Abstract
Carbon dioxide enters metabolism via six known CO 2 fixation pathways, of which only one is linear, exergonic in the direction of CO 2‐assimilation, and present in both bacterial and archaeal anaerobes – the Wood‐Ljungdahl (WL) or reductive acetyl‐CoA pathway. Carbon monoxide (CO) plays a central role in the WL pathway as an energy rich intermediate. Here, we scan the major biochemical reaction databases for reactions involving CO and CO 2. We identified 415 reactions corresponding to enzyme commission (EC) numbers involving CO 2, which are non‐randomly distributed across different biochemical pathways. Their taxonomic distribution, reversibility under physiological conditions, cofactors and prosthetic groups are summarized. In contrast to CO 2, only 15 reaction classes involving CO were detected. Closer inspection reveals that CO interfaces with metabolism and the carbon cycle at only two enzymes: anaerobic carbon monoxide dehydrogenase (CODH), a Ni‐ and Fe‐containing enzyme that generates CO for CO 2 fixation in the WL pathway, and aerobic CODH, a Mo‐ and Cu‐containing enzyme that oxidizes environmental CO as an electron source. The CO‐dependent reaction of the WL pathway involves carbonyl insertion into a methyl carbon‐nickel at the Ni‐Fe‐S A‐cluster of acetyl‐CoA synthase (ACS). It appears that no alternative mechanisms to the CO‐dependent reaction of ACS have evolved in nearly 4 billion years, indicating an ancient and mechanistically essential role for CO at the onset of metabolism.
Keywords: carbon dioxide, carbon monoxide, CODH/ACS, enzymatic reactions, metabolic networks
Abbreviations
- ACS
acetyl‐CoA synthase
- CODH
carbon monoxide dehydrogenase
- EC
enzyme commission
- Gt
gigatonne
- LUCA
last universal common ancestor
- PAP
presence‐absence pattern
- rTCA
reverse citric acid
- WL
Wood‐Ljungdahl
Introduction
In autotrophs, carbon dioxide enters metabolism mainly via six known pathways of CO2 fixation 1, 2, 3, 4, 5. In discussions about novel synthetic CO2 fixation pathways 6, 7, 8, 9, 10, it is often overlooked that heterotrophs also harbor a number of metabolic reactions that incorporate CO2. For example, carbon atoms from CO2 end up in the purine and pyrimidine rings during de novo nucleobase biosynthesis, from prokaryotes to humans 11, and CO2 assimilation into membrane lipids has been measured as a proxy of metabolic activity in different heterotrophic bacteria 12. Of the six natural pathways of autotrophic CO2 fixation, only one involves CO as an intermediate – the Wood‐Ljungdahl (WL) pathway, also called the reductive acetyl‐CoA pathway.
Among CO2 assimilation pathways, the WL pathway is unique in being the only linear pathway of carbon fixation that can occur exergonically 3, 13, 14. Phylogenetic evidence traces the pathway to the genome of the Last Universal Common Ancestor (LUCA) 15. It is the only known pathway of core CO2 fixation present in both bacteria and archaea 2, 3. Gene distributions for both the enzymes of the pathway and the synthesis of its salient pterin cofactors – tetrahydrofolate (H4F) in bacteria and tetrahydromethanopterin (H4MPT) in archaea – testify to the antiquity of the WL pathway 3, 16, which is closely aligned with theories that posit a chemolithoautotrophic origin of life 17, 18, 19. Its basic chemistry, the reduction in CO2 to organic one‐carbon (C1) moieties, occurs as spontaneous geochemical reactions in hydrothermal systems 20, 21. The WL pathway entails oxygen sensitive catalysts, as its enzymes are replete with iron and nickel sulfur centers essential for electron transfer and catalysis 14, 22. CO2‐reducing reactions of the WL pathway occur readily in the laboratory in the presence of native metals 23, 24. The WL pathway is the only pathway known that fixes CO2 while conserving energy as ATP, the mechanisms of energy conservation entailing chemiosmotic coupling and flavin‐based electron bifurcation 3, 25. The simplicity of the WL pathway 13, 22, its antiquity 13, 14, 15, 16, 26, 27, favorable energetics in the CO2‐reducing direction 3, 25 and chemical similarity to exergonic geochemical reactions in hydrothermal vents 20, 21 forge chemical links between early earth geochemistry and the biochemistry of the first cells.
The WL pathway works in a conceptually simple but chemically demanding manner – one carbon at a time 22. The enzymology of the pathway has been reviewed 3, 19, 22, 28, 29. In comparisons of the archaeal and bacterial pathway, the enzymes of the methyl synthesis branch show no sequence conservation across the prokaryotic domain divide 16, whereby the CO synthesis and thioesther synthesis are catalyzed by an enzyme well conserved between archaea and bacteria: bifunctional carbon monoxide dehydrogenase/acetyl CoA synthase (CODH/ACS). CODH catalyzes the reversible, ferredoxin‐dependent interconversion of CO and CO2 30. In the WL pathway, CO is generated as an intermediate of CO2 fixation, but environmental CO can also enter the pathway as a carbon and electron source 3, 31, 32. Both CODH and CO are central to carbon and energy metabolism in methanogens (archaea) 33, hydrogenogens, acetogens 22, 34, some solventogenic bacteria, such as ethanol‐producing Clostridium ljungdahlii 35 and other anaerobes including sulfate reducers 36, as reviewed in 32, 37. CODH contains FeS clusters, the active site contains an FeNiS cluster 38, 39, 40. An anaerobic CODH preparation containing copper in the active site was reported 41, but the enzyme was inactive. The CODH enzyme of the WL pathway is oxygen sensitive. ACS catalyzes the cleavage and synthesis of acetyl‐CoA, releasing or consuming CO, respectively. In Moorella thermoacetica, CO is carried inside the enzyme through a hydrophobic tunnel as proposed by scavenging experiments using hemoglobin 42 and subsequently supported by isotope exchange data 43 and structural data 44. Some facultative aerobes, as Rhodospirillum rubrum, have the anaerobic CODH but no ACS, and use it to conserve energy in the reverse direction through CO oxidation 32.
In other aerobic and facultative aerobic bacteria, CO oxidation can also be catalyzed by an oxygen tolerant enzyme that shares no sequence similarity with CODH of the WL pathway. The oxygen tolerant CO oxidizing enzyme is encoded by the cox operon 45. It is typically called aerobic CODH 45, but for clarity we will refer to it here by the name of its catalytic subunit, coxL. Importantly, coxL enzymes are not related to the CODH of the WL pathway, rather they are related to molybdenum hydroxylases 45, 46. The metals involved in coxL catalysis are molybdenum and copper 38, 46, 47, 48. Cox gene products only perform the oxidation of CO to CO2, which in some species of Proteobacteria, Firmicutes and Actinobacteria can then be fixed via the Calvin cycle 45, 47. In aerobes that use coxL enzymes, CO is typically a source of electrons for respiratory processes coupled with exogenous electron acceptors such as oxygen 45, 49, sulfate 50, anthraquinone disulfonate and fumarate 51.
Various lines of evidence point to the importance of CO in primordial metabolism 52, 53, 54, 55, 56. Here, we queried large and well curated biochemical databases – KEGG and BRENDA – to investigate the number and nature of entry points of CO and CO2 into metabolism.
Results and Discussion
CO2 is everywhere in metabolism, CO is rare
The KEGG and BRENDA have different reaction nomenclatures, therefore to compare their content it is convenient to use Enzyme Commission (EC) numbers, which also link metabolic data with taxonomy and other catalysis metadata. Figure 1 shows the content of both databases regarding enzyme classes that use CO2 and CO. Both databases reveal that CO is very rare in metabolism, whereas CO2 is very common. KEGG contained 390 EC numbers involving CO2, BRENDA Natural (a subset of BRENDA including only reactions tested in vivo) contained 323 EC numbers and both databases returned 13 EC numbers involving CO (Fig. 1).
Figure 1.
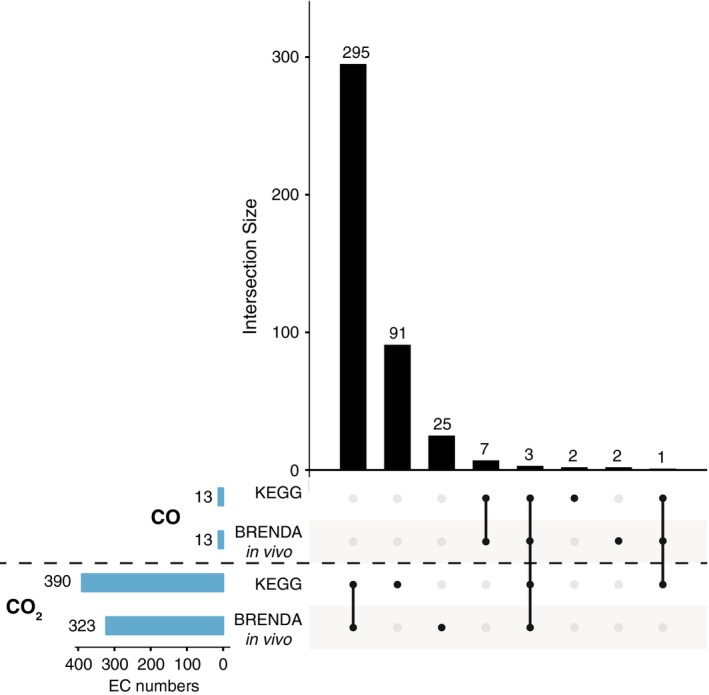
Enzyme Commission (EC) numbers involving CO 2 and CO in KEGG and BRENDA (only in vivo reactions) and their overlaps. The horizontal bars display the total of EC numbers for each molecule in each database. The vertical bars display the size of the overlaps (intersections) between the databases.
The results obtained from both databases are not completely overlapping (Fig. 1). In KEGG, there are 91 EC numbers involving CO2 that are not found in BRENDA. Conversely, 25 EC numbers involving CO2 are found in BRENDA but not in KEGG. Regarding CO, each database has two unique EC numbers: in BRENDA an additional dioxygenase, http://www.chem.qmul.ac.uk/iubmb/enzyme/EC1/13/11/54.html, and an additional heme oxygenase, http://www.chem.qmul.ac.uk/iubmb/enzyme/EC1/14/99/48.html, are listed as producing CO. In KEGG one of these non‐overlapping EC numbers is a misannotation: http://www.chem.qmul.ac.uk/iubmb/enzyme/EC2/1/1/258.html, a 5‐methyltetrahydrofolate: corrinoid/iron‐sulfur protein Co‐methyltransferase, represents a reaction of the Wood‐Ljungdahl pathway that does not involve CO as substrate or product 29. The second CO involving reaction unique to KEGG is http://www.chem.qmul.ac.uk/iubmb/enzyme/EC4/1/99/5.html, an O2‐dependent aldehyde oxygenase. O2‐dependent reactions cannot be primordial, because O2 is the product of cyanobacterial metabolism (see Conclusion).
Eleven EC numbers that involve CO occur in both databases. Of those 11, seven entail CO only as a by‐product of an O2‐dependent enzyme: two heme oxygenases, http://www.chem.qmul.ac.uk/iubmb/enzyme/EC1/14/14/18.html and http://www.chem.qmul.ac.uk/iubmb/enzyme/EC1/14/15/20.html; four dioxygenases: http://www.chem.qmul.ac.uk/iubmb/enzyme/EC1/13/11/24.html, http://www.chem.qmul.ac.uk/iubmb/enzyme/EC1/13/11/47.html, http://www.chem.qmul.ac.uk/iubmb/enzyme/EC1/13/11/48.html and http://www.chem.qmul.ac.uk/iubmb/enzyme/EC1/13/11/53.html and one synthase http://www.chem.qmul.ac.uk/iubmb/enzyme/EC4/1/99/17.html. The remaining four EC numbers involving CO all trace directly to CODH. The first is http://www.chem.qmul.ac.uk/iubmb/enzyme/EC1/2/2/4.html, aerobic CODH with cytochrome b‐561 as an electron acceptor. This reaction is disputed, however, as some authors argue that no cytochromes are involved in the aerobic CODH reaction 57, contrary to the original proposal 49. The second is http://www.chem.qmul.ac.uk/iubmb/enzyme/EC1/2/5/3.html, aerobic CODH with quinones as an electron acceptor. The third is EC. http://www.chem.qmul.ac.uk/iubmb/enzyme/EC1/2/7/4.html, anaerobic CODH with ferredoxin. The fourth is http://www.chem.qmul.ac.uk/iubmb/enzyme/EC2/3/1/169.html, the CODH/ACS combined reaction, which in BRENDA is considered as including only the second step of acetyl‐CoA synthesis and not the CO2 fixation step.
CO2 for all trades, CO only for CODH
CO2 is involved throughout all major functional pathways in KEGG, while CO is assigned to only 7 (Fig. 2A). Each EC number had from 0 to a maximum of 11 KEGG pathways assigned. Multifunctionality is a known and important characteristic of enzymes, so the functional analysis done here preserved all classifications assigned to all enzymes, except for the large generalist categories (‘Biosynthesis of antibiotics’, ‘Biosynthesis of secondary metabolites’, ‘Microbial metabolism in diverse environments’ and ‘Metabolic pathways’), which were discarded. A large number of enzymes do not have any pathways assigned (not shown in the plot) ‐ 113 involving CO2 and 5 involving CO. The functions of these 5 EC numbers involving CO were searched manually in the literature (see legend of Fig. 2; Table 1). All EC numbers involving CO as a substrate are assigned (or, if assigned Unknown, could be manually assigned) to ‘carbon fixation pathways in prokaryotes’ and ‘energy metabolism’ through the CODH reaction. Other pathways involve CO always as a byproduct, with the exception of the additional assignments of anaerobic CODH http://www.chem.qmul.ac.uk/iubmb/enzyme/EC1/2/7/4.html to ‘methane metabolism’ (in the methanogen pathway) and (through a reaction that does not involve CO) to ‘nitrotoluene degradation’.
Figure 2.
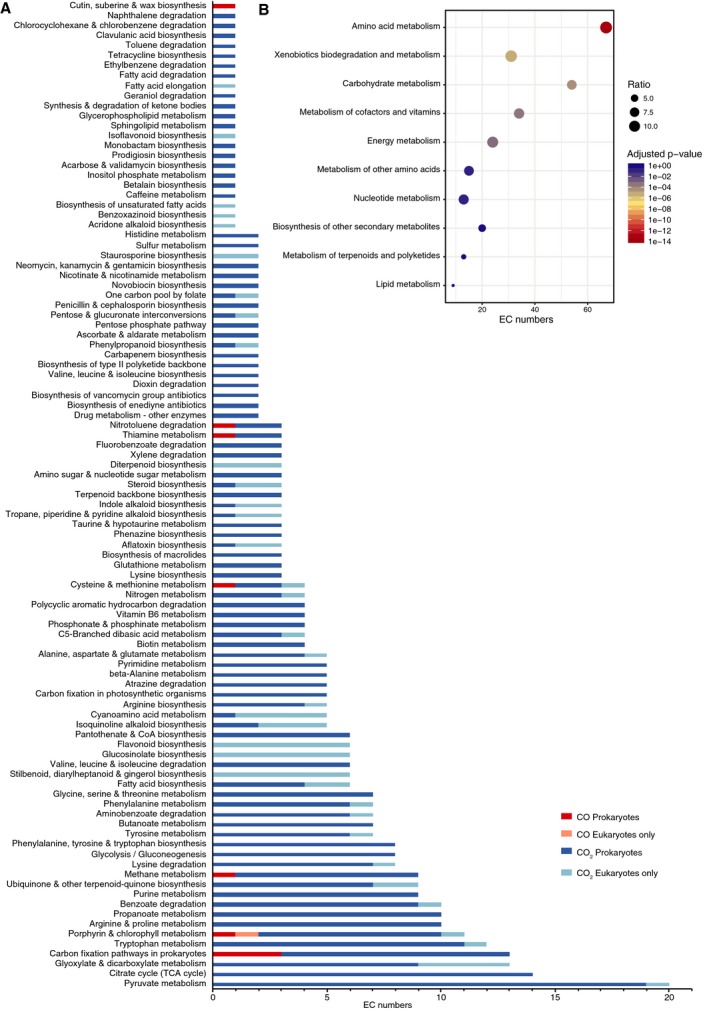
Functional analysis of EC numbers involving CO 2 and CO. (A) EC numbers involving CO 2 (dark and light blue for those in prokaryotes and in eukaryotes only, respectively) and CO (dark and light red, accordingly). (B) Enrichment analysis (Fisher's exact test with adjusted p‐values by the Bonferroni correction) for high‐level functional categories of EC numbers involving CO 2 (prokaryotes only).
Table 1.
Enzyme commission numbers associated with carbon monoxide
| EC number | Functional classifications in KEGG | Name and description | Proposed functional classification |
|---|---|---|---|
| http://www.chem.qmul.ac.uk/iubmb/enzyme/EC1/2/2/4.html | Unknown | CO dehydrogenase (cytochrome b 561); although present in strict aerobes, O2 is not required for the reaction. CO is oxidized to CO2 with water as the oxidant 49 | Energy Metabolism; Carbon fixation pathways in prokaryotes |
| http://www.chem.qmul.ac.uk/iubmb/enzyme/EC1/2/5/3.html | Unknown | Aerobic Carbon Monoxide dehydrogenase (quinone) | Energy Metabolism; Carbon fixation pathways in prokaryotes |
| http://www.chem.qmul.ac.uk/iubmb/enzyme/EC1/2/7/4.html | Carbon fixation pathways in prokaryotes; Methane metabolism; Nitrotoluene degradation | Anaerobic carbon‐monoxide dehydrogenase (ferredoxin) | |
| http://www.chem.qmul.ac.uk/iubmb/enzyme/EC2/3/1/169.html | Carbon fixation pathways in prokaryotes | CO‐methylating acetyl‐CoA synthase | |
| http://www.chem.qmul.ac.uk/iubmb/enzyme/EC2/1/1/258.html | Carbon fixation pathways in prokaryotes | 5‐methyltetrahydrofolate:corrinoid/iron‐sulfur protein Co‐methyltransferase; two step reaction: Tetrahydrofolate + acetyl‐CoA ↔ 5‐methyltetrahydrofolate + CoA + CO | |
| http://www.chem.qmul.ac.uk/iubmb/enzyme/EC1/13/11/24.html | Unknown | Quercetin 2,3‐dioxygenase. CO is a byproduct in this reaction. | Xenobiotics biodegradation and metabolism |
| http://www.chem.qmul.ac.uk/iubmb/enzyme/EC1/13/11/47.html | Unknown | 3‐hydroxy‐4‐oxoquinoline 2,4‐dioxygenase. CO is a byproduct in this reaction. | Xenobiotics biodegradation and metabolism |
| http://www.chem.qmul.ac.uk/iubmb/enzyme/EC1/13/11/48.html | Unknown | 3‐hydroxy‐2‐methylquinolin‐4‐one 2,4‐dioxygenase. CO is a byproduct in this reaction. | Xenobiotics biodegradation and metabolism |
| http://www.chem.qmul.ac.uk/iubmb/enzyme/EC1/13/11/53.html | Cysteine and methionine metabolism | Acireductone dioxygenase (Ni2+‐requiring). CO is a byproduct in this reaction. Unknown function; the same enzyme, when binding iron, is the one leading to the salvage of methionine (http://www.chem.qmul.ac.uk/iubmb/enzyme/EC1/13/11/54.html) 97, 98. | |
| http://www.chem.qmul.ac.uk/iubmb/enzyme/EC1/14/14/18.html | Porphyrin and chlorophyll metabolism | Heme oxygenase (biliverdin‐producing). CO is a byproduct in this reaction. | |
| http://www.chem.qmul.ac.uk/iubmb/enzyme/EC1/14/15/20.html | Porphyrin and chlorophyll metabolism | Heme oxygenase (biliverdin‐producing, ferredoxin). CO is a byproduct in this reaction. | |
| http://www.chem.qmul.ac.uk/iubmb/enzyme/EC4/1/99/5.html | Cutin, suberine and wax biosynthesis | Aldehyde oxygenase (deformylating) | |
| http://www.chem.qmul.ac.uk/iubmb/enzyme/EC4/1/99/17.html | Thiamine metabolism | Phosphomethylpyrimidine synthase. CO is a byproduct in this reaction. |
Each reaction in our set was annotated in a range from 1 to a maximum of 4320 taxa (species) in KEGG as per its occurrence, either through the corresponding gene in KEGG genomes or manually assigned upon examination of the literature (see Materials and methods). Out of the total 399 EC numbers gathered for CO2 and CO, 99 reactions were found to be annotated only in eukaryotes, only one of which involves CO (a mammalian heme oxygenase that produces CO, http://www.chem.qmul.ac.uk/iubmb/enzyme/EC1/14/14/18.html, annotated in 110 KEGG genomes). In prokaryotes, 292 ECs involved with CO2 were annotated, versus only 12 with CO.
To investigate the distribution of reactions across pathways where CO2 was involved, a higher‐level categorization was performed, using the KEGG pathway hierarchy, for prokaryotic EC numbers (Fig. 2B). A Fisher's exact test for enrichment of each pathway indicates amino‐acid metabolism as highly enriched for CO2–involving reactions, as well as a significant enrichment for xenobiotics biodegradation and metabolism, carbohydrate metabolism, metabolism of cofactors and vitamins and energy metabolism (adjusted p‐values of 2.49 × 10−14, 6.01 × 10−6, 4.44 × 10−5, 3.21 × 10−4 and 7.10 × 10−4, respectively).
The ubiquity of CO2 in metabolism is more clearly seen by highlighting CO2‐dependent reactions on the KEGG map ‘Metabolic Pathways’ (Fig. S1; portion shown in Fig. 3). The KEGG metabolic map used is the largest available for depiction, however it can still only plot 40% of the 292 prokaryotic EC numbers for CO2. The chemical reactions in the map are all theoretically reversible, however not all can be reversibly catalyzed by the same enzyme under the same physiological conditions. We cross‐checked all KEGG EC numbers involving CO2 against the information regarding reversibility in BRENDA. KEGG reactions have no direct information regarding reversibility – all are assigned as reversible. Reversibility information in BRENDA is two‐fold: (a) there is a direct assignment of compounds involved in the reaction as substrates or products and (b) each reaction assigned to an EC has an independent reversibility classification, which is assigned manually by the database curators as ‘reversible’, ‘irreversible’ or ‘unknown’. In Figs S1 and 3, EC numbers involving CO2 are highlighted according to the reversibility of the reactions they encode in BRENDA. When looking at all reactions, including those not in the map, 65% were classified as producing CO2 with reversibility unknown; 24% as utilizing CO2 or reversibly producing it; 11% as irreversibly producing CO2.
Figure 3.
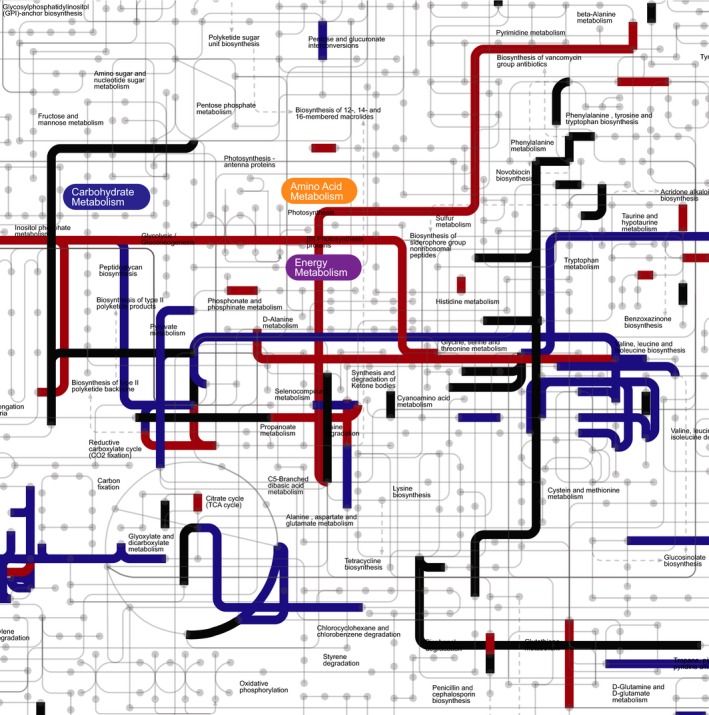
CO 2 in a section of a global metabolic map (full map provided as Fig. S1). A portion of the KEGG map ‘01100 – metabolic pathways’ with reactions involving CO 2 highlighted, portraying different directionality and reversibility assignments in BRENDA. In black, reactions not in BRENDA or where CO 2 is a product in BRENDA but reversibility is unknown; in blue, reactions where CO 2 is a substrate or it is a product and the reaction is classified as reversible in at least one study; in red, reactions classified as irreversible where CO 2 is a product.
Metals and cofactors
The cofactors and metals involved in CO and CO2 metabolism are different. We analyzed the number of studies reporting metal and cofactor utilization in BRENDA in vivo, only for EC numbers where CO and CO2 are assigned as substrates, and only for anaerobic, prokaryotic reactions (Fig. 4).
Figure 4.
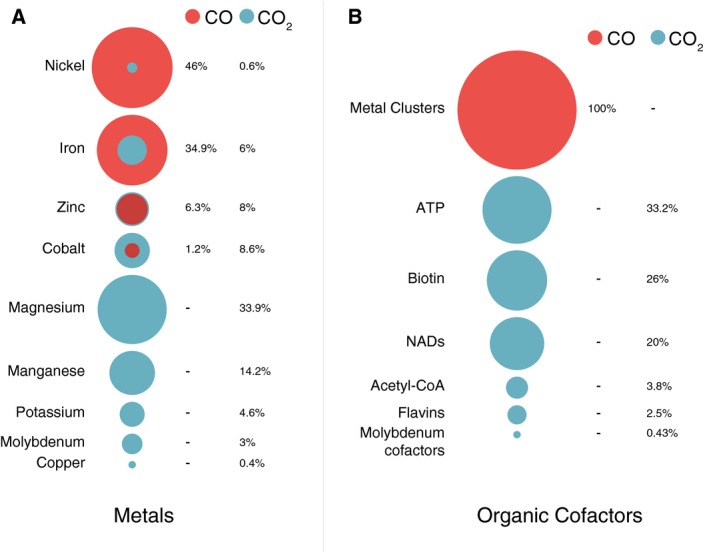
Metals and organic cofactors in reactions that consume CO or CO 2. Percentage of entries of experimental evidence in BRENDA demonstrating the participation of different (A) metals and (B) cofactors in the catalytic activity of enzymes that use CO (red) or CO 2 (blue) as substrates.
For CO, 63 entries for metal utilization were retrieved linked with the only EC number where CO is an in vivo substrate in BRENDA (anaerobic CODH reaction, http://www.chem.qmul.ac.uk/iubmb/enzyme/EC1/2/7/4.html). Nickel and iron are by far the most commonly reported metals, occurring in 46 and 34.9% of all 63 entries, respectively. For CO2, of the total 499 entries retrieved, magnesium and manganese are by far the preferred metals – 33.9 and 14.2%, respectively). Regarding organic cofactors, ATP, biotin and NADs are the most common for CO2 utilization – 33.2, 26 and 20%, respectively from a total of 235 entries – whereas for CO nickel‐iron‐sulfur clusters are the only reported cofactors. Different types of Ni‐Fe‐S clusters have been synthesized in the laboratory 58, 59, although none have yet been shown to catalyze the interconversion of CO2 and CO.
CODH/ACS: Archaea and bacteria, but not aerobes
An earlier paper plotted the evolutionary distribution of the archaeal type and bacterial type CODH and ACS enzymes across genomes 16 demonstrating the antiquity of the enzyme. Single gene phylogenies also trace CODH and ACS to the universal common ancestor 15, 26, 27. A fundamental limitation to gene phylogenies as a proxy of prokaryotic gene evolution is however that phylogenies only show in which lineages the gene is present, not the lineages in which it is missing. Plots of gene distributions reveal where genes are lacking. Figure 5A shows the current gene distribution at the prokaryotic phylum level for CODH and ACS as proxies for capacity to harness CO in metabolism and to fix it as acetyl‐CoA. As the query sequences, homologues from eight prokaryotes were used to obtain insights into the distribution of the catalytic domains.
Figure 5.
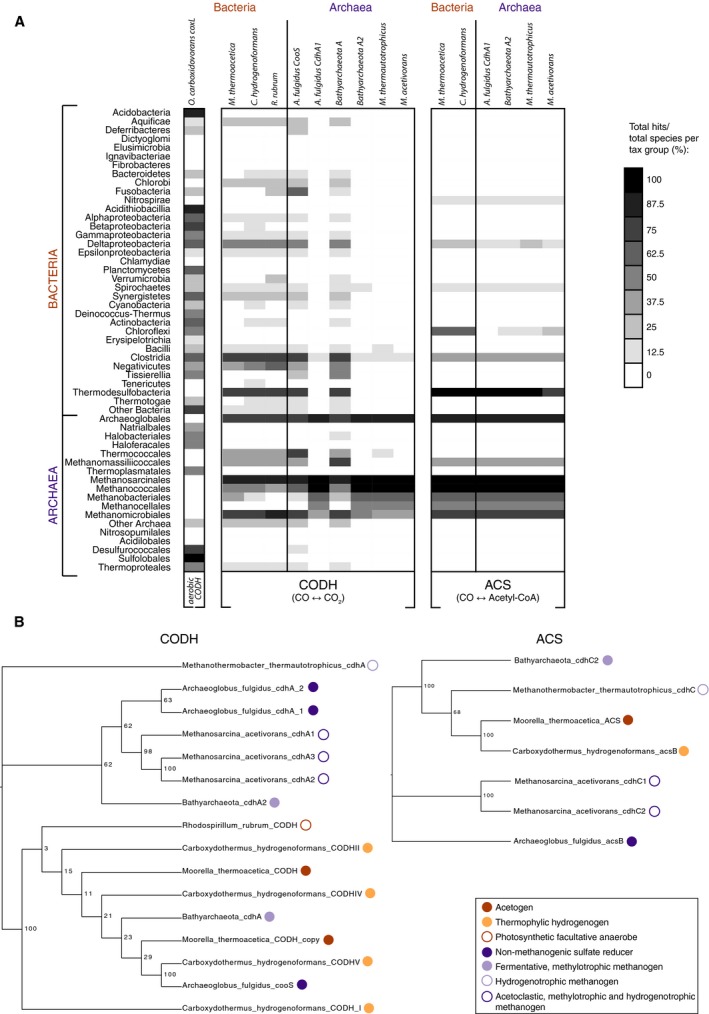
Phylogenomic analysis of CO‐interconverting enzymes. (A) Distribution of genes encoding the CODH and ACS reactions. The left part of the figure lists the taxonomic groups from 5655 completed sequenced genomes (212 archaeal and 5443 bacterial). The presence‐absence patterns (PAPs) represent the proportion of genomes within a taxonomic group where each gene is present according to the discrete grey scale‐bar of binned intervals (top right, value indicates upper value of each bin). Each column represents a different gene selected from a different query species capable of performing the aerobic CODH (oxidative) reaction, the anaerobic CODH or both the (anaerobic) CODH and the ACS reactions (Oligotropha carboxidovorans, Moorella thermoacetica, Carboxydothermus hydrogenoformans, Rhodospirillum rubrum, Archaeoglobus fulgidus, Candidatus Bathyarchaeota archaeon BA1, Methanothermobacter thermautotrophicus and Methanosarcina acetivorans). Homologous proteins were predicted by BLAST with an E‐value threshold of 10−5 and filtering for global amino acid identities of at least 20% with Powerneedle (see Materials and methods). (B) Phylogenetic trees of the query sequences of CODH (left) and ACS (right) used to BLAST the RefSeq Database to build the PAPs in (A), numbers at branches are bootstrap values. Metabolic modes of the different species are marked in front of the respective sequences with colored circles according to the legend (bottom right).
The main observation from Fig. 5 is that CODH and ACS are typically distributed among anaerobic autotrophs. Some diversity is seen in the bacterial copy of the enzyme – the presence/absence patterns obtained with the different queries are not fully identical – indicating divergence after duplication. This contrasts with the archaeal forms, with one interesting exception. In both A. fulgidus and Bathyarchaeota, there is one copy of CODH with the same distribution as the bacterial CODH. This suggests interdomain lateral gene transfer for this CODH subunit (Fig. 5B). Gene transfers from bacteria to archaea are very common in evolution 60, 61. The distribution of ACS is clearer, and uniform within both domains, showing some homology in‐between domains for the clostridial enzymes and Methanomicrobiales (Fig. 5).
CO forms stronger bonds to metals than CO2
The large difference in the numbers of metabolic reactions that involve either the utilization or production of CO and CO2 is striking. A closer look at the chemistry regarding the interaction of both compounds with metals provides further detail (Fig. 6). The orbitals in carbon atoms of both carbon monoxide and carbon dioxide are sp‐hybridized, such that both molecules are linear. At the level of electron configurations, however, CO and CO2 differ quite noticeably. In particular, the free electron pair of CO enables two complementing mechanisms (σ and π) that lead to very strong and short bonds with metals (Fig. 6A). The empty π‐orbitals of CO support backbonding with metals, which results in a very high affinity to nickel and iron in particular 62. The high affinity of CO for nickel leads to the facile formation of nickel carbonyl, Ni(CO)4 (a volatile liquid), which formed the basis of the Mond process, an early method for industrial nickel preparation 63. The strong affinity of CO to transition metals is the basis of its extreme toxicity to humans, it bonds with the iron in hemoglobin more strongly than does O2.
Figure 6.
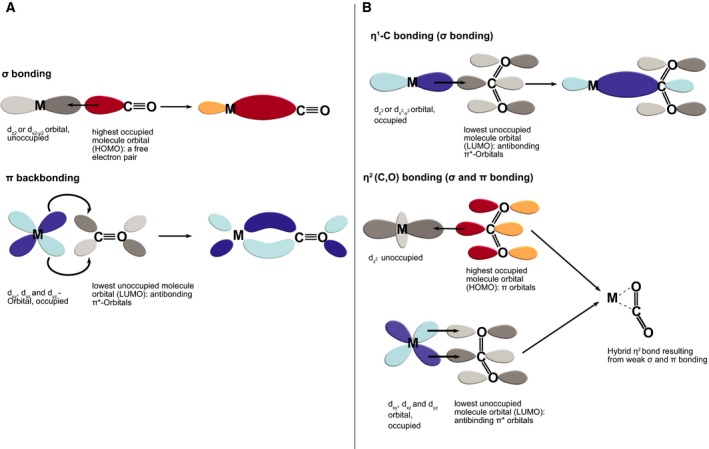
CO (A) and CO 2 (B) bonding to transition metals. (A) CO binds to a transition metal (M) via the free electron pair of its carbon atom. The electron density in this orbital (red = positive phase, yellow = negative phase) can be placed into empty metal d orbitals forming a σ bond. Concurrently, a π bond is formed between an occupied d orbital and the antibonding empty π* orbital of CO (darker grey = positive phase, lighter grey = negative phase), so called ‘π backbonding’. (B) Different bonding modes between CO 2 and transition metals include η1‐C coordination, which mostly happens with electron‐rich metals (i.e. lower oxidation states), as they can transfer charge from the dz 2 orbitals to the antibonding π* orbitals of CO 2. A double bond‐like interaction (dashed line) can also occur between a transition metal, carbon and oxygen, η2‐(C,O) bonding: an empty dz 2 orbital of a metal can take electron density from the π orbital of the CO 2 orbital (red/yellow), while electron density can also be transferred from occupied d orbitals (blue) into the antibonding π* orbitals of CO 2 (comparable to the backbonding of CO, but weaker).
By contrast, there are various bonding modes of CO2 to transition metals (Fig. 6B) which depend mostly on whether the metal is rich or poor in electrons. In general, the bonds that CO2 forms with metals are not as strong as those formed by CO. This can be a virtue in metabolism, as the rather weak bonds of CO2 to metals permit faster and more versatile catalytic reactions than those of CO. Nevertheless, the special bond between CO and transition metals also enables carbonyl insertion, both in industrial chemistry (heterogenic catalysis) 64, and in one very ancient and important biological reaction – CODH/acetyl‐CoA synthase 65, which requires the essential Ni‐Fe‐S cluster for achieving the slow reduction of CO2 to CO. Recent studies showing that CO2 is efficiently reduced by native metals to acetyl and pyruvoyl moieties entail metal bound carbonyl groups and carbonyl insertions in the proposed reaction mechanisms 23. This parallels the Fischer‐Tropsch type reaction mechanisms suggested for geochemical CO2 reduction processes giving rise to abiotic organic molecules in hydrothermal vents 66, 67.
Conclusion
For soil environments, it has been estimated that 0.2 gigatonnes (Gt) of CO is consumed each year globally 68 mainly through CO aerobic oxidation 69. During methanogenesis in anoxic environments 70, about 0.6 Gt of CH4 is produced annually from acetate 71, 72, a process that generates one mol of CO as a pathway intermediate per mol of acetate cleaved 71, 72, corresponding to roughly 1 Gt methanogenesis‐dependent CO synthesis per year. Based on reviews of CO metabolism 22, 37, and on our metabolic database search, it appears that CO interfaces with metabolism (the biotic segment of the carbon cycle) at only two enzymes: the anaerobic CODH, a Ni‐ and Fe‐containing enzyme, and the aerobic CODH, a Mo‐ and Cu‐containing enzyme. Although they catalyze the same reversible reaction (Eq. (1)),
| (1) |
the aerobic and anaerobic CODH enzymes have different subunit structures, different cofactors and are not related at the amino acid sequence or structural level [37, 39, 40, 41, 45]. Prokaryotes that use aerobic CODH use CO as a source of electrons in energy metabolism, are typically aerobes or facultative aerobes and tend to transfer the electrons from CO to high potential acceptors such as O2 or acceptors derived from it such as nitrate (NO3 −) 45, 73. Because O2 is a product of cyanobacterial metabolism 74, such high potential acceptors are latecomers in evolution, as current geochemical data have it that cyanobacterial O2 first appeared about 2.5 billion years ago 74, 75, 76.
From the standpoint of thermodynamics, it is well‐known that the WL pathway is the most favorable of the six known CO2 fixation pathways 3, 77:
| (2) |
The reaction is exergonic when H2 is the electron donor, which allows some acetogens and some methanogens to generate ion gradients and ATP at the expense of CO2 fixation. All other pathways of CO2 fixation require ATP hydrolysis to go forward. Recent findings show that the reverse citric acid (rTCA) cycle in some thermophiles requires hydrolysis of only one ATP to go forward 78, 79, but that ATP must still be generated by an independent energy metabolism. The reductive acetyl‐CoA pathway is simultaneously a source of carbon and energy, a strong argument in favor of its ancestral status among carbon assimilation pathways 2, 3. CO2‐fixation via the rTCA cycle could have arisen via closure of the incomplete (horseshoe) version of the rTCA cycle 80 (starting from acetyl‐CoA supplied by the WL‐pathway) as it occurs in some acetogens and methanogens 18, 81, 82.
Its linear nature, chemical simplicity, favorable energetics, and occurrence among both Bacteria and Archaea set it apart from other pathways of CO2 fixation and suggest that the WL is the most ancient of CO2 fixation pathways 3, 4. Strong evidence supporting the antiquity of the WL pathway comes from new findings showing that its main reactions are facile, with its central intermediates including pyruvate arising spontaneous in laboratory reactions overnight from CO2 and water at temperatures of 30–100 °C in the absence of enzymes, with native metals such as Fe0 and Ni0 functioning as catalysts and reductants 23. From the standpoint of energetics, there is something very special about the reductive acetyl‐CoA pathway among metabolic pathways. The involvement of CO as a reaction intermediate able to undergo carbonyl insertion might be the essential property that renders Ni‐dependent C—C bond formation in the CODH/ACS reaction mechanism apparently immune to substitution by organic cofactors or alternative enzymes over the last 4 billion years. In physiological evolution, it appears that there is something very special about CO.
Materials and methods
Data retrieval and integration
Both KEGG and BRENDA databases were scanned for classes of reactions involving CO and/or CO2 by parsing Enzyme Commission (EC) numbers. From BRENDA, we took only the subset of reactions tested in vivo. EC numbers involving bicarbonate () were also retrieved. Because of the chemical equilibrium between CO2 and and their rapid interconversion by carbonic anhydrases 83, which are widely distributed enzymes, throughout this work CO2 and were considered to be identical in database parsing procedures. EC numbers and the current list of KEGG organisms with the corresponding taxonomic classification were downloaded using the KEGG Rest API (http://www.kegg.jp/kegg/rest/keggapi.html), July 2017. The EC numbers from BRENDA were retrieved with the SOAP API Python interface. All integration was performed with Python scripts.
Taxonomy annotation
The taxonomic assignment of EC numbers was retrieved from the annotated genomes in the KEGG database. Among all 399 KEGG EC numbers used, 114 had no gene associated, and these were manually checked: for each EC number we checked the original literature linked in the KEGG entry to find the corresponding taxon where the EC number was identified. For 53 out of these 114 EC numbers, the taxon retrieved from the literature was not present in KEGG genomes. In these cases, a close phylogenetic cousin was assigned to the EC number so that it could be automatically assigned to the Prokaryotic or Eukaryotic domains.
Statistical analysis and metabolic maps
All the statistical analyses, including the Fisher's exact test for significance and Bonferroni correction, were performed with the package RPy2, that provides an interface between Python and the r statistical software. Overlapping sets of EC numbers were analyzed and plotted with UpSetR 84. The metabolic map with highlighted reactions was produced with iPath v2.0 85.
Analysis of distributions of CO enzymes
The query sequences for the catalytic domain of CODH and the catalytic domain of ACS were manually selected from nine different species (four archaea and five bacteria) that have been studied with respect to CO utilization. All annotated copies for both genes were taken for each genome. This exercise resulted in the collection of a total of 25 queries from: (bacterial) an acetogen, Moorella thermoacetica, with two copies of CODH and one copy of ACS 86; a thermophilic hydrogenogen, Carboxydothermus hydrogenoformans with four CODH copies and one ACS 87; a photosynthetic facultative anaerobe, Rhodospirillum rubrum with a single copy of CODH, capable of growth on carbon monoxide as sole energy source 88; two aerobes with one CODH each, Oligotropha carboxidovorans (coxL I) and Bradyrhizobium sp. CPP (coxL II) 89 – the latter with a similar pattern to the former (data not shown); (archaeal) a non‐methanogenic sulfate reducer, Archaeoglobus fulgidus, with 3 copies of CODH and one ACS 90; a recently identified, fermentative and possibly methylotrophic methanogen, Candidatus Bathyarchaeota archaeon BA1 with two copies of CODH and one of ACS 91, 92; one hydrogenotrophic methanogen, Methanothermobacter thermautotrophicus with one copy of each enzyme 93 and finally an acetoclastic methylotrophic, hydrogenotrophic methanogen, Methanosarcina acetivorans with three copies of CODH and two of ACS 94. Representative queries were taken from each genome when they were significantly similar. The queries were aligned with ClustalW 95 and phylogenetic inferences were made with RAxML 96.
To characterize CODH and ACS gene distribution, a BLAST search was performed against all prokaryotic genomes in RefSeq (NCBI, version September 2016), of which the primary hits (e‐value ≤ 1 × 10−5) were selected. A pairwise global ‘Needleman & Wunsch’ – alignment was then performed with these sequences against the whole database of prokaryotes again to filter for hits with global identity >20%.
Author contributions
JCX collected and analyzed the data from KEGG and BRENDA. MP analyzed the chemical configurations of CO2 and CO. WFM designed and supervised the study. The manuscript was written and proofread by all authors.
Supporting information
Fig. S1. CO2 in a global metabolic map. KEGG map ‘01100 – metabolic pathways’ with reactions involving CO2 highlighted, portraying different directionality and reversibility assignments in BRENDA.
Acknowledgements
This work was supported by grants from the European Research Council (666053), the Volkswagen Foundation (93 046) to WFM and by a cooperation grant from the Deutsche Forschungsgemeinschaft to WFM (MA1426/21‐1) and to Harun Tüysüz (TU 315/8‐1), Max Planck Institute for Coal Research, Mülheim.
References
- 1. Tabita FR (1988) Molecular and cellular regulation of autotrophic carbon dioxide fixation in microorganisms. Microbiol Rev 52, 155–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Berg IA (2011) Ecological aspects of the distribution of different autotrophic CO2 fixation pathways. Appl Environ Microbiol 77, 1925–1936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Fuchs G (2011) Alternative pathways of carbon dioxide fixation: insights into the early evolution of life? Annu Rev Microbiol 65, 631–658. [DOI] [PubMed] [Google Scholar]
- 4. Hügler M & Sievert SM (2011) Beyond the Calvin cycle: autotrophic carbon fixation in the ocean. Ann Rev Mar Sci 3, 261–289. [DOI] [PubMed] [Google Scholar]
- 5. Appel AM, Bercaw JE, Bocarsly AB, Dobbek H, DuBois DL, Dupuis M, Ferry JG, Fujita E, Hille R, Kenis PJA et al (2013) Frontiers, opportunities, and challenges in biochemical and chemical catalysis of CO2 fixation. Chem Rev 113, 6621–6658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Jajesniak P, Eldin H, Omar M & Wong TS (2014) Carbon dioxide capture and utilization using biological systems : opportunities and challenges. Bioprocess Biotech 4, 15. [Google Scholar]
- 7. Gong F, Liu G, Zhai X, Zhou J, Cai Z & Li Y (2015) Quantitative analysis of an engineered CO2‐fixing Escherichia coli reveals great potential of heterotrophic CO2 fixation. Biotechnol Biofuels 8, 10.1186/s13068-015-0268-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Antonovsky N, Gleizer S, Noor E, Zohar Y, Herz E, Barenholz U, Zelcbuch L, Amram S, Wides A, Tepper N et al (2016) Sugar synthesis from CO2 in Escherichia coli . Cell 166, 115–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Schwander T, Schada von Borzyskowski L, Burgener S, Cortina NS & Erb TJ (2016) A synthetic pathway for the fixation of carbon dioxide in vitro. Science 354, 900–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Claassens NJ (2017) A warm welcome for alternative CO2 fixation pathways in microbial biotechnology. Microb Biotechnol 10, 31–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bolton E, Abelson P & Aldous E (1952) Utilization of carbon dioxide in the synthesis of nucleic acid by Escherichia coli . J Biol Chem 198, 179–185. [PubMed] [Google Scholar]
- 12. Roslev P, Larsen MB, Jørgensen D & Hesselsoe M (2004) Use of heterotrophic CO2 assimilation as a measure of metabolic activity in planktonic and sessile bacteria. J Microbiol Methods 59, 381–393. [DOI] [PubMed] [Google Scholar]
- 13. Fuchs G & Stupperich E (1985) Evolution of autotrophic CO2 fixation In Evolution of Prokaryotes. FEMS Symposium No. 29 (Schleifer K. & Stackebrandt E, eds), pp. 235–251. Academic Press, London. [Google Scholar]
- 14. Russell MJ & Martin W (2004) The rocky roots of the acetyl‐CoA pathway. Trends Biochem Sci 29, 358–363. [DOI] [PubMed] [Google Scholar]
- 15. Weiss MC, Sousa FL, Mrnjavac N, Neukirchen S, Roettger M, Nelson‐Sathi S & Martin WF (2016) The physiology and habitat of the last universal common ancestor. Nat Microbiol 1, 10.1038/nmicrobiol.2016.116 [DOI] [PubMed] [Google Scholar]
- 16. Sousa FL & Martin WF (2014) Biochemical fossils of the ancient transition from geoenergetics to bioenergetics in prokaryotic one carbon compound metabolism. Biochim Biophys Acta – Bioenerg 1837, 964–981. [DOI] [PubMed] [Google Scholar]
- 17. Huber C & Wächtershäuser G (1997) Activated acetic acid by carbon fixation on (Fe, Ni)S under primordial conditions. Science 276, 245–247. [DOI] [PubMed] [Google Scholar]
- 18. Martin W & Russell MJ (2007) On the origin of biochemistry at an alkaline hydrothermal vent. Philos Trans R Soc B Biol Sci 362, 1887–1926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Berg IA, Kockelkorn D, Ramos‐Vera WH, Say RF, Zarzycki J, Hügler M, Alber BE & Fuchs G (2010) Autotrophic carbon fixation in archaea. Nat Rev Microbiol 8, 447–460. [DOI] [PubMed] [Google Scholar]
- 20. Schrenk MO, Brazelton WJ & Lang SQ (2013) Serpentinization, carbon, and deep life. Rev Mineral Geochemistry 75, 575–606. [Google Scholar]
- 21. McCollom TM (2016) Abiotic methane formation during experimental serpentinization of olivine. Proc Natl Acad Sci USA 113, 13965–13970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ragsdale SW & Pierce E (2008) Acetogenesis and the Wood‐Ljungdahl pathway of CO2 fixation. Biochim Biophys Acta – Proteins Proteomics 1784, 1873–1898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Varma SJ, Muchowska KB, Chatelain P & Moran J (2018) Native iron reduces CO2 to intermediates and end‐products of the acetyl‐CoA pathway. Nat Ecol Evol 2, 1019–1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sousa FL, Preiner M & Martin WF (2018) Native metals, electron bifurcation, and CO2 reduction in early biochemical evolution. Curr Opin Microbiol 43, 77–83. [DOI] [PubMed] [Google Scholar]
- 25. Buckel W & Thauer RK (2018) Flavin‐based electron bifurcation, ferredoxin, flavodoxin, and anaerobic respiration with protons (Ech) or NAD+(Rnf) as electron acceptors: a historical review. Front Microbiol 9, 10.3389/fmicb.2018.00401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Adam PS, Borrel G & Gribaldo S (2018) Evolutionary history of carbon monoxide dehydrogenase/acetyl‐CoA synthase, one of the oldest enzymatic complexes. Proc Natl Acad Sci USA 115, E1166–E1173 (erratum appears in Proc Natl Acad Sci USA 115, E5836–E5837). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Techtmann SM, Lebedinsky AV, Colman AS, Sokolova TG, Woyke T, Goodwin L & Robb FT (2012) Evidence for horizontal gene transfer of anaerobic carbon monoxide dehydrogenases. Front Microbio 3, 10.3389/fmicb.2012.00132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Vorholt J, Kunow J, Stetter KO & Thauer RK (1995) Enzymes and coenzymes of the carbon monoxide dehydrogenase pathway for autotrophic CO2 fixation in Archaeoglobus lithotrophicus and the lack of carbon monoxide dehydrogenase in the heterotrophic A. profundus . Arch Microbiol 163, 112–118. [Google Scholar]
- 29. Ragsdale SW (2008) Enzymology of the Wood‐Ljungdahl pathway of acetogenesis. Ann N Y Acad Sci 1125, 129–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ragsdale SW (2004) Life with carbon monoxide. Crit Rev Biochem Mol Biol 39, 165–195. [DOI] [PubMed] [Google Scholar]
- 31. Sokolova TG, Henstra A‐M, Sipma J, Parshina SN, Stams AJM & Lebedinsky AV (2009) Diversity and ecophysiological features of thermophilic carboxydotrophic anaerobes. FEMS Microbiol Ecol 68, 131–141. [DOI] [PubMed] [Google Scholar]
- 32. Diender M, Stams AJM & Sousa DZ (2015) Pathways and bioenergetics of anaerobic carbon monoxide fermentation. Front Microbiol 6, 10.3389/fmicb.2015.01275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Borrel G, Adam PS & Gribaldo S (2016) Methanogenesis and the Wood‐Ljungdahl pathway: an ancient, versatile, and fragile association. Genome Biol Evol 8, 1706–1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Schuchmann K & Müller V (2014) Autotrophy at the thermodynamic limit of life: a model for energy conservation in acetogenic bacteria. Nat Rev Microbiol 12, 809–821. [DOI] [PubMed] [Google Scholar]
- 35. Kopke M, Held C, Hujer S, Liesegang H, Wiezer A, Wollherr A, Ehrenreich A, Liebl W, Gottschalk G & Durre P (2010) Clostridium ljungdahlii represents a microbial production platform based on syngas. Proc Natl Acad Sci USA 107, 13087–13092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Jansen K, Thauer RK, Widdel F & Fuchs G (1984) Carbon assimilation pathways in sulfate reducing bacteria. Formate, carbon dioxide, carbon monoxide, and acetate assimilation by Desulfovibrio baarsii . Arch Microbiol 138, 257–262. [Google Scholar]
- 37. Oelgeschläger E & Rother M (2008) Carbon monoxide‐dependent energy metabolism in anaerobic bacteria and archaea. Arch Microbiol 190, 257–269. [DOI] [PubMed] [Google Scholar]
- 38. Bender G, Pierce E, Hill JA, Darty JE & Ragsdale SW (2011) Metal centers in the anaerobic microbial metabolism of CO and CO2 . Metallomics 3, 797–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Can M, Armstrong FA & Ragsdale SW (2014) Structure, function, and mechanism of the nickel metalloenzymes, CO dehydrogenase, and acetyl‐CoA synthase. Chem Rev 114, 4149–4174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Gregg CM, Goetzl S, Jeoung JH & Dobbek H (2016) AcsF catalyzes the ATP‐dependent insertion of Nickel into the Ni, Ni‐[4Fe4S] cluster of Acetyl‐CoA synthase. J Biol Chem 291, 18129–18138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Doukov TI, Iverson TM, Seravalli J, Ragsdale SW & Drennan CL (2002) A Ni‐Fe‐Cu center in a bifunctional carbon monoxide dehydrogenase/acetyl‐CoA synthase. Science 298, 567–572. [DOI] [PubMed] [Google Scholar]
- 42. Maynard EL & Lindahl PA (1999) Evidence of a molecular tunnel connecting the active sites for CO2 reduction and acetyl‐CoA synthesis in acetyl‐CoA synthase from Clostridium thermoaceticum . J Am Chem Soc 121, 9221–9222. [Google Scholar]
- 43. Seravalli J & Ragsdale SW (2000) Channeling of carbon monoxide during anaerobic carbon dioxide fixation. Biochemistry 39, 1274–1277. [DOI] [PubMed] [Google Scholar]
- 44. Gong W, Hao B, Wei Z, Ferguson DJ, Tallant T, Krzycki JA & Chan MK (2008) Structure of the alpha2epsilon2 Ni‐dependent CO dehydrogenase component of the Methanosarcina barkeri acetyl‐CoA decarbonylase/synthase complex. Proc Natl Acad Sci USA 105, 9558–9563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. King GM & Weber CF (2007) Distribution, diversity and ecology of aerobic CO‐oxidizing bacteria. Nat Rev Microbiol 5, 107–118. [DOI] [PubMed] [Google Scholar]
- 46. Dobbek H, Gremer L, Meyer O & Huber R (1999) Crystal structure and mechanism of CO dehydrogenase, a molybdo iron‐sulfur flavoprotein containing S‐selanylcysteine. Proc Natl Acad Sci USA 96, 8884–8889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Wilcoxen J & Hille R (2013) The hydrogenase activity of the molybdenum/copper‐containing carbon monoxide dehydrogenase of Oligotropha carboxidovorans . J Biol Chem 288, 36052–36060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Hille R, Dingwall S & Wilcoxen J (2015) The aerobic CO dehydrogenase from Oligotropha carboxidovorans . J Biol Inorg Chem 20, 243–251. [DOI] [PubMed] [Google Scholar]
- 49. Meyer O, Jacobitz S & Krüger B (1986) Biochemistry and physiology of aerobic carbon monoxide‐oxidizing bacteria. FEMS Microbiol Rev 39, 161–179. [Google Scholar]
- 50. Parshina SN, Sipma J, Henstra AM & Stams AJM (2010) Carbon monoxide as an electron donor for the biological reduction of sulphate. Int J Microbiol 2010, 10.1155/2010/319527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Henstra AM & Stams AJM (2004) Novel physiological features of Carboxydothermus hydrogenoformans and Thermoterrabacterium ferrireducens . Appl Environ Microbiol 70, 7236–7240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. King GM (2015) Carbon monoxide as a metabolic energy source for extremely halophilic microbes: implications for microbial activity in Mars regolith. Proc Natl Acad Sci USA 112, 4465–4470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Nava‐Sedeño JM, Ortiz‐Cervantes A, Segura A & Domagal‐Goldman SD (2016) Carbon monoxide and the potential for prebiotic chemistry on habitable planets around main sequence M stars. Astrobiology 16, 10.1089/ast.2015.1435 [DOI] [PubMed] [Google Scholar]
- 54. Miyakawa S, Yamanashi H, Kobayashi K, Cleaves HJ & Miller SL (2002) Prebiotic synthesis from CO atmospheres: implications for the origins of life. Proc Natl Acad Sci USA 99, 14628–14631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Aylward N & Bofinger N (2001) The reactions of methanimine and cyanogen with carbon monoxide in prebiotic molecular evolution on earth. Orig Life Evol Biosph 31, 481–500. [DOI] [PubMed] [Google Scholar]
- 56. Cody GD, Boctor NZ, Filley TR, Hazen RM, Scott JH & Yoder HS Jr (2000) The primordial synthesis of carbonylated iron‐sulfur clusters and the synthesis of pyruvate. Science 289, 1337–1340. [DOI] [PubMed] [Google Scholar]
- 57. Wilcoxen J, Zhang B & Hille R (2011) Reaction of the molybdenum‐ and copper‐containing carbon monoxide dehydrogenase from Oligotropha carboxydovorans with quinones. Biochemistry 50, 1910–1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Panda R, Zhang Y, McLauchlan CC, Rao PV, Tiago De Oliveira FA, Münck E & Holm RH (2004) Initial structure modification of tetrahedral to planar nickel(II) in a nickel‐iron‐sulfur cluster related to the C‐cluster of carbon monoxide dehydrogenase. J Am Chem Soc 126, 6448–6459. [DOI] [PubMed] [Google Scholar]
- 59. Song LC, Li YL, Li L, Gu ZC & Hu QM (2010) Synthetic and structural investigations of linear and macrocyclic nickel/iron/sulfur cluster complexes. Inorg Chem 49, 10174–10182. [DOI] [PubMed] [Google Scholar]
- 60. Nelson‐Sathi S, Sousa FL, Roettger M, Lozada‐Chávez N, Thiergart T, Janssen A, Bryant D, Landan G, Schönheit P, Siebers B et al (2015) Origins of major archaeal clades correspond to gene acquisitions from bacteria. Nature 517, 77–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Wagner A, Whitaker RJ, Krause DJ, Heilers J‐H, van Wolferen M, van der Does C & Albers S‐V (2017) Mechanisms of gene flow in archaea. Nat Rev Microbiol 15, 492–501. [DOI] [PubMed] [Google Scholar]
- 62. Jitaru M (2007) Electrochemical carbon dioxide reduction ‐ fundamental and applied topics. J Univ Chem Technol Metall 42, 333–344. [Google Scholar]
- 63. Mond L, Langer C & Quincke F (1890) Action of carbon monoxide on nickel. J Chem Soc Trans 57, 749–753. [Google Scholar]
- 64. Tchougreeff AL, Gulevich YV, Misurkin IA & Beletskaya IP (1993) A model for CO insertion in transition metal complexes. J Organomet Chem 455, 261–270. [Google Scholar]
- 65. Evans DJ (2005) Chemistry relating to the nickel enzymes CODH and ACS. Coord Chem Rev 249, 1582–1595. [Google Scholar]
- 66. McCollom TM & Seewald JS (2013) Serpentinites, hydrogen, and life. Elements 9, 129–134. [Google Scholar]
- 67. McCollom TM (2013) Miller‐Urey and beyond: What have we learned about prebiotic organic synthesis reactions in the past 60 years? Annu Rev Earth Planet Sci 41, 207–229. [Google Scholar]
- 68. King GM (1999) Characteristics and significance of atmospheric carbon monoxide consumption by soils. Chemosphere 1, 53–63. [Google Scholar]
- 69. Conrad R & Seiler W (1980) Role of microorganisms in the consumption and production of atmospheric carbon monoxide by soil. Appl Environ Microbiol 40, 437–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Thauer RK, Kaster A‐K, Seedorf H, Buckel W & Hedderich R (2008) Methanogenic archaea: ecologically relevant differences in energy conservation. Nat Rev Microbiol 6, 579–591. [DOI] [PubMed] [Google Scholar]
- 71. Thauer RK (1998) Biochemistry of methanogenesis: a tribute to Marjory Stephenson. 1998 Marjory Stephenson Prize Lecture. Microbiology 144, 2377–2406. [DOI] [PubMed] [Google Scholar]
- 72. Ferry JG (2010) How to make a living by exhaling methane. Annu Rev Microbiol 64, 453–473. [DOI] [PubMed] [Google Scholar]
- 73. Meyer O & Schlegel HG (1983) Biology of aerobic carbon monoxide‐oxidizing bacteria. Annu Rev Microbiol 37, 277–310. [DOI] [PubMed] [Google Scholar]
- 74. Fischer WW, Hemp J & Johnson JE (2016) Evolution of oxygenic photosynthesis. Annu Rev Earth Planet Sci 44, 647–683. [Google Scholar]
- 75. Lyons TW, Reinhard CT & Planavsky NJ (2014) The rise of oxygen in Earth's early ocean and atmosphere. Nature 506, 307–315. [DOI] [PubMed] [Google Scholar]
- 76. Allen JF (2016) A proposal for formation of Archaean stromatolites before the advent of oxygenic photosynthesis. Front Microbiol 7, 10.3389/fmicb.2016.01784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Fuchs G (1994) Variations of the acetyl‐CoA pathway in diversely related microorganisms that are not acetogens In Acetogenesis (Drake HL, ed.), pp. 507–520. Springer US, Boston, MA. [Google Scholar]
- 78. Mall A, Sobotta J, Huber C, Tschirner C, Kowarschik S, Bačnik K, Mergelsberg M, Boll M, Hügler M, Eisenreich W et al (2018) Reversibility of citrate synthase allows autotrophic growth of a thermophilic bacterium. Science 359, 563–567. [DOI] [PubMed] [Google Scholar]
- 79. Nunoura T, Chikaraishi Y, Izaki R, Suwa T, Sato T, Harada T, Mori K, Kato Y, Miyazaki M, Shimamura S et al (2018) A primordial and reversible TCA cycle in a facultatively chemolithoautotrophic thermophile. Science 359, 559–563. [DOI] [PubMed] [Google Scholar]
- 80. Giovannelli S, Sievert SM, Hügler M, Markert S, Becher D, Schweder T & Vetriani C (2017) Insight into the evolution of microbial metabolism from the deep‐branching bacterium, Thermovibrio ammonificans . eLife 6, e18990 10.7554/eLife.18990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Simpson PG & Whitman WB (1993) Anabolic pathways in methanogens In Methanogenesis (Ferry JG, ed.), pp. 445–472. Chapman & Hall Microbiology Series (Physiology/Ecology/Molecular Biology/Biotechnology). Springer US, Boston, MA. [Google Scholar]
- 82. Furdui C & Ragsdale SW (2000) The role of pyruvate ferredoxin oxidoreductase in pyruvate synthesis during autotrophic growth by the Wood‐Ljungdahl pathway. J Biol Chem 275, 28494–28499. [DOI] [PubMed] [Google Scholar]
- 83. Tresguerres M, Buck J & Levin LR (2010) Physiological carbon dioxide, bicarbonate, and pH sensing. Pflügers Arch – Eur J Physiol 460, 953–964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Lex A, Gehlenborg N, Strobelt H, Vuillemot R & Pfister H (2014) UpSet: visualization of intersecting sets. IEEE Trans Vis Comput Graph 20, 1983–1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Yamada T, Letunic I, Okuda S, Kanehisa M & Bork P (2011) iPath2.0: interactive pathway explorer. Nucleic Acids Res 39, W412–W415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Pierce E, Xie G, Barabote RD, Saunders E, Han CS, Detter JC, Richardson P, Brettin TS, Das A, Ljungdahl LG et al (2008) The complete genome sequence of Moorella thermoacetica (f. Clostridium thermoaceticum). Environ Microbiol 10, 2550–2573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Wu M, Ren Q, Durkin AS, Daugherty SC, Brinkac LM, Dodson RJ, Madupu R, Sullivan SA, Kolonay JF, Haft DH et al (2005) Life in hot carbon monoxide: the complete genome sequence of Carboxydothermus hydrogenoformans Z‐2901. PLoS Genet 1, 10.1371/journal.pgen.0010065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Munk AC, Copeland A, Lucas S, Lapidus A, Del Rio TG, Barry K, Detter JC, Hammon N, Israni S, Pitluck S et al (2011) Complete genome sequence of Rhodospirillum rubrum type strain (S1T). Stand Genomic Sci 4, 293–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. King GM (2003) Molecular and culture‐based analyses of aerobic carbon monoxide oxidizer diversity. Appl Environ Microbiol 69, 7257–7265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Klenk H‐P, Clayton RA, Tomb J‐F, White O, Nelson KE, Ketchum KA, Dodson RJ, Gwinn M, Hickey EK, Peterson JD et al (1997) The complete genome sequence of the hyperthermophilic, sulphate‐reducing archaeon Archaeoglobus fulgidus . Nature 390, 364–370. [DOI] [PubMed] [Google Scholar]
- 91. Evans PN, Parks DH, Chadwick GL, Robbins SJ, Orphan VJ, Golding SD & Tyson GW (2015) Methane metabolism in the archaeal phylum Bathyarchaeota revealed by genome‐centric metagenomics. Science 350, 434–438. [DOI] [PubMed] [Google Scholar]
- 92. Vanwonterghem I, Evans PN, Parks DH, Jensen PD, Woodcroft BJ, Hugenholtz P & Tyson GW (2016) Methylotrophic methanogenesis discovered in the archaeal phylum Verstraetearchaeota . Nat Microbiol 1, 10.1038/nmicrobiol.2016.170 [DOI] [PubMed] [Google Scholar]
- 93. Smith DR, Doucette‐Stamm LA, Deloughery C, Lee H, Dubois J, Aldredge T, Bashirzadeh R, Blakely D, Cook R, Gilbert K et al (1997) Complete genome sequence of Methanobacterium thermoautotrophicum deltaH: functional analysis and comparative genomics. J Bacteriol 179, 7135–7155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Galagan JE, Nusbaum C, Roy A, Endrizzi MG, Macdonald P, FitzHugh W, Calvo S, Engels R, Smirnov S, Atnoor D et al (2002) The genome of M. acetivorans reveals extensive metabolic and physiological diversity. Genome Res 12, 532–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Larkin MA, Blackshields G, Brown NP, Chenna R, McGettigan PA, McWilliam H, Valentin F, Wallace IM, Wilm A, Lopez R et al (2007) Clustal W and Clustal X version 2.0. Bioinformatics 23, 2947–2948. [DOI] [PubMed] [Google Scholar]
- 96. Stamatakis A (2014) RAxML version 8: a tool for phylogenetic analysis and post‐analysis of large phylogenies. Bioinformatics 30, 1312–1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Dai Y, Wensink PC & Abeles RH (1999) One protein, two enzymes. J Biol Chem 274, 1193–1195. [DOI] [PubMed] [Google Scholar]
- 98. Allpress CJ, Grubel K, Szajna‐Fuller E, Arif AM & Berreau LM (2013) Regioselective aliphatic carbon‐carbon bond cleavage by a model system of relevance to iron‐containing Acireductone dioxygenase. J Am Chem Soc 135, 659–668. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. CO2 in a global metabolic map. KEGG map ‘01100 – metabolic pathways’ with reactions involving CO2 highlighted, portraying different directionality and reversibility assignments in BRENDA.


