Abstract
Prolactin and placental lactogens control mammary development and lactation as well as play an important role in maternal behaviors. However, the molecular mechanisms in the brain responsible for this regulation remain largely unknown. Therefore, the present study investigated whether Signal Transducer and Activator of Transcription 5 (STAT5) signaling in the brain, the key transcriptional factor recruited by prolactin receptor and other hormones, is required for postpartum maternal behavior, maintenance of lactation and offspring growth. Neuronal ablation of STAT5 impaired the control of prolactin secretion and reduced the hypothalamic expression of suppressors of cytokine signaling (i.e., SOCS3 and CISH). In addition, neuronal STAT5 deletion attenuated the hyperphagia commonly observed during lactation by decreasing the hypothalamic expression of orexigenic neurotransmitters such as the neuropeptide Y and agouti-related protein. The lower food intake of lactating neuron-specific STAT5 knockout females resulted in reduced milk production and offspring growth. Unexpectedly, postpartum maternal behavior expression was not impaired in neuron-specific STAT5 knockout females. On the contrary, the latency to retrieve and group the pups into the nest was reduced in mutant dams. Finally, we demonstrated that approximately 30% of recorded neurons in the medial preoptic area were acutely depolarized by prolactin suggesting that fast STAT5-independent signaling pathways may be involved in the regulation of maternal behaviors. Overall, our results revealed important information about the molecular mechanisms recruited by hormones to orchestrate the activation of neural circuitries engaged in the induction of maternal care.
Keywords: Prolactin, Hypothalamus, Signaling pathways, Preoptic region
Introduction
The induction of maternal behaviors depends on environmental cues including olfactory and other sensory information, as well as internal signals such as changing levels of several hormones (i.e., estradiol, progesterone, prolactin, placental lactogens and oxytocin) (Dobolyi et al., 2014; Dulac et al., 2014). Most of these hormones are already altered during pregnancy priming the neural circuitries engaged in the regulation of maternal behaviors for the upcoming need to nurture the offspring (Augustine et al., 2008). Prolactin and placental lactogens, which bind to the same receptor, are particularly important to induce maternal behaviors in rodents (Kelly et al., 2001; Larsen and Grattan, 2012). For example, central administration of prolactin or placental lactogens stimulates maternal behaviors (Bridges et al., 1985, 1990, 1996, 1997; Bridges and Freemark, 1995; Mann and Bridges, 2001). In addition, prolactin receptor (PrlR) knockout mouse exhibits a deficiency in the expression of these behaviors (Lucas et al., 1998). Moreover, central infusion of prolactin receptor antagonist delays the onset of maternal behavior in rats (Bridges et al., 2001).
PrlR is member of the type I cytokine receptor family and therefore recruits different intracellular signaling pathways such as the Phosphatidylinositol-4,5-bisphosphate 3-kinase (PI3K) and the Signal Transducer and Activator of Transcription 5 (STAT5). It is believed that STAT5 is the key transcriptional factor activated by the PrlR to induce its classical effects as several studies have shown similar phenotypes between Prlr and Stat5a/b knockout mice such as impaired mammary gland development (Bole-Feysot et al., 1998; Cui et al., 2004; Liu et al., 1997; Ormandy et al., 1997; Teglund et al., 1998). Furthermore, STAT5 signaling modulates the activity of tuberoinfundibular dopaminergic (TIDA) neurons which control pituitary prolactin secretion by negative feedback (Grattan et al., 2001; Ma et al., 2005). When activated, the PrlR induces STAT5 phosphorylation (pSTAT5) which is then translocated to the nucleus to regulate the transcription of target genes (Bole-Feysot et al., 1998). Taking advantage of the fact that peripheral or central prolactin infusion induces the nuclear expression of pSTAT5 in specific brain areas, previous studies were able to identify the distribution of prolactin-responsive neurons in the rostral and mediobasal hypothalamus (Brown et al., 2010; Furigo et al., 2014; Nagaishi et al., 2014; Sapsford et al., 2012). The expression of prolactin-induced pSTAT5 immunoreactive neurons is particularly abundant in the preoptic region including the medial preoptic area (MPA), the anteroventral periventricular nucleus and the medial preoptic nucleus (Brown et al., 2010; Furigo et al., 2014). This finding is in accordance with the critical role of the preoptic region in the regulation of maternal behaviors (Numan, 2006; Numan and Nagle, 1983; Numan et al., 1977). For example, infusion of prolactin or placental lactogens into the MPA stimulates maternal behaviors in rats (Bridges and Freemark, 1995; Bridges et al., 1997; Numan, 2006; Numan and Nagle, 1983; Numan et al., 1977).
Although previous studies have established the role of several hormones in the expression of maternal behaviors (Dulac et al., 2014), the molecular mechanisms in the brain responsible for this regulation remain poorly known. Therefore, the objective of the present study was to investigate if central STAT5 signaling, as the major pathway recruited by PrlR (Bole-Feysot et al., 1998), is required for the expression of postpartum maternal behavior, lactation and consequently offspring growth. For this purpose, we generated neuron-specific STAT5 knockout (N-STAT5 KO) mice and physiologic, molecular and behavior studies were performed on females during the lactation period. In addition, we determined whether cells in the preoptic region were able to acutely respond to prolactin through STAT5-independent pathways.
Material and methods
Generation of neuron-specific STAT5 knockout mice
All animal procedures were approved by the Ethics Committee on the Use of Animals of the Institute of Biomedical Sciences, University of São Paulo (protocol number 12, approved on 03/19/2013). To induce neuronal deletion of Stat5a and Stat5b genes, the Nestin-Cre strain (B6.Cg-Tg(Nes-cre)1Kln/J, Jackson Laboratories) was bred with mice carrying loxP-flanked Stat5a/b alleles as previously described (Cui et al., 2004; Lee et al., 2008). Mice carrying neuronal deletion of STAT5 were homozygous for the loxP-flanked Stat5a/b alleles and hemizygous for the Nestin-Cre transgene, whereas the control group was composed of littermate animals carrying only the loxP-flanked Stat5a/b alleles. Mice were weaned at 3–4 weeks of age and the genomic DNA was extracted from tail tip for genotyping through PCR using a commercially available kit (Sigma). To confirm the efficacy of the deletion, mice were perfused, as described later, 90 min after receiving an intraperitoneal (i.p.) injection of ovine prolactin (5 μg/g, Sigma). We assessed the ability of prolactin to induce pSTAT5-immunoreactivity (pSTAT5-ir) in the brain of control and N-STAT5 KO females. In addition, the hypothalamus of an additional group of control and N-STAT5 KO females was collected to determine the expression of STAT5a and STAT5b mRNA levels.
Evaluation of lactation, postpartum maternal behavior and offspring growth
Control and N-STAT5 KO females mated with sexually-experienced wild-type C57BL/6 males. After confirming the pregnancy, the mice were single-housed and monitored daily to determine the day of birth which was considered day 1 of lactation (L1). Their body weight and food intake were determined at L1, L2, L5, L8 and L10. To guarantee comparable metabolic demands during lactation, we standardized 5 pups per litter at L2. The offspring mass was determined at L2, L5, L8, L10 and L14. Maternal behavior was assessed at L5 and L8. Before the test, the litter (5 pups) was weighed and separated from the mother for 4 h. After that, the litter was weighed again and distributed in the corners of the female’s cage. We evaluated the latency to contact, to retrieve and to group the pups into the nest and to crouch over them. After 1 h from the beginning of the test, the offspring were weighed and the difference between the mass in the beginning and end of the test represented the milk production which was expressed as g/pup/h. A group of lactating control and N-STAT5 KO females were euthanized at L10 to analyze serum prolactin levels by ELISA (RAB0408; Sigma) and the relative gene expression in the hypothalamus and mammary gland. In addition, pSTAT5 expression was assessed in the mammary gland by immunoblotting using a protocol described previously (Pedroso et al., 2014; Zampieri et al., 2013). The females were kept with their litter until the moment of the euthanasia.
Perfusion and tissue processing
Mice were deeply anesthetized and perfused transcardially with saline followed by a 10% buffered formalin solution (150–200 mL per mouse). Brains were collected and post-fixed in the same fixative for 1–2 h and cryoprotected overnight at 4 °C in 0.1 M PBS with 20% sucrose, pH 7.4. Brains were cut (30-μm thick sections) in the frontal plane using a freezing microtome. Four series of tissue were collected in antifreeze solution and stored at −20 °C.
Immunohistochemistry
Brain sections were rinsed in 0.02 M potassium PBS, pH 7.4 (KPBS), followed by a pretreatment in an alkaline (pH > 13) water solution containing 1% hydrogen peroxide and 1% sodium hydroxide for 20 min. After rinsing in KPBS, sections were incubated in 0.3% glycine and0.03% lauryl sulfate for 10 min each. Next, sections were blocked in 3% normal donkey serum for 1 h, followed by incubation in anti-pSTAT5Tyr694 primary antibody (1:1000; Cell Signaling; #9351) for 40 h. Subsequently, sections were incubated for 1 h in biotin-conjugated secondary antibody (1:1000, Jackson Laboratories) and next for 1 h with an avidin–biotin complex (1:500, Vector Labs). The peroxidase reaction was performed using 0.05% 3,3′-diaminobenzidine (DAB), 0.25% nickel sulfate and 0.03% hydrogen peroxide. Photomicrographs of brain sections were acquired with a Zeiss Axiocam HRc camera adapted to a Zeiss Axioimager A1 microscope (Zeiss, Munich, Germany). Images were digitized using Axiovision software (Zeiss). Photoshop image-editing software was used to combine photomicrographs into plates. Only sharpness, contrast and brightness were adjusted.
Relative gene expression
Total RNA from the hypothalamus and mammary gland was extracted with TRIzol reagent (Invitrogen). Assessment of RNA quantity and quality was performed with an Epoch Microplate Spectrophotometer (Biotek). Total RNA was incubated in DNase I RNase-free (Roche Applied Science). Reverse transcription was performed with 2 μg of total RNA with SuperScript II Reverse Transcriptase (Invitrogen) and random primers p(dN)6 (Roche Applied Science). Real-time polymer-ase chain reaction was performed using the 7500 Fast Real-Time PCR System (Applied Biosystems) and Power SYBR Green PCR Master Mix (Applied Biosystems). Specific primers were designed for each target gene according to sequences taken from GenBank. Melt curve analysis was conducted to validate the specificity of the primers. Relative quantification of mRNA was calculated by 2−ΔΔCt. Data were normalized to geometric average of GAPDH and cyclophilin A, and reported as fold changes compared to values obtained from the control group (set at 1.0).
Whole-cell recording
Whole-cell patch-clamp recordings were performed in neurons of the MPA in brain slices of N-STAT5 KO female mice on diestrus and during lactation (at day 5 or 6). During the recordings, neurons were maintained in hypothalamic slice preparations and data analyses were performed as previously described (Frazao et al., 2013). Mice were decapitated and the entire brain was removed. After removal, brains were immediately submerged in ice-cold, carbogen-saturated (95% O2 and 5% CO2) artificial cerebrospinal fluid (aCSF; 126 mM NaCl, 2.8 mM KCl, 1.2 mM MgCl2, 2.5 mM CaCl2, 1.25 mM NaH2PO4, 26 mM NaHCO3, and 5 mM glucose). Coronal sections from a hypothalamic block (250 μM thick) were cut with a Leica VT1000S vibratome and then incubated in oxygenated aCSF at room temperature for at least 1 h before recording. Slices were transferred to the recording chamber and allowed to equilibrate for 10–20 min before recording. The slices were bathed in oxygenated aCSF (30 °C–32 °C) at a flow rate of ~2 mL/min. The pipette solution for whole-cell recording was modified to include an intracellular dye (Alexa Fluor 488) for whole-cell recording: 120 mM K-gluconate, 10 mM KCl, 10 mM HEPES, 5 mM EGTA, 1 mM CaCl2, 1 mM MgCl2, 2 mM (Mg)-ATP, and 0.03 mM Alexa Fluor 488 hydrazide dye, pH 7.3. Infrared differential interference contrast was used to target and obtain the whole-cell recording of neurons at the MPA (Leica DM6000 FS equipped with a fixed stage and a Leica DFC360 FX high speed monochrome fluorescence digital camera). Electrophysiological signals were recorded using an Axopatch 700B amplifier (Molecular Devices), low-pass filtered at 2–5 kHz, and analyzed offline on a PC with pCLAMP programs (Molecular Devices). Recording electrodes had resistances of 2.5–5 MΩ when filled with the K-gluconate internal solution. Input resistance was assessed by measuring voltage deflection at the end of the response to a hyperpolarizing rectangular current pulse (500 ms of −10 to −50 pA). Membrane potential values were compensated for junction potential (−8 mV). Solutions containing ovine prolactin (250 nM; Sigma) were typically perfused for 5 min as previously described (Lyons et al., 2012). Alexa Fluor 488 hydrazide dye was used to determine the position of the recorded cells related to the third ventricle.
Statistical analysis
The results are expressed as the mean ± SEM. The differences between the groups were compared using an unpaired two-tailed Student’s t-test. For the electrophysiological studies, we used the paired two-tailed Student’s t test to compare the data before and after the drug application. Statistical analyses were performed using GraphPad Prism software. Degrees of freedom (df) for t statistics were marked as t(df). We used the Cohen’s d to calculate the effect size estimates as d = (t ∗ 2) / square root (df). We considered p values less than 0.05 to be statistically significant.
Results
Validation of neuron-specific STAT5 knockout mice
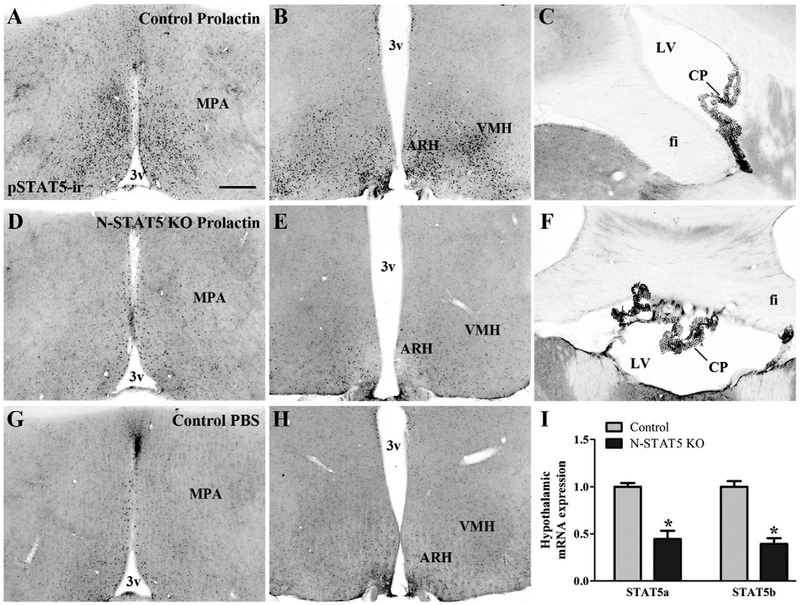
As previously demonstrated (Brown et al., 2010; Furigo et al., 2014; Nagaishi et al., 2014; Sapsford et al., 2012), prolactin administration induced nuclear pSTAT5-ir in several hypothalamic areas (Figs. 1A–C) compared to mice injected with PBS (Figs. 1G–H). For example, a large number of pSTAT5 immunoreactive neurons were observed in the preoptic region (Fig. 1A), in the arcuate nucleus of the hypothalamus (ARH; Fig. 1B) and in the ventromedial nucleus of the hypothalamus (Fig. 1B) of prolactin-injected control mice. However, neuron-specific STAT5 knockout (N-STAT5 KO) mice showed a striking reduction in the number of prolactin-induced pSTAT5 immunoreactive cells in the entire hypothalamus (Figs. 1D–E). Interestingly, choroid plexus showed similar pSTAT5-ir in both control (Fig. 1C) and N-STAT5 KO (Fig. 1F) mice indicating that some non-neuronal cells still express functional STAT5 genes in our conditional knockout model. Furthermore, gene expression analysis indicated significant reductions in STAT5a mRNA (t(10) = 4.99, d = 3.15, p = 0.0005) and STAT5b mRNA (t(10) = 6.81, d = 4.31, p < 0.0001) expression in the hypothalamus of N-STAT5 KO females compared to control mice (Fig. 1I). The fact that these animals still showed some mRNA expression is in accordance with the fact that STAT5 deletion occurred specifically in neurons, sparing other cell types (i.e., glia, epithelial and blood cells), since gene expression analyses were performed in the whole hypothalamus. Overall, our results indicate the efficacy of neuron-specific deletion of STAT5 genes.
Fig. 1.
Validation of neuron-specific STAT5 knockout mice. A–H. Photomicrographs of brain sections showing the pSTAT5-ir in prolactin-treated control (A–C) and N-STAT5 KO (D–F) mice as well as PBS-treated control mice (G–H). I. Bar graphs showing the expression of STAT5a and STAT5b mRNA in the hypothalamus of control (n = 5) and STAT5 KO (n = 7) mice. Abbreviations: 3v, third ventricle; ARH, arcuate nucleus of the hypothalamus; CP, choroid plexus; fi, fimbria of the hippocampus; LV, lateral ventricle; MPA, medial preoptic area; VMH, ventromedial nucleus of the hypothalamus. Scale bar = 200 μm. *p < 0.05 compared to the control group.
Normal gestation, but reduced food intake during lactation in neuron-specific STAT5 knockout females
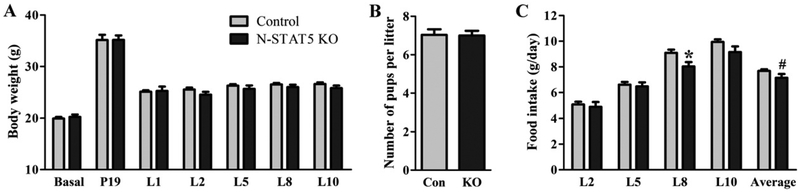
Since earlier studies found that neuronal deletion of STAT5 leads to late-onset obesity (Lee et al., 2008), we studied young adult N-STAT5 KO females and their respective littermate controls. Baseline body weight (before mating) was similar between groups (t(25) = 0.52, d = 0.21, p = 0.6084; Fig. 2A). There were no changes in fertility given that every female in this experiment, both control and N-STAT5 KO, got pregnant. In addition, N-STAT5 KO females went through gestation without expressing any noticeable alterations, and their body weight at the end of pregnancy (P19) was similar to that of control animals (t(14) = 0.01, d = 0.00, p = 0.9947; Fig. 2A). Control and N-STAT5 KO females gave birth to a similar number of pups (t(56) = 0.07, d = 0.02, p = 0.9452; Fig. 2B). During lactation, no significant changes in body weight of control and N-STAT5 KO females were found (Fig. 2A). However, the typical hyperphagia observed during lactation was attenuated in N-STAT5 KO females because these mice showed reduced food intake at day 8 of lactation (t(22) = 2.54, d = 1.08, p = 0.0189) and on average during the first 10 days of lactation (t(22) = 1.96, d = 0.84, p = 0.0623; Fig. 2C).
Fig. 2.
Differences in body weight, food intake and litter size between control and N-STAT5 KO mice. A. Changes in the body weight before pregnancy, at day 19 of pregnancy and during lactation (n = 10–17/group). B. Litter size at birth (n = 23–35/group). C. Daily food intake during lactation (n = 8–16/group). *p < 0.05 compared to the control group. #p = 0.0623 compared to the control group.
Decreased offspring growth in neuron-specific STAT5 knockout mice is caused by reduction in milk production
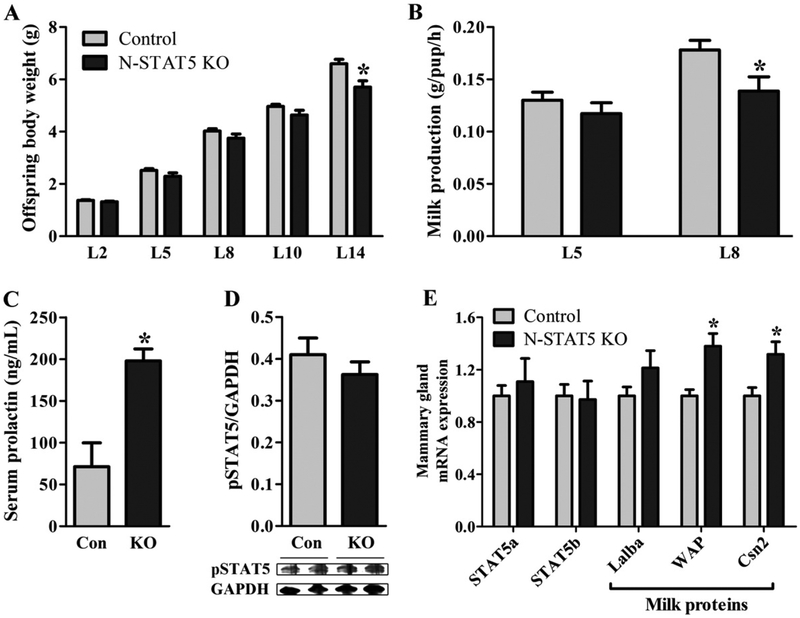
A similar percentage (approximately 80%) of primiparous control and N-STAT5 KO females was able to successfully support their off-spring. We assessed offspring growth as an indirect indicator of lactation performance. Although the litters from N-STAT5 KO females exhibited similar body weight until day 10 (t(25) = 1.88, d = 0.75, p = 0.0717), we observed a progressively lower weight gain rate when compared to control animals (Fig. 3A). Consequently, litters from N-STAT5 KO mice showed significantly lower body weight by day 14 than those from control dams (t(8) = 3.05, d = 2.15, p =0.0159; Fig. 3A). To investigate possible factors that could contribute to the decreased litter growth, we evaluated milk production during lactation. No significant changes in milk production were observed at day 5 (t(22) = 0.97, d = 0.42, p = 0.3405; Fig. 3B). However, N-STAT5 KO dams had a decreased milk production at day 8 (t(22) = 2.41, d = 1.03, p = 0.0247), when the demand for milk is higher due to the fast offspring growth (Fig. 3B). Since prolactin is a key hormone to stimulating milk production, we assessed serum prolactin levels during lactation. N-STAT5 KO females showed a 2.5 fold increase in serum prolactin levels compared to control mice (t(16) = 4.22, d = 2.11, p =0.0006; Fig. 3C). Therefore, reduced milk production is not caused by prolactin deficiency in N-STAT5 KO females. To confirm that the mammary glands of lactating N-STAT5 KO females were not affected by the genetic deletion, we determined pSTAT5 levels in the mammary tissue of lactating N-STAT5 KO females and no differences were found compared to control group (t(10) = 0.94, d = 0.60, p = 0.3678; Fig. 3D). STAT5a and STAT5b mRNA levels were also assessed in the mammary tissue and no significant differences between groups were observed (STAT5a: t(10) = 0.48, d = 0.31, p = 0.6389; STAT5b: t(10) = 0.16, d = 0.10, p = 0.8797; Fig. 3E), indicating that reduced milk production was not caused by changes in STAT5 signaling in the mammary gland. Interestingly, we observed an increased expression of mRNAs that codify milk proteins in the mammary tissue of lactating N-STAT5 KO females including the whey acidic protein (WAP; t(10) = 3.10, d = 1.96, p = 0.0113) and casein beta (Csn2; t(10) = 2.53, d = 1.60, p = 0.0298), but not the alpha-lactalbumin (Lalba; t(10) = 1.27, d = 0.81, p = 0.2319; Fig. 3E).
Fig. 3.
Offspring growth, milk production, prolactin levels during lactation and gene expression in the mammary gland. A. Changes in the offspring body weight (n = 10–17/group). B. Milk production at the days 5 and 8 of lactation (n = 8–16/group). C. Serum prolactin levels at L10 (n = 8–10/group). D. Phosphorylation of STAT5 (pSTAT5) in the mammary tissue of females at day 10 of lactation (n = 6/group). E. mRNA expression in the mammary tissue of females at day 10 of lactation (n = 5–7/group). *p < 0.05 compared to the control group.
Neuronal STAT5 signaling is not required for the expression of postpartum maternal behaviors
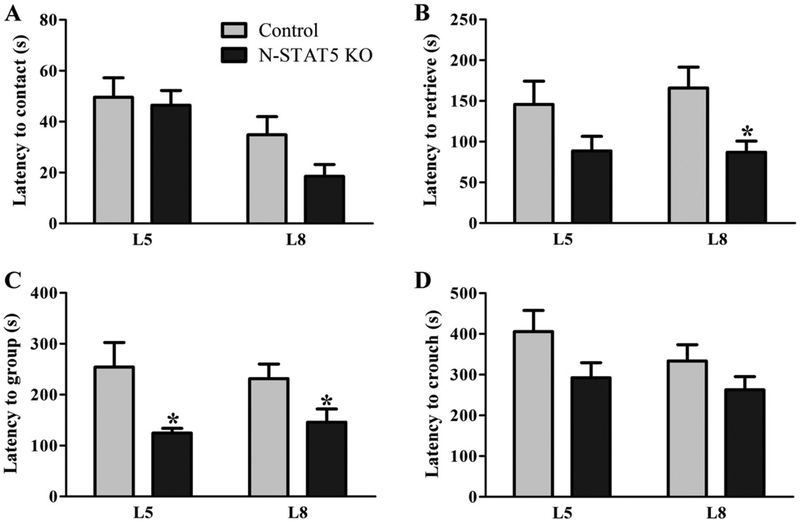
To evaluate maternal behaviors, we first determined the latency to contact the pups. No significant changes were observed between the two groups (L5: t(22) = 0.27, d = 0.11, p = 0.7895; L8: t(21) = 1.44, d = 0.63, p = 0.1652; Fig. 4A). Then, we assessed the latency to retrieve all pups into the nest, to group them and to crouch over to initiate feeding. Surprisingly, N-STAT5 KO females exhibited improved maternal behavior because they showed reduced latency to retrieve all pups (L5: t(21) = 1.69, d = 0.74, p = 0.1055; L8: t(20) = 2.73, d = 1.22, p = 0.013; Fig. 4B) and to group them into the nest (L5: t(16) = 2.64, d = 1.32, p = 0.018; L8: t(22) = 2.20, d = 0.94, p = 0.0383; Fig. 4C). No statistically significant difference between groups was observed in the latency to crouch over (L5: t(24) = 1.57, d = 0.64, p = 0.1307; L8: t(24) = 1.174, d = 0.48, p = 0.252; Fig. 4D).
Fig. 4.
Maternal behavior expression in lactating control and N-STAT5 KO mice. A–D. Latency to contact and retrieve all pups, to group them into the nest and to crouch over (n = 10–17/group). *p < 0.05 compared to the control group.
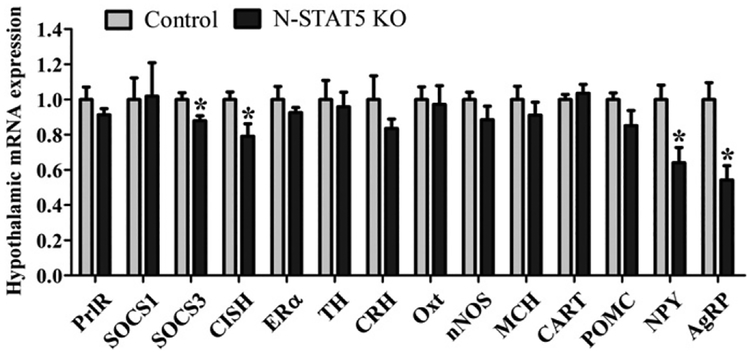
Reduced hypothalamic expression of Socs3, Cish, Npy and Agrp in lactating N-STAT5 KO females
To understand the physiological and behavioral changes observed in lactating N-STAT5 KO females, we assessed their hypothalamic gene expression at day 10 of lactation (Fig. 5). Initially, we investigated possible changes in the expression of genes related with PrlR signaling. No changes in the PrlR mRNA (t(10) = 1.21, d = 0.77, p = 0.2522) and suppressor of cytokine signaling 1 (SOCS1; t(10) = 0.07, d = 0.05, p = 0.9441) mRNA expression were observed between the groups (Fig. 5). However, N-STAT5 KO females exhibited reduced hypothalamic expression of SOCS3 (t(10) = 2.59, d = 1.64, p = 0.027) and cytokineinducible SH2-containing protein (CISH; t(10) = 2.29, d = 1.45, p =0.0453) mRNA compared to control mice (Fig. 5). Regarding neurotransmitters involved in the regulation of behavior expression, we did not observe significant differences between the groups in mRNA levels of estrogen receptor α (ERα; t(10) = 1.05, d = 0.67, p = 0.317), tyrosine hydroxylase (TH; t(10) = 0.32, d = 0.20, p = 0.7589), corticotropinreleasing hormone (CRH; t(10) = 1.29, d = 0.81, p = 0.2272), oxytocin (Oxt; t(10) = 0.20, d = 0.13, p = 0.8436), neuronal nitric oxide synthase (nNOS; t(10) = 1.16, d = 0.74, p = 0.2715), melanin-concentrating hormone (MCH; t(10) = 0.82, d = 0.52, p = 0.429) and cocaine and amphetamine regulated transcript (CART; t(10) = 0.55, d = 0.35, p = 0.595; Fig. 5). Because N-STAT5 KO females showed reduced food intake during lactation we also assessed the mRNA expression of neurotransmitters involved in the regulation of the energy balance such as pro-opiomelanocortin (POMC), neuropeptide Y (NPY) and agouti-related protein (AgRP). Although no changes were observed in POMC expression (t(8) = 1.34, d = 0.95, p = 0.2175), NPY (t(10) = 2.89, d = 1.82, p = 0.0163) and AgRP (t(10) = 3.64, d = 2.30, p = 0.0046) expression were significantly reduced in the hypothalamus of N-STAT5 KO females compared to controls (Fig. 5).
Fig. 5.
Hypothalamic mRNA expression analysis of control (n = 5) and N-STAT5 KO (n = 7) mice at day 10 of lactation. *p < 0.05 compared to the control group.
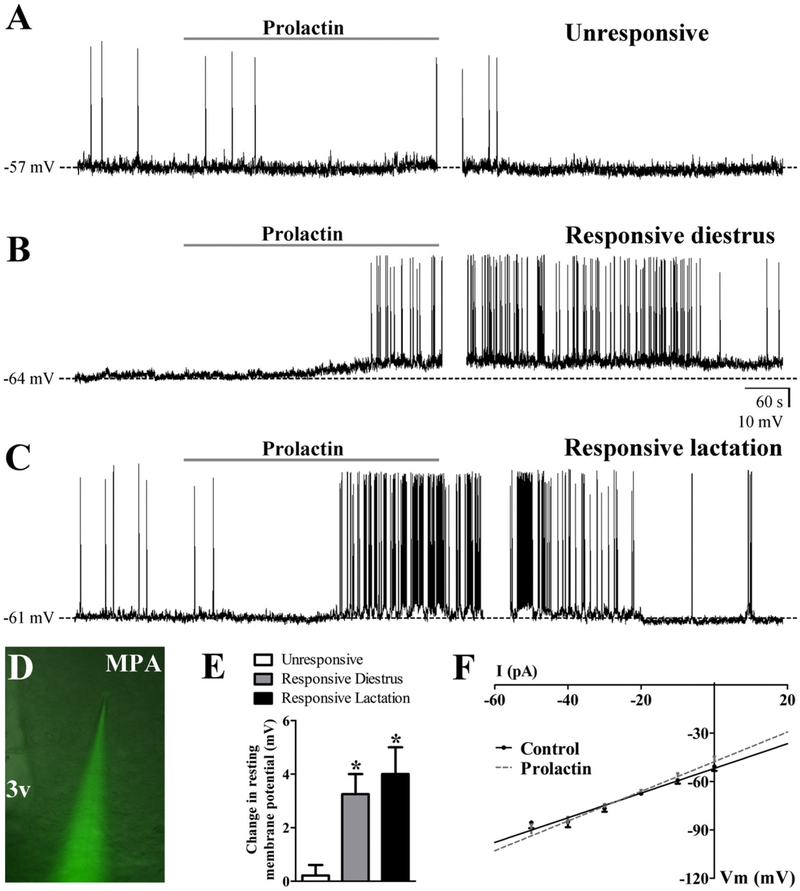
PrlR recruits fast STAT5-independent signaling pathways to acutely modify the activity of neurons in the preoptic region
Since the classic genomic STAT5 signaling pathway is not required for the expression of postpartum maternal behavior, we investigated if PrlR acutely activates alternative signaling pathways in the brain. For this purpose, we determined possible prolactin-induced changes in the cellular activity of neurons located in the preoptic region which is a critical site involved in prolactin’s effects on maternal behavior expression (Bridges and Freemark, 1995; Bridges et al., 1997; Numan, 2006; Numan and Nagle, 1983; Numan et al., 1977). We studied brain slices of N-STAT5 KO females on diestrus and during lactation to investigate possible STAT5-independent prolactin-induced acute responses. In current-clamp mode, MPA neurons were recorded under zero current injection (I = 0) in whole-cell patch-clamp configuration (Fig. 6). The average resting membrane potential (RMP) of all MPA neurons recorded in females on diestrus was −58.2 ± 1.7 mV (range from −49 to −68 mV, 12 cells out of 6 animals). Regarding lactating females, the RMP of the MPA neurons was similar to that observed in females on diestrus (−57.9 ± 3.1 mV, range from −44 to −73 mV, 8 cells out of 4 animals). The MPA neurons had an average steady-state capacitance of 12.4 ± 1.5 pF in females on diestrus (n = 12 cells) and 13.1 ±2.1 pF in lactating females (n = 5 cells). In both groups (diestrus and lactation), most of the MPA neurons recorded (n = 16 out of 20 cells) exhibited overshooting action potentials (APS) with an irregular firing pattern (1.2 ± 0.3 Hz). Therefore, based on the biophysical parameters analyzed, MPA neurons of females on diestrus exhibited similar basal characteristics compared to the MPA neurons recorded in lactating mice. In current clamp mode, the resting period was typically observed during 15–20 min before drug application. Notably, prolactin evoked a similar response in the MPA neurons of females on diestrus or during lactation. Regarding that, we observed a depolarization from rest in 4 out of 12 MPA neurons of females on diestrus (33%, RMP before prolactin: −60.0 ± 3.5 mV; after prolactin: −56.7 ± 3.6 mV; change in RMP: +3.3 ± 0.7 mV; Figs. 6B,E) and in 2 out 8 MPA neurons of lactating mice (25%, RMP before prolactin: −56.0 ± 5.0 mV; after prolactin: −52.0 ± 4.0 mV; change in RMP: +4.0 ± 1.0 mV; Figs. 6C,E). Since the overall response was similar when comparing females on diestrus or during lactation, the remaining results were presented together. The depolarization was accompanied by an increase in the whole-cell input resistance (before prolactin: 0.84 ± 0.14 GΩ; after prolactin, 0.95 ± 0.15 GΩ; n = 6 out of 20 cells, t(5) = 5.55, d = 4.97, p = 0.0026). MPA neurons that were depolarized in response to prolactin were subjected to a rectangular current step protocol (500 ms; ±10–50 pA) to obtain an I–V plot (Fig. 6F). Extrapolation of the slope conductance in the control and prolactin-containing aCSF revealed a reversal potential of −30.9 ± 7.7 mV (n = 6). Seventy percent of the MPA neurons (14 out of 20 cells) were unaffected in response to prolactin (change in RMP: 0.2 ± 0.4 mV; Input resistance, before prolactin: 1.1 ± 0.1 GΩ; after prolactin, 1.1 ±0.1 GΩ, t(26) = 0.11, d = 0.05, p = 0.4737; Figs. 6A,E). Of note, the steady-state capacitance, APS frequency or resting input resistance were not statistically different comparing responsive or unresponsive MPA neurons (capacitance, responsive cells: 15.7 ± 2.7 pF; unresponsive: 11.8 ± 1.3; t(13) = 1.46, d = 0.81, p = 0.1682; APS frequency, responsive: 0.8 ± 0.5 Hz; unresponsive: 1.3 ± 0.4 Hz; t(14) = 0.67, d = 0.36, p = 0.511; input resistance: responsive: 0.8 ± 0.1 GΩ; unresponsive: 1.1 ± 0.1 GΩ; t(18) = 1.191, d = 0.56, p = 0.2491), suggesting that the recordings were performed in a homogeneous population of neurons, at least regarding the selected biophysical parameters.
Fig. 6.
PrlR recruits fast STAT5-independent signaling pathways to acutely modify the biophysical properties of cells in the preoptic region. A–C. Current-clamp recordings of MPA neurons unresponsive to prolactin (A), responsive of a female on diestrus (B) and responsive of a lactating N-STAT5 KO female (C). Dashed lines indicate the resting membrane potential. D. Identification of prolactin excited neuron in the MPA for whole-cell patch-clamp recording (low magnification for anatomical reference). E. Bar graphs showing the change in the membrane potential of unresponsive (n = 14) and responsive (female on diestrus, n = 4; lactating female, n = 2) MPA neurons after prolactin application. F. I–V plot from MPA neurons responsive to prolactin (n = 14) illustrating the characteristic reduction in input resistance during the prolactin-induced depolarization (reversal = −31 mV). Abbreviations: 3v, third ventricle. *p < 0.01 compared to unresponsive cells (one-way ANOVA followed by Newman–Keuls test).
Discussion
Previous studies have described the key role of PrlR signaling for the expression of maternal behaviors (Bridges et al., 1985, 1990, 1996, 1997, 2001; Bridges and Freemark, 1995; Dulac et al., 2014; Kelly et al., 2001; Larsen and Grattan, 2012; Lucas et al., 1998; Mann and Bridges, 2001). However, the molecular mechanisms involved in this regulation remain largely unknown. Thus, the major objective of the present study was to investigate if central STAT5 signaling, as the main pathway recruited by the PrlR (Bole-Feysot et al., 1998), is required for the expression of post-partum maternal behaviors and for maintaining lactation and offspring growth. For this purpose, we generated mice lacking both Stat5a and Stat5b genes only in neurons since whole-body Stat5a/b knockout mice are infertile and, consequently, cannot be studied during lactation (Teglund et al., 1998). Unexpectedly, we found that postpartum maternal behavior expression was not impaired in neuron-specific STAT5 knockout females. These results indicate that other signaling pathways are responsible to induce maternal behavior expression. Accordingly, prolactin acutely depolarized neurons in the preoptic region, a critical hypothalamic area for the expression of maternal behaviors (Bridges and Freemark, 1995; Bridges et al., 1997; Numan, 2006; Numan and Nagle, 1983; Numan et al., 1977). Of note, the basal biophysical characteristics of MPA neurons and their response to prolactin were very similar when comparing N-STAT5 KO females on diestrus or during lactation. Overall, our findings suggest the participation of fast nongenomic signaling pathways since the prolactin’s effect was observed in the brain of N-STAT5 KO females and required only a few minutes to take place which is incompatible with the time required for observing effects through transcription factors.
Surprisingly, the latency to retrieve and group the pups into the nest was reduced in lactating N-STAT5 KO females. These results suggest that neuronal STAT5 deletion may have sensitized the activation of signaling pathways that are in fact involved in the expression of maternal behaviors. In accordance with this hypothesis, neuronal ablation of STAT5 caused a reduction in the hypothalamic expression of suppressors of cytokine signaling such as the SOCS3 and CISH. Interestingly, the expression of SOCS3 or CISH can be induced by the same receptors that are inhibited by them which include the PrlR. Therefore, these proteins act as a negative feedback loop regulating cytokine receptor signaling (Krebs and Hilton, 2001). However, the induction of SOCS expression requires the activation of STAT proteins (Krebs and Hilton, 2001). For example, wild-type mice show a STAT5-dependent CISH expression in the corpora lutea whereas no CISH expression is detected in the ovary of Stat5a/b mutants (Teglund et al., 1998). Therefore, neuronal STAT5 deletion probably led to a reduction in SOCS3 and CISH expression making the affected cells more apt to activate other signaling pathways that are usually inhibited by these proteins. A similar compensatory effect has been observed for other cytokines. For example, neuronal deletion of SOCS3 increases leptin-dependent PI3K signaling (Metlakunta et al., 2011). Conversely, enhanced STAT3 activation, which produces an up-regulation of SOCS3 expression, causes an inhibition of leptin-induced PI3K signaling (Ernst et al., 2009). Therefore, we hypothesize that the improved postpartum maternal behavior observed in N-STAT5 KO females was caused by an increased activation of signaling pathways that became less inhibited by SOCS3 and CISH reduction. Of note, although our results indicate that central STAT5 signaling is not required for the induction of maternal behaviors, we could not identify which signaling pathways are necessary for the expression of these behaviors. Previous studies have suggested the participation of the cyclic AMP response element-binding protein or the extracellular signal regulated kinase for the regulation of parental behaviors (Jin et al., 2005; Kuroda et al., 2007). Further experiments are still necessary to understand the exact role of each signaling pathway.
Lactation is an energy-demanding activity. Therefore, lactating females must increase their food intake in order to sustain milk production. The suckling stimulus increases the expression of NPY in the ARH and in the dorsomedial nucleus of the hypothalamus (Li et al., 1999). Additionally, AgRP is co-expressed in NPY neurons of the ARH (Morton et al., 2014). Both NPY and AgRP act as potent activators of hunger and contribute to the hyperphagia observed during lactation (Chen and Smith, 2004; Garcia et al., 2003; Li et al., 1999; Morton et al., 2014; Phillips and Palmiter, 2008). Our results revealed that lactating N-STAT5 KO females exhibited lower hypothalamic expression of NPY and AgRP as well as decreased food intake. Reduced levels of SOCS proteins in the hypothalamus of lactating N-STAT5 KO females may explain the observed changes in feeding behavior. In mice exposed to high-fat diets, increased hypothalamic expression of SOCS3 decreases leptin sensitivity, increases the food intake and favors the development of diet-induced obesity (Briancon et al., 2010; Mori et al., 2004; Pedroso et al., 2014). In addition, our group recently showed that SOCS3 deficiency in leptin receptor-expressing cells blunts the increased food intake typically observed during pregnancy and lactation (Zampieri et al., 2015). Since leptin inhibits food intake in part by reducing the activity of NPY/AgRP neurons (Morton et al., 2014), the reduced hypothalamic SOCS3 expression may have favored the anorexigenic effects of leptin during lactation resulting in lower expression of NPY and AgRP which, in turn, led to the decreased food consumption.
The decreased food intake observed in lactating N-STAT5 KO females was mild and did not affect their body weight or milk production during the first days of lactation. However, this condition may have compromised the energy supply to produce milk at the peak of lactation when the demand is higher due to the fast offspring growth. It has been known for decades that litter growth and milk production are negatively affected by limited food availability in rodents (Krackow, 1989; Taylor et al., 1986). This hypothesis is supported by the fact that reduced milk production in lactating N-STAT5 KO females was caused neither by prolactin deficiency nor by the lack of STAT5 signaling in the mammary tissue. Therefore, the reduced weight gain rate of N-STAT5 KO litters was likely secondary to the decreased food intake of their mothers leading to a lower capacity to maintain milk production. Interestingly, NSTAT5 KO females exhibited increased levels of transcripts that codify milk proteins in the mammary tissue despite their lower food intake during lactation. Milk protein genes have STAT5 response elements that are important to induce their transcription (Liu et al., 1995). Since STAT5 signaling was intact in the mammary tissue of mutant dams, their higher prolactin levels may have increased the transcription of milk protein genes.
The lower food intake may have influenced the expression of maternal behaviors of N-STAT5 KO females. Previous studies have shown that mild food restriction during lactation increases nursing behaviors in rats compared to ad libitum fed animals (McGuire et al., 1995; Pachon et al., 1995). This behavioral change may represent a compensation for the lower energy availability and a way to improve litter growth under unfavorable conditions. The energy demand to produce milk increases dams’ metabolic rate and their body temperature (Gamo et al., 2013). Since some evidence suggests that elevations in body temperature during lactation may limit nursing behaviors in rodents (Gamo et al., 2013; Leon et al., 1978; Woodside et al., 2012), the lower food intake and milk production presented by lactating N-STAT5 KO females possibly reduced their metabolic rate compared to control animals which, in turn, may have favored a decrease in the latencies to retrieve and group the pups.
Prolactin secretion is regulated by negative feedback in which increases in prolactin levels induce the activation of TIDA neurons resulting in dopamine release into the hypophyseal portal system and the consequent inhibition of pituitary lactotrophs (Bole-Feysot et al., 1998). In addition, the anterior pituitary gland also expresses the PrlR. Thus, prolactin may have direct effects on lactotrophs (Ferraris et al., 2012). Of note, Stat5b deficient mice display hyperprolactinemia supporting the role of this signaling pathway in the regulation of prolactin secretion (Grattan et al., 2001). Our results confirm these earlier findings and provide new evidence indicating that this regulation occurs at the brain level since neuron-restricted STAT5 deletion recapitulates this phenotype of hyperprolactinemia. Prolactin also evokes fast non-genomic effects on TIDA neurons indicating the involvement of other signaling pathways in this feedback circuit (Brown et al., 2012; Lyons et al., 2012). As the magnitude of the hyperprolactinemia caused by neuronal STAT5 deficiency seems to be lower than that caused by PrlR mutation (Schuff et al., 2002), it is very likely that PrlR recruits different signaling pathways, including STAT5, to regulate the activity of hypothalamic neurons in order to modulate prolactin circulating levels.
Conclusions
Neuronal ablation of STAT5 impaired the control of prolactin secretion and reduced the hypothalamic expression of suppressors of cytokine signaling. In addition, STAT5 neuronal deletion attenuated the hyperphagia observed during lactation by decreasing the hypothalamic expression of orexigenic neurotransmitters. The lower food intake of lactating N-STAT5 KO females resulted in reduced milk production and offspring growth. Importantly, neuronal STAT5 signaling is not required for postpartum maternal behavior, even though this pathway is considered the major signal transducer of key hormones that regulate the expression of parental behaviors such as prolactin or placental lactogens (Bole-Feysot et al., 1998; Bridges et al., 1996). Finally, we demonstrated that MPA neurons were acutely depolarized by prolactin suggesting that fast STAT5-independent signaling pathways may be involved in the regulation of maternal behaviors. Overall, our study revealed important information about the molecular mechanisms recruited by hormones to orchestrate the activation of neural circuitries engaged in the induction of maternal care.
Acknowledgments
We thank Ana M.P. Campos for the technical assistance and the São Paulo Research Foundation (FAPESP-Brazil, 10/18086, 12/12202–4, 13/21722–4 and 14/11752–6) and CAPES (Coordenação de Aperfeiçoamento de Pessoal de Nível Superior) for the financial support and fellowships. LH was supported by the Intramural Research Program of the NIH, The National Institute of Diabetes, Digestive and Kidney Diseases (NIDDK).
Footnotes
Competing interests
The authors declare no conflicts of interest.
References
- Augustine RA, Ladyman SR, Grattan DR, 2008. From feeding one to feeding many: hormone-induced changes in bodyweight homeostasis during pregnancy. J. Physiol 586, 387–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bole-Feysot C, Goffin V, Edery M, Binart N, Kelly PA, 1998. Prolactin (PRL) and its receptor: actions, signal transduction pathways and phenotypes observed in PRL receptor knockout mice. Endocr. Rev 19, 225–268. [DOI] [PubMed] [Google Scholar]
- Briancon N, McNay DE, Maratos-Flier E, Flier JS, 2010. Combined neural inactivation of suppressor of cytokine signaling-3 and protein-tyrosine phosphatase-1B reveals additive, synergistic, and factor-specific roles in the regulation of body energy balance. Diabetes 59, 3074–3084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridges RS, Freemark MS, 1995. Human placental lactogen infusions into the medial preoptic area stimulate maternal behavior in steroid-primed, nulliparous female rats. Horm. Behav 29, 216–226. [DOI] [PubMed] [Google Scholar]
- Bridges RS, DiBiase R, Loundes DD, Doherty PC, 1985. Prolactin stimulation of maternal behavior in female rats. Science 227, 782–784. [DOI] [PubMed] [Google Scholar]
- Bridges RS, Numan M, Ronsheim PM, Mann PE, Lupini CE, 1990. Central prolactin infusions stimulate maternal behavior in steroid-treated, nulliparous female rats. Proc. Natl. Acad. Sci. U. S. A 87, 8003–8007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridges RS, Robertson MC, Shiu RP, Friesen HG, Stuer AM, Mann PE, 1996. Endocrine communication between conceptus and mother: placental lactogen stimulation of maternal behavior. Neuroendocrinology 64, 57–64. [DOI] [PubMed] [Google Scholar]
- Bridges RS, Robertson MC, Shiu RP, Sturgis JD, Henriquez BM, Mann PE, 1997. Central lactogenic regulation of maternal behavior in rats: steroid dependence, hormone specificity, and behavioral potencies of rat prolactin and rat placental lactogen I. Endocrinology 138, 756–763. [DOI] [PubMed] [Google Scholar]
- Bridges R, Rigero B, Byrnes E, Yang L, Walker A, 2001. Central infusions of the recombinant human prolactin receptor antagonist, S179D-PRL, delay the onset of maternal behavior in steroid-primed, nulliparous female rats. Endocrinology 142, 730–739. [DOI] [PubMed] [Google Scholar]
- Brown RS, Kokay IC, Herbison AE, Grattan DR, 2010. Distribution of prolactin-responsive neurons in the mouse forebrain. J. Comp. Neurol 518, 92–102. [DOI] [PubMed] [Google Scholar]
- Brown RS, Piet R, Herbison AE, Grattan DR, 2012. Differential actions of prolactin on electrical activity and intracellular signal transduction in hypothalamic neurons. Endocrinology 153, 2375–2384. [DOI] [PubMed] [Google Scholar]
- Chen P, Smith MS, 2004. Regulation of hypothalamic neuropeptide Y messenger ribonucleic acid expression during lactation: role of prolactin. Endocrinology 145, 823–829. [DOI] [PubMed] [Google Scholar]
- Cui Y, Riedlinger G, Miyoshi K, Tang W, Li C, Deng CX, Robinson GW, Hennighausen L, 2004. Inactivation of Stat5 in mouse mammary epithelium during pregnancy reveals distinct functions in cell proliferation, survival, and differentiation. Mol. Cell. Biol 24, 8037–8047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobolyi A, Grattan DR, Stolzenberg DS, 2014. Preoptic inputs and mechanisms that regulate maternal responsiveness. J. Neuroendocrinol 26, 627–640. [DOI] [PubMed] [Google Scholar]
- Dulac C, O’Connell LA, Wu Z, 2014. Neural control of maternal and paternal behaviors.Science 345, 765–770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst MB, Wunderlich CM, Hess S, Paehler M, Mesaros A, Koralov SB, Kleinridders A, Husch A, Munzberg H, Hampel B, Alber J, Kloppenburg P, Bruning JC, Wunderlich FT, 2009. Enhanced Stat3 activation in POMC neurons provokes negative feedback inhibition of leptin and insulin signaling in obesity. J. Neurosci 29, 11582–11593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferraris J, Boutillon F, Bernadet M, Seilicovich A, Goffin V, Pisera D, 2012. Prolactin receptor antagonism in mouse anterior pituitary: effects on cell turnover and prolactin receptor expression. Am. J. Physiol. Endocrinol. Metab 302, E356–E364. [DOI] [PubMed] [Google Scholar]
- Frazao R, Cravo RM, Donato J Jr., Ratra DV, Clegg DJ, Elmquist JK, Zigman JM, Williams KW, Elias CF, 2013. Shift in Kiss1 cell activity requires estrogen receptor alpha. J. Neurosci 33, 2807–2820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furigo IC, Kim KW, Nagaishi VS, Ramos-Lobo AM, de Alencar A, Pedroso JA, Metzger M, Donato J Jr., 2014. Prolactin-sensitive neurons express estrogen receptor-alpha and depend on sex hormones for normal responsiveness to prolactin. Brain Res. 1566, 47–59. [DOI] [PubMed] [Google Scholar]
- Gamo Y, Troup C, Mitchell SE, Hambly C, Vaanholt LM, Speakman JR, 2013. Limits to sustained energy intake. XX. Body temperatures and physical activity of female mice during lactation. J. Exp. Biol 216, 3751–3761. [DOI] [PubMed] [Google Scholar]
- Garcia MC, Lopez M, Gualillo O, Seoane LM, Dieguez C, Senaris RM, 2003. Hypothalamic levels of NPY, MCH, and prepro-orexin mRNA during pregnancy and lactation in the rat: role of prolactin. FASEB J. 17, 1392–1400. [DOI] [PubMed] [Google Scholar]
- Grattan DR, Xu J, McLachlan MJ, Kokay IC, Bunn SJ, Hovey RC, Davey HW, 2001. Feedback regulation of PRL secretion is mediated by the transcription factor, signal transducer, and activator of transcription 5b. Endocrinology 142, 3935–3940. [DOI] [PubMed] [Google Scholar]
- Jin SH, Blendy JA, Thomas SA, 2005. Cyclic AMP response element-binding protein is required for normal maternal nurturing behavior. Neuroscience 133, 647–655. [DOI] [PubMed] [Google Scholar]
- Kelly PA, Binart N, Lucas B, Bouchard B, Goffin V, 2001. Implications of multiple phenotypes observed in prolactin receptor knockout mice. Front. Neuroendocrinol 22, 140–145. [DOI] [PubMed] [Google Scholar]
- Krackow S, 1989. Effect of food restriction on reproduction and lactation in house mice mated post partum. J. Reprod. Fertil 86, 341–347. [DOI] [PubMed] [Google Scholar]
- Krebs DL, Hilton DJ, 2001. SOCS proteins: negative regulators of cytokine signaling. Stem Cells 19, 378–387. [DOI] [PubMed] [Google Scholar]
- Kuroda KO, Meaney MJ, Uetani N, Fortin Y, Ponton A, Kato T, 2007. ERK-FosB signaling in dorsal MPOA neurons plays a major role in the initiation of parental behavior in mice. Mol. Cell. Neurosci 36, 121–131. [DOI] [PubMed] [Google Scholar]
- Larsen CM, Grattan DR, 2012. Prolactin, neurogenesis, and maternal behaviors. Brain Behav. Immun 26, 201–209. [DOI] [PubMed] [Google Scholar]
- Lee JY, Muenzberg H, Gavrilova O, Reed JA, Berryman D, Villanueva EC, Louis GW, Leinninger GM, Bertuzzi S, Seeley RJ, Robinson GW, Myers MG, Hennighausen L, 2008. Loss of cytokine-STAT5 signaling in the CNS and pituitary gland alters energy balance and leads to obesity. PLoS One 3, e1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leon M, Croskerry PG, Smith GK, 1978. Thermal control of mother–young contact in rats. Physiol. Behav 21, 79O–811O. [DOI] [PubMed] [Google Scholar]
- Li C, Chen P, Smith MS, 1999. Neuropeptide Y and tuberoinfundibular dopamine activities are altered during lactation: role of prolactin. Endocrinology 140, 118–123. [DOI] [PubMed] [Google Scholar]
- Liu X, Robinson GW, Gouilleux F, Groner B, Hennighausen L, 1995. Cloning and expression of Stat5 and an additional homologue (Stat5b) involved in prolactin signal transduction in mouse mammary tissue. Proc. Natl. Acad. Sci. U. S. A 92, 8831–8835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu XW, Robinson GW, Wagner KU, Garrett L, WynshawBoris A, Hennighausen L, 1997. Stat5a is mandatory for adult mammary gland development and lactogenesis. Genes Dev. 11, 179–186. [DOI] [PubMed] [Google Scholar]
- Lucas BK, Ormandy CJ, Binart N, Bridges RS, Kelly PA, 1998. Null mutation of the prolactin receptor gene produces a defect in maternal behavior. Endocrinology 139, 4102–4107. [DOI] [PubMed] [Google Scholar]
- Lyons DJ, Hellysaz A, Broberger C, 2012. Prolactin regulates tuberoinfundibular dopamine neuron discharge pattern: novel feedback control mechanisms in the lactotrophic axis. J. Neurosci 32, 8074–8083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma FY, Anderson GM, Gunn TD, Goffin V, Grattan DR, Bunn SJ, 2005. Prolactin specifically activates signal transducer and activator of transcription 5b in neuroendocrine dopaminergic neurons. Endocrinology 146, 5112–5119. [DOI] [PubMed] [Google Scholar]
- Mann PE, Bridges RS, 2001. Lactogenic hormone regulation of maternal behavior. Prog. Brain Res 133, 251–262. [DOI] [PubMed] [Google Scholar]
- McGuire MK, Pachon H, Butler WR, Rasmussen KM, 1995. Food restriction, gonadotropins, and behavior in the lactating rat. Physiol. Behav 58, 1243–1249. [DOI] [PubMed] [Google Scholar]
- Metlakunta AS, Sahu M, Yasukawa H, Dhillon SS, Belsham DD, Yoshimura A, Sahu A, 2011. Neuronal suppressor of cytokine signaling-3 deficiency enhances hypothalamic leptin-dependent phosphatidylinositol 3-kinase signaling. Am. J. Physiol. Regul. Integr. Comp. Physiol 300, R1185–R1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori H, Hanada R, Hanada T, Aki D, Mashima R, Nishinakamura H, Torisu T, Chien KR, Yasukawa H, Yoshimura A, 2004. Socs3 deficiency in the brain elevates leptin sensitivity and confers resistance to diet-induced obesity. Nat. Med 10, 739–743. [DOI] [PubMed] [Google Scholar]
- Morton GJ, Meek TH, Schwartz MW, 2014. Neurobiology of food intake in health and disease. Nat. Rev. Neurosci 15, 367–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagaishi VS, Cardinali LI, Zampieri TT, Furigo IC, Metzger M, Donato J Jr., 2014. Possible crosstalk between leptin and prolactin during pregnancy. Neuroscience 259, 71–83. [DOI] [PubMed] [Google Scholar]
- Numan M, 2006. Hypothalamic neural circuits regulating maternal responsiveness toward infants. Behav. Cogn. Neurosci. Rev 5, 163–190. [DOI] [PubMed] [Google Scholar]
- Numan M, Nagle DS, 1983. Preoptic area and substantia nigra interact in the control of maternal behavior in the rat. Behav. Neurosci 97, 120–139. [DOI] [PubMed] [Google Scholar]
- Numan M, Rosenblatt JS, Komisaruk BR, 1977. Medial preoptic area and onset of maternal behavior in the rat. J. Comp. Physiol. Psychol 91, 146–164. [DOI] [PubMed] [Google Scholar]
- Ormandy CJ, Camus A, Barra J, Damotte D, Lucas B, Buteau H, Edery M, Brousse N, Babinet C, Binart N, Kelly PA, 1997. Null mutation of the prolactin receptor gene produces multiple reproductive defects in the mouse. Genes Dev. 11, 167–178. [DOI] [PubMed] [Google Scholar]
- Pachon H, McGuire MK, Rasmussen KM, 1995. Nutritional status and behavior during lactation. Physiol. Behav 58, 393–400. [DOI] [PubMed] [Google Scholar]
- Pedroso JA, Buonfiglio DC, Cardinali LI, Furigo IC, Ramos-Lobo AM, Tirapegui J, Elias CF, Donato J Jr., 2014. Inactivation of SOCS3 in leptin receptor-expressing cells protects mice from diet-induced insulin resistance but does not prevent obesity. Mol. Metab 3, 608–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips CT, Palmiter RD, 2008. Role of agouti-related protein-expressing neurons in lactation. Endocrinology 149, 544–550. [DOI] [PubMed] [Google Scholar]
- Sapsford TJ, Kokay IC, Ostberg L, Bridges RS, Grattan DR, 2012. Differential sensitivity of specific neuronal populations of the rat hypothalamus to prolactin action. J. Comp. Neurol 520, 1062–1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuff KG, Hentges ST, Kelly MA, Binart N, Kelly PA, Iuvone PM, Asa SL, Low MJ, 2002. Lack of prolactin receptor signaling in mice results in lactotroph proliferation and prolactinomas by dopamine-dependent and -independent mechanisms. J. Clin. Invest 110, 973–981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor JB, Calvert CC, Baldwin RL, Sainz RD, 1986. Effects of dietary protein, fat and restriction on body composition and energy balance in lactating rats. J. Nutr 116, 1519–1528. [DOI] [PubMed] [Google Scholar]
- Teglund S, McKay C, Schuetz E, van Deursen JM, Stravopodis D, Wang DM, Brown M, Bodner S, Grosveld G, Ihle JN, 1998. Stat5a and Stat5b proteins have essential and nonessential, or redundant, roles in cytokine responses. Cell 93, 841–850. [DOI] [PubMed] [Google Scholar]
- Woodside B, Budin R, Wellman MK, Abizaid A, 2012. Many mouths to feed: the control of food intake during lactation. Front. Neuroendocrinol 33, 301–314. [DOI] [PubMed] [Google Scholar]
- Zampieri TT, Pedroso JA, Furigo IC, Tirapegui J, Donato J Jr., 2013. Oral leucine supplementation is sensed by the brain but neither reduces food intake nor induces an anorectic pattern of gene expression in the hypothalamus. PLoS One 8, e84094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zampieri TT, Ramos-Lobo AM, Furigo IC, Pedroso JA, Buonfiglio DC, Donato J Jr., 2015. SOCS3 deficiency in leptin receptor-expressing cells mitigates the development of pregnancy-induced metabolic changes. Mol. Metab 4, 237–245. [DOI] [PMC free article] [PubMed] [Google Scholar]