Abstract
Background & Aims
The use of non‐selective beta‐blockers has been associated with lower rates of infection and reduced infection‐associated morbidity in patients with cirrhosis. However, it is unknown if these drugs modify the systemic inflammatory response to circulating bacterial DNA.
Methods
Sixty‐three patients with cirrhosis were included during an episode of decompensation by ascites. Thirty of those patients were on beta‐blockers. Blood samples were obtained after each patient had been in the supine position for at least 30 minutes in a quiet atmosphere. Bacterial DNA, serum cytokines, nitric oxide, and LPS were determined. Phagocytic and oxidative burst activities were determined in polymorphonuclear cells from the patients.
Results
The detection rate of bacterial DNA in the blood was the same (33%) for patients not treated and treated with non‐selective beta‐blockers. Patients naive to non‐selective beta‐blockers showed significantly higher serum levels of IL6, IFN‐gamma and IL10 in response to the presence of bacterial DNA. Patients treated with non‐selective beta‐blockers showed higher basal inflammatory activity that did not change with the presence of bacterial DNA. Monocytes and granulocytes from patients treated with non‐selective beta‐blockers showed a significantly increased phagocytic capacity in the presence of bacterial DNA.
Conclusions
In patients with cirrhosis, chronic treatment with beta‐blockers is associated with a higher unstimulated production of serum cytokines and an increased phagocytic activity in the presence of bacterial DNA.
Keywords: bacterial DNA, cirrhosis, inflammation, non‐selective beta‐blockers, sympathetic nervous system
Abbreviations
- AF
ascitic fluid
- bactDNA
bacterial DNA
- BT
bacterial translocation
- ELISAs
enzyme‐linked immunosorbent assays
- fMLP
N‐formylmethionyl‐leucyl‐phenylalanine
- LAL
limulus amebocyte lysate
- LPS
gram‐negative bacterial endotoxin
- MFI
mean fluorescence intensity
- NO
nitric oxide
- NSBB
non‐selective beta‐blocker
- PAMPs
pathogen‐associated molecular patterns
- PCR
polymerase chain reaction
- PMA
phorbol‐12‐myristate‐13‐acetate
- PMN
polymorphonuclear
- PPIs
proton pump inhibitors
- ROS
reactive oxygen species
- SBP
spontaneous bacterial peritonitis
- SID
selective intestinal decontamination
- SNS
sympathetic nervous system
- TIPS
transjugular intrahepatic portosystemic shunt
- WBCs
white blood cells
Key Points.
The detection rate of bacterial DNA in the blood was the same (33%) for patients not treated and treated with non‐selective beta‐blockers.
Patients with cirrhosis chronically treated with non‐selective beta‐blockers show an increased systemic inflammatory response during an ascites episode.
Chronic treatment with beta‐blockers increases the phagocytic capacity of monocytes and granulocytes in response to the presence of bacterial DNA.
1. INTRODUCTION
Patients with cirrhosis and portal hypertension develop a hyperdynamic circulatory state characterized by splanchnic and peripheral vasodilation and increased plasma volume as a result of the excessive production of vasodilators.1 The progression of the disease is followed by an increased activity of the sympathetic nervous system (SNS) and the renin‐angiotensin‐aldosterone system that act as counter‐regulatory mechanisms2 that fail to reverse the vasodilation due to the important and sustained increase of vasodilator agents such as nitric oxide (NO)1 and a loss of alpha and beta adrenergic response.3, 4
Spontaneous bacterial peritonitis (SBP) is a bacterial infection frequently observed in patients with cirrhosis.5 This infection is due to bacterial translocation (BT), defined as the passage of gram‐negative bacteria or their products, which act as pathogen‐associated molecular patterns (PAMPs), from the intestinal lumen through the intestinal wall to the mesenteric lymph nodes.6
Our group has shown that the existence of bacterial DNA (bactDNA) in the blood and ascitic fluid (AF) of hospital patients with cirrhosis and noninfected ascites7, 8 constitutes a marker of BT.9 The presence of bactDNA is associated with a higher peritoneal nitric oxide (NO) production,10 an activation of the complement system11 and, in general, a higher soluble immune response not related to endotoxin12 similar to that observed in patients with SBP.13 Patients who present with bactDNA in blood during an episode of ascites have reduced survival in the next year.14
Non‐selective beta‐blockers (NSBBs) remain the main treatment of portal hypertension because of their efficacy at preventing variceal bleeding.15 The treatment with NSBB of patients with cirrhosis has been associated with lower rates of infections,16 decreased abnormal intestinal permeability and serum levels of LBP and IL‐6,17 reduced severity of systemic inflammation and improved survival of patients with acute‐on‐chronic liver failure.18 It is unknown if treatment with these drugs also modifies the inflammatory response to the presence of PAMPs without an overt clinical infection. Conversely, it has been shown that NSBB treatment increased the risks for hepatorenal syndrome and acute kidney injury in severely infected patients with cirrhosis.19 In these patients, a high production of proinflammatory cytokines seems to play a role in the worsening of liver function.20 Experimental models of bacterial peritonitis or splanchnic sympathectomy have shown that SNS activation is followed by lower levels of peritoneal secretion of TNF‐alpha and limited phagocytic activity of peritoneal macrophages,21 while SNS suppression is associated with a reduced rate of Escherichia coli bacterial translocation.22 The SNS is known to produce most of these effects through beta‐adrenergic receptors.23
Then, the SNS blockade could explain part of the reduction in mortality of patients with cirrhosis treated with NSBB due to the down‐regulation of bacterial translocation rates,22, 24 in addition to the haemodynamic effects or the ability of the SNS to modulate other systems.
The present study was designed to investigate the effects of beta‐blockers on the systemic immune response to the presence of bactDNA in patients with cirrhosis and ascites.
2. PATIENTS AND METHODS
We conducted a prospective trial in patients with cirrhosis and ascites. Cirrhosis was diagnosed by histology or by clinical, laboratory, and/or ultrasonographic findings. Patients were included during an episode of ascites decompensation. Exclusion criteria were the presence of culture‐positive blood or AF, temperature >38°C, white blood cells (WBCs) >12 000/mm3, neutrocytic ascites (>250 polymorphonuclear [PMN] cells/μL), infection treated with antibiotics in the preceding 4 weeks, hepatorenal syndrome or renal insufficiency, multinodular hepatocellular carcinoma and/or portal thrombosis, previous liver transplantation, transjugular intrahepatic portosystemic shunt (TIPS), alcoholic hepatitis, and refusal to participate in the study. The Institutional Review Board of the Hospital General Universitario de Alicante approved the study protocol, and all patients provided informed consent for inclusion in the study.
Patients were studied in the course of their admission to the hospital for an episode of ascitic decompensation. After signing the informed consent, blood samples were obtained after the patient had been in the supine position for at least 30 minutes in a quiet atmosphere. An automated measurement of blood pressure and heart rate was taken, and heart rate variability was recorded for 30 minutes (S810i Polar heart rate monitor; software RHRV version 4.0).
Blood samples were inoculated under aseptic conditions in rubber‐sealed sterile Vacutainer SST II tubes (BD Diagnostics, Belgium) that were never exposed to free air. Serum and plasma samples were stored at −80°C until the analyses.
To detect and identify the presence of bactDNA fragments in the blood, a broad‐range polymerase chain reaction (PCR) and partial nucleotide sequencing analysis was performed according to the methodology described previously.7 PCR amplicons were loaded onto DNA Laboratory‐on‐a‐chips® (Agilent Technologies, Palo Alto, CA, USA) and quantified with an Agilent 2100 BioAnalyzer (Agilent Technologies). Samples and reagents were handled in an airflow chamber and processed with pyrogen‐free material tested by the manufacturers.
The endpoint chromogenic limulus amebocyte lysate (LAL) test was used to quantify gram‐negative bacterial endotoxin (LPS) in the serum using a commercially available kit (QCL‐1000, Lonza Group Ltd., Basel, Switzerland).
Enzyme‐linked immunosorbent assays (ELISAs) for the quantitative measurement of TNF‐alpha, IFN‐gamma, IL‐10 and IL‐6 levels were performed with the serum of patients with Human Quantikine kits (R&D Systems, Minneapolis, MN, USA), according to the manufacturer's instructions. All samples were tested in triplicate and read at 490 nm in a microplate reader. The lower limits of detection of all cytokine assays were between 0.5–5 pg/mL.
NO levels in serum samples were calculated by measuring the conversion of nitrate to nitrite by the enzyme nitrate reductase using an ELISA (KGE001, R&D Systems) based on the Griess reaction.25
The phagocytic activity was evaluated using a Phagotest kit (Orpegen Pharma, Heidelberg, Germany) following the manufacturer's protocol. Two cellular activities are measured in this assay, the in vitro percentage of granulocytes and monocytes that are able to ingest fluorescein‐isothiocyanate (FITC)‐labelled opsonized E. coli bacteria, and their phagocytic capacity (total number of bacteria that are ingested per cell). Phagocytosis was expressed as a percentage of activated phagocytes and as the mean fluorescence intensity (MFI).
The oxidative burst was measured with a Phagoburst kit (Orpegen Pharma) according to the manufacturer's protocol. Like the phagocytic assay, this kit measures both the percentage of granulocytes and monocytes that generate reactive oxygen species (ROS) and their oxidative burst activity (number of ROS per cell) in response to the presence of unlabelled opsonized E. coli, N‐formylmethionyl‐leucyl‐phenylalanine (fMLP) and phorbol‐12‐myristate‐13‐acetate (PMA).
2.1. Statistical analysis
Continuous variables are reported as the mean ± standard deviation or median (quartiles 25‐75), and categorical variables are expressed as frequencies or percentages. The statistical differences between groups were analysed using the Chi‐square test for categorical data and the Mann‐Whitney U test for quantitative data. Statistical differences between 3 or more groups were analysed using the Kruskal‐Wallis test. Bivariate correlations between continuous variables were calculated using the Spearman test. Multiple comparisons were analysed with pairwise comparisons using the Mann‐Whitney U test and the Bonferroni correction to determine if the post hoc tests were significant. Univariate and multiple linear regression analyses were calculated to predict NO, TNF‐alpha, IFN‐gamma, IL10 or IL6 levels based on NSBB, bactDNA, LPS and clinical variables. Additionally, univariate and multivariate regression analyses were used to study the associations between phagocytic and oxidative cell activities and the levels of serum markers of inflammatory activation. All reported P values are 2‐sided, and P values less than .05 are considered to indicate significance. All analyses were carried out in R software (Core Team (2014). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. URL http://www.R-project.org/).
3. RESULTS
Sixty‐three consecutively admitted patients with cirrhosis were included in the study during episodes of decompensation by ascites. Thirty patients (46%) were being treated with NSBBs (28 with propranolol at a dose ranging from 20 to 120 mg/d [10 patients with doses lower than 60 mg/d, 16 with doses between 60 and 80 mg/d and 2 with doses higher than 80 mg/d] and 2 with nadolol at a dose of 40 mg/d) at enrolment (see flow chart in Figure 1). Median NSBB treatment duration was 15 months (minimum 5 months and maximum 66 months), and the reasons for NSBB treatment were the existence of varices and/or previous episodes of upper digestive bleeding in 29 patients and arrhythmia in 1 patient. The clinical and analytical characteristics of the patients included in the study are detailed in Table 1. Patients treated with NSBBs were younger, more frequently had gastroesophageal varices, and had lower counts of platelets in the blood and leukocytes in the ascitic fluid than patients not treated with these drugs.
Figure 1.
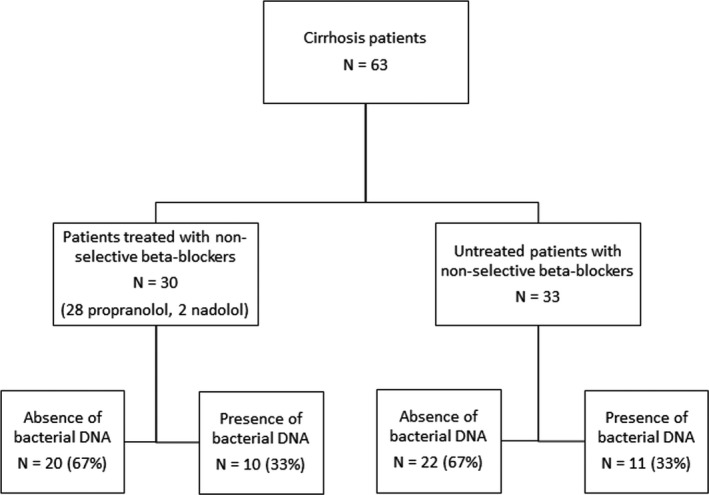
Patient flow chart
Table 1.
Clinical and analytical characteristics of patients included in the study according to beta‐blocker treatment and the presence of circulating bacterial DNA fragments
| No NSBB treatment | NSBB treatment | P value (NSBB vs No NSBB) | |||
|---|---|---|---|---|---|
| Absence of bactDNA | Presence of bactDNA | Absence of bactDNA | Presence of bactDNA | ||
| N | 22 | 11 | 20 | 10 | |
| Age (y) | 67 ± 9 | 69 ± 10 | 61 ± 10 | 62 ± 9 | .04 |
| Male sex N (%) | 14 (64) | 7 (64) | 16 (80) | 6 (60) | .44 |
| Aetiology of cirrhosis (Alcohol/Viral/Others) | 13/4/5 | 5/2/4 | 10/7/3 | 5/4/1 | .15 |
| Refractory ascites | 3 (14) | 2 (18) | 3 (15) | 1 (10) | .11 |
| Previous episodes of | |||||
| Encephalopathy (N %) | 17 (77) | 7 (64) | 15 (75) | 10 (100) | .32 |
| Upper digestive bleeding (N %) | 12 (55) | 7 (64) | 11 (55) | 7 (70) | .53 |
| Ascites (N %) | 18 (82) | 11 (100) | 19 (95) | 10 (100) | .16 |
| Hepatocellular carcinoma (N %) | 3 (14) | 4 (36) | 4 (2) | 0 (0) | .67 |
| Gastroesophageal varices (N %) | 15 (68) | 9 (82) | 18 (90) | 8 (80) | .04 |
| Child‐Pugh score | 9.0 ± 1.0 | 8.4 ± 2.0 | 8.4 ± 2.0 | 8.3 ± 2.0 | .42 |
| PPI at inclusion (N %) | 10 (45) | 6 (55) | 10 (50) | 7 (70) | .59 |
| SID at inclusion (N %) | 10 (45) | 6 (54) | 11 (55) | 4 (40) | .37 |
| Furosemide at inclusion (N %) | 14 (64) | 10 (91) | 12 (60) | 9 (90) | .95 |
| Total bilirubin (mg/dL) | 1.5 ± 1 | 1.2 ± 1 | 1.8 ± 2 | 1.7 ± 2 | .53 |
| Albumin (g/dL) | 2.8 ± 0.6 | 3.1 ± 0.5 | 3.1 ± 0.5 | 3.4 ± 0.5 | .10 |
| Total proteins (g/dL) | 5.9 ± 0.7 | 5.8 ± 0.8 | 5.5 ± 0.7 | 5.7 ± 0.9 | .61 |
| Quick (%) | 62 ± 16 | 61 ± 13 | 71 ± 14 | 66 ± 16 | .10 |
| Creatinine (mg/dL) | 1.2 ± 1.0 | 1.1 ± 0.9 | 1.3 ± 1.0 | 1.2 ± 0.3 | .35 |
| Haemoglobin (g/dL) | 10 ± 2 | 10 ± 2 | 11 ± 3 | 10 ± 1 | .51 |
| Haematocrit (%) | 32 ± 5 | 31 ± 5 | 32 ± 7 | 30 ± 3 | .89 |
| Platelets (x109/mm3) | 95 ± 61 | 133 ± 61 | 83 ± 49 | 61 ± 37 | .03 |
| Leukocytes (/mm3) | 3618 ± 2348 | 4639 ± 2680 | 4343 ± 2237 | 3024 ± 1665 | .96 |
| Glucose (mg/dL) | 116 ± 56 | 106 ± 27 | 110 ± 35 | 129 ± 49 | .68 |
| Serum sodium (mEq/L) | 136 ± 5 | 136 ± 4 | 134 ± 5 | 135 ± 4 | .24 |
| ALT (UI/L) | 21 ± 14 | 21 ± 21 | 55 ± 45 | 39 ± 51 | .05 |
| AF albumin (g/dL) | 0.9 ± 2.8 | 1.1 ± 1.6 | 0.6 ± 0.2 | 0.9 ± 0.2 | .06 |
| AF total proteins (g/dL) | 1.7 ± 0.2 | 1.7 ± 1.1 | 1.5 ± 1.1 | 1.6 ± 1.2 | .39 |
| AF Leukocytes (/mm3) | 232 ± 137 | 258 ± 297 | 109 ± 73 | 143 ± 195 | .01 |
| AF PMN (%) | 8.2 ± 4.1 | 13 ± 7.2 | 15.0 ± 20.1 | 15.1 ± 26.0 | .84 |
| AF Glucose (mg/dL) | 124 ± 28 | 123 ± 32 | 117 ± 26 | 153 ± 55 | .92 |
AF, ascitic fluid; ALT, alanine aminotransferase; bactDNA, bacterial DNA; NSBB, non‐selective beta‐blockers; PMN, polymorphonuclears; PPI, proton pump inhibitor; SID, selective intestinal decontamination.
Data are mean ± standard deviation or frequencies.
As expected, patients treated with NSBBs showed a significantly lower heart rate (73 ± 9 vs 84 ± 18; P value = .01) and longer mean normal to normal intervals (627 ± 170 vs 764 ± 170 ms; P value = .04). No other significant differences in heart rate variability were observed according to NSBB treatment. Mean arterial pressure was similar in patients treated and not treated with NSBBs (93 ± 13 mm Hg vs 91 ± 15 mm Hg; P value = .18).
The detection rate of bactDNA fragments was the same (33%) for patients not treated (N = 11) and treated with NSBBs (N = 10). The identified bacterial species were E. coli (N = 9), Proteus mirabilis (N = 1) and Streptococcus pneumoniae (N = 1) in patients not treated with NSBBs and E. coli (N = 6), Klebsiella pneumoniae (N = 2), Enterococcus faecalis (N = 1) and S. pneumoniae (N = 1) in those treated with NSBB. Only patients naive to NSBB showed increased levels of LPS in the presence of bactDNA, as shown in Table 2. Specifically, it was this group of patients who had significantly higher serum concentrations of the proinflammatory cytokines IL6 and IFN‐gamma and the anti‐inflammatory cytokine IL10 (Table 2). On the other hand, patients treated with NSBB showed a different activation profile characterized by higher basal levels of inflammatory activity expressed by increased levels of serum IL6 that did not change in response to the presence of bactDNA (Table 2).
Table 2.
Immune activation and LPS levels according to NSBB treatment and presence of circulating bactDNA fragments
| No NSBB treatment | NSBB treatment | P value (NSBB vs No NSBB) | |||
|---|---|---|---|---|---|
| Absence of bactDNA | Presence of bactDNA | Absence of bactDNA | Presence of bactDNA | ||
| N | 22 | 11 | 20 | 10 | |
| LPS (UI/L) | 0.29 [0.15‐0.34] | 0.72 [0.56‐0.90]a | 0.47 [0.34‐0.52]b | 0.39 [0.37‐0.62] | .17 |
| Serum markers of Inflammatory activation | |||||
| IFN‐gamma (pg/mL) | 6.0 [1.1‐9.0] | 11.0 [9.1‐14.0]a | 9.1 [6.7‐10.0] | 9.4 [7.0‐11.0] | .62 |
| IL10 (pg/mL) | 7.2 [1.3‐10.0] | 18.0 [12.0‐23.0]a | 11.0 [3.2‐16.0] | 14.0 [11.0‐16.0] | .17 |
| IL6 (pg/mL) | 18.0 [13.0‐21.0] | 34.0 [27.0‐38.0]a | 32.0 [23.0‐47.0]b | 30.0 [24.0‐32.0] | .002 |
| TNF‐alpha (pg/mL) | 22.0 [19.0‐24.0] | 21.0 [18.0‐29.0] | 19.0 [16.0‐22.0] | 20.0 [17.0‐26.0] | .15 |
| NO (pg/mL) | 85.0 [62.0‐97.0] | 81.0 [59.0‐116.0] | 80.0 [45.0‐94.0] | 101.0 [70.0‐120.0] | .51 |
bactDNA, bacterial DNA; INF, interferon; LPS, gram‐negative bacterial endotoxin; NO, nitric oxide; NSBB, non‐selective beta‐blocker; TNF, tumour necrosis factor.
Data are expressed as median [Q25‐Q75].
P < .05 vs absence of bactDNA.
P < .05 vs equivalent bactDNA group in patients non‐treated with BB.
In multiple linear regression analyses, serum IL6 levels were only significantly related with NSBB. On the other hand, IL10 levels were significantly associated with LPS concentrations, while IFN‐gamma concentrations were related with the Child‐Pugh score (Table 3).
Table 3.
Univariate and multivariate linear regression analyses
| Dependent variable | Independent variables | Univariate analysis | Multivariate analysis | R 2 | ||||
|---|---|---|---|---|---|---|---|---|
| Beta | 95% CI (lower, upper) | P | Beta | 95% CI (lower, upper) | P | |||
| IFN‐gamma | Child‐Pugh score | −0.8 | (−1.5, −0.07) | .032 | −0.8 | (−1.4, −0.1) | .030 | |
| Bacterial DNA | 3.2 | (0.8, 5.5) | .009 | 1.3 | (−1.0, 3.9) | .249 | ||
| LPS | 4.9 | (0.9, 8.8) | .016 | 2.9 | (−0.7, 7.4) | .102 | ||
| Leukocytes | 5 × 10−4 | (5 × 10−5, 1 × 10−3) | .029 | 4 × 10−4 | (6 × 10−6, 9 × 10−4) | .050 | 0.19 | |
| IL10 | Bacterial DNA | 8.8 | (3.3, 14.3) | .002 | 5.5 | (−0.4, 11.4) | .065 | |
| LPS | 16.6 | (7.6, 25.6) | .001 | 12.7 | (2.9, 22.4) | .012 | 0.21 | |
| IL6 | NSBB | 15.3 | (1.0, 29.5) | .036 | 15.7 | (2.2, 29.2) | .023 | |
| Leukocytes | 3 × 10−3 | (4 × 10−5, 6 × 10−3) | .047 | 2 × 10−3 | (6 × 10−4, 5 × 10−3) | .118 | 0.19 | |
INF, interferon; LPS, gram‐negative bacterial endotoxin; NO, nitric oxide; NSBB, non‐selective beta‐blocker; TNF, tumour necrosis factor.
Univariate linear regression analyses were calculated to predict NO, TNF‐alpha, IFN‐gamma, IL10 or IL6 based on NSBB, bacterial DNA, LPS and variables shown in Table 1. Only significant variables in the univariate analysis were included in the multivariate analysis and are shown in the table.
The in vitro percentage of granulocytes and monocytes that can ingest fluorescein‐isothiocyanate (FITC)‐labelled opsonized E. coli bacteria was similar in patients treated and not treated with NSBB with or without circulating bactDNA (percentages ranged between 73% to 88%). However, the total number of ingested bacteria per monocyte or granulocyte (the phagocytic capacity) was significantly increased by the presence of bactDNA in patients treated with NSBB but not in patients not treated with NSBB (Figure 2A,B). Treatment with NSBB was the only variable significantly associated with monocyte and granulocyte phagocytic activity (P = .024 and P = .029, respectively).
Figure 2.
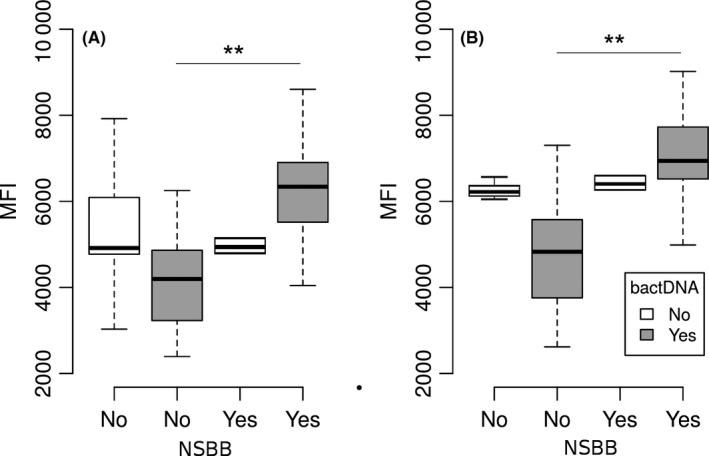
Phagocytic capacity of polymorphonuclear cells from patients treated with or without non‐selective beta‐blockers, according to the presence of bacterial DNA represented by the mean fluorescence intensity (MFI) measured by flow cytometry. A, Monocytes. B, Granulocytes. NSBB, non‐selective beta‐blocker; bactDNA, bacterial DNA; **P < .01
Phorbol‐12‐myristate‐13‐acetate and E. coli elicited a 4‐fold increase in ROS generation response. This increase in oxidative burst was observed in cells from both types of patients, those treated and those not treated with NSBB. In general, the generation of ROS was decreased in cells from patients with circulating fragments of bactDNA in the blood (Figure 3A,B). In linear regression analyses, the significant predictor of oxidative activity in monocytes was the presence of bactDNA in response to PMA (P = .0014, R 2 = 0.22) or E. coli (P = .0001, R 2 = 0.27). In the case of granulocytes, the significant and independent predictor of oxidative response was also bactDNA for both stimuli, PMA (P = .002, R 2 = 0.20) and E. coli (P = .001, R 2 = 0.24).
Figure 3.
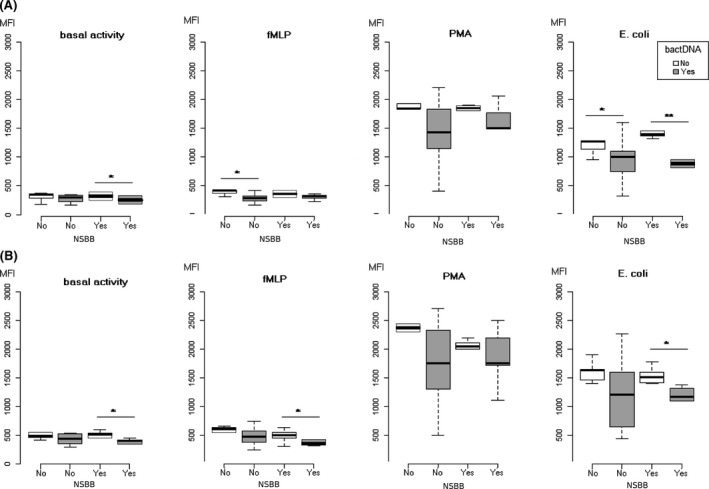
Oxidative burst of polymorphonuclear cells induced by fMLP, PMA and Escherichia coli stimuli from patients treated with or without non‐selective beta‐blockers, according to the presence of bacterial DNA (grey boxes) represented by the mean fluorescence intensity (MFI) measured by flow cytometry. A, Monocytes. B, Granulocytes. NSBB, non‐selective beta‐blocker; bactDNA, bacterial DNA; *P < .05, **P < .01
4. DISCUSSION
In this investigation, we provide evidence that patients with cirrhosis chronically treated with NSBBs respond differently during an episode of decompensation by ascites than non‐treated patients to the presence of PAMPs. The chronic use of NSBBs in patients without bactDNA is associated with higher basal levels of cytokines IL6, IFN‐gamma and IL10, similar to those observed in patients not treated with NSBBs but exposed to bactDNA. These results are in agreement with the proposed immune inhibitory effect of SNS through beta‐adrenoceptors that would be reversed by treatment with NSBB.23, 26
In our study, multivariate analysis showed that NSBB treatment is significantly associated with higher concentrations of serum IL6 independent of the presence of PAMPs. Cirrhotic patients with bacterial infections display marked and sustained increases in TNF‐alpha and IL‐6 blood levels that have been related to the development of cirrhotic decompensation and poor survival independent of liver dysfunction.27 NSBB treatment increases the risks for hepatorenal syndrome and acute kidney injury in severely infected patients with cirrhosis19 and high production levels of proinflammatory cytokines.20 These data suggest that the increase in IL‐6 associated with treatment with NSBB could be implicated in the worst prognosis of these patients after developing an infection, even though the NSBBs reduce the risk of infection. This effect would be independent of the mechanism by which the NSBBs reduce the risk of infection in patients with cirrhosis. The significance of these findings in patients with the presence of PAMPs is unknown and should be evaluated in future studies.
Potentially beneficial new therapeutic uses of NSBB have been proposed, such as the prevention of hepatocarcinoma or the reduction of bacterial infection episodes.16, 28 Merli et al16 found that the use of NSBBs was a protective factor against overt clinical infections. Reiberger et al17 showed that in cirrhotic patients, NSBB treatment decreased abnormal intestinal permeability and serum levels of LBP and IL‐6. Our data support the existence of different immune activation in cirrhotic patients treated with NSBB who showed higher serum levels of IL6, IFN‐gamma and IL10 in the absence of infection or circulating PAMPs, which could favour the existence of an unfavourable environment for the development of an infectious process.
However, in our study, the bacterial DNA detection rate was similar in patients treated and not treated with NSBB, suggesting a null effect of NSBB treatment on bacterial translocation rates. We do not know if differences in the BT rate according to NSBB treatment would have be found if we had included patients with cirrhosis without ascitic decompensation, a situation that favours BT, or if we had used a surrogate marker of BT other than bactDNA.
This study had limitations related to our study population. Patients treated with NSBB were significantly younger and showed lower counts of ascitic fluid leukocytes than non‐treated patients. Immune responses against foreign antigens seem to be impaired with age because of the reductions in the number and functions of most immune cells involved in innate immunity, such as monocytes/macrophages and natural killer cells.29 Therefore, we cannot disregard the possibility that the lower values of IL6, IL10 and IFN‐gamma in patients not treated with NSBBs and without bactDNA could be influenced by their greater age, even considering that multivariate analysis showed NSBB treatment as the only factor significantly associated with IL6 levels.
Elevated ascites PMN counts of more than 100 cells/μL has been associated with a significant risk for SBP development.30 In our study, patients treated with NSBBs showed a significantly lower count of leukocytes in ascitic fluid despite a lack of reduction in blood leukocytes. Total PMN counts in AF were similar in patients treated and not treated with NSBB and were clearly below the cut‐point of 100 cells/μL previously associated with the risk of developing SBP. Whether differences in AF leukocyte levels could be explained by a leukocyte redistribution influenced by NSBB treatment, as has been described in heart failure, has yet to be confirmed in specific studies.31
Beta‐adrenoceptor activation of human monocytes has been associated with a reduction of C. albicans phagocytosis.32 In our study, the phagocytic ability of the monocytes and granulocytes in response to bactDNA was increased only in patients treated chronically with NSBBs, suggesting a restitution of phagocytic capacity through the antagonism of beta‐adrenoceptor activation.
Desensitization phenomena have been described related to the production of anti‐inflammatory cytokines, such as IL‐10,33 decreasing the ROS generation, as we have observed in cells from patients with bactDNA in response to E. coli irrespective of treatment with NSBB. This regulatory mechanism could protect PMN by reducing apoptosis, thus increasing the anti‐infective potential of these cells.34
In summary, patients with cirrhosis chronically treated with NSBBs show a different immune response to PAMPs. The presence of circulating bactDNA in the absence of NSBB treatment is associated with increased serum levels of cytokines and decreased ROS generation. On the other hand, PMN phagocytic activity is recovered in response to the presence of bacterial DNA in patients treated with NSBBs. Additionally, NSBB treatment increases the basal cytokine production, regardless of bactDNA presence. Together, these data suggest a state of increased immune activation maintained over time in patients treated with NSBBs that should be explored in further studies.
CONFLICT OF INTEREST
The authors do not have any disclosures to report.
Gimenez P, Garcia‐Martinez I, Francés R, et al. Treatment with non‐selective beta‐blockers affects the systemic inflammatory response to bacterial DNA in patients with cirrhosis. Liver Int. 2018;38:2219–2227. 10.1111/liv.13890
Funding information
This work was supported by grants PI11/0962 and PI14/01090 from the Instituto de Salud Carlos III, Madrid, Spain; PROMETEO/2016/001 from Generalitat Valenciana, Valencia, Spain; and FEDER funds, EU.
Handling Editor: Dominique Thabut
REFERENCES
- 1. Iwakiri Y, Groszmann RJ. The hyperdynamic circulation of chronic liver diseases: from the patient to the molecule. Hepatology. 2006;43:S121‐S131. [DOI] [PubMed] [Google Scholar]
- 2. Floras JS, Legault L, Morali GA, Hara K, Blendis LM. Increased sympathetic outflow in cirrhosis and ascites: direct evidence from intraneural recordings. Ann Intern Med. 1991;114:373‐380. [DOI] [PubMed] [Google Scholar]
- 3. Ramond MJ, Comoy E, Lebrec D. Alterations in isoprenaline sensitivity in patients with cirrhosis: evidence of abnormality of the sympathetic nervous activity. Br J Clin Pharmacol. 1986;21:191‐196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hernández FT, Zapater P, De‐Madaria E, et al. Functional status of beta‐2‐adrenoceptor in isolated membranes of mature erythrocytes from patients with cirrhosis and oesophageal varices. Vascul Pharmacol. 2006;44:464‐468. [DOI] [PubMed] [Google Scholar]
- 5. Poca M, Alvarado‐Tapias E, Concepción M, et al. Predictive model of mortality in patients with spontaneous bacterial peritonitis. Aliment Pharmacol Ther. 2016;44:629‐637. [DOI] [PubMed] [Google Scholar]
- 6. Wiest R, Garcia‐Tsao G. Bacterial translocation (BT) in cirrhosis. Hepatology. 2005;41:422‐433. [DOI] [PubMed] [Google Scholar]
- 7. Such J, Francés R, Muñoz C, et al. Detection and identification of bacterial DNA in patients with cirrhosis and culture‐negative, nonneutrocytic ascites. Hepatology. 2002;36:135‐141. [DOI] [PubMed] [Google Scholar]
- 8. Francés R, Benlloch S, Zapater P, et al. A sequential study of serum bacterial DNA in patients with advanced cirrhosis and ascites. Hepatology. 2004;39:484‐491. [DOI] [PubMed] [Google Scholar]
- 9. Guarner C, González‐Navajas JM, Sánchez E, et al. The detection of bacterial DNA in blood of rats with CCl4‐induced cirrhosis with ascites represents episodes of bacterial translocation. Hepatology. 2006;44:633‐639. [DOI] [PubMed] [Google Scholar]
- 10. Francés R, Muñoz C, Zapater P, et al. Bacterial DNA activates cell mediated immune response and nitric oxide overproduction in peritoneal macrophages from patients with cirrhosis and ascites. Gut. 2004;53:860‐864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Francés R, González‐Navajas JM, Zapater P, et al. Bacterial DNA induces the complement system activation in serum and ascitic fluid from patients with advanced cirrhosis. J Clin Immunol. 2007;27:438‐444. [DOI] [PubMed] [Google Scholar]
- 12. González‐Navajas JM, Bellot P, Francés R, et al. Presence of bacterial‐DNA in cirrhosis identifies a subgroup of patients with marked inflammatory response not related to endotoxin. J Hepatol. 2008;48:61‐67. [DOI] [PubMed] [Google Scholar]
- 13. Francés R, Zapater P, González‐Navajas JM, et al. Bacterial DNA in patients with cirrhosis and noninfected ascites mimics the soluble immune response established in patients with spontaneous bacterial peritonitis. Hepatology. 2008;47:978‐985. [DOI] [PubMed] [Google Scholar]
- 14. Zapater P, Francés R, González‐Navajas JM, et al. Serum and ascitic fluid bacterial DNA: a new independent prognostic factor in noninfected patients with cirrhosis. Hepatology. 2008;48:1924‐1931. [DOI] [PubMed] [Google Scholar]
- 15. Reiberger T, Mandorfer M. Beta adrenergic blockade and decompensated cirrhosis. J Hepatol. 2017;66:849‐859. [DOI] [PubMed] [Google Scholar]
- 16. Merli M, Lucidi C, Di Gregorio V, et al. The chronic use of beta‐blockers and proton pump inhibitors may affect the rate of bacterial infections in cirrhosis. Liver Int. 2015;35:362‐369. [DOI] [PubMed] [Google Scholar]
- 17. Reiberger T, Ferlitsch A, Payer BA, et al. Non‐selective betablocker therapy decreases intestinal permeability and serum levels of LBP and IL‐6 in patients with cirrhosis. J Hepatol. 2013;58:911‐921. [DOI] [PubMed] [Google Scholar]
- 18. Mookerjee RP, Pavesi M, Thomsen KL, et al. Treatment with non‐selective beta blockers is associated with reduced severity of systemic inflammation and improved survival of patients with acute‐on‐chronic liver failure. J Hepatol. 2016;64:574‐582. [DOI] [PubMed] [Google Scholar]
- 19. Mandorfer M, Bota S, Schwabl P, et al. Nonselective β blockers increase risk for hepatorenal syndrome and death in patients with cirrhosis and spontaneous bacterial peritonitis. Gastroenterology. 2014;146:1680‐1690.e1. [DOI] [PubMed] [Google Scholar]
- 20. Gustot T, Durand F, Lebrec D, Vincent J‐L, Moreau R. Severe sepsis in cirrhosis. Hepatology. 2009;50:2022‐2033. [DOI] [PubMed] [Google Scholar]
- 21. Straub RH, Pongratz G, Weidler C, et al. Ablation of the sympathetic nervous system decreases gram‐negative and increases gram‐positive bacterial dissemination: key roles for tumor necrosis factor/phagocytes and interleukin‐4/lymphocytes. J Infect Dis. 2005;192:560‐572. [DOI] [PubMed] [Google Scholar]
- 22. Worlicek M, Knebel K, Linde HJ, et al. Splanchnic sympathectomy prevents translocation and spreading of E. coli but not S. aureus in liver cirrhosis. Gut. 2010;59:1127‐1134. [DOI] [PubMed] [Google Scholar]
- 23. Elenkov IJ, Wilder RL, Chrousos GP, Vizi ES. The sympathetic nerve–an integrative interface between two supersystems: the brain and the immune system. Pharmacol Rev. 2000;52:595‐638. [PubMed] [Google Scholar]
- 24. Pérez‐Paramo M, Muñoz J, Albillos A, et al. Effect of propranolol on the factors promoting bacterial translocation in cirrhotic rats with ascites. Hepatology. 2000;31:43‐48. [DOI] [PubMed] [Google Scholar]
- 25. Wennmalm A, Benthin G, Edlund A, et al. Metabolism and excretion of nitric oxide in humans. An experimental and clinical study. Circ Res. 1993;73:1121‐1127. [DOI] [PubMed] [Google Scholar]
- 26. Scanzano A, Cosentino M. Adrenergic regulation of innate immunity: a review. Front Pharmacol. 2015;6:171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Byl B, Roucloux I, Crusiaux A, Dupont E, Devière J. Tumor necrosis factor alpha and interleukin 6 plasma levels in infected cirrhotic patients. Gastroenterology. 1993;104:1492‐1497. [DOI] [PubMed] [Google Scholar]
- 28. Thiele M, Albillos A, Abazi R, Wiest R, Gluud LL, Krag A. Non‐selective beta‐blockers may reduce risk of hepatocellular carcinoma: a meta‐analysis of randomized trials. Liver Int. 2015;35:2009‐2016. [DOI] [PubMed] [Google Scholar]
- 29. Tajiri K, Shimizu Y. Liver physiology and liver diseases in the elderly. World J Gastroenterol. 2013;19:8459‐8467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Schwabl P, Bucsics T, Soucek K, et al. Risk factors for development of spontaneous bacterial peritonitis and subsequent mortality in cirrhotic patients with ascites. Liver Int. 2015;35:2121‐2128. [DOI] [PubMed] [Google Scholar]
- 31. von Haehling S, Schefold JC, Jankowska E, et al. Leukocyte redistribution: effects of beta blockers in patients with chronic heart failure. PLoS One. 2009;4:e6411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Borda ES, Tenenbaum A, Sales ME, Rumi L, Sterin‐Borda L. Role of arachidonic acid metabolites in the action of a beta adrenergic agonist on human monocyte phagocytosis. Prostaglandins Leukot Essent Fatty Acids. 1998;58:85‐90. [DOI] [PubMed] [Google Scholar]
- 33. Dang PM‐C, Elbim C, Marie J‐C, Chiandotto M, Gougerot‐Pocidalo M‐A, El‐Benna J. Anti‐inflammatory effect of interleukin‐10 on human neutrophil respiratory burst involves inhibition of GM‐CSF‐induced p47PHOX phosphorylation through a decrease in ERK1/2 activity. FASEB J. 2006;20:1504. [DOI] [PubMed] [Google Scholar]
- 34. Geering B, Simon H‐U. Peculiarities of cell death mechanisms in neutrophils. Cell Death Differ. 2011;18:1457‐1469. [DOI] [PMC free article] [PubMed] [Google Scholar]


