Abstract
Neutrophils are one of the most important effector cells of the innate immune response (1). They are traditionally seen as a homogenous population of short‐lived cells mainly involved in the defence against extracellular microorganisms by phagocytosis and intracellular killing (1,2). The cells contain a large armamentarium that aids in this function and ranges from the production of reactive oxygen species by a membrane‐bound NADPH oxidase to cytotoxic proteins and peptides residing in the different granules present in the cytoplasm (3). Recently, the view of neutrophils belonging to a homogenous population of cells has been challenged, and several neutrophil phenotypes have been described that exhibit specialized functions, such as involvement in tissue repair, tumour killing and immune regulation (4). It is not clear whether these cells belong to separate parallel lineages originating from the bone marrow or that neutrophils become instructed in the distant tissues, thus changing their phenotypes. In addition, functional heterogeneity in a phenotypically homogenous population of neutrophils adds to the complexity of neutrophil phenotypes(5). This article will review the current literature describing the heterogeneity within the neutrophil compartment with respect to both phenotype and function in health and disease.
Keywords: challenge, immune, immune regulation, inflammation, neutrophil, origin, phenotype
1. INTRODUCTION: NEUTROPHILS AS EFFECTOR CELL OF THE INNATE IMMUNE SYSTEM
Neutrophils are evolutionary old cells that have evolved from specialized cells that gained the propensity to phagocytose targets. Such phagocytosing cells are found throughout the animal kingdom from corals to mammals.6 In man, neutrophils are the most abundant innate immune cells in the peripheral blood and comprise about 60%‐70% of all leucocytes in this compartment. The cells quickly respond to inflammatory cues coming from infectious or damaged areas outside the blood system.3 They express several receptors by which they can sense soluble inflammatory mediators, ranging from bioactive lipids to cytokines.1, 3 The first mechanism that is initiated by interaction with these mediators is a priming response by which the cells switch to a pre‐activated state.7 This process is reviewed by Vogt et al in this special issue. The priming response is also instrumental for the cells to be able to interact with the vessel wall and transmigrate through the endothelium.3 Transmigration is both mediated by upregulated surface expression of adhesion molecules8 and inside‐out activation of integrin receptors, normally present in an inactive configuration on unprimed cells.9 After extravasation, the cells will migrate along gradients of chemotactic signals and thereby find the area with infection and/or tissue damage.10 Here, the cells engage in killing of microorganisms and/or clearance of damaged tissue. Again, priming is important, as the killing mechanisms are greatly enhanced after stimulation by inflammatory mediators.11, 12 For example, priming of neutrophils with TNF‐α enhances ROS production, degranulation and chemotaxis in response to other activating stimuli.13 Besides phagocytosis and intracellular killing, also extracellular killing mechanisms are operational through release of ROS and degranulation of granule contents.14 In the last decade, another extracellular mechanism has been discovered by which neutrophils can trap microorganisms in nets of DNA through a process referred to as NETosis.15 This NETosis is reviewed in this special issue by Van Avondt and Hartl.
2. THE MARGINATED POOL
The number of neutrophils in the peripheral blood can change greatly by relatively small changes in homeostasis. It has already been described decades ago that treatment with stress hormones such as epinephrine and cortisol can immediately liberate many additional neutrophils to the peripheral blood16; cells with a seemingly identical phenotype compared to the cells present in the blood previous to the challenge.17 A similar situation can be evoked by exercise.18 Again, these “demarginated” cells seem to be identical to the pre‐exercise cells.17 The consensus in the field is that the marginated pool consists of cells interacting with the vessel wall and that the cells are in complete equilibrium with the free‐flowing cells.2 Therefore, marginated and demarginated cells are not seen as functional phenotypes and will not be dealt with in the remainder of this review.
3. PRIMING, ACTIVATION, DIFFERENTIATION, AGEING AND DEATH
Neutrophils are extremely sensitive for environmental cues and express a multitude of receptors transducing the presence of these signals to the intracellular milieu. The cells can integrate all these signals into a changed activation status ranging from priming to full activation.7 This can result in a spectrum of neutrophil subtypes described in this review, an overview is provided in Figure 1. It is essential to define the different states of the cells that can be found in health and disease:
Figure 1.
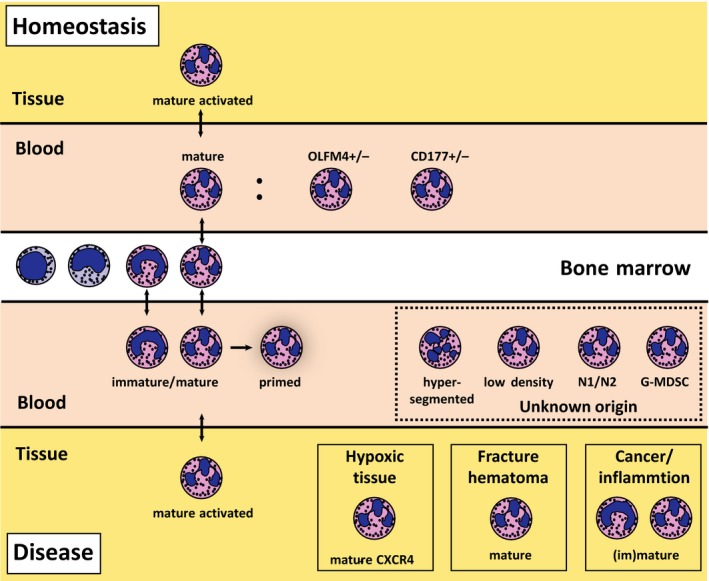
Neutrophil phenotypes in health and disease. Neutrophils develop in the bone marrow and leave the bone marrow after 5‐6 days.20 The cells circulate as a homogeneous population in the peripheral blood and can go to the tissues in small numbers under homeostatic conditions. In disease, neutrophil phenotypes redistribute and are present in peripheral blood and distant tissues. The origin of at least part of the additional blood neutrophil phenotypes remains to be established
Priming and activation are defined as a different state of cells belonging to the same phenotype. Priming and activation are typically not associated with differentiation and occur relatively fast.
A phenotype is defined as a neutrophil that has gained or lost a specialized function not shared with other phenotypes of cells. A clear example that will be reviewed in detail below is the propensity to inhibit T cells. Phenotypes likely originate from differences in differentiation of neutrophils in the bone marrow or in distant tissues. This process is relatively slow.
Obviously, it is difficult to discriminate between differentiation and activation, as the neutrophil compartment is highly dynamic with a clear exchange between blood, bone marrow and distant tissues.
Multiple studies have connected neutrophil age with specific markers and functions. The nuclear segmentation typical for neutrophils has been used as an indicator for their age. In a multitude of inflammatory conditions, immature neutrophils with a non‐segmented banded nucleus are released from the bone marrow, causing a so‐called left shift.19 Indeed, using an in vivo labelling approach, we have recently shown these banded neutrophils are younger than their segmented counterparts. However, unexpectedly, hypersegmented neutrophils, which are mobilized into the peripheral blood during acute inflammation, are not older than the cells with a normal nuclear morphology.20 The origin of neutrophils with a hypersegmented nucleus is still obscure, but the phenotype does not seem to be induced by activation, because the proteome is completely different and the cells are characterized by a marked downregulation of the protein translation machinery.20 It is clear that vitamin B12 deficiency is also associated with neutrophils with a hypersegmented nucleus, but at present, it is uncertain whether these cells belong to the same phenotype as the one found during acute inflammation.21
Besides nuclear morphology, several membrane markers have been associated with cellular age. These are typically differentially expressed between young neutrophils with a banded nucleus, and mature cells with a segmented nucleus. A clear example is the neutral endopeptidase CD10, which shows a lower expression on neutrophils with a banded nucleus.22 Unfortunately, these markers (see, eg, CD10, CD11b, CD15) are typically also induced on normal, mature cells by short‐term activation.22, 23, 24 Uhl et al25 used in vivo BrdU labelling of neutrophils in mice to show that aged neutrophils with a highly reactive phenotype are present in the circulation in an endotoxemia model. Comparable experiments in human are still missing, and the presence and function of aged neutrophils in inflammatory conditions thus remains uncertain.
At the end of life, neutrophils are generally thought to die by apoptosis in the tissues and to be cleared by phagocytosis by macrophages in a process generally referred to as efferocytosis.26 However, in vivo data supporting this concept are mainly from inflammatory conditions. The fate of neutrophils under homeostasis is much less clear, but there are solid data that describe the CXCR4/SDF axis as an important mechanism to clear aged neutrophils in the bone marrow27 (review by de Filippo and Rankin in this special issue).
Additionally, there is no clear consensus on the half‐life of neutrophils in peripheral blood, bone marrow and in the tissues. Novel methodology applying the stable isotope deuterium has led to conflicting results ranging from a relatively long lifespan of around 5 days28 to 19 hours.29 These conflicting results are caused by differences in the assumptions regarding the behaviour of progenitor cells in the bone marrow and the size of the neutrophil compartment.30
4. THE NEUTROPHIL IN TUMOUR SURVEILLANCE
The first clues that the neutrophil compartment was heterogeneous came from studies in the cancer field that identified myeloid‐derived suppressor cells (MDSCs31) and N1/N2 tumour‐associated neutrophils in mice.32 These experiments provided evidence that neutrophils could affect the immune response against tumours. Different neutrophil subsets can be involved in better tumour killing (N1 cells) and/or in suppressed immunity against tumours (N2 cells and MDSCs). Although understanding of these phenotypes holds great promise for cancer treatment and has rightfully raised great enthusiasm in the cancer immunity field, the concept is still in its early development. Additionally, until now, the existence of N1/N2 neutrophils in human is not shown.
The difference in N1/N2 cells has so far mainly been described in mice, and often in terms of expression of proteins that might be involved in anti‐ or pro‐tumour immune responses. Unfortunately, few mechanistic data are available obtained with isolated cells. MDSCs have mainly been studied in mice, and often, these cells are studied in bulk, encompassing both monocytes and neutrophils, and often not distinguishing this population from normal mature neutrophils.33 As neutrophils in tumour‐bearing mice display the typical left shift, it has been proposed that MDSCs are relatively young.34 However, Youn et al provided data that also neutrophils with a mature nucleus can act as MDSC.35 The field still awaits proper definition which cells in the MDSC pool are pro‐tumorigenic and which processes are operational in these cells.36 In the mouse models, arginase has been shown to play an important role, but the role of arginase in human MDSC is still conflicting.37, 38
It is important to emphasize that at least in vitro neutrophils are potent killers of tumour cells by antibody‐dependent cell‐mediated cytotoxicity (ADCC39) and trogocytosis.40 Moreover, the presence of these cells in the tumour tissue can be associated with a favourable outcome in the resolution of these tumours.32 This situation is a clear example for the dilemmas faced in the treatment of neutrophil‐mediated diseases. Some phenotypes are beneficial (microbe and tumour killing), whereas other are harmful (immune suppression or tumour promoting),32, 41 also see the review by Aarts and Kuiipers in this issue. Detailed analysis of the neutrophil phenotypes in time and place will allow more focused therapy directed against disease‐promoting phenotypes.
5. THE NEUTROPHIL IN IMMUNE REGULATION
Parallel to the description of the complex neutrophil response in tumour immunology, several studies have described compartmentalization of neutrophils in immune regulation under conditions of acute inflammation42 or during neutrophil mobilization by G‐CSF.43 These studies have in common that neutrophils with a more (hyper)segmented nucleus can suppress T‐cell activation and/or proliferation in vitro by cells with a more (hyper)segmented nucleus. T‐cell suppression is critically dependent on cell‐cell contact, as blocking antibodies directed against β2‐integrins could inhibit the response.42 The suppressing cells mobilized during acute inflammation are characterized by a normal expression of FcγRIII (CD16), low expression of L‐selectin (CD62L) and a high expression of Mac‐1 (CD11b) and p150.95 (CD11c).42
T cell‐suppressing neutrophils mobilized 5 days after G‐CSF mobilization have a high expression of CD10.43 As yet, it is unclear whether both suppressing neutrophils belong to the same phenotype or that cells mobilized for 3 hours by endotoxin or 5 days by G‐CSF belong to different phenotypes. Supportive for the latter hypothesis is the finding that G‐CSF‐mobilized cells use arginase for suppression, whereas cells during acute inflammation employ ROS for suppression. The arginase‐I inhibitor L‐arginine did not affect T‐cell suppression induced by the latter cells.
As mentioned also in the introduction, the origin of T cell‐suppressing neutrophils is not known. The fact that they are found in the blood within 3 hours post endotoxin challenge, and the marked difference in proteome argues against a short‐term activation‐induced change in functionality.20 Yet, kinetic experiments in the bone marrow and peripheral tissue have to be performed to identify the mechanism(s) and locations(s) for these immune regulatory neutrophils.
An interesting line of research carried out by the group of Takashima has identified a third immune regulatory neutrophil: a neutrophil‐dendritic cell hybrid. In mouse models, such cells were identified in different inflammation models.44 In vitro, these cells were clearly able to modulate T‐cell responses.44
Lastly, another potentially interesting neutrophil phenotype has been found in chronic inflammatory and infectious diseases, cancer and acute inflammation. This neutrophil phenotype is characterized by a relatively low buoyant density and is referred to as low‐density granulocyte (LDG).45 This “phenotype” has been described to be typically present in the mononuclear cell fraction of patients with a variety of acute and chronic disease. LDGs in SLE have been most thoroughly studied: these cells have an activated phenotype characterized by enhanced expression of activation markers and an increased propensity to form NETs.46 Unfortunately, these cells have been poorly characterized in terms of discrimination between short‐term activated cells or long‐term differentiated cells. In fact, the composition of the neutrophils that stay on top of the Ficoll layer is very heterogenous, and there is a marked difference in the numbers of and function attributed to the “LDGs” found under different conditions.45 Therefore, it seems too early to define a neutrophil phenotype solely on the characteristic of a low buoyant density.
6. THE NEUTROPHIL IN TISSUE REGENERATION AND REPAIR
It has already been suggested for decades that neutrophils play an important role in tissue repair. Most of the data supportive for this hypothesis are circumstantial, but nevertheless the findings in mouse models47, 48 and small children with leucocyte adhesion deficiency types I, II and III clearly show impaired tissue repair responses associated with the absence of neutrophils in the tissues and marked neutrophilia in blood and bone marrow.49, 50, 51 These data imply that the absence of neutrophils in the tissues due to aberrant homing leads to impaired tissue repair by as yet unidentified mechanisms. It is, however, important to emphasize that a CD18 deficiency also affects homing of monocytes to the tissues.47 These data are thus not sufficient to unequivocally place the neutrophil in tissue repair.
There is an interesting correlation between tissue repair, the presence of neutrophils and the absence of mononuclear cells in the early haematoma formed after bone fracture. Here, a highly regulated process is initiated upon fracture that leads to correct bone formation weeks after the event. In the early fracture haematoma, high numbers of neutrophils are found that contribute to so‐called emergency stroma. The cells produce a fibronectin mesh that is important for the later deposition of collagen by mononuclear cells.52 Removal of neutrophils from the early haematoma severely impairs bone formation in animal models.53
Intriguingly, normal uneventful fracture healing of large bones, such as tibia and femur, is associated with a clear neutrophilia 10‐12 days after the fracture.54 This neutrophilia is not associated with a detectable infection and/or sterile pathological inflammation. In fact, data in small cohorts of trauma patients with fractures of larger bones imply that this neutrophilia is important, as the absence of such neutrophilia is associated with poor bone formation and non‐union of the bone (pseudo‐arthrosis).53
Another neutrophil phenotype that is associated with tissue repair after hypoxia is the CXCR4high neutrophil.55 Massena et al showed that tissue VEGF‐A upregulation following hypoxia recruits a distinct subset of neutrophils to the lesion site through VEGFR1 and VEGFR2. Circulating CD49pos/CXCR4high/VEGFR1high neutrophils transmigrate and, at the site, promote neovascularization. Blocking of VEGFR1 or VEGFR2 impaired recruitment and subsequent angiogenesis and tissue repair. The angiogenic effect is thought to be due to an abundance of MMP‐9 delivered by these neutrophils.56 Combined, these data support the concept of the importance of neutrophils in normal tissue repair.
7. THE NEUTROPHIL IN ABERRANT PRO‐ AND ANTI‐INFLAMMATORY CONDITIONS
Most of the data described above focused on normal functioning of neutrophils in homeostatic and disease conditions. Under these conditions, the neutrophil responds correctly on disease cues. However, an increasing number of clinical conditions are found to be at least in part mediated by malfunctioning of these cells. Apart from the already mentioned pro‐tumorigenic action of certain neutrophil phenotypes, other diseases are mediated by both hyper‐ and hypo‐activity of these cells.49, 57, 58, 59, 60
7.1. Diseases associated with hyperactivation of neutrophil phenotypes
chronic inflammatory diseases (eg cystic fibrosis, chronic obstructive pulmonary disease), acute inflammation (systemic inflammatory response syndrome, acute respiratory distress syndrome, multi‐organ dysfunction) and reperfusion injury.61, 62, 63, 64 These conditions have in common that neutrophil‐driven tissue damage is a hall mark of the disease, and it is imperative that neutrophil responses responsible should be inhibited with future innovative treatments. Despite a general consensus that inhibition of neutrophils is essential in dealing with these diseases, the clinical options are very limited. This is mainly caused by the fact that neutrophils are notoriously resistant to currently available anti‐inflammatory drugs such as corticosteroids.65
7.2. Diseases associated with hypo‐activation or functional deficiency of neutrophil phenotypes
Neutrophil genetic deficiencies: The most common inherited neutrophil deficiency is chronic granulomatous disease caused by mutations in the subunits of the NADPH‐oxidase genes.66 CGD and the other rare neutrophil deficiencies are reviewed by van de Geer et al67 Little if anything is known of different neutrophil phenotypes in their functionality and occurrence in these diseases.
7.3. Acquired neutrophil hypo‐activation during compensatory anti‐inflammatory response syndrome (CARS) after major trauma and sepsis
Hypo‐activation of neutrophils is a much‐overlooked problem in severely injured patients. This injury is typically found in patients undergoing major operations or experiencing major trauma caused by accidents.60 The main and most important risk factors for mortality and morbidity in these patients are infectious complications that range from “simple” line infections to septic shock.68 These clinical conditions are associated with blood neutrophils that are refractory to activation.69, 70 This unresponsive neutrophil type is thought to contribute significantly to the occurrence of infections. CARS associated with neutrophil dysfunction normally resolves in a matter of days, but in certain ICU patients, this situation of immune suppression can last up to weeks and months.71 This clinical situation is now better recognized and is coined as persistent inflammation, immunosuppression and catabolism syndrome or PICS.72 Little is known about the role of different neutrophil phenotypes in mediating this immune suppression, but MDSCs have been implicated.73
8. NEUTROPHIL PHENOTYPES IN TISSUE AND BLOOD
Most of the data regarding neutrophils are coming from cells isolated from the peripheral blood. Yet, the main location for neutrophil function is in the tissues. It is unknown whether every neutrophil homes to the tissue and the topic of recirculation is still under discussion. Therefore, it is important to study the cells in the tissue as well. There are a few locations where the cells can be obtained in a relatively untouched way: bronchoalveolar lavage fluid (BAL,74), sputum,75 synovial fluid76 and oral neutrophils.77 Immunophenotyping of these cells invariantly shows activated cells with a mature appearance.76 The difficulty in the interpretation of this activation is whether the activation is the result of tissue homing per se, or that the disease associated with tissue neutrophilia is causing this phenotype, and/or is organ‐specific. Although few data are available for healthy individuals, neutrophil homing per se seems sufficient for the activation phenotype.78. Apart from tissue staining of N1/N2 cells, little is known regarding neutrophil phenotypes in different tissues in health and disease.79
9. NEUTROPHIL PHENOTYPES IN HOMEOSTASIS WITH UNKNOWN SIGNIFICANCE
In addition to neutrophil subsets present in disease, also neutrophil markers have been described that might represent distinct neutrophil subsets in homeostasis. The 2 markers known today are discussed below. For both, no specific function has been ascribed, yet they seem to play a role in pathogenesis of disease.
NB 1/CD177. Between 45 and 65% of circulating neutrophils are CD177‐positive.80 CD177 is a glycoprotein‐anchored receptor that among other functions regulates transmigration of neutrophils through binding of platelet endothelial cell adhesion molecule‐1.81, 82 CD177‐positive neutrophils seem to play a role in the pathogenesis of ANCA‐associated vasculitis. As CD177 serves as an anchor to proteinase 3 (PR3), all CD177‐positive neutrophils also selectively present PR3 on their cell surface,83 a known antigen for antineutrophil cytoplasmic antibodies. Stimulation by CD177‐activating antibodies indeed showed increased degranulation and ROS production.83
Olfactomedin‐4. Clemmensen et al84 reported OLFM‐4 expression that is post‐transcriptionally regulated and can serve to define subsets of neutrophils being OLFM‐4‐positive or OLFM‐4‐negative in health and disease. No clear functional differences have been described for neutrophils in vitro being positive or negative for this protein. However, OLFM‐4 has been shown to be linked to several immunological processes and clinical outcomes. For example, enhanced bactericidal capacity against Staphylococcus aureus and sepsis resistance have been described in OLFM‐4 knock‐out mice, whereas increased tumorigenesis is found in these mice.85, 87 In humans, high percentages of OLFM‐4‐positive neutrophils are associated with increased risk of multi‐organ failure and death in sepsis,88 and OLFM‐4 has been proposed as a biomarker for several malignancies.89 Additionally, several studies have shown a variety of pathways regulating OLFM‐4 expression, including those critically involved in carcinogenesis and inflammation.90
10. HETEROGENEITY OF CELLS IN HOMEOSTASIS: THE CONCEPT OF COMPETITIVE PHAGOCYTOSIS
The text above might give the impression that at least during homeostasis the neutrophil in the peripheral blood belongs to a homogenous population of cells. This seems true when the cells are phenotyped by flow cytometry for the expression of markers on their surface. However, when the cells are studied with respect to their phagocytic function, an important complexity emerges.5 It turns out that cells that are obtained from normal control individuals are very heterogeneous in their capacity to phagocytose bacteria. Hellebrekers et al came to the conclusion that all neutrophils can phagocytose bacteria, but some are much better than others. This implicates that these highly functional cells outcompete less functional cells in phagocytosis of their targets. It is as yet unknown whether the response to phagocytosis by highly functional cells is different from the response of low‐responding cells in terms of intracellular killing mechanisms. It is, however, conceivable that the highly functional cells will leave the blood first during inflammatory conditions, leaving behind the less functional cells. This concept is supported by studies showing hypo‐reactivity of neutrophils in injured patients69 and eosinophils in asthma patients.91 This is also seen in a murine model, where phagocytosis‐prone‐aged neutrophils transmigrated to tissues, leaving behind the less active neutrophils in the peripheral circulation.25 Whether such heterogeneity is also found in tissue neutrophils and/or is reflected in neutrophil phenotypes remains to be established.
11. CONCLUSIONS: REGAINING THE BALANCE IS THE SOLUTION
The neutrophil compartment is much more complex than ever anticipated. Keeping homeostasis in health and regaining homeostasis in disease are only reached when the balance of pro‐ and anti‐inflammatory functions of the cells is normalized. Simply inhibiting bulk neutrophils comes with an unpredictable risk of infectious complications. In fact, patients with neutrophil‐mediated diseases characterized by hypo‐reactivity of the cells, such as CARS and PICS, might even benefit from activation of the cells. Proof of principle for this latter hypothesis came from important work of the group of Pickkers et al, showing that treatment with IFN‐γ functionally antagonized LPS‐induced immune paralysis in humans.92 Therefore, clinical trials testing innovative new drugs targeting neutrophils should take into account that different phenotypes of these cells are operational at similar or different sites and at similar or different times.
Hellebrekers P, Vrisekoop N, Koenderman L. Neutrophil phenotypes in health and disease. Eur J Clin Invest. 2018;48(Suppl. 2):e12943 10.1111/eci.12943
REFERENCES
- 1. Borregaard N. Neutrophils, from marrow to microbes. Immunity. 2010;33:657‐670. [DOI] [PubMed] [Google Scholar]
- 2. Summers C, Rankin SM, Condliffe AM, Singh N, Peters AM, Chilvers ER. Neutrophil kinetics in health and disease. Trends Immunol. 2010;31:318‐324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kolaczkowska E, Kubes P. Neutrophil recruitment and function in health and inflammation. Nat Rev Immunol. 2013;13:159‐175. [DOI] [PubMed] [Google Scholar]
- 4. Silvestre‐Roig C, Hidalgo A, Soehnlein O. Neutrophil heterogeneity: implications for homeostasis and pathogenesis. Blood. 2016;127:2173‐2181. [DOI] [PubMed] [Google Scholar]
- 5. Hellebrekers P, Hietbrink F, Vrisekoop N, Leenen LPH, Koenderman L. Neutrophil functional heterogeneity: identification of competitive phagocytosis. Front Immunol. 2017;8:1‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Amulic B, Cazalet C, Hayes GL, Metzler KD, Zychlinsky A. Neutrophil function: from mechanisms to disease. Annu Rev Immunol. 2012;30:459‐489. [DOI] [PubMed] [Google Scholar]
- 7. Hallett MB, Lloyds D. Neutrophil priming: the cellular signals that say “amber” but not “green”. Immunol Today. 1995;16:264‐268. [DOI] [PubMed] [Google Scholar]
- 8. Lyck R, Enzmann G. The physiological roles of ICAM‐1 and ICAM‐2 in neutrophil migration into tissues. Curr Opin Hematol. 2015;22:53‐59. [DOI] [PubMed] [Google Scholar]
- 9. Margadant C, Monsuur HN, Norman JC, Sonnenberg A. Mechanisms of integrin activation and trafficking. Curr Opin Cell Biol. 2011;23:607‐614. [DOI] [PubMed] [Google Scholar]
- 10. Foxman EF, Campbell JJ, Butcher EC. Multistep navigation and the combinatorial control of leukocyte chemotaxis. J Cell Biol. 1997;139:1349‐1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ferrante A, Martin AJ, Bates EJ, et al. Killing of Staphylococcus aureus by tumor necrosis factor‐alpha‐activated neutrophils. The role of serum opsonins, integrin receptors, respiratory burst, and degranulation. J Immunol. 1993;151:4821‐4828. [PubMed] [Google Scholar]
- 12. Kumaratilake LM, Ferrante A, Jaeger T, Rzepczyk C. GM‐CSF‐induced priming of human neutrophils for enhanced phagocytosis and killing of asexual blood stages of Plasmodium falciparum: synergistic effects of GM‐CSF and TNF. Parasite Immunol. 1996;18:115‐123. [DOI] [PubMed] [Google Scholar]
- 13. Bajaj MS, Kew RR, Webster RO, Hyers TM. Priming of human neutrophil functions by tumor necrosis factor: enhancement of superoxide anion generation, degranulation, and chemotaxis to chemoattractants C5a and f‐Met‐Leu‐Phe. Inflammation. 1992;16:241‐250. [DOI] [PubMed] [Google Scholar]
- 14. Segal AW. How neutrophils kill microbes. Annu Rev Immunol. 2005;23:197‐223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Brinkmann V, Reichard U, Goosmann C, et al. Neutrophil extracellular traps kill bacteria. Science. 2004;303:1532‐1535. [DOI] [PubMed] [Google Scholar]
- 16. Davis JM, Albert JD, Tracy KJ, et al. Increased neutrophil mobilization and decreased chemotaxis during cortisol and epinephrine infusions. J Trauma. 1991;31:725‐732. [PubMed] [Google Scholar]
- 17. Athens JW, Raab SO, Mauer AM, Ashenbrucker H, Cartwright GE, Wintrobe MM. Leukokinetic studies IV. The total blood, circulating and marginal granulocyte pools and the granulocyte turnover rate in normal subjects. J Clin Invest. 1961;40:989‐995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Phillips D, Rezvani K, Bain BJ. Exercise induced mobilisation of the marginated granulocyte pool in the investigation of ethnic neutropenia. J Clin Pathol. 2000;53:481‐483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Seebach JD, Morant R, Rüegg R, Seifert B, Fehr J. The diagnostic value of the neutrophil left shift in predicting inflammatory and infectious diseases. Am J Clin Pathol. 1997;107:582‐591. [DOI] [PubMed] [Google Scholar]
- 20. Tak T, Wijten P, Heeres M, et al. Human CD62L dim neutrophils identified as a separate subset by proteome profiling and in vivo pulse‐chase labeling. Blood. 2018;129:3476‐3486. [DOI] [PubMed] [Google Scholar]
- 21. Thompson W, Cassino C, Babitz L, et al. Hypersegmented neutrophils and vitamin B12 deficiency. Hypersegmentation in B12 deficiency. Acta Haematol. 1989;81:186‐191. [DOI] [PubMed] [Google Scholar]
- 22. Orr Y, Taylor JM, Bannon PG, Geczy C, Kritharides L. Circulating CD10‐/CD16low neutrophils provide a quantitative index of active bone marrow neutrophil release. Br J Haematol. 2005;131:508‐519. [DOI] [PubMed] [Google Scholar]
- 23. van Lochem EG, van der Velden VHJ, Wind HK, te Marvelde JG, Westerdaal NAC, van Dongen JJM. Immunophenotypic differentiation patterns of normal hematopoiesis in human bone marrow: reference patterns for age‐related changes and disease‐induced shifts. Cytometry. 2004;60B:1‐13. [DOI] [PubMed] [Google Scholar]
- 24. Ssemaganda A, Kindinger L, Bergin P, et al. Characterization of neutrophil subsets in healthy human pregnancies. PLoS One. 2014;9:e85696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Uhl B, Vadlau Y, Zuchtriegel G, et al. Aged neutrophils contribute to the first line of defense in the acute inflammatory response. Blood. 2016;128:2327‐2338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Greenlee‐wacker MC. Clearance of apoptotic neutrophils and resolution of inflammation. Immunol Rev. 2016;273:357‐370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Casanova‐Acebes M, Pitaval C, Weiss LA, et al. Rhythmic modulation of the hematopoietic niche through neutrophil clearance. Cell. 2013;153:1025‐1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Pillay J, Den Braber I, Vrisekoop N, et al. In vivo labeling with 2‐H2O reveals a human neutrophil lifespan of 5.4 days. Blood. 2010;116:625‐627. [DOI] [PubMed] [Google Scholar]
- 29. Lahoz‐Beneytez J, Elemans M, Zhang Y, et al. Human neutrophil kinetics: modeling of stable isotope labeling data supports short blood neutrophil half‐lives. Blood. 2016;127:3431‐3438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Tak T, Tesselaar K, Pillay J, Borghans JAM, Koenderman L. What's your age again? determination of human neutrophil half‐lives revisited. J Leukoc Biol. 2013;94:595‐601. [DOI] [PubMed] [Google Scholar]
- 31. Nagaraj S, Gabrilovich DI. Myeloid‐derived suppressor cells. Adv Exp Med Biol. 2007;601:213‐223. [DOI] [PubMed] [Google Scholar]
- 32. Fridlender ZG, Sun J, Kim S, et al. Polarization of tumor‐associated neutrophil phenotype by TGF‐β: “N1” versus “N2” TAN. Cancer Cell. 2009;16:183‐194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Pillay J, Tak T, Kamp VM, Koenderman L. Immune suppression by neutrophils and granulocytic myeloid‐derived suppressor cells: similarities and differences. Cell Mol Life Sci. 2013;70:3813‐3827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Almand B, Clark JI, Nikitina E, et al. Increased production of immature myeloid cells in cancer patients: a mechanism of immunosuppression in cancer. J Immunol. 2001;166:678‐689. [DOI] [PubMed] [Google Scholar]
- 35. Youn J‐I, Collazo M, Shalova IN, Biswas SK, Gabrilovich DI. Characterization of the nature of granulocytic myeloid‐derived suppressor cells in tumor‐bearing mice. J Leukoc Biol. 2012;91:167‐181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Youn J‐I, Gabrilovich DI. The biology of myeloid‐derived suppressor cells: the blessing and the curse of morphological and functional heterogeneity. Eur J Immunol. 2010;40:2969‐2975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Highfill SL, Rodriguez PC, Zhou Q, et al. Bone marrow myeloid‐derived suppressor cells (MDSC) inhibit graft‐versus‐host (GVHD) disease via an arginase‐1 dependent mechanism that is upregulated by IL‐13. Blood. 2010;116:612‐626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Wu H, Zhen Y, Ma Z, et al. Arginase‐1‐dependent promotion of TH17 differentiation and disease progression by MDSCs in systemic lupus erythematosus. Sci Transl Med. 2016;8:1‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Albanesi M, Mancardi D, Jönsson F, et al. Neutrophils mediate antibody‐induced antitumor effects in mice. Blood. 2013;122:3160‐3164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Valgardsdottir R, Cattaneo I, Klein C, Introna M, Figliuzzi M, Golay J. Human neutrophils mediate trogocytosis rather than phagocytosis of CLL B cells opsonized with anti‐CD20 antibodies. Blood. 2017;129:2636‐2644. [DOI] [PubMed] [Google Scholar]
- 41. Shen M, Hu P, Donskov F, Wang G, Liu Q, Du J. Tumor‐associated neutrophils as a new prognostic factor in cancer: a systematic review and meta‐analysis. PLoS One. 2014;9:e98259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Pillay J, Kamp VM, Van Hoffen E, et al. A subset of neutrophils in human systemic inflammation inhibits T cell responses through Mac‐1. J Clin Invest. 2012;122:327‐336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Marini O, Costa S, Bevilacqua D, et al. Mature CD10+ and immature CD10‐ neutrophils present in G‐CSF – treated donors display opposite effects on T cells. Blood. 2017;129:1343‐1357. [DOI] [PubMed] [Google Scholar]
- 44. Geng S, Matsushima H, Okamoto T, et al. Emergence, origin, and function of neutrophil‐dendritic cell hybrids in experimentally induced inflammatory lesions in mice. Blood. 2013;121:1690‐1700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Carmona‐Rivera C, Kaplan MJ. Low‐density granulocytes: a distinct class of neutrophils in systemic autoimmunity. Semin Immunopathol. 2013;35:455‐463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Denny MF, Yalavarthi S, Zhao W, et al. A distinct subset of proinflammatory neutrophils isolated from patients with systemic lupus erythematosus induces vascular damage and synthesizes type I IFNs. J Immunol. 2010;184:3284‐3297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Peters T, Sindrilaru A, Hinz B, et al. Wound‐healing defect of CD18‐/‐ mice due to a decrease in TGF‐beta1 and myofibroblast differentiation. EMBO J. 2005;24:3400‐3410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Schruefer R, Sulyok S, Schymeinsky J, Peters T, Scharffetter‐Kochanek K, Walzog B. The proangiogenic capacity of polymorphonuclear neutrophils delineated by microarray technique and by measurement of neovascularization in wounded skin of CD18‐deficient mice. J Vasc Res. 2006;43:1‐11. [DOI] [PubMed] [Google Scholar]
- 49. Wada T, Tone Y, Shibata F, Toma T, Yachie A. Delayed wound healing in leukocyte adhesion deficiency type 1. J Pediatr. 2011;158:342. [DOI] [PubMed] [Google Scholar]
- 50. Etzioni A, Tonetti M. Leukocyte adhesion deficiency II‐from A to almost Z. Immunol Rev. 2000;178:138‐147. [DOI] [PubMed] [Google Scholar]
- 51. Kinashi T, Aker M, Sokolovsky‐Eisenberg M, et al. LAD‐III, a leukocyte adhesion deficiency syndrome associated with defective Rap1 activation and impaired stabilization of integrin bonds. Blood. 2004;103:1033‐1036. [DOI] [PubMed] [Google Scholar]
- 52. Bastian OW, Koenderman L, Alblas J, Leenen LPH, Blokhuis TJ. Neutrophils contribute to fracture healing by synthesizing fibronectin+extracellular matrix rapidly after injury. Clin Immunol. 2016;164:78‐84. [DOI] [PubMed] [Google Scholar]
- 53. Grundnes O, Reikeras O. The importance of the hematoma in fracture healing. Acta Orthop Scand. 1993;64:340‐342. [DOI] [PubMed] [Google Scholar]
- 54. Bastian OW, Kuijer A, Koenderman L, et al. Impaired bone healing in multitrauma patients is associated with altered leukocyte kinetics after major trauma. J Inflamm Res. 2016;9:69‐78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Massena S, Christoffersson G, Evelina V, et al. Identification and characterization of VEGF‐A – responsive neutrophils expressing CD49d, VEGFR1, and CXCR4 in mice and humans. Blood. 2016;126:2016‐2027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Christoffersson G, Vågesjö E, Vandooren J, et al. VEGF‐A recruits a proangiogenic MMP‐9–delivering neutrophil subset that induces angiogenesis in transplanted hypoxic tissue VEGF‐A recruits a proangiogenic MMP‐9 – delivering neutrophil subset that induces angiogenesis in transplanted hypoxic tissue. Blood. 2013;120:4653‐4662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Mishalian I, Bayuh R, Levy L, Zolotarov L, Michaeli J, Fridlender ZG. Tumor‐associated neutrophils (TAN) develop pro‐tumorigenic properties during tumor progression. Cancer Immunol Immunother. 2013;62:1745‐1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Wright HL, Moots RJ, Edwards SW. The multifactorial role of neutrophils in rheumatoid arthritis. Nat Rev Rheumatol. 2014;10:593‐601. [DOI] [PubMed] [Google Scholar]
- 59. Kang EM, Marciano BE, Deravin S, Zarember KA, Holland SM, Malech HL. Chronic granulomatous disease: overview and hematopoietic stem cell transplantation. J Allergy Clin Immunol. 2011;127:1319‐1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Lord JM, Midwinter MJ, Chen YF, et al. The systemic immune response to trauma: an overview of pathophysiology and treatment. Lancet. 2014;384:1455‐1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Hoenderdos K, Condliffe A. The neutrophil in chronic obstructive pulmonary disease: too little, too late or too much, too soon? Am J Respir Cell Mol Biol. 2013;48:531‐539. [DOI] [PubMed] [Google Scholar]
- 62. Graff I, Schram‐Doumont A, Szpirer C. Defective protein kinase C‐mediated actions in cystic fibrosis neutrophils. Cell Signal. 1991;3:259‐266. [DOI] [PubMed] [Google Scholar]
- 63. Fujishima S, Aikawa N. Neutrophil‐mediated tissue injury and its modulation. Intensive Care Med. 1995;21:277‐285. [DOI] [PubMed] [Google Scholar]
- 64. Yago T, Petrich BG, Zhang N, et al. Blocking neutrophil integrin activation prevents ischemia–reperfusion injury. J Exp Med. 2015;212:1267‐1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Bruijnzeel PLB, Uddin M, Koenderman L. Targeting neutrophilic inflammation in severe neutrophilic asthma: can we target the disease‐relevant neutrophil phenotype? J Leukoc Biol. 2015;98:549‐556. [DOI] [PubMed] [Google Scholar]
- 66. Roos D, Kuhns DB, Maddalena A, et al. Hematologically important mutations: X‐linked chronic granulomatous disease (third update). Blood Cells Mol Dis. 2010;45:246‐265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. van de Geer A, Gazendam RP, Kuijpers TW, Roos D. Neutrophil functional disorders. In: eLS. Chichester: John Wiley & Sons, Ltd; 2017. 10.1002/9780470015902.a0002182.pub3 [DOI]
- 68. Pories SE, Gamelli RL, Mead PB, Goodwin G, Harris F, Vacek P. The epidemiologic features of nosocomial infections in patients with trauma. Arch Surg. 1991;126:97‐99. [DOI] [PubMed] [Google Scholar]
- 69. Hietbrink F, Koenderman L, Althuizen M, Pillay J, Kamp V, Leenen LPH. Kinetics of the innate immune response after trauma. Shock. 2013;40:21‐27. [DOI] [PubMed] [Google Scholar]
- 70. Groeneveld KM, Koenderman L, Warren BL, Jol S, Leenen LPH, Hietbrink F. Early decreased neutrophil responsiveness is related to late onset sepsis in multitrauma patients: an international cohort study. PLoS One. 2017;12:e0180145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Gentile LF, Cuenca AG, Efron PA, et al. Persistent inflammation and immunosuppression: a common syndrome and new horizon for surgical intensive care. J Trauma Acute Care Surg. 2012;72:1491‐1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Vanzant EL, Lopez CM, Ozrazgat‐Baslanti T, et al. Persistent inflammation, immunosuppression, and catabolism syndrome after severe blunt trauma. J Trauma Acute Care Surg. 2014;76:21‐30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Mira JC, Brakenridge SC, Moldawer LL, Moore FA. Persistent inflammation, immunosuppression and catabolism syndrome. Crit Care Clin. 2017;33:245‐258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Thompson AB, Robbins RA, Ghafouri MA, Linder J, Rennard SI. Bronchoalveolar lavage fluid processing. Effect of membrane filtration preparation on neutrophil recovery. Acta Cytol. 1989;33:544‐549. [PubMed] [Google Scholar]
- 75. Gupta V, Singh D. Critical assessment of the value of sputum neutrophils. COPD. 2013;10:107‐114. [DOI] [PubMed] [Google Scholar]
- 76. Watson F, Robinson JJ, Phelan M, Bucknall RC, Edwards SW. Receptor expression in synovial fluid neutrophils from patients with rheumatoid arthritis. Ann Rheum Dis. 1993;52:354‐359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Ashkenazi M, Dennison DK. A new method for isolation of salivary neutrophils and determination of their functional activity. J Dent Res. 1989;68:1256‐1261. [DOI] [PubMed] [Google Scholar]
- 78. Fortunati E, Kazemier KM, Grutters JC, Koenderman L, Van Den Bosch VJMM. Human neutrophils switch to an activated phenotype after homing to the lung irrespective of inflammatory disease. Clin Exp Immunol. 2009;155:559‐566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Ma Y, Yabluchanskiy A, Iyer RP, et al. Temporal neutrophil polarization following myocardial infarction. Cardiovasc Res. 2016;110:51‐61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Stroncek DF, Shankar RA, Noren PA, Herr GP, Clement LT. Analysis of the expression of NB1 antigen using two monocloncal antibodies. Transfusion. 1996;36:168‐174. [DOI] [PubMed] [Google Scholar]
- 81. Sachs UJ, Andrei‐Selmer CL, Maniar A, et al. The neutrophil‐specific antigen CD177 is a counter‐receptor for platelet endothelial cell adhesion molecule‐1(CD31). J Biol Chem. 2007;282:23603‐23612. [DOI] [PubMed] [Google Scholar]
- 82. Bai M, Grieshaber‐Bouyer R, Wang J, et al. CD177 modulates human neutrophil migration through activation‐mediated integrin and chemoreceptor regulation. Blood. 2017;130:2092‐2100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Jerke U, Rolle S, Dittmar G, et al. Complement receptor Mac‐1 is an adaptor for NB1 (CD177)‐mediated PR3‐ANCA neutrophil activation. J Biol Chem. 2011;286:7070‐7081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Clemmensen SN, Bohr CT, Rorvig S, et al. Olfactomedin 4 defines a subset of human neutrophils. J Leukoc Biol. 2012;91:495‐500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Liu W, Yan M, Liu Y, McLeish KR, Coleman WG, Rodgers GP. Olfactomedin 4 inhibits cathepsin C‐mediated protease activities, thereby modulating neutrophil killing of Staphylococcus aureus and Escherichia coli in mice. J Immunol. 2012;189:2460‐2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Liu W, Yan M, Sugui JA, et al. Olfm4 deletion enhances defense against Staphylococcus aureus in chronic granulomatous disease. J Clin Invest. 2013;123:3751‐3755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Liu W, Li H, Hong SH, Piszczek GP, Chen W, Rodgers GP. Olfactomedin 4 deletion induces colon adenocarcinoma in ApcMin/+mice. Oncogene. 2016;35:5237‐5247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Alder MN, Opoka AM, Lahni P, Hildeman DA, Wong HR. Olfactomedin‐4 Is a candidate marker for a pathogenic neutrophil subset in septic shock. Crit Care Med. 2017;45:e426‐e432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Guette C, Valo I, Vétillard A, Coqueret O. Olfactomedin‐4 is a candidate biomarker of solid gastric, colorectal, pancreatic, head and neck, and prostate cancers. Proteomics Clin Appl. 2015;9:58‐63. [DOI] [PubMed] [Google Scholar]
- 90. Liu W, Rodgers GP. Olfactomedin 4 expression and functions in innate immunity, inflammation, and cancer. Cancer Metastasis Rev. 2016;35:201‐212. [DOI] [PubMed] [Google Scholar]
- 91. Johansson MW. Activation states of blood eosinophils in asthma. Clin Exp Allergy. 2014;44:482‐498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Leentjens J, Kox M, Koch RM, et al. Reversal of immunoparalysis in humans in vivo: a double‐blind, placebo‐controlled, randomized pilot study. Am J Respir Crit Care Med. 2012;186:838‐845. [DOI] [PubMed] [Google Scholar]


