Abstract
Purpose
It remains unclear whether eosinophilia is useful for in guiding inhaled corticosteroid (ICS) therapy in chronic obstructive pulmonary disease (COPD) patients. The goal of this study is to evaluate the risk of acute exacerbations, COPD‐related hospitalisations/accident and emergency visits, and all‐cause mortality with various levels of eosinophil counts among COPD patients using ICS.
Methods
A cohort study was conducted using the UK Clinical Practice Research Datalink. Patients were aged 40+ and had COPD (n = 32 693). Current users of ICS were stratified by relative and absolute eosinophil counts to determine the risk of outcomes with blood eosiniphilia using Cox regression analysis.
Results
Among COPD patients, current use of ICS was not associated with a reduced risk of acute COPD exacerbations, COPD‐related hospitalisations/accident and emergency visits, and all‐cause mortality. Stratification of ICS use by absolute or relative eosinophil counts did not result in significant differences in risk of COPD exacerbations or hospitalisations/accident and emergency visits. However, all‐cause mortality was reduced by 12% to 24% among patients with eosinophilia.
Conclusions
COPD‐related acute exacerbations or hospitalisations/accident and emergency visits were not reduced with eosinophilia among users of ICS with COPD. However, all‐cause mortality was reduced by 12% to 24%. These findings are potentially important and require further evaluation in prospective studies.
Keywords: chronic obstructive pulmonary disease, eosinophils, exacerbations, inhaled corticosteroids, pharmacoepidemiology
KEY POINTS.
We found no reduced risk of COPD‐related acute exacerbations or hospitalizations/A&E visits among patients with blood eosinophilia using ICS.
However, all-cause mortality was reduced among current ICS users with blood eosinophilia compared to current ICS users with low relative blood eosinophil count.
The findings have important implications on targeted prescribing of Inhaled corticosteroids among COPD patients based on blood eosinophil counts in general practice.
1. INTRODUCTION
Chronic obstructive pulmonary disease (COPD) is a major cause of morbidity and mortality worldwide and is defined by the presence of chronic respiratory symptoms and persistent airflow limitation.1 While bronchodilators are the cornerstone of pharmacological management of COPD, patients with frequent exacerbations are often additionally treated with inhaled corticosteroids (ICS).2 Exacerbations play a central role in the pathophysiology of COPD as they are related to lung function decline, poor health status, and increased mortality.3 While nonresponse to ICS therapy is common,4 potential side effects of ICS include fractures and pneumonia.
Clinical data have suggested that blood eosinophil count, which is present in up to 40% of COPD patients,5 is a promising biomarker of response to ICS in patients with COPD.6, 7, 8, 9 Eosinophilic airway inflammation has been associated with an increased risk of exacerbations, and patients with eosinophilic inflammation responded better to ICS therapy than noneosinophilic patients.6, 10 Pascoe and colleagues6 performed a post hoc analysis of data from 2 replicate, randomised, double‐blind trials with duration of 12 months. In the analysis, vilanterol 25 μg was compared with 25 μg vilanterol plus 50, 100, or 200 μg fluticasone furoate in patients with moderate‐to‐severe COPD. They observed that 68% of COPD patients had peripheral blood eosinophilia. Importantly, across all doses of ICS, fluticasone furoate and vilanterol reduced exacerbations by 29% compared with vilanterol alone in patients with eosinophil counts ≥2%, and by 10% in patients with eosinophil counts <2%. Analysis of data from the FLAME trial showed no significant reduction in exacerbation in indacaterol/gycopyrronium compared to salmeterol/fluticasone among patients with blood eosinophil levels of 150 to 300 cells/μL versus 300 cells/μL.8 In this study, we use the word “eosinophilia” to mean an elevated blood eosinophil count based on our defined cutoffs.
However, studies with real‐world evidence are currently limited and are needed to identify patients who will benefit from ICS use. Therefore, the aim of this study was to evaluate the risk of acute exacerbations, COPD‐related hospitalisations/accident and emergency visits, and all‐cause mortality, with various levels of eosinophil counts among COPD patients using ICS.
2. METHODS
2.1. Data source
This study was conducted with data obtained from the Clinical Practice Research Datalink (CPRD). It provides detailed information on drug prescriptions, clinical events, demographics, specialist referrals, hospital admissions, and electronic lab linked data of patients from 674 general practices, who are representative for 7% of the total British population.11, 12 Data collection started on January 1, 2005, corresponding to the introduction of the Quality and Outcomes Framework in April 2004, which improved routine recording of various diseases, including COPD.13 Routinely collected historical data were available, dating back to 1987. Previous studies with the CPRD have shown a high level of validity of recording of COPD11 and COPD exacerbations.14 Clinical Practice Research Datalink has previously been used to study COPD.15, 16, 17 The independent scientific advisory committee of MHRA database research approved the study protocol (17_065R).
2.2. Study population
We selected all patients aged ≥40 years with a diagnosis of COPD, as recorded by a first read code during valid data collection from January 1, 2005 through January 31, 2014. We excluded all patients with a diagnosis of COPD as recorded by validated read codes before January 1, 2005. Patients were followed from the date of their COPD diagnosis (index date) until the end of data collection, date of death, end of study (January 31, 2014) or when the outcome of interest occurred, whichever came first. The primary outcome of interest was an acute exacerbation of COPD, using validated read codes for acute exacerbation of COPD with 96% positive predictive value (PPV) in identifying acute exacerbation in the CPRD.18 Secondary outcomes were hospitalisation or accident and emergency (AE) visit for COPD and all‐cause mortality. Only patients with blood eosinophil counts measured during study period were included. Blood eosinophil count closest to index date (ie, start of follow‐up) was used in our analyses. Relatively stable blood eosinophil counts have been reported among COPD patients over time within the UK population, irrespective of exposure to systemic glucocorticoids or other factors.19, 20 We excluded patients with a history of asthma, those with COPD exacerbations, those with oral glucocorticoid in the past month, or those with ICS use in the past year.
2.3. Exposure
Exposure to ICS was determined time‐dependently during follow‐up. Each patient's follow‐up time was divided into fixed periods of 90 days, starting at index date. Prior to the start of each interval, ICS exposure was determined based on the date of the prescription, and classified as current, recent, past, or never use (patient without ICS exposure). Current users had received their most recent ICS prescription within the 30 days prior to the start of an interval, recent users were those with their most recent ICS prescription between 31 and 60 days prior, and past users were issued their most recent ICS prescription more than 60 days ago. Never users were those with no prior or current exposure to ICS at the start of an interval. At index date all patients were classified as never or current users, and could then move between exposure groups over time—meaning that, eg, past and recent users could become current users again when a new ICS prescription was issued. Current ICS users were stratified by serum count of eosinophils at baseline and classified as low (<2.0%), moderate (≥2.0 to 3.9%), high (4.0% to 5.9%), or very high (≥6.0%). We also stratified by absolute blood eosinophil count (<0.34 × 109 cells/L versus ≥0.34 × 109 cells/L). We chose this analysis approach because of its reliability in adequately assess exposure status and covariates essential to the validity of our results.21
2.4. Covariates
Potential confounders were assessed time‐dependently; with the exception of gender, smoking status, alcohol use, and body mass index, which were determined at baseline. The following confounders were considered as potential confounders, and identified at the start of each 90‐day interval: a history of congestive heart failure, anaemia, chronic liver disease, allergic rhinitis, all malignancies except nonmelanoma skin cancer, ischaemic heart disease, stroke, rheumatoid arthritis, diabetes mellitus, hypertension, pulmonary fibrosis, osteoporosis, and anxiety. Additionally, the use of antihistamines, oxygen, proton‐pump inhibitors, antipsychotics, antidepressants, or statins was identified in the past 6 months.22, 23, 24, 25, 26 We adjusted for proxy indicators of the severity of obstructive airway disease, as previously defined as dispensed prescriptions of short and long‐acting beta‐agonists, short and long acting muscarinic antagonists, xanthine derivatives, and oral corticosteroids.27, 28
2.5. Statistical analysis
We evaluated the risk of study outcomes stratified by ICS use, gender, and age using Cox regression analysis (SAS 9.4 PHREG procedure). Current ICS use was further stratified by blood eosinophil counts. The difference between each eosinophil count strata was tested using Wald's test for relative and absolute blood eosinophil counts. To avoid immortal time bias, all patients were classified as never users until the first ICS prescription after the index date, and all exposure categories of ICS use were incorporated into the statistical model time‐dependently. We ran separate models for age, gender, and absolute and relative counts avoiding collinearity between absolute and relative eosinophil counts. Potential confounders were included in the final models if they independently changed the β‐coefficient for current ICS exposure by at least 5%, or when consensus existed within the team of researchers.
3. RESULTS
We identified 213 561 patients with COPD, of whom 32 693 met the inclusion criteria (Figure 1). Table 1 shows the baseline characteristics of COPD patients, which were predominantly elderly males. At baseline, the mean blood eosinophil count was 3.1% (±2.7%), and 64.1% had a relative blood eosinophil count ≥2.0%. More than half of all patients were either overweight (31.9%) or obese (25.8%), and a considerable proportion of COPD patients suffered from ischemic heart disease (15.4%).
Figure 1.
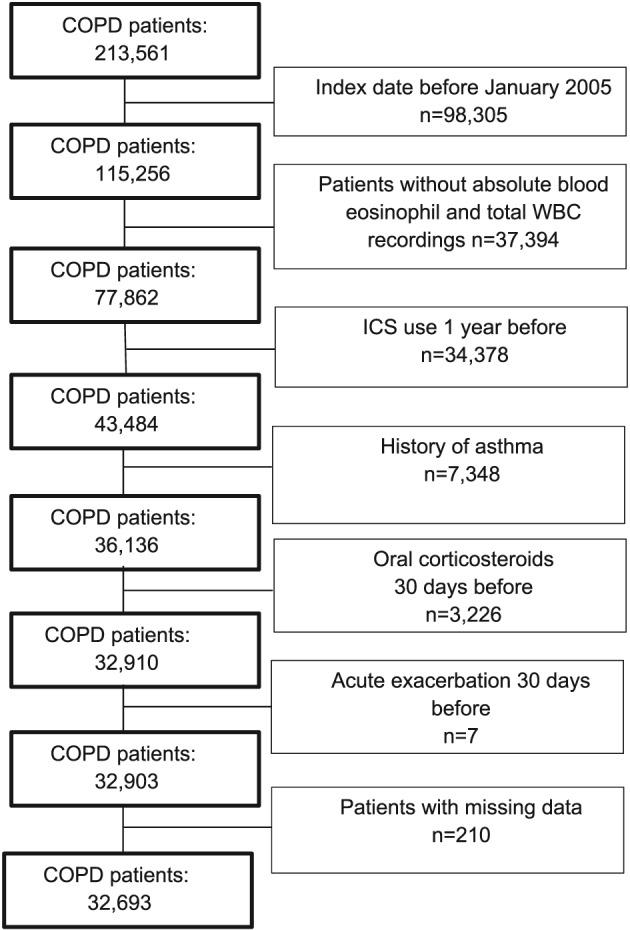
Flow chart showing the selection of eligible patients
Table 1.
Baseline characteristics
| COPD Patients (n = 32 693) | ||
|---|---|---|
| n | (%) | |
| Females | 14 551 | 44.5 |
| Mean age (years, SD) | 68.4 | 10.8 |
| Mean follow‐up time (years, SD) | 3.1 | 2.2 |
| Age category (years) | ||
| 40‐59 | 6902 | 21.1 |
| 60‐79 | 20 350 | 62.3 |
| 80+ | 5441 | 16.76 |
| BMI (kg/m2)* in the past 6 months | ||
| Underweight (BMI < 18.5 kg/m2) | 1704 | 5.2 |
| Normal weight (BMI 18.5‐24.9 kg/m2) | 11 286 | 34.5 |
| Overweight (BMI 25.0‐29.9 kg/m2) | 10 412 | 31.9 |
| Obese (BMI ≥ 30.0 kg/m2) | 8437 | 25.8 |
| Missing | 854 | 2.6 |
| Mean relative eosinophil count (%, SD) | 3.1 | 2.7 |
| Relative blood eosinophil count | ||
| Low (<2.0%) | 11 756 | 36.0 |
| Moderate (≥2.0%‐3.9%) | 13 059 | 39.9 |
| High (4.0%‐5.9%) | 5110 | 15.6 |
| Very high (≥6.0%) | 2768 | 8.5 |
| Absolute blood eosinophil count | ||
| <0.34 × 109 cells/L | 26 828 | 82.1 |
| ≥0.34 × 109 cells/L | 5865 | 17.9 |
| Smoking status at index date | ||
| Never | 3285 | 10.1 |
| Current | 14 522 | 44.4 |
| Former | 14 849 | 45.4 |
| Missing | 37 | 0.1 |
| Drug use (in the past 6 months) | ||
| SABAs | 15 307 | 46.8 |
| LABAs | 2724 | 8.3 |
| SAMAs | 2451 | 7.5 |
| LAMAs | 4432 | 13.6 |
| Xanthine derivatives | 97 | 0.3 |
| Antipsychotics | 352 | 1.1 |
| History of comorbidities | ||
| Diabetes mellitus | 4027 | 12.3 |
| Anxiety | 4865 | 14.8 |
| Osteoporosis | 1872 | 5.7 |
| Malignancies excluding nonmelanoma skin cancer | 4867 | 14.9 |
| Chronic liver disease | 110 | 0.3 |
| Ischaemic heart disease | 5027 | 15.4 |
Abbreviations: SD, standard deviation; COPD, chronic obstructive pulmonary disease; BMI, body mass index; SABAs, short‐acting beta‐2 agonists; LABAs, long‐acting beta‐2 agonists; SAMAs, short‐acting muscarinic antagonists; LAMAs, long‐acting muscarinic antagonists; ICS, inhaled corticosteroids.
3.1. Acute COPD exacerbations
Current use of ICS was not associated with a significant reduction in risk of acute exacerbations (adjusted hazard ratio [adj.HR] 1.15; 95% confidence interval [CI] 1.09‐1.21) (Table 2). The risk of acute COPD exacerbations was not significantly lower among current ICS users with relative eosinophil counts ≥2%, as compared to current users with relative counts <2%. This was similar when comparing absolute blood eosinophil counts ≥0.34 × 109 cells/L with counts <0.34 × 109 cells/L. There was no difference in risk of acute exacerbation between all relative and absolute eosinophil categories. Figure 2A shows life table analysis. When stratified by age and gender, no significant association with the risk of acute exacerbations was observed, using males or those aged 40 to 59 as the reference (Table 2).
Table 2.
Risk of acute COPD exacerbations with current use of ICS, stratified by eosinophil counts, gender, and age
| Exacerbations (n = 14 523)d | IR (/1000 PY) | Age and Gender‐Adjusted HR (95% CI) | Adjusted HR (95% CI)a | |
|---|---|---|---|---|
| ICS use | ||||
| Never | 8744 | 128.8 | Reference | Reference |
| Past | 2538 | 137.7 | 0.99 (0.99‐1.04) | 0.94 (0.90‐0.99) |
| Recent | 882 | 188.7 | 1.38 (1.28‐1.47) | 1.11 (1.03‐1.19) |
| Current | 2359 | 208.6 | 1.49 (1.41‐1.55) | 1.15 (1.09‐1.21) |
| By relative blood eosinophil count | ||||
| Low (<2.0%) | 838 | 207.5 | Reference | Reference |
| Moderate (≥2.0%‐3.9%) | 943 | 211.4 | 1.01 (0.92‐1.11) | 1.03 (0.93‐1.13) |
| High (4.0%‐5.9%) | 373 | 214.6 | 1.03 (0.91‐1.16) | 1.04 (0.92‐1.17) |
| Very high (≥6.0%) | 205 | 191.4 | 0.93 (0.79‐1.08) | 0.95 (0.81‐1.11) |
| By absolute blood eosinophil count | ||||
| <0.34 × 109 cells/L | 1907 | 208.5 | Reference | Reference |
| ≥0.34 × 109 cells/L | 452 | 209.0 | 1.00 (0.90‐1.11) | 0.99 (0.89‐1.09) |
| By gender | ||||
| Males | 1348 | 211.3 | Reference | Referenceb |
| Female | 1011 | 205.2 | 0.95 (0.88‐1.04) | 0.97 (0.89‐1.05) |
| By age categories | ||||
| 40‐59 years | 561 | 214.2 | Reference | Referencec |
| 60‐79 years | 1539 | 212.5 | 1.01 (0.92‐1.11) | 1.03 (0.93‐1.13) |
| 80+ years | 259 | 179.3 | 0.89 (0.77‐1.03) | 0.99 (0.85‐1.14) |
We evaluated the risk of study outcomes stratified by ICS use, gender, and age using Cox regression analysis.
Abbreviations: COPD, chronic obstructive pulmonary disease; ICS, Inhaled corticosteroids; IR, incidence rate; HR, hazard ratio; CI, confidence interval; PY, person‐years.
Adjusted for age, gender, smoking status, alcohol use, BMI, a history of heart failure, diabetes mellitus, chronic liver disease, pulmonary fibrosis, ischaemic heart disease, osteoporosis, anxiety, hypertension, anaemia, and the use of antipsychotics, statins, oxygen, proton‐pump inhibitors, antidepressants, or antipsychotics, long‐acting beta‐2 agonist, short‐acting beta‐2 agonist, short‐acting muscarinic agent, long‐acting muscarinic antagonist, xanthine derivatives, and oral corticosteroid use 6 months prior to the start of an interval.
Adjusted for all confounders under (a) except gender.
Adjusted for all confounders under (a) except age.
14 523 exacerbations were recorded among 32 693 patients with COPD.
Figure 2.
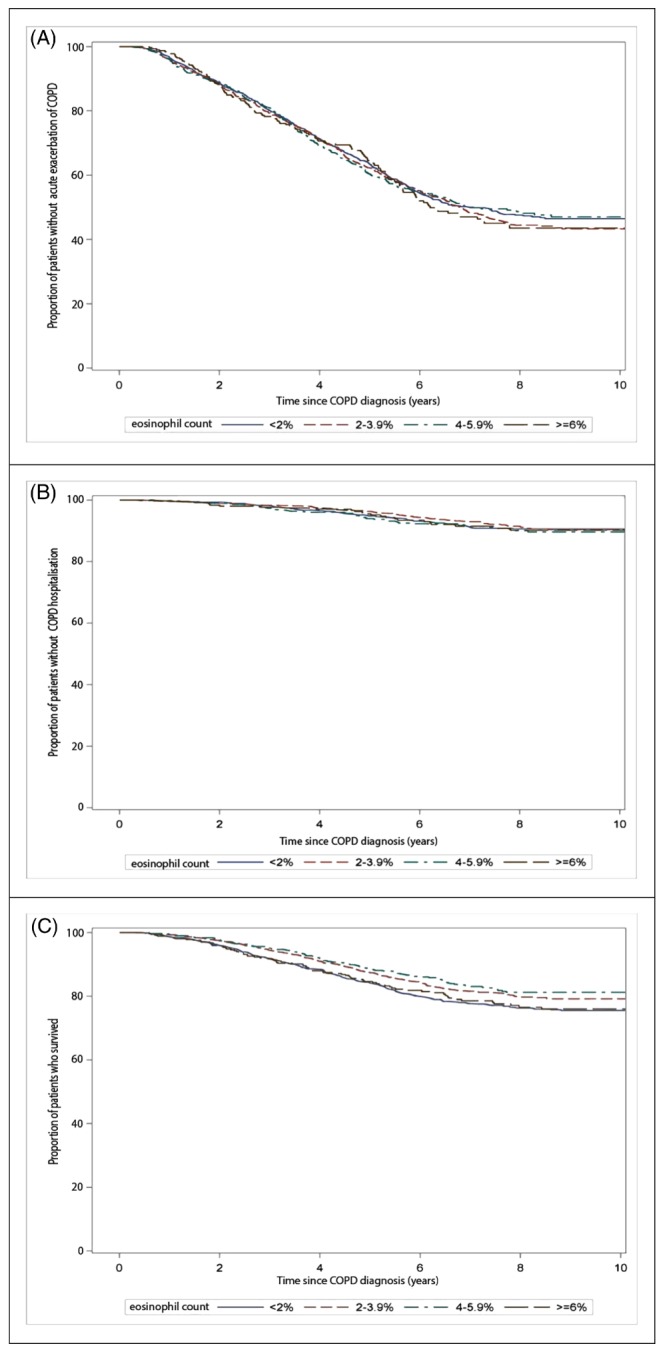
Kaplan‐Meier curve showing (A) the proportion of patients without exacerbations with current ICS users stratified by blood eosinophil counts, (B) the proportion of patients without COPD hospitalisations/accident and emergency visits among current ICS users stratified by blood eosinophil counts, and (C) the proportion of patients who survived among current ICS users stratified by relative blood eosinophil counts [Colour figure can be viewed at http://wileyonlinelibrary.com]
3.2. COPD‐related hospitalisations/accident and emergency visits
Current use of ICS was associated with a 1.2‐fold increased risk of COPD‐related hospitalisations/AE visits (adj.HR 1.17; 95% CI: 1.04‐1.32). Similar to acute COPD exacerbations, stratification by absolute or relative eosinophil count did not result in a significant difference in risk of COPD‐related hospitalisations/AE visits). We also found no difference in risk when we tested the difference in risk between absolute and relative blood eosinophil counts. Figure 2B shows the KM life table analysis curve. We did not identify an increased risk of hospitalisations with current female ICS users compared to current male ICS users (adj.HR 0.98; 95% CI 0.77‐1.11, Table 3), and there were no differences in the risk of hospitalisations with increasing age groups (Table 3).
Table 3.
Risk of COPD hospitalisations/AE visits with current use of ICS by eosinophil counts, gender, and age
| COPD Hospitalisations/AE Visits (n = 1987)d | IR (/1000 PY) | Age and Gender‐Adjusted HR (95% CI) | Adjusted HR (95% CI)a | |
|---|---|---|---|---|
| By ICS use | ||||
| Never | 973 | 11.9 | Reference | Reference |
| Past | 366 | 14.1 | 1.05 (0.93‐1.19) | 0.98 (0.87‐1.11) |
| Recent | 166 | 21.6 | 1.55 (1.31‐1.82) | 1.11 (0.93‐1.31) |
| Current | 482 | 24.5 | 1.71 (1.53‐1.91) | 1.17 (1.04‐1.32) |
| By relative blood eosinophil count | ||||
| Low (<2.0%) | 176 | 25.7 | Reference | Reference |
| Moderate (≥2.0%‐3.9%) | 183 | 23.5 | 0.91 (0.73‐1.11) | 0.93 (0.75‐1.14) |
| High (4.0%‐5.9%) | 78 | 25.1 | 0.96 (0.73‐1.26) | 0.99 (0.76‐1.29) |
| Very high (≥6.0%) | 45 | 23.9 | 0.93 (0.67‐1.29) | 0.97 (0.69‐1.35) |
| By absolute blood eosinophil count | ||||
| <0.34 × 109 cells/L | 395 | 25.0 | Reference | Reference |
| ≥0.34 × 109 cells/L | 87 | 22.5 | 0.89 (0.71‐1.13) | 0.89 (0.70‐1.12) |
| By gender | ||||
| Males | 258 | 23.5 | Reference | Referenceb |
| Females | 224 | 25.9 | 1.09 (0.91‐1.31) | 0.98 (0.77‐1.11) |
| By age categories | ||||
| 40‐59 years | 108 | 23.4 | Reference | Referencec |
| 60‐79 years | 326 | 25.4 | 1.11 (1.08‐1.58) | 1.18 (0.94‐1.48) |
| 80+ years | 48 | 21.8 | 1.17 (0.73‐1.44) | 1.29 (0.89‐1.85) |
We evaluated the risk of hospitalisation/AE visits for COPD stratified by ICS use, gender, and age using Cox regression analysis.
Abbreviations: COPD, chronic obstructive pulmonary disease; ICS, inhaled corticosteroids; IR, incidence rate; HR, hazard ratio; CI, confidence interval; PY, person‐years; AE, accident and emergency.
Adjusted for age, gender, smoking status, alcohol use, BMI, a history of heart failure, diabetes mellitus, chronic liver disease, pulmonary fibrosis, ischaemic heart disease, osteoporosis, anxiety, hypertension, anaemia, and the use of antipsychotics, statins, oxygen, proton‐pump inhibitors, antidepressants, antipsychotics, long‐acting beta‐2 agonist, short‐acting beta‐2 agonist, short‐acting muscarinic agent, long‐acting muscarinic antagonist, xanthine derivatives, and oral corticosteroid use 6 months prior to the start of an interval.
Adjusted for all confounders under (a) except gender.
Adjusted for all confounders under (a) except age.
1987 COPD hospitalisations/AE visits occurred in 32 693 patients with COPD.
3.3. All‐cause mortality
Current use of ICS was associated with a statistically significantly increased risk of all‐cause mortality (adj.HR 1.20; 95% CI 1.12‐1.29) compared to never ICS users (Table 4). In contrast to the previous outcomes, when current ICS users were stratified by relative blood eosinophil counts, we observed a decreased risk of all‐cause mortality among patients with increased eosinophil counts as compared to those with low (<2%) eosinophil counts. We found a difference in risk of all‐cause mortality between patients with low and moderate relative eosinophil counts (P = .001). No difference was found with absolute blood eosinophil counts. The KM curve shows differences in the proportion of patients with COPD who survived, stratified by relative blood eosinophil counts (Figure 2C). Stratification to by absolute blood eosinophil count did not show a significant association with all‐cause mortality adj HR 0.92; 95% CI (0.79‐1.06) for patients with absolute eosinophil counts ≥0.34 × 109 cells/L versus counts <0.34 × 109 cells/L. Female users of ICS had a 21% decreased risk of all‐cause mortality (adj.HR 0.79; 95% CI 0.69‐0.88) compared to males, and we found an increased risk of all‐cause mortality with age (Table 4).
Table 4.
Risk of all‐cause mortality with current use of ICS by baseline eosinophil counts, gender, and age
| All‐Cause Mortality (n = 6181)d | IR (/1000 PY) | Age and Gender‐Adjusted HR (95% CI) | Adjusted HR (95% CI)a | |
|---|---|---|---|---|
| By ICS use | ||||
| Never | 3160 | 38.3 | Reference | Reference |
| Past | 1343 | 49.8 | 1.32 (1.23‐1.39) | 1.25 (1.17‐1.33) |
| Recent | 484 | 59.7 | 1.67 (1.52‐1.84) | 1.36 (1.23‐1.49) |
| Current | 1194 | 57.4 | 1.56 (1.46‐1.67) | 1.20 (1.12‐1.29) |
| By relative blood eosinophil count | ||||
| Low (<2.0%) | 477 | 65.5 | Reference | Reference |
| Moderate (≥2.0%‐3.9%) | 435 | 52.9 | 0.81 (0.71‐0.92) | 0.88 (0.77‐0.99) |
| High (4.0%‐5.9%) | 168 | 51.2 | 0.73 (0.61‐0.87) | 0.79 (0.66‐0.94) |
| Very high (≥6.0%) | 114 | 56.7 | 0.71 (0.58‐0.87) | 0.76 (0.62‐0.93) |
| By absolute blood eosinophil count | ||||
| <0.34 × 109 cells/L | 959 | 57.4 | Reference | Reference |
| ≥0.34 × 109 cells/L | 235 | 57.1 | 0.90 (0.78‐1.04) | 0.92 (0.79‐1.06) |
| By gender | ||||
| Males | 712 | 60.9 | Reference | Referenceb |
| Females | 482 | 52.8 | 0.81 (0.72‐0.91) | 0.79 (0.69‐0.86) |
| By age categories | ||||
| 40‐59 years | 88 | 17.9 | Reference | Referencec |
| 60‐79 years | 729 | 53.6 | 2.97 (2.38‐3.70) | 2.38 (1.89‐3.00) |
| 80+ years | 377 | 164.5 | 9.31 (7.31‐11.74) | 5.07 (3.96‐6.49) |
Abbreviations: COPD, chronic obstructive pulmonary disease; ICS, Inhaled corticosteroids; IR, incidence rate; HR, hazard ratio; CI, confidence interval; PY, person‐years.
Adjusted for age, gender, smoking status, alcohol use, BMI, heart failure, atrial fibrillation, diabetes, anxiety, chronic liver disease, all cancers except nonmelanoma skin cancer, stroke, antipsychotics, long‐acting beta‐2 agonist, short‐acting beta‐2 agonist, short‐acting muscarinic agent, long‐acting muscarinic antagonist, xanthine derivatives and oral corticosteroid use 6 months prior to the start of an interval.
Adjusted for all confounders under (a) except gender.
Adjusted for all confounders under (a) except age.
6181 patients died after follow‐up of 32 693 patients with COPD.
4. DISCUSSION
We found no reduced risk of COPD‐related acute exacerbations or hospitalisations/AE visits among patients with blood eosinophilia among COPD patients using ICS. However, all‐cause mortality was reduced among current ICS users with blood eosinophilia compared to current ICS users with low relative blood eosinophil count. However, this effect could not be detected with absolute eosinophil counts.
4.1. ICS use and risk of exacerbations or COPD‐related hospitalisations/AE visits
As compared to never users, current users of ICS had a significantly increased risk of exacerbations or COPD‐related hospitalisations/AE visits, although the risk between recent and current users was comparable. Similarly, Melo et al found an increased risk of first exacerbation among COPD patients currently exposed to ICS.27 Since ICS are prescribed in order to prevent these events, confounding by disease severity may explain this, in particular because the association is also present among ICS users who have recently stopped taking the drugs (recent users). Furthermore, recent and current users of ICS in our study might represent a different phenotype of COPD patients, maybe the so‐called frequent exacerbators.29 Although, a multicentre 4‐year double blind study in COPD patients also reported an increased risk of first COPD exacerbation in patients exposed to any ICS compared to patients not exposed to ICS.30
4.2. Impact of blood eosinophil counts on exacerbations and COPD‐related hospitalisations/AE visits
Our findings are partially in line with previous research. While traditionally considered a characteristic of asthma, several studies have reported eosinophilic inflammation in patients with COPD.31, 32 A study by Pascoe and colleagues6 concluded that COPD patients treated with ICS with higher blood eosinophil counts had reduced exacerbations compared to those with low blood eosinophil counts. While our observational study results are in contrast to this, it is important to note that we excluded patients with a history of asthma while Pascoe and coworkers excluded only active asthma patients and included patients with a history of one or more exacerbations in the previous year.33 The FLAME trial evaluated the time to the first exacerbation in COPD patients exposed to indacaterol/gycopyrronium compared to salmeterol/fluticasone and found no significant risk reduction in exacerbation among patients with blood eosinophil levels of 150 up to 300 cells/μL, versus 300 cells/μL or more.8 When comparing our results to both previous studies, an important distinction was the comparator group, which may explain differences in results. Our findings were consistent with post hoc analyses from the ISOLDE trial.34 It showed no difference in the time‐to‐first exacerbation in patients with high or low eosinophil counts. It is important to note that our study also employed the time‐to‐first exacerbation approach. Similar to our study, 3 randomised clinical trials that studied blood eosinophils and ICS/long acting bronchodilator reported no statistically significant effects for time to first moderate/severe exacerbation in patients with relative eosinophils counts ≥2% versus counts <2%.35
4.3. Impact of blood eosinophil counts on all‐cause mortality
Our findings are in agreement with a study which evaluated the 3‐year survival using relative eosinophil count cutoff of ≥2%, which showed that COPD patients with higher blood eosinophil counts had a significantly decreased 3‐year mortality compared to COPD patients with blood eosinophil counts <2% exposed to ICS.36 However a randomised, double‐blind, placebo‐controlled study by Barnes and coworkers34 using subjects from the ISOLDE study found no difference in the risk of deaths in COPD patients exposed to ICS with different eosinophil counts. Similarly, a clinical study involving 303 patients with COPD found that elevated blood eosinophil counts (≥200, 300, 400 cells/μL) were not associated with mortality when compared with patients with decreased eosinophil counts.37
In recent years, various researchers have questioned the use of the 2% cutoff adopted by various studies to determine effective response to ICS in patients with COPD; others have argued in favour of the use of absolute blood eosinophil counts in making clinically beneficial decisions for patients with COPD.5, 38, 39 As absolute counts are less affected by total white blood cell count,8 Vedel‐Krogh et al suggested that 0.34 × 109 cells/L cutoff was appropriate in detecting COPD exacerbations because this cutoff was associated with increased risk of moderate and severe exacerbations in patients with COPD in population‐based settings.5 The exact mechanism underlying the presumed changes in COPD‐related exacerbation in patients with varying baseline blood eosinophil counts remains unclear.6, 7 However, it might be due to the non‐T helper 2 eosinophilic inflammation in COPD, induced by the epithelial innate lymphoid cell type 2 pathways. Innate lymphoid cell type 2 has been recognised in relation to the pathogenesis in severe nonallergic eosinophilic asthma.40
4.4. Strengths and limitations
A major strength to this study was the use of data from one of the world's largest primary care databases, thereby providing a very large population‐based cohort of COPD patients with eosinophil measurements. Second, we used a validated definition for an acute exacerbation of COPD, using read codes which were reported to show a 96% PPV of identifying an acute exacerbation within the CPRD.18 Nevertheless, we may have missed a considerable amount of exacerbations that may be miscoded, eg, as respiratory tract infections or pneumonia. Third, classification of exposure to ICS and covariates time‐dependently during follow‐up allowed us to conduct an “on treatment analysis,” which results in less nondifferential misclassification of exposure than in an “intention to treat analysis” which ignores ICS exposure during follow‐up. Fourth, we eliminated patients with a history of asthma to avoid patients with reversible airflow limitation from our study. Lastly, we had information on important confounding factors such as smoking status, BMI, and other comorbidities and drug use.
Despite numerous strengths, this study also had limitations. In addition to those already mentioned, there is a potential for residual confounding as we lacked information on the disease severity and previous exacerbations. Although the positive predictive value of identifying patients with COPD in the CPRD is 90%,11 lung function data were unavailable. While we excluded asthma patients, it was impossible to rule out the inclusion of patients with reversible airflow limitation.41 We lacked information on cause‐specific mortality; as such, we could not perform detailed analysis on the cause of death. Eosinophil counts are not been routinely collected as part of diagnosis of COPD patients, and thus, the patient population in this study may not be representative of all COPD patients. Moreover, this could have masked a true association between eosinophil counts and various outcomes. Inhaled corticosteroid inhalers are known to last for 25 to 100 days depending on the active ingredient and the dose prescribed by the GP42; this might have led to a nondifferential misclassification of ICS exposure, masking the true effect (bias towards the null) leading to insignificant findings. Without this potential distortion, the risk of acute exacerbations or hospitalisation/AE visits might have been lower for patients with elevated blood eosinophil counts. However, we detected a significant risk of all‐cause mortality irrespective of blood eosinophil counts; we do not believe that our results are influenced by the potential of a nondifferential misclassification.
In conclusion, among COPD patients, we did not find a reduced risk of COPD‐related acute exacerbations or hospitalisations/AE visits among patients with blood eosinophilia in COPD patients using ICS. However, all‐cause mortality was reduced among current ICS users with blood eosinophilia compared to current ICS users with low relative blood eosinophil count. However, this effect could not be detected with the absolute eosinophil counts. There is an increasing body of evidence linking blood eosinophil counts to clinically relevant outcomes in patients with COPD. As such, these findings are potentially important and require further evaluation in prospective studies.
ETHICS STATEMENT
The authors state that no ethical approval was needed.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
AUTHOR CONTRIBUTIONS
Conception or design: All authors equally contributed.
Analysis of data: OO and AB
Interpretation of data: All authors equally contributed.
Drafting the work OO, FV, FF, and AB
Revising the work critically for important intellectual content: All authors equally contributed.
Final approval of the version to be published: All authors equally contributed.
Oshagbemi OA, Franssen FME, Braeken DCW, et al. Blood eosinophilia, use of inhaled corticosteroids, and risk of COPD exacerbations and mortality. Pharmacoepidemiol Drug Saf. 2018;27:1191–1199. 10.1002/pds.4655
REFERENCES
- 1. Vogelmeier CF, Criner GJ, Martinez FJ, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease 2017 report. GOLD executive summary. Am J Respir Crit Care Med [Internet]. 2017;195(5):557‐582. Available from: doi: 10.1164/rccm.201701-0218PP [DOI] [PubMed] [Google Scholar]
- 2. Calverley PMA, Anderson JA, Celli B, et al. Salmeterol and fluticasone propionate and survival in chronic obstructive pulmonary disease. N Engl J Med [internet]. 2007;356(8):775‐789. Available from: http://www.ncbi.nlm.nih.gov/26641631/17314337 [DOI] [PubMed] [Google Scholar]
- 3. Wedzicha JA, Seemungal TA. COPD exacerbations: defining their cause and prevention. Lancet. 2007;370(9589):786‐796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kew KM, Seniukovich A. Inhaled steroids and risk of pneumonia for chronic obstructive pulmonary disease. Cochrane Database Syst Rev [Internet]. 2014;3:CD010115 Available from: 10.1002/14651858.CD010115.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Vedel‐Krogh S, Nielsen SF, Lange P, Vestbo J, Nordestgaard BG. Blood eosinophils and exacerbations in COPD: the Copenhagen General Population Study. Am J Respir Crit Care Med [Internet]. 2015;rccm.201509‐1869OC. Available from: http://www.atsjournals.org/doi/10.1164/rccm.201509-1869OCn http://www.ncbi.nlm.nih.gov/26585525/26641631 [DOI] [PubMed] [Google Scholar]
- 6. Pascoe S, Locantore N, Dransfield MT, Barnes NC, Pavord I. Blood eosinophil counts as markers of response to inhaled corticosteroids in COPD? Authors' reply. Lancet Respir Med. 2015;3(8):e27. [DOI] [PubMed] [Google Scholar]
- 7. Watz H, Tetzlaff K, Wouters EFM, et al. Blood eosinophil count and exacerbations in severe chronic obstructive pulmonary disease after withdrawal of inhaled corticosteroids: a post‐hoc analysis of the WISDOM trial. Lancet Respir Med. 2016;4(5):390‐398. [DOI] [PubMed] [Google Scholar]
- 8. Roche N, Chapman KR, Vogelmeier CF, et al. Blood eosinophils and response to maintenance COPD treatment: data from the FLAME trial. Am J Respir Crit Care Med [Internet]. 2017;Available from: doi: 10.1164/rccm.201701-0193OC;195(9):1189‐1197. [DOI] [PubMed] [Google Scholar]
- 9. Calverley PMA, Tetzlaff K, Vogelmeier C, et al. Eosinophilia, frequent exacerbations, and steroid response in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2017;(ja);196(9):1219‐1221. [DOI] [PubMed] [Google Scholar]
- 10. Price D, Wilson AM, Chisholm A, et al. Predicting frequent asthma exacerbations using blood eosinophil count and other patient data routinely available in clinical practice. J Asthma Allergy [Internet]. 2016;9:1‐12. Available from: https://www.dovepress.com/predicting-frequent-asthma-exacerbations-using-blood-eosinophil-count--peer-reviewed-article-JAA [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Quint JK, Müllerova H, DiSantostefano RL, et al. Validation of chronic obstructive pulmonary disease recording in the Clinical Practice Research Datalink (CPRD‐GOLD). BMJ Open [Internet]. 2014;4(7). Available from: http://bmjopen.bmj.com/content/4/7/e005540.abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Herrett E, Gallagher AM, Bhaskaran K, et al. Data resource profile: Clinical Practice Research Datalink (CPRD). Int J Epidemiol. 2015;44(3):827‐836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Taggar JS, Coleman T, Lewis S, Szatkowski L. The impact of the Quality and Outcomes Framework (QOF) on the recording of smoking targets in primary care medical records: cross‐sectional analyses from The Health Improvement Network (THIN) database. BMC Public Health [Internet]. 2012;12(1):1‐11. Available from: doi: 10.1186/1471-2458-12-329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Rothnie K, Mullerova H, Hurst J, et al. Validation of the recording of acute exacerbations of COPD in the clinical practice research datalink: Phase 1 results. Eur Respir J [Internet]. 2015;46(suppl 59). Available from: http://erj.ersjournals.com/content/46/suppl_59/PA4067 [Google Scholar]
- 15. Barakat MF, McDonald HI, Collier TJ, Smeeth L, Nitsch D, Quint JK. Acute kidney injury in stable COPD and at exacerbation. Int J Chron Obstruct Pulmon Dis [Internet]. 2015;10(1):2067‐2077. Available from: http://www.scopus.com/inward/record.url?eid=2-s2.0-84943158973&partnerID=tZOtx3y1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Mullerova H, Shukla A, Hawkins A, Quint J. Risk factors for acute exacerbations of COPD in a primary care population: a retrospective observational cohort study. BMJ Open. 2014;4(12):e006171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wurst KE, Shukla A, Muellerova H, Davis KJ. Respiratory pharmacotherapy use in patients newly diagnosed with chronic obstructive pulmonary disease in a primary care setting in the UK: a retrospective cohort study. COPD [Internet]. 2014;11(5):521‐530. Available from: http://search.proquest.com/docview/1561971611?accountid=14732%5Cnhttp://bd9jx6as9l.search.serialssolutions.com/?ctx_ver=Z39.88-2004&ctx_enc=info:ofi/enc:UTF-8&rfr_id=info:sid/ProQ:medlineshell&rft_val_fmt=info:ofi/fmt:kev:mtx:journal&rft.genre=article&rft [DOI] [PubMed] [Google Scholar]
- 18. Rothnie KJ, Müllerová H, Hurst JR, et al. Validation of the recording of acute exacerbations of COPD in UK primary care electronic healthcare records. PLoS One. 2016;11(3):e0151357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Oshagbemi OA, Burden AM, Braeken DCW, et al. Stability of blood eosinophils in patients with chronic obstructive pulmonary disease and in control subjects, and the impact of sex, age, smoking, and baseline counts. Am J Respir Crit Care Med. 2017;195(10):1402‐1404. [DOI] [PubMed] [Google Scholar]
- 20. Landis SH, Suruki R, Hilton E, Compton C, Galwey NW. Stability of blood eosinophil count in patients with COPD in the UK clinical practice research Datalink. COPD J Chronic Obstr Pulm Dis. 2017;14(4):382‐388. [DOI] [PubMed] [Google Scholar]
- 21. European Medicines Agency . The European Network of Centres for Pharmacoepidemiology and Pharmacovigilance (ENCePP) Guide on Methodological Standards in Pharmacoepidemiology (Revision 4). 2015;44(June):1–77.
- 22. Cavaillès A, Brinchault‐Rabin G, Dixmier A, et al. Comorbidities of COPD. Eur Respir Rev. 2013;22(130):454‐475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Chatila WM, Thomashow BM, Minai OA, Criner GJ, Make BJ. Comorbidities in chronic obstructive pulmonary disease. Proc Am Thorac Soc. 2008;5(4):549‐555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Dal Negro RW, Bonadiman L, Turco P. Prevalence of different comorbidities in COPD patients by gender and GOLD stage. Multidiscip Respir Med [Internet]. 2015;10(1):24 Available from: http://www.26585525central.nih.gov/articlerender.fcgi?artid=4525744%26;tool=pmcentrez%26;rendertype=abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Franssen FME, Rochester CL. Comorbidities in patients with COPD and pulmonary rehabilitation: do they matter? Eur Respir Rev. 2014;23(131):131‐141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Divo M, Cote C, De Torres JP, et al. Comorbidities and risk of mortality in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2012;186(2):155‐161. [DOI] [PubMed] [Google Scholar]
- 27. de Melo MN, Ernst P, Suissa S. Inhaled corticosteroids and the risk of a first exacerbation in COPD patients. Eur Respir J [Internet]. 2004;23(5):692‐697. Available from: http://www.scopus.com/inward/record.url?eid=2-s2.0-2442613152&partnerID=40&md5=6f084b2640a022de897acb2bc1456a14 [DOI] [PubMed] [Google Scholar]
- 28. Ernst P, Gonzalez AV, Brassard P, Suissa S. Inhaled corticosteroid use in chronic obstructive pulmonary disease and the risk of hospitalization for pneumonia. Am J Respir Crit Care Med. 2007;176(2):162‐166. [DOI] [PubMed] [Google Scholar]
- 29. Hurst JR, Vestbo J, Anzueto A, et al. Susceptibility to exacerbation in chronic obstructive pulmonary disease. N Engl J Med. 2010;363(12):1128‐1138. [DOI] [PubMed] [Google Scholar]
- 30. Morjaria JB, Rigby A, Morice AH. Inhaled corticosteroid use and the risk of pneumonia and COPD exacerbations in the UPLIFT study. Lung. 2017;195(3):281‐288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bafadhel M, McKenna S, Terry S, et al. Acute exacerbations of chronic obstructive pulmonary disease: identification of biologic clusters and their biomarkers. Am J Respir Crit Care Med. 2011;184(6):662‐671. [DOI] [PubMed] [Google Scholar]
- 32. Singh D, Kolsum U, Brightling CE, Locantore N, Agusti A, Tal‐Singer R. Eosinophilic inflammation in COPD: prevalence and clinical characteristics. Eur Respir J [Internet]. 2014. Available from: http://erj.ersjournals.com/content/early/2014/10/16/09031936.00162414 [DOI] [PubMed] [Google Scholar]
- 33. Groenke L, Disse B. Blood eosinophil counts as markers of response to inhaled corticosteroids in COPD? Lancet Respir Med. 2015;3(8):e26. [DOI] [PubMed] [Google Scholar]
- 34. Barnes NC, Sharma R, Lettis S, Calverley PMA. Blood eosinophils as a marker of response to inhaled corticosteroids in COPD. Eur Respir J [Internet]. 2016;47(5):1374‐1382. Available from: http://erj.ersjournals.com/content/early/2016/02/25/13993003.01370-2015.abstract. [DOI] [PubMed] [Google Scholar]
- 35. Pavord ID, Agusti A. Blood eosinophil count: a biomarker of an important treatable trait in patients with airway disease. Eur Respir J [Internet]. 2016;47(5):1299 LP‐1303 Available from: http://erj.ersjournals.com/content/47/5/1299.abstract [DOI] [PubMed] [Google Scholar]
- 36. Zysman M, Burgel PR, Paillasseur JL, et al. Relationship between blood eosinophil count (Eos), clinical characteristics and mortality of patients with COPD. Eur Respir J [Internet]. 2016;48(suppl 60). Available from: http://erj.ersjournals.com/content/48/suppl_60/PA4614.abstract [Google Scholar]
- 37. Marin Trigo JM, Martinez M, Cubero P, Forner M, Saetta M, Cosio MG. Blood eosinophils and outcomes in COPD. Eur Respir J [Internet]. 2016;48(suppl 60). Available from: http://erj.ersjournals.com/content/48/suppl_60/PA4624.abstract [Google Scholar]
- 38. Pavord ID, Lettis S, Locantore N, et al. Blood eosinophils and inhaled corticosteroid/long‐acting β‐2 agonist efficacy in COPD. Thorax [Internet]. 2016;71(2):118‐125. Available from: http://www.ncbi.nlm.nih.gov/pubmed/26585525\n http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=PMC4752631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Iyer AS, Dransfield MT. Serum eosinophils as a COPD biomarker: ready for prime time? Lancet Respir Med [Internet]. 2016. [cited 2016 Nov 11];4(5):341‐343. Available from: http://thelancet.com/article/S2213260016300406/fulltext [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Cheng S‐L, Lin C‐H. Effectiveness using higher inhaled corticosteroid dosage in patients with COPD by different blood eosinophilic counts. Int J Chron Obstruct Pulmon Dis [Internet]. 2016. [cited 2017 Feb 15];11(1):2341‐2348. Available from: https://www.dovepress.com/effectiveness-using-higher-inhaled-corticosteroid-dosage-in-patients-w-peer-reviewed-article-COPD [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Gorska K, Krenke R, Korczynski P, Kosciuch J, Domagala‐Kulawik J, Chazan R. Eosinophilic airway inflammation in chronic obstructive pulmonary disease and asthma. J Physiol Pharmacol [internet]. 2008;59(Suppl 6):261‐270. Available from: http://www.ncbi.nlm.nih.gov/pubmed/19218650 [PubMed] [Google Scholar]
- 42. NHS . Inhaler Repeat Prescriptions [Internet]. 2002 [cited 2017 Jan 4];Available from: http://www.neneccg.nhs.uk/resources/uploads/files/Inhaler-Repeat-Prescriptions_Patient information leaflet.pdf


