Abstract
Antiplatelet treatment is a potential therapeutic approach for sickle cell disease (SCD). Ticagrelor inhibits platelet aggregation and is approved for adults with acute coronary syndrome and following myocardial infarction. HESTIA1 (NCT02214121) was a 2‐part, phase 2 dose‐finding study generating ticagrelor exposure, platelet inhibition, and safety data in children with SCD (3‐17 years). In part A (n = 45), patients received 2 ticagrelor single doses, 0.125‐2.25 mg/kg (washout ≥7 days), then 7 days of twice‐daily (bid) dosing with 0.125, 0.563, or 0.75 mg/kg. In the 4‐week blinded Part B extension (optional), patients received ticagrelor (0.125, 0.563, or 0.75 mg/kg bid; n = 16) or placebo (n = 7). Platelet reactivity decreased from baseline to 2 hours postdosing, and returned to near baseline after 6 hours postdosing. Dose‐dependent platelet inhibition was seen with ticagrelor; mean relative P2Y12 reaction unit inhibition 2 hours after a single dose ranged from 6% (0.125 mg/kg) to 73% (2.25 mg/kg). Ticagrelor plasma exposure increased approximately dose proportionally. No patients experienced a hemorrhagic event during treatment. No differences were seen between groups in pain ratings and analgesic use during Part B. Ticagrelor was well tolerated with no safety concerns, no discontinuations due to adverse events (AEs), and reported AEs were mainly due to SCD. In conclusion, a dose‐exposure‐response relationship for ticagrelor was demonstrated in children with SCD for the first time. These data are important for future pediatric studies of the efficacy and safety of ticagrelor in SCD.
1. INTRODUCTION
Current understanding of sickle cell disease (SCD) includes pathophysiology beyond the red blood cell, for example, inflammation, oxidant stress, vasoregulation, endothelial dysfunction, and thrombosis.1, 2, 3, 4, 5 Platelet activation is at the cross‐roads of most of these pathophysiologic pathways, both acute and chronic. In SCD, during the non‐crisis “steady state” platelets are activated, and during painful episodes, platelets are further activated.6 This process promotes adherence of sickle cells to vessel walls thereby contributing to vaso‐occlusion. Furthermore, platelets have a pivotal role in adhesion of neutrophils during vaso‐occlusive crises (VOCs).7 Based on these findings, inhibiting platelet activation maybe a potential therapeutic option to reduce VOC risk in SCD. However, a recent, phase 3 trial (DOVE) with the antiplatelet agent prasugrel in 341 children and adolescents with SCD, reported a small numerical reduction of VOC events with prasugrel (328 events, 2.30 events/person‐year) vs placebo (408 events, 2.77 events/person‐year) and no increased bleeding risk with prasugrel.8 In addition, the reduction in VOC rate did not reach statistical significance in the DOVE trial.8 A potential limitation of the DOVE trial was that the observed level of platelet inhibition (P2Y12 reaction units [PRU]) was only ~20% from baseline.9 Thus, it is of interest to assess the potential clinical consequences of a higher level of platelet inhibition in patients with SCD.
The direct‐acting antiplatelet agent ticagrelor binds reversibly to the P2Y12 receptor and inhibits adenosine diphosphate‐induced platelet aggregation.10, 11, 12 Ticagrelor also reduces cellular uptake of adenosine via inhibition of nucleoside transporter 1;12, 13, 14 this effect may have vasodilatory or anti‐inflammatory effects in ischemic tissues during impending or ongoing VOC. Based on the results of 2 phase 3 trials in adults with coronary artery disease (PLATO study, PEGASUS‐TIMI 54 study),15, 16 ticagrelor with low‐dose aspirin (75‐100 mg/d) is currently indicated to reduce the rate of cardiovascular death, myocardial infarction, and stroke in adult patients with acute coronary syndrome or a history of myocardial infarction.17 In adults, the pharmacokinetics (PKs) and pharmacodynamics (PDs) of ticagrelor and its active metabolite (AR‐C124910XX) have been extensively evaluated.18, 19, 20, 21 However, little is known about the dose to exposure relationship for ticagrelor and AR‐C124910XX, and the dose to platelet inhibition response relationship in children. To further explore the use of ticagrelor in other indications and for pediatric use (an unmet need), the HESTIA clinical development program is investigating the potential use of ticagrelor in patients with SCD, given the promising data on the role of platelet inhibition in SCD.
The primary objective of this 2‐part, multicenter dose‐finding study (HESTIA1; NCT02214121) of ticagrelor in children with SCD was to demonstrate the relationship between ticagrelor dose and inhibition of platelet aggregation in children with SCD. Secondary objectives included assessments of ticagrelor plasma exposure, safety and tolerability. An exploratory efficacy analysis was to assess the potential impact of ticagrelor on pain‐related measures, and investigation of ticagrelor palatability in the pediatric population.
2. METHODS
2.1. Patients
Key eligibility criteria included patients with a diagnosis of homozygous sickle cell (hemoglobin SS) or sickle beta‐zero (hemoglobin Sβ0) thalassemia, aged ≥2 to <18 years, and body weight >16 kg. Patients receiving hydroxyurea were required to have a stable dose for 1 month before enrollment. Patients on chronic red blood cell transfusion therapy were excluded. The major exclusion criteria were factors that would elevate risk of bleeding: history of transient ischemic attack or cerebrovascular accident; severe head trauma; intracranial hemorrhage; intracranial neoplasm; arteriovenous malformation or aneurysm; abnormal transcranial Doppler (TCD) flow velocities (middle cerebral artery and the internal carotid artery); use of nonsteroidal antiinflammatory drugs for >3 d/wk; treatment with anticoagulants or antiplatelet drugs that could not be discontinued; moderate or severe hepatic impairment; active pathological bleeding or increased risk of bleeding complications. Patients were required to have had a normal TCD (if aged ≤16 years) and an ophthalmological examination (if aged ≥6 years) within the previous 12 months. Females of child‐bearing potential had to use a highly effective contraception method or sexual abstinence, be nonpregnant, not breast feeding, and not planning a pregnancy during the study.
Patients were randomized at 17 centers across North America, Europe, Africa, and the Middle East, chosen to reflect the global ethnic diversity of SCD. Signed, informed consent was obtained from the parent(s)/legal guardian, and assent/consent was obtained from each child/adolescent prior to any study‐specific procedures according to local requirements. The final study protocol, amendments, and informed consent documentation were approved by an Ethics Committee and/or Institutional Review Board. The study was conducted in accordance with the Declaration of Helsinki and the International Conference on Harmonization/Good Clinical Practice Guidelines, and followed applicable regulatory requirements and the AstraZeneca policy on bioethics.
2.2. Study design and treatments
HESTIA1 was a phase 2 multicenter study consisting of 2 parts (Supporting information Figure S1).
Part A was an open‐label, dose‐ranging phase. Eligible patients were randomized (using an Interactive Voice Response System/Interactive Web Response System) 1:1 to receive 1 of 2 dosing schedules; an initial single ticagrelor dose of 0.125 mg/kg followed at least 7 days later by a second single dose at either 0.375 or 0.563 mg/kg. Single doses were followed by repeated dosing with open‐label ticagrelor 0.125 mg/kg b.i.d. for 1 week. Doses were increased after a prespecified interim analysis conducted after 12 patients had completed dosing in part A. In the second dosing schedule in part A, patients received an initial ticagrelor dose of 0.75 mg/kg followed 7 days later by a single dose of ticagrelor at 1.125 or 2.25 mg/kg then followed by open‐label ticagrelor at 0.563 or 0.75 mg/kg b.i.d. for 1 week. Ticagrelor treatment was discontinued in patients who had a repeated pronounced PD response despite a reduced dose.
Part B was an optional, double‐blind, placebo‐controlled extension phase for patients who completed part A. Eligible patients were randomized 2:1 to ticagrelor or matching placebo for 4 weeks. Patients randomized to ticagrelor received 0.125, 0.563, or 0.75 mg/kg b.i.d.
In both parts, ticagrelor (or matching placebo) was administered as an oral suspension. All patients were followed up 30‐35 days after the last dose of study drug.
2.3. Platelet inhibition and plasma exposure assessments
In part A, 4 mL blood samples for PRU assessments were collected predose and 2 hours postdosing and at 6 hours (patients >21 kg), after single ticagrelor doses. At the end of 7 days of repeated dosing, PRU samples were collected predose and 2 hours postdose. In the optional part B, sampling for PRU assessments could be collected after 4 weeks pre and postdose. PRU assessments were measured in each study center following blood collection, using the VerifyNow system that has been validated for direct clinical use and is a commercially available point‐of‐care assay, according to the manufacturer's instructions (Accumetrics, Inc., San Diego, California).
Blood samples for quantification of plasma concentrations of ticagrelor and AR‐C124910XX were collected up to 6 or 8 hours postdose after a single ticagrelor dose, and predose and 2 hours postdose after 7 days repeated dosing of ticagrelor in part A. In the optional part B, only limited blood samples could be collected after 4 weeks for assessment of exposure. Plasma samples were stored at −20°C in an upright position within 30 minutes of preparation and kept frozen until shipment. The frozen samples were shipped (on dry ice) to and analyzed by the central analytical laboratory (Indianapolis, Indiana).
Ticagrelor and the active metabolite were quantified in plasma using a fully validated liquid chromatography tandem mass spectrometry method.22 Ranges of the calibration curves were 1‐2000 ng/mL (ticagrelor) and 2.5‐1000 ng/mL (AR‐C124910XX) with a 100 μL sample volume.
2.4. Safety
Safety was assessed throughout both parts of the study by monitoring adverse events (AEs) and serious adverse events (SAEs), including bleeding events. AEs were coded according to the Medical Dictionary for Regulatory Activities version 19.0. Patients underwent physical examination at enrollment and follow‐up. Vital signs were checked at each study visit. 12‐lead electrocardiograms (ECGs) were collected at enrollment and after 7 days of dosing in part A. Standard clinical laboratory testing (hematology, clinical chemistry, and urinalysis) was conducted at enrollment, after 7 days of dosing in part A and at the end of treatment in part B.
2.5. Exploratory efficacy analyses and palatability assessments
In both parts of the study, patients ≥4‐years old completed an electronic diary on a handheld device (with the help of a parent/guardian as appropriate) daily to record pain (including intensity), analgesic use (including opioids), and days absent from school/work (ages ≥6 years only). From these diaries, the following efficacy variables were derived: days with pain, intensity of pain, days of analgesic use, days of opioid analgesic use, and days of absence from school or work.
Investigational product palatability was assessed qualitatively in a standardized way by a nurse at the study site based on observation of willingness to swallow the study drug and negative response behavior in children <6‐years old, and was patient‐reported using the Hedonic Faces Scale (5‐point scale ranging from “dislike very much” to “like very much”) in patients ≥6 years.
2.6. Descriptive data analyses
No formal sample size calculation was performed. The sample size was selected to generate sufficient data for a population PK‐PD modeling‐ and simulation‐based dose selection in subsequent efficacy studies, whilst exposing a minimum number of pediatric patients to ticagrelor.
Absolute values and relative change from baseline of PRU by dose group were evaluated graphically (box plots) and with descriptive statistics (mean and SD) at 2 hours postdose. Ticagrelor and AR‐C124910XX plasma concentrations (geometric mean) were evaluated graphically over time after dosing and by dose group (box plots) at 2 hours postdose. AEs were summarized by number and percent of patients. Efficacy analyses were based on randomized treatment, and safety analyses were based on actual treatment received.
3. RESULTS
Data underlying the findings described in this article may be obtained in accordance with AstraZeneca's data sharing policy available at https://astrazenecagrouptrials.pharmacm.com/ST/Submission/Disclosure.
3.1. Patient disposition and demographics
A total of 18 centers screened patients (see acknowledgments), and 17 sites randomized patients in the USA (6), UK (4), Kenya (2), Lebanon (2), South Africa (2), and Canada (1) between September 11, 2014 (first patient randomized) and February 27, 2017 (last patient completed the study).
In part A, 46 patients were randomized, 45 received at least 1 ticagrelor dose, and 39 patients completed this part (Supporting Information Figure S2). The discontinuation reasons were: patient decision (n = 2; 1 of these patients had insufficient venous access to obtain a predose blood sample and was not dosed; neither withdrawal was due to AEs); development of study‐specific withdrawal criteria (ie, repeated low PRU values; n = 4). A total of 25 patients were randomized, 23 received the relevant study treatment, and 21 patients completed part B (Supporting Information Figure S2). The discontinuation reasons were: patient decision (n = 1; ticagrelor); development of study‐specific withdrawal criteria (ie, repeated low PRU values; n = 2; ticagrelor); and lost to follow‐up (n = 1; placebo).
Key demographic/baseline characteristics for patients dosed with ticagrelor in part A are shown in Table 1. Mean patient age was 11.2 years, and most patients (77.8%) were Black or African American. Twenty‐four patients (53.3%) were female, and about two‐thirds (66.7%) of patients had experienced a prior SCD complication (Supporting Information Table S1). Approximately 80% of patients were being treated with hydroxyurea.
Table 1.
Patient baseline characteristics of ticagrelor‐dosed patients in part A
| Characteristic | Ticagrelor (n = 45) |
|---|---|
| Age | |
| Mean (range) (years) | 11.2 (3–17) |
| 2‐11 years, n (%) | 24 (53.3) |
| 12‐18 years, n (%) | 21 (46.7) |
| Sex, n (%) | |
| Male | 21 (46.7) |
| Female | 24 (53.3) |
| Race, n (%) | |
| White | 10 (22.2) |
| Black or African American | 35 (77.8) |
Data shown for randomized patients taking at least 1 dose of ticagrelor.
Treatment compliance was high; median compliance with dosing was 100% in part A and 97.4% (ticagrelor) and 98.3% (placebo) in part B. In the 4‐week long part B, patients took the study drug for a mean (SD) of 27.4 (6.4) and 29.4 (3.4) days in the ticagrelor and placebo groups, respectively.
3.2. Platelet inhibition response
A clear dose–response relationship was seen, and the effect on both absolute and relative PRU is shown in Figure 1. As expected, due to the reversible mode of action of ticagrelor, PRU values returned towards baseline levels with the decline in ticagrelor plasma exposure at 6‐8 postdose (data not shown). In part A, PRUs measured prior to dosing were similar for each ticagrelor dose group (Supporting Information Table S2). PRU inhibition at 2 hours after single ticagrelor doses ranged from 6% (0.125 mg/kg) to 73% (2.25 mg/kg) (Supporting Information Table S2). With twice‐daily ticagrelor doses in part A, mean (SD) PRU inhibition at 2 hours postdose relative to baseline were −11.1% (21.8), −58.0% (29.8), and −45.2% (27.2) for ticagrelor 0.125, 0.536, and 0.75 mg/kg bid, respectively (Supporting Information Table S2).
Figure 1.
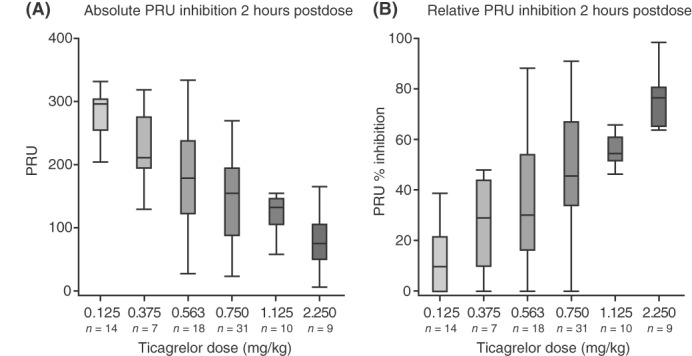
Boxplots of absolute (A) and relative (B) PRU at 2 hours postdose after single ticagrelor doses in part A. A, the observed PRU values at 2 hours post a single dose at both visits 2 and 3. PRU % inhibition was calculated by the dividing the observed PRU value at 2 hours by the individual baseline PRU value (1−[observed PRU/baseline PRU]) × 100, values above 100% and below 0% were imputed to 100% and 0%, respectively (Figure B only). For patients missing a visit 2 baseline PRU, the visit 3 predose value (following a 1‐week wash‐out) was used. The boxplot whiskers display the 5th and 95th percentile of the data, values beyond this (outliers) are excluded from the graphs. Abbreviation: PRU, P2Y12 reactivity unit
3.3. Ticagrelor and AR‐C124910XX plasma exposure
Exposure to ticagrelor and AR‐C124910XX increased approximately linearly with ticagrelor dose (Figure 2). Mean plasma concentration and time profiles for each of the single ticagrelor doses are shown in Supporting Information Figure S3. Mean 2‐hour ticagrelor plasma concentrations after a single ticagrelor dose were 13.6, 45.2, 86.9, 132, 216, and 478 ng/mL for the 0.125, 0.375, 0.563, 0.75, 1.125, and 2.25 mg/kg doses, respectively. At each dose level, plasma concentrations of AR‐C124910XX were approximately one‐third of ticagrelor concentrations (Figure 2).
Figure 2.
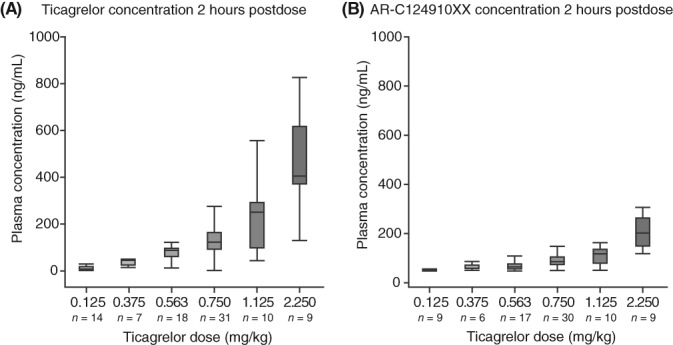
Boxplots of observed plasma concentrations at 2 hours postdose of (A) ticagrelor and (B) AR‐C124910XX following a single ticagrelor dose. The boxplot whiskers display the 5th and 95th percentile of the data, values beyond this (outliers) are excluded from the graphs. Values below the limit of quantification of the bioanalysis method are not included
At steady‐state in part A, mean ticagrelor plasma concentrations 2 hours postdosing (on day 7) were 18.1, 123, and 121 ng/mL for the 0.125, 0.563, and 0.75 mg/kg bid doses, respectively.
3.4. Safety and tolerability
No deaths or AEs leading to discontinuation of the study drug or to patient withdrawal occurred during either part of the study. The proportion of patients experiencing at least one AE during treatment was similar across all tested ticagrelor doses. SAEs developed in 5 patients in each of parts A and B of the study (Table 2). The most commonly reported AEs in part A were sickle cell anemia with crisis (ie, vaso‐occlusive pain), abdominal pain, arthralgia, and pain in extremity (Table 2). Similarly, these AEs were also the most common in part B in both the ticagrelor and placebo groups (Table 2). No patients experienced a hemorrhagic event during treatment, although 1 patient in the ticagrelor group in part B reported a mild spontaneous epistaxis on day 29 after the last ticagrelor dose during the follow‐up period, which was considered to be a minor bleed.
Table 2.
AEs regardless of causality
| AEs, n (%)a | Part A | Part B | |
|---|---|---|---|
| Ticagrelor (n = 45) | Ticagrelor (n = 16) | Placebo (n = 7) | |
| Any AE | 30 (66.7) | 13 (81.3) | 6 (85.7) |
| Any SAE | 5 (11.1) | 4 (25.0) | 1 (14.3) |
| Any AE leading to dose interruption | 1 (2.2) | 1 (6.3) | 1 (14.3) |
| AEs considered related to study drug | 2 (4.4) | 0 | 0 |
| AEs occurring in ≥2 patients in any arm | |||
| Sickle cell anemia with crisisb | 9 (20.0) | 4 (25.0) | 2 (28.6) |
| Abdominal pain | 6 (13.3) | 3 (18.8) | 2 (28.6) |
| Arthralgia | 5 (11.1) | 4 (25.0) | 2 (28.6) |
| Pain in extremity | 5 (11.1) | 3 (18.8) | 2 (28.6) |
| Back pain | 3 (6.7) | 2 (12.5) | 1 (14.3) |
| Headache | 4 (8.9) | 0 | 2 (28.6) |
| Facial pain | 2 (4.4) | 1 (6.3) | 1 (14.3) |
| Noncardiac chest pain | 2 (4.4) | 1 (6.3) | 1 (14.3) |
| Vomiting | 0 | 3 (18.8) | 0 |
| Oropharyngeal pain | 1 (2.2) | 3 (18.8) | 0 |
| Upper abdominal pain | 0 | 2 (12.5) | 0 |
| Pyrexia | 1 (2.2) | 2 (12.5) | 0 |
| Musculoskeletal pain | 1 (2.2) | 2 (12.5) | 1 (14.3) |
| Cough | 1 (2.2) | 2 (12.5) | 0 |
| SAEs | |||
| Sickle cell anemia with crisisb | 3 (6.7) | 3 (18.8) | 1 (14.3) |
| Gastroenteritis viral | 1 (2.2) | 0 | 0 |
| Acute chest syndrome | 1 (2.2) | 1 (6.3) | 0 |
| Hemorrhagic events | 0 | 0 | 0 |
Events occurring during follow‐up are not shown.
Abbreviations: AE, adverse event; MedDRA, Medical Dictionary for Regulatory Activities; SAE, serious adverse event.
Safety analysis is based on the actual treatment received.
Preferred term according to MedDRA version 19.1.
Two patients in part A developed AEs that were considered to be treatment related as judged by the investigator: 1 patient had abdominal pain, and the other patient had headache and jaundice. No treatment‐related AEs were reported in part B.
Most AEs were mild‐to‐moderate in intensity. Three patients in each part of the study had severe AEs as judged by the investigator, none of which were considered related to ticagrelor: sickle cell anemia with crisis (n = 2) and acute chest syndrome (n = 1) in part A, and sickle cell anemia with crisis (n = 2; ticagrelor group) and acute chest syndrome (n = 1; ticagrelor group) in part B.
There were no clinically meaningful differences across the groups in the physical examinations, vital signs, ECGs, or changes from baseline in laboratory parameters (hematology, clinical chemistry, and urinalysis).
3.5. Exploratory efficacy analyses and palatability
During part A, 43 of 44 children ≥4‐years old reported in the electronic diary. The mean (SD) proportion of self‐reported days with pain was 25.1% (31.1). In part B, the mean (SD) proportion of self‐reported days with pain was similar between ticagrelor (n = 14) and placebo (n = 8), that is, 27.0% (24.1) and 31.8% (23.7), respectively. There were no clinically relevant findings or differences between ticagrelor and placebo in any of the other exploratory efficacy variables assessed (data not shown).
Palatability was generally good. Children <6‐years old swallowed the ticagrelor suspension without problem and did not exhibit any negative response behavior. For children ≥6 years, the number self‐reporting “dislike very much”, “dislike a little”, “not sure”, “like a little”, and “like very much” was 1 (2.3%), 12 (27.9%), 10 (23.3%), 11 (25.6%), and 6 (14.0%), respectively.
4. DISCUSSION
HESTIA1 is the first pediatric study to demonstrate the relationship between ticagrelor dose, exposure and inhibition of platelet aggregation in children with SCD. PRU values decreased after ticagrelor dosing (predose to 2 hours postdosing). Mean PRU decreased in a dose‐dependent manner, ranging from a mean PRU reduction at 2 hours of 73% after a single dose of 2.25 mg/kg to 6% after a single ticagrelor dose of 0.125 mg/kg. As expected, ticagrelor plasma exposure appeared to increase approximately proportionally with increasing ticagrelor doses and was predictable. It is encouraging to note that ticagrelor was well tolerated in this cohort of children with SCD, and few patients withdrew from the study. Importantly, ticagrelor did not increase the risk of bleeding in this study population, and no safety concerns were raised from the evaluated dose range given as single doses and repeated doses. Treatment compliance was in excess of 90%. The ticagrelor oral suspension used in this study appeared to be palatable to the patients in this study.
Time‐matched plasma concentration and PRU measurements were collected to enable evaluation of ticagrelor exposure to the observed effect on PRU. The ticagrelor:metabolite ratio of 3:1 was also similar in children with SCD compared with adult healthy volunteers.23 Limited accumulation of plasma concentrations was observed when comparing the 2‐hour plasma exposure after a single ticagrelor dose and at steady state following twice‐daily dosing. The relationship between ticagrelor exposure and PD effect in this population is in line with that observed in young adult (18‐30 years) SCD patients (AstraZeneca data on file), and in other adults (healthy volunteers and patients with acute coronary syndromes).21
A similar platelet inhibition response in different age groups is consistent with the results of an in‐vitro investigation comparing the anti‐platelet potency of ticagrelor in children and adults.24 In this in vitro assessment, ticagrelor (0.01‐10 μmol/L) was added to platelet‐rich plasma from infants and children (0‐2, 2‐6 months, 6 months‐2 years, 2‐6, and 6‐12 years) and adults (≥18 years), and platelet inhibition was evaluated. For all infant and child age groups, the potency of ticagrelor was comparable with that seen in adults. These findings suggest that at equivalent levels of drug exposure, children and adults would have a comparable antiplatelet response to ticagrelor, and that platelets in children do not react differently to ticagrelor,24 which are supported by our current findings in pediatric patients with SCD.
HESTIA1 did include limited secondary/exploratory efficacy assessments. Due to the small number of patients analyzed for these efficacy variables and the fact that most patients randomized to ticagrelor received a dose providing only minor platelet inhibition, the ability to draw conclusions was limited with regard to clinical efficacy. A further constraint of this analysis was the short study duration. A longer study with a larger group of patients would be required to evaluate the potential effects of ticagrelor on VOCs in SCD.
There is a clear physiological rationale for studying antiplatelet agents in SCD. Interactions between sickle cells, endothelial cells, and plasma constituents initiate and sustain vaso‐occlusion.2 Platelet activation is increased in SCD patients even in the basal state, and increases further during VOC.6 Not only does platelet activation directly increase blood coagulability, it also potentiates or activates other clotting pathways that contribute to intravascular thrombosis and vasculopathy.6 Thus, inhibition of platelet activation has potential as a therapeutic option in SCD, and may result in decreased incidence and severity of vaso‐occlusion and modification of other disease manifestations related to microvascular occlusion.
To date, there is some clinical support for the potential use of agents inhibiting platelet activation or agents affecting down‐stream effects of activated platelets in SCD, such as a ticlopidine study showing a reduction in VOC rate of >60%25 and the recent study with crizanlizumab,26 a monoclonal antibody against P‐selectin, which is expressed on the surface of activated platelets and promotes cell aggregation during sickling,27 demonstrating >50% reduction in pain crises compared with placebo. In the recent phase 3 trial DOVE evaluating prasugrel in children and adolescents (2‐17 years), numerical reductions were seen for prasugrel compared with placebo in VOC event rate8 at a modest platelet inhibition.9 The present HESTIA1 study evaluated a broad range of platelet inhibition which was well tolerated with no safety concerns raised for the ticagrelor dose range evaluated. These findings, therefore, pave the way for future studies with ticagrelor to evaluate the efficacy and safety of a higher degree of platelet inhibition than in previous outcome studies in SCD with platelet inhibitors.
No safety concerns were raised in pediatric patients receiving single and multiple doses of ticagrelor in the present study, and ticagrelor had an acceptable tolerability profile in this age group. No bleeding events were seen during treatment with ticagrelor. Indeed, most of the reported AEs in HESTIA1 are consistent with common medical issues in children with SCD.28, 29, 30, 31 However, the safety profile of ticagrelor in this population will need further investigation in subsequent studies to establish the efficacy‐safety profile.
Our study is not without limitations. A small number of patients were randomized, although it is not appropriate to use large numbers of children at the early dose‐ranging phase of clinical research. The observed PRU values were variable, as seen in the baseline samples and following placebo treatment. The observed higher platelet inhibition with 0.563 mg/kg bid compared with 0.75 mg/kg bid ticagrelor may be attributed to the relatively small number of patients and the observed variability in the PRU assay. The study also had a short duration, and was not statistically powered to detect differences in clinical outcomes (ie, endpoints evaluated in the exploratory analyses reported herein) and safety vs placebo. However, this study was conducted at multiple centers around the world and ticagrelor was evaluated on a background of hydroxyurea in most patients, capturing data from a population representative of the global SCD population.
In conclusion, HESTIA1 is the first study to assess the dose to ticagrelor and AR‐C124910XX exposure, and dose to platelet inhibition response profile in children with SCD. The sensitivity to ticagrelor on platelet inhibition in these children appeared to be similar to that observed in healthy adults and adult patients with acute coronary syndrome/coronary artery disease. Overall, no safety concerns were raised from treating pediatric patients with a broad range of single and repeated doses of ticagrelor during the study. The potential of ticagrelor to impact SCD‐related pain crises warrants further evaluation in this population, and a phase 3 study (HESTIA3) is planned.32
CONFLICT OF INTEREST
LLH received funding from AstraZeneca for conducting the clinical trial and for serving on the Steering Committee, and was an investigator on HESTIA1. SS has attended advisory boards for AstraZeneca, and served on the HESTIA1 Steering Committee and was an investigator on HESTIA1. SW served on the HESTIA1 Steering Committee and was an investigator on HESTIA1. CA, JW, and AB are employees of AstraZeneca, and hold stocks and shares in AstraZeneca.
Supporting information
SUPPORTING INFORMATION TABLE S1 Patient baseline SCD characteristics of ticagrelor‐dosed patients in part A
SUPPORTING INFORMATION TABLE S2 Summary of absolute value and percent change from baseline in PRU by actual treatment and dose in part A
SUPPORTING INFORMATION FIGURE S1 Study design
SUPPORTING INFORMATION FIGURE S2 Patient disposition
SUPPORTING INFORMATION FIGURE S3 Plasma concentration time profiles for single doses of ticagrelor
ACKNOWLEDGMENTS
The authors on this article are the Study Steering Committee members for HESTIA1 and selected people from the AZ/Sponsor team. All authors recognize and appreciate the contribution made to this study by all of the investigators, staff, the patients and their families at the other participating centers who screened/randomized patients for HESTIA1 ‐ USA: Penn State Hershey Children's Hospital, Hershey, PA; Medical University of South Carolina, Charleston, SC; Children's Hospital of Philadelphia, Philadelphia, PA; Children's Hospital of Orange County, Orange, CA; UK: University Health Board, Cardiff; Evelina London Children's Hospital, St. Thomas Hospital, London; Royal London Hospital‐Barts Health NHS Trust, London; Royal Manchester Children's Hospital‐Central Manchester University Hospital NHS Foundation Trust, Manchester; Kenya: Gertrude's Childrens’ Hospital, University of Nairobi, Nairobi; KEMRI ‐ US Army ‐ Kenya, Medical Research, Kisumu; Lebanon: Rafik Hariri University Hospital, Bir Hasan, Beirut; American University of Beirut Medical Center, Pediatrics/Adolescent Medicine Department, Hamra, Beirut; Nini Hospital, Tripoli; South Africa: Red Cross War Memorial Childrens' Hospital, Cape Town, Western Cape; Tygerberg Hospital, University of Stellenbosch, Parow Valley, Cape Town. The authors also thank Jackie Phillipson from Zoetic Science (UK), who provided medical writing support (outline, drafts, assembling tables and figures, collating author comments, grammatical editing, and referencing) funded by AstraZeneca. This study was funded by AstraZeneca.
AUTHOR CONTRIBUTIONS
Contributed to conception and design (protocol development and/or design advice), acquisition of data, data analysis and data interpretation.: LLH, SS, SW, CA.
Contributed to acquisition of data and data interpretation: JW, AB.
Hsu LL, Sarnaik S, Williams S, Amilon C, Wissmar J, Berggren A, on behalf of the HESTIA1 Investigators:. A dose‐ranging study of ticagrelor in children aged 3‐17 years with sickle cell disease: A 2‐part phase 2 study. Am J Hematol. 2018;93:1493–1500. 10.1002/ajh.25273
Funding information: AstraZeneca
REFERENCES
- 1. Ataga KI, Brittain JE, Desai P, et al. Association of coagulation activation with clinical complications in sickle cell disease. PLoS One. 2012;7:e29786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Zhang D, Xu C, Manwani D, Frenette PS. Neutrophils, platelets, and inflammatory pathways at the nexus of sickle cell disease pathophysiology. Blood. 2016;127:801‐809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Owusu‐Ansah A, Ihunnah CA, Walker AL, Ofori‐Acquah SF. Inflammatory targets of therapy in sickle cell disease. Transl Res. 2016;167:281‐297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sparkenbaugh E, Pawlinski R. Prothrombotic aspects of sickle cell disease. J Thromb Haemost. 2017;15:1307‐1316. [DOI] [PubMed] [Google Scholar]
- 5. Ansari J, Moufarrej YE, Pawlinski R, Gavins FNE. Sickle cell disease: a malady beyond a hemoglobin defect in cerebrovascular disease. Expert Rev Hematol. 2018;11:45‐55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Noubouossie D, Key NS, Ataga KI. Coagulation abnormalities of sickle cell disease: relationship with clinical outcomes and the effect of disease modifying therapies. Blood Rev. 2016;30:245‐256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bennewitz MF, Jimenez MA, Vats R, et al. Lung vaso‐occlusion in sickle cell disease mediated by arteriolar neutrophil‐platelet microemboli. JCI Insight. 2017;2:e89761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Heeney MM, Hoppe CC, Abboud MR, et al. A multinational trial of prasugrel for sickle cell vaso‐occlusive events. N Engl J Med. 2016;374:625‐635. [DOI] [PubMed] [Google Scholar]
- 9. Jakubowski JA, Hoppe CC, Zhou C, et al. Real‐time dose adjustment using point‐of‐care platelet reactivity testing in a double‐blind study of prasugrel in children with sickle cell anaemia. Thromb Haemost. 2017;117:580‐588. [DOI] [PubMed] [Google Scholar]
- 10. Husted S, van Giezen JJ. Ticagrelor: the first reversibly binding oral P2Y12 receptor antagonist. Cardiovasc Ther. 2009;27:259‐274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. van Giezen JJ, Nilsson L, Berntsson P, et al. Ticagrelor binds to human P2Y(12) independently from ADP but antagonizes ADP‐induced receptor signaling and platelet aggregation. J Thromb Haemost. 2009;7:1556‐1565. [DOI] [PubMed] [Google Scholar]
- 12. Nylander S, Schulz R. Effects of P2Y12 receptor antagonists beyond platelet inhibition – comparison of ticagrelor with thienopyridines. Br J Pharmacol. 2016;173:1163‐1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Armstrong D, Summers C, Ewart L, Nylander S, Sidaway JE, van Giezen JJ. Characterization of the adenosine pharmacology of ticagrelor reveals therapeutically relevant inhibition of equilibrative nucleoside transporter 1. J Cardiovasc Pharmacol Ther. 2014;19:209‐219. [DOI] [PubMed] [Google Scholar]
- 14. Nylander S, Femia EA, Scavone M, et al. Ticagrelor inhibits human platelet aggregation via adenosine in addition to P2Y12 antagonism. J Thromb Haemost. 2013;11:1867‐1176. [DOI] [PubMed] [Google Scholar]
- 15. Wallentin L, Becker RC, Budaj A, et al. Ticagrelor versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2009;361:1045‐1057. [DOI] [PubMed] [Google Scholar]
- 16. Bonaca MP, Bhatt DL, Cohen M, et al. Long‐term use of ticagrelor in patients with prior myocardial infarction. N Engl J Med. 2015;372:1791‐1800. [DOI] [PubMed] [Google Scholar]
- 17.Brilinta (ticagrelor) tablets, for oral use. US prescribing Information. Revised March 2018. Available at: http://www.azpicentral.com/brilinta/brilinta.pdf. Last accessed April 19, 2018.
- 18. Butler K, Teng R. Pharmacokinetics, pharmacodynamics, safety and tolerability of multiple ascending doses of ticagrelor in healthy volunteers. Br J Clin Pharmacol. 2010;70:65‐77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Teng R, Butler K. Pharmacokinetics, pharmacodynamics, tolerability and safety of single ascending doses of ticagrelor, a reversibly binding oral P2Y(12) receptor antagonist, in healthy subjects. Eur J Clin Pharmacol. 2010;66:487‐496. [DOI] [PubMed] [Google Scholar]
- 20. Husted S, Emanuelsson H, Heptinstall S, Sandset PM, Wickens M, Peters G. Pharmacodynamics, pharmacokinetics, and safety of the oral reversible P2Y12 antagonist AZD6140 with aspirin in patients with atherosclerosis: a double‐blind comparison to clopidogrel with aspirin. Eur Heart J. 2006;27:1038‐1047. [DOI] [PubMed] [Google Scholar]
- 21. Teng R. Ticagrelor: pharmacokinetic, pharmacodynamic and pharmacogenetic profile: an update. Clin Pharmacokinet. 2015;54:1125‐1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sillén H, Cook M, Davis P. Determination of ticagrelor and two metabolites in plasma samples by liquid chromatography and mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci. 2010;878:2299‐2306. [DOI] [PubMed] [Google Scholar]
- 23. Teng R, Oliver S, Hayes MA, Butler K. Absorption, distribution, metabolism, and excretion of ticagrelor in healthy subjects. Drug Metab Dispos. 2010;38:1514‐1521. [DOI] [PubMed] [Google Scholar]
- 24. Söderlund F, Asztély A‐K, Jeppsson A, et al. In vitro anti‐platelet potency of ticagrelor in blood samples from infants and children. Thromb Res. 2015;136:620‐624. [DOI] [PubMed] [Google Scholar]
- 25. Cabannes R, Lonsdorfer J, Castaigne JP, Ondo A, Plassard A, Zohoun I. Clinical and biological double‐blind‐study of ticlopidine in preventive treatment of sickle‐cell disease crises. Agents Actions Suppl. 1984;15:199‐212. [PubMed] [Google Scholar]
- 26. Ataga KI, Kutlar A, Kanter J, et al. Crizanlizumab for the prevention of pain crises in sickle cell disease. N Engl J Med. 2017;376:429‐439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Matsui NM, Borsig L, Rosen SD, Yaghmai M, Varki A, Embury SH. P‐selectin mediates the adhesion of sickle erythrocytes to the endothelium. Blood. 2001;98:1955‐1962. [DOI] [PubMed] [Google Scholar]
- 28. Ware RE, de Montalembert M, Tshilol L, et al. Sickle cell disease. Lancet. 2017;390:311‐323. [DOI] [PubMed] [Google Scholar]
- 29. Novelli EM, Gladwin MT. Crises in sickle cell disease. Chest. 2016;149:1082‐1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Subramaniam S, Chao JH. Managing acute complication of sickle cell disease in pediatric patients. Pediatr Emerg Med Pract. 2013;13:1‐28. [PubMed] [Google Scholar]
- 31. Chakravorty S, Williams TN. Sickle cell disease: a neglected chronic disease of increasing global health importance. Arch Dis Child. 2015;100:48‐53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Heeney MM, Abboud MR, Amilon C, et al. Ticagrelor versus placebo for the reduction of vaso‐occlusive crises in pediatric sickle cell disease: design of a randomized, double‐blind, parallel‐group, multicenter, phase 3 study (HESTIA3). HemaSphere. 2018;2(S1):669 abstract PS1460. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
SUPPORTING INFORMATION TABLE S1 Patient baseline SCD characteristics of ticagrelor‐dosed patients in part A
SUPPORTING INFORMATION TABLE S2 Summary of absolute value and percent change from baseline in PRU by actual treatment and dose in part A
SUPPORTING INFORMATION FIGURE S1 Study design
SUPPORTING INFORMATION FIGURE S2 Patient disposition
SUPPORTING INFORMATION FIGURE S3 Plasma concentration time profiles for single doses of ticagrelor


