Abstract
Spontaneous preterm birth (SPTB), defined as delivery before 37 weeks gestation, remains a significant obstetric dilemma even after decades of research in this field. Although trends from 2007 to 2014 showed the rate of preterm birth slightly decreased, the CDC recently reported the rate of preterm birth has risen for two consecutive years since 2014. Currently, 1 in 10 pregnancies in the US still end prematurely. In this chapter, we will focus on the “compartment” of the cervix. The goal is to outline the current knowledge of normal cervical structure and function in pregnancy and the current knowledge of how the cervix malfunctions leading to SPTB. We will review the mechanisms by which our current interventions are hypothesized to work. Lastly, we will outline gaps in knowledge as well as future research directions that may lead to novel and effective interventions to prevent premature cervical failure and SPTB.
Keywords: cervix, premature cervical remodeling, short cervix, preterm birth
THE PERSISTENT PROBLEM OF SPONTANEOUS PRETERM BIRTH
Spontaneous preterm birth (SPTB), defined as delivery before 37 weeks gestation, remains a significant obstetric dilemma even after decades of research in this field. Although trends from 2007 to 2014 showed the rate of preterm birth slightly decreased, the CDC recently reported the rate of preterm birth has risen for two consecutive years since 2014. [1] Currently, 1 in 10 pregnancies in the US still end prematurely. [1] For providers on the frontlines providing obstetrical care, it remains a frustrating experience to explain to patients that our armamentarium of interventions to prevent SPTB is limited and not entirely effective. In fact, approximately 95% of cases of SPTB are intractable to current therapies. [2] When one witnesses the enormity of the emotional and financial burden that patients must endure, particularly when babies deliver at the cusp of viability or in the severe preterm period, it is clear the obstetric/research fields must do more to comprehend how the reproductive organs function in normal pregnancy and how they malfunction resulting in SPTB. The fact that in this day and age where cancers are being cured with cutting edge therapeutics but we do not even understand how normal labor starts is exasperating.
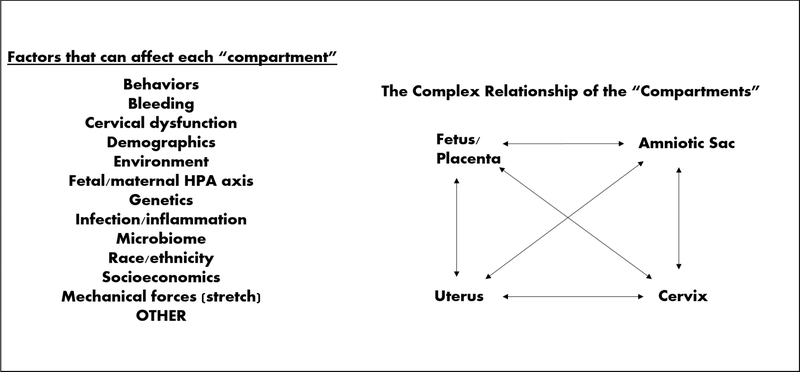
The fundamental problem of delineating why SPTB occurs and why our interventions fail to effectively and reliably prevent SPTB is three-fold. First, we do not fully understand the normal building blocks or tissue properties of the human uterus and cervix – this is because it is challenging to obtain human tissue samples during pregnancy. Thus our knowledge of how the essential building blocks change during pregnancy is extremely limited. Further, without a solid understanding of what occurs normally, it is exceedingly difficult to determine what “goes awry” or what is pathophysiological leading to malfunction and SPTB. An analogy to this clinical scenario is: how does one fix a broken car when one does not have the manufacturer’s manual detailing the car parts and how they assemble and work together? Second, the hypothesized etiologies of SPTB are incredibly diverse and complex. These include, but are not limited to, underling genetic predispositions, ethnic differences, environmental factors, hormonal causes, mechanical properties (e.g. tissue mechanical strength/stretch), immune factors, microbial players, and trauma. (Figure 1) Lastly, although we tend to think of pregnancy as a whole entity, there are four different “compartments” (uterus, amniotic membranes, fetus/placenta, cervix) that must cohesively interact and create a healthy symbiotic relationship with each other and the rest of the female body in order to produce a successful, term pregnancy. Although extensive research has been done on every one of these etiologies or “compartments” in relation to SPTB, to fully understand what occurs in normal pregnancies and those that result in SPTB, we must start to understand the complex interactions that occur in each “compartment”, between “compartments”, and between “compartments” and the various etiologies. (Figure 1) To do this, there needs to be collaborative efforts and engagement from clinicians and experts in all these various fields of research.
FIGURE 1:
Diagram showing the complexity of sPTB. This figures lists various factors that can “trigger” or “activate” each compartment (uterus, cervix, fetus/placenta, amniotic sac) which can ultimately lead to sPTB
In this chapter, we will focus on the “compartment” of the cervix. The goal is to outline the current knowledge of normal cervical structure and function in pregnancy and the current knowledge of how the cervix malfunctions leading to SPTB. We will review the mechanisms by which our current interventions are hypothesized to work. Lastly, we will outline gaps in knowledge as well as future research directions that may lead to novel and effective interventions to prevent premature cervical failure and SPTB.
NORMAL HUMAN CERVICAL TISSUE STRUCTURE AND REMODELING IN PREGNANCY
The cervix is the structure located at caudal end of the uterus whose function is to keep the fetus in utero until term. Once labor starts, the cervix dilates to allow for delivery of the fetus and then within minutes the internal os closes which, along with uterine contraction, achieves hemostasis. To date, although excellent research has investigated the process of cervical remodeling, knowledge on how the human cervix goes from a firm, strong structure that can withstand the increasing load of a growing pregnancy to one that is soft and compliant to allow for delivery of an infant and then closes within minutes remains limited.
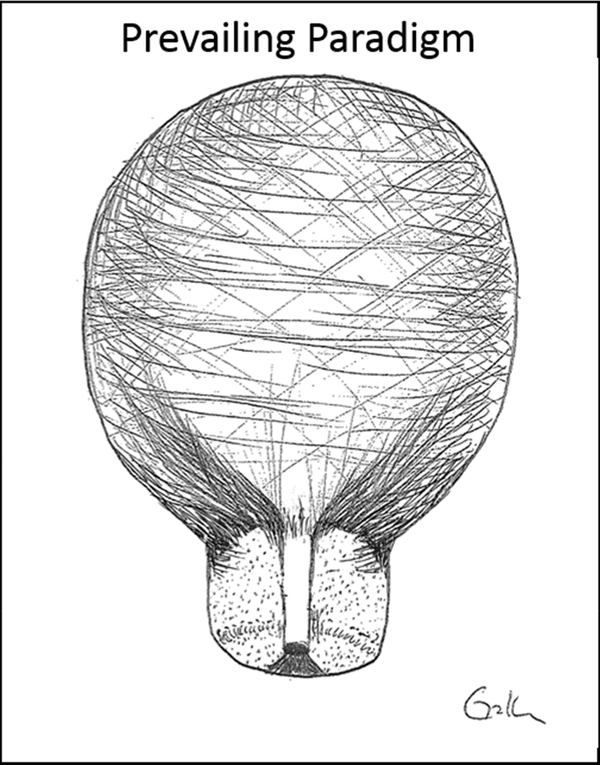
Until recently, our knowledge of human cervical tissue architecture has been based on work from the 1940s. This work suggests that unlike the uterus, which is the muscular powerhouse, the cervix is a hydrated, mainly collagenous (80–90% collagen) structure with very minimal cellular content (10–15%). The stromal extracellular matrix (ECM) has also been found to contain matricellular proteins, proteoglycans, glycosaminoglycans and a small amount of elastin fibers. [3-6; Figure 2] Since the cervix was thought to be mainly collagen/ECM, the working paradigm has been that the cervix is a “passive bystander” in the process of parturition. Somehow the collagen matrix remodels itself and the cervix starts to passively dilate due to the strength of uterine contractions. As such, many studies since the 1940s have focused on how the collagen network provides the mechanical strength to the human cervix and how the collagen/ECM network changes in pregnancy.
FIGURE 2:
Image adapted from Vink et al showing the prevailing paradigm of cervical tissue structure which states the cervix is mainly a homogenous collagenous structure with minimal cellular content. (6)
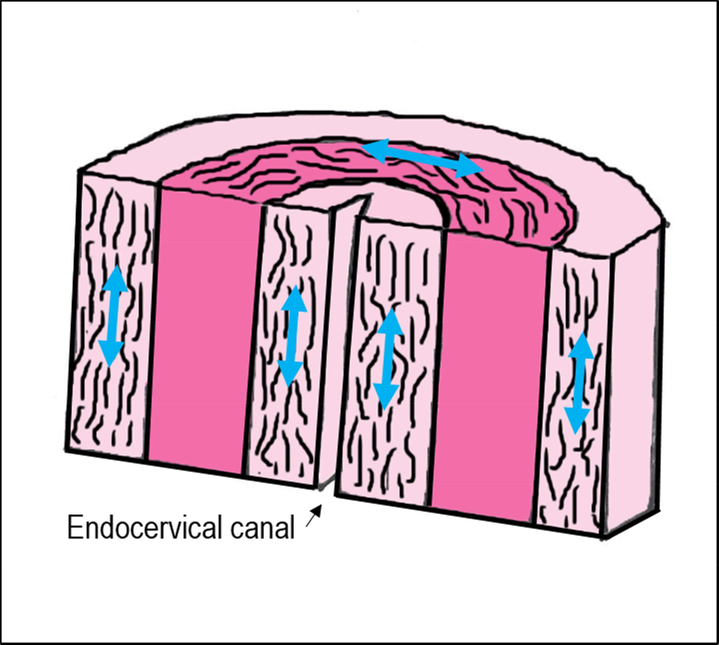
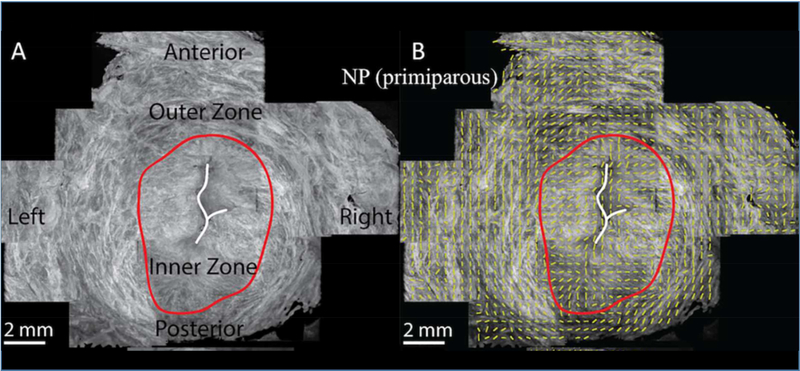
The mechanical strength of the cervix in pregnancy has been thought to depend on two main components of cervical tissue architecture: 1) the type/ organization of the collagen network and 2) the degree of tissue hydration. Early studies in the 1970s and 1980s of human cervical tissue samples reported the organization of the collagen network, or scaffold in the cervix, had a particular anatomical organization. Specifically, these early studies described three distinct “zones of collagen” in the cervix, which are thought to provide tissue strength. These studies found a middle zone in the stroma where collagen fibers are oriented circumferentially around the endocervical canal. This zone is hypothesized to prevent cervical dilation. These studies also reported two zones (one along the endocervical canal and one along the outer edge of the cervix towards the vagina) that run parallel to the endocervical canal. These zones are thought to attach the cervix with the ECM/collagen network in the uterus to ensure the cervix stays attached to the uterus (Figure 3). [7–10] Recent work suggests these collagen “zones” may not be as distinct as previously thought, and the collagen scaffold may be more complex with interweaving zones that depend on the geographic region in the cervix (i.e. the internal os appears different than the external os). [11, 12] For example, Yao et al suggests the circumferential zone of collagen, found in the earlier studies, is not just limited to the middle stroma but extends to the outer border of the cervix (Figure 4). [11] Continued studies characterizing the directionality of the collagen fiber network accompanied with detailed mechanical test are needed to understand the structure-function relationship of the cervix and its corresponding mechanical performance.
FIGURE 3:
The proposed three zones of collagen organization which consists of an outer and inner zone of collagen fibers that run parallel to the endocervical canal and a middle zone of fibers that are circumferentially organized around the endocervical canal. (7–10)
FIGURE 4:
Images adapted from Yao et al showing optical coherence tomography images of a transverse slice of human nonpregnant cervical tissue at level of internal os showing there is an outer zone of circumferentially oriented collagen fibers. (11)
At the collagen fiber level, several studies in rodents have described that in the nonpregnant and early pregnant state, the collagen fibers are neatly packed creating organized scaffold that likely provides stability and strength to the tissue – similar to a well-organized, tightly packed Jenga® puzzle. As the cervix remodels in pregnancy, the tissue is thought to soften in distinct phases by increasing disorganization and instability of this collagen scaffold. Specifically, rodent studies have shown that as pregnancy progresses, there is increased spacing between collagen fibers as well as changes in the shape and size of collagen fibers. [13–23] The mechanism by which the collagen network changes and becomes disorganized is thought to involve an influx of immune cells (a sterile inflammatory response) and release of matrix metalloproteinases (MMPs), enzymes that degrade collagen/ECM. [24–39] However, what triggers this sterile inflammatory response and remodeling of the collagen matrix remains unclear.
New data suggests that in addition to the overall organizational structure of the collagen scaffold, mechanical strength of collagen network is also directly related to the type and degree of collagen crosslinking between collagen fibrils. Yoshida et al demonstrated as pregnancy progresses in mice, there appears to be a decrease in the collagen crosslink maturity ratio (the ratio of mature to immature collagen crosslink densities) which correlates to softer, more compliant cervical tissue. [40] We have shown collagen crosslinks can be measured in the human cervix and regional differences exist in the cervix. [41] However, how collagen crosslinking changes in human cervical tissue during pregnancy remains unknown.
The second factor that is thought to influence to the mechanical strength of the cervix is the degree of tissue hydration. Rodent studies have shown that as the cervix remodels/softens in pregnancy, there is an increase in water content in cervical tissue. The increase in hydration is thought to be an additional mechanism to disrupt the stable, strong collagen scaffold resulting in a disorganized/weaker tissue. [13,15–17, 42,43] Studies have shown that as hydration increases in cervical tissue in pregnancy, there is a concomitant increase in hyaluronic acid (HA) content, a glycosaminoglycan that is thought to increase hydration in tissues. However, a recent study by Akgul et al found that HA is not essential to normal cervical ripening. [42] Thus, although tissue hydration seems to play an important factor in cervical softening, the exact mechanism of what triggers the influx of water into the cervical tissue is unknown. [44]
Recent studies have also discovered there are smaller ECM components that appear to be critical to the mechanical strength of a tissue. These include matricellular proteins and proteoglycans (ie decorin, versican, fibromodulin, biglycan, asporin) known to assist in organizing collagen fibrils in the collagen network. [20, 45–50] Matricellular proteins (i.e. tenascins, thrombospondins, SPARC proteins) are rapidly turned over during cervical remodeling in rodent pregnancies and these proteins are thought to be important in regulating ECM production as well as play a role in cell-ECM interactions. [45,51].
This chapter provides a brief overview of the prevailing concepts of normal cervical tissue structure and how the cervix remodels/softens in pregnancy. Extensive research has been done, particularly in rodents, regarding this remodeling process. Please consider the outstanding reviews by Timmons et al [16], Word et al [13], Nallasamy et al [52], House et al [14], Mahendroo et al [15] and for further complete details about this process and the distinct remodeling phases reported in rodent cervix. Although rodent studies are critical to understanding changes in pregnancy (as human tissues are challenging to obtain in pregnancy), we must remember that the findings in rodent studies have not been confirmed in human cervical tissue samples. In addition, we must keep in mind that the reproductive organs as well as hormonal milieu in rodents do not fully mimic humans. Lastly, as quadrupedal animals, the uterus/cervix in a rodent does not share the same gravitational force exposures as bipedal humans.
AN UPDATED MODEL OF CERVICAL TISSUE STRUCTURE AND FUNCTION IN PREGNANCY – IS THE INTERNAL OS A SPECIALIZED SPHINCTER?
In 1996, the landmark study by Iams et al established that a short cervical length as measured by transvaginal ultrasound is an important predictor of SPTB. [53] Since then studies have shown that as a cervix starts to fail, there appears to be funneling or dilation that seems to start at the internal os. [54] In addition, it is common obstetric knowledge that a “multiparous” cervix can be characterized as one where the external os is soft and dilated where the internal os is tightly closed. From a research/tissue architecture perspective, these regional differences in cervical tissue function do not make sense if we use the prevailing paradigm that stated the cervix is a homogenous collagenous structure. If the cervix were a homogenous structure it should in theory act the same regardless of regional location (ie internal vs external os). The prevailing paradigm that characterizes the cervix as homogenous, collagenous, “passive bystander” in the process of parturition also does not explain how the cervix, within minutes after delivery of a fetus, actively closes at the internal os, while the external os can stay dilated for hours/days/weeks. Collagen is not fully elastic and does not have the ability to retract a cervix that is ten centimeters dilated to one that is closed in that short timeframe.
To investigate why these regional differences in function existed, our lab recently reevaluated the architecture of human cervical tissue with specific attention to geographic differences between the internal and external os. We found that the area of the internal os histologically looks completely different than the external os. Unlike the external os which appears to be mostly collagen with a small amount (about 10%) of smooth muscle cells that were scattered in the stroma, the internal os contains approximately 50–60% smooth muscle. There also appeared to be a pattern to the smooth muscle distribution at the internal os. Specifically, the smooth muscle bundles were circumferentially oriented around the endocervical canal. [6] This orientation of smooth muscle persisted until the mid-cervix from which the smooth muscle content gradually decreased (Figure 5). [6] In addition, we found that when stimulating human cervical tissue with oxytocin, cervical tissue obtained from the area of the internal os was more contractile than tissue obtained from the external os. [6]
FIGURE 5:
Image adapted from Vink et al showing the updated paradigm of cervical tissue structure which states the upper half of the cervix contains a significant amount of smooth muscle that are circumferentially oriented around the endocervical canal. (6)
When taking into consideration these findings as well as the clinical knowledge that the internal os appears to be the critical area that keeps the cervix closed (ie in multiparous women) and fails in women with premature cervical shortening, we began to wonder if the area of the internal os is a specialized sphincter. If the internal os is indeed a specialized sphincter, we then contemplated the question, “is premature cervical failure at the internal os in women with cervical shortening actually evidence of the sphincter failing to keep the pregnancy in utero?” Similarly, this concept of a specialized sphincter could explain how the internal os closes so rapidly after delivery of a fetus. When one considers the bladder and rectum, which are pelvic organs that share similar functions to the uterus (ie they must retain a product and when full or at certain pressure threshold release this product), it would make biologic sense that the uterus has a specialized sphincter that functions to keep its product (the fetus) in utero until it is triggered at a certain threshold to deliver its contents.
The idea of a sphincter at the internal os is actually an idea that was introduced decades ago but over time faded from focus. In 1931, Ivy et al demonstrated that during pregnancy, macaques develop a strong sphincter at the internal os. [55] Schild et al demonstrated in the 1950s that the cervix can contract independently from the uterus when stimulated by various contractile agonists. [56] Similarly, studies in the 1980s and 1990s demonstrated that electromyography (EMG) activity can be detected in the human cervix and suggested that contractility of the human cervix can prolong the length latent labor. [57–63] It is unclear why the concept of a sphincter at the internal os faded from focus. However, the re-emergence of the idea of a specialized sphincter in the cervix now opens a vast new horizon of possibilities to explain how a cervix functions and malfunctions in pregnancy.
EVALUATING THE MECHANSIMS OF PREMATURE CERVICAL REMODELING AND CURRENT TREATMENT OPTIONS TO PREVENT SPTB
Since the prevailing paradigm noted the cervix was a mostly collagenous structure, most of the studies to date have focused on identifying a “collagen defect” which leads to a weak cervix that fails prematurely in pregnancy. To date, although various studies have been published, results have been mixed in terms of finding a clear identifiable “collagen defect” in the cervix in women with cervical insufficiency. [64–71] The inability to find a clear “collagen defect” in women with cervical insufficiency likely lies in the following issues. First, the criteria to define “cervical insufficiency” in these studies were not standardized. Second, the methods of obtaining cervical samples from the human cervix were not standardized. Many studies sampled the external os (which we now know is histologically different than the internal os) and most studies sampled the cervix either in the nonpregnant state or after delivery which may not fully capture the pathophysiologic state of the failing cervix. Lastly, the challenging obstacle to performing human cervical tissue studies is finding appropriate gestational-age matched normal controls. Most of the referenced studies used either nonpregnant patients or non-gestational age matched patients as controls. [72]
The second mechanism frequently hypothesized as an etiology of premature cervical remodeling is infection. Many studies have evaluated this concept in rodent studies with the introduction of an infectious/inflammatory agent (ie lipopolysaccharide) as the trigger for the inflammatory process leading to premature cervical remodeling. [52, 73–78] Emerging fields that study the microbiome of the vagina also may begin to elucidate the microbial causes of premature cervical remodeling. [79–82] However in many cases, no clear infectious or microbial etiologies are found. [83] Thus, the third hypothesis being explored is one where the normal sterile inflammatory process, which remodels the cervix at term, is triggered prematurely. [26] Unfortunately, the trigger that starts the possible sterile inflammatory process remains unknown. The main obstacle to date of understanding how a cervix prematurely remodels lies in the fact that we do not understand how a cervix remodels in normal pregnancies. With this lack of fundamental knowledge, it is incredibly difficult to know what changes are “pathologic” and what triggers true premature cervical remodeling.
TREATMENT OPTIONS FOR PREMATURE CERVICAL REMODELING
The mainstay of current treatment options for women who are faced with a prematurely failing cervix (as identified by a short or dilated cervix) or a history of premature cervical failure leading to SPTB lies in progesterone therapy, cerclage or possibly the cervical pessary.
Progesterone
Progesterone therapy appears to be effective in preventing SPTB in a subset of high-risk women. As other chapters in this issue will focus specifically on progesterone therapy to reduce SPTB a brief overview will be reviewed here. In the seminal trial by Meis et al, weekly intramuscular injections of hydroxyprogesterone caproate from 16 to 20 weeks of gestation until 36 weeks in singleton pregnancies significantly decreased the rate of SPTB by approximately one-third in women with a prior SPTB. [84] Additionally, another seminal trial by da Fonseca et al showed that women at risk for SPTB who received to daily vaginal progesterone suppositories from 24 until 34 weeks of gestation had decreased rates of SPTB compared to patients receiving placebo. [85] Recently, the OPPTIMUM trial showed that vaginal progesterone did not significantly reduce the primary obstetric (fetal death or birth before 34 weeks) or neonatal composite outcomes (death, brain injury, or bronchopulmonary dysplasia). [86] A subgroup analysis of women with a history of a prior SPTB, found that vaginal progesterone had no significant effect on both obstetric and childhood outcomes, but it did show a possible treatment effect on composite neonatal outcome (OR 0.48, 95% CI 0.29–0.79; P-interaction 0.053). [86]
In women with an incidental short cervix (no prior history of SPTB, current singleton pregnancy), several trials have shown that vaginal progesterone may decrease the rate of SPTB in this cohort of women. [87,88] A recent systematic review and meta-analysis of individual patient data from randomized trials which included data from the OPPTIMUM trial found that vaginal progesterone supplementation significantly reduced the risk of preterm birth and neonatal morbidity/mortality in singletons with a cervical length ≤25 mm. [89] In contrast to vaginal progesterone, trials which randomly assigned weekly intramuscular hydroxyprogesterone caproate (250 mg or 500 mg) or placebo to singleton pregnancies complicated with a short cervix found that hydroxyprogesterone caproate did not reduce the risk of preterm birth. [90,91] As such, weekly intramuscular hydroxyprogesterone caproate is not recommended for women with a short cervix without a history of prior preterm birth.
Given these findings, the American College of Obstetricians and Gynecologists (ACOG) and the Society for Maternal Fetal Medicine (SMFM) have published guidelines on which patients should receive progesterone treatment. [92,93] However, although some studies have shown benefit, progesterone therapy should not be thought of as a cure-all for every woman at risk for SPTB. Several studies have shown that even if all pregnant women in the U.S. who have a history of preterm birth receive progesterone, the risk of SPTB would only be reduced by a mere 20 percent. The absolute preterm birth rate would only be reduced by a shocking 0.01 percent. [84, 85, 94, 95] In addition, if women with an incidental short cervix were effectively treated with progesterone therapy, we would only see an additional absolute risk reduction of only 0.02 percent. [95]
The reason why progesterone is not effective in every treated patient remains unclear. One reason may lie in the fact that we do not fully comprehend how progesterone works – specifically in the compartment of the cervix. Studies have suggested that progesterone may regulate cervical remodeling through different methods. [13,15] Progesterone may influence cell function that may in turn modulate the components in the cervical ECM (ie collagen synthesis [96], glycosaminoglycans [97]) that can in turn influence ECM organization. Progesterone withdrawal also appears to be essential to the cervical ripening phase that occurs just prior to delivery. Mice that have an abnormally high level of progesterone at the end of pregnancy (due to abnormalities in progesterone metabolism) have been found to have impaired cervical ripening. [98] In addition, mifepristone to women induces cervical ripening. [99,100] Lastly, since cervical remodeling is thought to involve an inflammatory process, progesterone is thought to have an anti-inflammatory effect that may mitigate the remodeling process. However, the data on how progesterone may counteract inflammation has been mixed. [101] Further, if there is in fact a specialized sphincter at the internal os as the updated paradigm of cervical tissue structure suggests and progesterone is thought to “quiet” smooth muscle (ie prevent premature activation of the myometrium and labor), would progesterone be in fact “relaxing” the sphincter? To date, no studies have evaluated the effect of progesterone on cervical smooth muscle function. Cleary, further studies are needed to first understand the mechanisms behind normal cervical remodeling in pregnancy as well as understanding pathogenic states of premature cervical remodeling. Once these mechanisms are understood we may then finally begin to understand why progesterone works in some but not all women with premature cervical remodeling to prevent SPTB.
Cerclage
Cervical cerclage is a procedure that involves placing a suture around the cervix in order to mechanically keep the cervix closed during pregnancy. To date a variety of studies have been performed to evaluate the effectiveness of cerclage in preventing SPTB. Currently both ACOG and SMFM have suggested guidelines as to which patient populations are candidates for cerclage placement. [93,102] ACOG states a history-indicated cerclage, placed in the late first or early second trimester, can be considered in a patient with cervical insufficiency (defined as a “history of unexplained second-trimester loss or preterm delivery in the absence of labor or abruptio placentae”). [102] This recommendation is based on three randomized controlled trials. Although two of the three the trials that no significant improvement in perinatal outcomes in women who underwent cerclage placement [103, 104], the third trial by the Medical Research Council/Royal College of Obstetricians and Gynaecologists found positive results. Specifically, this study which evaluated women with singleton pregnancies at high risk for SPTB, found a decreased rate of SPTB at less than 33 weeks in women who underwent cerclage placement (83 [13%] vs 110 [17%], P=.03). [105]
Women with a history of prior SPTB who are currently carrying singleton gestations also have the option of undergoing cervical length screening from 16 until 23+6 weeks gestation and undergoing an ultrasound cerclage placement if the cervical length shortens to ≤25mm. [93,102] In this cohort of women, cerclage has been shown to be associated with improved perinatal outcomes and composite neonatal morbidity and mortality. [106,107] Women who present with asymptomatic advanced cervical dilation without evidence of infection have historically been candidates for physical examindicated cerclage (also known as emergency or rescue cerclage). [102] ACOG states, however, that “given the lack of larger randomized trials that have demonstrated clear benefit, these women should be counseled about the potential for associated maternal and perinatal morbidity”.[102]
Although multiple randomized clinical trials and retrospective studies have evaluated the efficacy of cerclage in the above mentioned patient cohorts, it is unclear why cerclage works in some women and not in others. Could it be that there is a critical threshold of “softness” after which a cerclage is no longer able to hold the cervix closed? For instance, if the cervical tissue was “moderately” remodeled (ie had the consistency of recently chewed gum) would the cerclage hold better than in a cervix that had undergoing extensive remodeling and now had the consistency of butter? If so, what is that critical “softness” threshold? In addition, are there certain conditions where a cerclage may actually enhance the remodeling process via triggering an inflammatory response? An intriguing study by Kindinger et al recently evaluated the effect of braided vs monofilament cerclage suture on perinatal outcomes and vaginal microbiome. The study found that braided cerclage was associated with increased intrauterine death and preterm birth vs monofilament suture. Braided suture was also associated with a persistent shift toward vaginal microbiome dysbiosis (characterized as “reduced Lactobacillus spp. and enrichment of pathobionts”) which was associated with excretion of inflammatory cytokines into cervicovaginal fluid. [108] There is still much more work to be done to understand the effect of cervical tissue consistency on how well the cerclage will hold and also the effect of cerclage placement at a cellular and microscopic level.
This data may allow us to better optimize and risk stratify which women would benefit from this therapeutic intervention.
Pessary
The cervical pessary has been tested in several studies as a possible intervention to prevent SPTB in singleton pregnancies. Although there are a few studies which show evidence of benefit [109–111], a recent large, multicenter trial of women with short cervices demonstrated no overall benefit of pessary in preventing SPTB. [112] Studies, are currently being conducted by Maternal Fetal Medicine Units Network through the Eunice Kennedy Shriver National Institute of Child Health and Human Development to further evaluate the efficacy of pessary in preventing SPTB. Interestingly, there is a paucity of data that evaluates how the pessary may work. The prevailing thought is that the pessary works similar to a cerclage in that it is a mechanical device that keeps the cervix closed during pregnancy. It has also been suggested that the pessary may alleviate the amount of pressure on the cervix by shifting the angle of the cervix. [113] However, no studies to date have evaluated what the pessary does to the cervix at a biochemical/cellular level in the cervix. In addition, similar to the cerclage scenario, is there a critical threshold at which the cervix is “too remodeled” or “too soft” where the pessary won’t be effective. Perhaps there are other characteristics about the cervix outside of a “short cervix” that may help us better risk stratify patients to optimize outcomes.
Lastly, not every woman’s pelvis or reproductive organs are identical in terms of geometric shape. Is it possible that the current available pessaries are not a “good geometric fit” to certain women’s pelvises? Our lab has started investigating this “geometric” question by creating personalized finite element computer simulation models of pregnancy.(114-116) These specialized computer models take into account factors such as maternal tissue properties (ie strength of uterine tissue, fetal membranes, cervical tissue), maternal pelvic geometry (ie cervical and uterine dimensions, including cervical angle) and intrauterine pressure to simulate and predict how the cervix will mechanically function in pregnancy. Specifically, these specialized computer models determine how much stretch is present within the uterus, fetal membranes, and the cervix, giving us insight into which structural factors drive tissue stretching at the internal os. These computer simulation models will be critical in helping us understand how the various “compartments” interact to influence cervical function, elucidate how certain interventions (ie the pessary) may work in keeping the cervix closed, and develop personalized therapeutics to enhance the mechanical performance of the cervix.
Treatment options for a failing cervix in multiple gestations
To date, treatment options for multiple gestations that encounter premature cervical failure remains a conundrum. Cerclage may increase the risk of SPTB in these women and is not currently recommended by ACOG. [102, 117] Although a recent meta-analysis of individual patient data showed that vaginal progesterone decreased the rate of SPTB < 33 weeks in women with twin gestations and a short cervix (≤25 mm), current ACOG guidelines do not recommend treatment. [90] SMFM states in twin pregnancies with a short cervical length (≤25mm before 24 weeks of gestation), vaginal progesterone may decrease the rate of adverse perinatal outcome but does not give specific guidelines. [93] Current guidelines regarding the use of the pessary in multiple gestations are lacking.
FUTURE RESEARCH AND TREATMENT OPTIONS
We are currently in a very exciting time in Obstetrics. The updated paradigm of cervical tissue structure that includes the possibility of a specialized sphincter in the cervix finally opens a vast array of mechanistic possibilities to explain how the cervix functions in normal pregnancy as well as how it may malfunction causing premature cervical failure. This paradigm suggests that cervix is not a “passive bystander” in the process of normal parturition but may indeed be an active player – possibly even the quarterback that regulates the entire process. As we move forward, areas of active interest will lie in trying to understand the role of cervical smooth muscle cells in regulating cervical function. For instance, what influences contractility of this possible sphincter? We know the cervix remains highly innervated during pregnancy while innervation of the uterus decreases. [72] Is there neuronal control of this specialized sphincter? If so, could there be an imbalance of neuronal signals that cause the sphincter to malfunction? Further, smooth muscle bundles in the possible sphincter need a supply of blood/oxygen/nutrients. How does the blood supply to the cervix change in pregnancy and do changes influence smooth muscle function? To date, our knowledge of cervical vasculature and perfusion in pregnancy is lacking. Lastly, we know from literature on other body systems such as vasculature and uterus that smooth muscle cells are mechanosensitive and mechanical factors such as stretch can trigger the secretion of enzymes involved in remodeling ECM. [72] As a pregnancy grows, there is an increasing amount of pressure and stretch that occurs at the internal os. [115] Could there be a critical level of stretch of the smooth muscle cells in the possible specialized sphincter that triggers cervical tissue remodeling?
As we start to investigate these novel areas, it is imperative that we remember to first understand “normal” human tissue structure/function prior to delineating what is “abnormal”. In order to understand how pregnancy functions or malfunctions as a whole it will also be critical that experts from each of the various “compartments” and etiologies (i.e. uterus, cervix, fetal membranes, fetus/placenta, immune/vascular/nervous systems, environment etc.) to engage in open, multidisciplinary collaboration and communication. Lastly, as more attention is paid to the cervix, it may be time that we stop lumping patients into categories such as “long, normal” and “short, abnormal”. Given that not all women with a “short” cervix behave the same, is it time that we develop a way to better categorize or phenotype women who have a failing cervix? This new categorization system may include factors such as length, consistency (tissue strength and/or hydration), muscle function and/or inflammatory profiles. Although more work will need to be done to accurately and reliably evaluate these factors, with such an approach, we may finally be able to perform better risk-stratification of patients as well as develop novel therapeutics that may one day finally solve the persistent problem of spontaneous preterm birth.
SUMMARY:
This chapter reviews the current knowledge of human cervical tissue function and introduces a revised paradigm which includes the possibility of a sphincter at the internal os. We also review current treatment options for women whose pregnancies are complicated by a prematurely failing cervix as well as current gaps in knowledge regarding how these treatment options may work to prevent SPTB.
PRACTICE POINTS:
An updated paradigm of cervical tissue structure and function introduces the possibility of a specialized sphincter at the internal os.
Our current understanding of how the human cervix remodels in pregnancy is limited.
Current treatment options for singleton pregnancies with a prior SPTB include hydroxyprogesterone caproate from 16 to 20 weeks until 36 weeks gestation. These patients may also undergo cervical length surveillance starting at 16 weeks until 24 weeks gestation and undergo an ultrasound-indicated cerclage placement if diagnosed with a cervical length ≤ 25mm prior to 24 weeks gestation.
For singleton pregnancies without a history of prior SPTB who are found to have an incidentally short cervix (< 20mm), daily vaginal progesterone is recommended.
Women with a history of cervical insufficiency (defined as “history of unexplained second-trimester loss or preterm delivery in the absence of labor or abruptio placentae”) may undergo history-indicated cerclage placement.
Current recommendations for the use of the cervical pessary in singletons are lacking.
Current treatment guidelines for multiple gestations who present with premature cervical failure are lacking.
RESEARCH AGENDA:
Understand the profile of proteins that exist in the normal human cervix, how these proteins change as the cervix remodels in pregnancy and their influence on resident cell function in the cervix
Role of cervical smooth muscle in cervical function in pregnancy
Role of neuronal and vascular changes in the cervix as the cervical remodels in pregnancy
Understand how “geometric” factors such as uterine/cervix shape/angle, tissue mechanical properties etc influence pregnancy outcomes
Further investigate how currently available treatment options affect cervical tissue at the cell/matrix level.
ACKNOWLEDGEMENT:
The research included in this review was supported financially by the Society for Maternal Fetal Medicine/ American Association of Obstetricians & Gynecologists Foundation Scholarship, The Louis J. Gerstner, Jr. Foundation, National Science Foundation, March of Dimes Prematurity Research Center at the University of Pennsylvania, and K08HD088758 from the Eunice Kennedy Shriver National Institute of Child Health and Human Development. The content is solely the responsibility of the authors and does not necessarily represent the official views of the funding agencies listed here.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DISCLOSURE: The authors have nothing to disclose.
REFERENCES
- 1.Centers for Disease Control and Prevention (Accessed December 31, 2017, at http://www.cdc.gov/reproductivehealth//MaternalInfantHealth/PretermBirth.htm)
- 2.Norman JE, Shennan AH. Prevention of preterm birth—why can’t we do any better? The Lancet. 2013;381:184–185. [DOI] [PubMed] [Google Scholar]
- 3.Danforth D The fibrous nature of the human cervix and its relation to the isthmic segment in gravid and nongravid uteri. Proc Inst Med Chic. 1947. January 15;16(10):295. [PubMed] [Google Scholar]
- 4.Danforth D The fibrous nature of the human cervix, and its relation to the isthmic segment in gravid and nongravid uteri. Am J Obstet Gynecol. 1947. April;53(4):541–60. [DOI] [PubMed] [Google Scholar]
- 5.Danforth DN. The morphology of the human cervix. Clin Obstet Gynecol. 1983. March;26(1):7–13. [DOI] [PubMed] [Google Scholar]
- 6.Vink JY, Qin S, Brock CO, Zork NM, Feltovich HM, Chen X, et al. A new paradigm for the role of smooth muscle cells in the human cervix. Am J Obstet Gynecol. 2016. October;215(4):478.e1–478.e11. [DOI] [PubMed] [Google Scholar]
- 7.Vink J, Feltovich H. Cervical etiology of spontaneous preterm birth. Semin Fetal Neonatal Med. 2016. Apr;21(2):106–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dubrauszky V, Schwalm H, Fleischer M. Fibre system of connective tissue in childbearing age, menopause and pregnancy. Archiv Gynakol. 1971;210:276–92. [DOI] [PubMed] [Google Scholar]
- 9.Aspden RM. Collagen organization in the cervix and its relation to mechanical function. Coll Relat Res. 1988;8:103–12. [DOI] [PubMed] [Google Scholar]
- 10.Weiss S, Jaermann T, Schmid P, Staempfli P, Boesiger P, Niederer P, et al. Three-dimensional fiber architecture of the nonpregnant human uterus determined ex vivo using magnetic resonance diffusion tensor imaging. Anat Rec A Discov Mol Cell Evol Biol. 2006. January; 288(1):84–90. [DOI] [PubMed] [Google Scholar]
- 11.Yao W, Gan Y, Myers KM, Vink JY, Wapner RJ, Hendon CP. Collagen Fiber Orientation and Dispersion in the Upper Cervix of Non-Pregnant and Pregnant Women. PLoS One. 2016. November 29;11(11):e0166709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Reusch LM, Feltovich H, Carlson LC, Hall G, Campagnola PJ, Eliceiri KW,.et al. Nonlinear optical microscopy and ultrasound imaging of human cervical structure. J Biomed Opt. 2013;18:031110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Word RA, Li XH, Hnat M, Carrick K. Dynamics of cervical remodeling during pregnancy and parturition: mechanisms and current concepts. Semin Reprod Med. 2007. January;25(1):69–79. [DOI] [PubMed] [Google Scholar]
- 14.House M, Kaplan DL, Socrate S. Relationships between mechanical properties and extracellular matrix constituents of the cervical stroma during pregnancy. Semin Perinatol. 2009. October;33(5):300–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mahendroo M Cervical remodeling in term and preterm birth: insights from an animal model. Reproduction. 2012. April;143(4):429–38. [DOI] [PubMed] [Google Scholar]
- 16.Timmons B, Akins M, Mahendroo M. Cervical remodeling during pregnancy and parturition. Trends Endocrinol Metab. 2010. June;21(6):353–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Read CP, Word RA, Ruscheinsky MA, Timmons BC, Mahendroo MS. Cervical remodeling during pregnancy and parturition: molecular characterization of the softening phase in mice. Reproduction. 2007;134:327–340. [DOI] [PubMed] [Google Scholar]
- 18.Bryant MW, Greenwell A, and Weeks P Alterations in collages organization during dilation of the cervix uteri. Surg. Gynecol. Obstet 1968;126,27. [PubMed] [Google Scholar]
- 19.Buckingham J, Selden R, and Danforth D Connective tissue changes in the cervix during pregnancy and labor. Ann. NY Acad. Sci 1962;97,733–742 [DOI] [PubMed] [Google Scholar]
- 20.Leppert PC Anatomy and physiology of cervical ripening. Clin. Obstet. Gynecol 1995;38,267–279. [DOI] [PubMed] [Google Scholar]
- 21.Rimmer D The effect of pregnancy on the collagen of the uterine cervix of the mouse. J. Endocrinol 1973;57,413–418. [DOI] [PubMed] [Google Scholar]
- 22.Theobald P, Rath W, Kuhnle H, Kuhn W Histological and electron microscopic examinations of collagenous connective tissue of the non-pregnant cervix, pregnant cervix, and the pregnant prostaglandin-treated cervix. Arch. Gynecol 1982;231,241–245. [DOI] [PubMed] [Google Scholar]
- 23.Winkler M and Rath W Changes in the cervical extracellular matrix during pregnancy and parturition. J. Perinat. Med 1999;27,45–61. [DOI] [PubMed] [Google Scholar]
- 24.Gonzalez JM, Franzke CW, Yang F, Romero R, Girardi G. Complement activation triggers metalloproteinases release inducing cervical remodeling and preterm birth in mice. Am J Pathol. 2011. August;179(2):838–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stygar D, Wang H, Vladic YS, Ekman G, Eriksson H, Sahlin L. Increased level of matrix metalloproteinases 2 and 9 in the ripening process of the human cervix. Biol Reprod. 2002. September;67(3):889–94. [DOI] [PubMed] [Google Scholar]
- 26.Dubicke A, Ekman-Ordeberg G, Mazurek P, Miller L, Yellon SM. Density of Stromal Cells and Macrophages Associated With Collagen Remodeling in the Human Cervix in Preterm and Term Birth. Reprod Sci. 2016. May;23(5):595–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dobyns AE, Goyal R, Carpenter LG, Freeman TC, Longo LD, Yellon SM. Macrophage gene expression associated with remodeling of the prepartum rat cervix: microarray and pathway analyses. PLoS One. 2015. March 26;10(3):e0119782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Payne KJ, Clyde LA, Weldon AJ, Milford TA, Yellon SM. Residency and activation of myeloid cells during remodeling of the prepartum murine cervix. Biol Reprod. 2012. November 1;87(5):106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mackler AM, Iezza G, Akin MR, McMillan P, Yellon SM. Macrophage trafficking in the uterus and cervix precedes parturition in the mouse. Biol Reprod. 1999. October;61(4):879–83. [DOI] [PubMed] [Google Scholar]
- 30.Yellon SM, Mackler AM, Kirby MA. The role of leukocyte traffic and activation in parturition. J Soc Gynecol Investig. 2003. September;10(6):323–38. [DOI] [PubMed] [Google Scholar]
- 31.Yellon SM, Ebner CA, Sugimoto Y. Parturition and recruitment of macrophages in cervix of mice lacking the prostaglandin F receptor. Biol Reprod. 2008. March;78(3):438–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liggins GC. Cervical ripening as an inflammatory reaction In The Cervix in Pregnancy and Labour, Clinical and Biochemical Investigation, Ellwood D &Anderson ABM. Edinburgh: Churchill Livingston; 1981 [Google Scholar]
- 33.Junqueira LC, Zugaib M, Montes GS, Toledo OM, Krisztán RM, Shigihara KM. Morphologic and histochemical evidence for the occurrence of collagenolysis and for the role of neutrophilicpolymorphonuclear leukocytes during cervical dilation. Am J Obstet Gynecol. 1980. October 1;138(3):273–81. [DOI] [PubMed] [Google Scholar]
- 34.Young A, Thomson AJ, Ledingham M, Jordan F, Greer IA, Norman JE. Immunolocalization of proinflammatory cytokines in myometrium, cervix, and fetal membranes during human parturition at term. Biology of Reproduction. 2002;66: 445–449. [DOI] [PubMed] [Google Scholar]
- 35.Osman I, Young A, Ledingham MA, Thomson AJ, Jordan F, Greer IA, et al. Leukocyte density and pro-inflammatory cytokine expression in human fetal membranes, decidua, cervix and myometrium before and during labour at term. Molecular Human Reproduction. 2003. 9: 41–45. [DOI] [PubMed] [Google Scholar]
- 36.Hirsch E, Filipovich Y, Mahendroo M. Signaling via the type I IL-1 and TNF receptors is necessary for bacterially induced preterm labor in a murine model. Am J Obstet Gynecol. 2006;194: 1334–1340. [DOI] [PubMed] [Google Scholar]
- 37.Gonzalez JM, Xu H, Chai J, Ofori E, Elovitz MA. Preterm and term cervical ripening in CD1 Mice (Musmusculus): similar or divergent molecular mechanisms? Biol Reprod. 2009. December;81(6):1226–32. [DOI] [PubMed] [Google Scholar]
- 38.Timmons BC, Fairhurst AM, Mahendroo MS. Temporal changes in myeloid cells in the cervix during pregnancy and parturition. J Immunol. 2009. March 1;182(5):2700–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Timmons BC, Mahendroo MS. Timing of neutrophil activation and expression of proinflammatory markers do not support a role for neutrophils in cervical ripening in the mouse. Biol Reprod. 2006. February;74(2):236–45. [DOI] [PubMed] [Google Scholar]
- 40.Yoshida K, Jiang H, Kim M, et al. Quantitative evaluation of collagen crosslinks and corresponding tensile mechanical properties in mouse cervical tissue during normal pregnancy. PLoS One. 2014;9:e112391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zork NM, Myers KM, Yoshida K, et al. A systematic evaluation of collagen crosslinks in the human cervix. Am J Obstet Gynecol. 2015;212:321.e1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Akgul Y, Word RA, Ensign LM, Yamaguchi Y, Lydon J, Hanes J, Mahendroo M.Hyaluronan in cervical epithelia protects against infection-mediated preterm birth. J Clin Invest. 2014. December;124(12):5481–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Holt R, Timmons B, Akgul Y, Akins M, Mahendroo M. The molecular mechanisms of cervical ripening differ between term and preterm birth. Endocrinology. 2011;152:1036–1046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Straach KJ, Shelton JM, Richardson JA, Hascall VC, Mahendroo MS. Regulation of hyaluronan expression during cervical ripening. Glycobiology. 2005;15(1):55–65 [DOI] [PubMed] [Google Scholar]
- 45.Akins ML, Luby-Phelps K, Bank RA, Mahendroo M. Cervical softening during pregnancy: regulated changes in collagen cross-linking and composition of matricellular proteins in the mouse. Biol Reprod. 2011. May;84(5):1053–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Danforth DN, Veis A, Breen M, Weinstein HG, Buckingham JC, Manalo P. The effect of pregnancy and labor on the human cervix: changes in collagen, glycoproteins, and glycosaminoglycans. Am J Obstet Gynecol 1974; 120: 641–651 [DOI] [PubMed] [Google Scholar]
- 47.Norman M, Ekman G, Ulmsten U, Barchan K, Malmstrom A. Proteoglycan metabolism in the connective tissue of pregnant and non-pregnant human cervix. An in vitro study. Biochem J 1991;275 (Pt 2): 515–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Osmers R, Rath W, Pflanz MA, Kuhn W, Stuhlsatz HW, Szeverenyi M. Glycosaminoglycans in cervical connective tissue during pregnancy and parturition. Obstet Gynecol 1993; 81: 88–92 [PubMed] [Google Scholar]
- 49.Westergren-Thorsson G, Norman M, Bjornsson S, Endresen U, Stjernholm Y, Ekman G, et al. Differential expressions of mRNA for proteoglycans, collagens and transforming growth factor-beta in the human cervix during pregnancy and involution. Biochim Biophys Acta 1998; 1406: 203–213 [DOI] [PubMed] [Google Scholar]
- 50.Akgul Y, Holt R, Mummert M, Word A, Mahendroo M. Dynamic changes in cervical glycosaminoglycan composition during normal pregnancy and preterm birth. Endocrinology. 2012. July;153(7):3493–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bornstein Paul. Matricellular proteins: an overview. J Cell Commun Signal. 2009. December;3(3–4):163–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nallasamy S, Mahendroo M. Distinct Roles of Cervical Epithelia and Stroma in Pregnancy and Parturition. Semin Reprod Med. 2017. March;35(2):190–200. [DOI] [PubMed] [Google Scholar]
- 53.Iams JD, Goldenberg RL, Meis PJ, Mercer BM, Moawad A, Das A, et al. The length of the cervix and the risk of spontaneous premature delivery. NICHD Maternal Fetal Medicine Unit Network. N Engl J Med. 1996. February 29;334(9):567–72 [DOI] [PubMed] [Google Scholar]
- 54.Mancuso MS, Szychowski JM, Owen J, Hankins G, Iams JD, Sheffield JS, et al. Vaginal Ultrasound Trial Consortium. Cervical funneling: effect on gestational length and ultrasound-indicated cerclage in high-risk women. Am J Obstet Gynecol. 2010. September;203(3):259.e1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ivy A, Hartman C, and Koff A The contractions of the monkey uterus at term. Am J Obstet Gynecol. 1931;22:388–399 [Google Scholar]
- 56.Schild HO, Fitzpatrick RI, Nixon WC. Activity of the human cervix and corpus uteri. Their response to drugs in early pregnancy. Lancet. 1951;1:250–253 [DOI] [PubMed] [Google Scholar]
- 57.Pajntar M, Leskosek B, Rudel D, Verdenik I Contribution of cervical smooth muscle activity to the duration of latent and active phases of labor. Br J Obstet Gynaecol. 2001. 108:533–538 [DOI] [PubMed] [Google Scholar]
- 58.Rudel D, Pajntar M Active contractions of the cervix in the latent phase of labor. Br J Obstet Gynaecol. 1999. 106:446–452 [DOI] [PubMed] [Google Scholar]
- 59.Pajntar M, Verdenik I, Pusenjak S, Rudel D, Leskosek B Activity of smooth muscles in human cervix and uterus. Eur J Obstet Gynecol Reprod Biol. 1998;79:199–204 [DOI] [PubMed] [Google Scholar]
- 60.Pajntar M, Verdenik I Electromyographic activity in cervices with very low Bishop score during labor. Int J Gynaecol Obstet. 1995;49:277–281 [DOI] [PubMed] [Google Scholar]
- 61.Pajntar M, Rudel D Changes in electromyographic activity of the cervix after stimulation of labor with oxytocin. Gynecol Obstet Invest. 1991;31:204–207 [DOI] [PubMed] [Google Scholar]
- 62.Pajntar M, Roskar E, Rudel D Longitudinally and circularly measured EMG activity in the human uterine cervix during labor. Acta Physiol Hung. 1988;71:497–502 [PubMed] [Google Scholar]
- 63.Pajntar M, Roskar E, Rudel D Electromyographic observations on the human cervix during labor. Am J Obstet Gynecol. 1987;156:691–697 [DOI] [PubMed] [Google Scholar]
- 64.Roddick JW Jr, Buckingham JC, Danforth DN. The muscular cervix--a cause of incompetency in pregnancy. Obstet Gynecol. 1961. May;17:562–5. [PubMed] [Google Scholar]
- 65.Buckingham JC, Buethe RA Jr, Danforth DN. Collagen-Muscle Ratio In Clinically Normal And Clinically Incompetent Cervices. Am J Obstet Gynecol. 1965. January 15;91:232–7. [DOI] [PubMed] [Google Scholar]
- 66.Leppert PC, Yu SY, Keller S, Cerreta J, Mandl I. Decreased elastic fibers and desmosine content in incompetent cervix. Am J Obstet Gynecol. 1987. November;157(5):1134–9. [DOI] [PubMed] [Google Scholar]
- 67.Rechberger T, Uldbjerg N, Oxlund H. Connective tissue changes in the cervix during normal pregnancy and pregnancy complicated by cervical incompetence. Obstet Gynecol. 1988. April;71(4):563–7. [PubMed] [Google Scholar]
- 68.Petersen LK, Uldbjerg N. Cervical collagen in non-pregnant women with previous cervical incompetence. Eur J Obstet Gynecol Reprod Biol. 1996. July;67(1):41–5. [DOI] [PubMed] [Google Scholar]
- 69.Oxlund BS, Ørtoft G, Brüel A, Danielsen CC, Oxlund H, Uldbjerg N. Cervical collagen and biomechanical strength in non-pregnant women with a history of cervical insufficiency. Reprod Biol Endocrinol. 2010. July 30;8:92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gedikbasi A, Yücel B, Arslan O, Giris M, Gedikbasi A, Abbasoglu SD. Dynamic collagen changes in cervix during the first trimester and decreased collagen content in cervical insufficiency. J Matern Fetal Neonatal Med. 2016. September;29(18):2968–72. [DOI] [PubMed] [Google Scholar]
- 71.Sundtoft I, Langhoff-Roos J, Sandager P, Sommer S, Uldbjerg N. Cervical collagen is reduced in non-pregnant women with a history of cervical insufficiency and a short cervix. Acta Obstet Gynecol Scand. 2017. April 4. [DOI] [PubMed] [Google Scholar]
- 72.Vink J, Mourad M.The pathophysiology of human premature cervical remodeling resulting in spontaneous preterm birth: Where are we now? Semin Perinatol. 2017. November;41(7):427–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Stafford GP, Parker JL, Amabebe E, Kistler J, Reynolds S, Stern V, et al. Spontaneous Preterm Birth Is Associated with Differential Expression of Vaginal Metabolites by Lactobacilli-Dominated Microflora. Front Physiol. 2017. August 23;8:615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Willcockson AR, Nandu T, Liu CL, Nallasamy S, Kraus WL, Mahendroo M. Transcriptome signature identifies distinct cervical pathways induced in LPS-mediated preterm birth. Biol Reprod. 2017. December 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Timmons BC, Reese J, Socrate S, Ehinger N, Paria BC, Milne GL, et al. Prostaglandins are essential for cervical ripening in LPS-mediated preterm birth but not term or antiprogestin-driven preterm ripening. Endocrinology. 2014. January;155(1):287–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Nold C, Anton L, Brown A, Elovitz M. Inflammation promotes a cytokine response and disrupts the cervical epithelial barrier: a possible mechanism of premature cervical remodeling and preterm birth.. Am J Obstet Gynecol. 2012. March;206(3):208.e1–7. [DOI] [PubMed] [Google Scholar]
- 77.McGee D, Smith A, Poncil S, Patterson A, Bernstein AI, Racicot K. Cervical HSV-2 infection causes cervical remodeling and increases risk for ascending infection and preterm birth. PLoS One. 2017. November 30;12(11):e0188645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Racicot K, Cardenas I, Wünsche V, Aldo P, Guller S, Means RE, Romero R, Mor G. Viral infection of the pregnant cervix predisposes to ascending bacterial infection. J Immunol. 2013. July 15;191(2):934–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Dunn AB, Dunlop AL, Hogue CJ, Miller A, Corwin EJ. The Microbiome and Complement Activation: A Mechanistic Model for Preterm Birth. Biol Res Nurs. 2017. May;19(3):295–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Vinturache AE, Gyamfi-Bannerman C, Hwang J, Mysorekar IU, Jacobsson B; Preterm BirthInternational Collaborative (PREBIC). Maternal microbiome - A pathway to preterm birth. Semin Fetal Neonatal Med. 2016. April;21(2):94–9. [DOI] [PubMed] [Google Scholar]
- 81.Prince AL, Antony KM, Chu DM, Aagaard KM. The microbiome, parturition, and timing of birth: more questions than answers.J Reprod Immunol. 2014. October;104–105:12–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kindinger LM, Bennett PR, Lee YS, Marchesi JR, Smith A, Cacciatore S, et al. The interaction between vaginal microbiota, cervical length, and vaginal progesterone treatment for preterm birth risk. Microbiome. 2017. January 19;5(1):6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Romero R, Miranda J, Chaiworapongsa T, Chaemsaithong P, Gotsch F, Dong Z, et al. Sterile intra-amniotic inflammation in asymptomatic patients with a sonographic short cervix: prevalence and clinical significance. J Matern Fetal Neonatal Med. 2014. September 24:1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Meis PJ, Klebanoff M, Thom E, Dombrowski MP, Sibai B, Moawad AH, et al. Prevention of recurrent preterm delivery by 17 alpha-hydroxyprogesterone caproate. NICHD Maternal-Fetal Medicine Units Network. N Engl J Med 2003;348:2379–85. [DOI] [PubMed] [Google Scholar]
- 85.da Fonseca EB, Bittar RE, Carvalho MH, Zugaib M. Prophylactic administration of progesterone by vaginal suppository to reduce the incidence of spontaneous preterm birth in women at increased risk: a randomized placebo-controlled doubleblind study. Am J Obstet Gynecol. 2003. February;188(2):419–24. [DOI] [PubMed] [Google Scholar]
- 86.Norman JE, Marlow N, Messow CM, Shennan A, Bennett PR, Thornton S, et al. Vaginal progesterone prophylaxis for preterm birth (the OPPTIMUM study): a multicentre, randomised, double-blind trial. Lancet. 2016. May 21;387(10033):2106–2116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Fonseca EB, Celik E, Parra M, Singh M, Nicolaides KH. Progesterone and the risk of preterm birth among women with a short cervix. Fetal Medicine Foundation Second Trimester Screening Group. N Engl J Med 2007;357:462–9. [DOI] [PubMed] [Google Scholar]
- 88.Hassan SS, Romero R, Vidyadhari D, Fusey S, Baxter JK, Khandelwal M, et al. Vaginal progesterone reduces the rate of preterm birth in women with a sonographic short cervix: a multicenter, randomized, double-blind, placebo-controlled trial. PREGNANT Trial. Ultrasound Obstet Gynecol 2011;38:18–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Romero R, Conde-Agudelo A, Da Fonseca E, O’Brien JM, Cetingoz E, Creasy GW, et al. Vaginal Progesterone for Preventing Preterm Birth and Adverse Perinatal Outcomes in Singleton Gestations with a Short Cervix: A Meta-Analysis of Individual Patient Data. Am J Obstet Gynecol. 2017. November 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Grobman WA, Thom EA, Spong CY, Iams JD, Saade GR, Mercer BM, et al. 17 alpha-hydroxyprogesterone caproate to prevent prematurity in nulliparas with cervical length less than 30 mm. Am J Obstet Gynecol 2012; 207:390.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Winer N, Bretelle F, Senat MV, Bohec C, Deruelle P, Perrotin F, et al. 17 alpha-hydroxyprogesterone caproate does not prolong pregnancy or reduce the rate of preterm birth in women at high risk for preterm delivery and a short cervix: a randomized controlled trial. Am J Obstet Gynecol 2015; 212:485.e1. [DOI] [PubMed] [Google Scholar]
- 92.Committee on Practice Bulletins—Obstetrics, The American College of Obstetricians and Gynecologists. Practice bulletin no. 130: prediction and prevention of preterm birth. Obstet Gynecol. 2012. October;120(4):964–73. [DOI] [PubMed] [Google Scholar]
- 93.Society for Maternal and Fetal Medicine (accessed on December 31,2017 at https://www.smfm.org/publications/231-smfm-preterm-birth-toolkit)
- 94.Petrini JR, Callaghan WM, Klebanoff M, et al. Estimated effect of 17 alpha-hydroxyprogesterone caproate on preterm birth in the United States. Obstet Gynecol 2005; 105:267. [DOI] [PubMed] [Google Scholar]
- 95.Norman JE, Bennett P. Preterm birth prevention-Time to PROGRESS beyond progesterone. PLoS Med 2017; 14:e1002391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.House M, Tadesse-Telila S, Norwitz ER, Socrate S, Kaplan DL. Inhibitory effect of progesterone on cervical tissue formation in a three-dimensional culture system with human cervical fibroblasts. Biol Reprod. 2014. January 30;90(1):18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Carbonne B, Dallot E, Haddad B, Ferré F, Cabrol D. Effects of progesterone on prostaglandin E(2)-induced changes in glycosaminoglycan synthesis by human cervical fibroblasts in culture. Mol Hum Reprod. 2000. July;6(7):661–4. [DOI] [PubMed] [Google Scholar]
- 98.Mahendroo MS, Porter A, Russell DW, Word RA. The parturition defect in steroid 5alpha-reductase type 1 knockout mice is due to impaired cervical ripening. Mol Endocrinol. 1999. June;13(6):981–92. [DOI] [PubMed] [Google Scholar]
- 99.Rådestad A, Christensen NJ, Strömberg L. Induced cervical ripening with Mifepristone in first trimester abortion. A double-blind randomized biomechanical study.Contraception. 1988. September;38(3):301–12. [DOI] [PubMed] [Google Scholar]
- 100.Wing DA, Fassett MJ, Mishell DR. Mifepristone for preinduction cervical ripening beyond 41 weeks’ gestation: a randomized controlled trial. Obstet Gynecol. 2000. October;96(4):543–8. [DOI] [PubMed] [Google Scholar]
- 101.Nold C, Maubert M, Anton L, Yellon S, Elovitz MA. Prevention of preterm birth by progestational agents: what are the molecular mechanisms? Am J Obstet Gynecol. 2013. March;208(3):223.e1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.American College of Obstetricians and Gynecologists. ACOG Practice Bulletin No. 142. Cerclage for the management of cervical insufficiency. Obstet Gynecol. 2014. February;123(2 Pt 1):372–9. [DOI] [PubMed] [Google Scholar]
- 103.Lazar P, Gueguen S, Dreyfus J, Renaud R, Pontonnier G, Papiernik E. Multicentred controlled trial of cervical cerclage in women at moderate risk of preterm delivery. Br J Obstet Gynaecol 1984;91:731–5. [DOI] [PubMed] [Google Scholar]
- 104.Rush RW, Isaacs S, McPherson K, Jones L, Chalmers I, Grant A. A randomized controlled trial of cervical cerclage in women at high risk of spontaneous preterm delivery. Br J Obstet Gynaecol 1984;91:724–30. [DOI] [PubMed] [Google Scholar]
- 105.Final report of the Medical Research Council/Royal College of Obstetricians and Gynaecologists multicentre randomised trial of cervical cerclage. MRC/RCOG Working Party on Cervical Cerclage. Br J Obstet Gynaecol 1993;100:516–23. [DOI] [PubMed] [Google Scholar]
- 106.Berghella V, Rafael TJ, Szychowski JM, Rust OA, Owen J. Cerclage for short cervix on ultrasonography in women with singleton gestations and previous preterm birth: a meta-analysis. Obstet Gynecol 2011;117:663–71. [DOI] [PubMed] [Google Scholar]
- 107.Owen J, Hankins G, Iams JD, Berghella V, Sheffield JS, Perez-Delboy A, et al. Multicenter randomized trial of cerclage for preterm birth prevention in high-risk women with shortened midtrimester cervical length. Am J Obstet Gynecol 2009;201:375.e1–375.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Kindinger LM, MacIntyre DA, Lee YS, Marchesi JR, Smith A, McDonald JA, et al. Relationship between vaginal microbial dysbiosis, inflammation, and pregnancy outcomes in cervical cerclage. Sci Transl Med. 2016. August 3;8(350):350ra102. [DOI] [PubMed] [Google Scholar]
- 109.Goya M, Pratcorona L, Merced C, Rodó C, Valle L, Romero A, et al. Cervical pessary in pregnant women with a short cervix (PECEP): an open-label randomised controlled trial. Lancet. 2012. May 12;379(9828):1800–6. [DOI] [PubMed] [Google Scholar]
- 110.Hui SY, Chor CM, Lau TK, Lao TT, Leung TY. Cerclage pessary for preventing preterm birth in women with a singleton pregnancy and a short cervix at 20 to 24 weeks: a randomized controlled trial. Am J Perinatol. 2013. April;30(4):283–8. [DOI] [PubMed] [Google Scholar]
- 111.Saccone G, Maruotti GM, Giudicepietro A, Martinelli P; Italian Preterm Birth Prevention (IPP) Working Group. Effect of Cervical Pessary on Spontaneous Preterm Birth in Women With Singleton Pregnancies and Short Cervical Length: A Randomized Clinical Trial. JAMA. 2017. December 19;318(23):2317–2324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Nicolaides KH, Syngelaki A, Poon LC, Picciarelli G, Tul N, Zamprakou A, et al. A Randomized Trial of a Cervical Pessary to Prevent Preterm Singleton Birth. N Engl J Med. 2016. March 17;374(11):1044–52. doi: 10.1056/NEJMoa1511014. [DOI] [PubMed] [Google Scholar]
- 113.Cannie MM, Dobrescu O, Gucciardo L, Strizek B, Ziane S, Sakkas E, et al. Arabin cervical pessary in women at high risk of preterm birth: a magnetic resonance imaging observational follow-up study. Ultrasound Obstet Gynecol. 2013. October;42(4):426–33. [DOI] [PubMed] [Google Scholar]
- 114.Westervelt AR, Fernandez M, House M, Vink J, Nhan-Chang CL, Wapner R, et al. A Parameterized Ultrasound-Based Finite Element Analysis of the Mechanical Environment of Pregnancy. J Biomech Eng. 2017. May 1;139(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Westervelt AR, Myers KM. Computer modeling tools to understand the causes of preterm birth. Semin Perinatol. 2017. December;41(8):485–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Myers KM, Hendon CP, Gan Y, Yao W, Yoshida K, Fernandez M, et al. A continuous fiber distribution material model for human cervical tissue. J Biomech. 2015. June 25;48(9):1533–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Berghella V, Odibo AO, To MS, Rust OA, Althuisius SM. Cerclage for short cervix on ultrasonography: meta-analysis of trials using individual patient-level data. Obstet Gynecol 2005;106:181–9. [DOI] [PubMed] [Google Scholar]