Abstract
Human T helper 17 (TH17) cells regulate host defense, autoimmunity, and tumor immunity. Although cytokines that control human TH17 cell development have been identified, the costimulatory molecules important for TH17 cell generation are unknown. Here, we found that the inducible costimulator (ICOS) was critical for the differentiation and expansion of human TH17 cells. Human cord blood contained a subset of CD161+CD4+T cells that were recent emigrants from the thymus, expressed ICOS constitutively, and were imprinted as TH17 cells through ICOS signaling. ICOS stimulation induced c-MAF, RORC2, and T-bet expression in these cells, leading to increased secretion of interleukin-21 (IL-21), IL-17, and interferon-γ (IFN-γ) compared with cells stimulated with CD28. Conversely, CD28 ligation abrogated ICOS costimulation, dampening RORC2 expression while promoting the expression of the aryl hydrocarbon receptor, which led to reduced secretion of IL-17 and enhanced production of IL-22 compared with cells stimulated with ICOS. Moreover, ICOS promoted the robust expansion of IL-17+IFN-γ+ human T cells, and the antitumor activity of these cells after adoptive transfer into mice bearing large human tumors was superior to that of cells expanded with CD28. The therapeutic effectiveness of ICOS-expanded cells was associated with enhanced functionality and engraftment in vivo. These findings reveal a vital role for ICOS signaling in the generation and maintenance of human TH17 cells and suggest that components of this pathway could be therapeutically targeted to treat cancer or chronic infection and, conversely, that interruption of this pathway may have utility in multiple sclerosis and other autoimmune syndromes. These findings have provided the rationale for designing new clinical trials for tumor immunotherapy.
INTRODUCTION
CD4+ T cells are important in regulating immunity to pathogens, allergic responses, asthma, and immunity to self or tumor tissues (1–3). Depending on the microenvironmental cues present, naїve CD4+ T cells may differentiate into one of several T helper (TH) cell lineages, including TH1, TH2, TH17, TH22, and regulatory T (Treg) cells (4, 5). TH1 and TH2 cells are effector cells that express T-bet and GATA-3, respectively (1). In contrast, Treg cells suppress effector T cell functions and are essential for regulating autoimmune responses (6), and the recently described TH22 cells secrete interleukin-22 (IL-22) and might be a subset of skin-homing cells responsible for inflammation (7, 8). TH17 cells augment host defense, have a major role in mucosal immunity, enhance a number of autoimmune diseases, and release cytokines, including IL-17A and IL-17F (9). The contribution of TH17 cells to tumor immunity varies, showing the potential for both antitumorigenic and protumorigenic activity (10). Therefore, identification of the mechanisms that control TH17 responses is essential to understand tumor immunity.
The functions of cytokines [for example, transforming growth factor-β (TGF-β), IL-6, IL-1β, IL-21, and IL-23] and transcription factors (such as RORC2 and RORα) in human TH17 cell development are distinct from TH1 and TH2 effector cells (11–14). Further, natural agonists for the aryl hydrocarbon receptor (AHR) augment murine TH17 cell differentiation (15). However, the specific costimulatory pathways that may influence TH17 generation and stability remain to be elucidated.
Antigen-specific and antigen-nonspecific costimulatory signals from antigen-presenting cells (APCs) are necessary for the activation, differentiation, and function of T lymphocytes (16). CD28 is considered to be the primary co-signaling molecule on CD4+ T cells because of its early expression, and it is often used to generate IL-17-producing lymphocytes (12–14, 17–19). However, in addition to CD28, signaling via the inducible costimulator (ICOS, also called CD278) is required for optimal cytokine secretion, because both molecules are essential for optimal IL-17A secretion by murine TH17 cells (20). Recent findings in murine models have revealed that ICOS amplifies TH17 responses by inducing the expression of the transcription factor c-MAF and therefore transactivating IL-21 production (21). Although both CD28 and ICOS are important for the generation of murine TH17 cells, their particular roles in regulating key genes in human TH17 cells remain to be identified.
Here, we show that the nature of costimulation during CD4+ T cell activation critically regulates human TH17 cell differentiation. ICOS, but not CD28, is necessary for optimal expansion and function of human TH17 cells. Surprisingly, CD28 ligation abrogated the effects of ICOS costimulation. These data are surprising given that CD28 is often used to expand human TH17 cells, and they raise the possibility that the full inflammatory potential of human TH17 cell in vivo has not been fully reflected by previous in vitro studies. Of clinical relevance, genetically reprogrammed human TH17 cells expanded with ICOS mediated superior regression of human tumors compared to cells expanded with CD28. These findings reveal a key role for ICOS signaling in human TH17 cell development and suggest new therapeutic approaches.
RESULTS
ICOS and CD28 have distinct effects on human CD4+ T cell subsets
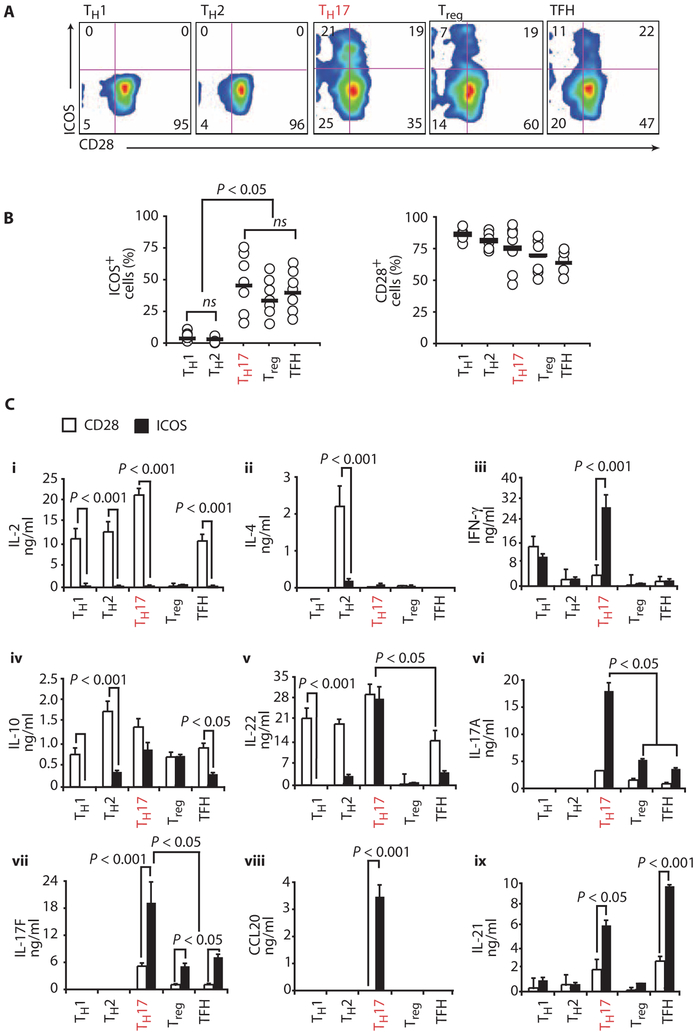
ICOS was originally identified as a molecule expressed on T cells only after activation (22). Constitutive expression of ICOS was later found on a subpopulation of resting murine effector memory T cells, Treg cells, and follicular helper T (TFH) cells (23–25). Given the recent identification of human TH17 cells, we sought to examine whether ICOS was also constitutively expressed on these cells. We sorted resting peripheral blood CD4+ T cells into various subsets based on their expression of chemokine receptors and other cell surface molecules. This strategy yielded TH1 (CXCR3+CCR4−CCR6−), TH2 (CCR4+CXCR3−CCR6−), TH17 (CCR4+CCR6+), Treg (CD25+CD127lo), and TFH (CXCR5+CD45RO+) subsets (18, 26, 27). Surprisingly, ~40% of cells in the TH17 subset constitutively expressed ICOS, whereas the TH1 and TH2 subsets did not express ICOS (Fig. 1, A and B). As expected, Treg and TFH subsets constitutively expressed ICOS (23–25), whereas all subsets constitutively expressed CD28 at high levels (Fig. 1, A and B).
Fig. 1.
Distinct expression and function of ICOS and CD28 on human CD4+ T cell subsets. (A) The expression of ICOS and CD28 costimulatory molecules was assessed on resting human peripheral blood CD4+ T cell subsets, consisting of CXCR3+CCR4−CCR6+ TH1, CCR4+CXCR3−CCR6− TH2, CCR4+CCR6+ TH17, CD25+CD127loFoxP3+ Treg, and CXCR5+CD45RO+ TFH cells. (B) Flow cytometric quantification of ICOS and CD28 on different subsets from several normal donors (n = 7). Horizontal bars indicate mean. ns, not significant. (C) Cytokines IL-2 (i), IL-4 (ii), IFN-γ (iii), IL-10 (iv), IL-22 (v), IL-17A (vi), IL-17F (vii), CCL20 (viii), and IL-21 (ix) secreted from various sorted cells activated with antibodies to CD3/CD28 or CD3/ICOS beads and measured on day 3 by ELISA. Representative of three experiments. Statistics were corrected for multiple comparisons with the ANOVA Scheffé test. TFH, follicular helper T.
Given that human T cell subsets constitutively express varying amounts of ICOS and CD28, we sought to evaluate the functional effects of signaling via these particular molecules on each subset. Thus, subsets were sorted as described above and then stimulated with antibodies to CD3/CD28 or CD3/ICOS beads. IL-2, IL-4, interferon-γ (IFN-γ), IL-10, IL-22, IL-17A, IL-17F, CCL20, and IL-21 production was measured by enzyme-linked immunosorbent assay (ELISA) (Fig. 1C). As expected, all subsets except Treg cells secreted substantial amounts of IL-2 after CD28 costimulation (Fig. 1C, i). In contrast, ICOS costimulation did not trigger IL-2 secretion, corroborating our previous finding that CD28, but not ICOS, mediates IL-2 production by T cells (28, 29). Furthermore, CD28, but not ICOS, induced IL-4 production by TH2 cells (Fig. 1C, ii). IL-10 and IL-22 secretion was triggered by both CD28 and ICOS costimulation in a subset-specific manner, although in most subsets CD28 costimulation induced higher amounts of these cytokines (Fig. 1C, iv and v). In contrast, ICOS costimulation of TH17 cells resulted in significantly higher production of IL-17A, IL-17F, CCL20, and IL-21 compared with CD28 costimulation (Fig. 1C, vi to ix). Notably, ICOS-stimulated TH17 cells also produced greater amounts of IFN-γ than CD28-stimulated TH1 cells, a subset reported to be a dominant source of IFN-γ secretion (Fig. 1C, iii). Although ICOS costimulation augments TH17 cell function, it is interesting that this signal did not amplify TH1 or TH2 cell function, likely because these cells lack ICOS.
ICOS drives human TH17 cell differentiation
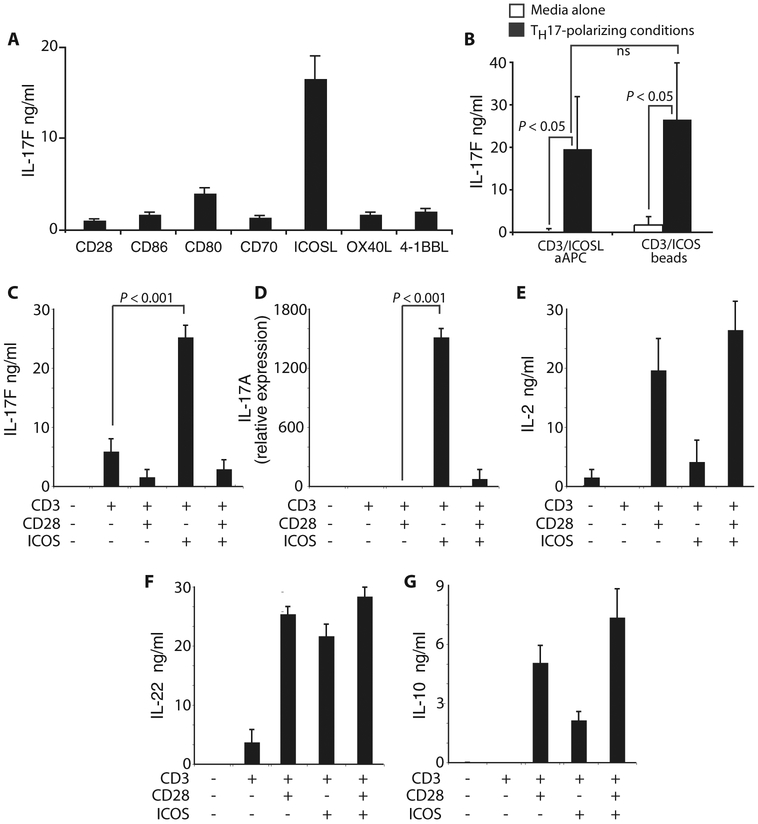
Costimulatory molecules play critical roles in initiating T cell responses (16, 30), but their individual influence on human TH17 functionality remains unknown. To understand their respective impact on TH17 function, we activated peripheral blood CD4+ T cells with OKT3-loaded artificial APCs (aAPCs) engineered to express CD86, CD80, CD70, ICOSL, OX40L, or 4-1BBL and then cultured the cells in TH17-polarizing conditions (IL-6, IL-1β, IL-23, neutralizing IFN-γ, and neutralizing IL-4 antibodies in serum containing endogenous sources of TGF-β). Only ICOS costimulation reproducibly induced IL-17F secretion (Fig. 2A), supporting the notion that ICOS might play a unique role in human TH17 cell development.
Fig. 2.
ICOS augments cytokine production by human TH17 cells. (A) IL-17F production was assessed by peripheral blood CD4+ T cells differentiated to a TH17 phenotype with TH17-polarizing conditions (IL-6, IL-1β, IL-23, neutralizing IFN-γ, and neutralizing IL-4 antibodies in serum containing TGF-β, a cytokine required for inducing TH17 differentiation) and activated with either aAPCs expressing CD86, CD80, CD70, ICOSL, OX40L, or 4-1BBL or with beads bearing antibodies to CD3 and CD28 on day 3 by ELISA. (B) IL-17F production was assessed by peripheral blood CD4+ T cells cultured with or without TH17-polarizing conditions and activated with aAPC engineered to express ICOSL or with beads bearing antibodies to CD3/ICOS on day 3. (C to G) Using ELISA or reverse transcription PCR (RT-PCR), we measured (C) IL-17F, (D) IL-17A, (E) IL-2, (F) IL-22, and (G) IL-10 secretion or expression by TH17-polarized CD4+ T cells activated with beads bearing antibodies to CD3, CD28, and/or ICOS on day 3. Representative of two experiments.
We next asked whether ICOS engagement alone might be sufficient to induce IL-17F secretion by bulk unpolarized CD4+ T cells. We found that ICOS engagement was not sufficient to promote significant IL-17F production in the absence of TH17-polarizing conditions. However, in the presence of TH17-polarizing conditions, ICOS induced IL-17F secretion from bulk CD4+ T cells (Fig. 2B). Delivery of the ICOS signal via either beads or aAPCs was equally effective at inducing IL-17F secretion (Fig. 2B). Thus, although ICOS was sufficient to augment IL-17F secretion in already differentiated CCR4+CCR6+ TH17 cells (Fig. 1C), it was not capable of inducing IL-17F secretion by bulk CD4+ T cells in the absence of TH17-polarizing conditions (Fig. 2B). This inability to detect IL-17F may be, in part, due to the low frequency of TH17 cells in bulk CD4+ T cells.
ICOS and CD28 costimulation are both required for the differentiation of murine TH17 cells (20). Therefore, we suspected that they would also augment human TH17 function in combination. Conversely, the addition of CD28 with ICOS markedly reduced IL-17F secretion (Fig. 2C) and IL-17A messenger RNA (mRNA) expression (Fig. 2D). Yet, combining these signals did not exert a similar “veto effect” on IL-2, IL-10, or IL-22 secretion (Fig. 2, E to G). These data are surprising given that CD28 is often used to expand human TH17 cells.
ICOS expands the population of IL-17A+IFN-γ+ human CD4+ T cells
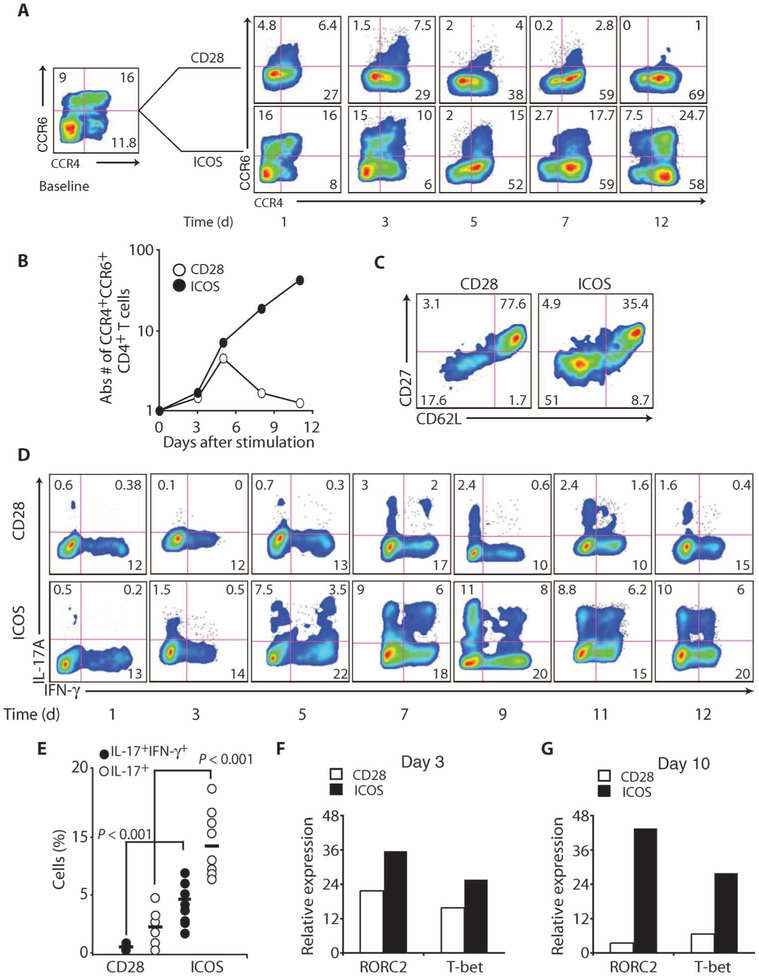
Although ICOS augmented human TH17 cell function at early time points (day 3 after activation), it remained unclear whether ICOS supported their long-term development. To address this question, we measured the frequency and absolute numbers of CCR4+CCR6+CD4+ T cells throughout their primary expansion. At baseline, the frequency of CCR4+CCR6+CD4+ T cells was ~16% (Fig. 3A). However, a progressive decrease in the frequency of these cells was observed in the CD28-costimulated culture. In contrast, the frequency of CCR4+CCR6+CD4+ T cells was stable, and even increased slightly, in the ICOS-costimulated culture. The selective outgrowth of these cells by ICOS was apparent when their absolute numbers were compared to those expanded with CD28 (Fig. 3B). In the ICOS-stimulated culture, the number of CCR4+CCR6+CD4+ T cells increased by more than 30-fold, whereas in the CD28-stimulated culture, their number increased for 5 days and then returned to baseline. Cultures driven by CD28 had a greater frequency of cells with a central memory–like (CD62LhiCD27hi) phenotype, as reported (31), whereas ICOS-driven cultures contained a higher frequency of cells with an effector memory–like (CD62LloCD27lo) phenotype (Fig 3C).
Fig. 3.
ICOS is critical for the expansion of human TH17 cells. (A and B) The frequency (A) and absolute number (B) of CCR4+CCR6+CD4+ T cells over time were assessed by flow cytometry from peripheral blood CD4+ T cells cultured in TH17-polarizing conditions and activated with antibodies to CD3/CD28 or CD3/ICOS beads. (C) CD27 and CD62L expression was measured on day 10 on these cells with flow cytometry. (D) On the days indicated, CD28- or ICOS-engaged TH17-polarized CD4+ T cells were stimulated with PMA-ionomycin and the frequency of cells secreting IL-17A and IFN-γ was assessed via flow cytometry. (E) The frequency of CD28- or ICOS-engaged TH17-polarized cells coproducing IL-17A and/or IFN-γ was determined at the end of their primary expansion (ranging from days 9 to 14) in several different normal donors (n = 8). (F and G) RORC2 and T-bet expression in these treated cells was measured with RT-PCR on days 3 and 10. Representative of two to three experiments.
We next evaluated the effects of CD28 or ICOS on human TH17 cell function over time. In cultures costimulated with CD28, TH17-polarized CD4+ T cells produced IL-17A after the first 5 to 7 days of expansion (Fig. 3D), consistent with previous reports. However, the frequency of CD28-engaged TH17-polarized cells producing IL-17A or both IL-17A and IFN-γ declined nearly to baseline levels by the end of their primary expansion. In contrast, the frequency of these cells increased over time in ICOS-costimulated cultures (Fig. 3D), a finding reproduced in several independent cultures. Cells engaged with ICOS coexpressed both transcription factors RORC2 and T-bet (Fig. 3, F and G), master regulators of TH17 and TH1 differentiation, at greater mRNA concentrations than cells engaged with CD28 over time. Thus, ICOS expands the population of IL-17A+IFN-γ+CD4+ T cells (Fig. 3E) and this correlates to induction of RORC2 and T-bet.
ICOS and CD28 have distinct roles in development of TH17 cells derived from cord blood
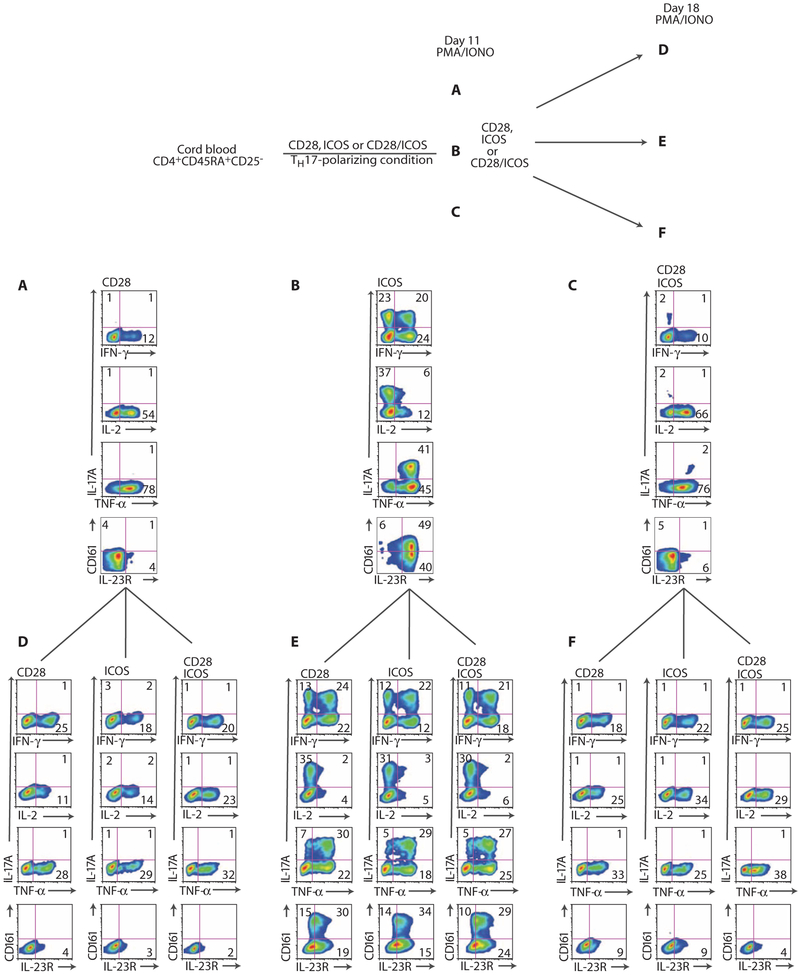
The above data indicated that ICOS preferentially expands effector human TH17 cells, but these data did not discern whether ICOS supports their development from naїve CD4+ T cells. Bauquet and coworkers reported that ICOS was crucial for the expansion but not the development of murine TH17 cells (21). Thus, we sought to determine whether naїve CD4+ T cells preferentially differentiate into TH17 cells via ICOS signaling. To test this, we sorted naїve CD45RA+CD25−CD4+ T cells from umbilical cord blood (UCB), cultured them in TH17-polarizing conditions, and activated them with an antibody to CD3 beads bearing antibodies to CD28 and/or ICOS. The function and phenotype of the cultures were assessed after primary (day 11) and secondary (day 18) stimulation (Fig. 4 scheme). IL-17A, IFN-γ, IL-2, and tumor necrosis factor–α (TNF-α) were measured after phorbol 12-myristate 13-acetate (PMA)–ionomycin activation. We found that >40% of cells engaged with ICOS produced IL-17A alone or IFN-γ alone and that ~20% of ICOS-engaged cells secreted both cytokines. In contrast, few cells engaged with CD28 produced IL-17A (Fig. 4, A and B). CD28 was indeed functional under these conditions because ~10% of these cells produced IFN-γ and >50% of these cells produced IL-2 after CD28 or CD28 plus ICOS costimulation (Fig. 4, A and C). Yet, only ~10% of cells secreted IL-2 after ICOS costimulation alone (Fig. 4B). Combining CD28 with ICOS costimulation prevented IL-17A production, and IFN-γ was produced by these cells at similar levels to CD28 stimulation alone (Fig. 4C). Primary engagement of cells with ICOS but not CD28 induced substantial TNF-α and IL-17A coexpression. CD161 expression was assessed as well, because human TH17 cells originate from CD161+CD4+ T cell precursors in UCB (32). Nearly half of cells engaged with ICOS coexpressed CD161 and IL-23 receptor (IL-23R) (Fig. 4B), whereas <5% of cells engaged with CD28 or CD28 plus ICOS were IL-23R– and CD161-positive (Fig. 4, A and C) and resting CD4+CD45RA+CD25− T cells contain <0.5% of these cells (fig. S1). Examination of cells after secondary expansion revealed that cells originally stimulated with ICOS continued to secrete high amounts of IL-17A, IFN-γ, and TNF-α, and this was independent of the mode of secondary costimulation (Fig. 4E). Likewise, ~30% of these cells continued to coexpress IL-23R and CD161. However, virtually no UCB TH17-polarized cells initially stimulated with CD28 or with CD28 plus ICOS secreted IL-17A, even after a restimulation with ICOS (Fig. 4, D and F). Thus, CD28 costimulation does not block IL-17A secretion after primary induction by unopposed ICOS costimulation (Fig. 4, B and E). These data suggest an important role for ICOS in programming TH17 development from naїve human UCB CD4+ T cells.
Fig. 4.
ICOS drives rapid TH17 cell differentiation from naive UCB CD4+ T cells. (A to C) UCB CD45RA+CD25−CD4+ T cells were cultured with TH17-polarizing conditions and expanded with antibodies to CD3/CD28, CD3/ICOS, or CD3/CD28/ICOS beads. Starting on day 3, IL-2 (50 lU/ml) was added to the cultures. Cultures were stimulated with PMA-ionomycin (IONO) and the intracellular expression of IL-17A, IFN-γ, IL-2, and TNF-α and the extracellular expression of IL-23R and CD161 were assessed on day 11. Cells from (A) to (C) were reactivated with antibodies to CD3-coupled beads bearing antibodies to CD28 and/or ICOS. (D to F) Cultures were restimulated with PMA-ionomycin and the intracellular expression of IL-17A, IFN-γ, IL-2, and TNF-α and the extracellular expression of IL-23R and CD161 were assessed on day 18. Representative of two experiments.
ICOS augments human TH17 function by inducing c-MAF and IL-21
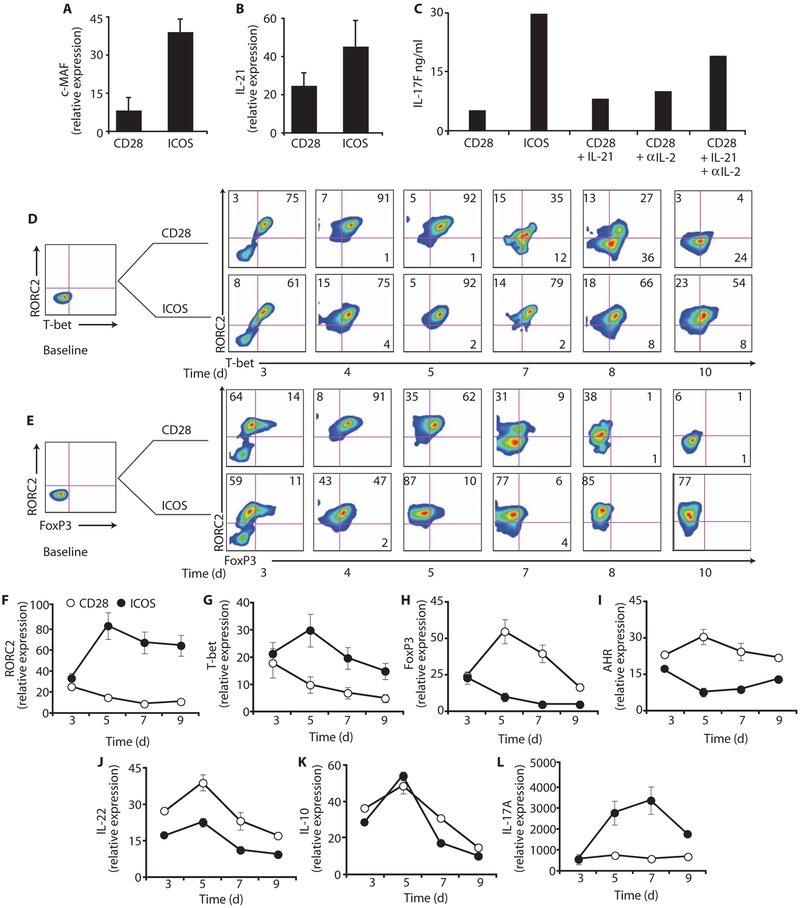
We next investigated the mechanisms underlying enhanced human TH17 cell functionality via ICOS. In mice, ICOS induces the transcription factor c-MAF, which, in turn, transactivates IL-21 and augments TH17 function (21). We investigated whether ICOS also induces c-MAF in human TH17 cells, given that ICOS increases IL-21 secretion (Fig. 1C, ix). Human UCB CD4+ T cells polarized toward a TH17 phenotype expressed considerably higher mRNA concentrations of c-MAF and IL-21 upon ICOS versus CD28 costimulation (Fig. 5, A and B). Similar results were observed in peripheral blood human TH17 cells (fig. S2). Thus, ICOS induced greater amounts of c-MAF expression than CD28, corresponding with increased IL-21 expression by ICOS-stimulated human TH17 cells.
Fig. 5.
CD28 and ICOS differentially regulate c-MAF, RORC2, and T-bet expression in UCB TH17 cells. UCB CD4+T cells were cultured in TH17-polarizing conditions and expanded with antibodies to CD3/CD28 or CD3/ICOS beads. IL-2 (50 IU/ml) was added on day 3. (A and B) On day 5, mRNA expression of c-MAF and IL-21 in CD28- or ICOS-stimulated cells was measured by RT-PCR. (C) On day 5, IL-17F production in CD28-stimulated cells cultured with exogenous IL-21 and IL-2 neutralization was measured by ELISA. (D to L) On the days indicated, RORC2, T-bet, FoxP3, AHR, IL-22, IL-10, and IL-17A production in CD28- or ICOS-stimulated cells was measured by flow cytometry and RT-PCR. Representative of two to three experiments.
We hypothesized that IL-21 induced by ICOS was partially responsible for enhanced human TH17 cell functionality. Thus, we assessed whether adding exogenous IL-21 to CD28-stimulated TH17-polarized UCB CD4+ T cells would increase their potential to secrete IL-17F. Consistent with previous studies (13), adding IL-21 to CD28-stimulated TH17-polarized UCB cells modestly increased their capacity to secrete IL-17F but not to the level attained by ICOS-stimulated TH17-polarized UCB cells (Fig. 5C). Given that TH17 cells costimulated with CD28 secrete significantly higher amounts of IL-2 than those stimulated with ICOS, we hypothesized that IL-2 might be responsible for the reduced functionality observed in CD28-stimulated TH17-polarized UCB cells. Indeed, IL-17F production was increased in the cultures where IL-2 was neutralized. Furthermore, exogenous IL-21 together with IL-2 neutralization in the culture of CD28-stimulated TH17-polarized UCB cells further increased IL-17F production, but it still did not induce IL-17F secretion to a level comparable to that elicited by ICOS stimulation (Fig. 5C). Thus, in addition to c-MAF–mediated IL-21 production, other factors are likely involved in mediating the ICOS-enhanced function of human TH17-polarized UCB cells.
ICOS induces RORC2 expression
To better understand the mechanisms underlying how ICOS signaling augmented the functionality of human TH17 cells, we investigated how ICOS regulates the cell expression of RORC2 (RORγt), T-bet (Tbx21), and FoxP3, master regulators of TH17, TH1, and Treg cells (1), respectively. Thus, RORC2, T-bet, and FoxP3 were measured in naїve UCB CD25−CD4+ T cells cultured in TH17-polarizing conditions over time via flow cytometry. At baseline, the cells expressed virtually no RORC2, T-bet, or FoxP3; there was a transient activation-associated increase in their expression in each culture at 3 to 5 days after stimulation (Fig. 5, D and E). However, by the end of their primary expansion, we found that >75% of ICOS-stimulated cells expressed RORC2 (Fig. 5, D and E, days 7 to 10). In contrast, the frequency of CD28-expanded cells expressing RORC2, T-bet, and FoxP3 progressively declined (Fig. 5, D and E). Likewise, ICOS induced greater mRNA expression of RORC2 and T-bet than CD28 (Fig. 5, F and G), whereas CD28 induced greater yet transient mRNA expression of FoxP3 than ICOS in these cells (Fig. 5H). Similar to peripheral blood data (Fig. 2F and fig. S3), CD28 induced higher expression of the AHR transcripts than ICOS (Fig. 5I), likely resulting in their heightened production of IL-22 (Fig. 5J). These data are consistent with findings in mice showing that AHR correlates with IL-22 production by T cells (15, 33). IL-10 expression was comparable in cells stimulated with either CD28 or ICOS (Fig. 5K), whereas IL-17A expression was significantly higher in cells stimulated with ICOS versus CD28 over time (Fig. 5L). RORC2 transcripts were stably induced at high amounts throughout the culture compared to T-bet and FoxP3 transcripts in cells stimulated with ICOS (Fig. 5, F to H).
We suspected that the amounts of IL-17A with CD28 costimulation might be low because the cells were differentiated in serum without the addition of TGF-β. Indeed, titrating TGF-β into the culture over a 3-log10 range of concentration increased the amount of IL-17A produced by TH17-polarized CD4+ T cells expanded with the CD28 signal but not to the amounts reached by ICOS-stimulated cells (fig. S4). These data underscore the notion that CD28-costimulated T cells are composed of TH17 cells that have not reached their full inflammatory potential. Further, they reveal the importance of the availability of TGF-β in the microenvironment as well as CD28 “veto signaling” (Fig. 2, C and D), which have the potential to regulate the inflammatory potential of TH17 cells.
UCB CD161+CD4+ T cells constitutively express ICOS
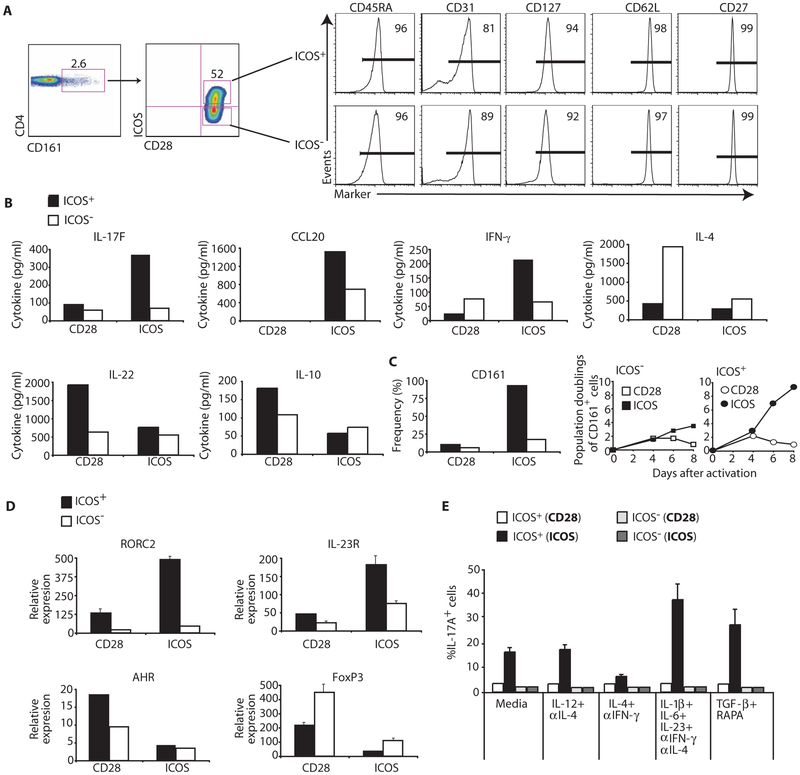
Given that TH17 cells originate from a CD161+CD4+UCB T cell precursor (32) and that ICOS is critical for augmenting their function, we investigated whether these cells express ICOS constitutively. Similar to peripheral blood CCR4+CCR6+CD4+ TH17 cells (Fig. 1, A and B), ~50% of resting CD161+CD4+ cord blood T cells expressed ICOS (Fig. 6A). Thus, we investigated whether CD161+CD4+ T cells that constitutively express ICOS were phenotypically different from ICOS−CD161+CD4+ T cells. Given that ICOS+ cells from peripheral blood are largely effector memory cells, we hypothesized that ICOS+CD161+CD4+ cord blood T cells would be a more differentiated subset than ICOS−CD161+CD4+ T cells. Unexpectedly, ICOS+CD161+CD4+ and ICOS−CD161+CD4+ T cells shared a similar naїve phenotype (Fig. 6A), as indicated by comparable high expression of CD45RA, CD127, CD62L, and CD27, and bright expression of CD31, which is typical of recent thymic emigrants (34).
Fig. 6.
Human TH17 cells originate from ICOS+CD161+CD4+T cell precursors. (A) CD45RA, CD31, CD127, CD62L, and CD27 expression was assessed on ICOS+CD161+CD4+ and ICOS−CD161+CD4+ T cells from the UCB via flow cytometry. (B) IL-17F, CCL20, IFN-γ, IL-4, IL-22, and IL-10 secretion by sorted ICOS+CD161+CD4+ and ICOS−CD161+CD4+ T cells cultured with TH17-polarizing conditions and expanded with antibodies to CD3/CD28 or CD3/ICOS beads was assessed on day 4 by ELISA. (C) The frequency and absolute number of CD161+ cells cultured with TH17-polarizing conditions and expanded with antibodies to CD3/CD28- or CD3/ICOS-coated beads was determined on day 4 or on the days indicated, respectively. (D) RORC2, IL-23R, AHR, and FoxP3 mRNA expression in sorted ICOS+CD161+CD4+ and ICOS−CD161+CD4+ T cells cultured with TH17-polarizing conditions and expanded with antibodies to CD3/CD28- or CD3/ICOS-coated beads was assessed on day 7 by RT-PCR. (E) On day 7, ICOS+CD161+CD4+ and ICOS−CD161+CD4+ T cells cultured in media alone or in TH1-, TH2-, TH17-, and Treg-polarizing conditions and expanded with antibodies to CD3/CD28- or CD3/ICOS-coated beads were then stimulated with PMA-ionomycin, and IL-17A secretion was assessed by flow cytometry. Representative of two to three experiments.
ICOS+CD161+CD4+ T cells are imprinted as TH17 cells via ICOS signaling
We next investigated whether CD161+CD4+ cord blood T cells that express ICOS differentiate into human TH17 cells via ICOS signaling. Thus, we examined the function of ICOS+CD161+CD4+ versus ICOS−CD161+CD4+ T cells sorted from UCB that were stimulated with antibodies to either CD3/CD28 or CD3/ICOS beads under TH17-polarizing conditions. ICOS+CD161+CD4+ T cells secreted higher amounts of IL-17F, CCL20, and IFN-γ upon ICOS engagement compared to ICOS−CD161+CD4+ T cells (Fig. 6B). In contrast, CD28 engagement mediated slightly greater secretion of IL-10 and IL-22 by ICOS+CD161+CD4+ than by ICOS−CD161+CD4+ T cells. Further, CD28 engagement induced IL-4 secretion by ICOS−CD161+CD4+ T cells. Notably, ICOS but not CD28 engagement promoted the sustained expansion of ICOS+CD161+CD4+ T cells, as indicated via their greater frequency and overall yields (Fig. 6C).
It has been reported that CD161+CD4+ T cells constitutively express RORC2 and IL-23R and that TH17-polarizing conditions further up-regulate expression of these molecules (32). Given our findings, we hypothesized that CD161+CD4+ T cells that constitutively express ICOS would express higher mRNA amounts of RORC2 and IL-23R than ICOS−CD161+CD4+ T cells. Moreover, we suspected that ICOS engagement would further increase RORC2 and IL-23R mRNA expression in ICOS+CD161+CD4+ T cells. Indeed, resting ICOS+CD161+CD4+ UCB T cells expressed higher mRNA amounts of RORC2 and IL-23R than resting ICOS−CD161+CD4+ or bulk UCB T cells (fig. S5). Furthermore, ICOS engagement induced greater expression of RORC2 and IL-23R mRNA in ICOS+CD161+CD4+ versus ICOS−CD161+CD4+ T cells (Fig. 6D), corresponding with their increased IL-17F and CCL20 secretion (Fig. 6B). In contrast, CD28 engagement induced higher mRNA expression amounts of AHR in ICOS+CD161+CD4+ T cells (Fig. 6D), consistent with their enhanced IL-22 production (Fig. 6B). Thus, in addition to CD161, ICOS might be a surface marker for UCB CD4+ T cells that develop into TH17 cells.
Given that costimulation of ICOS+CD161+CD4+ T cells with ICOS specifically induced RORC2 and IL-17A, we hypothesized that these cells were imprinted as TH17 cells via the ICOS signal, and consequently, even in the presence of TH1-, TH2-, and Treg-polarizing conditions, these cells would continue to secrete IL-17A and resist differentiation into TH1, TH2, or Treg cells, respectively. To test this notion, we sorted ICOS+CD161+CD4+ and ICOS−CD161+CD4+ T cells, stimulated them with antibodies to CD3/CD28- or CD3/ICOS-coated beads, and then cultured them in media alone or in TH1-, TH2-, TH17-, and Treg-polarizing conditions. ICOS costimulation of ICOS+CD161+CD4+ T cells induced IL-17A secretion even under TH1-, TH2-, or Treg-polarizing conditions, although at varying amounts (Fig. 6E). In contrast, costimulation through CD28 induced modest amounts of IL-17A secretion, even in the presence of TH17-polarizing conditions (Fig. 6E). Conditions that polarize bulk UCB CD4+ T cells toward a TH1, TH2, TH17, and Treg cell phenotype were effective because they promoted IFN-γ, IL-4, IL-17A secretion, or FoxP3 expression, respectively (fig. S6). In contrast, ICOS costimulation of ICOS+CD161+CD4+ T cells was unable to elicit IL-4 secretion and failed to promote FoxP3 expression when cultured in conditions that fostered their TH2 or Treg development (fig. S6). Regardless of the T cell subset–polarizing conditions and the mode of costimulation, we found that less than 5% of ICOS−CD161+CD4+ T cells produced IL-17A (Fig. 6E). Thus, our results indicate that cells with the potential to differentiate into TH17 cells are largely confined to the ICOS+ subset of CD161+CD4+ UCB T cells and are rapidly imprinted as TH17 cells via ICOS signaling.
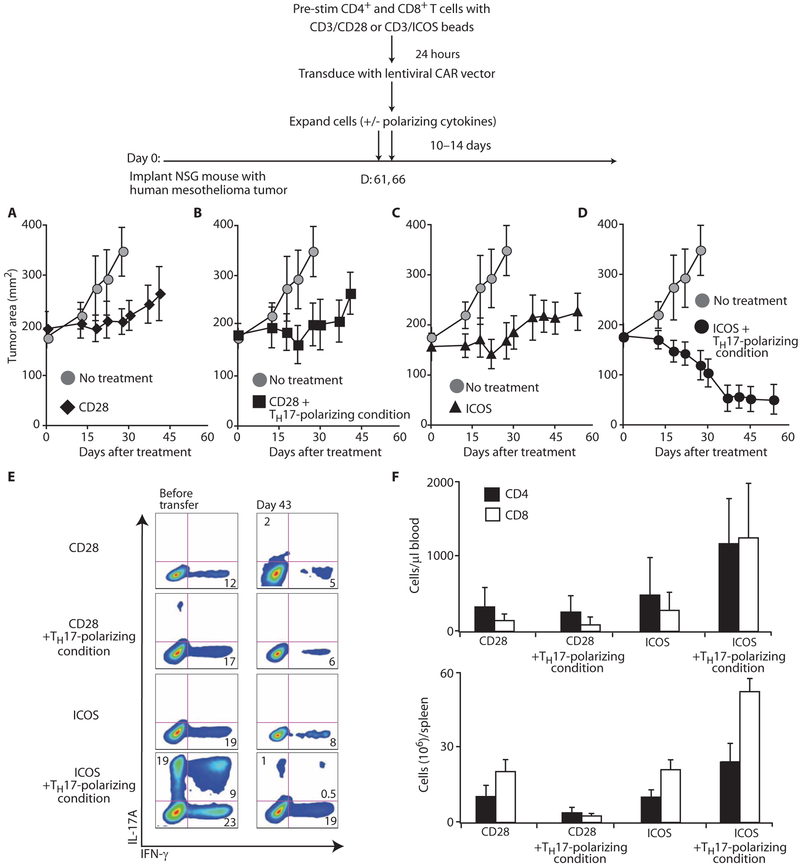
ICOS augments T cell–mediated tumor immunity
We have reported that genetically redirected peripheral blood T cells expanded with antibodies to CD3/CD28 beads mediate robust antitumor effects after infusion into mice bearing human tumor xenografts (35). Given our finding that ICOS costimulation in the presence of TH17-polarizing conditions generates IL-17A+IFN-γ+ T lymphocytes in vitro, we wished to investigate how these cells, upon genetic redirection, would affect the growth of human tumors. To test this question, we expanded bulk peripheral blood T cells with antibodies to CD3/CD28 or CD3/ICOS beads in the presence or absence of TH17-polarizing conditions and genetically modified them with a chimeric antigen receptor (CAR) to confer specificity for mesothelin-expressing tumors (Fig. 7 scheme). NOD/scid/IL-2Rγnull mice were injected in the flank with the human mesothelioma cell line M108 and were injected intratumorally with the redirected cells beginning on day 61 after tumor challenge. We found that mice treated with ICOS-stimulated T cells polarized with TH17 cytokines experienced superior tumor regression compared with all other treatment groups (P < 0.005; Fig. 7, D versus A to C). Only cells stimulated with ICOS in the presence of TH17-polarizing conditions were able to mediate regression of large tumors (Fig. 7D). Cells stimulated by CD28 alone or by CD28 plus TH17-polarizing conditions were able to slow tumor progression, but were unable to mediate long-lasting tumor regression (Fig. 7, A to C). The therapeutic effectiveness of polarized cells stimulated with ICOS may be a consequence of their enhanced IFN-γ secretion upon antigen recognition ex vivo (Fig. 7E) and increased engraftment in vivo (Fig. 7F). Our findings identify ICOS and its downstream signaling pathways as a target for the development of cancer immunotherapy to modify TH17 cell function and numbers.
Fig. 7.
ICOS augments T cell-mediated tumor immunity. As shown schematically, human CD4+ and CD8+ T cells were stimulated with antibodies to CD3/CD28 or CD3/ICOS beads and cultured with or without TH17-polarizing conditions. One day later, bead-activated T cells were genetically redirected with a CAR that binds mesothelin. After their primary expansion, the genetically redirected cells (two administrations, 8 × 106 cells total) were infused into mice bearing a large human mesothelin (M108) tumor preestablished for 61 days (n = 8 mice per group). (A to D) Tumor growth was measured in mice infused with genetically redirected cells expanded with the ICOS or CD28 signal with or without TH17-polarizing conditions. Tumor growth was analyzed with a linear mixed-effects model and by applying a conservative Bonferroni correction approach (mean ± SEM). (E) Redirected T cells were isolated from the mouse spleens (on day 43) and cultured with irradiated aAPCs bearing mesothelin. IL-17A and IFN-γ secretion was analyzed by flow cytometry 24 hours later. (F) The absolute number of CD4+ and CD8+ T cells was determined in the blood and spleen on days 21 and 43, respectively. Representative of two experiments.
DISCUSSION
Phylogenetic studies indicate that the co-signaling molecule ICOS arose as a duplication of CD28 and that this event was coincident with the appearance of high-affinity memory antibody responses (36). Although many aspects of the ICOS and CD28 paralogs are conserved, a number of important differences have emerged. For example, the expression pattern of human and mouse CD28 in thymus and peripheral T cells is considerably different (28, 37, 38). We have uncovered a difference between ICOS expression in human and mouse CD4+ T cells, where, unlike humans, ICOS is not expressed on recent thymic emigrants in the mouse (23). ICOS-deficient humans have few TFH cells (39) and impaired TH1, TH2, and TH17 responses (40), suggesting that ICOS signaling has nonredundant roles for the homeostasis of multiple human CD4+ T cell subsets. Our data suggest that some of these differences between mice and humans could be a result of the earlier expression of ICOS during lymphocyte ontogeny in humans than in mice.
Our data suggest that CD28 and ICOS ligands, in concert with the cytokine milieu, critically dictate the fate of TH17 cells. Previous studies have shown that CD28 costimulation can provide short-term expansion of TH17 cells, and our results are consistent with those findings. However, using an ICOS-based culture system, we have identified conditions that permit sustained expansion of human TH17 cells. Given that ICOSL is constitutively expressed in many tissues, and ICOSL overexpression can result in autoimmunity (41, 42), our findings raise a question as to how TH17 cell expansion is controlled. Our results may address this paradox in that CD28 ligands temper the growth and inflammatory potential of TH17 cells. These data are particularly interesting in light of recent data describing a new human T cell lineage called TH22 cells, which are characterized by their ability to produce IL-22 but nominal amounts of IL-17A and IFN-γ (7). Our data suggest that CD28 may transition TH17 cells into TH22 cells, whereas ICOS transitions them into TH1/TH17 cells. Additional experiments to understand the potential impact of CD28 signaling on TH22 cell development will be required. Our data support the idea that the fate of T cell subsets, particularly TH17 cells, appears more flexible in humans than previously appreciated (5).
There are several therapeutic implications from these findings. A number of autoimmune and inflammatory conditions are associated with increased TH17 cells and their associated cytokines. For example, skin lesions in psoriasis show substantial up-regulation of CCL20 and CCR6 (43). In multiple sclerosis, a subset of patients has disease that is dominated by TH17 cells, and this biomarker predicts the lack of response to subsequent therapy with IFN-β (44). The relative balance of APCs with ligands for ICOS and CD28 is likely to play a role in the homeostasis of pathogenic and regulatory TH17 cell populations. Thus, modulation of ICOS function may have therapeutic utility in certain autoimmune disorders.
TH17 cells can also promote antitumor immunity in mice and humans (10, 45, 46). For adoptive therapy, the use of defined cell culture conditions to control CD28 and ICOSL availability may permit the selective growth or depletion of TH17 cells to abrogate chronic inflammation or enhance antitumor immunity, as demonstrated here. We have shown that ICOS stimulation can be used to generate clinically relevant numbers of human TH17 lymphocytes with potent antitumor activities. New tumor immunotherapy clinical trials are currently being designed on the basis of the findings reported here that will test the antitumor effects of genetically reprogrammed TH17 cells.
MATERIALS AND METHODS
Cell purification
Blood samples were obtained from the Human Immunology Core of the University of Pennsylvania. Peripheral blood CD4+ T cells were negatively isolated and >95% pure adult subsets of TH1, TH2, TH17, Treg, and TFH CD4+ T cells were further purified as described (18, 26, 27).
T cell activation with beads or aAPCs
For stimulation, 1 × 106 CD4+ T cells were cultured with either 3 × 106 activating beads coated with antibodies to CD3, CD28, and/or ICOS or with 0.5 × 106 CD32-transduced aAPCs bearing CD80, CD86, CD70, ICOSL, OX40L, or 4-1BBL. The methods of aAPC generation and T cell expansion are described elsewhere (29, 47). Cultures were monitored for cell volume and enumerated via Coulter Multisizer 3 (Beckman Coulter).
Cell culture and TH1, TH2, TH17, and Treg cell polarization
Cells were cultivated in RPMI 1640 culture media as described previously in a 37°C and 5% CO2 incubator (37). For polarization experiments, cells were seeded with antibody-coated beads or aAPCs. IL-2 (50 to 100 IU/ml) was added at day 3 and media were replaced as described previously (47, 48). For TH17 cell polarization, as indicated, IL-1β (10 ng/ml), IL-6 (10 ng/ml), IL-23 (20 ng/ml), and neutralizing antibodies (10 μg/ml) against IL-4 and IFN-γ (eBioscience) were added at day 0 and maintained throughout the experiment. Experiments were conducted with fetal calf serum containing endogenous sources of TGF-β. In experiments indicated, IL-21 (25 ng/ml) (eBioscience) and an antibody to IL-2 (5 μg/ml) (R&D Systems) were added to TH17-polarized T cells. For TH1 cell polarization, IL-12 (5 ng/ml) and neutralizing antibodies against IL-4 (eBioscience) were added at day 0. For TH2 cell polarization, IL-4 (5 ng/ml) and neutralizing antibodies against IFN-γ (eBioscience) were added at day 0 and maintained throughout the experiment. For Treg cell polarization, TGF-β (5 ng/ml) and rapamycin (50 ng/ml) were added at day 0 and maintained throughout the experiment. Cells and supernatant were harvested at various days throughout short- and long-term primary and secondary cultures for intracellular staining and/or ELISA.
Real-time polymerase chain reaction
RNA was extracted with the RNAqueous isolation kit (Ambion), and then complementary DNA (cDNA) was transcribed with iScript cDNA Synthesis (Bio-Rad) and used as a template for Taqman polymerase chain reaction (PCR) from the specified samples. Expression of RORC2, Tbx21(T-bet), FoxP3, AHR, c-MAF, IL-17A, IL-21, and IL-23R was assessed with specific primers and probes (Applied Biosystems) via the Applied Biosystems 7500 Fast System. Gene expression was normalized to expression of the human gene β-actin. Relative quantitation was performed with unmanipulated CD4+ T cells as a reference.
Surface and intracellular staining
For intracellular cytokine staining, cells were incubated for 5 hours with PMA (20 ng/ml) (Sigma) and ionomycin (2 μg/m1) (Sigma) and GolgiStop (BD). Surface staining was performed, followed by intracellular staining, as described previously, with an LSR II (BD Biosciences) flow cytometer and FlowJo software (Tree Star Inc.). RORC2, T-bet, and FoxP3 were stained with FoxP3 staining buffers (eBioscience).
Mice
The University of Pennsylvania Institutional Animal Care and Use Committee approved all animal experiments. NSG mice were purchased from The Jackson Laboratory and bred in the vivarium at the University of Pennsylvania. The mice were housed under specific pathogen-free conditions in microisolator cages and given ad libitum access to autoclaved food and acidified water.
In vivo assessment of anti-mesothelin CAR T cells
A chimeric anti-mesothelin single-chain variable fragment (scFv) fusion protein containing the 4-1BB and T cell receptor Z (TCRZ) signaling domains was generated as described previously (35). M108 xenograft tumors were established as described previously (35) in NSG mice before adoptive transfer of TH17 cells. Tumors were measured with calipers, and their area was calculated by multiplying the length by the width.
Statistical analysis
Tumor growth data were analyzed by life table methods with a linear mixed-effects model via a conservative Bonferroni correction approach. Values of P < 0.005 were considered statistically significant. Other data were analyzed by analysis of variance (ANOVA) Scheffé test or Student’s t test. Values of P = 0.05 were considered statistically significant.
SUPPLEMENTARY MATERIAL
Supplemental Figure 1. UCB CD45RA+CD25−CD4+ T cells contain few CD161+IL−23R+ cells. The expression of CD161 and IL-23R surface markers on CD45RA+CD25−CD4+ T cells was assessed on human umbilical cord blood cells using flow cytometry. Data representative of 2 experiments.
Supplemental Figure 2. ICOS induces c-MAF and IL-21. PB CD4+ T cells were cultured in TH17 polarizing conditions (IL-1β, IL-6, IL-23, plus neutralizing anti-IFN-γ and anti-IL-4) and activated with anti-CD3 beads bearing either anti-CD28 or anti-ICOS antibodies. After their primary expansion, their c-MAF and IL-21 expression mRNA levels was assessed by RT-PCR. Data representative of 2 experiments.
Supplemental Figure 3. CD28 induces expression of the aryl hydrocarbon receptor. PB CD4+ T cells were programmed toward a TH17 phenotype and activated with anti-CD3 beads bearing either anti-CD28 or anti-ICOS antibodies. After their primary expansion, their mRNA expression level of AHR relative to β-actin was assessed by RT-PCR. Data representative of 2 experiments.
Supplemental Figure 4. Exogenous TGF-β augments the inflammatory potential of human TH17 cells. PB CD4+T cells were programmed toward a TH17 phenotype and activated with anti-CD3 beads bearing either anti-CD28 or 2 anti-ICOS antibodies in media containing serum and the indicated supplemental TGF-β (from 0.1-10 ng/ml) was added to the culture on day 1. IL-17A secretion by cells was measured on day 5 post-activation by ELISA. Data representative of 2 experiments.
Supplemental Figure 5. ICOS+CD161+CD4+ T cells from UCB constitutively express RORC2 and IL23R. CD4+, ICOS+CD161+CD4+ and ICOS−CD161+CD4+ T cells were sorted and their mRNA expression level of RORC2 and IL-23R relative to β-actin was measured by RT-PCR. Data representative of 2 experiments.
Supplemental Figure 6. ICOS+CD161+CD4+T cells are imprinted as TH17 cells. CD4+ and ICOS+CD161+CD4+T cells from UCB were sorted and cultured in various polarizing conditions as indicated. The frequency of IFN-γ+(A), IL-4+(B), IL-17A+(C) or FoxP3+(D) cells was measured after their primary expansion with anti-CD3 beads bearing anti-CD28 or anti-ICOS antibodies. As a control, companion control cultures of bulk UCB CD4 T cells were stimulated with anti-CD3/CD28 beads. Cytokines and FoxP3 were measured by flow cytometry or ELISA on day 7 of culture post-stimulation with PMA/ionomycin. Data representative of 2 experiments.
Acknowledgments:
We thank S. McGettigan, K. Haines, A. Mexas, M. Frigault, S.Guedan, and M. Eghbal for reading this manuscript and/or providing technical assistance. This work is dedicated to the remembrance of R.G.C.
Funding: This work was supported by NIH grants 5R01CA105216, 1R01CA120409, and 5P01CA066726 (to C.H.J.) and by NIH training grant (to C.M.P.).
Footnotes
Competing interests: C.M.P. and J.L.R. have filed a patent for the expansion of TH17 cells using ICOSL-expressing aAPCs based on work reported in this article.
REFERENCES
- 1.Zhu J, Yamane H, Paul WE, Differentiation of effector CD4 T cell populations. Annu. Rev. Immunol 28, 445–489 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Muranski P, Restifo NP, Adoptive immunotherapy of cancer using CD4+ T cells. Curr. Opin. Immunol 21, 200–208 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhu J, Paul WE, CD4 T cells: Fates, functions, and faults. Blood 112, 1557–1569 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.O’Shea JJ, Paul WE, Mechanisms underlying lineage commitment and plasticity of helper CD4+ T cells. Science 327, 1098–1102 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Murphy KM, Stockinger B, Effector T cell plasticity: Flexibility in the face of changing circumstances. Nat. Immunol 11, 674–680 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tang Q, Bluestone JA, Regulatory T-cell physiology and application to treat autoimmunity. Immunol. Rev 212, 217–237 (2006). [DOI] [PubMed] [Google Scholar]
- 7.Duhen T, Geiger R, Jarrossay D, Lanzavecchia A, Sallusto F, Production of interleukin 22 but not interleukin 17 by a subset of human skin-homing memory T cells. Nat. Immunol 10, 857–863 (2009). [DOI] [PubMed] [Google Scholar]
- 8.Trifari S, Kaplan CD, Tran EH, Crellin NK, Spits H, Identification of a human helper T cell population that has abundant production of interleukin 22 and is distinct from TH-17, TH1 and TH2 cells. Nat. Immunol 10, 864–871 (2009). [DOI] [PubMed] [Google Scholar]
- 9.Korn T, Bettelli E, Oukka M, Kuchroo VK, IL-17 and Th17 cells. Annu. Rev. Immunol 27, 485–517 (2009). [DOI] [PubMed] [Google Scholar]
- 10.Zou W, Restifo NP, TH17 cells in tumour immunity and immunotherapy. Nat. Rev. Immunol 10, 248–256 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhou L, Littman DR, Transcriptional regulatory networks in Th17 cell differentiation. Curr. Opin. Immunol 21, 146–152 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Manel N, Unutmaz D, Littman DR, The differentiation of human TH-17 cells requires transforming growth factor-β and induction of the nuclear receptor RORγt. Nat. Immunol 9, 641–649 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yang L, Anderson DE, Baecher-Allan C, Hastings WD, Bettelli E, Oukka M, Kuchroo VK, Hafler DA, IL-21 and TGF-β are required for differentiation of human TH17 cells. Nature 454, 350–352 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Volpe E, Servant N, Zollinger R, Bogiatzi SI, Hupé P, Barillot E, Soumelis V, A critical function for transforming growth factor-β, interleukin 23 and proinflammatory cytokines in driving and modulating human TH-17 responses. Nat. Immunol 9, 650–657 (2008). [DOI] [PubMed] [Google Scholar]
- 15.Veldhoen M, Hirota K, Christensen J, O’Garra A, Stockinger B, Natural agonists for aryl hydrocarbon receptor in culture medium are essential for optimal differentiation of Th17 T cells. J. Exp. Med 206, 43–49 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Greenwald RJ, Freeman GJ, Sharpe AH, The B7 family revisited. Annu. Rev. Immunol 23, 515–548 (2005). [DOI] [PubMed] [Google Scholar]
- 17.Acosta-Rodriguez EV, Napolitani G, Lanzavecchia A, Sallusto F, Interleukins 1β and 6 but not transforming growth factor-β are essential for the differentiation of interleukin 17–producing human T helper cells. Nat. Immunol 8, 942–949 (2007). [DOI] [PubMed] [Google Scholar]
- 18.Acosta-Rodriguez EV, Rivino L, Geginat J, Jarrossay D, Gattorno M, Lanzavecchia A, Sallusto F, Napolitani G, Surface phenotype and antigenic specificity of human interleukin 17–producing T helper memory cells. Nat. Immunol 8, 639–646 (2007). [DOI] [PubMed] [Google Scholar]
- 19.Wilson NJ, Boniface K, Chan JR, McKenzie BS, Blumenschein WM, Mattson JD, Basham B, Smith K, Chen T, Morel F, Lecron JC, Kastelein RA, Cua DJ, McClanahan TK, Bowman EP, de Waal Malefyt R, Development, cytokine profile and function of human interleukin 17–producing helper T cells. Nat. Immunol 8, 950–957 (2007). [DOI] [PubMed] [Google Scholar]
- 20.Park H, Li Z, Yang XO, Chang SH, Nurieva R, Wang YH, Wang Y, Hood L, Zhu Z, Tian Q, Dong C, A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat. Immunol 6, 1133–1141 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bauquet AT, Jin H, Paterson AM, Mitsdoerffer M, Ho IC, Sharpe AH, Kuchroo VK, The costimulatory molecule ICOS regulates the expression of c-Maf and IL-21 in the development of follicular T helper cells and TH-17 cells. Nat. Immunol 10, 167–175 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hutloff A, Dittrich AM, Beier KC, Eljaschewitsch B, Kraft R, Anagnostopoulos I, Kroczek RA, ICOS is an inducible T-cell co-stimulator structurally and functionally related to CD28. Nature 397, 263–266 (1999). [DOI] [PubMed] [Google Scholar]
- 23.Burmeister Y, Lischke T, Dahler AC, Mages HW, Lam KP, Coyle AJ, Kroczek RA, Hutloff A, ICOS controls the pool size of effector-memory and regulatory T cells. J. Immunol 180, 774–782 (2008). [DOI] [PubMed] [Google Scholar]
- 24.Ito T, Hanabuchi S, Wang YH, Park WR, Arima K, Bover L, Qin FX, Gilliet M, Liu YJ, Two functional subsets of FOXP3+ regulatory T cells in human thymus and periphery. Immunity 28, 870–880 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.King C, Tangye SG, Mackay CR, T follicular helper (TFH) cells in normal and dysregulated immune responses. Annu. Rev. Immunol 26, 741–766 (2008). [DOI] [PubMed] [Google Scholar]
- 26.Liu W, Putnam AL, Xu-Yu Z, Szot GL, Lee MR, Zhu S, Gottlieb PA, Kapranov P, Gingeras TR, Fazekas de St Groth B, Clayberger C, Soper DM, Ziegler SF, Bluestone JA, CD127 expression inversely correlates with FoxP3 and suppressive function of human CD4+ T reg cells. J. Exp. Med 203, 1701–1711 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rasheed AU, Rahn HP, Sallusto F, Lipp M, Müller G, Follicular B helper T cell activity is confined to CXCR5hiICOShi CD4 T cells and is independent of CD57 expression. Eur. J. Immunol 36, 1892–1903 (2006). [DOI] [PubMed] [Google Scholar]
- 28.Riley JL, June CH, The CD28 family: A T-cell rheostat for therapeutic control of T-cell activation. Blood 105, 13–21 (2005). [DOI] [PubMed] [Google Scholar]
- 29.Parry RV, Rumbley CA, Vandenberghe LH, June CH, Riley JL, CD28 and inducible costimulatory protein Src homology 2 binding domains show distinct regulation of phosphatidylinositol 3-kinase, Bcl-xL, and IL-2 expression in primary human CD4 T lymphocytes. J. Immunol 171, 166–174 (2003). [DOI] [PubMed] [Google Scholar]
- 30.Smith CA, Farrah T, Goodwin RG, The TNF receptor superfamily of cellular and viral proteins: Activation, costimulation, and death. Cell 76, 959–962 (1994). [DOI] [PubMed] [Google Scholar]
- 31.Bondanza A, Valtolina V, Magnani Z, Ponzoni M, Fleischhauer K, Bonyhadi M, Traversari C, Sanvito F, Toma S, Radrizzani M, La Seta-Catamancio S, Ciceri F, Bordignon C, Bonini C, Suicide gene therapy of graft-versus-host disease induced by central memory human T lymphocytes. Blood 107, 1828–1836 (2006). [DOI] [PubMed] [Google Scholar]
- 32.Cosmi L, De Palma R, Santarlasci V, Maggi L, Capone M, Frosali F, Rodolico G, Querci V, Abbate G, Angeli R, Berrino L, Fambrini M, Caproni M, Tonelli F, Lazzeri E, Parronchi P, Liotta F, Maggi E, Romagnani S, Annunziato F, Human interleukin 17–producing cells originate from a CD161+CD4+ T cell precursor. J. Exp. Med 205, 1903–1916 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Veldhoen M, Hirota K, Westendorf AM, Buer J, Dumoutier L, Renauld JC, Stockinger B, The aryl hydrocarbon receptor links TH17-cell-mediated autoimmunity to environmental toxins. Nature 453, 106–109 (2008). [DOI] [PubMed] [Google Scholar]
- 34.Köhler A, Schmithorst V, Filippi MD, Ryan MA, Daria D, Gunzer M, Geiger H, Altered cellular dynamics and endosteal location of aged early hematopoietic progenitor cells revealed by time-lapse intravital imaging in long bones. Blood 114, 290–298 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Carpenito C, Milone MC, Hassan R, Simonet JC, Lakhal M, Suhoski MM, Varela-Rohena A, Haines KM, Heitjan DF, Albelda SM, Carroll RG, Riley JL, Pastan I, June CH, Control of large, established tumor xenografts with genetically retargeted human T cells containing CD28 and CD137 domains. Proc. Natl. Acad. Sci. U.S.A 106, 3360–3365 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bernard D, Hansen JD, Du Pasquier L, Lefranc MP, Benmansour A, Boudinot P, Costimulatory receptors in jawed vertebrates: Conserved CD28, odd CTLA4 and multiple BTLAs. Dev. Com. Immunol 31, 255–271 (2007). [DOI] [PubMed] [Google Scholar]
- 37.Turka LA, Ledbetter JA, Lee K, June CH, Thompson CB, CD28 is an inducible T cell surface antigen that transduces a proliferative signal in CD3+ mature thymocytes. J. Immunol 144, 1646–1653 (1990). [PubMed] [Google Scholar]
- 38.Gross JA, Callas E, Allison JP, Identification and distribution of the costimulatory receptor CD28 in the mouse. J. Immunol 149, 380–388 (1992). [PubMed] [Google Scholar]
- 39.Bossaller L, Burger J, Draeger R, Grimbacher B, Knoth R, Plebani A, Durandy A, Baumann U, Schlesier M, Welcher AA, Peter HH, Warnatz K, ICOS deficiency is associated with a severe reduction of CXCR5+CD4 germinal center Th cells. J. Immunol 177, 4927–4932 (2006). [DOI] [PubMed] [Google Scholar]
- 40.Takahashi N, Matsumoto K, Saito H, Nanki T, Miyasaka N, Kobata T, Azuma M, Lee SK, Mizutani S, Morio T, Impaired CD4 and CD8 effector function and decreased memory T cell populations in ICOS-deficient patients. J. Immunol 182, 5515–5527 (2009). [DOI] [PubMed] [Google Scholar]
- 41.Tafuri A, Shahinian A, Bladt F, Yoshinaga SK, Jordana M, Wakeham A, Boucher LM, Bouchard D, Chan VS, Duncan G, Odermatt B, Ho A, Itie A, Horan T, Whoriskey JS, Pawson T, Penninger JM, Ohashi PS, Mak TW, ICOS is essential for effective T-helper-cell responses. Nature 409, 105–109 (2001). [DOI] [PubMed] [Google Scholar]
- 42.Yu D, Tan AH, Hu X, Athanasopoulos V, Simpson N, Silva DG, Hutloff A, Giles KM, Leedman PJ, Lam KP, Goodnow CC, Vinuesa CG, Roquin represses autoimmunity by limiting inducible T-cell co-stimulator messenger RNA. Nature 450, 299–303 (2007). [DOI] [PubMed] [Google Scholar]
- 43.Homey B, Dieu-Nosjean MC, Wiesenborn A, Massacrier C, Pin JJ, Oldham E, Catron D, Buchanan ME, Müller A, deWaal Malefyt R, Deng G, Orozco R, Ruzicka T, Lehmann P, Lebecque S, Caux C, Zlotnik A, Up-regulation of macrophage inflammatory protein-3α/CCL20 and CC chemokine receptor 6 in psoriasis. J. Immunol 164, 6621–6632 (2000). [DOI] [PubMed] [Google Scholar]
- 44.Axtell RC, de Jong BA, Boniface K, van der Voort LF, Bhat R, De Sarno P, Naves R, Han M, Zhong F, Castellanos JG, Mair R, Christakos A, Kolkowitz I, Katz L, Killestein J, Polman CH, de Waal Malefyt R, Steinman L, Raman C, T helper type 1 and 17 cells determine efficacy of interferon-β in multiple sclerosis and experimental encephalomyelitis. Nat. Med 16, 406–412 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Martin-Orozco N, Muranski P, Chung Y, Yang XO, Yamazaki T, Lu S, Hwu P, Restifo NP, Overwijk WW, Dong C, T helper 17 cells promote cytotoxic T cell activation in tumor immunity. Immunity 31, 787–798 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Muranski P, Boni A, Antony PA, Cassard L, Irvine KR, Kaiser A, Paulos CM, Palmer DC, Touloukian CE, Ptak K, Gattinoni L, Wrzesinski C, Hinrichs CS, Kerstann KW, Feigenbaum L, Chan C, Restifo NP, Tumor-specific Th17-polarized cells eradicate large established melanoma. Blood 112, 362–373 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Suhoski MM, Golovina TN, Aqui NA, Tai VC, Varela-Rohena A, Milone MC, Carroll RG, Riley JL, June CH, Engineering artificial antigen-presenting cells to express a diverse array of co-stimulatory molecules. Mol. Ther 15, 981–988 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Maus MV, Thomas AK, Leonard DG, Allman D, Addya K, Schlienger K, Riley JL, June CH, Ex vivo expansion of polyclonal and antigen-specific cytotoxic T lymphocytes by artificial APCs expressing ligands for the T-cell receptor, CD28 and 4-1BB. Nat. Biotechnol 20, 143–148 (2002). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. UCB CD45RA+CD25−CD4+ T cells contain few CD161+IL−23R+ cells. The expression of CD161 and IL-23R surface markers on CD45RA+CD25−CD4+ T cells was assessed on human umbilical cord blood cells using flow cytometry. Data representative of 2 experiments.
Supplemental Figure 2. ICOS induces c-MAF and IL-21. PB CD4+ T cells were cultured in TH17 polarizing conditions (IL-1β, IL-6, IL-23, plus neutralizing anti-IFN-γ and anti-IL-4) and activated with anti-CD3 beads bearing either anti-CD28 or anti-ICOS antibodies. After their primary expansion, their c-MAF and IL-21 expression mRNA levels was assessed by RT-PCR. Data representative of 2 experiments.
Supplemental Figure 3. CD28 induces expression of the aryl hydrocarbon receptor. PB CD4+ T cells were programmed toward a TH17 phenotype and activated with anti-CD3 beads bearing either anti-CD28 or anti-ICOS antibodies. After their primary expansion, their mRNA expression level of AHR relative to β-actin was assessed by RT-PCR. Data representative of 2 experiments.
Supplemental Figure 4. Exogenous TGF-β augments the inflammatory potential of human TH17 cells. PB CD4+T cells were programmed toward a TH17 phenotype and activated with anti-CD3 beads bearing either anti-CD28 or 2 anti-ICOS antibodies in media containing serum and the indicated supplemental TGF-β (from 0.1-10 ng/ml) was added to the culture on day 1. IL-17A secretion by cells was measured on day 5 post-activation by ELISA. Data representative of 2 experiments.
Supplemental Figure 5. ICOS+CD161+CD4+ T cells from UCB constitutively express RORC2 and IL23R. CD4+, ICOS+CD161+CD4+ and ICOS−CD161+CD4+ T cells were sorted and their mRNA expression level of RORC2 and IL-23R relative to β-actin was measured by RT-PCR. Data representative of 2 experiments.
Supplemental Figure 6. ICOS+CD161+CD4+T cells are imprinted as TH17 cells. CD4+ and ICOS+CD161+CD4+T cells from UCB were sorted and cultured in various polarizing conditions as indicated. The frequency of IFN-γ+(A), IL-4+(B), IL-17A+(C) or FoxP3+(D) cells was measured after their primary expansion with anti-CD3 beads bearing anti-CD28 or anti-ICOS antibodies. As a control, companion control cultures of bulk UCB CD4 T cells were stimulated with anti-CD3/CD28 beads. Cytokines and FoxP3 were measured by flow cytometry or ELISA on day 7 of culture post-stimulation with PMA/ionomycin. Data representative of 2 experiments.