Abstract
BI 655064 is a humanized antagonistic anti‐cluster of differentiation (CD) 40 monoclonal antibody that selectively blocks the CD40‐CD40L interaction. The CD40‐CD40L pathway is a promising treatment target for autoimmune diseases such as rheumatoid arthritis, systemic lupus erythematosus, and lupus nephritis. The safety, tolerability, pharmacokinetics, and pharmacodynamics of repeated once‐weekly BI 655064 subcutaneous dosing over 4 weeks were evaluated in a multiple‐dose study in healthy subjects. Subjects (N = 40) were randomized 4:1 to four sequential BI 655064 dose groups (80, 120, 180, 240 mg) or to placebo. Safety and tolerability, plasma exposure, CD40 receptor occupancy, and CD40L‐induced CD54 upregulation were assessed over 64 and 78 days for the 80‐ to 180‐mg and 240‐mg dose groups, respectively. BI 655064 exposure increased in a supraproportional manner, due to target‐mediated drug clearance, for doses between 80 mg and 120 mg, but was near proportional for doses greater than 120 mg. Terminal half‐life ranged between 6 and 8 days. Dose‐dependent accumulation of BI 655064 supports the use of a loading dose in future clinical studies. Following 4 weeks of dosing, >90% CD40 receptor occupancy and inhibition of CD54 upregulation were observed at all dose levels, lasting for 17 days after the last dose. BI 655064 was generally well tolerated. There were no serious adverse events and the frequency and intensity of adverse events were similar for BI 655064 and placebo; no dose relationship or relevant signs of an acute immune reaction were observed. These findings support further investigation of BI 655064 as a potential treatment for autoimmune diseases.
Keywords: anti‐CD40, multiple rising dose, rheumatoid arthritis, systemic lupus erythematosus, lupus nephritis, healthy subjects
Despite therapeutic progress in recent years, there is still an unmet need for new treatments for autoimmune diseases such as rheumatoid arthritis (RA), systemic lupus erythematosus (SLE), and lupus nephritis.1, 2, 3 The interaction of the cell surface receptor cluster of differentiation (CD) 40 and its ligand CD40L (CD154) is known to play a central role in the regulation of humoral and cellular immunity, and in the pathogenesis of these autoimmune diseases.3, 4, 5 Therefore, the CD40‐CD40L interaction is an attractive target for the modulation of autoimmune diseases.6
CD40 is a cell surface receptor that belongs to the tumor necrosis factor receptor family and is expressed on B cells, dendritic cells, monocytes, macrophages, kidney cells, and other nonimmune cells.3, 7 CD40 is a key costimulatory molecule involved in the development of antigen‐driven acquired immunity by activating B cells and other antigen‐presenting cells (APCs), including dendritic cells and macrophages, but is also involved in the activation of nonimmune resident cells.4 CD40L is a member of the tumor necrosis factor superfamily that is expressed primarily by activated T cells, as well as by activated B cells and platelets.5 Binding of CD40 to CD40L results in the upregulation of E‐selectin (CD62E), vascular cell adhesion molecule‐1 (CD106), and intercellular adhesion molecule‐1 (CD54), thereby increasing leukocyte margination and diapedesis.8
The CD40‐CD40L interaction appears to be required for optimal APC‐T‐cell activation.9 The CD40‐CD40L pathway is thought to be particularly important for amplification of the T‐cell response and is involved in several autoimmune diseases.10 Blocking the CD40 signaling pathway has been shown to inhibit T helper 1 cell differentiation and maintenance of the immune response.11 Increased expression of CD40 and CD40L is associated with active disease in patients with RA.12, 13 Elevated levels of CD40L on B and T cells are associated with disease activity in SLE, and renal CD40 expression on mesangial cells is upregulated in patients with class III and class IV lupus nephritis.10, 14, 15
Previous clinical development of monoclonal antibodies against CD40L failed due to incidents of thromboembolism, which were initiated by the activation and aggregation of platelets, possibly due to the fragment crystallizable (Fc) region of anti‐CD40L antibodies activating the FcγRIIa (CD32a) platelet receptor.6, 16, 17, 18 Recent studies indicate that antibodies lacking a functional Fc region do not induce thromboembolic events, fail to activate platelets, and retain pharmacologic activity,6, 17, 19 as well as clinical activity.20
BI 655064 is a humanized antagonistic anti‐CD40 monoclonal antibody that selectively binds CD40 and blocks the CD40‐CD40L interaction; it was designed to have no agonistic activity and to prevent stimulating cytokine production.21 Two replacement mutations in the Fc region (Leu234Ala and Leu235Ala) were incorporated to prevent Fc‐mediated antibody‐dependent or complement‐mediated cellular cytotoxicity and platelet activation. BI 655064 demonstrated potent and comparable binding properties in both human (EC90 = 6.85 ± 0.74 nM) and cynomolgus monkey B cells, and potent inhibition of CD40L‐induced peripheral blood mononuclear cell proliferation without agonism. When bound to platelets, BI 655064 does not appear to alter platelet activation, aggregation, or function.21 In preclinical assessments in cynomolgus monkeys, with multiple doses up to 50 mg/kg BI 655064 for 26 weeks, reversible decreases in B‐cell levels, reversible reduction of lymphoid organ germinal centers, and good general tolerability without thromboembolic events or relevant cytokine release were demonstrated (21 and unpublished data). The no‐observed‐adverse‐effect level in these assessments was 50 mg/kg—the highest dose administered (unpublished data).
In a single rising dose study in healthy volunteers, increasing intravenous (IV) and subcutaneous (SC) single doses of up to 120‐mg BI 655064 were well tolerated and showed a high potential to block the CD40‐CD40L pathway. Dose‐related increases in CD40 receptor occupancy (RO) and inhibition of B cell activation (as measured by the inhibition of CD54 upregulation), after both IV and SC dosing of BI 655064, were observed.22
The objective of this randomized, placebo‐controlled, double‐blind study was to determine the safety, tolerability, pharmacokinetics (PK), and pharmacodynamics (PD) of 4 weeks of repeated, once‐weekly SC dosing of 80‐, 120‐, 180‐, or 240‐mg BI 655064 in healthy subjects.
Methods
Study Design
This phase 1 study was approved by the independent ethics committee of the participating center and the New Zealand health authority, and all participating subjects provided informed consent. The study was sponsored by Boehringer Ingelheim and conducted at a single trial center in Auckland, New Zealand, by Auckland Clinical Studies Ltd. The study registration identifier is ClinicalTrials.gov NCT01751776.
The study was a randomized, placebo‐controlled, double‐blind, within‐dose group study. Multiple rising SC doses of 80‐ to 240‐mg BI 655064 were tested in healthy subjects once weekly over a 4‐week treatment period.
Doses were selected based on safety, PK, and PD data from the single rising dose study.22 In that study, the maximal tested IV dose (120 mg) was well tolerated, and provided a 7‐fold higher maximum observed concentration (Cmax) and a 3‐fold higher area under the plasma drug concentration‐time curve (AUC) than the tested 120‐mg SC dose. Based on these PK data, it was calculated that the exposure from the 120‐mg IV dose in the single rising dose study would cover the exposure expected with a 240‐mg SC dose.
Eligible subjects were randomized to receive BI 655064 or placebo in a ratio of 4:1 via an Interactive Response Technology tool in four sequential SC dose groups (80, 120, 180, and 240 mg), with dose groups separated by at least 7 days. Each dose group comprised 10 subjects (8 active, 2 placebo) (Figure 1). Escalation to the next dose level was decided by an independent data monitoring committee based on the evaluation of safety, tolerability, PK, and PD data.
Figure 1.
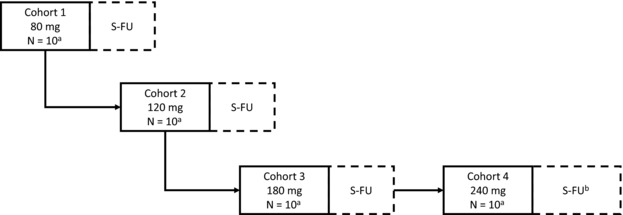
Study design. S‐FU, safety follow‐up.
a2 subjects randomized to placebo and 8 subjects randomized to BI 655064.
bS‐FU was longer for cohort 4 (56 days instead of 42 days).
All subjects received their last dose of treatment on day 22. Subjects in the 80‐, 120‐, and 180‐mg SC dose groups were followed for 42 days after their last dose, and the total study duration was 64 days. Subjects in the 240‐mg SC dose group were followed for 56 days, and the total study duration was 78 days.
Subjects, investigators, and sponsor staff remained blinded to study treatment. An initial database lock was performed after patients in the 80‐ to 180‐mg SC dose groups had completed the study, and all data were unblinded following completion of the 240‐mg SC dose group.
Blood samples (2.7 mL) for PK analysis were collected from a forearm vein using an indwelling catheter into tripotassium ethylenediaminetetraacetic acid anticoagulant tubes. Samples were collected before dosing and after dosing at 1, 8, and 12 hours, and on days 1, 2, 3 (morning and evening, 12 hours apart), 4 (morning and evening, 12 hours apart), 5, 6, 7 (before the second dose), 14 (before the third dose), 21 (before the fourth dose, and at 1 and 12 hours after the fourth dose), 22, 23, 24, 25, 26, 27, 28, 29, 31, 34, 38, 42, 49, 56, 63 (80‐ to 180‐mg dose groups only), and 77 (240‐mg dose group only) after the first dose.
Blood samples for assessment of anti‐BI 655064 antibodies (ADAs) were taken before dosing and after the first dose on days 21 (before the fourth dose), 38, 63 (80‐ to 180‐mg dose groups only), and 77 (240‐mg dose group only) after the first dose.
Blood samples were immediately placed on ice after collection, and centrifuged at 4°C for 10 minutes within 30 minutes of sample collection. Plasma was transferred into two polypropylene sample vials (0.5 mL each) and stored at –20°C before shipping to the analytical laboratory.
Blood samples (4.9 mL) for PD analysis were collected from a forearm vein using an indwelling catheter into heparin‐anticoagulant blood tubes before dosing and on days 3, 7 (before the second dose), 21 (before the fourth dose), 24, 28, 38, 63 (80‐ to 180‐mg dose groups only), and 77 (for the 240‐mg dose group only) after the first dose, and were delivered immediately to the laboratory for analysis. Assay validation for the CD40 RO and CD54 upregulation assays showed that whole blood could be left at room temperature for up to 24 hours and 6 hours, respectively, prior to analysis.
Study Participants
Eligible subjects aged 18‐60 years, with a body mass index of 18.5‐29.9 kg/m2, were enrolled. Female participants had to be postmenopausal, surgically sterilized, sexually abstinent, have a vasectomized sexual partner, or be practicing accepted methods of contraception for ≥30 days before study drug administration, and up to 30 days after study completion, and test negative for pregnancy before and during the study.
Subjects were excluded if they had any evidence of clinically significant abnormalities identified by medical examination or by laboratory testing; a concomitant disease; any gastrointestinal, hepatic, renal, respiratory, cardiovascular, metabolic, immunologic, or hormonal disorders; a disease of the central nervous system; orthostatic hypotension, fainting spells, or blackouts; or allergies or drug hypersensitivity reactions. Subjects were also excluded if they had taken any other medications with a long half‐life (t1/2; >24 hours) within 30 days or fewer than 10 half‐lives prior to randomization; had taken drugs that might have influenced the results of the study within 10 days prior to the first dosing day of the study; had received any investigational drug within 60 days prior to the first dosing day of the study; had donated blood within 30 days prior to the first dosing day of the study; had evidence of drug abuse or excessive alcohol or cigarette use; tested positive for human immunodeficiency virus, hepatitis B, hepatitis C, tuberculosis, or chronic or relevant acute infection; or had the intention of starting a new exercise regimen within 1 week prior to the first dosing day of the study. Women who were lactating or who were planning on becoming pregnant within 30 days after study completion were also excluded.
Analytical Methods
Plasma concentrations of BI 655064 were analyzed using a validated sandwich enzyme‐linked immunosorbent assay with a lower limit of quantitation of 30 ng/mL. The 96‐well microtiter plates were first coated with an anti‐BI 655064 antibody, blocked, and washed. The plates were then incubated with study samples, calibrators, or quality control samples, and washed again. Binding of BI 655064 was detected with a biotinylated anti‐BI 655064 antibody, followed by streptavidin conjugated with horseradish peroxidase, and finally with the peroxidase substrate tetramethylbenzidine. Plates were read colorimetrically, and the data were analyzed with a 5‐parameter logistic fit. The quantitative range was 30‐800 ng/mL. Adequate accuracy and precision were assessed during routine analysis with quality control samples at 3 concentrations: low (50 or 100 ng/mL), middle (126 or 200 ng/mL), and high (500 or 590 ng/mL). The reproducibility of the enzyme‐linked immunosorbent assay was tested by incurred sample reanalysis, in which 93% of samples passed the acceptance criteria (≤30% difference from mean).
Anti‐BI 655064 antibodies were analyzed in plasma samples using a validated bridging electrochemiluminescence method. All reported sample data met the assay‐specific acceptance criteria. Validation of the ADA assay demonstrated that 250 ng/mL of the positive control anti‐BI 655064 ADA could be detected in the presence of plasma concentrations of 50 μg/mL BI 655064. A true positive response in a subject was further characterized by additional titer assays. Titers were determined by analysis of serial 2‐fold dilutions of the sample. Reported titers were the highest fold dilution that produced a mean electrochemiluminescence value that was greater than or equal to the plate‐specific cut point.
Both the determination of BI 655064 concentrations and the ADA assessments were performed by Covance Laboratories, Inc. (Chantilly, Virginia).
For the measurement of CD40 RO, whole blood samples were incubated with an excess of fluorescein‐isothiocyanate–labeled BI 655064 and anti‐CD19‐allophycocyanin (APC; for gating on B cells) for 20 minutes at room temperature in the dark. Fluorescence‐activated cell sorting (FACS) lysing solution was added and tubes were incubated for 15 minutes at room temperature in the dark, followed by centrifugation (1300 rpm) at 4°C for 6 minutes, and removal of the supernatant. CellFix (Becton, Dickinson and Company, Eysins, Switzerland) was added, and tubes were vortexed and stored at 4°C in the dark until FACS analysis, which was performed within 24 hours of adding the CellFix. All samples were kept on ice during measurements.
For the measurement of the inhibition of CD54 upregulation, whole blood was either incubated with interleukin‐4 (IL‐4) alone (“nonstimulated FACS tubes”) or with MegaCD40L + IL‐4 (“stimulated FACS tubes”). The tubes were vortexed and incubated in the dark at 37°C in a humidified incubator for 23‐26 hours. Anti‐CD19‐APC and anti‐CD54‐phycoerythrin were added to each tube, and tubes were vortexed and incubated for 20 minutes in the dark at room temperature. FACS lysing solution was added and tubes were incubated for 15 minutes at room temperature in the dark, followed by centrifugation (1300 rpm) at 4°C for 6 minutes and removal of the supernatant. CellFix was added, and tubes were vortexed and stored at 4°C in the dark until FACS analysis, which was performed within 2 hours of adding the CellFix. All samples were kept on ice during measurements.
Both the CD40 RO and CD54 upregulation assays were quasi‐quantitative. Assay results were based on a percentage change (ie, on‐treatment samples were related to a predose sample).
To examine the potential for thromboembolic events, the following assessments were performed: prothrombin time‐international normalized ratio, activated partial thromboplastin time, antithrombin III, fibrinogen, protein S and C, platelet count, bleeding time (measured with the Duke method), and D‐dimers.
Pharmacokinetic Evaluation
Plasma BI 655064 concentration‐time data were analyzed by a noncompartmental approach using WinNonlinTM (version 5.02, Gary, North Carolina). Parameters determined included Cmax, time to achieve Cmax (tmax), terminal elimination constant (λz), and terminal t½ using the standard WinNonlinTM procedure. Area under the drug plasma concentration‐time curve over the uniform dosing interval τ (AUC0‐τ) after the last (fourth) dose were calculated using the linear‐up log‐down algorithm of WinNonlinTM. The accumulation ratios (RA,Cmax based on Cmax; RA,AUC based on AUC0‐τ) were calculated as the ratio of the value after the fourth dose to the value after the first dose.
Pharmacodynamic Evaluation
Pharmacodynamic assessment included the evaluation of CD40 RO by BI 655064 and inhibition of B‐cell activation, as measured by the megaCD40L‐induced upregulation of CD54 in whole blood using the aforementioned validated FACS assays. The relationships between the dose of BI 655064 and inhibition of CD40 RO and CD54 upregulation were previously explored using standard sigmoidal Emax models and reported by Albach et al.22
Safety and Tolerability
The safety and general tolerability of BI 655064 were assessed by monitoring treatment‐emergent adverse events (AEs), physical examinations, vital signs (blood pressure and pulse), 12‐lead electrocardiogram (ECG), and clinical laboratory tests (hematology, clinical chemistry, and urinalysis).
Statistical Analysis
No formal sample size determination was performed: 8 subjects per dose group were considered sufficient for the PK and safety analyses. Study results were analyzed using descriptive statistics for safety, PK, and PD. The safety population included all subjects who had received the study drug (BI 655064 or placebo). The PK and PD populations included all subjects who received study drug and who provided evaluable data for PK and PD analysis, respectively. Dose proportionality of AUC0‐τ and Cmax after the fourth dose was assessed using a power model. A 95%CI for the slope was computed. Perfect dose proportionality was defined by a slope parameter (β) of 1. A descriptive analysis, including graphical presentations of the concentration data, was performed to assess whether steady‐state was achieved.
Results
Subjects
In total, 40 healthy subjects were randomized and treated in the study. Subjects received repeated once‐weekly SC treatment with placebo (n = 8), 80‐mg BI 655064 (n = 8), 120‐mg BI 655064 (n = 8), 180‐mg BI 655064 (n = 8), or 240‐mg BI 655064 (n = 8) over a 4‐week period. All 40 subjects completed the planned observation period and there were no premature discontinuations. The majority of subjects were male (83%) and white (73%), with a mean (standard deviation [SD]) age of 30 (10.8) years, and a mean (SD) body mass index of 25 (3.1) kg/m2 (Table 1). There were no relevant demographic differences between the treatment groups.
Table 1.
Demographics and Baseline Characteristics
| BI 655064 | ||||||
|---|---|---|---|---|---|---|
| 80 mg (n = 8) | 120 mg (n = 8) | 180 mg (n = 8) | 240 mg (n = 8) | Placebo (n = 8) | Total (N = 40) | |
| Sex, n (%) | ||||||
| Male | 6 (75) | 8 (100) | 6 (75) | 7 (87.5) | 6 (75) | 33 (82.5) |
| Female | 2 (25) | 0 | 2 (25) | 1 (12.5) | 2 (25) | 7 (17.5) |
| Race, n (%) | ||||||
| White | 6 (75) | 7 (87.5) | 5 (62.5) | 6 (75) | 5 (62.5) | 29 (72.5) |
| Hawaiian/Pacific Islander | 0 | 0 | 3 (37.5) | 2 (25) | 2 (25) | 7 (17.5) |
| Asian | 2 (25) | 1 (12.5) | 0 | 0 | 1 (12.5) | 4 (10) |
| Mean age, years (SD) | 25.9 (4.1) | 31.4 (13.3) | 37.3 (10.8) | 29.5 (10.6) | 27.8 (12.1) | 30.4 (10.8) |
| Mean BMI, kg/m2 (SD) | 24.5 (2.0) | 24.4 (4.3) | 26.7 (2.4) | 24.2 (3.0) | 25.4 (3.1) | 25.0 (3.1) |
| Smoking history, n (%) | ||||||
| Never smoked | 7 (87.5) | 7 (87.5) | 6 (75) | 5 (62.5) | 7 (87.5) | 32 (80) |
| Ex‐smoker | 0 | 0 | 2 (25) | 0 | 0 | 2 (5) |
| Currently smokes | 1 (12.5) | 1 (12.5) | 0 | 3 (37.5) | 1 (12.5) | 6 (15) |
| Alcohol history, n (%) | ||||||
| Non‐drinker | 1 (12.5) | 2 (25) | 0 | 1 (12.5) | 1 (12.5) | 5 (12.5) |
| Drinkera | 7 (87.5) | 6 (75) | 8 (100) | 7 (87.5) | 7 (87.5) | 35 (87.5) |
BMI, body mass index; SD, standard deviation.
At a level that did not interfere with study participation, based on investigator assessment.
Pharmacokinetics
Geometric mean (gMean) selected PK parameters for BI 655064 following first SC dose (day 1) and last SC dose (fourth) administration are presented in Table 2. After the first dose, median tmax increased with each weekly dose, but tmax, did not show any clear dose relationship after the fourth dose (tmax, 4). The maximum plasma concentration and AUC normalized for administered dose (Cmax,norm, 4 and AUCτ,norm, 4, respectively) were lower for the 80‐mg BI 655064 dose group and similar for the 3 higher dose groups, suggesting a greater than proportional increase in exposure from 80 mg to 120 mg, but near dose‐proportional kinetics for doses >120 mg. Geometric mean accumulation ratios based on Cmax or AUC (RA,Cmax, 4 and RA,AUC, 4, respectively) were determined to assess accumulation of BI 655064 following 4 multiple doses. After 4 once‐weekly SC doses of 80 mg, RA,Cmax, 4 and RA,AUC, 4 values were 8.3‐ and 11.6‐fold higher, respectively, than after a single dose, indicating accumulation of BI 655064. The gMean accumulation was lower for the 3 higher doses (range: 3.7‐4 for RA,Cmax, 4 and 4.9‐5.8 for RA,AUC, 4). The terminal t1/2 of BI 655064 ranged from 156 to 199 hours (6‐8 days). Visual inspection of trough concentrations suggested that steady‐state was not achieved for any of the doses: trough plasma concentrations for all dose groups continued to increase with each subsequent dose (Figure 2).
Table 2.
Selected PK Parameters of BI 655064 Following First Dose (Day 1) and Last Dose (After 4 Once‐Weekly Subcutaneous Administrations)
| BI 655064 | ||||
|---|---|---|---|---|
| 80 mg (n = 8) | 120 mg (n = 8) | 180 mg (n = 8) | 240 mg (n = 8) | |
| After the first dose | ||||
| Cmax, μg/mL | 1.6 (492) | 7.7 (29.5) | 9.9 (67.8) | 18.0 (46.3) |
| Cmax,norm, μg/mL/mg | 0.02 (492) | 0.06 (29.5) | 0.05 (67.8) | 0.08 (46.3) |
| tmax, h | 66 (12‐168) | 78 (48‐108) | 108 (72‐144) | 156 (108‐168) |
| AUC0‐τ,a μg·h/mL | 154 (683) | 850 (34.4) | 919 (78.9) | 1630 (42.6) |
| AUC0‐τ, norm, μg·h/mL/mg | 1.9 (683) | 7.1 (34.4) | 5.1 (78.9) | 6.8 (42.6) |
| After the fourth dosea | ||||
| Cmax, 4, μg/mL | 13.1 (59.1) | 28.7 (35.6) | 39.8 (37.4) | 68.4 (21.9) |
| Cmax,norm, 4, μg/mL/mg | 0.16 (59.1) | 0.24 (35.6) | 0.22 (37.4) | 0.29 (21.9) |
| tmax, 4, h | 96.0 (96‐144) | 84.1 (12‐144) | 96.0 (72‐192) | 108 (96‐504) |
| AUC0‐τ, 4,b μg·h/mL | 1790 (56.4) | 4140 (35.4) | 5470 (37.4) | 9460 (22.4) |
| AUC0‐τ, norm, 4, μg·h/mL/mg | 22.3 (56.4) | 34.5 (35.4) | 30.4 (37.4) | 39.4 (22.4) |
| t½, 4, h | 186 (39.4) | 156 (28.3) | 171 (39.6) | 199 (28.4) |
| RA,Cmax, 4 | 8.3 (226) | 3.7 (31.9) | 4.0 (46.9) | 3.8 (23.4) |
| RA,AUC, 4 c | 11.6 (289) | 4.9 (27.7) | 6.0 (45.3) | 5.8 (25.4) |
Data are presented as geometric mean (geometric coefficient of variation, %), except data for tmax, which are presented as median (range). AUC, area under the drug plasma concentration‐time curve; Cmax, maximum observed concentration; PK, pharmacokinetic; RA, accumulation ratio; t½, half‐life; tmax, time to achieve Cmax.
PK parameters analyzed after the fourth dose of BI 655064 are indicated with a subscript 4 (eg, tmax, 4).
AUC0‐τ is synonymous with AUC0‐168h.
RA,AUC is equal to AUC0‐τ after the fourth dose divided by AUC0‐τ after the first dose.
Figure 2.
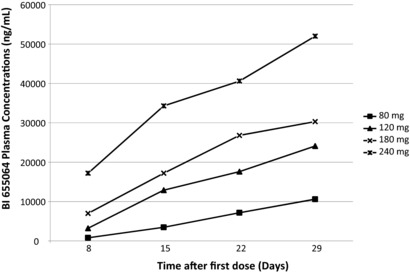
Predose concentrations of BI 655064 on days 8, 15, and 22 (Cpre) and trough concentration on day 29.
Analysis of dose proportionality over the SC dose range of 80‐240 mg showed that the slope for Cmax and AUC0‐τ were significantly different from unity, indicating that BI 655064 exposure was not proportional to dose (Cmax: power model slope β = 2.1 [95%CI, 1.2‐2.9] after first dose; slope β = 1.4 [95%CI, 1.1‐1.8] after last dose; n = 32; AUC0‐τ: slope β = 1.4 [95%CI, 1.1‐1.8] after last dose; n = 32). However, for the higher doses (120‐240 mg) a trend toward dose‐proportionality was observed.
Pharmacodynamics
Administration of BI 655064 resulted in dose‐dependent CD40 RO and inhibition of CD54 upregulation (Figure 3).
Figure 3.

Arithmetic mean percentage of CD40 receptor occupancy (A), and inhibition of CD54 upregulation (B). The time scales for individual doses have been staggered to provide clarity of overlapping SDs. Black arrows indicate dosing on days 0, 7, 14, and 21. SD, standard deviation.
After a single SC dose of BI 655064, arithmetic mean CD40 RO had already reached near‐maximal values for each dose level at the first postdose measurement (72 hours) (Figure 3A). At that time point, the 80‐mg dose resulted in approximately 89% CD40 RO. For the 120‐ to 240‐mg dose groups, the CD40 RO plateaued at 94%‐95%; because this was at the limit of detection for the assay, it was not possible to ascertain whether higher occupancy levels were achievable. These levels of CD40 RO were maintained for the remainder of the study.
After the last (fourth) once‐weekly SC administration of BI 655064, CD40 RO was >90% at all measured time points until day 39 (17 days after the last administration) for all doses (80‐240 mg). For the 180‐mg dose group, 79% (geometric coefficient of variation [gCV] 23%) CD40 RO was still detectable at day 64, and for the 240‐mg dose group, 68% (gCV 29%) CD40 RO was detectable at day 78, indicating long‐term persistent binding to the receptor, although variability was higher at these later time points. No noteworthy CD40 RO was observed in the placebo group.
Inhibition of CD54 upregulation after administration of a single dose of BI 655064 followed a similar pattern to that observed for CD40 RO, with the 80‐mg dose giving 87% inhibition at the first postdose measurement (72 hours), and a >90% inhibition observed for the higher doses at this time point (all dose groups; Figure 3B). For the 80‐mg dose group, inhibition increased further to 95% at day 7 after dosing, whereas for all other doses, 95% inhibition was observed from 72 hours onward; in the placebo group, inhibition of CD54 upregulation varied between –20% and 30%. After the last (fourth) once‐weekly SC administration of BI 655064, inhibition of CD54 upregulation was >90% for all doses, up to day 39 (17 days after the last administration). An inhibitory effect was still detectable for the 180‐mg group (89% at day 64) and for the 240‐mg group (51% at day 78).
Safety
The overall frequency and intensity of AEs were similar in the BI 655064 treatment groups (all BI 655064 doses [78%] and the placebo group [88%]; Table 3). No serious AEs, severe AEs, or AEs leading to discontinuation or death were reported. Although the subject numbers were small, there did not appear to be any relationship between BI 655064 dose, treatment‐related AEs, or the frequency and intensity of AEs. Infections were reported in 8 subjects (25%) receiving BI 655064 and in 5 subjects (63%) receiving placebo. There were no thromboembolic events. The most frequently reported treatment‐related AE was headache, in 4 subjects (13%) receiving BI 655064 and 2 subjects (25%) receiving placebo. All AEs were mild or moderate in intensity and all were resolved.
Table 3.
Summary of Adverse Events and Frequency of Treatment‐Related Adverse Events
| BI 655064 | ||||||
|---|---|---|---|---|---|---|
| Number of Subjects (%) | 80 mg (n = 8) | 120 mg (n = 8) | 180 mg (n = 8) | 240 mg (n = 8) | Placebo (n = 8) | Total (N = 32) |
| Any AEs | 7 (88) | 6 (75) | 6 (75) | 6 (75) | 7 (88) | 25 (78) |
| Any serious AEs | 0 | 0 | 0 | 0 | 0 | 0 |
| Any severe AEs | 0 | 0 | 0 | 0 | 0 | 0 |
| AEs leading to discontinuation | 0 | 0 | 0 | 0 | 0 | 0 |
| Any TRAEa | 2 (25) | 1 (13) | 4 (50) | 0 | 4 (50) | 7 (22) |
| Nervous system disorder | 2 (25) | 0 | 3 (38) | 0 | 2 (25) | 5 (16) |
| Headache | 1 (13) | 0 | 3 (38) | 0 | 2 (25) | 4 (13) |
| Lethargy | 1 (13) | 0 | 0 | 0 | 0 | 1 (3) |
| General disorders/administration site | ||||||
| conditions | 0 | 1 (13) | 1 (13) | 0 | 1 (13) | 2 (6) |
| Fatigue | 0 | 0 | 1 (13) | 0 | 1 (13) | 1 (3) |
| Injection site pain | 0 | 0 | 0 | 0 | 1 (13) | 0 |
| Injection site rash | 0 | 1 (13) | 0 | 0 | 0 | 1 (3) |
| Skin and subcutaneous tissue disorders | 0 | 1 (13) | 0 | 0 | 1 (13) | 1 (3) |
| Macular rash | 0 | 0 | 0 | 0 | 1 (13) | 0 |
| Pruritic rash | 0 | 0 | 0 | 0 | 1 (13) | 0 |
| Erythematous rash | 0 | 1 (13) | 0 | 0 | 0 | 1 (3) |
| Gastrointestinal disorders | 0 | 0 | 1 (13) | 0 | 0 | 1 (3) |
| Nausea | 0 | 0 | 1 (13) | 0 | 0 | 1 (3) |
AE, adverse event; TRAE, treatment‐related adverse event.
Defined by the investigator.
For individual subjects in the 80‐mg and 120‐mg treatment groups, substantial elevations in creatine kinase (CK) were observed both at baseline and following treatment with either BI 655064 or placebo (range, 1.1‐88 times upper limit of normal [ULN]). Overall, these elevations in CK were determined to be generally attributable to extensive exercise. Implementation of stricter exercise restrictions for the higher dose groups (180‐240 mg) resulted in a reduction in CK levels (maximum 3‐fold ULN).
Of all subjects treated with BI 655064, mild and transient leukopenia and neutropenia were observed in 12 (37.5%) and 14 (43.8%) subjects, respectively. Only 1 subject receiving placebo had mild and transient neutropenia. Values below the lower limit of normal were observed in 4 of the 12 subjects (33.3%) with mild transient leukopenia and in 5 of the 14 subjects (35.7%) with mild transient neutropenia prior to receiving treatment. In all subjects, white blood cell (WBC) and absolute neutrophil counts returned to within normal values (WBC normal range: 4‐11 × 109/L and absolute neutrophil normal range: 1.9‐7.5 × 109/L) or reached a pretreatment level by the end of the study, except for 1 subject who had a WBC count of 3.77 × 109/L at the end‐of‐study visit and 1 subject with a low WBC count and absolute neutrophil count of 3.58 × 109/L and 1.69 × 109/L, respectively, at the end‐of‐study visit. Furthermore, there was no increase in the number of subjects with leukopenia or neutropenia with increasing BI 655064 dose.
There were no clinically significant changes in bleeding time, platelet counts, or coagulation parameters, including D‐dimers, antithrombin III, fibrinogen, and protein S and C.
There were no clinically relevant findings or treatment differences between groups in vital signs, ECGs, or physical examinations. Assessments of local tolerability indicated that all doses of BI 655064 were well tolerated, and there was no difference compared with the placebo group.
Pre‐existing ADA responses were observed in 4 subjects (10%), 3 of whom subsequently received BI 655064 and 1 received placebo (Table 4). ADA titer was increased in only 1 of these subjects, dosed with 240‐mg BI 655064 (treatment‐boosted ADA [pre‐existing ADA that was boosted to a higher level following biologic administration]).23
Table 4.
Summary of ADA Responses
| Treatment, Dose | Total Number of Subjects With a Positive ADA Response | Time, h | Number of Subjects With a Positive ADA at Time Point | Titer, 1:x |
|---|---|---|---|---|
| Placebo (n = 8) | 1 | –0.5 | 1 | 4 |
| 503.5 | 1 | 4 | ||
| 912 | 1 | 4 | ||
| 1512 | 1 | 4 | ||
| BI 655064 80 mg (n = 8) | 6 | –0.5 | 1a | 80 |
| 503.5 | 1 | 20 | ||
| 912 | 1 | 10 | ||
| 1512 | 6 | 8‐400b | ||
| BI 655064 120 mg (n = 8) | 6 | 503.5 | 1c | 4 |
| 1512 | 6 | 2‐640b | ||
| BI 655064 180 mg (n = 8) | 3 | –0.5 | 1d | 8 |
| 1512c | 2 | 2‐4b | ||
| BI 655064 240 mg (n = 8) | 4 | –0.5 | 1e | 2 |
| 1848 | 4 | 4‐40b |
End‐of‐study samples were 1512 hours (63 days) after the first dose for the 80‐ to 180‐mg dose groups, and 1848 hours (77 days) for the 240‐mg dose group. ADA, anti‐drug antibody.
This subject had a positive ADA response at –0.5 hours and also at 503.5, 912, and 1512 hours after the first dose.
Data are presented as range (min‐max).
This subject had a positive ADA response at 503.5 hours and 1512 hours after the first dose.
In this subject, a positive ADA response was detected only at –0.5 hours.
This subject had a positive ADA response at –0.5 hours and at 1848 hours after the first dose.
Seroconversion was observed in 16 subjects (50%) following BI 655064 treatment; onset was mainly in the end‐of‐study samples (15 subjects [47%]). At that time, levels of BI 655064 were already very low (gMean BI 655064 plasma concentrations ranged from 0.182‐10.5 μg/mL for the 80‐ to 240‐mg dose groups).
Treatment‐induced ADA (ADA developing de novo following biologic administration) or treatment‐boosted ADA responses were observed in 5 subjects (62.5%) in the 80‐mg dose group and 6 subjects (75%) in the 120‐mg dose group. Overall median titers in the 80‐mg and 120‐mg dose groups were 20 and 8, respectively. Treatment‐induced or treatment‐boosted ADA responses were observed in fewer subjects in the higher dose groups: 2 (25%) and 4 (50%) subjects in the 180‐mg and 240‐mg dose groups, respectively, with overall median titers of 4 and 8, respectively. The maximum titer in an individual subject was 640, observed in the 120‐mg dose group.
Discussion
The objectives of this study were to investigate the effects of 4 weeks of rising SC doses of BI 655064 (80‐240 mg per week) in healthy subjects. Assessment of PK parameters indicated near proportional kinetics from SC doses of 120‐ to 240‐mg BI 655064, but supraproportional kinetics from SC doses of 80‐120 mg due to target‐mediated drug clearance, as was observed in a previous study, following single IV doses of BI 655064.22 The effect is enhanced by the wide distribution of CD40 receptors (particularly platelets, with their short t1/2), and has previously been reported in other studies with antagonistic anti‐CD40 antibodies.24, 25 A near proportional dose‐exposure relationship was observed over the SC dose range of 120‐ to 240‐mg BI 655064 for Cmax (after the first and the last doses) and for AUC0‐τ (after the last dose), with slope β = 1.2 for both parameters, possibly indicating that CD40 RO is nearing saturation at these doses. Plasma exposures achieved in this study after the first SC doses of 80‐mg and 120‐mg BI 655064 were similar to those observed in a previous single rising dose study in healthy volunteers, where gMean AUC values of 120 μg·h/mL and 888 μg·h/mL were obtained following a single SC dose of 80‐mg and 120‐mg BI 655064, respectively.22 Accumulation of BI 655064 was observed at all dose levels following multiple dosing compared with single dosing (first dose) administration. However, lower accumulation was observed for the 120‐ to 240‐mg doses, with relatively constant gMean RA,Cmax values of between 3.7 and 4, and gMean RA, AUC values between 4.9 and 6. Steady‐state was not achieved within the 4‐week period for any of the doses administered. Modeling showed that it may take up to 12 weeks to reach steady‐state when dosing 120‐mg BI 655064 once weekly (manuscript in preparation). The prediction to reach steady‐state within about 12 weeks has been confirmed by the treatment of patients with RA with 120‐mg BI 655064 SC once weekly for 12 weeks, showing that PK steady‐state is reached within about 10‐12 weeks.26 This therefore supports the use of a loading dose to achieve steady‐state more rapidly in future clinical studies. For the exposure parameters AUC and Cmax, interindividual variability was higher for the 80‐mg dose (gCVs: 56.4%‐59.1%), moderate for the 120‐mg and 180‐mg doses (gCVs: 35.4%‐37.4%), and lower for the 240‐mg dose (gCVs: 21.9%‐22.4%). BI 655064 was absorbed slowly from the site of SC injection, with a median tmax increasing with dose after the first dose; after the fourth dose there was no dose relationship with tmax, 4. Following multiple BI 655064 dosing, the estimated terminal t1/2 ranged between 6 and 8 days, with no apparent difference among doses.
Assessment of CD40 RO and inhibition of CD54 upregulation indicated that single SC doses between 120‐ and 240‐mg BI 655064 resulted in >90% CD40 RO and >90% inhibition of CD54 upregulation from 72 hours after dosing. Following the last (fourth) dose of BI 655064, >90% CD40 RO and inhibition of CD54 upregulation was maintained for at least 408 hours (17 days) after dosing over the SC dose range of 80‐240 mg. These results suggest the possibility of continuous complete inhibition of agonistic CD40 ligation with biweekly SC administrations of BI 655064. Modeling showed that dosing of BI 655064 120 mg SC once weekly for 3 weeks, followed by dosing once every second week, would result in a continuous >90% CD40 RO. It is expected that in patients with inflammatory diseases such as RA, SLE, or lupus nephritis, the CD40 receptor will be highly expressed and upregulated on a variety of immune cells and resident cells (eg, mesangial cells in lupus nephritis); thus, higher doses of BI 655064 than those which resulted in 90% receptor occupancy on B cells in healthy subjects might be needed to fully block the CD40 receptor in patients with autoimmune diseases. Clinical studies of BI 655064 in these patients will need to evaluate whether longer dosing intervals can achieve clinical efficacy or whether weekly dosing is needed.
Ascending multiple SC doses of BI 655064 were considered to be safe and showed good overall tolerability in healthy subjects. All AEs were mild or moderate in intensity and no AEs leading to discontinuation from the study were reported. Serum CK values elevated above the ULN were reported in some subjects at baseline and after treatment in both the BI 655064 and placebo groups, but these were attributable to excessive exercise, similarly to what has been reported previously in the literature.27, 28 Implementation of stricter exercise restrictions for the 180‐mg and 240‐mg dose groups resulted in reduced CK concentrations. Several subjects showed mild and transient leukopenia and neutropenia following treatment with BI 655064. However, values below the lower limit of normal were already observed prior to treatment in 33.3% of subjects with leukopenia and 35.7% with neutropenia. Transient neutropenia is very common in healthy subjects, and in some cases is associated with concurrent viral infections. Neutropenia has previously been reported in subjects performing high‐intensity sports, and the majority of subjects enrolled in this study performed intense physical activities, as supported by the observed substantial elevations of serum CK values.29 In addition, it has more recently been shown that exercise‐induced muscle damage initiates a rapid local inflammatory response, and that local accumulation of leukocytes is associated with muscle weakness.30 The elevated CK levels observed in this study indicate that these subjects had some muscular damage; therefore, a redistribution of leukocytes from the circulation toward the muscles may have contributed to the observed transient leukopenia and neutropenia. Taken together, there is no clear relationship between the observed neutropenia and treatment with BI 655064. However, changes in WBCs and neutrophils will be carefully monitored in subsequent clinical studies with BI 655064.
No clinically relevant vital signs, ECG assessment, or physical examination findings were reported. In line with observations after administration of single IV and SC doses of BI 655064,22 no thromboembolic events were reported during multiple dosing, and there were no clinically relevant changes in platelet or coagulation parameters. Previously, investigation into platelet aggregometry and binding studies with human platelets showed that blocking CD40 had no obvious impact on platelet function (unpublished data); in toxicology studies, it was demonstrated that BI 655064 bound to platelets in cynomolgus monkeys had no impact on platelet number or function.21 Taken together, these findings indicate that when bound to platelets, BI 655064 does not appear to alter platelet activation, aggregation, or function.21 These data, as well as data from other anti‐CD40 or anti‐CD40L antibodies lacking a functional Fc region,17, 19, 20 support the interpretation that the risk of thromboembolic events, as observed with earlier anti CD40L antibodies,16 can be avoided by eliminating the function of the Fc region of the antibodies.
Treatment‐induced or treatment‐boosted ADA responses in 50% of subjects receiving BI 655064 did not cause any clinical symptoms (no relationship with AEs or change in exposure) or lead to observable changes in PK after multiple doses. Leukocyte and neutrophil counts were unaffected and were within the normal range or had reached pretreatment levels except for 2 subjects (these 2 subjects were negative for ADAs). There was a higher occurrence of ADAs in the 80‐mg and 120‐mg dose groups than in the higher dose groups (180 mg and 240 mg). BI 655064 plasma concentrations were near the lower limit of assay quantification at the time of onset for ADA response (the end‐of‐study visit); BI 655064 had largely been eliminated, and circulating BI 655064 levels were below the drug tolerance of the ADA assay. Furthermore, the small number of subjects in this study did not allow for a definitive evaluation of BI 655064 dose or ADA occurrence or titer. Based on the mechanism of BI 655064 inhibiting CD40 receptors, and thus blocking the production of antibodies, the occurrence of ADAs after BI 655064 administration and antibody isotope switching would not be expected. At the 1512‐hour (63‐day) time point (80‐ to 180‐mg dose groups) or the 1848‐hour (77‐day) time point (240‐mg dose group), CD40 RO had already declined below 90%. Furthermore, BI 655064 levels are expected to be even lower in germinal centers; thus, the concentrations of BI 655064 might have been too low to block the formation of ADAs. This hypothesis is also supported by preclinical assessments with BI 655064 in cynomolgus monkeys, where all doses showed >90% CD40 RO for peripheral B cells, but the lowest group (1 mg/kg) did not display a full pharmacologic effect on germinal centers and developed ADAs (unpublished data).21
Conclusions
Following ascending multiple once‐weekly SC BI 655064 dosing over a 4‐week period in healthy subjects, PK increased supraproportionally due to target‐mediated clearance for doses between 80 mg and 120 mg, but was near proportional for doses >120 mg. Dose‐dependent accumulation of BI 655064 supports the use of a loading dose to achieve steady‐state sooner in future clinical studies. BI 655064 showed a high potential to block the CD40‐CD40L pathway, with persistent inhibition of CD40L‐induced CD54 upregulation. Thus, further studies will need to evaluate whether a longer dosing interval could be clinically efficient in patients with an autoimmune disease, such as RA, SLE, or lupus nephritis. Ascending multiple SC doses of BI 655064 over the range of 80‐240 mg were generally well tolerated, and no relevant signs of acute immune reaction were observed.
Acknowledgments
The authors wish to acknowledge Dr Anna Brooks, Dr Vaughan Feisst, and other staff at the School of Biological Sciences, University of Auckland, New Zealand, for assistance with the CD40 RO and CD54 upregulation analyses, and Dr Christine Grimaldi, DMPK, Boehringer‐Ingelheim Ridgefield CT, USA, for technical assistance with the ADA analysis.
Data Sharing
The authors confirm that the data supporting the findings of this study are available within the article. Further information or data in relation to this study are available on request from the corresponding author.
Disclosures
The study was sponsored by Boehringer Ingelheim. A.G.E., B.R., C. Schoelch, D.J., S.J.P., and J.S. are employees of Boehringer Ingelheim. C. Schwabe and P.H. are employees of Auckland Clinical Studies and contracted by Boehringer Ingelheim. P.R.D. is an employee of the University of Auckland and contracted by Boehringer Ingelheim. At the time of the study, T.D. and J.H. were employees of Boehringer Ingelheim.
Editorial assistance in the preparation of this manuscript was provided by Michèle Underhill and Leigh Church at SuccinctChoice Medical Communications (London, UK) and funded by Boehringer Ingelheim.
References
- 1. Kalden JR, Schulze‐Koops H. Immunogenicity and loss of response to TNF inhibitors: implications for rheumatoid arthritis treatment. Nat Rev Rheumatol. 2017;13(12):707–718. [DOI] [PubMed] [Google Scholar]
- 2. de Zubiria Salgado A, Herrera‐Diaz C. Lupus nephritis: an overview of recent findings. Autoimmune Dis. 2012;2012:849684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Peters AL, Stunz LL, Bishop GA. CD40 and autoimmunity: the dark side of a great activator. Semin Immunol. 2009;21(5):293–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Schonbeck U, Libby P. The CD40/CD154 receptor/ligand dyad. Cell Mol Life Sci. 2001;58(1):4–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Elgueta R, Benson MJ, de Vries VC, Wasiuk A, Guo Y, Noelle RJ. Molecular mechanism and function of CD40/CD40L engagement in the immune system. Immunol Rev. 2009;229(1):152–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Xie JH, Yamniuk AP, Borowski V, et al. Engineering of a novel anti‐CD40L domain antibody for treatment of autoimmune diseases. J Immunol. 2014;192(9):4083–4092. [DOI] [PubMed] [Google Scholar]
- 7. van Kooten C, Banchereau J. CD40‐CD40 ligand. J Leukoc Biol. 2000;67(1):2–17. [DOI] [PubMed] [Google Scholar]
- 8. Berner B, Wolf G, Hummel KM, Muller GA, Reuss‐Borst MA. Increased expression of CD40 ligand (CD154) on CD4+ T cells as a marker of disease activity in rheumatoid arthritis. Ann Rheum Dis. 2000;59(3):190–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Aarvak T, Natvig JB. Cell‐cell interactions in synovitis: antigen presenting cells and T cell interaction in rheumatoid arthritis. Arthritis Res. 2001;3(1):13–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Goules A, Tzioufas AG, Manousakis MN, Kirou KA, Crow MK, Routsias JG. Elevated levels of soluble CD40 ligand (sCD40L) in serum of patients with systemic autoimmune diseases. J Autoimmun. 2006;26(3):165–171. [DOI] [PubMed] [Google Scholar]
- 11. Howland KC, Ausubel LJ, London CA, Abbas AK. The roles of CD28 and CD40 ligand in T cell activation and tolerance. J Immunol. 2000;164(9):4465–4470. [DOI] [PubMed] [Google Scholar]
- 12. Yellin MJ, Winikoff S, Fortune SM, et al. Ligation of CD40 on fibroblasts induces CD54 (ICAM‐1) and CD106 (VCAM‐1) up‐regulation and IL‐6 production and proliferation. J Leukoc Biol. 1995;58(2):209–216. [DOI] [PubMed] [Google Scholar]
- 13. Guo Y, Walsh AM, Fearon U, et al. CD40L‐dependent pathway is active at various stages of rheumatoid arthritis disease progression. J Immunol. 2017;198(11):4490–4501. [DOI] [PubMed] [Google Scholar]
- 14. Desai‐Mehta A, Lu L, Ramsey‐Goldman R, Datta SK. Hyperexpression of CD40 ligand by B and T cells in human lupus and its role in pathogenic autoantibody production. J Clin Invest. 1996;97(9):2063–2073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Yellin MJ, D'Agati V, Parkinson G, et al. Immunohistologic analysis of renal CD40 and CD40L expression in lupus nephritis and other glomerulonephritides. Arthritis Rheum. 1997;40(1):124–134. [DOI] [PubMed] [Google Scholar]
- 16. Boumpas DT, Furie R, Manzi S, et al. A short course of BG9588 (anti‐CD40 ligand antibody) improves serologic activity and decreases hematuria in patients with proliferative lupus glomerulonephritis. Arthritis Rheum. 2003;48(3):719‐727. [DOI] [PubMed] [Google Scholar]
- 17. Shock A, Burkly L, Wakefield I, et al. CDP7657, an anti‐CD40L antibody lacking an Fc domain, inhibits CD40L‐dependent immune responses without thrombotic complications: an in vivo study. Arthritis Res Ther. 2015;17:234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sidiropoulos PI, Boumpas DT. Lessons learned from anti‐CD40L treatment in systemic lupus erythematosus patients. Lupus. 2004;13(5):391–397. [DOI] [PubMed] [Google Scholar]
- 19. Tocoian A, Buchan P, Kirby H, et al. First‐in‐human trial of the safety, pharmacokinetics and immunogenicity of a PEGylated anti‐CD40L antibody fragment (CDP7657) in healthy individuals and patients with systemic lupus erythematosus. Lupus. 2015;24(10):1045–1056. [DOI] [PubMed] [Google Scholar]
- 20. Chamberlain C, Colman PJ, Ranger AM, et al. Repeated administration of dapirolizumab pegol in a randomised phase I study is well tolerated and accompanied by improvements in several composite measures of systemic lupus erythematosus disease activity and changes in whole blood transcriptomic profiles. Ann Rheum Dis. 2017;76(11):1837–1844. [DOI] [PubMed] [Google Scholar]
- 21. Ralph K, Nicoletti A, Musvasva E, et al. THU0407 preclinical characterization of a highly selective and potent antagonistic anti‐CD40 mAb. Ann Rheum Dis. 2015;74(suppl 2):344. [Google Scholar]
- 22. Albach FN, Wagner F, Huser A, et al. Safety, pharmacokinetics and pharmacodynamics of single rising doses of BI 655064, an antagonistic anti‐CD40 antibody in healthy subjects: a potential novel treatment for autoimmune diseases. Eur J Clin Pharmacol. 2018;74(2):161–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Shankar G, Arkin S, Cocea L, et al. Assessment and reporting of the clinical immunogenicity of therapeutic proteins and peptides‐harmonized terminology and tactical recommendations. AAPS J. 2014;16(4):658–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bensinger W, Maziarz RT, Jagannath S, et al. A phase 1 study of lucatumumab, a fully human anti‐CD40 antagonist monoclonal antibody administered intravenously to patients with relapsed or refractory multiple myeloma. Br J Haematol. 2012;159(1):58–66. [DOI] [PubMed] [Google Scholar]
- 25. Goldwater R, Keirns J, Blahunka P, et al. A phase 1, randomized ascending single‐dose study of antagonist anti‐human CD40 ASKP1240 in healthy subjects. Am J Transplant. 2013;13(4):1040–1046. [DOI] [PubMed] [Google Scholar]
- 26. Daniluk SPR, Mueller‐Ladner U, Petrikova A, et al. Safety and efficacy of BI 655064, an antagonistic anti‐CD40 antibody in rheumatoid arthritis (RA) patients. Ann Rheum Dis. 2016;75(suppl 2):718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kindermann W. Creatine kinase levels after exercise. Dtsch Arztebl Int. 2016;113(19):344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Brancaccio P, Maffulli N, Limongelli FM. Creatine kinase monitoring in sport medicine. Br Med Bull. 2007;81‐82:209‐230. [DOI] [PubMed] [Google Scholar]
- 29. Davidson RJ, Robertson JD, Galea G, Maughan RJ. Hematological changes associated with marathon running. Int J Sports Med. 1987;8(1):19–25. [DOI] [PubMed] [Google Scholar]
- 30. Paulsen G, Crameri R, Benestad HB, et al. Time course of leukocyte accumulation in human muscle after eccentric exercise. Med Sci Sports Exerc. 2010;42(1):75–85. [DOI] [PubMed] [Google Scholar]


