Abstract
Objective
The present study was undertaken to determine the efficacy of coadministration of fingolimod with alteplase in acute ischemic stroke patients in a delayed time window.
Methods
This was a prospective, randomized, open‐label, blinded endpoint clinical trial, enrolling patients with internal carotid artery or middle cerebral artery proximal occlusion within 4.5 to 6 hours from symptom onset. Patients were randomly assigned to receive alteplase alone or alteplase with fingolimod. All patients underwent pretreatment and 24‐hour noncontrast computed tomography (CT)/perfusion CT/CT angiography. The coprimary endpoints were the decrease of National Institutes of Health Stroke Scale scores over 24 hours and the favorable shift of modified Rankin Scale score (mRS) distribution at day 90. Exploratory outcomes included vessel recanalization, anterograde reperfusion, and retrograde reperfusion of collateral flow.
Results
Each treatment group included 23 patients. Compared with alteplase alone, patients receiving fingolimod plus alteplase exhibited better early clinical improvement at 24 hours and a favorable shift of mRS distribution at day 90. In addition, patients who received fingolimod and alteplase exhibited a greater reduction in the perfusion lesion accompanied by suppressed infarct growth by 24 hours. Fingolimod in conjunction with alteplase significantly improved anterograde reperfusion of downstream territory and prevented the failure of retrograde reperfusion from collateral circulation.
Interpretation
Fingolimod may enhance the efficacy of alteplase administration in the 4.5‐ to 6‐hour time window in patients with a proximal cerebral arterial occlusion and salvageable penumbral tissue by promoting both anterograde reperfusion and retrograde collateral flow. These findings are instructive for the design of future trials of recanalization therapies in extended time windows. Ann Neurol 2018;84:725–736
Although pharmacologic and mechanical recanalization of an occluded cerebral artery is the standard‐of‐care treatment for acute ischemic stroke patients, >50% of such patients with successful recanalization still have an unfavorable outcome.1, 2, 3 The failure of perfusion within the microvascular bed downstream of an occlusion has been proposed as a leading cause of such "futile" recanalization.4, 5 In addition, such microvascular failure hampers collateral circulation and results in infarct growth.6, 7, 8
Cerebral ischemia‐induced cell death swiftly activates the immune system and initiates inflammation within the brain.9, 10, 11, 12 In an early phase, these immune responses appear to exacerbate neurovascular dysfunction by promoting thrombus formation and accumulation of blood components in the cerebral microvasculature.13, 14 These changes subsequently exacerbate the ischemic cascade catalyzing neural cell death in the penumbra, resulting in the extension of infarction, which potentially limits the efficacy of pharmacologic or mechanical reperfusion.15, 16, 17
Fingolimod is a disease‐modifying drug for relapsing multiple sclerosis. Fingolimod targets sphingosine‐1‐phosphate receptors and inhibits the egress of lymphocytes from spleen and lymph nodes, thus reducing the numbers of circulating lymphocytes and inhibiting their subsequent homing to the brain. Several independent studies reported that fingolimod also attenuated microvascular thrombus formation and increased postischemic reperfusion in stroke models.15, 18 We previously reported that fingolimod limited the expansion of infarct volume and ameliorated hemorrhagic transformation in patients with acute ischemic stroke who received intravenous alteplase within 4.5 hours after stroke onset.19 However, it remains unclear whether fingolimod can enhance the efficacy of alteplase in a delayed time window and the mechanism governing the impact of fingolimod on alteplase treatment remains undefined. In the present study, we evaluated whether fingolimod administered in combination with alteplase improved clinical outcomes via improving anterograde reperfusion and retrograde reperfusion of collateral circulation in patients with anterior vessel occlusion and imaging mismatch within 4.5 to 6 hours of ischemia onset.
Subjects and Methods
Study Design and Participants
This is a prospective, multicenter, randomized, open‐label, blinded endpoint clinical trial. The main inclusion criteria were: acute ischemic stroke patients at 4.5 to 6 hours from symptom onset, age > 18 years, baseline National Institutes of Health Stroke Scale (NIHSS) > 4, perfusion computed tomography (PCT)‐defined mismatch ratio > 1.2, head and neck computed tomographic angiography (CTA) evidence of internal carotid artery proximal or middle cerebral artery occlusion, and no contraindications to alteplase. Patients who planned to undergo intra‐arterial treatment were excluded from the trial. Recruitment was carried out between June 2014 and May 2017 at 3 stroke centers in China. Written informed consent was provided by the patients or their health care proxies. The study protocol and informed consent procedures were approved by the ethics committee at each participating center. This study is registered with https://ClinicalTrials.gov (identifier: NCT02002390).
Patients were randomly assigned in a 1:1 ratio to receive alteplase plus fingolimod or alteplase alone by means of a central telephone service; the alteplase group received 0.9mg/kg alteplase with the first 10% injected intravenously as a bolus followed by the remainder administered over a 1‐hour period (maximum dose was 90mg); the fingolimod plus alteplase group was given intravenous alteplase and 0.5mg fingolimod (Gilenya; Novartis, Basel, Switzerland) administered orally, daily for 3 consecutive days (Fig 1A). The first dose of fingolimod was given immediately after computed tomographic (CT) scanning. Counts of circulating lymphocyte subsets were monitored with fluorescence‐activated cell sorting to confirm the biological activity of fingolimod as previously described.20, 21, 22
Figure 1.
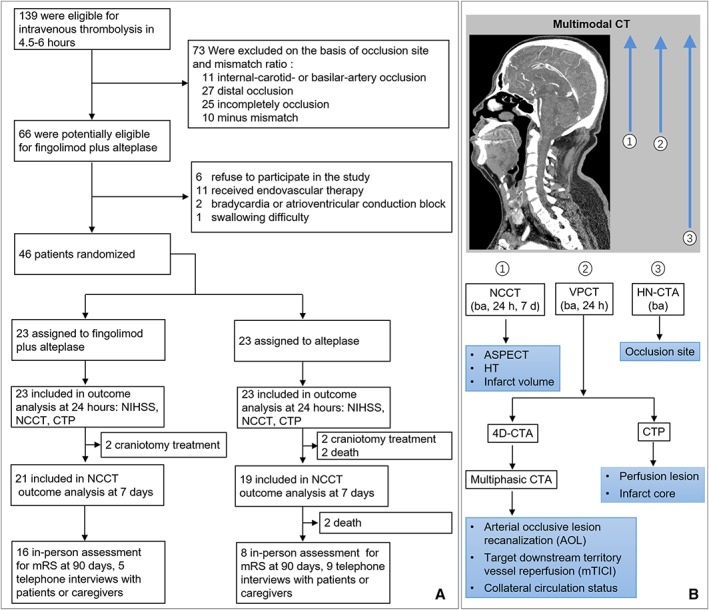
Trial profile. (A) Patient enrollment and follow‐up. Due to death or craniectomy, modified Rankin Scale (mRS) scores of 6 patients in the alteplase group and 2 patients in the fingolimod plus alteplase group were recorded as 6 at day 90 assessments. For the rest of these patients, 16 patients received in‐person assessment for mRS at 90 days, and 5 patients or caregivers were interviewed by telephone in fingolimod plus alteplase group; face‐to‐face assessments were performed in 8 patients, and 9 others received telephone follow‐up in the alteplase group. (B) Processing and analysis of multimodal computed tomographic (CT) scans. Noncontrast CT (NCCT) was evaluated for ischemic severity (Alberta Stroke Program Early CT score), infarct lesion and hemorrhagic transformation (HT), head–neck CT angiography (HN‐CTA) for site of occlusion, and volume perfusion CT (VPCT) for ischemic core and perfusion lesion. In addition, VPCT data were reconstructed to multiphasic 4‐dimensional CTA (4D‐CTA): phase 1 of 4D‐CTA (arterial peak), phase 2 of 4D‐CTA (8 seconds after peak attenuation), and phase 3 of 4D‐CTA (16 seconds after peak attenuation). Target arterial lesion recanalization (Arterial Occlusive Lesion Scale [AOL]) and target downstream territory reperfusion (modified Thrombolysis in Cerebral Ischemia [mTICI]) were evaluated on the imaging of phase 1 of 4D‐CTA. Phases 1 to 3 of 4D‐CTA were used to assess circulation flow with the 6‐point University of Calgary ordinal scale. ba = baseline; CTP = CT perfusion; NIHSS = National Institutes of Health Stroke Scale.
Clinical Evaluation
Clinical assessments were performed at baseline, 24 hours, discharge, and 90 days. If some of these data were missing, data from the last observation were used. If patients died or underwent decompressive craniectomy, a modified Rankin Scale (mRS) score of 6 was used. Clinical evaluations included the NIHSS score for assessing neurologic deficits and mRS for assessing global disability. Clinical assessments were performed by experienced neurologists blinded to patients’ enrollment status.
Imaging Evaluation
Examinations were performed on a third‐generation dual‐source multidetector CT hardware platform (Somatom Force; Siemens Healthcare, Erlangen, Germany). All patients underwent standard noncontrast CT with 5mm section thickness followed by volume PCT (VPCT) and head and neck CTA at baseline (see Fig 1B). Follow‐up imaging consisted of noncontrast CT and VPCT at 24 hours and noncontrast CT at 7 days for assessment of image outcomes.
VPCT consisted of 27 consecutive spiral acquisitions of the brain (115mm in the z‐axis, 6‐second delay after start of contrast medium injection, 37‐second total imaging duration, 70kV, 120mA, maximum pitch = 0.5, rotation time = 0.25 milliseconds, collimation = 2 × 64 × 0.6mm). A 40ml bolus of contrast medium (iopamidol 370; Braccosine, Shanghai, China) was used at a flow rate of 6ml/s, followed by a 40ml saline chaser at 6ml/s. Head and neck CTA from the aortic arch to the vertex (70kV, 120mA) was performed after the administration of iopamidol at the same volume and rate as VPCT. The effective dose amounted to 1.0mSv for noncontrast CT, 2.1mSv for VPCT, and 1.6mSv for head and neck CTA acquisition.
Noncontrast CT scans were assessed for the presence of early parenchymal ischemic changes using the Alberta Stroke Program Early CT score at baseline, hemorrhage transformation at 24 hours, and infarct volume at day 1 and day 7. Symptomatic intracranial hemorrhage was defined as large parenchymal hematoma combined with a significant clinical deterioration of ≥4 points on the NIHSS. Head and neck CTA data reconstructed with a section width of 0.75mm and increments of 0.4mm were used to determine occlusion site.
VPCT data were reconstructed with a section width/increments of 1.5mm/1mm for 4‐dimensional CTA (4D‐CTA) and 5mm/3mm for perfusion parameter maps. 4D‐CTA was processed using a commercial software package (Dynamic Angio; Siemens Healthcare Sector, Munich, Germany) as we previously described.23 To define different phases on 4D‐CTA, the point of peak arterial opacification during the 37‐second imaging time was identified on the time attenuation curve in the unaffected middle cerebral artery. Consecutively, axial maximum intensity projections were reconstructed from images at peak attenuation (phase 1), 8 seconds (phase 2), and 16 seconds (phase 3) after peak attenuation with 10mm slice thickness and 1mm increments. Target arterial lesion recanalization was evaluated with the Arterial Occlusive Lesion Scale (AOL) on phase 1 of 4D‐CTA of Willis circle. Recanalization was defined as AOL ≥ 2. Anterograde reperfusion of target downstream territory was evaluated with the modified Thrombolysis in Cerebral Ischemia (mTICI) score in patients with recanalization on phase 1 of 4D‐CTA. Using phases 1 to 3 of 4D‐CTA, we assessed retrograde reperfusion changes of secondary collateral flow through leptomeningeal arteries among patients with no recanalization and used a validated 6‐point pial arterial filling ordinary score established at the University of Calgary.24
CT perfusion (CTP) was processed offline with the commercial software MiStar (Apollo Medical Imaging Technology, Melbourne, Victoria, Australia). The volume of the acute perfusion lesion was defined as tissue with relative delay time > 3 seconds, and the threshold of acute ischemic core was relative cerebral blood flow < 30% of contralateral as we previously described.25 Mismatch ratio was defined as perfusion lesion/ischemic core.
Outcomes
The primary outcomes evaluated were the decrease of NIHSS scores over 24 hours and the proportion of patients whose mRS score was 0, 1, or 2 at day 90. Secondary outcomes included the relative decrease of perfusion lesion (1 − [perfusion lesion volume at 24 hours / perfusion lesion volume at baseline]), the relative infarct lesion growth ([infarct lesion volume of noncontrast CT at 24 hours / ischemic core volume of CTP at baseline] − 1), and the favorable shift of hemorrhage transformation type distribution over 24 hours (no hemorrhage transformation, asymptomatic intracranial hemorrhage, symptomatic intracranial hemorrhage), as well as relative infarct lesion growth between day 7 and 24 hours. Exploratory outcome included the favorable shift of recanalization level distribution on target arterial lesion at 24 hours (none, AOL = 0–1; incomplete, AOL = 2; complete, AOL = 3), the favorable shift of anterograde reperfusion distribution level of target downstream territory (no perfusion, mTICI = 0; incomplete perfusion, mTICI = 2 or 2a; complete reperfusion, mTICI = 2b or 3) at 24 hours, and the change of retrograde reperfusion of collateral flow over 24 hours. Sustained collateral circulation means the change of pial arterial filling ordinary score over 24 hours is ≥ 0.
Statistical Analysis
The primary hypothesis was that a combination of alteplase and fingolimod would improve 1 or both coprimary outcomes (the change of NIHSS score at 24 hours and the proportion of patients whose mRS score was 0, 1, or 2 at day 90) relative to alteplase alone. We calculated the sample size for the current study on the basis of our pilot study,20 with power set at 80% and an assumption of superiority with respect to 1 of the 2 coprimary endpoints at an alpha level of 0.025. The change of NIHSS score at 24 hours was tested by means of Wilcoxon rank sum test. mRS at day 90 was tested by using an unadjusted chi‐squared test of proportions. Secondary outcomes and exploratory outcomes with a normal distribution were tested with the use of Student t test; for nonparametric distributions, the Wilcoxon rank sum test was used, and categorical variables were compared by using the chi‐squared test of proportions or Fisher exact test. All analyses were repeated after adjustment for age and baseline NIHSS to demonstrate the effect of fingolimod treatment on outcomes with multiple linear regression, binary logistic regression, median regression, or multivariate ordinal logistic regression. SPSS for Windows version 22.0 software (IBM, Armonk, NY) was used for the analysis.
Results
Patient Characteristics and Pharmaceutical Effect of Fingolimod
From 2014 through 2017, a total of 139 patients within 4.5 to 6 hours after the onset of ischemic stroke were screened. Finally, 46 patients were enrolled in the trial; 23 were randomly assigned to the fingolimod plus alteplase group and 23 to the alteplase group (see Fig 1). Reasons for exclusion are detailed in Figure 1. Baseline characteristics were balanced between the 2 groups. Participants assigned to the fingolimod plus alteplase group had an older mean age (67 vs 65 years), a higher median NIHSS (14 vs 11), a larger median core volume (27 vs 24ml), and a longer median interval between the time that a patient was last known to be well and treatment with intravenous alteplase (320 vs 313 minutes), although these potential differences were not statistically significant (Table 1).
Table 1.
Patient Baseline Characteristics
| Characteristic | Alteplase, n = 23 | Fingolimod + Alteplase, n = 23 | p |
|---|---|---|---|
| Mean age (SD), yr | 65 (13.0) | 67 ( 6.8) | 0.431 |
| Sex, male, n (%) | 7 (30) | 9 (39) | 0.758 |
| Medical history | |||
| Hypertension, n (%) | 13 (57) | 19 (83) | 0.108 |
| Diabetes mellitus, n (%) | 5 (22) | 5 (22) | 1.000 |
| Mean blood glucose (SD), mmol/l | 7.2 (2.0) | 7.7 (2.4) | 0.539 |
| Hyperlipidemia, n (%) | 6 (26) | 6 (26) | 1.000 |
| Arial fibrillation, n (%) | 6 (26) | 7 (30) | 1.000 |
| Current smoking, n (%) | 9 (39) | 9 (39) | 1.000 |
| Medication, n (%) | 0.373 | ||
| Antiplatelet agent | 3 (13) | 5 (22) | |
| Anticoagulant agent | 1 (4) | 3 (13) | |
| Median time to alteplase treatment (IQR), min | 313 (296–340) | 320 (300–330) | 0.895 |
| Median NIHSS score at baseline (IQR) | 11 (5–19) | 14 (8–24) | 0.508 |
| Imaging | |||
| Median ischemic core volume at baseline (IQR), ml | 24 (12–41) | 27 (16–53) | 0.429 |
| Median perfusion lesion volume at baseline (IQR), ml | 83 (60–145) | 93 (54–126) | 0.809 |
| Median score of leptomeningeal collateral circulationa | 3 (1–4) | 3 (1–4) | 0.124 |
| Occlusion site, n (%) | 0.769 | ||
| Terminal internal carotid artery | 2 (9) | 4 (17) | |
| First segment of middle cerebral artery | 9 (40) | 8 (35) | |
| Second segment of middle cerebral artery | 12 (51) | 11 (48) | |
| ASPECT score | 7 (5–7) | 6 (5–7) | 0.162 |
Hypertension was defined as systolic blood pressure ≥ 140mmHg and/or diastolic blood pressure ≥ 90mmHg. Diabetes was defined with fasting plasma glucose ≥ 7.0mmol/l and/or any time plasma glucose ≥ 11.1mmol/l, and hyperlipidemia was defined as serum total cholesterol levels of >5.72mmol/l and/or triglycerides > 1.7mmol/l.
University of Calgary scoring on multiphase computed tomographic angiography was used to estimate leptomeningeal collateral circulation status, which ranged from 0 (no vessels in any phase within the occluded vascular territory) to 5 (no delay and normal extent vessel within the occluded arterial territory), with lower scores indicating worse collateral circulation status.
ASPECT = Alberta Stroke Program Early CT; IQR = interquartile range; NIHSS = National Institutes of Health Stroke Scale; SD = standard deviation.
Lymphocyte subset counts were assessed to monitor the biological activity of fingolimod as we previously described.19, 20, 21 At baseline, the lymphocyte subset counts were similar between the 2 groups. Compared with the alteplase group, the numbers of all lymphocyte subsets showed decreases as early as 1 day after treatment with fingolimod, and this trend persisted to 3 and 7 days after stroke onset (Fig 2).
Figure 2.
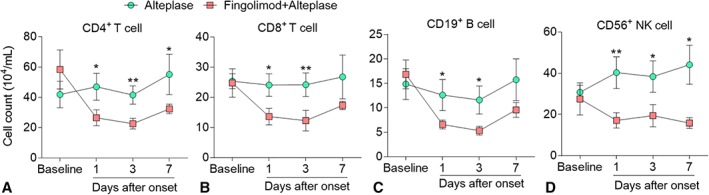
Lymphocyte counts in acute ischemic stroke patients decreased after fingolimod treatment. Whole blood was drawn from all patients at baseline and 1, 3, and 7 days after treatment. Mononuclear cells were purified and stained with antibodies to individual cell types. Percentages of CD4+ T cells (A), CD8+ T cells (B), CD19+ B cells (C), and CD3−CD56+ natural killer (NK) cells (D) were determined by flow cytometry; absolute numbers were calculated and are displayed as ×104/ml blood from patients. Data are shown as mean ± standard error. Comparisons were performed by 2‐way analysis of variance test followed by Bonferroni post hoc test; *p < 0.05, **p < 0.01 compared with control group.
Fingolimod Improved Alteplase Efficacy
All 46 patients enrolled were available for evaluation of clinical and imaging outcomes, except for final infarct volume on day 7, which included 40 patients (21 patients in the fingolimod plus alteplase and 19 patients in the alteplase group; see Fig 1). The first primary outcome of the median change in NIHSS score at 24 hours was 4.0 in the fingolimod plus alteplase group as compared with 0 in the alteplase group (p = 0.004; Fig 3A). There was also a shift in the distribution of the mRS scores in favor of the fingolimod intervention (mRS = 0–2/mRS = 3–4/mRS = 5–6: 57%/34%/9% vs 22%/39%/39%, p = 0.037; see Fig 3B).
Figure 3.
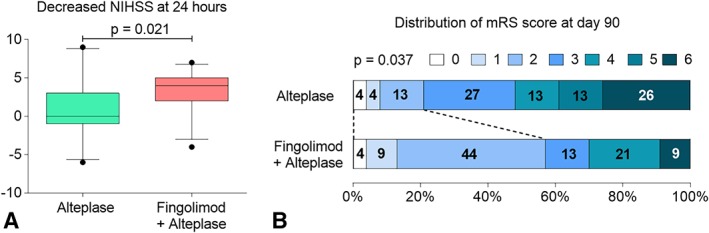
Fingolimod decreased National Institutes of Health Stroke Scale (NIHSS) scores at 24 hours and facilitated a favorable shift in the distribution of modified Rankin Scale (mRS) at day 90 among patients receiving intravenous alteplase. (A) Decrease in the NIHSS score at 24 hours. The horizontal line inside each box indicates the median, the top and bottom of the box represent the interquartile range, bars show the 5th and 95th percentiles, and circles designate outliers. (B) Distribution of mRS at day 90.
With respect to secondary endpoints, fingolimod added further benefit to alteplase in decreasing the size of the perfusion lesion (relative median perfusion lesion decrease: 0.7 vs 0.1, p < 0.001) and inhibiting infarct expansion (relative median infarct lesion growth: 1.4 vs 0.1, p < 0.001); this benefit persisted to the 7th day. The relative infarct volume growth between day 7 and 24 hours was smaller in the fingolimod plus alteplase group than the alteplase group (0.2 vs 0.4, p = 0.031; Fig 4A–D). Although the incidence of asymptomatic intracranial hemorrhage was increased (61% vs 8%) at 24 hours, there was no significant difference in the incidence of symptomatic intracranial hemorrhage (4% vs 8%) between groups (see Fig 4E). The magnitude and significance of improvement in clinical status and tissue lesion size in the fingolimod plus alteplase group did not change after correction, except for the relative infarct volume growth between day 7 and 24 hours (Table 2).
Figure 4.
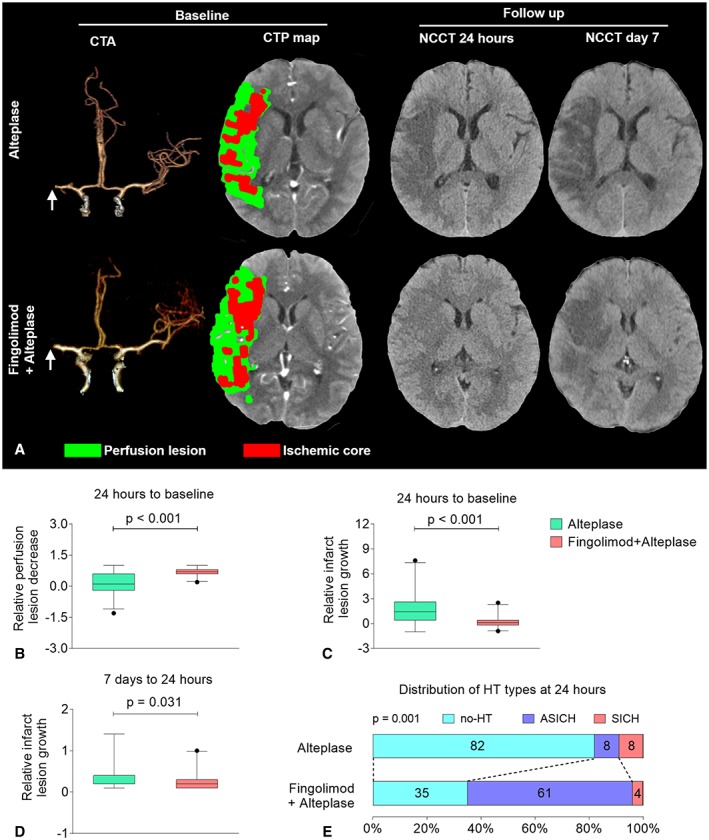
Fingolimod decreased perfusion lesions and restrained infarct growth at 24 hours among patients with intravenous alteplase treatment. (A) Representative multimodal computed tomographic (CT) scans of patients with alteplase treatment (upper panel) and fingolimod plus alteplase treatment (lower panel). At baseline, although the site of artery occlusions (arrows) and the mismatch status were the same, striking differences were evident at follow‐up. The progression of infarct volume was restrained in fingolimod‐treated patients. (B) The decrease in perfusion lesion at 24 hours. The relative perfusion lesion decrease was defined as 1 − (perfusion lesion volume at 24 hours / perfusion lesion volume at baseline). Positive values for the relative perfusion lesion decrease rate indicate improvement. (C) The growth in the infarct lesion at 24 hours. The relative infarct lesion growth was defined as (infarct lesion volume of noncontrast CT [NCCT] at 24 hours / ischemic core volume of CT perfusion [CTP] at baseline) − 1. Negative values for the relative infarct lesion growth rate indicate improvement. (D) The growth of the infarct lesion from day 7 to 24 hours. The relative infarct lesion growth was defined as (infarct lesion volume of NCCT at day 7 / infarct volume of NCCT at 24 hours) − 1. Negative values for the relative infarct lesion growth rate indicate improvement. The horizontal line inside each box indicates the median, the top and bottom of the box indicate the interquartile range, the bars indicate the 5th and 95th percentiles, and the circle indicates an outlier. (E) The distribution of hemorrhagic transformation (HT) types at 24 hours. The numbers in the bars are percentages of patients who had the same type. Symptomatic intracranial hemorrhage (SICH) was defined as large parenchymal hematoma combined with a significant clinical deterioration of ≥4 points on the National Institutes of Health Stroke Scale (NIHSS). Asymptomatic intracranial hemorrhage (ASICH) was defined as a small parenchymal hematoma combined with clinical deterioration of <4 points on the NIHSS. CTA = CT angiography.
Table 2.
Trial Outcomes
| Outcomes | Differences (95% CI)a | Effect Variable | Unadjusted Value (95% CI) | Unadjusted p | Adjusted Value (95% CI) | Adjusted p |
|---|---|---|---|---|---|---|
| Primary outcome | ||||||
| NIHSS score improvement between 24 hours and baseline, medianb | 4 (0 to 5) | Median difference | 4 (0 to 5) | 0.021 | 4 (0 to 5) | 0.018 |
| The proportion of mRS = 0–2 at 90 daysc | 34.8 (0.9 to 60.2) | Odds ratio | 4.7 (1.3 to 17.0) | 0.037 | 8.6 (1.8 to 40.1) | 0.007 |
| Secondary outcome | ||||||
| Relative perfusion lesion decreases from 24 hours to baseline, meand | 0.5 (0.3 to 0.8) | Beta coefficient | 0.5 (0.3 to 0.8) | <0.001 | 0.5 (0.2 to 0.7) | <0.001 |
| Relative Infarct lesion growth from 24 hours to baseline, meand | −1.7 (−2.8 to −0.8) | Beta coefficient | −1.7 (−2.8 to −0.8) | 0.001 | −1.7 (−2.7 to −0.7) | 0.002 |
| Relative infarct volume growth from day 7 to 24 hours, meand | −0.2 (−0.4 to 0.0) | Beta coefficient | −0.2 (−0.4 to −0.0) | 0.067 | −0.2 (−0.4 to −0.1) | 0.057 |
| The hemorrhage transformation type distribution at 24 hourse | Common odds ratio | 0.1 (0 to 0.6) | 0.005 | 0.1 (0 to 0.5) | 0.004 | |
| Exploratory outcome | ||||||
| The recanalization distribution at 24 hours: AOL score distributione | Common odds ratio | 0.8 (0.3 to 2.6) | 0.734 | 0.9 (0.3 to 2.8) | 0.814 | |
| The antegrade reperfusion distribution of patients with recanalization at 24 hours: mTICI score distributione | Common odds ratio | 0.1 (0.0 to 0.7) | 0.019 | 0.1 (0.0 to 0.9) | 0.041 | |
| Collateral flow level change of patients with no recanalization at 24 hours: pial arterial filling score change, meane | 2.9 (2.0 to 3.8) | Beta coefficient | 2.9 (2.0 to 3.8) | <0.001 | 2.9 (2.1 to 3.8) | <0.001 |
Four patients in the control group did not undergo noncontrast computed tomography at 7 days owing to brain herniation or death. mTICI score was assessed among patients with recanalization. Collateral flow was assessed among patients with no recanalization. Estimates were adjusted for age and baseline NIHSS.
Differences (intervention group − control group) are shown as percentage points, median or mean.
Median regression was used for outcome adjustment.
Binary logistic regression model was used for outcome adjustment.
The multiple linear regression was used for outcome adjustment.
The multivariate ordinal logistic regression model was used for outcome adjustment.
AOL = Arterial Occlusive Lesion Scale; CI = confidence interval; mRS = modified Rankin Scale; mTICI = modified Thrombolysis in Cerebral Ischemia; NIHSS = National Institutes of Health Stroke Scale.
Fingolimod Promoted Anterograde Reperfusion of Target Downstream Territory and Improved Retrograde Collateral Flow
Fingolimod did not significantly affect the target arterial lesion recanalization at 24 hours (10 [41%] vs 9 [39%], p = 0.938; Fig 5). However, in patients with recanalization, fingolimod improved anterograde reperfusion of the target downstream territory assessed by mTICI scores (mTICI = 2b–3: 80% vs 33%, p = 0.023) at 24 hours. Surprisingly, we found that fingolimod prevented collateral circulation failure in the patients without recanalization (University of Calgary pial arterial filling ordinary score = 2 vs −1, p < 0.001). The magnitude and significance of anterograde reperfusion and collateral circulation improvement in the fingolimod plus alteplase groups did not change after correction (see Table 2). Complete recanalization (mTICI = 2b–3) or sustained collateral circulation (change of pial arterial filling ordinary score ≥ 0) were strongly associated with good recovery (mRS = 0–2) at 90 days (odds ratio = 4.9, 95% confidence interval = 1.0–23.3, p = 0.044).
Figure 5.
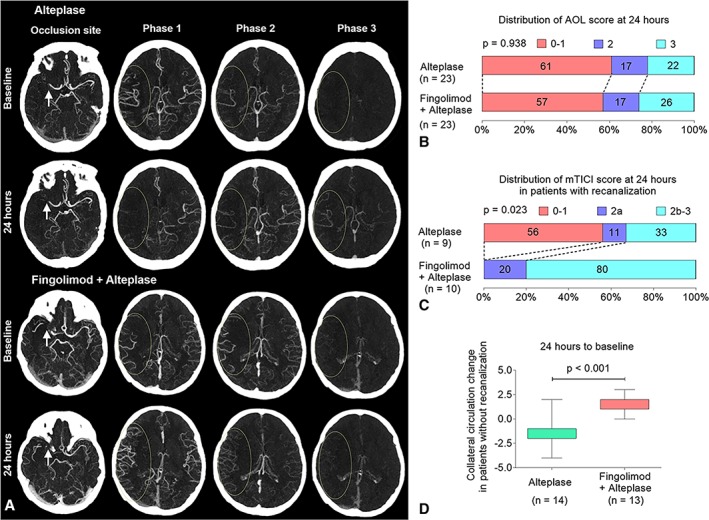
Fingolimod improved target downstream territory reperfusion and reversed the failure of collateral flow at 24 hours among patients with intravenous alteplase treatment. (A) Representative multiphase 4‐dimensional computed tomographic angiography (4D‐CTA) of patients with alteplase treatment (upper panel) and fingolimod plus alteplase treatment (lower panel). Although the recanalization status of target arterial lesion (arrows) was the same (first row), striking differences were evident at retrograde reperfusion of target downstream territory (ellipses) on phases 1 to 3 of 4D‐CTA. Fingolimod reversed the deterioration of collateral circulation. (B) The distribution of recanalization on target arterial lesion at 24 hours (no recanalization, Arterial Occlusive Lesion Scale (AOL) = 0–1; incomplete recanalization, AOL = 2; complete recanalization, AOL = 3). The numbers in the bars are percentages of patients who had the same grade. (C) The distribution of the anterograde reperfusion of target downstream territory at 24 hours in patients with recanalization (no perfusion, modified Thrombolysis in Cerebral Ischemia [mTICI] = 0; incomplete perfusion, mTICI = 1 or 2a; complete reperfusion, mTICI = 2b or 3). The numbers in the bars are percentages of patients who had the same grade. (D) The change in the collateral circulation of patients without recanalization at 24 hours. The collateral circulation change was defined as (collateral circulation score at 24 hours) − (collateral circulation score at baseline). Positive values for the collateral circulation change indicate collateral circulation improvement. The top and bottom of the boxes indicate the interquartile range, and the bars indicate the 5th and 95th percentiles.
Safety
The complications and adverse events are summarized in Table 3. Six patients (26%) in the alteplase group experienced severe cerebral edema and brain herniation, 2 of whom underwent decompressive craniectomy on day 3; 4 others refused surgery and died. Two patients (9%) treated with fingolimod plus alteplase underwent craniectomy, but neither died. Signs of infection did not differ significantly between the 2 groups. Electrocardiographic monitoring, which identified paroxysmal atrial flutter in 2 patients with atrial fibrillation during fingolimod treatment, normalized following intravenous amiodarone administration. Another 2 patients in the fingolimod plus alteplase group experienced thrombocytopenia and recovered without treatment.
Table 3.
Complications and Adverse Events
| Outcome | Alteplase, n = 23 | Fingolimod + Alteplase, n = 23 | p |
|---|---|---|---|
| Complications, n (%) | |||
| Deaths | 4 (17) | 0 (0) | 0.109 |
| Myocardial infarctions | 0 (0) | 0 (0) | 1.000 |
| Recurrent strokes | 0 (0) | 1 (4) | 1.000 |
| Cerebral herniation | 6 (26) | 2 (9) | 0.243 |
| Hemorrhage of digestive tract | 0 (0) | 0 (0) | 1.000 |
| Fever, > 38° | 1 (8) | 0 (0) | 1.000 |
| Adverse events, n (%) | |||
| Any adverse event leading to discontinuation | 0 (0) | 0 (0) | 1.000 |
| Any serious adverse event | 0 (0) | 0 (0) | 1.000 |
| Other adverse events of interest, n (%) | |||
| Suspected lung infection | 4 (17) | 3 (13) | 1.000 |
| Urinary tract infection | 0 (0) | 0 (0) | 1.000 |
| Herpes virus infection | 0 (0) | 0 (0) | 1.000 |
| Abnormal laboratory liver function test | 0 (0) | 0 (0) | 1.000 |
| Gastrointestinal disorders | 0 (0) | 0 (0) | 1.000 |
| Thrombocytopenia | 0 (0) | 2 (9) | 0.489 |
| Bradycardia | 0 (0) | 0 (0) | 1.000 |
| Atrial flutter | 0 (0) | 2 (9) | 0.489 |
| Macular edema | 0 (0) | 0 (0) | 1.000 |
Discussion
In this homogeneous group of ischemic stroke patients with a proximal vessel occlusion in the anterior circulation and perfusion mismatch in the 4.5‐ to 6‐hour time window, we found that fingolimod plus alteplase treatment was superior to alteplase alone with respect to the coprimary endpoints of early clinical improvement at 24 hours and functional recovery at 90 days. Additionally, fingolimod administration in combination with alteplase was associated with greater perfusion lesion reduction and suppressed infarct growth.
The magnitude of the clinical benefit of alteplase treatment in our study was smaller than that in the EPITHET trial26 (which expanded the alteplase treatment time window to 3–6 hours based on mismatch), despite similar clinical severities and mismatched characteristics (mRS = 0–2 at 90 days: 22% vs 41%; median relative growth at 24 hours: 1.4 vs 0.2). Key differences between our study and the EPITHET trial include involvement of patients with middle cerebral artery or internal carotid artery proximal occlusion, longer time to the onset of treatment (313 vs 293 minutes), larger ischemic core (24 vs 18ml), and imaging method (multimodal CT vs multimodal magnetic resonance imaging). However, the outcomes of combined fingolimod and alteplase treatment in our study were better than those of the placebo group and alteplase group of the EPITHET trial (mRS = 0–2 at 90 days: 57% vs 45% vs 41%; median relative growth at 24 hours: 0.1 vs 0.2 vs 0.8).
Whereas the absolute difference in mRS = 0–2 observed in this study was similar to the difference between endovascular thrombectomy and control, the early NIHSS change was less impressive in this study compared to the thrombectomy trials.3 The reasons for this discrepancy are unknown. Preservation of collateral circulation and attenuation of secondary inflammatory damage as a result of fingolimod treatment during the acute and subacute periods may contribute to better outcome,15, 19 which needs to be confirmed in future studies.
Compared with a conventional CTA, multiphase CTA acquires 2 extra cerebral images with 8‐ and 16‐second delays. In this way, the evaluation of vascular filling and collateral circulation at 3 different time points is possible.24 More recently, the ESCAPE trialists developed a 6‐point score system for pial arterial filling using multiphase CTA, which showed a good interrater reliability and ability to help determine clinical outcome.24, 27 In this unique study, featuring coadministration of an immune modulator with alteplase for acute stroke patients in whom multiphase 4D‐CTA was obtained at baseline and again 24 hours later, the influence of fingolimod on arterial recanalization, anterograde reperfusion, and retrograde reperfusion of collateral flow were explored based on multiphase 4D‐CTA. In agreement with our previous study, fingolimod had little effect on target vessel recanalization. After alteplase thrombolysis, > 50% of the patients had complete recanalization failure, which is in accordance with results from other studies.28, 29 However, we found a significant improvement in downstream anterograde reperfusion and retrograde reperfusion from fingolimod treatment. The incidence of asymptomatic hemorrhagic infarction was greater in the fingolimod intervention group than in the control group, possibly owing to the early reperfusion. However, the rate of more serious parenchymal hematomas or symptomatic hemorrhage was not higher in the intervention group than in the control group.
Successful recanalization does not consistently lead to better outcomes in stroke patients, as <50% of patients with successful thrombolysis or thrombectomy have good outcomes (mRS = 0–2).1, 2 In our study, 10 patients responded with successful recanalization after fingolimod treatment, and 8 (80%) patients experienced good outcomes, which is higher when compared with the alteplase alone group (30%) or in other studies evaluating pharmacologic or mechanical recanalization (50%).2 This result suggests that fingolimod relieved futile recanalization in patients with causative clot dissolution. In addition, 17% of patients with collateral perfusion in the alteplase group and 61% of patients with collateral perfusion in the fingolimod plus alteplase group were observed 24 hours after the onset of stroke among patients with no recanalization. Those percentages are also higher than the 30% previously observed.30 The action of fingolimod in reversing the failure of microvascular circulation is believed to be responsible for this phenomenon.
No‐reflow phenomenon4 and the failure to recruit collateral circulation6, 7 may occur in patients with severely damaged microcirculation, thus precluding the potential benefit of reperfusion strategies.16, 17 By binding to the sphingosine‐1‐phosphate receptors of lymphocytes, fingolimod is capable of inhibiting the egress of lymphocytes from lymph nodes, thereby reducing the number of circulating lymphocytes. Consequently, fingolimod not only inhibits such cells from infiltrating into the brain parenchyma and reduces secondary injury to brain tissues, but also, at an earlier phase, reduces the number of cells accumulating simultaneously in the brain's microvasculature.15, 18 The latter action could inhibit inflammatory thrombosis in capillaries and maintain the potency of brain vasculature. Thus, fingolimod may also preserve microvasculature function by reducing vascular inflammation, in turn sustaining the perfusion of brain tissue and saving the penumbra in a prolonged time interval to thrombolysis. In that context, the reduction of cell migration to the brain achieved by fingolimod may have important implications for reperfusion therapies.
This study has several notable limitations, including a small sample size, a lack of blinded treatment administration, and lack of an independent data safety and monitoring entity. In addition, we utilized strict inclusion criteria based on CTA and CTP to identify patients who would be most likely to benefit from alteplase treatment within 4.5 to 6 hours, which limited the study to centers with a single model of CT scanner, and excluded patients who underwent mechanical thrombectomy. The cost of these strict criteria is lack of generalizability. Owing to the absence of a control group without intravenous alteplase treatment, we also could not conclude that fingolimod extends the therapeutic window for alteplase in acute ischemic stroke when documenting the benefits or risks of fingolimod with alteplase coadministration.
Although mechanical thrombectomy has recently been established as the standard of care for selected patients with large vessel occlusions, <10% of all stroke patients are currently eligible for mechanical thrombectomy based on current guidelines.31 Thrombectomy requires highly specialized centers that are unevenly distributed among countries and regions. In some regions of China, centers capable of mechanical thrombectomy are a long distance away, resulting in significant delays in patient transfer, which may make patients ineligible for thrombectomy. In this regard, intravenous thrombolysis with alteplase remains a viable treatment for the majority of acute ischemic stroke patients at many centers,26, 32, 33, 34, 35 and efforts to extend the therapeutic window for intravenous thrombolytics have the potential to positively impact outcome for a significant proportion of stroke patients. Along these lines, intravenous thrombolysis within 6 hours has been tested in several completed and ongoing trials that have utilized imaging to select those patients who exhibit a mismatch between infarcted tissue and perfusion deficits.26, 32, 33, 36 The results of these studies are preliminary but promising, suggesting that intravenous thrombolysis improves outcome if administered out to 6 hours in patients who exhibit mismatch on perfusion imaging. Additionally, a clinical trial evaluating fingolimod in conjunction with thrombectomy for patients with proximal large vessel occlusion has been initiated.37
Author Contributions
F.‐D.S. and Y.F. formulated the conception and design of this study; D.‐C.T., Z.Z., J.Y., X.Y., L.S., S.Z., and M.Z. contributed to data acquisition; all authors analyzed the data; Y.F., D.‐C.T., K.S., A.F.D., and F.D.S. made critical revisions of the manuscript; F.‐D.S., K.S., D.‐C.T., and A.F.D. drafted the manuscript and prepared the figures.
Potential Conflicts of Interest
Nothing to report.
Acknowledgment
This study was supported in part by the National Science Foundation of China (grant 91642205) and funds of Advanced Innovation Center for Human Brain Protection, Capital Medical University, Beijing, China.
We thank our patients for participating in this study, the members of the teams at our enrolling sites, and S. Shi and P. Minick for editorial assistance.
References
- 1. Rha JH, Saver JL. The impact of recanalization on ischemic stroke outcome: a meta‐analysis. Stroke 2007;38:967–973. [DOI] [PubMed] [Google Scholar]
- 2. Espinosa de Rueda M, Parrilla G, Manzano‐Fernandez S, et al. Combined multimodal computed tomography score correlates with futile recanalization after thrombectomy in patients with acute stroke. Stroke 2015;46:2517–2522. [DOI] [PubMed] [Google Scholar]
- 3. Goyal M, Menon BK, van Zwam WH, et al. Endovascular thrombectomy after large‐vessel ischaemic stroke: a meta‐analysis of individual patient data from five randomised trials. Lancet 2016;387:1723–1731. [DOI] [PubMed] [Google Scholar]
- 4. Stoll G, Kleinschnitz C, Nieswandt B. Molecular mechanisms of thrombus formation in ischemic stroke: novel insights and targets for treatment. Blood 2008;112:3555–3562. [DOI] [PubMed] [Google Scholar]
- 5. Yemisci M, Gursoy‐Ozdemir Y, Vural A, et al. Pericyte contraction induced by oxidative‐nitrative stress impairs capillary reflow despite successful opening of an occluded cerebral artery. Nat Med 2009;15:1031–1037. [DOI] [PubMed] [Google Scholar]
- 6. Campbell BC, Christensen S, Tress BM, et al. Failure of collateral blood flow is associated with infarct growth in ischemic stroke. J Cereb Blood Flow Metab 2013;33:1168–1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Yeo LL, Paliwal P, Low AF, et al. How temporal evolution of intracranial collaterals in acute stroke affects clinical outcomes. Neurology 2016;86:434–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Makris N, Chamard L, Mikkelsen IK, et al. Acute reperfusion without recanalization: serial assessment of collaterals within 6 h of using perfusion‐weighted magnetic resonance imaging. J Cereb Blood Flow Metab 2017. 10.1177/0271678X17744716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gan Y, Liu Q, Wu W, et al. Ischemic neurons recruit natural killer cells that accelerate brain infarction. Proc Natl Acad Sci U S A 2014;111:2704–2709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Liu Q, Jin WN, Liu Y, et al. Brain ischemia suppresses immunity in the periphery and brain via different neurogenic innervations. Immunity 2017;46:474–487. [DOI] [PubMed] [Google Scholar]
- 11. Shi K, Wood K, Shi FD, et al. Stroke‐induced immunosuppression and poststroke infection. Stroke Vasc Neurol 2018;3:34–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Fu Y, Liu Q, Anrather J, Shi FD. Immune interventions in stroke. Nat Rev Neurol 2015;11:524–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Langhauser F, Gob E, Kraft P, et al. Kininogen deficiency protects from ischemic neurodegeneration in mice by reducing thrombosis, blood‐brain barrier damage, and inflammation. Blood 2012;120:4082–4092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kleinschnitz C, Kraft P, Dreykluft A, et al. Regulatory T cells are strong promoters of acute ischemic stroke in mice by inducing dysfunction of the cerebral microvasculature. Blood 2013;121:679–691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kraft P, Gob E, Schuhmann MK, et al. FTY720 ameliorates acute ischemic stroke in mice by reducing thrombo‐inflammation but not by direct neuroprotection. Stroke 2013;44:3202–3210. [DOI] [PubMed] [Google Scholar]
- 16. Zhang L, Zhang ZG, Chopp M. The neurovascular unit and combination treatment strategies for stroke. Trends Pharmacol Sci 2012;33:415–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Fisher M, Saver JL. Future directions of acute ischaemic stroke therapy. Lancet Neurol 2015;14:758–767. [DOI] [PubMed] [Google Scholar]
- 18. Schuhmann MK, Krstic M, Kleinschnitz C, Fluri F. Fingolimod (FTY720) reduces cortical infarction and neurological deficits during ischemic stroke through potential maintenance of microvascular patency. Curr Neurovasc Res 2016;13:277–282. [DOI] [PubMed] [Google Scholar]
- 19. Zhu Z, Fu Y, Tian D, et al. Combination of the immune modulator fingolimod with alteplase in acute ischemic stroke: a pilot trial. Circulation 2015;132:1104–1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Fu Y, Hao J, Zhang N, et al. Fingolimod for the treatment of intracerebral hemorrhage: a 2‐arm proof‐of‐concept study. JAMA Neurol 2014;71:1092–1101. [DOI] [PubMed] [Google Scholar]
- 21. Fu Y, Zhang N, Ren L, et al. Impact of an immune modulator fingolimod on acute ischemic stroke. Proc Natl Acad Sci U S A 2014;111:18315–18320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Zhang J, Shi K, Li Z, et al. Organ‐ and cell‐specific immune responses are associated with the outcomes of intracerebral hemorrhage. FASEB J 2018;32:220–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zhang S, Lai Y, Ding X, et al. Absent filling of ipsilateral superficial middle cerebral vein is associated with poor outcome after reperfusion therapy. Stroke 2017;48:907–914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Menon BK, d'Esterre CD, Qazi EM, et al. Multiphase CT angiography: a new tool for the imaging triage of patients with acute ischemic stroke. Radiology 2015;275:510–520. [DOI] [PubMed] [Google Scholar]
- 25. Yu Y, Han Q, Ding X, et al. Defining core and penumbra in ischemic stroke: a voxel‐ and volume‐based analysis of whole brain CT perfusion. Sci Rep 2016;6:20932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Davis SM, Donnan GA, Parsons MW, et al. Effects of alteplase beyond 3 h after stroke in the Echoplanar Imaging Thrombolytic Evaluation Trial (EPITHET): a placebo‐controlled randomised trial. Lancet Neurol 2008;7:299–309. [DOI] [PubMed] [Google Scholar]
- 27. Goyal M, Demchuk AM, Menon BK, et al. Randomized assessment of rapid endovascular treatment of ischemic stroke. N Engl J Med 2015;372:1019–1030. [DOI] [PubMed] [Google Scholar]
- 28. Albers GW, von Kummer R, Truelsen T, et al. Safety and efficacy of desmoteplase given 3‐9 h after ischaemic stroke in patients with occlusion or high‐grade stenosis in major cerebral arteries (DIAS‐3): a double‐blind, randomised, placebo‐controlled phase 3 trial. Lancet Neurol 2015;14:575–584. [DOI] [PubMed] [Google Scholar]
- 29. Huang X, Cheripelli BK, Lloyd SM, et al. Alteplase versus tenecteplase for thrombolysis after ischaemic stroke (ATTEST): a phase 2, randomised, open‐label, blinded endpoint study. Lancet Neurol 2015;14:368–376. [DOI] [PubMed] [Google Scholar]
- 30. Cho TH, Nighoghossian N, Mikkelsen IK, et al. Reperfusion within 6 hours outperforms recanalization in predicting penumbra salvage, lesion growth, final infarct, and clinical outcome. Stroke 2015;46:1582–1589. [DOI] [PubMed] [Google Scholar]
- 31. Leira EC, Savitz SI. In the era of thrombectomy, let us also protect the majority of patients with stroke who only require medical treatment! Stroke 2018;49:1538–1540. [DOI] [PubMed] [Google Scholar]
- 32. Parsons M, Spratt N, Bivard A, et al. A randomized trial of tenecteplase versus alteplase for acute ischemic stroke. N Engl J Med 2012;366:1099–1107. [DOI] [PubMed] [Google Scholar]
- 33. Ma H, Parsons MW, Christensen S, et al. A multicentre, randomized, double‐blinded, placebo‐controlled phase III study to investigate EXtending the time for Thrombolysis in Emergency Neurological Deficits (EXTEND). Int J Stroke 2012;7:74–80. [DOI] [PubMed] [Google Scholar]
- 34. Amiri H, Bluhmki E, Bendszus M, et al. European Cooperative Acute Stroke Study‐4: extending the time for thrombolysis in emergency neurological deficits ECASS‐4: ExTEND. Int J Stroke 2016;11:260–267. [DOI] [PubMed] [Google Scholar]
- 35. Wardlaw JM, von Kummer R, Carpenter T, et al. Protocol for the perfusion and angiography imaging sub‐study of the Third International Stroke Trial (IST‐3) of alteplase treatment within six‐hours of acute ischemic stroke. Int J Stroke 2015;10:956–968. [DOI] [PubMed] [Google Scholar]
- 36. De Silva DA, Brekenfeld C, Ebinger M, et al. The benefits of intravenous thrombolysis relate to the site of baseline arterial occlusion in the Echoplanar Imaging Thrombolytic Evaluation Trial (EPITHET). Stroke 2010;41:295–299. [DOI] [PubMed] [Google Scholar]
- 37. Zhang S, Zhou Y, Zhang R, et al. Rationale and design of combination of an immune modulator Fingolimod with Alteplase bridging with Mechanical Thrombectomy in Acute Ischemic Stroke (FAMTAIS) trial. Int J Stroke 2017;12:906–909. [DOI] [PubMed] [Google Scholar]


