intoxication (hospitalisation and death) and unspecified death.
4.2. Comparison with previous studies
This real‐life safety cohort study is the first of its kind to examine baclofen used for AUD. The results therefore cannot be directly compared with those of experimental studies, in which, unlike in real‐life, patients generally receive treatment within a standardised framework. Furthermore, no experimental study has been designed to measure and compare risks of all‐cause hospitalisation or death between users of various drugs for AUD.
The available safety data concern specific adverse events. Three trials of high‐dose baclofen, reported by Müller (2015),15 Beraha (2016),17 and Reynaud (2017),18 using doses of 30 to 270, 150, and 180 mg/day, included 28, 89, and 158 patients treated with baclofen, respectively. These trials reported a higher incidence of adverse effects in the baclofen arm, particularly somnolence, asthenia, dizziness, and headache. Reynaud18 also reported three hospitalisations for overdose in the baclofen arm versus none in the placebo arm.
Much of the currently available safety data for baclofen for AUD are derived from recent French observational studies. Retrospective data from French poison centres, published by Pélissier (2017), included 294 cases of poisoning with baclofen between 2008 and 2013, including nine deaths, particularly in the context of suicide, and cardiorespiratory phenomena at high doses.36 In 2016, Olivier reported four cases of severe central sleep apnoea in the absence of the other risk factors commonly associated with this disease.20
Our findings concerning specific causes of hospitalisation and death of patients treated with baclofen, highlighting the risks of self‐poisoning, and the increased risk of all‐cause hospitalisation and death, are consistent with the results of these studies.
4.3. Strengths and limitations
This study was conducted on almost 47 000 patients living in France, treated with baclofen for AUD, including more than 9000 patients taking mean doses ≥75 mg/day (60 times more patients on high‐dose baclofen than in the most powerful clinical trial18). Robust and objective measures were used to detect events (hospitalisations, deaths), as the coding of these events is subject to strict and controlled procedures.24, 25, 26
No patients were lost to follow‐up in this study, which is an important point when assessing serious adverse events. All hospitalised patients and all deaths in France were systematically recorded. Assessment of all‐cause mortality was preferred in order to take all beneficial and adverse effects of each drug into account.
The two groups presented different characteristics, as is often the case in observational studies. Patients on baclofen globally presented slightly more favourable medical and social characteristics. The effect of complete adjustment for patient characteristics, especially history of hospitalisation for alcohol‐related problems, resulted in a higher estimated risk of all‐cause hospitalisation in the baclofen group. Similarly, adjustment also led to an increased risk of death among people exposed to baclofen vs approved drugs for AUD.
This study also comprises several limitations. As is often the case in medical administrative database studies, exposure could only be evaluated based on medication dispensing data, which does not guarantee that the medication was actually taken. Similarly, SNIIRAM dispensing data cannot be used to precisely estimate the doses of medication, as the dosage and number of days of treatment are not directly available, but are calculated. Although the estimates necessary to calculate the mean daily dose are probably adequate to measure a dose effect, they cannot be used to precisely define risk levels. The results presented according to the dose categories used in this study must therefore be interpreted with caution.
Another limitation of SNIIRAM data concerns the absence of detailed socioeconomic variables and the absence of reliable measures of obesity and smoking, suggesting a possible risk of residual confounding related to these variables.
The off‐label indication for baclofen cannot be determined directly from the SNIIRAM database. Following mediatisation of the use of baclofen for AUD and in view of its central neurological action, the rare use of baclofen has also been reported in bulimia,37 cocaine addiction and stuttering.38 Some patients treated with baclofen may also have been included in the cohort for muscle contractures secondary to sciatica, despite the exclusion of private rheumatologist‐initiated treatments, which could explain the increased risk of hospitalisation, mainly at low doses <30 mg/day, for dorsalgia (code: M54), or pain (code: R52). However, the low frequency of these hospitalisations possibly not related to the consequences of AUD would have a negligible impact on estimation of the risks of all‐cause hospitalisation and death.
The risks associated with all‐cause “hospitalisation” and “death” events are competitive risks. However, “death” events probably had very little impact on estimation of the risk of hospitalisation because of their respective frequencies: 16 226 hospitalisations vs 556 death. Deaths occurring during the 30 days after a hospitalisation were taken into account in the estimation of the risk of death. Analysis of the link between exposure to drugs for AUD and deaths more than 30 days after hospitalisation became less clinically relevant.
The main limitation of this study is the lack of data about alcohol. Data concerning the types and severity of alcoholism and alcohol consumption were not available in the SNIIRAM database (only hospital stays for alcohol‐related problems were identified), and the indications for each drug for AUD, unknown, can range from abstinence to reduction of alcohol consumption or craving. Baclofen for AUD is an off‐label drug promoted by patients and prescribers, unlike approved drugs. AUD could differ depending on these drugs, as the medical care of patients could temper the strength of risk associations and, more specifically, the dose‐response relationship.
However, baclofen exposure increased the risks of hospitalisation or death, irrespective of the dose class, and the similarity of the main results in the more homogeneous population restricted to subjects with a history of hospitalisation for alcohol‐related problems, supports the absence of major residual confounding in this population of patients, under the age of 70 years, with no major comorbidities.
4.4. Conclusion
This study raises concerns about the safety profile of baclofen for AUD, particularly at high doses, with higher risks of hospitalisation and mortality than approved drugs.
ETHICS STATEMENT
The study was approved by the French Data Protection Agency (regulatory decision DE‐2016‐72).
CONFLICT OF INTEREST
The authors declare no conflict of interest.
Supporting information
Data S1. S1. SNIIRAM, PMSI and CépiDc databases
Table S2. Impact of cofactors on the risk of hospitalisation or death in patients initiating approved drugs (acamprosate, naltrexone, nalmefene) or baclofen for alcohol use disorders
Table S3. HR for baclofen vs approved drugs, stratified by age and gender ± another covariate (HRstrat), and then adjusted for all other covariates† (HRstrat+ad), for all‐cause mortality
Table S4. HR for baclofen vs approved drugs, stratified by age and gender ± another covariate (HRstrat), and then adjusted for all other covariates† (HRstrat+ad), for all‐cause hospitalisations
Table S5. Risks of specific‐cause hospitalisation of patients initiating approved drugs (acamprosate, naltrexone, nalmefene) or baclofen for alcohol use disorders
Table S6. Risks of specific‐cause death of patients initiating approved drugs (acamprosate, naltrexone, nalmefene) or baclofen for alcohol use disorders
Table S7. Risks of specific‐cause hospitalisation of patients with a history of hospitalisation for an alcohol‐related problem initiating approved drugs (acamprosate, naltrexone, nalmefene) or baclofen for alcohol use disorders
Table S8. Risks of specific‐cause death of patients with a history of hospitalisation for an alcohol‐related problem initiating approved drugs (acamprosate, naltrexone, nalmefene) or baclofen for alcohol use disorders
ACKNOWLEDGEMENTS
All authors are employees of public institutions and received no specific funding for this study. The authors thank Karim Bounebache for his help in use of the CépiDc database and Anthony Saul for English revision of the manuscript.
Chaignot C, Zureik M, Rey G, Dray‐Spira R, Coste J, Weill A. Risk of hospitalisation and death related to baclofen for alcohol use disorders: Comparison with nalmefene, acamprosate, and naltrexone in a cohort study of 165 334 patients between 2009 and 2015 in France. Pharmacoepidemiol Drug Saf. 2018;27:1239–1248. 10.1002/pds.4635
All authors are employees of public institutions and received no specific funding for this study. This original research has not been previously reported (congress, abstract). A report with a broader purpose (use, persistence, and safety of baclofen) contains some of the data reported in this study but is only available in French.
REFERENCES
- 1. Addolorato G, Caputo F, Capristo E, et al. Baclofen efficacy in reducing alcohol craving and intake: a preliminary double‐blind randomized controlled study. Alcohol Alcohol. 2002;37(5):504‐508. [DOI] [PubMed] [Google Scholar]
- 2. Ameisen O. Complete and prolonged suppression of symptoms and consequences of alcohol‐dependence using high‐dose baclofen: a self‐case report of a physician. Alcohol Alcohol. 2005;40(2):147‐150. [DOI] [PubMed] [Google Scholar]
- 3. Addolorato G, Leggio L, Ferrulli A, et al. Effectiveness and safety of baclofen for maintenance of alcohol abstinence in alcohol‐dependent patients with liver cirrhosis: randomized, double‐blind controlled study. Lancet. 2007;370(9603):1915‐1922. [DOI] [PubMed] [Google Scholar]
- 4. Garbutt JC, Kampov‐Polevoy AB, Gallop R, Kalka‐Juhl L, Flannery BA. Efficacy and safety of baclofen for alcohol dependence: a randomized, double‐blind, placebo‐controlled trial. Alcohol Clin Exp Res. 2010;34(11):1849‐1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Addolorato G, Leggio L, Ferrulli A, et al. Dose‐response effect of baclofen in reducing daily alcohol intake in alcohol dependence: secondary analysis of a randomized, double‐blind, placebo‐controlled trial. Alcohol Alcohol. 2011;46(3):312‐317. [DOI] [PubMed] [Google Scholar]
- 6. Rigal L, Alexandre‐Dubroeucq C, de Beaurepaire R, Le Jeunne C, Jaury P. Abstinence and ‘low‐risk’ consumption 1 year after the initiation of high‐dose baclofen: a retrospective study among ‘high‐risk’ drinkers. Alcohol Alcohol. 2012;47(4):439‐442. [DOI] [PubMed] [Google Scholar]
- 7. de Beaurepaire R. Suppression of alcohol dependence using baclofen: a 2‐year observational study of 100 patients. Front Psych. 2012;3:103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ameisen O. The End of my Addiction. New York: SCrichton Books; 2008. [Google Scholar]
- 9. Ameisen O. Le Dernier Verre. Paris: Denoël; 2008. [Google Scholar]
- 10. Dupouy J, Fournier JP, Jouanjus E, et al. Baclofen for alcohol dependence in France: incidence of treated patients and prescription patterns—a cohort study. Eur Neuropsychopharmacol. 2014;24(2):192‐199. [DOI] [PubMed] [Google Scholar]
- 11. Chaignot C, Weill A, Ricordeau P, Alla F. [Use in France of baclofen for alcohol dependence from 2007 to 2013: cohort study based on the databases SNIIRAM and PMSI.] Therapie. 2015;70(5):443‐453. [DOI] [PubMed] [Google Scholar]
- 12. Auffret M et al. On‐the‐ground application of the ‘temporary recommendation for use’ regulatory measure on off‐label use of baclofen for alcohol dependence in France: a regional survey of community pharmacies. Fundam Clin Pharmacol. 2017;1‐5. [DOI] [PubMed] [Google Scholar]
- 13. Caisse nationale de l'assurance maladie . Open MEDIC: base complète sur les dépenses de médicaments interrégimes. http://open-data-assurance-maladie.ameli.fr/medicaments/index.php#Open_MEDIC.
- 14. French National Agency for Medicines and Health Products Safety (ANSM) . Recommandation Temporaire d'Utilisation du baclofène dans la prise en charge des patients alcoolo‐dépendants—2014. Available at: http://ansm.sante.fr/S-informer/Points-d-information-Points-d-information/Une-recommandation-temporaire-d-utilisation-RTU-est-accordee-pour-le-baclofene-Point-d-information.
- 15. Müller CA, Geisel O, Pelz P, et al. High‐dose baclofen for the treatment for alcohol dependence (BACLAD study): a randomized, placebo‐controlled trial. Eur Neuropsychopharmacol. 2015;25(8):1167‐1177. [DOI] [PubMed] [Google Scholar]
- 16. Ponizovsky A, Rosca P. Baclofen as add‐on to standard psychosocial treatment for alcohol dependence: a randomised, double‐blind, placebo‐controlled trial with 1 year follow‐up. J Subst Abuse Treat. 2015;52:24‐30. [DOI] [PubMed] [Google Scholar]
- 17. Beraha EM, Salemink E, Goudriaan AE, et al. Efficacy and safety of high‐dose baclofen for the treatment for alcohol dependence: a multicentre, randomised, double‐blind controlled trial. Eur Neuropsychopharmacol. 2016;26(12):1950‐1959. [DOI] [PubMed] [Google Scholar]
- 18. Reynaud M, Aubin HJ, Trinquet F, et al. A randomized, placebo‐controlled study of high‐dose baclofen in alcohol‐dependent patients—the ALPADIR study. Alcohol Alcohol. 2017;52(4):439‐446. [DOI] [PubMed] [Google Scholar]
- 19. French National Agency for Medicines and Health Products Safety (ANSM) . Réunion du Comité technique de Pharmacovigilance—CT012016083 (Séance du mardi 11 octobre 2016) http://ansm.sante.fr/content/download/102997/1305695/version/1/file/CR_CT_Pharmacovigilance_CT012016083_11-10-2016.pdf.
- 20. Olivier PY, Joyeux‐Faure M, Gentina T, et al. Severe central sleep apnea associated with chronic baclofen therapy: a case series. Chest. 2016;149(5):e127‐e131. [DOI] [PubMed] [Google Scholar]
- 21. Guillou‐Landreat M, Victorri Vigneau C, Gerardin M. Gambling disorder: a side effect of an off‐label prescription of baclofen—literature review. BMJ Case Rep. 2017;10. 2017. pii: bcr2016217506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Liu J, Wang LN. Baclofen for alcohol withdrawal. Cochrane Database Syst Rev. 2017;20(4). CD008502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Palpacuer C, Duprez R, Huneau A, et al. Pharmacologically controlled drinking in the treatment of alcohol dependence or alcohol use disorders: a systematic review with direct and network meta‐analyses on nalmefene, naltrexone, acamprosate, baclofen and topiramate. Addiction. 2018;113(2):220‐237. [DOI] [PubMed] [Google Scholar]
- 24. Tuppin P, Rudant J, Constantinou P, et al. Value of a national administrative database to guide public decisions: from the système national d'information interrégimes de l'Assurance Maladie (SNIIRAM) to the système national des données de santé (SNDS) in France. Rev Epidemiol Sante Publique. 2017;65(Suppl 4):S149‐S167. [DOI] [PubMed] [Google Scholar]
- 25. Bezin J, Duong M, Lassalle R, et al. The national healthcare system claims databases in France, SNIIRAM and EGB: powerful tools for pharmacoepidemiology. Pharmacoepidemiol Drug Saf. 2017;26(8):954‐962. [DOI] [PubMed] [Google Scholar]
- 26. Moulis G, Lapeyre‐Mestre M, Palmaro A, Pugnet G, Montastruc JL, Sailler L. French health insurance databases: what interest for medical research? Rev Med Interne. 2015. Jun;36(6):411‐417. [DOI] [PubMed] [Google Scholar]
- 27. Polard E, Nowak E, Happe A, Biraben A, Oger E. For the GENEPI study group. Brand name to generic substitution of antiepileptic drugs does not lead to seizure‐related hospitalization: a population‐based case‐crossover study. Pharmacoepidemiol Drug Saf. 2015;24(11):1161‐1169. [DOI] [PubMed] [Google Scholar]
- 28. Basson M, Mezzarobba M, Weill A, et al. Severe intestinal malabsorption associated with olmesartan: a French nationwide observational cohort study. Gut. 2016;65(10):1664‐1669. [DOI] [PubMed] [Google Scholar]
- 29. Maura G, Blotière P‐O, Bouillon K, et al. Comparison of the short‐term risk of bleeding and arterial thromboembolic events in nonvalvular atrial fibrillation patients newly treated with dabigatran or rivaroxaban versus vitamin K antagonists: a French nationwide propensity‐matched cohort study. Circulation. 2015;132(13):1252‐1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Weill A, Dalichampt M, Raguideau F, et al. Low dose oestrogen combined oral contraception and risk of pulmonary embolism, stroke, and myocardial infarction in five million French women: cohort study. BMJ. 2016;353:i2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Miranda S, Chaignot C, Collin C, Dray‐Spira R, Weill A, Zureik M. Human papillomavirus vaccination and risk of autoimmune diseases: a large cohort study of over 2million young girls in France. Vaccine. 2017;35(36):4761‐4768. [DOI] [PubMed] [Google Scholar]
- 32. Rey G. Death certificate data in France: production process and main types of analyses. Rev Med Interne. 2016;37(10):685‐693. [DOI] [PubMed] [Google Scholar]
- 33. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373‐383. [DOI] [PubMed] [Google Scholar]
- 34. Bannay A, Chaignot C, Blotière PO, et al. The best use of the Charlson comorbidity index with electronic health care database to predict mortality. Med Care. 2016;54(2):188‐194. [DOI] [PubMed] [Google Scholar]
- 35. Rey G, Jougla E, Fouillet A, Hémon D. Ecological association between a deprivation index and mortality in France over the period 1997‐2001: variations with spatial scale, degree of urbanicity, age, gender and cause of death. BMC Public Health. 2009;9(1):33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Pelissier F, de Haro L, Cardona F, et al. Self‐poisoning with baclofen in alcohol‐dependent patients: national reports to French poison control centers, 2008‐2013. Clin Toxicol (Phila). 2017;55(4):275‐284. [DOI] [PubMed] [Google Scholar]
- 37. Weibel S, Lalanne L, Riegert M, Bertschy G. Efficacy of high‐dose baclofen for alcohol use disorder and comorbid bulimia: a case report. J Dual Diagn. 2015;11(3–4):203‐204. [DOI] [PubMed] [Google Scholar]
- 38. Beraha E, Bodewits P, van den Brink W, Wiers R. Speaking fluently with baclofen? BMJ Case Rep. 2017. May 11;2017. pii: bcr‐2016‐218714 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1. S1. SNIIRAM, PMSI and CépiDc databases
Table S2. Impact of cofactors on the risk of hospitalisation or death in patients initiating approved drugs (acamprosate, naltrexone, nalmefene) or baclofen for alcohol use disorders
Table S3. HR for baclofen vs approved drugs, stratified by age and gender ± another covariate (HRstrat), and then adjusted for all other covariates† (HRstrat+ad), for all‐cause mortality
Table S4. HR for baclofen vs approved drugs, stratified by age and gender ± another covariate (HRstrat), and then adjusted for all other covariates† (HRstrat+ad), for all‐cause hospitalisations
Table S5. Risks of specific‐cause hospitalisation of patients initiating approved drugs (acamprosate, naltrexone, nalmefene) or baclofen for alcohol use disorders
Table S6. Risks of specific‐cause death of patients initiating approved drugs (acamprosate, naltrexone, nalmefene) or baclofen for alcohol use disorders
Table S7. Risks of specific‐cause hospitalisation of patients with a history of hospitalisation for an alcohol‐related problem initiating approved drugs (acamprosate, naltrexone, nalmefene) or baclofen for alcohol use disorders
Table S8. Risks of specific‐cause death of patients with a history of hospitalisation for an alcohol‐related problem initiating approved drugs (acamprosate, naltrexone, nalmefene) or baclofen for alcohol use disorders


