Abstract
Background
During the last decade, the number of people with ≥1 tattoo has increased noticeably within the European population. Despite this, limited safety information is available for tattoo inks.
Objectives
To test the skin sensitization potential of 5 tattoo inks in vitro by using reconstructed human skin (RHS) and the contact sensitization biomarker interleukin (IL)‐18.
Methods
Two red and 3 black tattoo inks, 1 additive (Hamamelis virginiana extract) and 1 irritant control (lactic acid) were tested. The culture medium of RHS (reconstructed epidermis on a fibroblast‐populated collagen hydrogel) was supplemented with test substances in a dose‐dependent manner for 24 hours, after which cytotoxicity (histology; thiazolyl blue tetrazolium bromide assay) and skin sensitization potential (IL‐18 secretion; enzyme‐linked immunosorbent assay) were assessed.
Results
All but 1 ink showed cytotoxicity. Notably, 1 red ink and 1 black ink were able to cause an inflammatory response, indicated by substantial release of IL‐18, suggesting that these inks may be contact sensitizers.
Conclusions
The in vitro RHS model showed that 4 tattoo inks were cytotoxic and 2 were able to cause an inflammatory IL‐18 response, indicating that an individual may develop allergic contact dermatitis when exposed to these tattoo inks, as they contain contact sensitizers.
Keywords: allergic contact dermatitis, human reconstructed skin, IL‐18, in vitro, safety assessment, skin sensitization, tattoo ink
Abbreviations
- AOP
adverse outcome pathway
- CI
colour index
- EC50
effective chemical concentration to reduce cell viability by 50%
- HPLC
high‐performance liquid chromatography
- IL
interleukin
- LC
Langerhans cell
- NA
not available
- PAA
primary aromatic amine
- PAH
polycyclic aromatic hydrocarbon
- RHE
reconstructed human epidermis
- RHS
reconstructed human skin
- SEM
standard error of the mean
- SI
stimulation index
1. INTRODUCTION
During the last decade, the percentage of Europeans with ≥1 tattoo has increased noticeably, from 5% to 10% in 2003 to 12% in 2016.1 Simultaneously, the number of patients with tattoo‐related complications visiting the dermatologist indicates a substantial health risk. Allergic reactions are reported to be mostly associated with red tattoos, which may cause itching, and also, sometimes, plaque‐like infiltration, confined to uniformly coloured sites of the tattoo.2, 3 Contact allergy occurring in a tattoo can ultimately manifest itself as hyperkeratosis, or even ulceration and necrosis of the skin.3, 4, 5 The time of onset of complications may differ substantially, from immediately after application of the tattoo up to years or decades afterwards.1 Occasionally, the allergic reaction can manifest simultaneously in older, similarly coloured tattoos.3 The disease burden of a chronic allergic reaction in a tattoo is high, and significantly reduces quality of life.6 Therefore, the generation of in vitro toxicity data is of utmost importance if we are to gain a better understanding of safety regarding tattoo inks. Recent publications concerning the health hazard of tattoo inks have provided insights into cytotoxicity, genotoxicity and reactive oxygen species production in the skin following tattoo ink exposure.7, 8, 9, 10 However, in vitro data on the sensitization potential of tattoo inks are scarce.
The pigments in tattoo ink are not strictly intended for intradermal use, but for other (industrial) uses, such as applications in textiles, lacquer, inks, or plastics. Therefore, these pigments are not specifically assessed for injection into and permanent application on the human body.11, 12 Currently, azo dyes are the chemicals that are most commonly used as colorants in tattoo inks. They have replaced the traditional pigments such as cinnabar (red), chromium (green), cobalt (blue), cadmium (yellow) or manganese (purple) derivatives.1 Azo dyes are present in many consumer products, such as textiles and leather clothing, and may cause allergy.13, 14, 15 Metabolism or chemical degradation of the azo bond may result in the formation of primary aromatic amines, some of which have been proven to possess mutagenic and carcinogenic or sensitizing properties.11
Black inks usually contain pigments of natural origin, such as Carbon Black (Pigment Black 6/7). This pigment consists mainly of elemental carbon (>97%). The Scientific Committee for Consumer Safety (formerly the SCCP) concluded, in their scientific opinion, that this Carbon Black pigment, when considered in its nanostructured form, can be regarded as being safe at concentrations up to 10% in consumer products.16 However, polycyclic aromatic hydrocarbons (PAHs) may be present as contaminants in this pigment, and several of these have been classified as carcinogenic by the International Agency for Research on Cancer.8, 17
The adverse outcome pathway (AOP) for the process of skin sensitization defines 1 molecular initiating event and a number of key events, as follows18: key event 1 (initial event)—covalent binding of a xenobiotic chemical to a skin protein, forming a hapten, and penetration of the hapten through the stratum corneum into the viable layers of the underlying epidermis; key event 2—activation of keratinocytes, resulting in secretion of cytokines, for example, interleukin (IL)‐1a, IL‐18, and tumour necrosis factor‐α; key event 3—Langerhans cell (LC) activation (migration and maturation), directly or by a hapten‐carrier protein complex; and key event 4—presentation of the antigen by matured LCs to antigen‐responsive T lymphocytes in the draining lymph nodes, resulting in the formation of primed effector and memory T lymphocytes.18, 19
It has been observed that sensitization potency is related to the irritant properties of the chemical, and that this irritancy results in an innate immune inflammatory response (ie, xenoinflammation).20 New insights into the mechanism of xenoinflammation have identified IL‐18 (among other cytokines) as playing a pivotal role in key event 2 of skin sensitization and allergic contact dermatitis, but, surprisingly, not in respiratory sensitization or irritant contact dermatitis.20, 21, 22 IL‐18 is therefore considered to be the key link between xenoinflammation, which may be caused by irritants as well as contact allergens, and migration of the dendritic cells.20 Hence, this cytokine can be considered to be a specific biomarker that is upregulated by chemicals that have the potential to be skin sensitizers.
We have previously developed an in vitro assay to distinguish contact sensitizers from irritants on the basis of the release of IL‐18 from reconstructed human epidermis (RHE).23, 24 The assay is currently undergoing validation in Europe, Asia, and America, and can be used with both commercially available RHE and in‐house academic RHE. An IL‐18 stimulation index (SI) equal to or above a threshold value (to be defined for each type of RHE) is strongly indicative of a skin sensitizer. For our in‐house RHE, an SI of ≥5 predicted skin sensitizers as opposed to non‐sensitizers with 95% accuracy.23 Note that, in the RHE model, chemicals were applied topically to the stratum corneum, thus mimicking topical exposure in humans.
The aim of this study was to determine whether an organotypic three‐dimensional (3D) reconstructed human skin (RHS) model could serve as a screening tool for determining the skin sensitization potential of tattoo inks. RHS consists of a reconstructed differentiated epidermis grown on a fibroblast‐populated collagen hydrogel at the air‐liquid interface.25 As tattoo inks are permanently injected into the dermis, topical application was considered not to be the best application method. Therefore, instead of topical exposure, tattoo inks were added to the culture medium to mimic intradermal exposure. IL‐18 release and cytotoxicity were determined in vitro for 2 red and 3 black tattoo inks, in order to obtain information on sensitization potential. These red inks were chosen because they were associated with allergic reactions in patients visiting the tattoo outpatient clinic of VU University Medical Centre, Amsterdam UMC.
2. MATERIALS AND METHODS
2.1. RHC culture
Human foreskin was obtained from healthy human donors and was used anonymously, in compliance with the VU University Medical Centre, Amsterdam UMC's ethical guidelines and the “Code for Proper Use of Human Tissues” as formulated by the Dutch Federation of Medical Scientific Organizations (see http://www.fmwv.nl). RHS was constructed as described previously.26 In short, dermal fibroblasts and epidermal keratinocytes were isolated from foreskins and cultured until 90% confluent. Passage 1 cells were used to construct RHS in 2.5‐cm‐diameter transwells (pore size of 0.4 μm; Corning, New York, New York). Keratinocytes (5 × 104 cells) were seeded on top of the fibroblast‐populated collagen hydrogels (1 × 105 fibroblasts per gel).
RHS was initially cultured submerged for 3 days, and was then cultured for a further 14 days at the air‐liquid interface, with the culture medium only in contact with the underside of RHS via the porous transwell membrane, in order to promote epidermal differentiation, in Dulbecco's modified Eagle's medium (Lonza, Basel, Switzerland) and Ham‐F12 (Gibco, Grand Island, Nebraska) (3:1) containing 0.2% UltroserG (BioSepra, Cergy‐Saint‐Christophe, France), 1% penicillin‐streptomycin, 1 μM hydrocortisone, 1 μM isoproterenol, 0.1 μM insulin, 2 ng/mL keratinocyte growth factor, 0.5 ng/mL epidermal growth factor, 1.0 × 10−5 M l‐carnitine, and 1.0 × 10−2 M l‐serine, and supplemented with a lipid mixture (25 μM palmitic acid, 15 μM linoleic acid, and 7 μM arachidonic acid), 50 μg/mL ascorbic acid, and vitamin E. Medium was changed twice weekly. For the exposure, cultures were incubated overnight in the above‐mentioned medium, in the absence of hydrocortisone.25 All substances were from Sigma Chemical (St Louis, Missouri) unless stated otherwise.
2.2. Chemical exposure
Two red inks and 3 black inks that are commercially available from Intenze (Intenze Products, Kalsdorf, Austria), Eternal Ink (Eternal Ink, Brighton, UK) and Carbon Black (H‐A‐N, Esslingen, Germany) were selected for testing in the RHS model (Table 1). Hamamelis virginiana extract was also tested, as it is added to 2 of the 5 tattoo inks investigated in this study as an “anti‐inflammatory agent” (Table 1). Isopropanol (1% wt/wt) was used as the vehicle for diluting Eternal Ink Light Red and Carbon Black No. 13, as this is a component of the undiluted ink, and glycerol (1% wt/wt) was used as the vehicle for the other inks, as glycerin (not isopropanol) was a component of these inks (Table 1). Furthermore, a non‐sensitizing irritant control was tested in order to obtain a sensitization threshold level for the SI IL‐18 parameter, namely, lactic acid (Table 2).
Table 1.
Commercially available tattoo inks and the relevant hazard identification of these substances according to CLPa
| Tattoo inkb | Batch number | Chemical listed in ink | CI No. | Pigment | CAS no. | Hazard identification |
|---|---|---|---|---|---|---|
| Intenze Gold Label Bright Red | RD69Y79O75IMX40 | NA | CI 12 477 | Red 210 | 61932‐63‐6 | Results in o‐anisidine (CAS no. 90‐04‐0) after amide hydrolysis, Acute Tox. 3, Carc. 1B, Muta. 2 |
| 2‐([4‐Methoxy‐2‐nitrophenyl]azo)‐N‐(2‐methoxyphenyl)‐3‐oxobutyramide | CI 11 740 | Yellow 65 | 6528‐34‐3 | NA | ||
| 4,4′‐([3,3′‐Dichloro(1,1′‐biphenyl)‐4,4′‐diyl]bis[azo])bis(2,4‐dihydro‐5‐methyl‐2‐phenyl‐3H‐pyrazol‐3‐one) | CI 21 110 | Orange 13 | 3520‐72‐7 | Results in 3,3′‐chlorobenzidine (CAS no. 91‐94‐1) after cleavage of azo bond, Carc. 1B, Skin Sens. 1 | ||
| Hamamelis virginiana extract | NA | NA | 84696‐19‐5 | NA | ||
| Diazolidinyl urea | NA | NA | 78491‐02‐8 | Formaldehyde (CAS no. 50‐00‐0)‐releasing preservative, Carc. 1B, Muta 2, Skin Sens. 1, Acute Tox. 3, and Skin Corr. 1B | ||
| Eternal Ink Light Red | NA | 4‐([4‐(Aminocarbonyl)phenyl]azo)‐N‐(2‐ethoxyphenyl)‐3‐hydroxynaphthalene‐2‐carboxamide | CI 12 475 | Red 170 | 2786‐76‐7 | Skin Sens. 1c |
| Isopropanol | NA | NA | 67‐63‐0 | Eye irrit. 2 | ||
| Hamamelis virginiana water | NA | NA | 84696‐19‐5 | NA | ||
| Intenze Sculpting Black | BK76DIS | Carbon Black | CI 77 266 | Black 6/7 | 1333‐86‐4 | Carc. 2c |
| Distilled water | NA | NA | 7732‐18‐5 | NA | ||
| Isopropanol | NA | NA | 67‐63‐0 | Eye irrit. 2 | ||
| Glycerin | NA | NA | 56‐81‐5 | NA | ||
| Intenze True Black | BLK1301MX40‐GE | Carbon Black | CI 77 266 | Black 6/7 | 1333‐86‐4 | Carc. 2c |
| Distilled water | NA | NA | 7732‐18‐5 | NA | ||
| Isopropanol | NA | NA | 67‐63‐0 | Eye irrit. 2 | ||
| Glycerin | NA | NA | 56‐81‐5 | NA | ||
| Carbon Black No. 13 Blackout | A0000585 | Carbon Black | CI 77 266 | Black 6/7 | 1333‐86‐4 | Carc. 2c |
| Ammonium acrylate copolymer | NA | NA | NA | NA | ||
| Propylene glycol | NA | NA | 57‐55‐6 | NA | ||
| Poloxamer 331 | NA | NA | NA | NA | ||
| Poloxamer 188 | NA | NA | NA | NA | ||
| Isopropanol | NA | NA | 67‐63‐0 | Eye irrit. 2 |
Abbreviations: CI, colour index; NA, not available.
The 2 red inks and 3 black inks were commercially available from Intenze (Intenze Products, Kalsdorf, Austria), Eternal Ink (Eternal Ink, Brighton, UK), and Carbon Black (H‐A‐N, Esslingen, Germany).
Classification, Labelling and Packaging of Substances and Mixtures Regulation (CLP, Regulation [EC] No. 1272/2008).
The ingredients mentioned on the label of the tattoo ink bottle.
Self‐classified by the registrant under REACH (Registration, Evaluation, Authorization or Restriction of Chemicals Regulation [EC] No. 1907/2006).
Table 2.
The hazard identification of the vehicles, additives and irritants according to CLPa
| Chemical name | CAS no. | Hazard identification |
|---|---|---|
| Isopropanol | 67‐63‐0 | Eye irrit. 2 |
| Glycerol | 56‐81‐5 | NA |
| Hamamelis virginiana extract | 84696‐19‐5 | NA |
| Lactic acid | 79‐33‐4 | Eye dam. 1b, Skin Irrit. 2b, Eye Irrit. 2b, Skin Corr. 1Bb |
Abbreviation: NA, not available.
Classification, Labelling and Packaging of Substances and Mixtures Regulation (CLP, Regulation [EC] No. 1272/2008).
Self‐classified by the registrant under REACH (Registration, Evaluation, Authorization, and Restriction of Chemicals Regulation [EC] No. 1907/2006).
The culture medium of RHS was supplemented with test substances at final concentrations of 10%, 1%, 0.1%, and 0.01%, or as stated otherwise, for 24 hours. Lactic acid and H. virginiana extract were diluted in culture medium to the required concentrations. RHS was then harvested. RHS biopsies were taken and processed immediately: (1) with the thiazolyl blue tetrazolium bromide (MTT) assay to determine mitochondrial activity; and (2) for histology. In addition, culture supernatant obtained from underneath the air‐exposed RHS was harvested and stored at −20°C until further enzyme‐linked immunosorbent assay (ELISA) analysis.
2.3. MTT assay
Mitochondrial activity, as an indicator of cell viability, was determined with the MTT assay.27 For each RHS, a punch biopsy (diameter of 3 mm) was taken, rinsed in phosphate‐buffered saline (PBS) to remove any excess ink from the underside of the culture, and transferred to a 96‐well culture plate containing 200 μL of MTT diluted in PBS (2 mg/mL) and further processed as described in Gibbs et al.23 To determine whether the tattoo inks were able to interfere with the MTT assay, 10% of each tattoo ink was tested in the absence of RHS. No colour change at 570 nm was observed, and it was therefore concluded that the inks did not interfere with the MTT assay at the concentrations used for RHS exposure. This is the method recommended in OECD TG 431 and 439 for the in vitro skin corrosion test (epiCS 2012).
2.4. Haematoxylin and eosin paraffin staining assay
For light microscopic examination, RHS samples were fixed in 4% paraformaldehyde and embedded in paraffin. Subsequently, thin sections (5 μm) were cut and stained with haematoxylin and eosin.
2.5. IL‐18 ELISA
After exposure, the amount of IL‐18 in the culture supernatant was measured with a commercially available specific sandwich ELISA kit according to the manufacturer's instructions (MBL, Nagoya, Japan). Cytokine levels determined in the exposed RHS were transformed into an SI relative to the vehicle (fold increase), according to the procedure described in reference 23.
2.6. High‐performance liquid chromatography (HPLC)
Reversed‐phase HPLC (Jasco, Tokyo, Japan) was performed with an aSymmetry C18 column (100 Å, 5 μm, 3.9 × 150 mm; Waters, Milford, Massachusetts) containing dimethyloctadecylsilyl‐bonded amorphous silica to detect the presence of PAHs in the black inks. A PAH identification mixture and a benzo[a]pyrene standard were obtained from Sigma Chemical. To prepare the benzo[a]pyrene standard, the chemical was dissolved in methanol to a concentration of 10 μg/mL. Each black ink (1 mL) was extracted overnight with 5 mL of dinitrochloromethane (Biosolve, Valkenswaard, The Netherlands), and dried in a vacuum concentrator (RVC‐2‐25 Co plus; M. Christ, Osterode, Germany). Subsequently, ink samples were dissolved in methanol. Samples were filtered over a polytetrafluoroethylene 0.2‐μm membrane filter, and elution was performed with a linear gradient from 40% to 85% acetonitrile (Actu‐ALL chemicals, Oss, The Netherlands) containing 0.1% trifluoroacetic acid (Biosolve) for 45 minutes at a flow rate of 1 mL/min. The PAH identification mixture was used for comparison with peaks obtained from the tattoo inks. For identification of benzo[a]pyrene, 20 μL of 10 μg/mL benzo[a]pyrene standard was analysed separately, and 90 μL of 100 μg/mL benzo[a]pyrene standard was then spiked with 10 μL of the extracted Intenze Sculpting Black tattoo ink fraction.
2.7. Data analysis
The data represent at least 3 independent experiments with an intra‐experiment duplicate. For each experiment, RHS constructed from a different skin donor was used. The cell viability and IL‐18 secretion results were analysed with 1‐way ANOVA followed by Dunn's multiple comparison test (graphpad prism, version 7.0). A difference was considered to be significant when the P value was <.05, as compared with the vehicle‐exposed control for IL‐18 SI and the negative control for cell viability.
3. RESULTS
3.1. Tattoo inks show irritant properties when exposed to RHS
Five tattoo inks were selected for testing in the RHS model in order to determine their skin irritation and sensitization potential. Furthermore, 2 vehicles (glycerol and isopropanol), 1 tattoo ink additive (H. virginiana extract) and 1 irritant (lactic acid) were tested (Tables 1 and 2). The substances were added to the culture medium of RHS, and tested in a dose‐dependent manner for 24 hours.
Clearly deleterious effects were observed in the tissue architecture of the RHS exposed to the tattoo inks Eternal Ink Light Red, Intenze Gold Label Bright Red and Intenze Sculpting Black at a concentration of 10% (Figure 1). Cytotoxicity was indicated by an increase in the number of vacuoles, shrinkage of nuclei, and detachment of the epidermis. Carbon Black No. 13 Blackout and Intenze True Black had no visible effects on tissue histology. As expected, the irritant control (lactic acid) was clearly cytotoxic at a concentration of 0.06%, and H. virginiana extract had no effect on tissue architecture.
Figure 1.
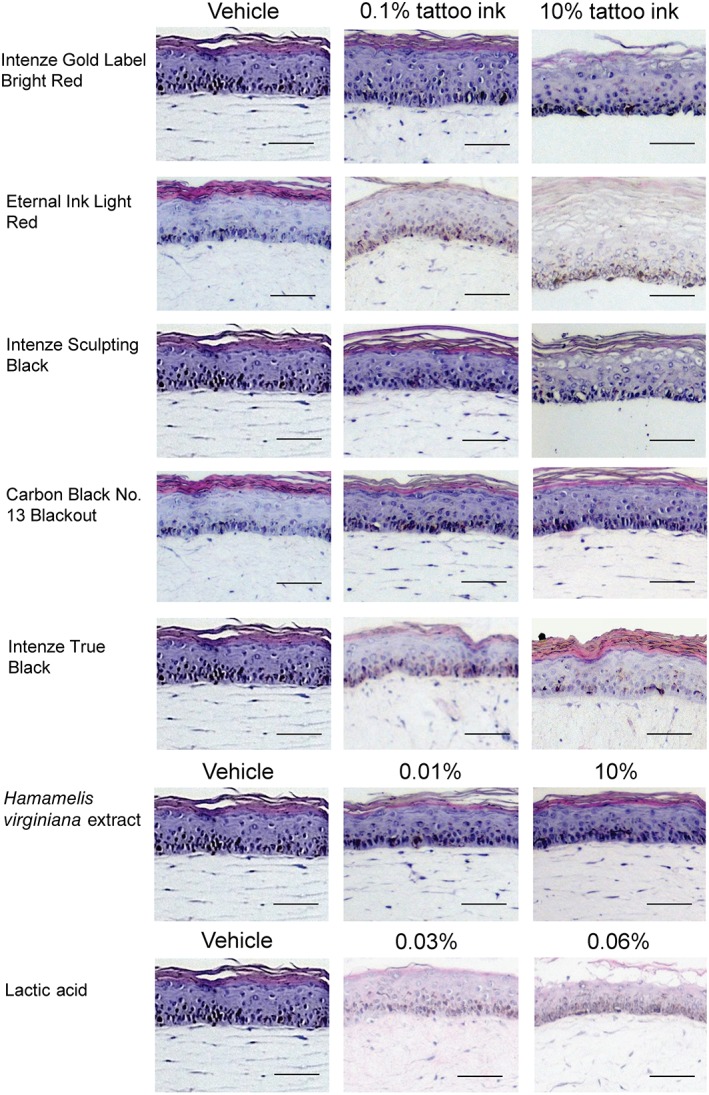
Histology of reconstructed human skin (RHS) exposed to tattoo inks, Hamamelis virginiana extract, the vehicles glycerol and isopropanol, and lactic acid. Test substances were added to the culture medium for 24 hours. For Eternal Ink Light Red and Carbon Black No. 13 Blackout, isopropanol (1% wt/wt) was used as the vehicle, and for all other substances, glycerol was used as the vehicle (1% wt/wt). Representative haematoxylin and eosin staining of 5‐μm paraffin‐embedded RHS tissue sections is shown. Magnification: ×200. Scale bar: 50 μm
The MTT assay was performed in order to determine the test substance concentration that reduces RHS viability by 50% (EC50 value). This value relates to the cytotoxic/irritant potential of the substances (a low EC50 value corresponds to strong irritant potency). With the exception of Intenze True Black and H. virginiana extract, RHS viability was reduced in a dose‐dependent manner (Figure 2; Table 3). Ranking of the inks, additive and irritant in order of most toxic to non‐toxic on the basis of their EC50 values showed the following: Eternal Ink Light Red (0.04% wt/wt) > lactic acid (0.05% wt/wt) > Intenze Sculpting Black (0.09% wt/wt) > Intenze Gold Label Bright Red (6.3% wt/wt) > Carbon Black No. 13 Blackout (12% wt/wt) > Intenze True Black (not reached) > H. virginiana extract (100% wt/wt). Clearly, 4 of the 5 inks have cytotoxic and irritant in vitro properties, with Eternal Ink Light Red and Intenze Sculpting Black being highly cytotoxic, in the same order of magnitude as lactic acid.
Figure 2.
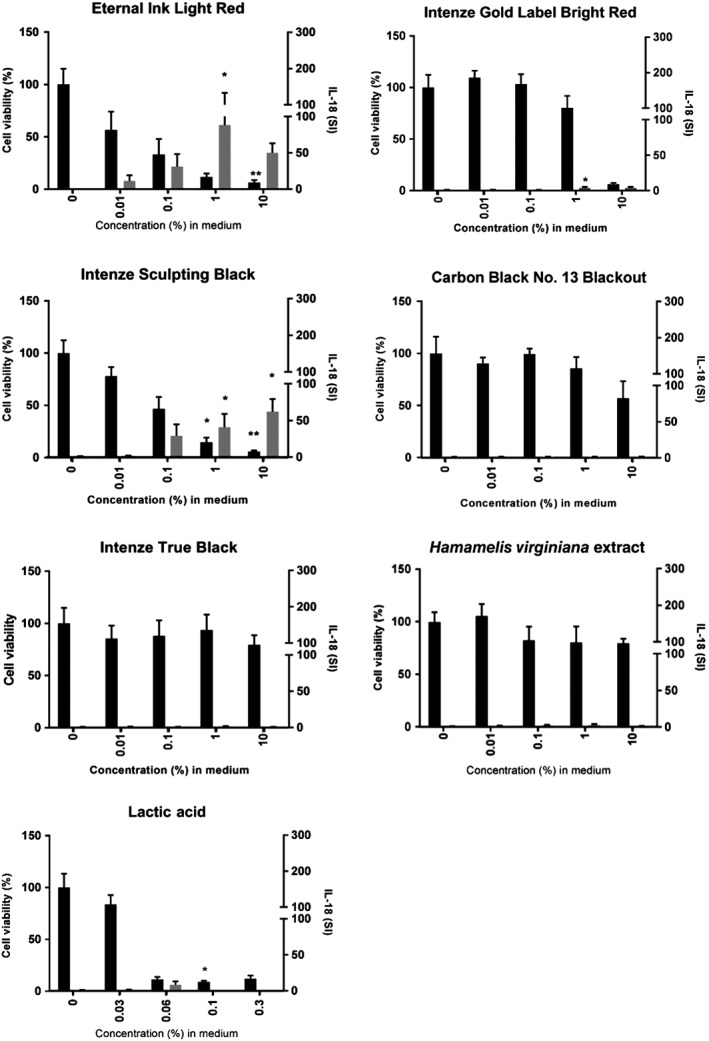
Tattoo inks are cytotoxic and result in interleukin (IL)‐18 release from reconstructed human skin (RHS). RHS was exposed to test substances for 24 hours, culture supernatants were analysed with a specific IL‐18 enzyme‐linked immunosorbent assay, and RHS cell viability was then determined with the thiazolyl blue tetrazolium bromide assay. IL‐18 stimulation index (SI) (grey bars) and cell viability (black bars) are shown as mean ± standard error of the mean (SEM), with each experiment (n) representing a different batch of RHS constructed from a different donor skin for Eternal Ink Light Red (n = 5), Intenze Gold Label Bright Red (n = 5), Intenze Sculpting Black (n = 5), Carbon Black No. 13 Blackout (n = 4), Intenze True Black (n = 4), Hamamelis virginiana extract (n = 4), and lactic acid (n = 3). Statistical significance was determined with 1‐way ANOVA followed by Dunn's multiple comparison test. *P < .05 and **P < .01 as compared with the vehicle‐exposed RHS
Table 3.
Summary results of cell viability and interleukin (IL)‐18 secretion for inks, additive, and irritant
| Concentration (%) | Cell viability ± SEM (%) | EC50 (%) | IL‐18 SI ± SEM | N | |
|---|---|---|---|---|---|
| Tattoo ink | |||||
| Eternal Ink Light Red | 0.01 | 57 ± 16 | 0.04 | 11 ± 7.6 | 5 |
| 0.1 | 33 ± 13 | 31 ± 17 | |||
| 1 | 12 ± 3.0 | 88 ± 45 | |||
| 10 | 6.4 ± 2.2 | 50 ± 13 | |||
| Intenze Gold Label Bright Red | 0.01 | 110 ± 6.1 | 6.3 | 1.2 ± 0.48 | 5 |
| 0.1 | 103 ± 8.5 | 0.9 ± 0.15 | |||
| 1 | 80 ± 11 | 4.1 ± 1.3 | |||
| 10 | 6.4 ± 1.6 | 4.1 ± 1.4 | |||
| Intenze Sculpting Black | 0.01 | 78 ± 7.7 | 0.09 | 1.9 ± 0.37 | 5 |
| 0.1 | 47 ± 10 | 29 ± 14 | |||
| 1 | 15 ± 4.0 | 41 ± 16 | |||
| 10 | 5.8 ± 1.0 | 62 ± 15 | |||
| Carbon Black No. 13 Blackout | 0.01 | 90 ± 5.0 | 12 | 1.4 ± 0.23 | 4 |
| 0.1 | 99 ± 4.6 | 1.5 ± 0.23 | |||
| 1 | 86 ± 9.4 | 1.5 ± 0.28 | |||
| 10 | 57 ± 14 | 1.3 ± 0.56 | |||
| Intenze True Black | 0.01 | 85 ± 11 | NR | 1.1 ± 0.19 | 4 |
| 0.1 | 88 ± 13 | 0.8 ± 0.18 | |||
| 1 | 94 ± 13 | 1.4 ± 0.50 | |||
| 10 | 79 ± 7.6 | 0.7 ± 0.28 | |||
| Additive | |||||
| Hamamelis virginiana extract | 0.01 | 105 ± 10 | 100 | 1.6 ± 0.35 | 4 |
| 0.1 | 82 ± 11 | 2.5 ± 0.39 | |||
| 1 | 80 ± 13 | 2.8 ± 1.12 | |||
| 10 | 79 ± 3.8 | 1.2 ± 0.25 | |||
| Control irritant | |||||
| Lactic acid | 0.03 | 84 ± 7.4 | 0.05 | 1.4 ± 0.24 | 3 |
| 0.06 | 11 ± 1.9 | 8.3 ± 3.9 | |||
| 0.1 | 9 ± 1.0 | 0.1 ± 0.02 | |||
| 0.3 | 12 ± 2.7 | 0.0 ± 0.01 | |||
Abbreviations: N, number of experiments; NR, not reached; SEM, standard error of the mean; SI, stimulation index.
Eternal Ink Light Red and Carbon Black No. 13 Blackout exposure conditions are expressed relative to the vehicle isopropanol (1% wt/wt); all other exposure conditions are expressed relative to the vehicle glycerol (1% wt/wt). IL‐18 SIs of ≥10 are indicated in bold, and are indicative of the substance being a skin sensitizer, as this value is higher than that obtained for lactic acid.
3.2. Exposure to Eternal Ink Light Red and Intenze Sculpting Black increases the release of the sensitization biomarker IL‐18 from RHS
Having determined the cytotoxic and irritant potential of the 5 tattoo inks, we next determined whether the dyes could also result in an increase in release of the sensitization biomarker IL‐18 from RHS. Two inks in particular, Eternal Ink Light Red (SI: 88 ± 45) and Intenze Sculpting Black (SI: 62 ± 15), resulted in substantial release of IL‐18 into the culture supernatant of RHS as compared with the other 3 tattoo inks, indicating that these inks may have sensitization potential (Figure 2; Table 3). The tattoo ink vehicles (glycerol and isopropanol), H. virginiana extract and lactic acid did not increase IL‐18 release, as expected.
3.3. The extremely strong sensitizer benzo[a]pyrene is present in Intenze Sculpting Black
To explain the differences in IL‐18 release and cytotoxicity observed between Intenze Sculpting Black and the other 2 black inks, HPLC was performed to screen for PAHs (Figure 3). Special attention was paid to benzo[a]pyrene, because this compound is classified as an extremely strong sensitizer according to the local lymph node assay (LLNA).28 HPLC analysis indicated that Intenze Sculpting Black contains 3 major compounds that co‐eluted with peaks 1, 3 and 11 from the PAH identification mixture. Intenze True Black contains mainly compounds that co‐eluted with peaks 4 and 13, whereas Carbon Black mainly contains compound 4. Interestingly, compound 11 being benzo[a]pyrene (as determined by spiking the ink with a benzo[a]pyrene standard see Materials and Methods section 2.6) was present only in Intenze Sculpting Black.
Figure 3.
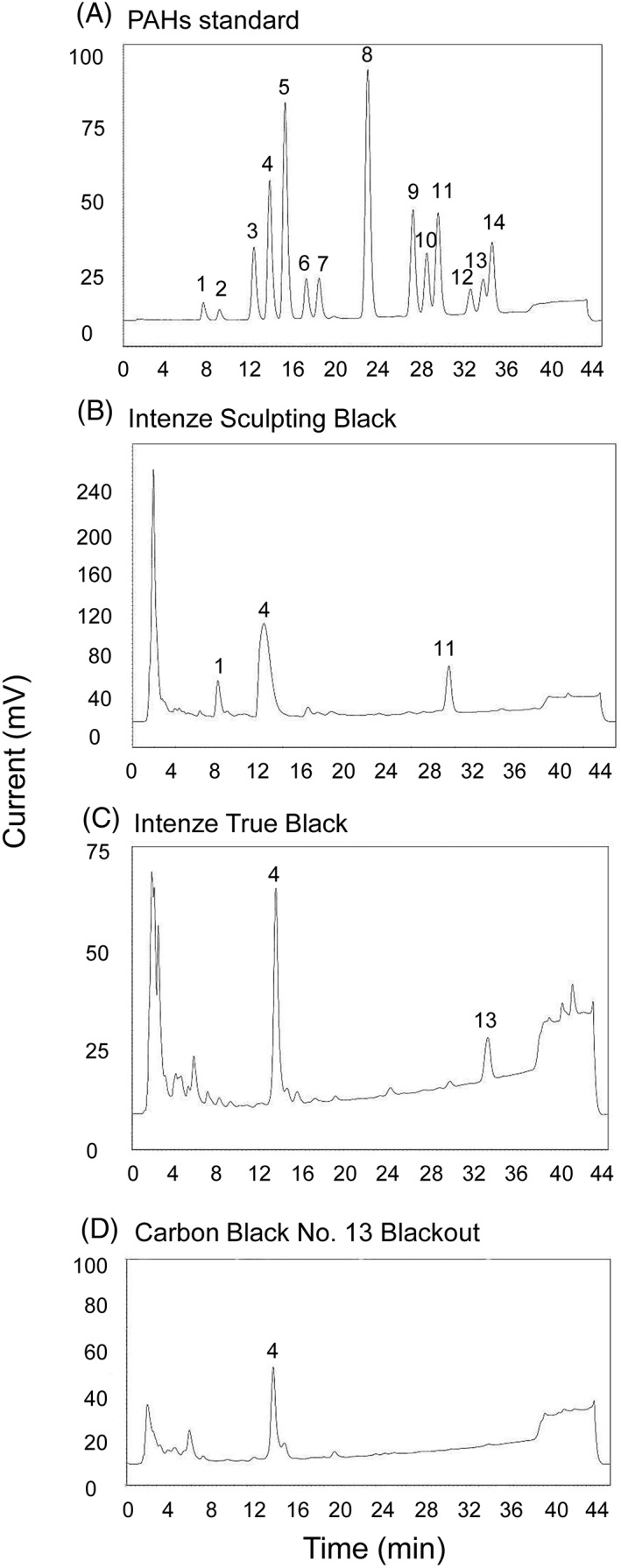
Black tattoo inks contain polycyclic aromatic hydrocarbons (PAHs). Comparative high‐performance liquid chromatography analysis was performed on the black tattoo inks. (A), PAH identification mixture. Peaks correspond to the following substances: (1) naphthalene; (2) acenaphthylene; (3) fluorene; (4) phenanthrene; (5) anthracene; (6) fluoranthene; (7) pyrene; (8) benz[a]anthracene/chrysene; (9) benzo[b]fluoranthene; (10) benzo[k]fluoranthene; (11) benzo[a]pyrene; (12) dibenz[ah]anthracene; (13) benzo[ghi]perylene; (14) indeno(1,2,3‐cd)pyrene. (B), Intenze Sculpting Black contains naphthalene, fluorene, and benzo[a]pyrene. (C), Intenze True Black contains phenanthrene and benzo[ghi]perylene. (D), Carbon Black No. 13 Blackout contains phenanthrene
4. DISCUSSION
From our clinical experience and from the scientific literature it can be concluded that tattoo inks may have deleterious health effects in the skin.1, 3 This finding calls for much stricter safety assessment of tattoo inks in the future. In this study, using the 3D organotypic RHS model and the sensitization biomarker IL‐18, we clearly show that 4 of 5 tested inks are cytotoxic (and therefore have irritant properties) and that 2 inks may have sensitization potential. Currently, in vitro analysis regarding the toxicity of tattoo inks has focused on cytotoxicity, genotoxicity, and reactive oxygen species production.7, 8, 9, 10 Our research is therefore the first to use an advanced in vitro RHS model to study tattoo ink‐induced complications observed in patients.
It is now generally accepted that skin sensitization will not be established without xenoinflammation, which involves triggering the inflammasome and the innate immune system (key events 2 and 3 in the AOP).20 Furthermore, the irritant potency of a chemical has been shown in vivo and in vitro to be directly related to sensitization potency, and it has also been shown that skin irritation is related to the development and severity of allergic contact dermatitis.29, 30, 31, 32 In this study, we have shown that the inks vary considerably in their irritant potency, as illustrated by the broad range of EC50 values obtained after addition of inks to the culture medium of RHS (EC50 0.04% to EC50 not reached). Notably, Eternal Ink Light Red and Intenze Sculpting Black had EC50 values in the same range as lactic acid (EC50 < 0.1%), suggesting that both inks have strong skin irritant and corrosive properties. Furthermore, these 2 inks were able to cause significant IL‐18 secretion, which is a key biomarker for the onset of skin sensitization (key event 2).
In our RHS (and in our RHE) assay, cytotoxicity is required, as this results in cell membrane permeability, which ensures the release of all intracellularly accumulated IL‐18 into the culture supernatant.23 A background level of IL‐18 can be measured after exposure to irritants and exposure to contact sensitizers. However, only upon exposure to a contact sensitizer can neosynthesis and intracellular accumulation of IL‐18 occur in the cell. Therefore, an increase in IL‐18 concentration is indicative of a skin sensitizer in both the RHS model and the RHE model.23 As our previous studies used RHE (rather than RHS), we cannot directly use the same prediction model for labelling and classifying sensitizers.22, 23 The current results indicate that the threshold for distinguishing between sensitizers and irritants is slightly higher with RHS than the RHE IL‐18 SI threshold of 5, as lactic acid in RHS has an IL‐18 SI of 8.3 ± 3.9. This may be attributable to the presence of fibroblasts in RHS and the different method used for chemical exposure, namely, dermal rather than topical epidermal. However, it should be noted that, even for commercially available and in‐house RHE, each model needs to define its own threshold.23 Notably, in comparison with the other tattoo inks and lactic acid, Eternal Ink Light Red and Intenze Sculpting Black had very high RHS SI IL‐18 values (88 ± 45 and 62 ± 15, respectively), indicating that these inks were activating key event 2 of the sensitization AOP. Further investigation with a standard panel of chemicals, as was used in the RHE model,23 is now required to validate the RHS dermal exposure model for comparing sensitization and irritant potency.
The assessment of tattoo inks in the RHS model will remain semiquantitative, as it will not be possible to extrapolate the EC50 and SI IL‐18 values to the in vivo tattoo ink concentrations, as we have done in the past for chemical sensitizers, because, currently, no tattoo ink human or animal (LLNA) chemical concentration data are available for such correlations.23 The importance of developing a model that includes a dermal exposure route is shown by the clinical study performed by Serup and Carlsen,2 who patch tested 79 patients with suspected allergy to red inks with 9 red pigments. Only 1 ink was positive in 9 of the patients. This may be attributable to the topical application method used in patch testing, with the red pigments not being able to penetrate the stratum corneum to trigger an immune response, or it may be attributable to the pigments used in the study not being present in the red tattoos that triggered the allergic symptoms. For these reasons, in our study we chose to expose RHS via the more relevant dermal route, and to investigate the complete tattoo inks as obtained from the suppliers.
As shown in Table 1, Eternal Ink Light Red contains the skin sensitizer Pigment Red 170. Therefore, it was expected that this ink would score positive as a skin sensitizer in our RHS model (high IL‐18 SI: 88 ± 45). In comparison, Intenze Gold Label Bright Red, which also causes clinical allergy, does not contain any substances with skin sensitization potential according to its label. However, on the basis of the chemical compositions of both the preservative diazolidinyl urea and Pigment Orange 13 present in Intenze Gold Label Bright Red, one may expect the release of oxidation products with skin sensitization capacity over time (ie, formaldehyde and 3,3′‐chlorobenzidine). This may explain the difference that we found between Eternal Ink Light Red (positive score as a skin sensitizer) and Gold Label Bright Red (negative score as a skin sensitizer) in our RHS model. We are aware of the fact that 24 hours of exposure is a current limitation of our RHS model, as this excludes possible toxic effects of red ink pro‐electrophiles and pre‐electrophiles (OECD 2012). These processes can be studied in the RHS model in the future by extending the exposure time, or by co‐exposure with ultraviolet light.
In order to investigate the black inks further, HPLC was used to screen for PAHs. The black inks contained a number of compounds correlating with PAHs, with Intenze Sculpting Black containing 1 compound (peak 11), which was confirmed as being benzo[a]pyrene. Considering the sensitizing (and carcinogenic) properties of PAHs, and in particular of benzo[a]pyrene,17, 28 these PAHs may be related to the cytotoxicity and high IL‐18 release observed after RHS exposure to Intenze Sculpting Black. Although reactions to black inks have been reported, such as papular and nodular reactions and phototoxic reactions, allergic reactions to black tattoo ink rarely occur.1, 2, 33, 34 One possible explanation for this may be that the PAHs are removed from the skin with time, in contrast to the encapsulated ink pigments.35, 36 Also, whereas a substantial increase in IL‐18 secretion was observed in the RHS after exposure to Intenze Sculpting Black, our RHS IL‐18 assay represents only keratinocyte activation (key event 2 of the sensitization AOP), and further downstream key events, such as dendritic cell activation and T cell priming, may possibly not occur. This will be a subject for further investigation.
Tattoo inks currently do not fall under European harmonized legislation, but under national regulations based on resolution CoE ResAP (2008)1.12 These regulatory frameworks differ between countries: some EU countries do not have specific legislation regarding tattoo safety; some countries regulate tattooing practices, but do not transpose the CoE ResAP into the national legislation; and some countries have adopted either CoE ResAP (2003)2 or CoE ResAP (2008)1.37 The European Chemicals Agency is therefore preparing a dossier for the restriction of hazardous chemicals in tattoo inks under the Registration, Evaluation, Authorization, and Restriction of Chemicals Regulation (REACH, Regulation [EC] No. 1907/2006). This document will provide information on all of the required toxicology endpoints.
5. CONCLUSION
Our results contribute to a better understanding of the tattoo‐induced complications observed in our outpatient tattoo clinic, where we observed most allergic reactions in red pigmented tattoos.4, 5 The substantial increase in IL‐18 release that we observed in RHS exposed to Eternal Ink Light Red supports the clinical data in suggesting that this ink may be responsible for chronic allergic reactions. As the number of people with a tattoo has been increasing significantly during the past decade, it is important for adequate safety assessment of tattoo inks to be implemented.
ACKNOWLEDGEMENTS
The authors wish to acknowledge Dr J. G. M. Bolscher for critically reading this manuscript and for discussions. This study was financed by Amsterdam University Medical Centers (location VU university medical center).
Conflict of interest
The authors declare no potential conflict of interests.
Bil W, van der Bent SAS, Spiekstra SW, Nazmi K, Rustemeyer T, Gibbs S. Comparison of the skin sensitization potential of 3 red and 2 black tattoo inks using interleukin‐18 as a biomarker in a reconstructed human skin model. Contact Dermatitis. 2018;79:336–345. 10.1111/cod.13092
Funding information Amsterdam VU university medical centers
REFERENCES
- 1. Piccinini P, Pakalin S, Contor L, Bianchi L, Senaldi C. Safety of tattoos and permanent make‐up: final report. EUR27947, 2016.
- 2. Serup J, Carlsen KH. Patch test study of 90 patients with tattoo reactions: negative outcome of allergy patch test to baseline batteries and culprit inks suggests allergen(s) are generated in the skin through haptenization. Contact Dermatitis. 2014;71:255‐263. [DOI] [PubMed] [Google Scholar]
- 3. Serup J, Hutton Carlsen K, Sepehri M. Tattoo complaints and complications: diagnosis and clinical spectrum. Curr Probl Dermatol. 2015;48:48‐60. [DOI] [PubMed] [Google Scholar]
- 4. van der Bent SAS, Maijer KI, Rustemeyer T. Image gallery: hyperkeratotic hypersensitivity reaction to red pigment tattoo. Br J Dermatol. 2017;177:e350. [DOI] [PubMed] [Google Scholar]
- 5. van der Bent SA, Wolkerstorfer A, Rustemeyer T. Cutaneous adverse reactions to tattoos. Ned Tijdschr Geneeskd. 2016;160:A9808. [PubMed] [Google Scholar]
- 6. Carlsen KH, Serup J. Chronic tattoo reactions cause reduced quality of life equaling cumbersome skin diseases. Curr Probl Dermatol. 2015;48:71‐75. [DOI] [PubMed] [Google Scholar]
- 7. Falconi M, Teti G, Zago M, et al. Influence of a commercial tattoo ink on protein production in human fibroblasts. Arch Dermatol Res. 2009;301:539‐547. [DOI] [PubMed] [Google Scholar]
- 8. Regensburger J, Lehner K, Maisch T, et al. Tattoo inks contain polycyclic aromatic hydrocarbons that additionally generate deleterious singlet oxygen. Exp Dermatol. 2010;19:e275‐e281. [DOI] [PubMed] [Google Scholar]
- 9. Neale PA, Stalter D, Tang JYM, Escher BI. Bioanalytical evidence that chemicals in tattoo ink can induce adaptive stress responses. J Hazard Mater. 2015;296:192‐200. [DOI] [PubMed] [Google Scholar]
- 10. Wamer WG, Yin JJ. Photocytotoxicity in human dermal fibroblasts elicited by permanent makeup inks containing titanium dioxide. J Cosmet Sci. 2011;62:535‐547. [PubMed] [Google Scholar]
- 11. Piccinini P, Contor L, Pakalin S, Raemaekers T, Senaldi C. Safety of tattoos and permanent make‐up. State of play and trends in tattoo practices. EUR27528, 2015.
- 12. Laux P, Tralau T, Tentschert J, et al. A medical‐toxicological view of tattooing. Lancet. 2016;387:395‐402. [DOI] [PubMed] [Google Scholar]
- 13. Wijnhoven S W P, Ezendam J, Schuur A G, Loveren H V, Engelen J G M V. Allergens in consumer products. RIVM Report 320025001/2008, 2008.
- 14. Ryberg K, Agner T, Andersen KE, et al. Patch testing with a textile dye mix—a multicentre study. Contact Dermatitis. 2014;71:215‐223. [DOI] [PubMed] [Google Scholar]
- 15. Isaksson M, Ale I, Andersen KE, et al. Patch testing to a textile dye mix by the International Contact Dermatitis Research Group. Dermatitis. 2015;26:170‐176. [DOI] [PubMed] [Google Scholar]
- 16. SCCS . Opinion on Carbon Black (nano‐form). SCCS/1515/13, 2013.
- 17. International Agency for Research on Cancer, eds . Carbon black, titanium dioxide, and talc Monographs on the Evaluation of Carcinogenic Risk to Humans. Lyon, France: World Health Organization Press; 2010: 1‐466. [PMC free article] [PubMed] [Google Scholar]
- 18. OECD . The Adverse Outcome Pathway for Skin Sensitisation Initiated by Covalent Binding to Proteins. Part 1: Scientific Evidence. Paris: OECD Publishing; 2012. [Google Scholar]
- 19. Rovida C, Alepee N, Api AM, et al. Integrated Testing Strategies (ITS) for safety assessment. ALTEX. 2015;32:25‐40. [DOI] [PubMed] [Google Scholar]
- 20. Martin SF, Esser PR, Weber FC, et al. Mechanisms of chemical‐induced innate immunity in allergic contact dermatitis. Allergy. 2011;66:1152‐1163. [DOI] [PubMed] [Google Scholar]
- 21. Corsini E, Galbiati V, Mitjans M, Galli CL, Marinovich M. NCTC 2544 and IL‐18 production: a tool for the identification of contact allergens. Toxicol In Vitro. 2013;27:1127‐1134. [DOI] [PubMed] [Google Scholar]
- 22. Galbiati V, Papale A, Marinovich M, Gibbs S, Roggen E, Corsini E. Development of an in vitro method to estimate the sensitization induction level of contact allergens. Toxicol Lett. 2017;271:1‐11. [DOI] [PubMed] [Google Scholar]
- 23. Gibbs S, Corsini E, Spiekstra SW, et al. An epidermal equivalent assay for identification and ranking potency of contact sensitizers. Toxicol Appl Pharmacol. 2013;272:529‐541. [DOI] [PubMed] [Google Scholar]
- 24. Teunis M, Corsini E, Smits M, et al. Transfer of a two‐tiered keratinocyte assay: IL‐18 production by NCTC2544 to determine the skin sensitizing capacity and epidermal equivalent assay to determine sensitizer potency. Toxicol In Vitro. 2013;27:1135‐1150. [DOI] [PubMed] [Google Scholar]
- 25. Spiekstra SW, Toebak MJ, Sampat‐Sardjoepersad S, et al. Induction of cytokine (interleukin‐1alpha and tumor necrosis factor‐alpha) and chemokine (CCL20, CCL27, and CXCL8) alarm signals after allergen and irritant exposure. Exp Dermatol. 2005;14:109‐116. [DOI] [PubMed] [Google Scholar]
- 26. Kosten IJ, Buskermolen JK, Spiekstra SW, De Gruijl TD, Gibbs S. Gingiva equivalents secrete negligible amounts of key chemokines involved in Langerhans cell migration compared to skin equivalents. J Immunol Res. 2015;2015:627125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65:55‐63. [DOI] [PubMed] [Google Scholar]
- 28. Gerberick GF, Ryan CA, Kern PS, et al. Compilation of historical local lymph node data for evaluation of skin sensitization alternative methods. Dermatitis. 2005;16:157‐202. [PubMed] [Google Scholar]
- 29. Basketter DA, Kan‐King‐Yu D, Dierkes P, Jowsey IR. Does irritation potency contribute to the skin sensitization potency of contact allergens? Cutan Ocul Toxicol. 2007;26:279‐286. [DOI] [PubMed] [Google Scholar]
- 30. Bonneville M, Chavagnac C, Vocanson M, et al. Skin contact irritation conditions the development and severity of allergic contact dermatitis. J Invest Dermatol. 2007;127:1430‐1435. [DOI] [PubMed] [Google Scholar]
- 31. Grabbe S, Steinert M, Mahnke K, Schwartz A, Luger TA, Schwarz T. Dissection of antigenic and irritative effects of epicutaneously applied haptens in mice. Evidence that not the antigenic component but nonspecific proinflammatory effects of haptens determine the concentration‐dependent elicitation of allergic contact dermatitis. J Clin Invest. 1996;98:1158‐1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Mclelland J, Shuster S, Matthews JN. ‘Irritants’ increase the response to an allergen in allergic contact dermatitis. Arch Dermatol. 1991;127:1016‐1019. [PubMed] [Google Scholar]
- 33. Sepehri M, Hutton Carlsen K, Serup J. Papulo‐nodular reactions in black tattoos as markers of sarcoidosis: study of 92 tattoo reactions from a hospital material. Dermatology. 2016;232:679‐686. [DOI] [PubMed] [Google Scholar]
- 34. Carlsen KH, Serup J. Photosensitivity and photodynamic events in black, red and blue tattoos are common: A ‘Beach Study’. J Eur Acad Dermatol. 2014;28:231‐237. [DOI] [PubMed] [Google Scholar]
- 35. Lehner K, Santarelli F, Vasold R, et al. Black tattoos entail substantial uptake of genotoxic polycyclic aromatic hydrocarbons (PAH) in human skin and regional lymph nodes. PLoS One. 2014;9:e92787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kaur RR, Kirby W, Maibach H. Cutaneous allergic reactions to tattoo ink. J Cosmet Dermatol. 2009;8:295‐300. [DOI] [PubMed] [Google Scholar]
- 37. Piccinini P, Bianchi L, Pakalin S, Senaldi C. Safety of tattoos and permanent make‐up. Compilation of information on legislative framework and analytical methods. EUR27394, 2015.


