Abstract
Acto-myosin cross-bridge kinetics are important for beat-to-beat regulation of cardiac contractility; however, physiological and pathophysiological mechanisms for regulation of contractile kinetics are incompletely understood. Here we explored whether thin filament-mediated Ca2+ sensitization influences cross-bridge kinetics in permeabilized, osmotically compressed cardiac muscle preparations. We used a murine model of hypertrophic cardiomyopathy (HCM) harboring a cardiac troponin C (cTnC) Ca2+-sensitizing mutation, Ala8Val in the regulatory N-domain. We also treated wild-type murine muscle with bepridil, a cTnC-targeting Ca2+ sensitizer. Our findings suggest that both methods of increasing myofilament Ca2+ sensitivity increase cross-bridge cycling rate measured by the rate of tension redevelopment (kTR); force per cross-bridge was also enhanced as measured by sinusoidal stiffness and I1,1/I1,0 ratio from X-ray diffraction. Computational modeling suggests that Ca2+ sensitization through this cTnC mutation or bepridil accelerates kTR primarily by promoting faster cross-bridge detachment. To elucidate if myofilament structural rearrangements are associated with changes in kTR, we used small angle X-ray diffraction to simultaneously measure myofilament lattice spacing and isometric force during steady-state Ca2+ activations. Within in vivo lattice dimensions, lattice spacing and steady-state isometric force increased significantly at submaximal activation. We conclude that the cTnC N-domain controls force by modulating both the number and rate of cycling cross-bridges, and that the both methods of Ca2+ sensitization may act through stabilization of cTnC’s D-helix. Furthermore, we propose that the transient expansion of the myofilament lattice during Ca2+ activation may be an additional factor that could increase the rate of cross-bridge cycling in cardiac muscle. These findings may have implications for the pathophysiology of HCM.
Keywords: small-angle X-ray diffraction, kinetics of tension redevelopment, sinusoidal stiffness, myofilament lattice spacing, Ca2+ sensitivity, troponin C, bepridil, hypertrophic cardiomyopathy, thin filament
1. INTRODUCTION
Contraction of the heart depends on Ca2+ binding to the troponin complex. The troponin complex resides in the thin filament of striated myocytes in the heart and skeletal muscle. In cardiac myocytes, the troponin complex is comprised of the Ca2-binding subunit, cardiac troponin C (cTnC), the inhibitory subunit, cardiac troponin I (cTnI), and the tropomyosin-binding subunit, cardiac troponin T (cTnT). A transient rise in intracellular Ca2+ levels leads to a series of conformational changes in the thin filament, which ultimately promotes cyclic actin-myosin interactions and relative sliding of the thick (i.e., myosin) and thin (i.e., actin) filaments (1, 2). Considering that the regulatory N-terminal domain of cTnC is the Ca2+ sensitive switch, it is not surprising that it plays a central role in regulating force production in striated muscle.
It has been previously suggested that kinetics of cross-bridge cycling are influenced by Ca2+dependent activation in striated muscle across multiple vertebrate species (3–13). One of the first studies to demonstrate this relation used detergent skinned (permeabilized) rat ventricular trabeculae and showed that increasing Ca2+ concentrations resulted in a concomitant increase in the rate of tension redevelopment (kTR) (12). This Ca2+-dependence of kTR was subsequently reported in permeabilized rat cardiomyocytes (3) and permeabilized guinea pig trabeculae (13). Noteworthy, even in intact cardiac muscle, it was demonstrated that Ca2+ influences tension redevelopment (5). Small molecule cTnC Ca2+ sensitizers have also been shown to influence cross-bridge kinetics (9, 14). Although these studies point to a role for Ca2+ in regulating crossbridge mechanics beyond steric access to myosin-binding sites on actin, little is known about the direct role of cTnC in modulation of cross-bridge kinetics and the underlying mechanisms.
The sarcomeres of striated muscle cells form a well-ordered lattice comprising a double, hexagonal array of overlapping thin and thick filaments that are responsible for generating contractile force in the heart (1, 15). It has been previously shown that interfilament spacing modulates cross-bridge kinetics in Drosophila flight muscle (16). Mathematical modeling also supports the idea that myofilament lattice spacing may modulate cross-bridge kinetics in striated muscle (17). Furthermore, myofilament lattice spacing (18) and sarcomere length (19) are dynamic during the contraction-relaxation cycles of the heart in vivo. Therefore, lattice spacing may also have a functional role in regulating Ca2+-dependent cross-bridge kinetics in cardiac muscle.
In this study, we explored the effects of thin filament Ca2+ sensitization on cross-bridge mechanics in permeabilized cardiac ventricular muscle. For all of the experiments in this study, dextran T-500 was included in the solutions to osmotically compress the myofilament lattice to the dimensions found in vivo (18). We found that Ca2+-sensitization through the regulatory (N-terminal) domain of cTnC, by either a small molecule Ca2+ sensitizer, bepridil (Bep) (14), or a cTnC mouse model of HCM (20), influences both the number and kinetics of acto-myosin cross-bridges. To understand a potential molecular mechanism underlying the Ca2+-dependence of acto-myosin kinetics, we used small angle X-ray diffraction to simultaneously measure lattice spacing and steady-state, isometric force. Here we show that lattice spacing increases throughout isometric force development at physiologically-relevant Ca2+ concentrations. Expansion of the myofilament lattice may therefore constitute a novel molecular mechanism to mediate contractility in the heart.
2. MATERIALS AND METHODS
2.1. Experimental Animals
All protocols and experimental procedures followed NIH guidelines and were approved by Florida State University’s Animal Care and Use Committee (IACUC). Knock-in mice harboring the cTnC-A8V mutation in both alleles (KI-TnC-A8V+/+) and wild-type controls (“Ctrl”) were used in this study. These mice have been previously characterized and published by our group (20, 21). KI-TnC-A8V+/+ mice were shown to develop HCM in the adult stage (20, 22) and are therefore denoted as “HCM.” All animals used in this study ranged from 7–10 months of age.
2.2. Calcium Solutions
Relaxing and activating solutions were calculated using the pCa Calculator (23) and contained: 20 mM 3-[N-morpholino]propanesulfonic acid (MOPS), 7 mM ethylene glycol-bis(2aminoethylether)-N,N,N′,N′-tetraacetic acid (EGTA), 15 mM phosphocreatine, 15 units mL−1 creatine phosphokinase, 2.5 mM MgATP2-, 1 mM free Mg2+, ionic strength maintained constant at 150 mM by adding Kpropionate (KPr), varying [Ca2+], pH 7.0. The anion of choice for the pCa solutions was propionate (e.g., CaPr2, MgPr2 and KPr). All solutions were made and experiments were performed at room temperature (20–21°C). All solutions contained 3% (w:v) dextran Mr 450,000–650,000 (Sigma-Aldrich, cat. 31392), referred to as “dextran T-500”.
2.3. Cardiac Muscle Preparations
Cardiac permeabilized muscles were prepared as previously described (24). For muscle mechanics studies, excised Ctrl hearts (4 male and 1 female) and HCM hearts (2 males and 2 females) were opened along the septum and pinned onto a Sylgard-coated plate (Sylgard 184 Silicone Elastomer) to expose the left ventricle (LV) to non-ionic detergent 1% Triton X-100 (v:v) in a relaxing pCa 8 solution (10−8 M, 150 mM ionic strength, 2.5 mM MgATP2-, pH 7). After 4 hrs incubation in Triton X-100 skinning solution at 4°C, the solution was changed to a relaxing pCa 8 solution containing 51% glycerol (v:v) and stored at -20°C. Isolated papillary muscles were then utilized for mechanical studies within one week. For small angle X-ray diffraction studies, excised Ctrl hearts (2 male, 2 female) and HCM hearts (3 female, 2 male) were cut along the left ventricle parallel to the septum and pinned onto Sylgard-coated plates exposing the left ventricle to non-ionic detergent 1% Triton X-100 and 0.2 mg/ml saponin in relaxing pCa 8 solution for 4 hrs at 4oC. Papillary muscles and trabeculae were used for lattice spacing measurements within 12 hrs of isolation. Permeabilized Cardiac Muscle Preparations (CMPs) were dissected and their ends attached to aluminum T-clips. For muscle mechanics experiments, end compliance was minimized by chemically fixing the ends using 1% glutaraldehyde (25). Sarcomere length (SL) at pCa 8 was set to 2.1 μm using HeNe laser diffraction. Prior to the start of both mechanical and X-ray diffraction experiments, permeabilized CMPs were incubated for 1 hr in pCa 8 with 3% dextran T-500 solution to compress the myofilament lattice to physiological dimensions.
2.4. Muscle Mechanics
Force was measured using a force transducer (Aurora Scientific Inc. Model 403A) and length was controlled using a high-speed servomotor (Aurora Scientific Inc. Model 322C). Measurements of kTR were obtained by shortening CMPs (~ 1 mm in length and 0.15 ± 0.01 mm in diameter for WT and 0.16 ± 0.01 mm in diameter for HCM) 20% of L0 (initial CMP length at pCa 8) for 20 ms at ramp shortening rate ~ Vmax, followed by a step 25% rapid re-stretch and release that returned CMP back to L0. The apparent rate constant was obtained from each tension recovery time course as previously described (8, 26). Briefly, the half-time (t1/2) of force recovery—an unbiased, model-independent estimate of a kinetic constant—was transformed according to kTR = τ−1 = -ln(t1/2)/0.5 and the rate was expressed in units of s−1. Conditions where steady-state, isometric force was below 15% of the maximal force were excluded from the kTR analysis. Sinusoidal Stiffness (SS) measurements were recorded as previously described (27). Briefly, CMP were oscillated ~ 0.2% L0 peak-to-peak at 100 Hz with a sampling rate of 1 kHz (i.e., 5-fold above the Nyquist frequency); this frequency was chosen to correspond to the highest frequency used in our previous study and is > 4-fold higher than the highest “dip” frequency at saturating Ca2+ in Bode plots of stiffness (21). SS data were analyzed using R Studio Fast Fourier transform and reported in units of MPa. Relative pCa-force and pCa-SS relationships were fit using a 2-parameter, sigmoidal Hill equation, and normalized pCa-force relationships were fit using a 3-parameter, sigmoidal Hill equation as previously described (28).
2.5. Small-Angle X-Ray Diffraction
X-ray diffraction experiments were conducted at the BioCAT undulator-based beamline 18ID at the Advanced Photon Source (Argonne National Labs, Lemont, IL) (29). The CMPs (~ 1 mm in length and 0.35 ± 0.02 mm in diameter for WT and 0.25 ± 0.02 mm in diameter for HCM) were carefully attached to aluminum T-clips between a force transducer (Aurora Scientific model 402A) and a servomotor (Cambridge Technology, model 308B; Aurora Scientific, Aurora, Canada), both mounted on micromanipulators. The CMPs were set to SL 2.1 μm at pCa 8 using HeNe laser diffraction. A custom experimental apparatus allowed simultaneous collection of the X-ray patterns and steady-state force during Ca2+ stepwise activation (30). Lattice spacing was measured as described previously (30). The sample-to-detector distance for the small-angle Xray diffraction camera was ~ 3 m and the focal spot size was about 150 × 50 μm in the detector plane. Diffraction images were collected on a CCD-based X-ray detector (Mar 165, Rayonix Inc. Evanston IL). Exposure times were ~ 0.5 s with an incident X-ray flux of ~ 1 × 1012 photons s−1. To minimize radiation damage to specific regions of each CMP, exposure was distributed along ~ 90% of the length of each CMP by oscillation of the muscle rig using a ZETA microstepping drive and motor (Compumotor Division of Parker Hannifin Corp, Rohnert Park, CA); motion was along the CMP’s longitudinal axis using the default waveform setting of the ZETA drive at a rate of 1 – 2 mm s−1. Lattice spacing (d1,0) was evaluated from the diffraction pattern using Bragg’s law. Intensities of the 1,0 and 1,1 equatorial reflections (I1,0 and I1,1, respectively) were determined from nonlinear least square fits to 1-dimensional projections of the integrated intensity along the equator (31).
2.6. Mathematical Modeling of Tension Redevelopment Kinetics
The relationship between isometric steady-state force and kTR was modeled using the 3-state model of muscle contraction that was originally described by Landesberg and Sideman (7), and subsequently validated for cardiac muscle (32, 33). The 3-state model for cardiac muscle is a simplified version of a 4-state model that has been used extensively for modeling force-kTR relations in skeletal muscle (9, 26, 32, 34). The coupled differential equations for the 3-state model were solved for a given set of parameters (f, g, kOFF and [Ca2+]kON) using MatLab (release R2016b 9.1.0.441655, The MathWorks, Inc., Natick, MA, USA) as described (33). Parameter estimates (f, g and kOFF) were iteratively modified from initial starting values to identify best fit values using the Simplex algorithm (35), implemented in MatLab; kON was held constant according to values obtained experimentally using WT- or HCM-containing thin filaments in the presence of myosin and MgATP (36) and pCa was varied between 9 and 4 in increments of 0.05 pCa units. Steady-state force and kTR were calculated for each of the predicted force time courses using algorithms equivalent to those applied to CMP data. Interpolative refinement was then used to estimate kTR and pCa where the predicted steady-state force matched the experimental force values. Best fit estimates of 3-state model parameters (f, g and kOFF) were identified according to the least squares criterion where the sum of squared deviations (SSD) to be minimized was modified to include not only the difference between the predicted and measured force-kTR relations but also the difference between the predicted and measured pCa50 for isometric force; SSD components were weighted to emphasize the maximum kTR, pCa50, and the maximum force normalized to Ctrl. A maximum relative tolerance of 10−5 was used to define convergence of the Simplex algorithm for each parameter estimate (f, g and kOFF) and also the corresponding modified sum of squares for each set of parameter estimates in the simplex.
2.7. Statistical Analysis
Data were reported as mean ± S.E. Statistical significance was tested using one-way ANOVA with post-hoc Student-Newman-Keuls test. When a group failed normality or equal variance, a non-parametric test was used. The specific statistical tests for each parameter can be found in the legends of figures and tables. p<0.05 was considered statistically significant. SigmaPlot version 12 was used for statistical analysis.
3. RESULTS
3.1. Myofilament Ca2+ sensitization through the N-domain of cTnC
Although our group has previously reported that KI-cTnC-A8V+/+ heart muscle exhibits enhanced Ca2+ sensitivity of steady-state isometric force production (20, 21), experiments in this present study differed by the presence of dextran T-500. Since it is well known that permeabilizing muscle induces swelling of the myofilament lattice, 3% dextran T-500 was included in all Ca2+ solutions to achieve physiological lattice spacing dimensions (18, 37, 38). Steady-state, isometric force was measured as a function of graded Ca2+ concentrations in permeabilized cardiac muscle preparations (CMPs) isolated from HCM mouse hearts at SL 2.1 μm in the presence of 3% dextran. Consistent with previous reports, HCM CMPs exhibited a significant leftward shift of the pCa-force relationship, indicating increased myofilament Ca2+ sensitivity of isometric force production (HCM vs. Ctrl ΔpCa50 = +0.61 pCa units) (Fig. 1A, 1B, 1D and Table 1).
Figure 1. pCa-force relationship and maximum force at SL 2.1 μm.
A) Normalized steady-state isometric force as a function of pCa. The force values were normalized to the maximum steady-state isometric force in Ctrl CMPs. B) Relative steady-state isometric force as a function of pCa. The force values were normalized to the maximum force of each individual CMPs. C) Maximum steady-state isometric force values for each CMPs. D) pCa50 values for each CMPs. Horizontal bars represent the mean. Data are shown as mean ± S.E. n = 19 Ctrl, 23 HCM and 10 Ctrl+Bep. *p<0.05, statistical tests are described in legend of Table 1.
Table 1.
Contractile Parameters for CMPs at SL 2.1 μm
| Ctrl |
HCM |
Ctrl+Bep |
|
|---|---|---|---|
| 42.6 ± 3.3 | 36.7 ± 2.6 | 39.2 ± 5.0 | |
| FpCa50 | 5.57 ± 0.02 | 6.18 ± 0.03*,# | 5.77 ± 0.04* |
| nHill | 3.0 ± 0.2 | 2.4 ± 0.4* | 1.9 ± 0.2* |
| ktr max (s−1) | 19.3 ± 1.1 | 24.8 ± 0.9*,# | 27.8 ± 1.0* |
| SSmax (MPa) | 2.3 ± 0.3 | 1.4 ± 0.1* | 2.2 ± 0.4 |
| SSpCa50 | 5.59 ± 0.02 | 6.28 ± 0.03*,# | 5.81 ± 0.04* |
Fmax = maximum steady-state isometric force; FpCa50 = pCa needed to reach 50% of the maximum steady-state isometric force; nHill = cooperativity of thin filament activation; ktr max = maximum rate of tension redevelopment; SSmax = maximum steady-state sinusoidal stiffness; SSpCa50 = pCa needed to reach 50% of the maximum steady-state sinusoidal stiffness.
p<0.05, HCM or Ctrl+Bep vs. Ctrl
p<0.05, HCM vs. Ctrl+Bep
The statistical significance for Fmax, FpCa50, ktr max, SSpCa50 was determined using one-way ANOVA with post-hoc Student-Newman-Keuls test.
The statistical significance for nHill, SSmax was determined using Kruskal-Wallis one-way analysis of variance on ranks.
Number of CMPs = 10–23 per parameter.
As an additional tool to examine the functional effects of Ca2+-sensitization through cTnC apart from HCM muscle, we treated Ctrl CMPs with Bep, a small molecule Ca2+ sensitizer that is known to bind directly to the regulatory domain of cTnC (14, 39, 40). Upon treatment with 300 μM Bep, Ctrl+Bep CMPs exhibited a significant leftward shift in the pCa-force relationship (Ctrl+Bep vs. Ctrl ΔpCa50 = +0.20 pCa units) (Fig. 1A, 1B, 1D and Table 1), indicating enhanced myofilament Ca2+ sensitivity during isometric contraction. These results followed the same myofilament Ca2+ sensitivity trend observed for HCM CMPs and are also consistent with previous reports (14). No significant differences in maximum steady-state force among the three preparations were observed (Fig. 1C, Table 1). The cooperativity of thin filament activation (nHill) was significantly reduced in both Ca2+-sensitized groups compared to Ctrl (HCM = 2.75 ± 0.55, Ctrl+Bep = 1.85 ± 0.16 and Ctrl = 3.34 ± 0.17) (Fig. 1A, 1B and Table 1). In the following experiments, we used HCM and Ctrl+Bep CMPs to probe the effects of cTnC-mediated Ca2+sensitization on various mechanical parameters of muscle contraction.
3.2. Two independent methods of increasing Ca2+ sensitivity through the N-domain of cTnC also increase kTR
Several reports have provided evidence showing that Ca2+ influences the rate of force production in cardiac muscle (3–7, 12, 41), but little is known about the direct role of cTnC in this process. To this end, we measured kTR to understand how enhanced myofilament Ca2+ sensitivity through cTnC influences contractile kinetics. In most physiologically relevant circumstances, activation-dependence of kTR provides insight into the actomyosin cross-bridge cycling kinetics at high levels of activation, and the dynamics of individual regulatory units on thin filaments at submaximal levels of activation (8, 9, 26, 32, 34). Representative force and length traces are shown in Figs. 2A-D. HCM CMPs exhibited significantly faster kTR at all levels of Ca2+activation compared to Ctrl CMPs (Fig. 2E, Table 1). Ctrl+Bep CMPs also exhibited significantly faster kTR at all levels of Ca2+-activation compared to Ctrl (Fig. 2E, Table 1). Furthermore, HCM and Ctrl+Bep CMPs display faster kTR at similar isometric force levels compared to Ctrl (Fig. 2F). These results suggest that the regulatory N-domain of cTnC could play an important role in determining cross-bridge kinetics in cardiac muscle.
Figure 2. The rate of tension redevelopment (kTR) at SL 2.1 μm.
A and B) Representative tracings of absolute force values as a function of time during the kTR protocol. C and D) Representative tracings of percentage length variation as a function of time during the kTR protocol. E) kTR as a function of increasing Ca2+ concentration. Dashed lines were drawn by eye. F) Normalized force vs. kTR. The force values were normalized to the maximum steady-state isometric force in Ctrl CMPs. Lines represent solutions to the 3-state model in Fig. 5; solid lines represent model solutions using the best fit parameters (f, g, kOFF) and kON listed in Table 2, and dotted lines represent solutions when all four parameters were either increased (upper line) or decreased (lower line) by 2%. Data are shown as mean ± S.E. n = 19 Ctrl, 22 HCM, and 10 Ctrl+Bep.
3.3. Mathematical modeling of kTR indicates that both methods of increasing Ca2+ sensitivity increase cross-bridge kinetics in addition to the expected changes in thin filament regulatory unit dynamics
To understand the source of faster kTR observed in HCM and Ctrl+Bep CMPs, we used a 3-state model of muscle contraction to explore the effects of HCM and Bep on the cross-bridge attachment (f) and detachment (g) rates (Fig. 2F). The best fit values for g, f and kOFF are reported in Table 2. Compared to Ctrl, the g values for HCM and Ctrl+Bep CMPs increased by a larger magnitude than f (Table 2). Because maximal kTR ∝ f + g (42), these analyses suggest that the increased kTR observed in HCM CMP is due to a faster cross-bridge detachment rate, g, while the increased kTR in Ctrl+Bep CMP comes from both faster cross-bridge attachment and detachment rates.
Table 2.
Optimized parameter estimates and predictions from the 3-state model depicted in Fig. 5
| CMPs | Bepridi l (μΜ) | 3-state model fitted parameters |
3-state model predictions | |||||
|---|---|---|---|---|---|---|---|---|
| Cross-bridge | Regulatory unit | |||||||
| f (s−1) | g (s−1) | kON (M−1 s−1) (36) | kOFF (s−1) | pCa50 | max force (norm.) | max kTR (s−1) | ||
| Ctrl | 0 | 11.8 | 8.3 | 1.84×108 | 1254.4 | 5.57 | 1.00 | 19.3 |
| HCM | 0 | 12.4 | 12.6 | 1.34×108 | 176.6 | 6.18 | 0.86 | 24.8 |
| Ctrl | 300 | 15.6 | 13.4 | 1.84×108 | 702.2 | 5.77 | 0.92 | 27.8 |
Best fit parameter estimates for three parameters (f, g and kOFF) were obtained using the Simplex method (see Methods section); values for kON were from measurements reported by Pinto et al. (36). These parameter sets were used to illustrate relations between kTR and steady-state, isometric force in Fig. 2F (solid lines in Fig. 2F); also see the figure for information on the influence of small deviations (+/− 2%) from the best fit parameter estimates on predicted kTR-force relations (dashed lines in Fig. 2F).
3.4. Ca2+ sensitization through the N-domain of cTnC may influence the number of crossbridges
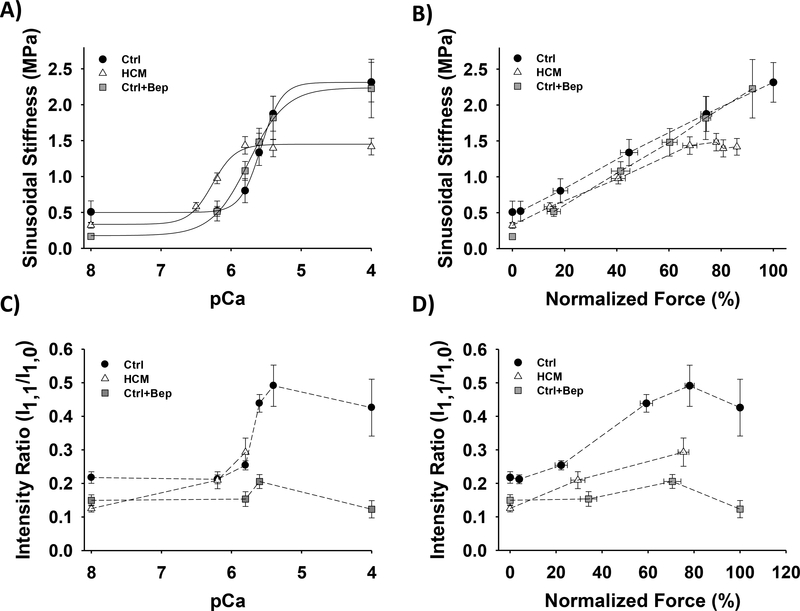
Considering the importance of actin-myosin interactions in the contraction-relaxation cycle of the heart, we sought to examine potential alterations in thin-thick filament interactions as a result of Ca2+ sensitization through cTnC. To accomplish this, we measured sinusoidal stiffness (SS) during steady-state, isometric contraction at all levels of Ca2+ activation (Fig. 3A and Table 1). Measurements of SS provide insight into the overall number of cross-bridges, although this is modulated by end compliance and compliances within the preparation (43–45) and can be further modulated by cross-bridge kinetics (21) and fundamental properties of the preparation (46). HCM CMPs and Ctrl+Bep CMPs exhibited leftward shifts of pCa-SS relations compared to Ctrl (HCM vs. Ctrl ΔSSpCa50 = +0.69 and Ctrl+Bep vs. Ctrl ΔSSpCa50 = +0.22 pCa units). The exhibited shifts in SSpCa50 were correlated to those observed from the corresponding pCa-force relations (Figs. 2 and 3A, and Table 1). Despite the differences in Ca2+ sensitivity, the relationships between SS and normalized force were generally similar for all three preparations across most levels of Ca2+-activation (Fig. 3B); the overall linearity of the relationships in Fig. 3B for most levels of Ca2+-activation indicates that the primary mechanism by which Ca2+ activates force is through recruitment of cross-bridges. At the highest levels of Ca2+ activation, however, maximum steady-state SS of HCM CMPs was significantly lower than Ctrl (Fig. 3A, 3B and Table 1), consistent with data at the highest frequency utilized in our prior study (21). In contrast, there was no significant difference in maximum steady-state SS between Ctrl+Bep vs.
Figure 3. Sinusoidal stiffness and small-angle X-ray diffraction analysis in CMPs at SL 2.1 μm.
A) Ca2+-dependence of steady-state sinusoidal stiffness. B) Normalized force vs. steady-state sinusoidal stiffness. The force values were normalized to the maximum steady-state isometric force in Ctrl CMPs. Dashed lines were drawn by eye. C) Equatorial diffraction intensity ratios (I1,1/I1,0) as a function of pCa. The equatorial diffraction intensities were obtained by small-angle X-ray diffraction. D) Normalized force vs. equatorial intensity ratios. The force values were normalized to the maximum steady-state isometric force in Ctrl CMP. Data are shown as mean ± S.E. n = 19 Ctrl, 23 HCM and 10 Ctrl for sinusoidal stiffness data. The number of CMPs and X-ray shots for the small-angle X-ray diffraction data is presented in supplementary Table 1.
Ctrl (Fig. 3A and Table 1). Assuming that the number of cross-bridges ∝ f/(f + g) (42), the measured differences in maximum SS for the three preparations (Fig. 3A, B) are in the direction predicted from values of f and g obtained from fitting force-kTR data (Fig. 2F) to the 3-state model (Table 2). The observation that Ctrl and Ca2+ sensitized HCM (and Ctrl+Bep) CMPs develop statistically indistinguishable levels of force at saturating Ca2+ (Fig. 1C and Table 1) with lower maximum steady-state isometric SS for HCM (Fig. 3A, 3B and Table 1) suggests not only that the number of cross-bridges may be lower in HCM CMPs, but also that the force per cross-bridge could be higher in Ca2+-sensitized HCM muscle.
To complement our findings from SS measurements, we also used small-angle X-ray diffraction to collect equatorial intensity ratios (I1,1/I1,0) during graded Ca2+ activation. Representative diffraction images and intensity profiles are shown in Supplemental Fig. 1. The I1,1/I1,0 intensity ratio provides information about mass transfer from thick to thin filaments, which can be used as an index for the number of cross-bridges formed during muscle activation. Consistent with Ctrl SS measurements (Figs. 3A, 3B), I1,1/I1,0 generally increased with activation level in Ctrl CMPs (Figs. 3C, 3D). Comparing I1,1/I1,0 in both HCM and Ctrl+Bep CMPs with Ctrl at similar levels of Ca2+-activated steady-state force, less mass transfer from thick to thin filaments was observed in the Ca2+ sensitized CMPs (Fig. 3C, 3D). Taken together, the X-ray and SS measurements both suggest that the number of cross-bridges and force per cross-bridge could be altered in HCM, and possibly Ctrl+Bep, Ca2+ sensitized CMPs. These findings therefore demonstrate that a single N-terminal domain point mutation in cTnC perturbs acto-myosin interactions in a Ca2+dependent manner. Furthermore, findings from the Ctrl+Bep CMPs support the importance of cTnC-mediated Ca2+ sensitization in perturbing thick-thin filament interactions.
3.5. Myofilament lattice spacing increases during Ca2+-activated isometric force production
Considering that we observed Ca2+-dependent effects on cross-bridge kinetics in both HCM and Bep-treated muscle, we hypothesized that alterations in myofilament lattice spacing may help explain some of these effects. To address this question, we used small angle X-ray diffraction to simultaneously measure isometric force and lattice spacing as a function of Ca2+ concentration. Representative diffraction images in various Ca2+ solutions are provided in Fig. 4A. It should be noted that there is an inverse relationship between the d1,0 equatorial distance in the diffraction patterns and myofilament lattice spacing. Starting with the lattice compressed to physiological diastolic dimensions (18, 37, 47) by 3% dextran in relaxing conditions (supplemental Fig. 2), lattice spacing of Ctrl CMPs increased in parallel with increased Ca2+-activated, isometric force (Fig. 4B). A similar force-lattice spacing trend was observed at most levels of Ca2+ activation for HCM (Fig. 4B) but not Ctrl+Bep CMPs (Fig. 4C), possibly due to a larger lattice spacing for Ctrl+Bep CMPs in relaxing conditions (supplemental Fig. 2). Beginning at equivalent lattice spacing dimensions compared to Ctrl and reaching comparable maximum lattice spacing upon Ca2+ activation, Ctrl and HCM CMPs demonstrated similar ranges of lattice spacing (Ctrl Δ = + 2.22 nm and HCM Δ = + 2.35 nm) (Fig. 4D). Bep-sensitized CMPs exhibited an attenuated, activation-associated increase in lattice spacing (Ctrl+Bep Δ = + 0.30 nm) (Fig. 4E and supplemental Fig. 2). Surprisingly, at the highest levels of Ca2+ activation, lattice spacing of both HCM and Ctrl+Bep CMPs collapsed while maintaining maximum isometric force (Fig. 4B-E). The physiological significance of this lattice collapse in maximally activated, Ca2+ sensitized preparations, is questionable because normal cardiomyocyte contraction occurs at submaximal Ca2+ concentrations in the beating heart. Strikingly, significantly larger lattice spacing was observed for both HCM and Ctrl+Bep CMPs compared to Ctrl at submaximal activation (pCa 5.8 and 5.6) due at least in part to increased Ca2+ sensitivity (Fig. 4D and 4E).
Figure 4. Simultaneous measurements of myofilament lattice spacing and steady-state isometric force at SL 2.1 μm.
A) Representative images of CMPs small-angle X-ray diffraction in various pCa solutions. The top horizontal line shows a deviation of the d1,0 as contraction develops and the bottom red line intersects each d1,0 intensity at the center to demonstrate the decrease in lattice spacing that is being reported. Note the reciprocal relationship between d1,0 equatorial distances and lattice spacing. B and C) Relative force vs. lattice spacing. The force values were normalized to the maximum force of each individual CMPs. D and E) Ca2+-dependence of myofilament lattice spacing. Dashed lines were drawn by eye. *p<0.05 vs. Ctrl, statistical significance was determined by Mann-Whitney U test. A minimum of 20 X-ray shots were obtained at each pCa, for exact number of CMPs and shots and refer to supplementary Table 2 Data are shown as mean ± S.E.
4. DISCUSSION
The major observations from this study using osmotically compressed, permeabilized muscle preparations from mouse heart are as follows. Increased Ca2+-sensitivity via the N-domain of cTnC (HCM or Bep) was associated with: (i) increased kTR at all Ca2+-activation levels, which was most readily explained by an increased rate of cross-bridge detachment (g); and (ii) reduced mass transfer from thick to thin filaments (I1,1/I1,0 ratio) upon Ca2+ activation, and also stiffnessto-force ratio. Myofilament lattice spacing increased with Ca2+-activation for both WT and HCM preparations. These results have implications for the pathophysiology associated with some HCM mutations and the physiological interrelationship among thin filament Ca2+activation, mechanical kinetics, and myofilament lattice spacing in cardiac muscle.
4.1. Interrelationship between thin filament Ca2+-sensitivity and mechanical kinetics
Consistent with our prior studies conducted in the absence of osmotic compression (20, 21, 48), the HCM mutation enhanced myofilament Ca2+ sensitivity of steady-state, isometric force in the presence of 3% dextran T-500 (Fig. 1, Table 1). The HCM mutation was also associated with increased Ca2+ sensitivity of kTR (Fig. 2E) and stiffness (Fig. 3A). Interestingly, kTR was faster at all levels of thin filament Ca2+-activation (Fig. 2E), and the increases were markedly beyond what could be attributed to the leftward shift of the force-kTR relationship (Fig. 2F). At saturating Ca2+, this HCM mutation was previously shown to be associated with increased myofibrillar ATPase activity (28, 49) and higher frequencies of oscillatory work during small amplitude, sinusoidal length perturbations (21). Both prior observations at maximal activation, together with the kTR measurements at maximal and submaximal levels of thin filament Ca2+-activation, are indicative of faster cross-bridge cycling. Faster cross-bridge kinetics may be consistent with expectations for an HCM mutant but, at first blush, are surprising for a point mutation on the thin filament. When the force-kTR and pCa50 data were simultaneously fit (as described in Materials and Methods) to the 3-state model (Fig. 5), we found that not only is kOFF reduced (7-fold) for HCM compared to WT—as required to explain the leftward shift of the force-pCa relationship— but the cross-bridge detachment rate (g) is increased by ~ 50% with little change in attachment rate (f) (Table 2). This inference from the Ca2+ activation-dependence of kTR is quantitatively consistent with our previous report that the HCM mutation increases cross-bridge detachment rate, as determined from sinusoidal perturbation analysis at maximal Ca2+ activation (21).
Figure 5. Schematic of the three-state model of striated muscle contraction.
Faster kTR at all levels of thin filament Ca2+-activation was similarly observed with an independent approach for enhancing myofilament Ca2+ sensitivity: treatment of control cardiac muscle with the small molecule Ca2+-sensitizer bepridil (Fig. 2). Mechanistically, Bep and HCM-associated A8V mutation augment myofilament Ca2+ sensitivity by increasing the Ca2+ binding affinity to Ca2+-binding regulatory site II in cTnC (14, 36, 50). As with the HCM mutation, fitting the data to the 3-state model (Fig. 5) indicated that not only is kOFF reduced by almost 2-fold for Bep compared to WT—as required to explain the leftward shift of the forcepCa relationship—but the cross-bridge detachment rate (g) is increased by ~ 60% (Table 2); with Bep, however, cross-bridge attachment rate (f) is increased ~ 30%. Although lower Bep concentrations were shown to have no effect on maximum kTR in skeletal muscle preparations (51, 52) and human atrial myofibrils (53), it is not clear which of the numerous experimental differences beyond Bep concentration—including species, muscle type, absence of osmotic compression, longer relaxed SL, lower temperature and slower maximal kTR—might contribute to this difference compared with our finding of a marked increase in both submaximal and maximal kTR upon Bep treatment of mouse ventricular tissue (Fig 2, Table 1). Intriguingly, a different Ca2+ sensitizer, calmidazolium, has also been shown to increase kTR at submaximal activation in skeletal muscle (9). Like Bep, calmidazolium functions through interactions with the regulatory N-domain of TnC (54).
The above findings suggest that enhanced myofilament Ca2+ sensitivity through cTnC may accelerate cross-bridge kinetics, at least under certain circumstances. In support of this possibility, engineered mutations in cTnC (F27W or the double mutant V44Q,F27W) that increase Ca2+-binding affinity also increase kTR at maximal Ca2+ activation of cardiac muscle (11); while the double mutant also increases kTR at submaximal Ca2+ activation, the increases at all levels of Ca2+ activation were smaller than reported here (Fig. 2). However, in contrast to our findings (Fig. 2), these engineered mutations in cTnC did not alter the force-kTR relation. Moreover, several mutations in the C-termini of cTnI or cTnT increase both the Ca2+ sensitivity of filament sliding in motility assays and also the maximum speed (55, 56), although kTR has not been measured with these mutations. In addition, although faster kTR was reported with a Ca2+sensitizing mutation in cTnT at submaximal Ca2+ activation (57), the increase was less than observed here with a cTnC mutation (Fig. 2) and was restricted to low levels of thin filament Ca2+ activation. These observations suggest there may be a mechanistic basis to a relationship between Ca2+-sensitization and faster cross-bridge cycling that involves the N-domain of cTnC. Bepridil binds at the N-domain of TnC (14, 39, 40), which is also the location of the HCMassociated A8V mutation used in our present study, the location of engineered mutations by Norman et al. (11), and also the domain to which the C-terminus of cTnI binds in the presence of Ca2+ (40, 58). However, a study by Kreutziger et al. (59), using an engineered Ca2+-sensitizing mutation in cTnC, L48Q which is also in the regulatory N-domain, showed at most a small but not significant increase in maximal kTR in mouse ventricular myofibrils, and increases in kTR only at a low level of Ca2+ activation in rat ventricular trabeculae. While the differences in Kreutziger et al.’s (59) study may be attributed to experimental variables—including extraction/reconstitution of cTnC, absence of osmotic compression, longer relaxed SL, lower temperature and slower maximal kTR—we sought a more specific structural feature that might reconcile our kTR results (Fig. 2) with those of Kreutziger et al. (59).
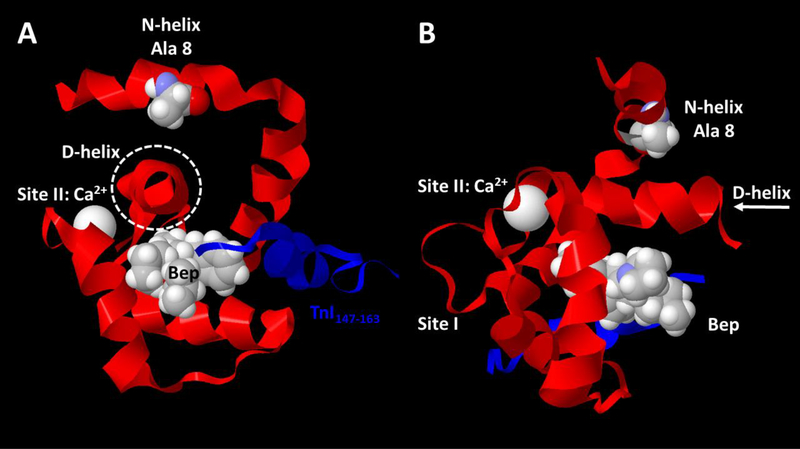
Examination of Wang et al.’s (40) NMR solution structure of cTnC’s N-domain complexed with Ca2+, cTnI switch peptide, and Bep (PDB 1LXF) reveals that, unlike Leu 48, Ala 8 and Bep both interact with the D-helix, albeit from opposite sides (Fig. 6). The sidechain of Ala 8 interacts with the sidechain of Val 82, and that interaction would be expected to strengthen with Ala → Val substitution in the HCM mutant, as suggested more generally for stabilization of protein tertiary structure by the branched sidechains in clusters of Ile-Leu-Val residues (60). Bepridil interacts with several residues in the D-helix as well as other parts of cTnC (39, 40). Thus both the HCM mutation and Bep would be expected to stabilize the D-helix relative to the rest of the N-domain. Ca2+ affinity at cTnC’s regulatory site could modulated via the D-helix because the D-helix connects directly to the C-terminal side of site II’s EF-hand. In addition, we note that the D-helix also connects to cTnC’s C-domain as part of the DE-helix linker. While the N- and C-domains of TnC are often considered to be independently mobile in the absence of TnI (and TnT) (61, 62), there appears to be some level of structural coordination between the N- and C-domains associated with divalent cation binding any of the functional EF-hands (63) and it is clear that there is functionally significant communication between the N- and C- domains of TnC (64, 65). The current work adds to the likelihood that coupling of the D-helix to site II, and by extension via entire DE-linker to the C-domain, may be important not only for determining Ca2+binding affinity of site II but also regulatory unit dynamics and contractile kinetics. Altogether, these findings raise the possibility of portions of the regulatory domain dynamics of cTnC in controlling cross-bridge detachment kinetics in heart muscle.
Figure 6. Structural evaluation suggests a common feature for Ca2+-sensitizers bepridil (Bep) and HCM mutation in the N-helix of cTnC, A8V: both interact with the D-helix of cTnC.
NMR structure of the regulatory N-domain of human cTnC complexed with Ca2+, Ca2+sensitizing molecule Bep, and the switch peptide from human cTnI was adapted from PDB 1LXF model 1 (40) using MatLab molviewer. A) View of cTnC N-domain looking down the Dhelix (within dashed circle) from the direction of cTnC’s C-domain (C-domain not shown). B) Side view of cTnC N-domain; arrow (right) indicates direction of view in panel A. In both panels, peptide backbones are shown as ribbons, with cTnC N-domain ribbon in red and cTnI switch peptide ribbon in blue; cTnC residue Ala 8 is emphasized with space-fill atoms. Ca2+ (bound at site II) and Bep are shown as space-fill atoms. Note that cTnC Ala 8 and Bep interact with opposite sides of cTnC’s D-helix which, along with the E-helix (not shown), connects the N- and C- domains.
The 3-state model (Fig. 5) for cardiac muscle and the related 4-state model for skeletal muscle (7, 32), while highly simplified, are capable of capturing the major features of force-kTR relations under a wide variety of conditions. A major advantage of this model’s simplicity is that the small number of free parameters (three in this study: f, g and kOFF) allows unambiguous identification of best-fit parameter estimates, as illustrated in Fig. 2F, and summarized in Table 2. We were able to reduce the number of free parameters for the 3-state model from four to three by constraining kON to that obtained experimentally (36). The 3-state model incorporates thin filament regulatory unit dynamics that were missing from the 2-state model used by Brenner in an initial investigation of the Ca2+-dependence of kTR (10). The highly simplified nature of the 3-state model, while advantageous in many respects, means that there are many factors that are not included. Experimental measurements and more detailed models suggest that additional parameters—particularly those that may vary with Ca2+-activation level such as cooperative interactions among myofilament proteins, activation of the thick filament by force, myofilament compliance, and lattice spacing—could influence the relationship between Ca2+, thin filament regulatory unit dynamics, kTR and force (44, 45, 66–70). We now consider myofilament filament lattice spacing, which we were able to measure during steady-state Ca2+-activations of cardiac muscle preparations (Fig. 4).
4.2. Can activation-dependent changes in myofilament lattice spacing influence mechanical kinetics?
Remarkably, the changes in myofilament lattice spacing with graded Ca2+ of Ctrl CMPs presented in this current report (Fig. 4D) are quantitatively comparable with those previously observed in living mouse heart (18). Thus, as previously noted, the lattice spacing measurements reported in our study are physiologically relevant, despite the use of permeabilized muscle. In addition to quantitatively similar lattice dimensions, the trend towards an increase in lattice spacing during contraction is supported by several studies (67, 71–73).
Myofilament lattice spacing is determined by the balance of opposing forces that favor lattice expansion against lattice compression. These forces include several components (15); because the preparations were permeabilized with detergent, the compressive force associated with a living cell’s membrane is not considered here.
First to consider is the compressive, osmotic pressure associated with dextran T-500. This high MW polymer is too large to enter the myofilament lattice, and a constant amount (3% w:v) was added to all bathing solutions to compress the filament lattice—which as noted above swells in the absence of the cell membrane and osmotic agents—to physiological dimensions. In the absence of osmotic compression, the lattice spacing of permeabilized muscle preparations— swollen in relaxed conditions—decreases upon Ca2+ activation (74). Compression of the filament lattice by osmotic agents, however, allows for activation of permeabilized muscle with no further reduction in lattice spacing (75). It is worth considering that, in addition to increasing osmolarity and thereby creating an osmotic pressure gradient between the bulk solution surrounding the permeabilized muscle preparation and the solution within that surrounds the myofilaments, high MW solutes (and solutes in general) alter properties of the bulk solution such as the activity of water (aW), viscosity (η), dielectric constant (ε), etc. In addition, macromolecule solutes can act as “crowders” that mimic the crowded intracellular environment (76). While these solution properties affect actomyosin affinity and kinetics when the solutes directly surround the proteins (77, 78), any changes associated with lattice compression by high MW solutes exclusively in the bulk solution are not quantitatively consistent with effects obtained when the solutes are in the solution surrounding actin and myosin. For example, adding dextrans or low MW solutes to the solution surrounding actin and myosin markedly increases solution viscosity and slows actomyosin kinetics in a manner that is inversely proportional to the increase in viscosity (33). Osmotic compression by dextran T-500 in the bulk solution surrounding permeabilized muscle preparations, however, either increases kTR (68) or causes at most a slight inhibition of kTR (41). Structural effects within the myofilament lattice of permeabilized muscle when dextran T-500 is added to the bulk solution, such as the closer association of myosin heads with the thick filament (31, 38), are most likely indirect effects, and can be mimicked by lowering the ionic strength and/or lowering the temperature (38, 79), or stretching to longer SL (30).
There are several additional determinants of lattice spacing to consider beyond osmotic compression. The second factor is the radial component of mechanical force due to structural proteins (e.g., titin and z-disc proteins) that are responsible for maintaining the structure of the sarcomere. For example, when the sarcomere is stretched along the long axis of a relaxed myocyte, these proteins impose a compressive, radial force such that the lattice volume remains almost constant (15, 37, 47). If the relationship between diastolic lattice spacing and SL (37, 47) applies during steady-state Ca2+-activation, then sarcomere shortening during contraction (due to end compliance) might be quantitatively estimated from changes in lattice spacing. However, additional factors that contribute to radial force may alter this relationship in actively contracting muscle. Third is the electrostatic force associated with surface charge of the myofilaments. This is an expansive force as the thick and thin filaments both have net negative surface charge. Myofilament surface charge can be modified—directly through alteration of net charge, or indirectly by changes in protein structure and the location of acidic and basic sidechains—by the binding of ions (80) and some covalent post-translational modifications (e.g., phosphorylation). Fourth, and finally, is the radial component of cross-bridge force. The main vector of crossbridge force is, of course, along the long axis of the myocyte (17, 75). The radial force of a single, attached cross-bridge varies with lattice spacing such that it can be compressive or expansive, and thus there is an equilibrium value (15, 17, 75, 81). Furthermore, the step size of cycling cross-bridges is predicted to vary in a biphasic manner with lattice spacing (i.e., there is an optimal lattice spacing for cross-bridge function) (17). The total radial force due to all crossbridges in an activated myocyte obviously depends on the number of cross-bridges. Taking into consideration all of these possible factors, it is apparent that the major change in net radial force upon Ca2+-activation—leading to the observed lattice expansion—would likely be due to recruited cross-bridges. It is not possible, however, to rule out contributions from components other than the osmotic compressive force due to Dextran T-500, which was constant throughout this study.
Myofilament lattice spacing may modulate cross-bridge kinetics in an invertebrate striated muscle (16), and the same could be true for vertebrate muscles including cardiac muscle. Indeed, measurements of lattice spacing in the heart in situ indicate dynamic changes during the contraction-relaxation cycle and suggest that the myofilament lattice does not maintain a constant volume during force generation (18, 47, 71). Studies in intact skeletal muscle fibers also show changes in lattice volume during contraction and rapid length changes (82). Therefore, it is not surprising that we observed a significant expansion of the myofilament lattice at submaximal Ca2+ activation in Ctrl and HCM CMPs, with increased Ca2+ sensitivity of HCM compared to Ctrl (Fig. 4D). This increased Ca2+ sensitivity of lattice spacing is comparable to that observed for force, kTR and SS (Figs. 1, 2E and 3A, and Table 1). It is tempting to speculate that lattice expansion (Fig. 4) may play a role in the observed activation-dependent increase of kTR (Fig. 2). In support of this idea, it was previously shown that osmotic compression of the myofilament lattice in skinned rat cardiac trabeculae by addition of 4% dextran to the bulk solution dampened the kTR-force relation compared to untreated muscle (41). In the absence of SL control, end compliance allows for sarcomere shortening, the extent to which depends on force and thus the level of Ca2+ activation, and thus at least some of the increase in kTR with Ca2+ activation (Fig. 2) could be attributed to sarcomere shortening. As discussed in more detail above, diastolic lattice spacing expands at shorter SL; while this would also be expected during active contraction, it is not clear that the relationship would be quantitatively the same in both systolic and diastolic conditions. Despite this uncertainty, these studies add support to the possibility that lattice spacing may play a role in modulation of contractile kinetics in striated muscle.
How might myofilament lattice spacing influence kTR if in fact it does so? At high levels of activation, kTR is primarily determined by cross-bridge cycling rates: kTR ∝ f + g (42). At low levels of Ca2+ activation, kTR is modulated by the dynamics of thin filament regulatory units, but f and/or g could also vary with activation level in a manner that is not inherent in the 3-state model. Strikingly, the effects of Ctrl+Bep and HCM CMPs on muscle mechanics were generally similar (Figs. 1, 2 and 3), with the exceptions of the force-lattice spacing relation (Fig. 4B, D) and rate of cross-bridge attachment (Table 2). Considering that we observed a larger lattice spacing at low Ca2+ levels in Ctrl+Bep CMPs which was not seen in HCM (Fig. 4), we therefore speculate that expansion of the lattice spacing may influence f rather than g. Bep binding to cTnC could have structural effects beyond those associated the N-domain (Fig. 6), as suggested in the structure of Li et al. (39), and it is possible that also Bep binds elsewhere in the sarcomere. However, the overall similarity between the effects of Bep and the HCM mutation on cardiac mechanics suggests that the primary effects of Bep occur through cTnC. The idea of lattice spacing controlling muscle kinetics is not entirely novel. Based on experimental approaches and computational modeling, it has been previously suggested that lattice spacing can influence either rate of cross-bridge attachment and/or detachment in cardiac and skeletal muscle (17, 38).
We cannot fully explain the precipitous collapse of lattice spacing observed at high levels of muscle activation in both Ca2+-sensitized CMPs, HCM and Ctrl+Bep (Fig. 4). Because this phenomenon was not observed for Ctrl CMPs (Fig. 4), whatever is responsible appears to be specifically attributable to thin filament Ca2+-sensitization. The myofilaments are negatively charged under physiological conditions, i.e. pH ~ 7, ionic strength 150–200 mM (15). At maximal activation (~ pCa 4), the thin filaments of the Ca2+-sensitized CMPs would theoretically be saturated with Ca2+ ions to a greater extent compared to Ctrl (i.e., non-sensitized) CMPs. In principle, this could reduce the net surface charge of the negatively charged thin filaments, thus reducing the expansive electrostatic force and ultimately leading to collapse of lattice spacing. Regardless of the mechanism, we argue against the physiological relevance of this observation, considering that cardiomyocytes do not operate in such ranges of Ca2+ concentrations in vivo (83).
4.3. Thin-to-thick filament force transmission and regulation
An interesting observation in this present study showed that the HCM-associated CMPs were able to achieve the same steady-state isometric force as control preparations with fewer cross-bridges, suggesting that individual myosin heads may generate greater force (Fig. 3B). This was also evident in Bep-treated cardiac muscle (Fig. 3), thus highlighting the contribution of TnCmediated Ca2+ sensitization in these effects. X-ray diffraction measurements of the equatorial intensity ratios, which are used as an index of degree of mass shift from thick to thin filaments, presumably due to recruitment of cross-bridges, also showed that HCM and Bep-treated muscle was able to achieve the same given force with a reduced number of cross-bridges (Fig. 3D). Altogether, these results suggest that myofilament Ca2+ sensitivity—as determined by stabilization of the D-helix within the regulatory N-domain of cTnC—may be an important determinant of not only cross-bridge kinetics, but also the number of cross-bridges recruited during contraction of the myocardium.
4.4. Implications for HCM pathophysiology
Missense mutations in cardiac thin filament regulatory proteins (e.g. troponin and tropomyosin) are known to cause heritable HCM (84–86). Despite the high prevalence and severe morbidity/mortality associated with HCM, the molecular mechanisms involved in HCM pathogenesis at the level of the sarcomere remain incompletely understood. In this study, we utilized our previously characterized knock-in mouse model of HCM, which harbors a single point mutation (Ala8Val) in cTnC; importantly, this mutation is clinically relevant, as it is associated with inherited forms of cardiomyopathies in human patients (48, 87) and a mouse model (20).
Intracellular Ca2+ concentrations in cardiac myocytes range from ~ 100 nM in diastole to ~ 1 μM in systole, which is approximately pCa 7 to pCa 5.8 (83). Remarkably, we found that lattice spacing, force, stiffness, and kTR were all increased within these physiologically relevant Ca2+ concentrations. Most if not all of the increases at a given Ca2+ concentration for force and stiffness are due to leftward shift along the pCa axis (increased Ca2+ sensitivity), but kTR is enhanced beyond what can be attributed to the leftward shift alone. Although the peak of the Ca2+ transient is reduced in HCM cardiomyocytes (20), this may occur possibly due to an increase in the amount of Ca2+ bound to the Ca2+ sensitized thin filaments; thus, peak force may still be enhanced in HCM (88). Moreover, we have previously shown that the cTnC-A8V HCM mutation exhibits elevated myofibrillar ATPase activity at physiologically relevant ranges of Ca2+ concentrations, with significantly increased activity at pCa 5.8 (49), consistent with the enhanced contractile kinetics observed in this present study. Because increased Ca2+ sensitivity is a hallmark of familial hypertrophic cardiomyopathies—not only for the mutation in this study, but in general (84)—any associated increase in cross-bridge cycling through more rapid detachment, and the accompanying increase in ATP utilization, could thus contribute to the hypercontractile phenotype. We conclude that increases in force, stiffness and kTR, and possibly also lattice spacing, would collectively contribute to the augmented contraction of the heart that is generally associated with HCM.
4.5. Limitations of this study
In the absence of real-time SL control, internal sarcomere shortening can occur (89). Given the inherent difficulties in monitoring SL during cardiac muscle activation, this approach was not utilized in our experiments. Shortening of SL below 2.1 μm (the initial value at pCa 8) due to end compliances could be anticipated to result in: reduced steady-state, Ca2+-activated, isometric force and stiffness; reduced Ca2+ sensitivity (and pCa50); faster kTR; and increased lattice spacing. As discussed in greater detail above (Section 4.2), a subset of our results might be explained, in part, by activation-dependent reductions in SL, although it is not possible to quantitatively predict for all of these parameters and at each level of Ca2+ activation the extent to which this might possibly be the case. While lattice dimensions are maintained during contraction if SL is held constant in intact cardiac muscle (90), it is important to recall, however, that SL changes dynamically during ventricular filling and ejection phases of the cardiac cycle (19). Therefore, if internal sarcomere shortening occurred in our preparations, our results are nonetheless physiologically relevant. What is more, despite monitoring and maintaining a constant SL using an iterative feedback control system, Moss and colleagues were still able to show a Ca2+dependent increase in kTR (12). Furthermore, we have previously reported that controlling SL has minimal effects on the Ca2+ dependence of kTR in skeletal muscle (8). Interestingly, it has also been reported that activating Ca2+, but not SL, influences cross-bridge kinetics (91). It is possible that an experimental approach to analyze cross-bridge kinetics (kTR vs. sinusoidal length perturbations) may yield conflicting results. In the aforementioned study, sinusoidal length perturbations were used an index of cross-bridge kinetics (91) rather than the kTR. However, we recently reported that the cTnC A8V HCM mouse model exhibits faster cross-bridge cycling kinetics despite using a different method of analysis (i.e. sinusoidal length perturbation) (21).
Detergent-permeabilized CMPs were used in this study rather than intact muscle, which may not accurately reflect the in vivo environment. However, despite skinning of muscle preparations, our lattice dimensions mirror those observed in vivo (18) owing to the use of 3% dextran in all of our experiments. Another limitation of this study is the use of a murine model to investigate the effects of an HCM-associated mutation, since it is well-known that there are differences in contractile kinetics and Ca2+ handling in mice compared to humans (92). However, similar changes in cross-bridge kinetics have been observed using explanted HCM human heart tissue (93, 94).
4.6. Conclusions
In this study, we provide evidence showing that sensitizing the myofilament to Ca2+ through stabilizing the D-helix within the regulatory N-domain of cTnC influences both the number and rate of cycling of cross-bridges in cardiac muscle. We also showed that isometric force generation was accompanied by an increase in myofilament lattice spacing. We favor a model in which cross-bridge kinetics and force production are modulated by expansion of the myofilament lattice during cardiac muscle contraction. Taken together, these findings provide novel insights into the molecular mechanisms underlying the contractile regulation of vertebrate myocardium and how these processes can be perturbed in the setting of HCM.
Supplementary Material
ACKNOWLEDGMENTS
Authors thank Rumit Mistry and Brittany Griffin for their technical assistance, and Dr. Campion K. P. Loong for MatLab code to efficiently calculate kTR time courses using the 3-state model (Fig. 5). This work was supported by the National Heart, Lung and Blood Institute HL128683 to JRP. This research used resources of the Advanced Photon Source, a U.S. Department of Energy (DOE) Office of Science User Facility operated for the DOE Office of Science by Argonne National Laboratory under Contract No. DE-AC02–06CH11357. "This project was supported by grant 9 P41 GM103622 from the National Institute of General Medical Sciences of the National Institutes of Health." The content is solely the responsibility of the authors and does not necessarily reflect the official views of the National Institute of General Medical Sciences or the National Institutes of Health.
Footnotes
DISCLOSURES
None.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Bagshaw CR (1993) Muscle contraction (Chapman & Hall, London; New York: ) 2nd Ed pp x–155 p. [Google Scholar]
- 2.Takeda S, Yamashita A, Maeda K, & Maeda Y (2003) Structure of the core domain of human cardiac troponin in the Ca(2+)-saturated form. Nature 424(6944):35–41. [DOI] [PubMed] [Google Scholar]
- 3.Vannier C, Chevassus H, & Vassort G (1996) Ca-dependence of isometric force kinetics in single skinned ventricular cardiomyocytes from rats. Cardiovascular research 32(3):580–586. [PubMed] [Google Scholar]
- 4.Saeki Y, et al. (2004) Role of Ca2+ in determining the rate of tension development and relaxation in rat skinned myocardium. Journal of molecular and cellular cardiology 36(3):371–380. [DOI] [PubMed] [Google Scholar]
- 5.Baker AJ, Figueredo VM, Keung EC, & Camacho SA (1998) Ca2+ regulates the kinetics of tension development in intact cardiac muscle. The American journal of physiology 275(3 Pt 2):H744–750. [DOI] [PubMed] [Google Scholar]
- 6.Araujo A & Walker JW (1994) Kinetics of tension development in skinned cardiac myocytes measured by photorelease of Ca2+. The American journal of physiology 267(5 Pt 2):H1643–1653. [DOI] [PubMed] [Google Scholar]
- 7.Landesberg A & Sideman S (1994) Coupling calcium binding to troponin C and crossbridge cycling in skinned cardiac cells. The American journal of physiology 266(3 Pt 2):H1260–1271. [DOI] [PubMed] [Google Scholar]
- 8.Chase PB, Martyn DA, & Hannon JD (1994) Isometric force redevelopment of skinned muscle fibers from rabbit activated with and without Ca2+. Biophysical journal 67(5):1994–2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Regnier M, Martyn DA, & Chase PB (1996) Calmidazolium alters Ca2+ regulation of tension redevelopment rate in skinned skeletal muscle. Biophysical journal 71(5):27862794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brenner B (1988) Effect of Ca2+ on cross-bridge turnover kinetics in skinned single rabbit psoas fibers: implications for regulation of muscle contraction. Proceedings of the National Academy of Sciences of the United States of America 85(9):3265–3269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Norman C, Rall JA, Tikunova SB, & Davis JP (2007) Modulation of the rate of cardiac muscle contraction by troponin C constructs with various calcium binding affinities. American journal of physiology. Heart and circulatory physiology 293(4):H2580–2587. [DOI] [PubMed] [Google Scholar]
- 12.Wolff MR, McDonald KS, & Moss RL (1995) Rate of tension development in cardiac muscle varies with level of activator calcium. Circulation research 76(1):154–160. [DOI] [PubMed] [Google Scholar]
- 13.Palmer S & Kentish JC (1998) Roles of Ca2+ and crossbridge kinetics in determining the maximum rates of Ca2+ activation and relaxation in rat and guinea pig skinned trabeculae. Circulation research 83(2):179–186. [DOI] [PubMed] [Google Scholar]
- 14.Solaro RJ, Bousquet P, & Johnson JD (1986) Stimulation of cardiac myofilament force, ATPase activity and troponin C Ca++ binding by bepridil. J Pharmacol Exp Ther 238(2):502–507. [PubMed] [Google Scholar]
- 15.Millman BM (1998) The filament lattice of striated muscle. Physiol Rev 78(2):359–391. [DOI] [PubMed] [Google Scholar]
- 16.Tanner BC, et al. (2012) Thick-to-thin filament surface distance modulates cross-bridge kinetics in Drosophila flight muscle. Biophysical journal 103(6):1275–1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Williams CD, Regnier M, & Daniel TL (2010) Axial and radial forces of cross-bridges depend on lattice spacing. PLoS computational biology 6(12):e1001018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Toh R, et al. (2006) An X-Ray diffraction study on mouse cardiac cross-bridge function in vivo: effects of adrenergic {beta}-stimulation. Biophysical journal 90(5):1723–1728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kobirumaki-Shimozawa F, et al. (2016) Nano-imaging of the beating mouse heart in vivo: Importance of sarcomere dynamics, as opposed to sarcomere length per se, in the regulation of cardiac function. J Gen Physiol 147(1):53–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Martins AS, et al. (2015) In Vivo Analysis of Troponin C Knock-In (A8V) Mice: Evidence that TNNC1 Is a Hypertrophic Cardiomyopathy Susceptibility Gene. Circulation. Cardiovascular genetics 8(5):653–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kawai M, et al. (2017) Myosin Rod Hypophosphorylation and CB Kinetics in Papillary Muscles from a TnC-A8V KI Mouse Model. Biophysical journal 112(8):1726–1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dossat AM, et al. (2017) Pathogenesis of depression- and anxiety-like behavior in an animal model of hypertrophic cardiomyopathy. FASEB J 31(6):2492–2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dweck D, Reyes-Alfonso A, Jr., &Potter JD (2005) Expanding the range of free calcium regulation in biological solutions. Anal Biochem 347(2):303–315. [DOI] [PubMed] [Google Scholar]
- 24.Baudenbacher F, et al. (2008) Myofilament Ca2+ sensitization causes susceptibility to cardiac arrhythmia in mice. The Journal of clinical investigation 118(12):3893–3903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chase PB & Kushmerick MJ (1988) Effects of pH on contraction of rabbit fast and slow skeletal muscle fibers. Biophysical journal 53(6):935–946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Regnier M, Rivera AJ, Chase PB, Smillie LB, & Sorenson MM (1999) Regulation of skeletal muscle tension redevelopment by troponin C constructs with different Ca2+ affinities. Biophysical journal 76(5):2664–2672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chase PB, Martyn DA, Kushmerick MJ, & Gordon AM (1993) Effects of inorganic phosphate analogues on stiffness and unloaded shortening of skinned muscle fibres from rabbit. The Journal of physiology 460:231–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Veltri T, et al. (2017) Hypertrophic Cardiomyopathy Cardiac Troponin C Mutations Differentially Affect Slow Skeletal and Cardiac Muscle Regulation. Frontiers in physiology 8:221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fischetti R, et al. (2004) The BioCAT undulator beamline 18ID: a facility for biological non-crystalline diffraction and X-ray absorption spectroscopy at the Advanced Photon Source. J Synchrotron Radiat 11(Pt 5):399–405. [DOI] [PubMed] [Google Scholar]
- 30.Farman GP, Walker JS, de Tombe PP, & Irving TC (2006) Impact of osmotic compression on sarcomere structure and myofilament calcium sensitivity of isolated rat myocardium. American journal of physiology. Heart and circulatory physiology 291(4):H1847–1855. [DOI] [PubMed] [Google Scholar]
- 31.Irving TC & Millman BM (1989) Changes in thick filament structure during compression of the filament lattice in relaxed frog sartorius muscle. J Muscle Res Cell Motil 10(5):385–394. [DOI] [PubMed] [Google Scholar]
- 32.Hancock WO, Huntsman LL, & Gordon AM (1997) Models of calcium activation account for differences between skeletal and cardiac force redevelopment kinetics. J Muscle Res Cell Motil 18(6):671–681. [DOI] [PubMed] [Google Scholar]
- 33.Loong CK, Takeda AK, Badr MA, Rogers JS, & Chase PB (2013) Slowed Dynamics of Thin Filament Regulatory Units Reduces Ca2+-Sensitivity of Cardiac Biomechanical Function. Cell Mol Bioeng 6(2):183–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Regnier M, Martyn DA, & Chase PB (1998) Calcium regulation of tension redevelopment kinetics with 2-deoxy-ATP or low [ATP] in rabbit skeletal muscle. Biophysical journal 74(4):2005–2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Caceci MS & Cacheris WP (1984) Fitting Curves to Data. Byte 9(5):340–&. [Google Scholar]
- 36.Pinto JR, et al. (2011) Strong cross-bridges potentiate the Ca(2+) affinity changes produced by hypertrophic cardiomyopathy cardiac troponin C mutants in myofilaments: a fast kinetic approach. The Journal of biological chemistry 286(2):1005–1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Irving TC, Konhilas J, Perry D, Fischetti R, & de Tombe PP (2000) Myofilament lattice spacing as a function of sarcomere length in isolated rat myocardium. American journal of physiology. Heart and circulatory physiology 279(5):H2568–2573. [DOI] [PubMed] [Google Scholar]
- 38.Martyn DA, et al. (2004) Response of equatorial x-ray reflections and stiffness to altered sarcomere length and myofilament lattice spacing in relaxed skinned cardiac muscle. Biophysical journal 86(2):1002–1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li Y, Love ML, Putkey JA, & Cohen C (2000) Bepridil opens the regulatory N-terminal lobe of cardiac troponin C. Proceedings of the National Academy of Sciences of the United States of America 97(10):5140–5145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang X, Li MX, & Sykes BD (2002) Structure of the regulatory N-domain of human cardiac troponin C in complex with human cardiac troponin I147–163 and bepridil. The Journal of biological chemistry 277(34):31124–31133. [DOI] [PubMed] [Google Scholar]
- 41.Adhikari BB, Regnier M, Rivera AJ, Kreutziger KL, & Martyn DA (2004) Cardiac length dependence of force and force redevelopment kinetics with altered cross-bridge cycling. Biophysical journal 87(3):1784–1794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Brenner B & Eisenberg E (1986) Rate of force generation in muscle: correlation with actomyosin ATPase activity in solution. Proceedings of the National Academy of Sciences of the United States of America 83(10):3542–3546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Martyn DA, Chase PB, Regnier M, & Gordon AM (2002) A simple model with myofilament compliance predicts activation-dependent crossbridge kinetics in skinned skeletal fibers. Biophysical journal 83(6):3425–3434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Daniel TL, Trimble AC, & Chase PB (1998) Compliant realignment of binding sites in muscle: transient behavior and mechanical tuning. Biophysical journal 74(4):1611–1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chase PB, Macpherson JM, & Daniel TL (2004) A spatially explicit nanomechanical model of the half-sarcomere: myofilament compliance affects Ca(2+)-activation. Ann Biomed Eng 32(11):1559–1568. [DOI] [PubMed] [Google Scholar]
- 46.Cecchi G, Griffiths PJ, & Taylor S (1986) Stiffness and force in activated frog skeletal muscle fibers. Biophysical journal 49(2):437–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yagi N, et al. (2004) Sarcomere-length dependence of lattice volume and radial mass transfer of myosin cross-bridges in rat papillary muscle. Pflugers Arch 448(2):153–160. [DOI] [PubMed] [Google Scholar]
- 48.Landstrom AP, et al. (2008) Molecular and functional characterization of novel hypertrophic cardiomyopathy susceptibility mutations in TNNC1-encoded troponin C. Journal of molecular and cellular cardiology 45(2):281–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zot HG, Hasbun JE, Michell CA, Landim-Vieira M, & Pinto JR (2016) Enhanced troponin I binding explains the functional changes produced by the hypertrophic cardiomyopathy mutation A8V of cardiac troponin C. Arch Biochem Biophys 601:97–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pinto JR, et al. (2009) A functional and structural study of troponin C mutations related to hypertrophic cardiomyopathy. The Journal of biological chemistry 284(28):1909019100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.de Tombe PP, et al. (2007) Myofilament calcium sensitivity does not affect cross-bridge activation-relaxation kinetics. Am J Physiol Regul Integr Comp Physiol 292(3):R11291136. [DOI] [PubMed] [Google Scholar]
- 52.Wahr PA & Metzger JM (1999) Role of Ca2+ and cross-bridges in skeletal muscle thin filament activation probed with Ca2+ sensitizers. Biophysical journal 76(4):2166–2176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Piroddi N, et al. (2007) Tension generation and relaxation in single myofibrils from human atrial and ventricular myocardium. Pflugers Arch 454(1):63–73. [DOI] [PubMed] [Google Scholar]
- 54.Reid DG, et al. (1990) A proton nuclear magnetic resonance and molecular modeling study of calmidazolium (R24571) binding to calmodulin and skeletal muscle troponin C. The Journal of biological chemistry 265(17):9744–9753. [PubMed] [Google Scholar]
- 55.Kohler J, et al. (2003) Familial hypertrophic cardiomyopathy mutations in troponin I (K183D, G203S, K206Q) enhance filament sliding. Physiological genomics 14(2):117–128. [DOI] [PubMed] [Google Scholar]
- 56.Brunet NM, et al. (2012) Micromechanical thermal assays of Ca2+-regulated thinfilament function and modulation by hypertrophic cardiomyopathy mutants of human cardiac troponin. J Biomed Biotechnol 2012:657523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Stelzer JE, et al. (2004) Expression of cardiac troponin T with COOH-terminal truncation accelerates cross-bridge interaction kinetics in mouse myocardium. American journal of physiology. Heart and circulatory physiology 287(4):H1756–1761. [DOI] [PubMed] [Google Scholar]
- 58.Farah CS & Reinach FC (1995) The troponin complex and regulation of muscle contraction. Faseb J 9(9):755–767. [DOI] [PubMed] [Google Scholar]
- 59.Kreutziger KL, et al. (2011) Calcium binding kinetics of troponin C strongly modulate cooperative activation and tension kinetics in cardiac muscle. Journal of molecular and cellular cardiology 50(1):165–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kathuria SV, Chan YH, Nobrega RP, Ozen A, & Matthews CR (2016) Clusters of isoleucine, leucine, and valine side chains define cores of stability in high-energy states of globular proteins: Sequence determinants of structure and stability. Protein science : a publication of the Protein Society 25(3):662–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Slupsky CM & Sykes BD (1995) NMR solution structure of calcium-saturated skeletal muscle troponin C. Biochemistry 34(49):15953–15964. [DOI] [PubMed] [Google Scholar]
- 62.Sia SK, et al. (1997) Structure of cardiac muscle troponin C unexpectedly reveals a closed regulatory domain. The Journal of biological chemistry 272(29):18216–18221. [DOI] [PubMed] [Google Scholar]
- 63.Badr MA, Pinto JR, Davidson MW, & Chase PB (2016) Fluorescent Protein-Based Ca2+ Sensor Reveals Global, Divalent Cation-Dependent Conformational Changes in Cardiac Troponin C. PloS one 11(10):e0164222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Marques MA, et al. (2017) Allosteric Transmission along a Loosely Structured Backbone Allows a Cardiac Troponin C Mutant to Function with Only One Ca2+ Ion. The Journal of biological chemistry 292(6):2379–2394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Veltri T, et al. (2017) Amide hydrogens reveal a temperature-dependent structural transition that enhances site-II Ca2+-binding affinity in a C-domain mutant of cardiac troponin C. Sci Rep 7(1):691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tanner BC, Daniel TL, & Regnier M (2012) Filament compliance influences cooperative activation of thin filaments and the dynamics of force production in skeletal muscle. PLoS computational biology 8(5):e1002506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Williams CD, Salcedo MK, Irving TC, Regnier M, & Daniel TL (2013) The lengthtension curve in muscle depends on lattice spacing. Proc Biol Sci 280(1766):20130697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.McDonald KS, Wolff MR, & Moss RL (1997) Sarcomere length dependence of the rate of tension redevelopment and submaximal tension in rat and rabbit skinned skeletal muscle fibres. J Physiol (Lond) 501(Pt 3):607–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Reconditi M, et al. (2017) Myosin filament activation in the heart is tuned to the mechanical task. Proceedings of the National Academy of Sciences of the United States of America 114(12):3240–3245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kampourakis T, Sun YB, & Irving M (2016) Myosin light chain phosphorylation enhances contraction of heart muscle via structural changes in both thick and thin filaments. Proceedings of the National Academy of Sciences of the United States of America 113(21):E3039–3047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Pearson JT, et al. (2004) In situ measurements of crossbridge dynamics and lattice spacing in rat hearts by x-ray diffraction: sensitivity to regional ischemia. Circulation 109(24):2976–2979. [DOI] [PubMed] [Google Scholar]
- 72.Bagni MA, et al. (1994) Lattice spacing changes accompanying isometric tension development in intact single muscle fibers. Biophysical journal 67(5):1965–1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Cecchi G, Griffiths PJ, Bagni MA, Ashley CC, & Maeda Y (1991) Time-resolved changes in equatorial x-ray diffraction and stiffness during rise of tetanic tension in intact length-clamped single muscle fibers. Biophysical journal 59(6):1273–1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Brenner B & Yu LC (1985) Equatorial x-ray diffraction from single skinned rabbit psoas fibers at various degrees of activation. Changes in intensities and lattice spacing. Biophysical journal 48(5):829–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Brenner B & Yu LC (1991) Characterization of radial force and radial stiffness in Ca(2+)-activated skinned fibres of the rabbit psoas muscle. The Journal of physiology 441:703–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zhou HX (2013) Polymer crowders and protein crowders act similarly on protein folding stability. FEBS letters 587(5):394–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Karatzaferi C, Chinn MK, & Cooke R (2004) The force exerted by a muscle cross-bridge depends directly on the strength of the actomyosin bond. Biophysical journal 87(4):25322544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Chase PB, Chen Y, Kulin KL, & Daniel TL (2000) Viscosity and solute dependence of Factin translocation by rabbit skeletal heavy meromyosin. Am J Physiol Cell Physiol 278(6):C1088–1098. [DOI] [PubMed] [Google Scholar]
- 79.Xu S, Martyn D, Zaman J, & Yu LC (2006) X-ray diffraction studies of the thick filament in permeabilized myocardium from rabbit. Biophysical journal 91(10):37683775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Naylor GR, Bartels EM, Bridgman TD, & Elliott GF (1985) Donnan potentials in rabbit psoas muscle in rigor. Biophysical journal 48(1):47–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Brenner B, Xu S, Chalovich JM, & Yu LC (1996) Radial equilibrium lengths of actomyosin cross-bridges in muscle. Biophysical journal 71(5):2751–2758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Cecchi G, Bagni MA, Griffiths PJ, Ashley CC, & Maeda Y (1990) Detection of radial crossbridge force by lattice spacing changes in intact single muscle fibers. Science 250(4986):1409–1411. [DOI] [PubMed] [Google Scholar]
- 83.Bers DM (2002) Cardiac excitation-contraction coupling. Nature 415(6868):198–205. [DOI] [PubMed] [Google Scholar]
- 84.Willott RH, et al. (2010) Mutations in Troponin that cause HCM, DCM AND RCM: what can we learn about thin filament function? Journal of molecular and cellular cardiology 48(5):882–892. [DOI] [PubMed] [Google Scholar]
- 85.Tardiff JC (2011) Thin filament mutations: developing an integrative approach to a complex disorder. Circulation research 108(6):765–782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Morimoto S (2008) Sarcomeric proteins and inherited cardiomyopathies. Cardiovascular research 77(4):659–666. [DOI] [PubMed] [Google Scholar]
- 87.Jaafar N, et al. (2015) Genetic profile of hypertrophic cardiomyopathy in Tunisia: Is it different? Glob Cardiol Sci Pract 2015:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kataoka A, Hemmer C, & Chase PB (2007) Computational simulation of hypertrophic cardiomyopathy mutations in troponin I: influence of increased myofilament calcium sensitivity on isometric force, ATPase and [Ca2+]i. J Biomech 40(9):2044–2052. [DOI] [PubMed] [Google Scholar]
- 89.de Tombe PP & ter Keurs HE (2016) Cardiac muscle mechanics: Sarcomere length matters. Journal of molecular and cellular cardiology 91:148–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Farman GP, Allen EJ, Gore D, Irving TC, & de Tombe PP (2007) Interfilament spacing is preserved during sarcomere length isometric contractions in rat cardiac trabeculae. Biophysical journal 92(9):L73–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wannenburg T, et al. (2000) Cross-bridge kinetics in rat myocardium: effect of sarcomere length and calcium activation. American journal of physiology. Heart and circulatory physiology 279(2):H779–790. [DOI] [PubMed] [Google Scholar]
- 92.Janssen PM, Biesiadecki BJ, Ziolo MT, & Davis JP (2016) The Need for Speed: Mice, Men, and Myocardial Kinetic Reserve. Circulation research 119(3):418–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Belus A, et al. (2008) The familial hypertrophic cardiomyopathy-associated myosin mutation R403Q accelerates tension generation and relaxation of human cardiac myofibrils. The Journal of physiology 586(15):3639–3644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Witjas-Paalberends ER, et al. (2014) Faster cross-bridge detachment and increased tension cost in human hypertrophic cardiomyopathy with the R403Q MYH7 mutation. The Journal of physiology 592(15):3257–3272. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.