Abstract
Aims
The PREVIEW lifestyle intervention study (http://clinicaltrials.gov Identifier: NCT01777893) is, to date, the largest, multinational study concerning prevention of type‐2 diabetes. We hypothesized that the initial, fixed low‐energy diet (LED) would induce different metabolic outcomes in men vs women.
Materials and methods
All participants followed a LED (3.4 MJ/810 kcal/daily) for 8 weeks (Cambridge Weight Plan). Participants were recruited from 8 sites in Europe, Australia and New Zealand. Those eligible for inclusion were overweight (BMI ≥ 25 kg/m2) individuals with pre‐diabetes according to ADA‐criteria. Outcomes of interest included changes in insulin resistance, fat mass (FM), fat‐free mass (FFM) and metabolic syndrome Z‐score.
Results
In total, 2224 individuals (1504 women, 720 men) attended the baseline visit and 2020 (90.8%) completed the follow‐up visit. Following the LED, weight loss was 16% greater in men than in women (11.8% vs 10.3%, respectively) but improvements in insulin resistance were similar. HOMA‐IR decreased by 1.50 ± 0.15 in men and by 1.35 ± 0.15 in women (ns). After adjusting for differences in weight loss, men had larger reductions in metabolic syndrome Z‐score, C‐peptide, FM and heart rate, while women had larger reductions in HDL cholesterol, FFM, hip circumference and pulse pressure. Following the LED, 35% of participants of both genders had reverted to normo‐glycaemia.
Conclusions
An 8‐week LED induced different effects in women than in men. These findings are clinically important and suggest gender‐specific changes after weight loss. It is important to investigate whether the greater decreases in FFM, hip circumference and HDL cholesterol in women after rapid weight loss compromise weight loss maintenance and future cardiovascular health.
Keywords: dietary intervention, obesity, prevention, weight loss, pre‐diabetes
1. INTRODUCTION
Type 2 diabetes mellitus is one of the fastest growing chronic diseases worldwide.1 We are aware of the major risk factors, including overweight or obesity, and know that achieving weight loss “prevents diabetes” in the sense that onset of new cases is delayed. The most recent paper exploring the dose‐response effect of weight loss shows that more than 4.3% weight loss is needed to prevent diabetes, for 3 years, in Japanese men.2 The PREVIEW intervention study (PREVention of diabetes through lifestyle Intervention and population studies in Europe and around the World; http://www.previewstudy.com) is, to date, the largest, multinational study that aims to prevent type 2 diabetes in overweight individuals with pre‐diabetes. Diet and physical activity are utilized, with changes being reinforced by behavior modification techniques.3 The study is an ongoing 3‐year multicentre, 2‐by‐2 factorial, randomized controlled trial, in which eligible adult participants initially followed an 8‐week low‐energy diet (LED). The aim was to induce weight loss of at least 8%, in order to qualify for inclusion in the randomized intervention where the focus is on long‐term weight loss maintenance.4
The majority of individuals who use weight loss programmes, including bariatric surgery, are women.5, 6 Investigating whether outcomes differ between men and women is import in developing gender‐specific treatment programmes, if required.6, 7, 8, 9, 10, 11, 12 Differences in outcome after weight loss have been reported previously, with men commonly losing more body weight and fat than women.13 This difference is mainly explained by the concept of the LED, in which a fixed daily energy intake is provided to both genders, despite men and women having significantly different energy requirements because men characteristically have a greater body mass. Notably, however, men may mobilize more intra‐abdominal fat than women during weight loss, whereas women may lose more subcutaneous fat.14, 15 The greater reduction in intra‐abdominal fat in men is accompanied by a more pronounced improvement in metabolic risk profile. Therefore, greater improvement in terms of risk factors in men is not only related to a greater negative energy balance, but also to a gender‐specific effect.16, 17
Of interest are the differences in glycaemia between overweight men and women. The prevalence of pre‐diabetes has been reported to be significantly higher in men than in women.18 Impaired fasting glucose (IFG), which is indicative of hepatic insulin resistance (IR), is also more common in men, typically 1.5‐3 times higher.19 Conversely, the prevalence of impaired glucose tolerance (IGT), which is indicative of skeletal muscle IR, has been reported to be higher in women than in men in almost all age groups.16
In this study, we aimed to compare the effects of an 8‐week LED‐induced weight loss on metabolic outcomes in a large group of men and women. The study included data from adult participants aged 25‐70 years who were enrolled in the PREVIEW diabetes prevention study.
2. MATERIALS AND METHODS
Adult participants were recruited to the PREVIEW study between August 2013 and March 2015 from eight intervention sites. The study sites were University of Copenhagen (UCPH), Denmark; University of Helsinki (HEL), Finland; University of Nottingham (UNOTT), UK; University of Maastricht (UM), The Netherlands; University of Navarra (UNAV), Spain; Medical University of Sofia (MU), Bulgaria; University of Auckland (UOA), New Zealand and University of Sydney (UNSYD), Australia. Overweight men and women with pre‐diabetes were eligible for inclusion. Participants were recruited via advertisements in newspapers and newsletters, radio and television advertisements/interviews and by contacting primary and occupational health care providers. Interested individuals were pre‐screened for eligibility by using the Finnish Diabetes Risk Score,20, 21 as well as the inclusion and exclusion criteria listed online (Appendix S1). Potentially eligible participants were then invited to an information meeting, where written and oral information was provided. Before continuing to the laboratory screening session, written informed consent was obtained. Criteria for assessing pre‐diabetes were those recommended by the American Diabetes Association (ADA),22 namely, fasting venous plasma glucose concentration of 5.6‐6.9 mmol/L (IFG) and/or venous plasma glucose concentration of 7.8‐11.0 mmol/L at 2 hours (IGT) after oral administration of 75 g glucose during an oral glucose tolerance test (OGTT), with fasting plasma glucose (FPG) less than 7.0 mmol/L. Haemoglobin A1c (HbA1c) was not used to determine eligibility. Other inclusion criteria for adult participants were age of 25‐70 years and body mass index (BMI) ≥ 25 kg/m2. Prior to screening and recruitment, the study protocol was approved by the Ethical Committees of participating countries.
2.1. Interventions
The PREVIEW study comprises 2 intervention phases. Phase 1 is an 8‐week, weight‐loss phase using a formula LED (3.4 MJ/d) intended to induce weight loss of ≥8% to qualify for the next phase. Phase 2 is an ongoing 148‐week randomized lifestyle intervention that focuses on diet, physical activity and behaviour modification for maintenance of weight loss. The LED was implemented by using a range of formula products of the Cambridge Weight Plan (Northants, UK). All intervention sites used the standard assortment from the Cambridge Weight Plan available in the UK to ensure that the nutritional content of sachets was identical. The sachets, which included soups, shakes and porridges, were provided to participants without charge. Participants were instructed to consume 4 sachets (4 × 40 g) per day. Of these, 3 sachets were to be dissolved in milk (3 × 250 mL low fat milk, total 750 mL/d) and 1 sachet in 250 mL of water. The fat content of the milk was ≤0.5 g/100 mL and the energy content ≤170 kJ (40 kcal)/100 mL. Participants with a BMI > 40 kg/m2 were encouraged to dissolve all 4 sachets in milk to increase intake of protein. In total, the LED provided an estimated 3.4 MJ/d (810 kcal/d), ∼85 g of protein, ∼5 g of essential fatty acids and the daily requirement for vitamins and minerals.23, 24
The macronutrient composition of the LED was as follows: 43.7 total energy % from protein, 41.2 total energy % from carbohydrate and 15.1 total energy % from fat. The fiber content of the LED was relatively low at 13.3 g/d. To avoid gastrointestinal side effects, psyllium fiber was recommended to participants, as well as sufficient water to remain hydrated. In addition to the sachets and milk, participants were permitted to consume 375 g of low‐starch vegetables such as tomatoes, cucumber and lettuce per day. Replacement of these additional foods by alternatives was not permitted. During the LED intervention, participants attended group visits at the intervention sites at weeks 2, 4, 6 and 8, where they were guided in the use of the LED by experienced dietitians or counsellors. Further information about the LED is available online (Appendix S2).
2.2. Outcomes
All outcomes were measured before and after the 8‐week intervention at clinical investigation days (CIDs) which participants attended in a fasting state (10‐12 hours). The main outcome of interest in this analysis was change in insulin resistance (IR), calculated by the Homeostasis Model for Assessment (HOMA). The equation used was (FSI * FPG)/22.5, where FSI is fasting serum insulin concentration (mU/L) and FPG is fasting plasma glucose (mmol/L).25 Other outcomes included changes in FPG, HbA1c, fasting insulin, C‐peptide, total cholesterol, high‐density lipoprotein (HDL) cholesterol, low‐density lipoprotein (LDL) cholesterol, triglycerides (TG), C‐reactive protein (CRP) and liver enzymes, alanine aminotransferase (ALT) and aspartate aminotransferase (AST).
Blood samples were drawn from the antecubital vein. Serum and whole blood samples were initially stored at −80 °C at the individual intervention sites, after which they were transported to a laboratory in Finland for central batch analyses (National Institute for Health and Welfare, Helsinki). The laboratory (T077) is accredited by the Finnish Accreditation Service and fulfils requirements of the standard SFS‐EN ISO/IEC 17025:2005. The scope of accreditation covers all analyses with the exception of those for AST and C‐Peptide. Laboratory measurements were performed on Architect ci8200 integrated system (Abbott Laboratories, Abbott Park, Illinois). Other outcomes were change in body weight, measured in a fasting state, with an empty bladder, wearing only underwear (or other light clothing). Two measurements were taken to the nearest 0.1 kg and the mean was calculated. For measurement of height, participants were required to remove shoes, and stand with their heels, buttocks and upper part of the back in contact with a wall‐mounted stadiometer. Height was measured to the nearest 0.5 cm and the mean of 2 measurements was calculated. Waist, hip and thigh circumference were measured to the nearest 0.5 cm with a non‐stretch tape, with the participant standing. Two measurements were recorded and the mean was calculated. Waist circumference (WC) was measured midway between the bottom of the rib cage (last floating rib) and the top of the iliac crest, at the end of expiration. Hip circumference was measured at the widest point between the hips and buttocks, following the same procedure as that for waist measurement. Mid‐thigh circumference was measured on the right side of the body, with the measuring tape placed horizontally around the thigh midway between the midpoint of the inguinal crease and the proximal border of the patella.
Measurements of body composition were performed by different methods at the different intervention sites (Appendix S3). Fertile women were tested for pregnancy before DXA. Outcomes of interest were fat mass (FM), fat‐free mass (FFM), bone mineral content (BMC) and bone mineral density (BMD).
Systolic (SBP) and diastolic (DBP) blood pressure and heart rate were measured using a validated automatic device on the right arm after 5‐10 minutes in a resting position. Measurements were performed 3 times with a 1‐minute rest between each recording and the mean value was recorded. Pulse pressure was calculated using the formula SBP minus DBP. Mean arterial pressure (MAP) was calculated using the formula 0.42 × SBP + 0.58 × DBP.26
Metabolic syndrome (MS) was evaluated using an MS Z‐score, which is a continuous score of the 5 MS variables, as reported previously.27 Gender‐specific Z‐scores were used to account for variations in criterion between men and women. The equations used were: MS Z‐score, ([50‐HDL]/14.1) + ([TG‐150]/81.0) + ([FPG‐100]/11.3) + ([WC‐88]/9.0) + ([MAP‐100]/9.1) for women, and Z‐score, ([40‐HDL]/9.0) + ([TG‐150]/81.0) + ([FPG‐100]/11.3) + ([WC‐102]/7.7) + ([MAP‐100]/9.1) for men.27
At all visits to the intervention sites, participants were asked whether they had experienced adverse events (AEs). Any reported AE was noted on a related form that captured onset, end, intensity, causality, action taken and outcome of the AE.
2.3. Statistical methods
Descriptive characteristics at CID1 and CID2 are summarized as mean ± SD. Differences between men and women were analysed using a linear mixed model, including intervention site as random effect. The estimate of mean difference at baseline is presented as mean ± SEM. All analyses were carried out as complete‐case analyses, that is, data from all participants who attended both the baseline visit (CID1) and the visit at Week 8 (CID2), independent of the amount of weight loss.
Count data, such as number of participants who dropped out or achieved a successful weight loss were analysed for group differences by simple 2 × 2 contingency tables and Chi‐square. For continuous outcomes, the mean gender difference was estimated using ANCOVA‐type linear mixed models, adjusting for fixed effects of baseline and age, and including centres as random effects. As the weight loss intervention provided 3.4 MJ/d (810 kcal/d), we anticipated that men would experience a larger energy deficit than women during the intervention and, therefore, would lose more weight. To adjust for weight loss difference between men and women, the same ANCOVA‐type linear model was applied for all outcome variables, while adjusting for weight loss percentage (%) as well. All statistical analyses and calculations were performed with the statistical program R version 3.3.2 and RStudio version 0.98.1028. A P‐value of <0.05 was considered significant.
3. RESULTS
The flow of participants is shown in Figure 1. A total of 2224 individuals (1504 women, 720 men) participated in the baseline visit (CID1) and began the LED phase. The majority of participants described themselves as Caucasian (1.949, 87.6%) and the remainder were Polynesian (92, 4.1%), Asian (59, 2.7%), Hispanic (44, 2.0%) or Black (38, 1.7%). A total of 42 individuals (1.9%) were classified as “other” and most were of mixed origin. On average, the age of included individuals was 51.6 ± 11.6 years, body weight was 100.1 ± 21.4 kg, BMI was 35.4 ± 6.6 kg/m2, HOMA‐IR was 3.75 ± 2.43 and FPG was 6.2 ± 0.7 mmol/L. Baseline characteristics are shown in Table 1.
Figure 1.
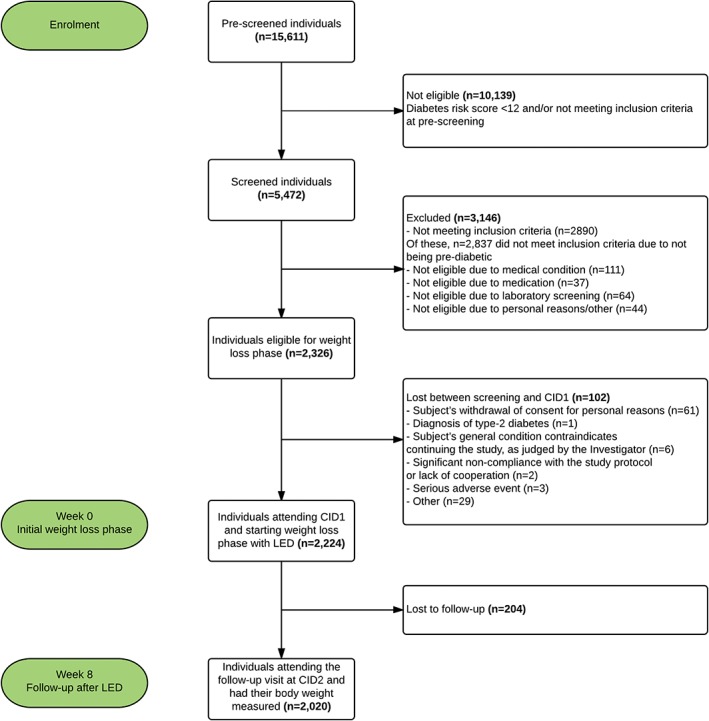
Trial flow chart. Pre‐screening, screening, individuals starting initial weight‐loss phase and follow‐up of study participants
Table 1.
Anthropometrical, metabolic and clinical characteristics of the participants at the baseline visit (CID1) and following 8 weeks (CID2)
| Variable | CID1 ‐ all (N = 2224) | CID1 ‐ women (N = 1504) | CID1 ‐ men (N = 720) | Mean difference between men and womena | CID2 ‐ all (N = 2020) | CID2 ‐ women (N = 1352) | CID2 ‐ men (N = 668) | Mean difference between men and womena |
|---|---|---|---|---|---|---|---|---|
| Age, y | 51.6 ± 11.6 (25.0‐70.0) | 51.0 ± 11.6 | 52.9 ± 11.6 | 1.1 ± 0.5 | ‐ | ‐ | ‐ | ‐ |
| Weight, kg | 100.1 ± 21.4 (58.4‐238.0) | 95.6 ± 19.8 | 109.4 ± 21.6 | 14.9 ± 0.9*** | 88.9 ± 19.2 | 85.2 ± 17.5 | 96.4 ± 20.2 | 12.3 ± 0.8*** |
| Height, cm | 168.0 ± 9.4 (139.0‐198.0) | 163.5 ± 6.7 | 177.4 ± 6.9 | 13.7 ± 2.9*** | ‐ | ‐ | ‐ | ‐ |
| BMI, kg/m2 | 35.4 ± 6.6 (24.7‐77.3) | 35.7 ± 6.7 | 34.7 ± 6.3 | −0.5 ± 0.3 | 31.4 ± 6.0 | 31.8 ± 6.0 | 30.6 ± 5.9 | −0.8 ± 0.3** |
| Primary outcome | ||||||||
| HOMA‐IRb | 3.75 ± 2.43 (0.23‐31.37) | 3.5 ± 2.2 | 4.24 ± 2.82 | 0.73 ± 0.11*** | 2.28 ± 1.61 | 2.26 ± 1.45 | 2.31 ± 1.90 | 0.12 ± 0.08 |
| Secondary outcomes | ||||||||
| Metabolic syndrome Z‐scorec | 2.6 ± 3.2 (−7.5‐18.2) | 2.4 ± 3.2 (−7.5‐18.2) | 2.9 ± 3.3 (−5.9‐14.0) | 0.5 ± 0.1*** | 0.1 ± 3.1 | 0.4 ± 2.9 | −0.6 ± 3.3 | −0.9 ± 0.1*** |
| Fasting glucose, mmol/L | 6.2 ± 0.7 (3.4‐13.6) | 6.1 ± 0.7 | 6.3 ± 0.7 | 0.2 ± 0.03*** | 5.8 ± 0.6 | 5.7 ± 0.6 | 5.8 ± 0.6 | 0.1 ± 0.03 |
| HbA1c, mmol/mol | 36.7 ± 4.1 (23.0‐79.0) | 36.7 ± 3.9 | 36.8 ± 4.4 | 0.2 ± 0.2 | 34.6 ± 3.4 | 34.7 ± 3.3 | 34.3 ± 3.6 | −0.3 ± 0.2 |
| HbA1c, % | 5.5 ± 0.4 (4.3‐9.4) | 5.5 ± 0.4 | 5.5 ± 0.4 | 0.02 ± 0.02 | 5.3 ± 0.3 | 5.3 ± 0.3 | 5.3 ± 0.3 | −0.03 ± 0.01* |
| Insulin, mU/L | 13.5 ± 8.0 (1.0‐97.5) | 12.8 ± 7.4 | 14.8 ± 8.9 | 2.1 ± 0.4*** | 8.7 ± 5.5 | 8.7 ± 5.0 | 8.8 ± 6.4 | 0.3 ± 0.3 |
| C‐peptide, pmol/L | 923.6 ± 349.3 (177.0‐3234.0) | 888.8 ± 328.2 | 996.3 ± 379.7 | 115.1 ± 15.7*** | 706.6 ± 302.4 | 713.4 ± 290.1 | 693.0 ± 325.3 | −7.9 ± 14.0 |
| Total cholesterol, mmol/L | 5.2 ± 1.0 (2.0‐10.3) | 5.3 ± 1.0 | 5.0 ± 1.0 | −0.2 ± 0.04*** | 4.3 ± 1.0 | 4.4 ± 0.9 | 4.1 ± 1.0 | −0.3 ± 0.04*** |
| LDL cholesterol, mmol/L | 3.2 ± 0.8 (0.7‐7.2) | 3.3 ± 0.8 | 3.2 ± 0.9 | −0.1 ± 0.04** | 2.6 ± 0.8 | 2.7 ± 0.8 | 2.5 ± 0.8 | −0.2 ± 0.04*** |
| HDL cholesterol, mmol/L | 1.3 ± 0.3 (0.6‐2.6) | 1.3 ± 0.3 | 1.1 ± 0.2 | −0.2 ± 0.01*** | 1.2 ± 0.2 | 1.2 ± 0.2 | 1.1 ± 0.2 | −0.1 ± 0.01*** |
| Triglycerides, mmol/L | 1.5 ± 0.8 (0.23‐11.5) | 1.4 ± 0.8 | 1.6 ± 0.8 | 0.2 ± 0.04*** | 1.1 ± 0.5 | 1.1 ± 0.5 | 1.1 ± 0.7 | 0.03 ± 0.02 |
| C‐reactive protein, mg/L | 5.4 ± 7.0 (0.1‐144.5) | 5.9 ± 7.3 | 4.3 ± 6.2 | −1.4 ± 0.3*** | 4.4 ± 6.4 | 4.5 ± 6.0 | 4.1 ± 7.1 | −0.3 ± 0.3 |
| ALT, U/L | 27.9 ± 16.3 (6.0‐182.0) | 25.1 ± 13.9 | 33.8 ± 19.2 | 9.6 ± 0.7*** | 35.7 ± 26.9 | 36.7 ± 28.9 | 33.6 ± 22.1 | −3.3 ± 1.3* |
| AST, U/L | 27.7 ± 10.7 (10.0‐140.0) | 26.4 ± 9.8 | 30.5 ± 11.9 | 4.4 ± 0.5*** | 28.6 ± 13.0 | 28.5 ± 14.2 | 28.7 ± 10.2 | 0.01 ± 0.6 |
| Anthropometry, body composition and blood pressure | ||||||||
| Waist circumference, cm | 110.4 ± 14.7 (71.0‐210.0) | 107.5 ± 14.0 | 116.7 ± 14.3 | 9.8 ± 0.6*** | 100.6 ± 13.7 | 98.2 ± 13.0 | 105.3 ± 13.8 | 7.5 ± 0.6*** |
| Hip circumference, cm | 118.5 ± 13.8 (80.5‐202.0) | 120.5 ± 14.1 | 114.2 ± 12.1 | −5.5 ± 0.6*** | 110.9 ± 12.7 | 112.7 ± 13.0 | 107.1 ± 11.2 | −4.8 ± 0.6*** |
| Thigh circumference, cm | 60.4 ± 7.3 (40.5‐99.0) | 61.0 ± 7.6 | 59.1 ± 6.6 | −1.8 ± 0.3*** | 56.6 ± 6.6 | 57.1 ± 6.9 | 55.5 ± 5.9 | −1.7 ± 0.3*** |
| Fat‐free mass, kg | 56.5 ± 12.0 (32.8‐138.4) | 50.8 ± 7.7 | 68.6 ± 10.3 | 18.7 ± 0.4*** | 53.6 ± 11.1 | 48.1 ± 6.9 | 65.0 ± 9.4 | 17.7 ± 0.4*** |
| Fat mass, kg | 43.0 ± 13.7 (7.7‐128.3) | 44.3 ± 13.1 | 40.3 ± 14.4 | −3.7 ± 0.6*** | 34.7 ± 12.9 | 36.7 ± 12.2 | 30.7 ± 13.5 | −5.5 ± 0.6*** |
| Fat % | 43.3 ± 7.6 (11.1‐61.3) | 46.4 ± 5.8 | 36.8 ± 6.6 | −10.0 ± 0.3*** | 39.3 ± 9.0 | 42.9 ± 6.9 | 31.7 ± 8.0 | −11.4 ± 0.3*** |
| Bone mineral content, g | 2877 ± 572 (1442‐5500) | 2631 ± 399 | 3424 ± 518 | 811 ± 21*** | 2826 ± 567 | 2579 ± 403 | 3366 ± 495 | 793 ± 23*** |
| Bone mineral density, g/cm2 | 1.3 ± 0.1 (0.9‐1.7) | 1.2 ± 0.1 | 1.3 ± 0.1 | 0.09 ± 0.007*** | 1.3 ± 0.1 | 1.2 ± 0.1 | 1.3 ± 0.1 | 0.1 ± 0.007*** |
| SBP, mm Hg | 129.1 ± 15.9 (80.7‐185.3) | 127.1 ± 16.0 | 133.2 ± 14.7 | 6.0 ± 0.7*** | 122.0 ± 15.8 | 120.8 ± 15.7 | 124.3 ± 15.6 | 3.4 ± 0.7*** |
| DBP, mm Hg | 78.1 ± 11.1 (38.0‐117.3) | 76.8 ± 11.4 | 80.9 ± 9.9 | 3.7 ± 0.4*** | 75.0 ± 9.9 | 74.7 ± 10.0 | 75.5 ± 9.6 | 0.6 ± 0.4 |
| Pulse pressure, mm Hg | 50.9 ± 12.4 (19.0‐100.3) | 50.3 ± 12.8 | 52.3 ± 11.2 | 2.3 ± 0.5* | 47.0 ± 11.4 | 46.2 ± 11.6 | 48.8 ± 10.8 | 2.8 ± 0.5*** |
| MAP, mm Hgd | 99.5 ± 11.8 (56.6‐137.1) | 97.9 ± 12.0 | 102.9 ± 10.8 | 4.6 ± 0.5*** | 94.7 ± 11.3 | 94.0 ± 11.3 | 96.0 ± 11.2 | 1.8 ± 0.5*** |
| Heart rate, bpm | 71.3 ± 10. 6 (35.0‐119.3) | 71.9 ± 10.3 | 70.0 ± 11.0 | −1.3 ± 0.5* | 65.8 ± 10.9 | 66.9 ± 10.6 | 63.6 ± 11.2 | −2.3 ± 0.5*** |
Abbreviations: ALT, Alanine aminotransferase; AST, Aspartate aminotransferase; BMI, Body mass index; CID, Clinical investigation day; DBP, Diastolic blood pressure; HDL, High‐density lipoprotein; HOMA‐IR, Homeostasis model of assessment insulin resistance; LDL, Low‐density lipoprotein; MAP, Mean arterial pressure; SBP, Systolic blood pressure; SD, Standard deviation.
Data for all men and women are presented as mean ± SD (min‐ ax).
For difference between men and women the estimate is given as mean difference ± SEM adjusted for site;
*P < 0.05, **P < 0.01, ***P < 0.001.
The formula to calculate the HOMA‐IR was: fasting insulin(mU/L) *fasting glucose (mmol/L) / 22.5.25
The metabolic syndrome was calculated as a Z score.27
The formula used to calculate MAP was 0.42 x SBP + 0.58 x DBP.26
Changes after the LED are shown in Table 2. A total of 2020 participants attended the CID2 visit, with a dropout rate during the 8 weeks of 9.2% (204 participants; 152 women, 52 men). The proportion of dropouts varied among centres (UCPH, 2.5%; HEL, 5.8%; UM, 7.4%; UNAV, 9.0%; UNSYD, 10.1%; MU, 12.1%; UOA, 12.7%; UNOTT, 14.4%). Proportionally, more women (10.1%) than men (7.2%) dropped out, leaving a risk difference of −2.9% points (95% confidence interval [CI], −0.5% to −5.6% points; P = 0.01). Among participants who began the LED phase, 1857 (83.5%) achieved the target of ≥8% weight loss at 8 weeks. Fewer women (82%) than men (86.5%) achieved target weight loss (difference of 4.5% points; 95% CI, 1.4‐7.7% points; P = .02).
Table 2.
Changes in anthropometry, HOMA‐IR and blood markers in participants meeting at the clinical investigation day after the LED (CID2)
| Variable | Alla (N = 2020) | P valuea | Womenb (N = 1352) | Menb (N = 668) | Mean difference between men and womenb | P valueb | Mean difference between men and womenc | P valuec |
|---|---|---|---|---|---|---|---|---|
| Primary outcome | ||||||||
| ΔWeight, kg | −10.7 ± 0.4 | <0.001 | −10.2 ± 0.4 | −11.8 ± 0.5 | −1.6 ± 0.1 | <0.001 | ‐ | ‐ |
| Weight loss (%) | 10.8 ± 3.1 | ‐ | 10.3 ± 2.8 | 11.8 ± 3.4 | 1.3 ± 0.1 | <0.001 | ‐ | ‐ |
| ΔHOMA‐IRd | −1.42 ± 0.15 | <0.001 | −1.35 ± 0.15 | −1.50 ± 0.15 | −0.15 ± 0.06 | Ns | 0.01 ± 0.06 | Ns |
| Secondary outcomes | ||||||||
| Metabolic syndrome Z‐scoree | −2.5 ± 0.2 | <0.001 | −2.1 ± 0.2 | −3.4 ± 0.2 | −1.3 ± 0.1 | <0.001 | −0.9 ± 0.1 | <0.001 |
| ΔFasting glucose, mmol/L | −0.44 ± 0.07 | <0.001 | −0.42 ± 0.07 | −0.46 ± 0.07 | −0.04 ± 0.02 | Ns | 0.02 ± 0.02 | Ns |
| ΔHbA1c, mmol/mol | −2.1 ± 0.2 | <0.001 | −1.93 ± 0.15 | −2.35 ± 0.16 | −0.42 ± 0.10 | <0.001 | −0.25 ± 0.10 | Ns |
| ΔHbA1c, % | −0.19 ± 0.02 | <0.001 | −0.17 ± 0.01 | −0.22 ± 0.01 | −0.05 ± 0.01 | <0.001 | −0.03 ± 0.01 | <0.05 |
| Δinsulin, mU/L | −4.51 ± 0.44 | <0.001 | −4.28 ± 0.44 | −4.83 ± 0.46 | −0.55 ± 0.21 | <0.05 | −0.03 ± 0.21 | Ns |
| ΔC‐peptide, pmol/L | −210.4 ± 18.7 | <0.001 | −183.7 ± 22.6 | −259.7 ± 23.4 | −76.0 ± 10.5 | <0.001 | −48.6 ± 10.3 | <0.001 |
| ΔTotal cholesterol, mmol/L | −0.93 ± 0.08 | <0.001 | −0.89 ± 0.09 | −1.02 ± 0.09 | −0.13 ± 0.03 | <0.001 | −0.04 ± 0.03 | Ns |
| ΔLDL cholesterol, mmol/L | −0.64 ± 0.06 | <0.001 | −0.60 ± 0.07 | −0.72 ± 0.07 | −0.11 ± 0.03 | <0.001 | −0.04 ± 0.03 | Ns |
| ΔHDL cholesterol, mmol/L | −0.12 ± 0.02 | <0.001 | −0.12 ± 0.02 | −0.10 ± 0.02 | 0.02 ± 0.01 | <0.05 | 0.03 ± 0.01 | <0.01 |
| ΔTriglycerides, mmol/L | −0.40 ± 0.04 | <0.001 | −0.39 ± 0.04 | −0.42 ± 0.04 | −0.02 ± 0.02 | Ns | 0.02 ± 0.02 | Ns |
| ΔC‐reactive protein, mg/L | −0.89 ± 0.17 | <0.001 | −0.97 ± 0.27 | −0.77 ± 0.31 | 0.20 ± 0.29 | Ns | 0.38 ± 0.29 | Ns |
| ΔALT, U/L | 7.6 ± 1.7 | <0.001 | 10.0 ± 2.3 | 2.2 ± 2.4 | −7.8 ± 1.3 | <0.001 | −8.3 ± 1.3 | <0.001 |
| ΔAST, U/L | 1.0 ± 0.6 | Ns | 1.5 ± 0.7 | −0.1 ± 0.8 | −1.6 ± 0.6 | <0.05 | −1.8 ± 0.6 | <0.05 |
| Anthropometry, body composition and blood pressure | ||||||||
| ΔWaist circumference, cm | −9.6 ± 0.4 | <0.001 | −9.2 ± 0.4 | −10.5 ± 0.4 | −1.4 ± 0.3 | <0.001 | −0.2 ± 0.2 | Ns |
| ΔHip circumference, cm | −7.1 ± 0.3 | <0.001 | −7.1 ± 0.2 | −7.2 ± 0. 3 | −0.04 ± 0.2 | Ns | 0.7 ± 0.2 | <0.001 |
| Δthigh circumference, cm | −3.7 ± 0.1 | <0.001 | −3.7 ± 0.1 | −3.9 ± 0.1 | −0.2 ± 0.2 | Ns | 0.2 ± 0.2 | Ns |
| ΔFat free mass, kg | −2.74 ± 0.37 | <0.001 | −3.17 ± 0.38 | −1.90 ± 0.40 | 1.26 ± 0.18 | <0.001 | 1.58 ± 0.17 | <0.001 |
| ΔFat mass, kg | −7.80 ± 0.39 | <0.001 | −7.09 ± 0.40 | −9.33 ± 0.41 | −2.23 ± 0.15 | <0.001 | −1.30 ± 0.12 | <0.001 |
| ΔFat % | −3.9 ± 0.5 | <0.001 | −3.7 ± 0.4 | −4.3 ± 0.4 | −0.6 ± 0.2 | <0.01 | −0.2 ± 0.2 | Ns |
| ΔBone mineral content, g | −45.3 ± 20.0 | <0.05 | −57.1 ± 19.4 | −8.6 ± 20.3 | 48.4 ± 9.3 | <0.001 | 53.8 ± 9.5 | <0.001 |
| ΔBone mineral density, g/cm2 | 0.004 ± 0.005 | Ns | 0.001 ± 0.004 | 0.007 ± 0.004 | 0.006 ± 0.002 | <0.05 | 0.005 ± 0.002 | Ns |
| ΔSBP, mm Hg | −7.5 ± 0.7 | <0.001 | −7.7 ± 1.3 | −7.8 ± 1.3 | −0.2 ± 0.6 | Ns | 0.6 ± 0.6 | Ns |
| ΔDBP, mm Hg | −3.5 ± 0.8 | <0.001 | −3.2 ± 0.9 | −4.5 ± 0.9 | −1.3 ± 0.4 | 0.001 | −0.8 ± 0.4 | Ns |
| ΔPulse pressure, mm Hg | −4.0 ± 0.9 | <001 | −4.5 ± 0.9 | −3.2 ± 0.9 | 1.4 ± 0.4 | <0.01 | 1.6 ± 0.4 | <0.001 |
| ΔMAP, mm Hgf | −5.2 ± 0.6 | <0.001 | −5.1 ± 0.9 | −5.9 ± 1.0 | −0.8 ± 0.4 | Ns | −0.2 ± 0.4 | Ns |
| ΔHeart rate, bpm | −5.4 ± 0.8 | <0.001 | −4.9 ± 1.1 | −6.3 ± 1.1 | −1.4 ± 0.4 | <0.001 | −1.1 ± 0.4 | <0.05 |
Abbreviations: ALT, Alanine aminotransferase; AST, Aspartate aminotransferase; BMI, Body mass index; CID, Clinical investigation day; DBP, Diastolic blood pressure; HDL, High‐density lipoprotein; HOMA‐IR, Homeostasis model of assessment insulin resistance; LDL, Low‐density lipoprotein; MAP, Mean arterial pressure; SBP, Systolic blood pressure; SE, Standard error.
Data are given as mean change ± SEM.
ANCOVA models include adjustment for intervention site.
ANCOVA models include adjustment for site, age, gender and baseline.
ANCOVA models include adjustment for site, age, gender, baseline and weight loss percentage.
The formula to calculate the HOMA‐IR was: fasting insulin(mU/L) *fasting glucose (mmol/L) / 22.5.25
The metabolic syndrome was calculated as a Z score.27
The formula used to calculate MAP was 0.42 x SBP + 0.58 x DBP.26
The mean LED weight loss (±SEM) in all participants was 10.7 ± 0.4 kg (10.8%; P < 0.001), with women losing 16% less weight than men (10.2 ± 0.4 kg [10.3%] vs 11.8 ± 0.5 kg [11.8%], respectively; P < .001). On average, HOMA‐IR decreased by 1.42 ± 0.15 units (P < 0.001) in all participants and was similar between women and men (1.35 ± 0.15 vs 1.50 ± 0.15, respectively; P, ns). The overall change in metabolic syndrome Z‐score was −2.5 ± 0.2 (P < 0.001), but the improvement was less in women than in men (−2.1 ± 0.2 vs −3.4 ± 0.2, respectively), with a mean difference of −1.3 ± 0.1 (P < 0.001). The difference remained highly significant after adjusting for differences in weight loss (%) (P < 0.001).
Of the 2224 participants who completed the baseline visit, 1429 (64.3%) had isolated IFG, 283 (12.7%) had isolated IGT and 512 (23.0%) had both IFG and IGT at the screening visit. Following weight loss as the result of the 8‐week LED, 694 participants (35.8%) had reverted to normo‐glycaemia based on FPG alone. This number increased to 40.2% among participants who met the target weight loss (ie, ≥8% of initial body weight).
Following the LED, FFM decreased more in women than in men (3.2 ± 0.4 kg vs 1.9 ± 0.4 kg, respectively [mean difference, 1.3 ± 0.2 kg; P < 0.001]). Conversely, FM decreased less in women than in men (7.1 ± 0.4 kg vs 9.3 ± 0.4 kg, respectively [mean difference, −2.2 ± 0.2 kg; P < 0.001). For both outcomes, the difference in changes between women and men remained highly significant after adjusting for weight loss (%).
A separate analysis of changes in anthropometry, HOMA‐IR and blood markers in female participants in different age groups is shown in Appendix S4. The younger age group (<45.9 years) experienced statistically different changes in HOMA‐IR, HbA1c, insulin, HDL cholesterol, ALT, thigh circumference, BMC, BMD and heart rate compared to the two older age groups (46‐54 years and > 55 years). Between the older age groups, changes in HbA1c, ALT, thigh circumference and BMD were statistically significantly different after the LED weight loss.
During the LED weight loss period, 961 AEs were reported across all sites. Of these, 10 events were reported as serious adverse events (SAEs). However, all SAEs were evaluated as unlikely to be related, or unrelated, to the study intervention and the LED weight loss. Women reported significantly more adverse events than men (Table 3). The main AEs were constipation, cold/influenza, muscular weakness and pain.
Table 3.
Adverse effects reported by the PREVIEW participants during and immediately after the weight‐loss period at the respective intervention sites
| Symptoms | All (n = 2224) | Women (n = 1504) | Men (n = 720) | Risk difference (95% CI) *P < 0.05 |
|---|---|---|---|---|
| Constipation | 169 (7.6%) | 129 (8.6%) | 40 (5.6%) | 0.030* (0.008;0.052) |
| Diarrhea | 34 (1.5%) | 26 (1.7%) | 8 (1.1%) | 0.006 (−0.004; 0.016) |
| Other gastrointestinal symptoms including feeling nausea, having pain, flatulence and vomiting | 84 (3.8%) | 67 (4.5%) | 17 (2.4%) | 0.021* (0.006; 0.036) |
| Having a cold/influenza | 121 (5.4%) | 85 (5.7%) | 36 (5.0%) | 0.007 (−0.013; 0.026) |
| Sore throat | 10 (0.4%) | 6 (0.4%) | 4 (0.6%) | −0.002 (−0.008; 0.005) |
| Dizziness | 44 (2.0%) | 27 (1.8%) | 17 (2.4%) | −0.006 (−0.019; 0.007) |
| Headaches and migraines | 66 (3.0%) | 56 (3.7%) | 10 (1.4%) | 0.023* (0.011; 0.036) |
| Muscular weakness and pain | 113 (5.0%) | 77 (5.1%) | 36 (5.0%) | 0.001 (−0.018; 0.021) |
| Allergic reaction | 8 (0.4%) | 6 (0.4%) | 2 (0.3%) | 0.001 (−0.004; 0.006) |
| Hair loss | 19 (0.9%) | 18 (1.2%) | 1 (0.1%) | 0.011* (0.004; 0.017) |
| Changes in menstrual symptoms, −cycle or postmenstrual symptoms | 15 (0.7%) | 15 (1.0%) | ‐ | |
| Various infections | 74 (3.3%) | 61 (4.1%) | 13 (1.8%) | 0.023* (0.009; 0.036) |
| Dry skin, eczema and other effects on skin | 23 (1.0%) | 17 (1.1%) | 6 (0.8%) | 0.003 (−0.006; 0.011) |
| Gout | 6 (0.3%) | 0 (0.0%) | 6 (0.8%) | −0.008* (−0.015; −0.002) |
| Other | 175 (7.9%) | 122 (8.1%) | 53 (7.4%) | 0.008 (−0.016; 0.031) |
| Total | 961 (43.2%) | 712 (47.3%) | 249 (34.6%) | 0.128* (0.085; 0.171) |
Abbreviation: CI, confidence interval.
Data are presented as numbers and proportions, no. (%); mean difference between women and men is estimated via the risk difference. *Analysed using chi‐square; P < 0.05.
4. DISCUSSION
In this worldwide intervention study, participants lost an average of 11% body weight and showed significant improvements in insulin resistance (change in HOMA‐IR, −1.4; P < 0.001) after an 8‐week LED. There were differences in other metabolic outcomes according to gender; men appeared to benefit more than women. Men lost significantly more body weight than women, and had larger reductions in metabolic syndrome Z‐score, C‐peptide, FM and heart rate, even after adjusting for differences in weight loss (%). In contrast, women had larger reductions in HDL cholesterol, hip circumference, BMC, FFM and pulse pressure than men, again after adjustment for differences in weight loss (%). As declines in HDL cholesterol, BMC and lean mass are generally not supportive of long‐term health, it is of general interest to determine whether rapid weight loss with a LED compromises the health of some women. Therefore, it is of importance to investigate whether the long‐term effects of rapid weight loss are indeed more beneficial for men than for women with regard to prevention of both type‐2 diabetes and cardiovascular disease.
Previous studies have reported that differences in metabolic outcome according to gender occur because men mobilize more intra‐abdominal fat than women during weight loss, and that this is accompanied by a more pronounced improvement in the metabolic risk profile.12, 14, 15 In the present study, we found important differences when comparing outcomes between women and men, both before and after adjusting for differences in weight loss (%). This suggests intrinsic differences in how men and women adapt to dietary energy deficits.
Following LED weight loss, the loss of FFM was, on average, 25% of the total weight loss. Changes in FFM of this magnitude are considered normal during LED weight loss.28, 29 Interestingly, however, women lost twice as much FFM as men (31.4% vs 16.1%, respectively), which is striking, as men had a larger energy deficit during the LED phase. It would be expected that men would have a larger requirement for dietary protein, as their FFM was much larger than that of women at baseline. Using the most recent Joint FAO/WHO/UNU Expert Consultation on Human Energy Requirements,30 it is possible to estimate the daily energy requirement of an average male PREVIEW participant (body weight, 109.4 kg) with a low daily physical activity level (PAL, 1.45) as 13 MJ (3086 kcal). In comparison, a female participant (body weight, 95.6 kg) with a similar activity level would have a daily energy requirement of approximately only 10 MJ (2353 kcal). However, despite this large difference in energy requirement, men managed to preserve more FFM during the LED than women. Looking at this from a compliance perspective, the daily provision of 3.4 MJ/d with the LED would leave men with an energy deficit of 9.6 MJ/d and women with a deficit of 6.5 MJ/d. After 8 weeks, these energy deficits should yield a weight loss of 18.3 kg for men and of 12.4 kg for women according to Westerterp et al.32 Considering the actual weight loss achieved, 11.8 kg for men and 10.2 kg for women, there is reason to believe that women were closer to their theoretically achievable weight loss target (82.2%) than men (64.5%). If we then evaluate and make the reverse calculation of achieved weight loss, it appears that the mean energy intake in men must have been 6.1 MJ/d and the mean energy intake in women must have been 4.55 MJ/d. This suggests that women were more compliant with the diet than men. Similar observations were made by Camps SG et al.31 It would be interesting to investigate differences between men and women in compliance with and adaptation to the LED phase as it may help explain the differences found in this analysis.
Physical activity (PA) and exercise training are associated with numerous health benefits.33 In the PREVIEW study, we did not measure the level of physical activity during or immediately after the LED weight‐loss phase. Differences in physical activity level between participants could have impacted some results presented in this paper; however, the strict inclusion criterion (absence of high PA) led to a narrower between‐person variance in PA, which decreased the likelihood that one could find an association between PA, weight loss and the related outcomes. The included participants were, more or less, physically inactive and no guidance concerning PA was given during the LED phase. Although we do not have direct evidence, it is unlikely that any major changes in PA occurred during the LED phase.
In the PREVIEW study, different equipment was used to measure body composition at the different intervention sites (Appendix S3); however, the same equipment was always used to measure a given participant. There are many body composition methods available to estimate different body compartments.34, 35 The more practical and acceptable methods that are frequently used to estimate body composition include Dual‐energy X‐ray absorptiometry (DXA) and bioimpedance analysis (BIA), which were primarily used in the current study. The validity of DXA and BIA has been debated previously; their accuracy can vary according to age, adiposity, etc.34, 36 In this study, using different equipment at the various sites may have introduced some variability in the data. However, as the same equipment was always used for a given participant, and as adjustments were made for the site in the analyses, we believe that we have limited the bias to the greatest extent possible while we acknowledge that not using the same equipment across all sites is a weakness of the trial.
Additionally, 87.6% of the study participants described themselves as Caucasian and the remaining participants were Polynesians Asian, Hispanic, Black or of mixed origin. Therefore, the ethnic diversity of PREVIEW participants does not allow generalization of the results to all ethnic groups but primarily to Caucasians.
Drop‐out rates during the LED were generally low but varied across centres, from 2.5% to 14.4%. The lower drop‐out rate in men might be explained by the greater, early success experienced by men using the LED. There can be many reasons for the difference in drop‐out rates across sites. At some sites, participants were not as familiar with using formula LEDs for weight loss as those at other sites; thus, cultural and social challenges varied. Differences in compliance and efficacy of the LED in different settings have also been reported in an earlier large‐scale study.37
As outlined in this discussion, many aspects of the study could have contributed to the gender‐specific effects that we found. Regional fat distribution is indeed different between men and women and, as described earlier, men may mobilize more intra‐abdominal fat than women, whereas women may lose more subcutaneous fat during weight loss.14, 15 However, our aim with these analyses was not to attempt to disentangle the various contributors to gender‐specific effects, that is, gender‐specific hormones, behaviour and compliance during the LED. Our aim was to assess gender‐specific effects as a whole and future analysis of our data could explore what constitutes these gender‐specific effects.
In the separate analysis investigating differences between age groups within the female population, we found several statistically significant findings. Whether these findings are clinically important or simply statistically significant findings is difficult to interpret.
Generally, weight loss is known to be associated with improvements in liver transaminases once weight stability has been achieved.38 However, our current study is consistent with the existing literature in showing that transient mild increases in liver enzymes can be observed in some individuals immediately after an LED period.39 Increments were significantly larger in women than in men. It has been reported in previous studies that values return to normal within a few weeks.24 The consequences of the changes are believed to be benign if the enzyme elevation is transient.39
An important strength of our study is the large sample size and the wide age span, in all sites in Europe, Australia and New Zealand. In addition, criteria for identifying pre‐diabetes were consistent from site to site as ADA criteria (IFG, 5.6‐6.9 mmol/L)22, 40 were used. The range for identifying IFG according to the World Health Organization is narrower (6.1‐6.9 mmol/L). 40 However, in the present study, following LED weight loss, more than 35% of the men and women with IFG at screening reverted to normo‐glycaemia. A recent systematic review and meta‐analysis41 concluded that the risk of cardiovascular disease was increased in individuals with FPG as low as 5.6 mmol/L. Concerning participants with IFG, according to WHO criteria (> 6.1 mmol/L; n = 790), 442 participants (55.9%) were no longer classified with pre‐diabetes after LED weight loss. This number increased to 62.6%, when including only those participants with successful weight loss (ie, ≥8% of initial body weight).
The results presented in this analysis provide data only on short‐term changes. Indeed, maintaining weight loss and the accompanying improvements is challenging.42, 43 Whether PREVIEW participants are able to maintain the weight loss and achieved metabolic responses, and whether differences between genders persist in the long term will be apparent once the trial is completed. However, the 8‐week LED in individuals with pre‐diabetes did result in the initial 10% weight loss needed to achieve major metabolic improvement in the first phase of a diabetes prevention programme.
In conclusion, an 8‐week LED was accompanied by significant improvements in anthropometry, blood pressure and metabolic profile in overweight women and men with pre‐diabetes. While HOMA‐IR improved in all participants, regardless of gender, men lost significantly more body weight than women and had larger reductions in metabolic syndrome Z‐score, C‐peptide and FM, even after adjusting for differences in weight loss (%). In contrast, women had larger reductions in HDL cholesterol, FFM and BMC that could be considered undesirable. These findings are clinically important and suggest gender‐specific differences between men and women after weight loss. It is of importance to investigate whether the greater reduction in FFM, BMC, hip circumference and HDL cholesterol in women after rapid weight loss is indeed beneficial or, rather, might compromise weight loss maintenance and future optimal/good cardiovascular health.
Supporting information
Appendix S1. Inclusion and exclusion criteria.
Appendix S2. Standard Operating Procedure (SOP): Guidelines to dieticians/diet‐instructors for instructing participants on how to follow the Low‐Energy Diet (LED).
Appendix S3. Body composition equipments.
Appendix S4. Changes in anthropometry.
ACKNOWLEDGMENTS
The following additional contributors assisted in conduct of the trial during recruitment, intervention and/or data collection. University of Copenhagen: Ulla Skovbæch Pedersen, Marianne Juhl Hansen, Bettina Belmann Mirasola, Maria Roed Andersen, Anne Wengler, Jane Jørgensen, Sofie Skov Frost, Eivind Bjørås, Grith Møller, Lone Vestergaard Nielsen. University of Helsinki: Elli Jalo, Saara Kettunen, Laura Korpipää, Tiia Kunnas, Heini Hyvärinen, Heikki Tikkanen, Sanna Ritola. University of Nottingham: Elizabeth Simpson, Shelley Archer, Natalie Bailey‐Flitter, Nicky Gilbert, Laura Helm, Sally Maitland, Melanie Marshall, Theresa Mellor, Grace Miller, Seodhna Murphy, Vicky Newman, Amy Postles, Jakki Pritchard, Maria Papageorgiou, Cheryl Percival, Clare Randall, Sue Smith, Sarah Skirrow. University of Maastricht: Tanja Adam. Universidad de Navarra: Blanca Martinez de Morentin Aldabe, María Hernández Ruiz de Eguilaz, Salomé Pérez Diez, Rodrigo San‐Cristobal, Maria dels Angels Batlle, Laura Moreno‐Galarraga, Alejandro Fernández‐Montero, Marian Nuin, Javier Baquedano, Maria Eugenia Ursúa, Francisco Javier Martinez Jarauta, Pilar Buil, Lourdes Dorronsoro, Juana María Vizcay, Teodoro Durá‐Travé, and all general practitioners and nurses from the Navarra Health Services who collaborated in recruitment of participants. Medical University of Sofia: Nadka Boyadjieva, Pavlina Gateva‐Andreeva, Georgi Bogdanov, Galina Dobrevska. University of Auckland: Amy Liu, Lindsay Plank, Anne‐Thea McGill, Madhavi Bollineni, Kelly Storey, Nicholas Gant, Jonathon Woodhead, Hannah Chisholm, Wonjoo Lee, Chelsea Cheah, Eric Hansen, Hacer Tekinkaya, Nadia Harvey. University of Sydney: Roslyn Muirhead, Kylie Simpson, Michele Whittle, Kirstine Bell. Lene Stevner, UCPH, assisted with advice on ethical issues, Good Clinical Practice and approval of the study protocol. Moreover, we would like to acknowledge all the additional people who have worked, and are currently working, for PREVIEW, including trainees, post‐graduate and undergraduate students. Finally, a respectful thank you to all the study participants of PREVIEW.
Conflict of interest
All authors have completed the ICMJE uniform disclosure form and make the following declarations. P. C. received travel grants to attend scientific meetings from The Cambridge Weight Plan, UK, outside the submitted work. T. M. L. reports personal fees from Sense Kost, outside the submitted work. M. W.‐P. has nothing to disclose. I. M. has nothing to disclose. J. A. M. has nothing to disclose. S. H. has nothing to disclose. S. P. holds the Fonterra Chair in Human Nutrition at the University of Auckland. S. H. has nothing to disclose. C. R. has nothing to disclose. A. A. reports personal fees from Basic Research, USA; Beachbody, USA; BioCare Copenhagen, Denmark; Crossfit, USA; Danish Agriculture and Food Council; Dutch Beer Institute, The Netherlands; Feast Kitchen A/S, Denmark; Groupe Éthique et Santé, France; McCain Foods Limited, USA; Nestlé Research Center, Switzerland; Novo Nordisk, Denmark; Pfizer, Germany; Saniona, Denmark; Sanofi‐Aventis, Germany; S‐Biotek, Denmark; Scandinavian Airlines System, Denmark; TetraPak, Sweden; Weight Watchers, USA; Zaluvida, Switzerland; Gelesis, USA; Personalized Weight Management Research Consortium ApS, Denmark and grants from Arla Foods, Denmark; Danish Dairy Research Council; Gelesis, USA; and Global Dairy Platform, outside the submitted work; has patents pending (University of Copenhagen, methods of inducing weight loss, treating obesity and preventing weight gain; licensee, Gelesis, USA) and (Biomarkers, predicting degree of weight loss (licensee Nestec SA, Switzerland); is co‐inventor of a number of other patents owned by the University in accordance with Danish law; receives royalties for the books “Verdens Bedste Kur”/Politikens Forlag, Denmark, 2012 (subsequently published in English as “World's Best Diet”/Penguin, Australia and “The Nordic Way”/Random House, USA), and “Spis dig slank efter dit blodsukker” (Eat according to your blood sugar and be slim)/Politikens Forlag, Denmark, 2017; is co‐author of several books in the pipeline about personlized nutrition for weight loss; is co‐owner and member of the board of the consultancy company Dentacom Aps, Denmark; is co‐founder and co‐owner and member of the board of UCPH spin‐outs Mobile Fitness A/S & Flaxslim ApS. L. P.‐S. has nothing to disclose. F. S.‐P. has nothing to disclose. K. H. P. was supported by the Academy of Finland (grants 272376, 314383 and 266286), the Finnish Diabetes Research Foundation, Novo Nordisk Foundation and Helsinki University Hospital Government funds. M. D. has nothing to disclose. M. A. T. has nothing to disclose. S. N.‐C. has nothing to disclose. T. H.‐D. has nothing to disclose. S. B. has nothing to disclose. M. P. S. has nothing to disclose. M. H.‐L. has nothing to disclose. J. B.‐M. has nothing to disclose. M. F. has nothing to disclose. A. R. has nothing to disclose.
1. Author contributions
The PREVIEW project was designed by A. R., J. B.‐M., M. W.‐P., M. F., Wolfgang Schlicht (W.S.) and Edith Feskens (E.F.) The PREVIEW intervention study for adult participants was designed by A. R., M. F. and T. M. L. All co‐authors contributed to implementation of the experimental trial and design of the protocol for intervention. It was the first author, P. C.’s idea to test the gender difference hypothesis reported in this paper. All authors contributed to analysis and interpretation of the data. P. C. drafted the manuscript. All authors contributed to critical revision of the manuscript for important intellectual content. The corresponding author, P.C., is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Christensen P, Meinert Larsen T, Westerterp‐Plantenga M, et al. Men and women respond differently to rapid weight loss: Metabolic outcomes of a multi‐centre intervention study after a low‐energy diet in 2500 overweight, individuals with pre‐diabetes (PREVIEW). Diabetes Obes Metab. 2018;20:2840–2851. 10.1111/dom.13466
Funding information Funding was received from the EU 7th Framework Programme (FP7/2007‐2013) under grant agreement no. 312057, The National Health and Medical Research Council ‐ EU Collaborative Grant, AUS, The NZ Health Research Council (14/191) and the UoA Faculty Research Development Fund. The Cambridge Weight Plan, UK kindly donated all products for the 8‐week Low‐Energy Diet period.
REFERENCES
- 1. WHO | Diabetes . World Health Organization; WHO [Internet]. 2016. Available at: http://www.who.int/mediacentre/factsheets/fs312/en Accessed July 12, 2017.
- 2. Iwahashi H, Noguchi M, Okauchi Y, Morita S, Imagawa A, Shimomura I. Extent of weight reduction necessary for minimization of diabetes risk in Japanese men with visceral fat accumulation and glycated hemoglobin of 5.6‐6.4%. J Diabetes Investig. 2015;6:553‐559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kahlert D, Unyi‐Reicherz A, Stratton G, et al. PREVIEW behavior modification intervention toolbox (PREMIT): a study protocol for a psychological element of a multicenter project. Front Psychol. 2016;7:1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Fogelholm M, Larsen T, Westerterp‐Plantenga M, et al. PREVIEW: Prevention of diabetes through lifestyle intervention and population studies in Europe and around the world. Design, methods, and baseline participant description of an adult cohort enrolled into a three‐year randomised clinical trial. Nutrients. 2017;9:632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kennedy‐Dalby A, Adam S, Ammori BJ, Syed AA. Weight loss and metabolic outcomes of bariatric surgery in men versus women ‐ a matched comparative observational cohort study. Eur J Intern Med. 2014;25:922‐925. [DOI] [PubMed] [Google Scholar]
- 6. Bhogal MS, Langford R. Gender differences in weight loss; evidence from a NHS weight management service. Public Health. 2014;128:811‐813. [DOI] [PubMed] [Google Scholar]
- 7. Coles LT, Fletcher EA, Galbraith CE, Clifton PM. Patient freedom to choose a weight loss diet in the treatment of overweight and obesity: a randomized dietary intervention in type 2 diabetes and pre‐diabetes. Int J Behav Nutr Phys Act. 2014;11:64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Caudwell P, Gibbons C, Finlayson G, Näslund E, Blundell J. Exercise and weight loss. Exerc Sport Sci Rev. 2014;42:92‐101. [DOI] [PubMed] [Google Scholar]
- 9. Alberti KGMM, Eckel RH, Grundy SM, et al. Harmonizing the metabolic syndrome: a joint interim statement of the international diabetes federation task force on epidemiology and prevention; National Heart, Lung, and Blood Institute; American Heart Association; world heart federation; international atherosclerosis society; and International Association for the Study of obesity. Circulation. 2009;120:1640‐1645. [DOI] [PubMed] [Google Scholar]
- 10. Biro SM, Olson DL, Garren MJ, Gould JC. Diabetes remission and glycemic response to pre‐bariatric surgery diet. J Surg Res. 2013;185:1‐1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Buse JB, Caprio S, Cefalu WT, et al. How do we define cure of diabetes? Diabetes Care. 2009;32:2133‐2135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Handjieva‐Darlenska T, Handjiev S, Larsen TM, et al. Initial weight loss on an 800‐kcal diet as a predictor of weight loss success after 8 weeks: the diogenes study. Eur J Clin Nutr. 2010;64:994‐999. [DOI] [PubMed] [Google Scholar]
- 13. Williams RL, Wood LG, Collins CE, Callister R. Effectiveness of weight loss interventions ‐ is there a difference between men and women: a systematic review. Obes Rev. 2015;16:171‐186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wirth A, Steinmetz B. Gender differences in changes in subcutaneous and intra‐abdominal fat during weight reduction: an ultrasound study. Obes Res. 1998;6:393‐399. [DOI] [PubMed] [Google Scholar]
- 15. Gasteyger C, Larsen TM, Vercruysse F, Pedersen D, Toubro S, Astrup A. Visceral fat loss induced by a low‐calorie diet: a direct comparison between women and men. Diabetes Obes Metab. 2009;11:596‐602. [DOI] [PubMed] [Google Scholar]
- 16. Singh GM, Danaei G, Farzadfar F, et al. The age‐specific quantitative effects of metabolic risk factors on cardiovascular diseases and diabetes: A pooled analysis. PLoS One. 2013;8:e65174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Saudek CD. Can diabetes be cured?: potential biological and mechanical approaches. JAMA. 2009;301:1588‐1590. [DOI] [PubMed] [Google Scholar]
- 18. Cowie CC, Rust KF, Ford ES, et al. Full accounting of diabetes and pre‐diabetes in the U.S. population in 1988‐1994 and 2005‐2006. Diabetes Care. 2009;32:287‐294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Unwin N, Shaw J, Zimmet P, Alberti KGMM. Impaired glucose tolerance and impaired fasting glycaemia: the current status on definition and intervention. Diabet Med. 2002;19:708‐723. [DOI] [PubMed] [Google Scholar]
- 20. Silventoinen K, Pankow J, Lindström J, Jousilahti P, Hu G, Tuomilehto J. The validity of the Finnish diabetes risk score for the prediction of the incidence of coronary heart disease and stroke, and total mortality. Eur J Cardiovasc Prev Rehabil. 2005;12:451‐458. [DOI] [PubMed] [Google Scholar]
- 21. Lindström J, Louheranta A, Mannelin M, et al. The Finnish diabetes prevention study (DPS): lifestyle intervention and 3‐year results on diet and physical activity. Diabetes Care. 2003;26:3230‐3236. [DOI] [PubMed] [Google Scholar]
- 22. American Diabetes Association . 2. Classification and diagnosis of diabetes. Diabetes Care. 2017;40:S11‐S24. [DOI] [PubMed] [Google Scholar]
- 23. Christensen P, Bartels EM, Riecke BF, et al. Improved nutritional status and bone health after diet‐induced weight loss in sedentary osteoarthritis patients: a prospective cohort study. Eur J Clin Nutr. 2012;66:504‐509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Christensen P, Bliddal H, Riecke BF, Leeds AR, Astrup A, Christensen R. Comparison of a low‐energy diet and a very low‐energy diet in sedentary obese individuals: a pragmatic randomized controlled trial. Clin Obes. 2011;1:31‐40. [DOI] [PubMed] [Google Scholar]
- 25. Wallace TM, Levy JC, Matthews DR. Use and abuse of HOMA modeling. Diabetes Care. 2004;27:1487‐1495. [DOI] [PubMed] [Google Scholar]
- 26. Papaioannou TG, Protogerou AD, Vrachatis D, et al. Mean arterial pressure values calculated using seven different methods and their associations with target organ deterioration in a single‐center study of 1878 individuals. Hypertens Res. 2016;39:640‐647. [DOI] [PubMed] [Google Scholar]
- 27. Johnson JL, Slentz CA, Houmard JA, et al. Exercise training amount and intensity effects on metabolic syndrome (from studies of a targeted risk reduction intervention through defined exercise). Am J Cardiol. 2007;100:1759‐1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Larsen TM, Dalskov S‐M, van Baak M, et al. Diets with high or low protein content and glycemic index for weight‐loss maintenance. N Engl J Med. 2010;363:2102‐2113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lantz H, Peltonen M, Agren L, Torgerson JS. Intermittent versus on‐demand use of a very low calorie diet: a randomized 2‐year clinical trial. J Intern Med. 2003;253:463‐471. [DOI] [PubMed] [Google Scholar]
- 30. Human energy requirements: report of a joint FAO/ WHO/UNU Expert Consultation. Food Nutr Bull. 2005;26:166. [PubMed] [Google Scholar]
- 31. Camps SG, Verhoef SP, Westerterp KR. Weight loss, weight maintenance, and adaptive thermogenesis. Am J Clin Nutr. 2013;97:990‐994. [DOI] [PubMed] [Google Scholar]
- 32. Westerterp KR, Donkers JH, Fredrix EW, Boekhoudt P. Energy intake, physical activity and body weight: a simulation model. Br J Nutr. 1995;73:337‐347. [DOI] [PubMed] [Google Scholar]
- 33. Haskell WL, Lee I‐M, Pate RR, et al. Physical activity and public health. Med Sci Sport Exerc. 2007;39:1423‐1434. [DOI] [PubMed] [Google Scholar]
- 34. Wells JCK, Fewtrell MS. Measuring body composition. Arch Dis Child. 2005;91:612‐617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Wells JC, Fuller NJ, Dewit O, Fewtrell MS, Elia M, Cole TJ. Four‐component model of body composition in children: density and hydration of fat‐free mass and comparison with simpler models. Am J Clin Nutr. 1999;69:904‐912. [DOI] [PubMed] [Google Scholar]
- 36. Ferri‐Morales A, Nascimento‐Ferreira MV, Vlachopoulos D, et al. Agreement between standard body composition methods to estimate percentage of body fat in young male athletes. Pediatr Exerc Sci. 2018;30:402‐410. [DOI] [PubMed] [Google Scholar]
- 37. Papadaki A, Linardakis M, Plada M, et al. A multicentre weight loss study using a low‐calorie diet over 8 weeks: regional differences in efficacy across eight European cities. Swiss Med Wkly. 2013;143:w13721. [DOI] [PubMed] [Google Scholar]
- 38. Katsagoni CN, Georgoulis M, Papatheodoridis GV, Panagiotakos DB, Kontogianni MD. Effects of lifestyle interventions on clinical characteristics of patients with non‐alcoholic fatty liver disease: a meta‐analysis. Metabolism. 2017;68:119‐132. [DOI] [PubMed] [Google Scholar]
- 39. Gasteyger C, Larsen TM, Vercruysse F, Astrup A. Effect of a dietary‐induced weight loss on liver enzymes in obese subjects. Am J Clin Nutr. 2008;87:1141‐1147. [DOI] [PubMed] [Google Scholar]
- 40. WHO . Definition and Diagnosis of Diabetes and Intermediate Hyperglycaemia. Geneva, Switzerland: WHO Document Production Services; 2006. [Google Scholar]
- 41. Huang Y, Cai X, Mai W, Li M, Hu Y. Association between prediabetes and risk of cardiovascular disease and all cause mortality: systematic review and meta‐analysis. BMJ. 2016;355:i5953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Christensen P, Henriksen M, Bartels EM, et al. Long‐term weight‐loss maintenance in obese patients with knee osteoarthritis: a randomized trial. Am J Clin Nutr. 2017;106:755‐763. [DOI] [PubMed] [Google Scholar]
- 43. Kushner RF, Ryan DH. Assessment and lifestyle Management of Patients with Obesity. JAMA. 2014;312:943‐952. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. Inclusion and exclusion criteria.
Appendix S2. Standard Operating Procedure (SOP): Guidelines to dieticians/diet‐instructors for instructing participants on how to follow the Low‐Energy Diet (LED).
Appendix S3. Body composition equipments.
Appendix S4. Changes in anthropometry.


