Abstract
Objective
To investigate consistency of lymphocyte immunophenotype between labial gland and peripheral blood in patients with primary Sjogren's syndrome (pSS).
Methods
Seventy‐one pSS patients and 35 patients with maxillofacial trauma were included in the study. Based on the ratio of CD20 to CD3 in labial gland from 71 pSS patients, they were divided into the high and (n = 48) and low CD20 expression group (n = 23). Lymphocyte immunophenotypes in labial glands, course of disease, erythrocyte sedimentation rate (ESR), C‐reactive protein, immunoglobulin, and complement levels were analyzed.
Results
In the labial gland, the levels of IgG, IgA, IgM, and C3c were higher, but C1q was lower in the pSS group than in the control group (all P < .05). CD20 was detected in labial gland samples of all pSS patients, in which CD3 was positive in 66 (93.0%) patients, and negative in 5 (7.0%). The plasma levels of IgG, IgA, IgM, and CRP, and ESR were higher, but serum C4 level was lower in pSS patients than in the control group (all P < .01). Serum IgG level, ESR, and labial gland CD20 were higher in the high CD20 expression group than the low expression group (all P < .05).
Conclusion
Primary Sjogren's syndrome patients had a higher expression of CD20 positive infiltrating lymphocytes of the labial gland, accompanied with the changes of immunoglobulins, and complements in both the labial gland and peripheral blood.
Keywords: CD20, CD3, complement, immunoglobulin, labial gland, primary Sjogren's syndrome
1. INTRODUCTION
Primary Sjögren's Syndrome (pSS) is an autoimmune disease characterized by exocrine gland hypofunction. Many studies analyzed the prevalence and characteristics of systemic involvement in pSS.1, 2 The European League against Rheumatism (EULAR) task force on SS developed the EULAR‐SS disease activity index (ESSDAI), which includes specific organ‐by‐organ definitions. Clinically, the ESSDAI provides a reliable picture of systemic involvement in pSS.3, 4 The evaluation of systemic involvement in pSS in a cohort of 921 Spanish patients using the ESSDAI definitions showed that pSS is undeniably a systemic disease, with the joints, lungs, skin, and peripheral nerves being the most frequently involved organs. Cytopenias, hypocomplementaemia, and cryoglobulinaemia at diagnosis strongly correlated with higher cumulative ESSDAI scores in the clinical domains.5
The lacrimal gland and salivary gland are involved mostly in pSS, leading to xerophthalmia or xerostomia, accompanied with hypergammaglobulinemia and increased multiple autoantibodies.6 The gold standard for diagnosis of pSS is to confirm lymphocyte infiltration in salivary gland with histopathological examination,7 in which lacrimal gland biopsy is the most common. However, it has been debated whether T lymphocytes or B lymphocytes are predominant in the lymphocyte infiltrate in the salivary gland and whether there is a consistency on the immunophenotype of lymphocytes of the salivary gland and blood circulation.
T lymphocytes mainly mediate cellular immunity. Mature T lymphocytes, based on their surface CD markers, have been divided into CD4+ and CD8+ cell subjects; both express CD3+ marker. CD3+ is a common differentiation antigen of mature T cells, is expressed on the surface of all mature T cells, and, thus, represents total T lymphocytes in a tissue.8 CD20 antigen of human B lymphocytes is a nonglycosylated phosphoric acid protein. B cells at different developmental stages, from pre‐B cells, immature B cells, mature B cells, to activated B cells, express CD20, but plasmacytes do not express CD20.9 The main function of CD20 is to regulate activation, proliferation, and differentiation of B cells. Therefore, CD20 has been used to locate B cells.
In this study, we analyzed infiltrating lymphocytes in the labial gland and the peripheral blood of pSS patients to confirm whether there are the deposits of immunoglobulins and complements in the labial gland and whether immune impairment in the labial gland is associated with the hypergammaglobulinemia.
2. SUBJECTS AND METHODS
2.1. Subjects
The prospective study was approved by the Ethics Committee of our hospital. In the pSS group, 71 pSS patients (5 males, 66 females; and mean age 46.2 ± 13.2 years) were hospitalized in the Department of Rheumatology, General Hospital at Tianjin Medical University from August 2013 to April 2016. The patients were diagnosed according to the ACR/EULAR 2016 classification criteria for pSS.10 All patients were newly diagnosed without any previous treatment (including adrenal glucocorticoid and immunosuppressive agents). Patients with lymphoma were excluded. Informed consent was offered by each patient. In the control group, 35 patients (7 males, 28 females, and mean age 43.7 ± 8.1 years) underwent traumatically maxillofacial surgery, and the labial gland specimens were collected from 23 of them. They were confirmed not to suffer from other diseases including infectious and autoimmune diseases.
2.2. Specimen collection and patient grouping
Four milliliters of venous blood were extracted from the elbow vein of each patient at fasting. The serum samples were separated by centrifuging 1006 g for 10 minutes and kept in −70°C. Local infiltration anesthesia was performed in lower lip mucosa. The labial gland was removed by making a 2‐cm transverse incision in the location of vestibule ditch to the middle of the red line, slightly left or right. Specimens were fixed using 10% formaldehyde solution, embedded in paraffin, and sectioned with 4 μm thickness.
In the pSS group, specimens of both labial gland and serum were obtained from each pSS patient. However, in the control group, 35 serum specimens and only 23 labial gland specimens were obtained from 35 patients. The expression of CD20 in the labia gland was detected in 71 pSS patients, 66 of which also showed CD3 positive expression, and 5 of which were CD3 negative. Based on the ratio of CD20/CD3 level, the 48 patients were assigned to the high CD20 expression group, because their CD20 was higher than their CD3 level (Figures 1, 2, 3), while 23 patients were assigned to the low CD20 expression group, because 20 out of the 23 patients showed the same level of CD20 and CD3 and 3 showed their CD20 were lower than their CD3 levels (Figures 1, 2, 3). In the control group, only a few scattered lymphocytes were seen infiltrating the labial gland, and the expressions of CD3 and CD20 were not detectable.
Figure 1.
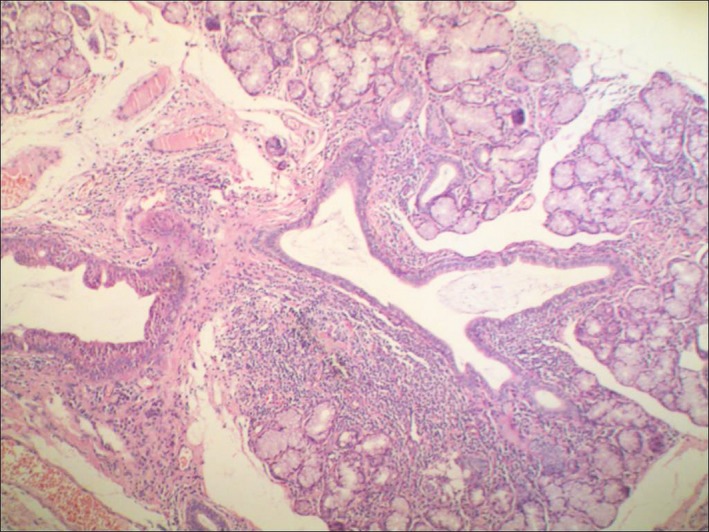
H&E staining of labial glands in PSS patients (×100): the salivary gland shows diffuse infiltration of ductal lymphoid tissue (including lymphocytes and plasma cells), irregular expansion, and peripheral acinar destruction with mild eosinophilic changes in the duct
Figure 2.
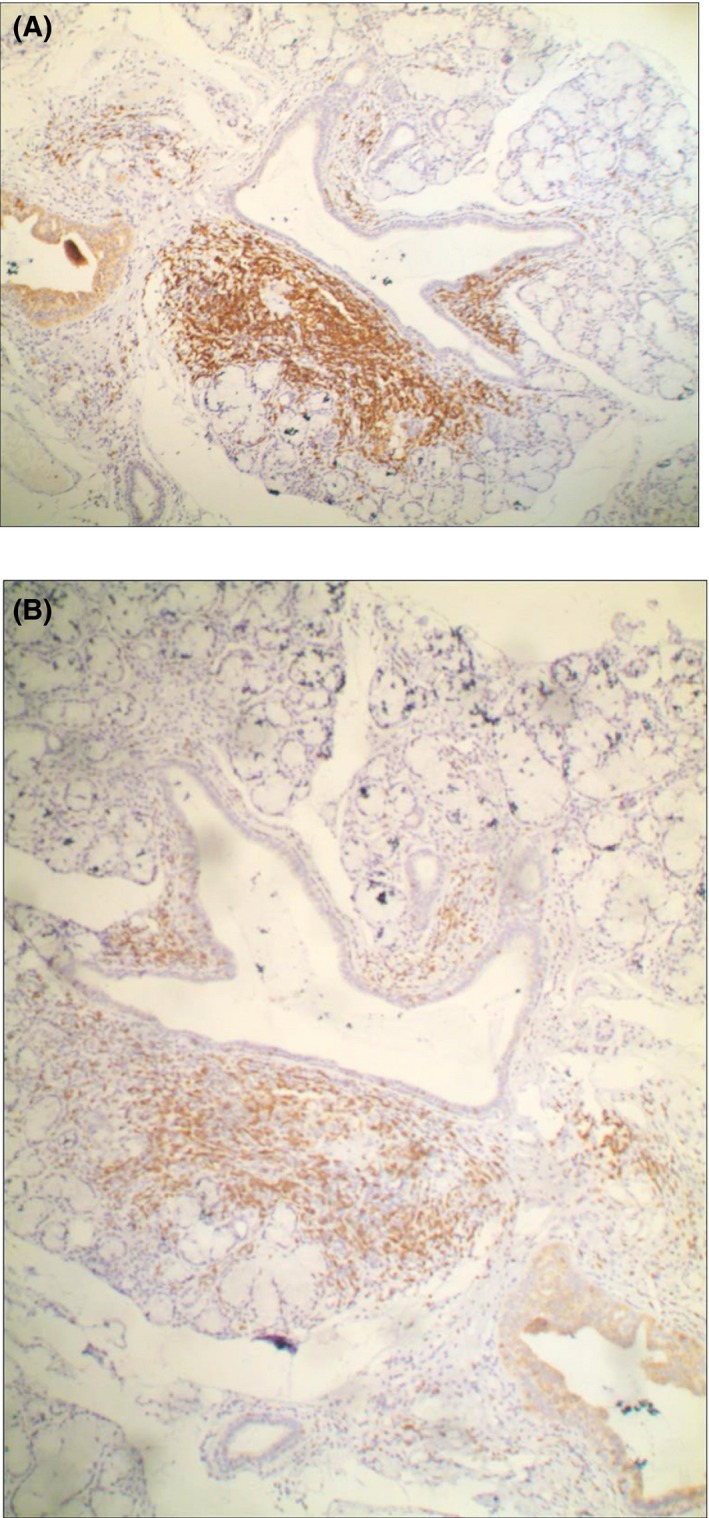
Immunohistochemical staining of labial gland in pSS patients (×100): CD20 expression of lymphocyte tissue (A, showing more brown staining) was significantly higher than CD3 expression (B, showing brown staining less than A)
Figure 3.
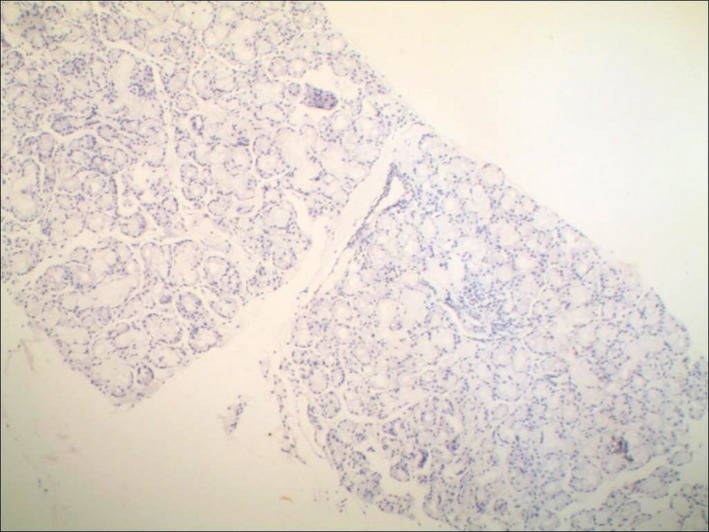
The interstitia of the labial gland in the control group showed negative immunohistochemical staining (×100): Scattered lymphocyte infiltration was found, but no positive staining for CD3 and Cd20 (brown color) was observed
2.3. Immunohistochemical staining and biochemistry measurements
The expressions of CD20, CD3, IgG, IgA, IgM, C1q, and C3c in each specimen were detected with immunohistochemical universal 2‐step method. The monoclonal antibodies of rabbit anti‐human CD20, rabbit antihuman CD3 and the polyclonal antibodies of rabbit antihuman IgG, IgA, IgM, complement C3c, and complement C1q were purchased from Beijing Zhongjin Jinqiao Biotechnology Co. (Beijing, China); PBS buffer replaced the primary antibody as a negative control; results were stained with 3,3′‐diaminobenzidine (DAB).
According to the color intensity and distribution on each slice to determine the expression volume of each target material, it was defined: no color as the negative “‐”; partial coloring/light yellow to be the weak positive “+”; diffuse coloring/brown judged as positive “++”; diffuse coloring/dark brown as the strong positive “+++”. Serum IgG, IgA, IgM, C3, C4, C‐reactive protein (CRP), and erythrocyte sedimentation rate (ESR) were measured by routine biochemistry methods.
Pathological examination was carried out by 3 experienced pathologists independently.
2.4. Statistical analysis
Continuous variables in line with the normal distribution were expressed with mean ± SD and the t test was used to compare 2 independent samples. Continuous variables which do not conform to the normal distribution were expressed with median (IQR) and the Wilcoxon two‐sample test was used to compare between the 2 groups. Countable variables between 2 groups were compared with chi‐square test. The graded data such as each immune parameter in immunohistochemical test between 2 groups were performed with Wilcoxon rank sum test. The SPSS 19.0 of Windows statistical software package was used for statistical analysis, all tests were two sided, and P < .05 meant that the difference was statistically significant.
3. RESULTS
3.1. The general clinic information of collected 71 pSS patients
We collected general clinic information from all of 71 pSS patients, including disease onset, immune serology, inflammatory markers, and blood parameters (Table 1).
Table 1.
Comparison of clinical characteristics between CD20 low expression patients and high expression patients, IQR, interquartile range
| Variables | Low CD20 expression, (n = 23) | High CD20 expression (n = 48) | Total | P |
|---|---|---|---|---|
| Age (years), mean ± SD | 48.48 ± 12.97 | 45.17 ± 13.3 | 46.24 ± 13.20 | .326 |
| RF (IU/mL), median (IQR) | 20.2 (20.0,149.0) | 162.5 (55.1,379.5) | 109.0 (20.2,323.0) | .004 |
| IL‐2 (pg/mL), median (IQR) | 61.0 (44.0,66.0) | 45.5 (38.5,59.5) | 50.0 (40.0,63.0) | .017 |
| IL‐2R (ng/L, median (IQR) | 94.0 (70.0,120.0) | 72.5 (58.0,104.0) | 80.0 (60.0,110.0) | .117 |
| IL‐6 (ng/L), median (IQR) | 12.5 (10,17.5) | 11.3 (10,12.5) | 12.5 (10.0, 12.5) | .545 |
| IL‐6R (ng/L), median (IQR) | 152.0 (110.0,201.0) | 143.5 (110.5,180.0) | 144 (110.0,187.0) | .641 |
| BAFF (ng/L), median (IQR) | 704 (451 991) | 951 (609 1240) | 906 (556 1200) | .041 |
| BAFF‐R (pg/mL), median (IQR) | 300 (260 391) | 291 (241 362) | 300 (246 373) | .465 |
| Disease course (y), median (IQR) | 2.0 (0.5,5.0) | 3.0 (1.0,6.0) | 3.0 (1.0,5.0) | .315 |
| WBC decrease, n (%) | ||||
| No | 17 (73.91) | 27 (56.25) | 44 (61.97) | .151 |
| Yes | 6 (26.09) | 21 (43.75) | 27 (38.03) | |
| Xerophthalmia, n (%) | ||||
| − | 7 (30.43) | 12 (25.00) | 19 (26.76) | .628 |
| + | 16 (69.57) | 36 (75.00) | 52 (73.24) | |
| SSB, n (%) | ||||
| − | 1 (7.14) | 4 (10.81) | 5 (9.80) | 1.000 |
| + | 13 (92.86) | 33 (89.19) | 46 (90.20) | |
| SSA, n (%) | ||||
| − | 0 (0.00) | 1 (2.17) | 1 (1.45) | 1.000 |
| + | 23 (100.0) | 45 (97.83) | 68 (98.55) | |
3.2. Comparison of lymphocyte immunophenotypes from the labial glands between the pSS and control groups
The highest grading with 3 plus signs of IgG, IgA, IgM, and C3c in the labial glands was significantly higher in the pSS group than in the control group, respectively (all P < .05). However, the highest grading with 3 plus signs of C1q levels from the labial glands was significantly lower in the pSS group than in the control group (P < .01) (Table 2).
Table 2.
Gradings of immunoglobulins and complements from labial glands of pSS and Control groups
| Immunity index | Grading | pSS group (n = 71) | Control group (n = 23) | P |
|---|---|---|---|---|
| IgG | + | 2 (2.82) | 16 (69.57) | <.001 |
| ++ | 37 (52.11) | 7 (30.43) | ||
| +++ | 32 (45.07) | 0 (0.00) | ||
| IgA | + | 14 (19.72) | 9 (39.13) | .046 |
| ++ | 21 (29.58) | 7 (30.43) | ||
| +++ | 36 (50.70) | 7 (30.43) | ||
| IgM | + | 1 (1.41) | 7 (30.43) | <.001 |
| ++ | 30 (42.25) | 16 (69.57) | ||
| +++ | 40 (56.34) | 0 (0.00) | ||
| C3c | + | 1 (1.41) | 4 (17.39) | <.001 |
| ++ | 3 (4.23) | 19 (82.61) | ||
| +++ | 67 (94.37) | 0 (0.00) | ||
| C1q | + | 14 (19.72) | 0 (0.00) | <.001 |
| ++ | 57 (80.28) | 16 (69.57) | ||
| +++ | 0 (0.00) | 7 (30.43) |
3.3. Feature comparisons of blood index between the pSS and control groups
The levels of blood IgG, IgA, IgM, ESR, and CRP were higher in the pSS group than in the control group (P < .01), the level of C4 was significantly lower in the pSS group than in the control group (P < .01), and there was a similar level of C3 between 2 groups (Table 3).
Table 3.
Levels of blood immunoglobulin, complement, ESR, and CRP in the pSS and control groups
| Immunity Index (mean ± SD) | pSS group (n = 71) | Control group (n = 35) | P |
|---|---|---|---|
| IgG (mg/dL) | 2299.2 ± 806.3 | 1156.3 ± 129.0 | <.001 |
| IgA (mg/dL) | 370.6 ± 140.1 | 238.9 ± 80.2 | <.001 |
| IgM (mg/dL) | 157.6 ± 130.5 | 92.1 ± 38.9 | .005 |
| C3 (mg/dL) | 94.1 ± 24.4 | 100.0 ± 11.8 | .345 |
| C4 (mg/dL) | 17.9 ± 6.3 | 24.9 ± 4.1 | .001 |
| ESR (mm/h) | 37.4 ± 14.1 | 14.4 ± 4.04 | <.001 |
| CRP (mg/dL) | 0.6 ± 0.9 | 0.25 ± 0.12 | .024 |
3.4. Comparisons of all measurements between the high and low CD20 expression groups
The mean age of the high CD20 expression group was 45.17 ± 13.30 years, and the lower expression group was 48.48 ± 12.97 years. The course of disease was 4.59 ± 5.48 years in the high CD20 expression group and 3.53 ± 4.61 years in the low CD20 expression group. There was no statistically significant difference on the mean age and the course of disease between 2 groups (P > .05). The other clinical characteristics between low and high CD20 expression patients were also prepared and no statistically significant difference was found (Table 1).
Unlike the patterns of lymphocyte immune phenotypes from the labial glands between the pSS and control groups, the highest grading with 3 plus signs of IgG and the second highest grading with 2 plus signs of C1q were significantly higher in the high CD20 expression group than in the low CD20 expression group (P < .01) (Figures 4 and 5). But no differences were shown on IgA, IgM, and C3c measurements between the 2 groups (Table 4).
Figure 4.
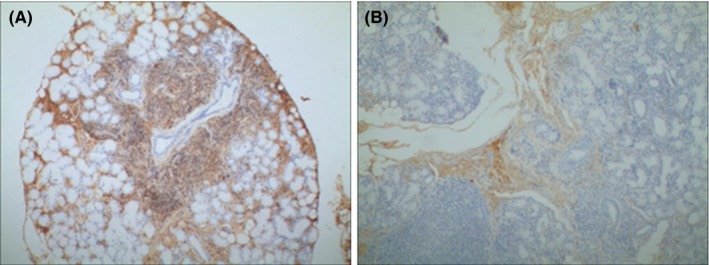
Expresison of IgG in the labial gland of pSS paitents. Immunohistochemistry showed that the IgG level was apparently higher in the high CD20 expresison group (A) than the low CD20 expresison group (B). ×100
Figure 5.
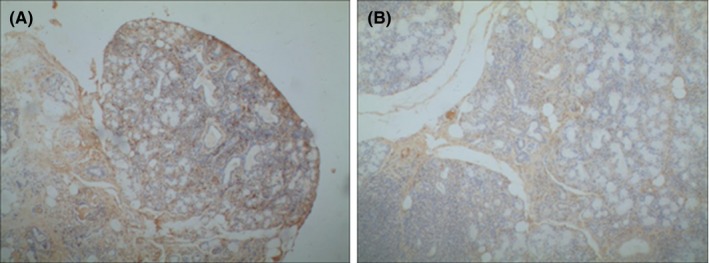
Expresison of C1q in the labial gland of pSS paitents. Immunohistochemistry showed that the C1q level was apparently higher in the high CD20 expresison group (A) than the low CD20 expresison group (B). ×100
Table 4.
Immunoglobulin and complement contents in the labial gland in the CD20 high expression group (n = 48) and the CD20 low expression group (n = 23)
| Immunity index | Grading | High CD20 expression, n (%) | Low CD20 expression, (%) | P |
|---|---|---|---|---|
| IgG | + | 0 (0.00) | 2 (8.70) | <.001 |
| ++ | 16 (33.33) | 21 (91.30) | ||
| +++ | 32 (66.67) | 0 (0.00) | ||
| IgA | + | 13 (27.08) | 1 (4.35) | .563 |
| ++ | 10 (20.83) | 11 (47.83) | ||
| +++ | 25 (52.08) | 11 (47.83) | ||
| IgM | + | 0 (0.00) | 1 (4.35) | .261 |
| ++ | 19 (39.58) | 11 (47.83) | ||
| +++ | 29 (60.42) | 11 (47.83) | ||
| C3c | + | 0 (0.00) | 1 (4.35) | .061 |
| ++ | 1 (2.08) | 2 (8.7) | ||
| +++ | 47 (97.92) | 20 (86.96) | ||
| C1q | + | 2 (4.17) | 12 (52.17) | <.001 |
| ++ | 46 (95.83) | 11 (47.83) | ||
| +++ | 0 (0.00) | 0 (0.00) |
There were no statistically significant differences in mean age and in the course of disease between 2 groups, respectively (P > .05). The levels of blood IgG and ESR were higher in high expression group than in low expression group (both P < .05). However, there was no significant difference in serum IgA, IgM, C3, C4, and CRP levels between 2 groups (Table 5).
Table 5.
Blood immunoglobulin, complement, ESR, and CRP measuring in CD20 high (n = 48) and low expression (n = 23) groups
| Immunity Index | High CD20 expression, (mean ± SD) | Low CD20 expression, (mean ± SD) | P |
|---|---|---|---|
| IgG (mg/dL) | 2497.1 ± 751.3 | 1886.1 ± 773.8 | .002 |
| IgA (mg/dL) | 375.9 ± 134.0 | 359.6 ± 154.6 | .648 |
| IgM (mg/dL) | 149.7 ± 96.8 | 174.1 ± 183.7 | .465 |
| C3 (mg/dL) | 93.6 ± 24.9 | 95.2 ± 23.8 | .807 |
| C4 (mg/dL) | 17.4 ± 6.6 | 19.0 ± 5.5 | .324 |
| ESR (mm/h) | 39.8 ± 13.3 | 32.3 ± 14.7 | .035 |
| CRP (mg/dL) | 0.61 ± 0.91 | 0.56 ± 0.81 | .823 |
4. DISCUSSION
The current study showed that pSS patients had significantly higher serum IgG, IgA, IgM, and CRP and ESR than controls, suggesting an abnormal humoral immune response in the peripheral blood of pSS patients. Moreover, patients with high CD20 expression had significantly higher levels of serum IgG and ESR than those with low CD20 expression, suggesting higher disease activity in the high CD20 expression group.
Infiltrating lymphocytes in the lower lip salivary gland of pSS patients include T cells, B cells, and plasma cells, with activated CD4+ T cells predominantly in the early stage of the disease.11, 12 It has been shown that the infiltrating lymphocytes in the salivary glands of SS patients are mainly CD3+ cells, with a ratio of CD4+ T cells:CD8+ T cells of 2:1.13 Subsequent studies suggested that pSS also exhibits features of B cell activation, as demonstrated by formation of various autoantibodies.
Chistodoulou et al. reported that B cell incidence was positively correlated with infiltration grade and biopsy focus score.14 CD20+ B lymphocytes were found in the infiltrating lymphocytes of small salivary glands in pSS patients,15 including autoimmune B cells.16 Jin et al. found that CD4(+)CXCR5(+) Tfh cells were significantly increased in the salivary gland and peripheral blood of pSS patients, with aberrant CD19(+)CD27(+) memory B cells and CD19(+)CD27(high) plasma cells, suggesting that CD4(+)CXCR5(+) Tfh cells may contribute to the pathogenesis of pSS by promoting maturation of B cells.17 These findings may explain the higher levels of IgG, IgA, and IgM in the labial gland and serum of pSS patients in the current study. Therefore, Tfh cells may be a potential prognostic marker for pSS patients.
Our results differ somewhat from the previous literature. CD20 antigen was present in all labial gland samples from 71 pSS patients; 66 of them were CD3 positive, and only 5 patients were CD3 negative. The fact that labial gland infiltrating lymphocytes in pSS patients primarily expressed CD20 may be due to severity of hospitalized patients. Pijpe et al. showed that parotid lymphocyte infiltration was significantly reduced at 12 weeks after rituximab treatment, with a decreased ratio of B:T cells.18 Thus, the infiltration of lymphocytes in the glands of pSS patients may be a dynamic process, with different cells involved at different disease stages. CD4+ T cells may be predominant in mildly damaged glands, but infiltrating inflammatory cells in severely damaged glands are mainly B cells. Therefore, the analysis of lymphocyte subsets in pSS labial gland specimens will help to understand disease development. This result is consistent with the study of the EULAR Sjogren's syndrome group, suggesting that CD3 and CD20 staining of lip glands should be performed in clinical studies and the ratio of B/T cells in lymphocytic infiltrate should also be delineated.19
Ectopic germinal center was present in the exocrine glands of 25% pSS patients.20 A study showed that only 8 out of 54 healthy persons had focal lymphocytic infiltration, salivary gland inflammation, and FS ≥ 1, but they did not have xerophthalmia and xerostomia.21 Therefore, the level of lymphocyte infiltration in pSS exocrine glands cannot directly reflect the decline of glandular function. We also found no significant difference in extraglandular manifestations of pSS patients in the high and low CD20 expression groups in terms of xerophthalmia and leukocyte reduction. However, we observed that pSS patients had significantly higher levels of IgG, IgM, and IgA in the labial gland than in the control patients; noticeably, pSS patients with high CD20 expression had appreciably higher IgG levels than those with low CD20 expression. The result indicated that not only lymphocyte infiltration but also immunoglobulin deposition was present in the exocrine glands of pSS patients, which may indicate that IgG predominant pSS is severe.
C3 has been shown to play a role in the occurrence and development of Sjogren's syndrome, especially in early SS. We found that the level of serum C4 was lower in the pSS group than the control group. Furthermore, the C3 level in the labial gland was higher in the pSS group than the control group. Clq is the initiating factor of the classical complement activation pathway. We found that C1q level was lower in the pSS group than the control group, suggesting that complement activation in pSS patients may proceed via the alternative activation pathway. Notably, patients with high CD20 expression had higher C1q levels than patients with low CD20 expression, suggesting that the classic pathway may also cause tissue injuries when the infiltrating lymphocytes are predominantly CD20 positive cells. In severe pSS, the classic and bypass pathways may be activated simultaneously in the labial glands. Immunoglobulin codeposition may activate the classical complement pathway and contribute to immune damage in labial gland.
A study of 921 pSS patients using the ESSDAI5 showed that cytopenias, hypocomplementaemia, and cryoglobulinaemia at diagnosis strongly correlated with higher cumulative ESSDAI scores in the clinical domains. The data from our current research not only match the ESSDAI but also provide more detailed information on hypocomplementaemia and cryoglobulinaemia to complement these diagnostic criteria. This result is consistent with the study of the EULAR Sjogren's syndrome group, which suggests that CD3 and CD20 staining of lip glands should be performed in clinical studies and the ratio of B/T cells in lymphocytic infiltrate should also be reported. Brito‐Zeron et al. thought that the main prognostic factors for an adverse outcome of pSS were vasculitis, severe involvement in parotid scintigraphy, hypocomplementaemia, and/or cryoglobulins at diagnosis. Patients with at least 2 of these factors need a closer follow‐up.22 Therefore, based on the current result, it is necessary to follow up the CD20 expression in infiltrating lymphocytes in the labial gland, as well as changes of immunoglobulins and complements in both the labial gland and peripheral blood of pSS patients.
We also noted that patients with high CD20 expression had significantly lower levels of serum IL‐2 and IL‐2R than those with low CD20 expression, while IL‐6 and IL‐6R levels were comparable between the 2 groups. Tishler et al. found that SS patients had significantly higher salivary levels of IL‐2R than dry mouth syndrome patients but noted no significant difference in serum levels of IL‐2R between the 2 groups.23 Tomas et al. also showed that SS patients had significantly higher serum IL‐2R levels, but they found no correlation of serum IL‐2R levels with clinical activities of SS.24 Akiyama et al. have recently shown that serum IL‐2R levels correlated with ESSDAI scores and the number of affected organs in pSS.25 The significance of sera IL‐2 and IL‐2R levels in pSS patients remains to be further defined.
In conclusion, both CD3+ T lymphocytes and CD20+ B lymphocytes were found in the labial gland of pSS patients and CD20+ B lymphocytes may be predominant in patients with a longer disease course. Furthermore, deposits of immunoglobulin and complement were present in the labial glands of pSS patients.
CONFLICT OF INTEREST
All the authors declare that they have no conflict of interest.
Sun W, Zhang N, Zhang Y, Shao Z, Gong L, Wei W. Immunophenotypes and clinical features of lymphocytes in the labial gland of primary Sjogren's syndrome patients. J Clin Lab Anal. 2018;32:e22585 10.1002/jcla.22585
REFERENCES
- 1. Friedman JA, Miller EB, Green L, Huszar M, Schattner A. A community‐based cohort of 201 consecutive patients with primary Sjogren's syndrome in Israel: Ashkenazi patients compared with those of Sephardic descent. Clin Exp Rheumatol. 2006;24:274‐280. [PubMed] [Google Scholar]
- 2. Ioannidis JP, Vassiliou VA, Moutsopoulos HM. Long‐term risk of mortality and lymphoproliferative disease and predictive classification of primary Sjogren's syndrome. Arthritis Rheum. 2002;46:741‐747. [DOI] [PubMed] [Google Scholar]
- 3. Seror R, Bootsma H, Bowman SJ, et al. Outcome measures for primary Sjogren's syndrome. J Autoimmun. 2012;39:97‐102. [DOI] [PubMed] [Google Scholar]
- 4. Seror R, Ravaud P, Bowman SJ, et al., Force ESsT . EULAR Sjogren's syndrome disease activity index: development of a consensus systemic disease activity index for primary Sjogren's syndrome. Ann Rheum Dis 2010;69:1103‐1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ramos‐Casals M, Brito‐Zeron P, Solans R, et al., Autoimmune Diseases Study Group of the Spanish Society of Internal M . Systemic involvement in primary Sjogren's syndrome evaluated by the EULAR‐SS disease activity index: analysis of 921 Spanish patients (GEAS‐SS Registry). Rheumatology (Oxford) 2014;53:321‐331. [DOI] [PubMed] [Google Scholar]
- 6. Fox RI, Michelson P. Approaches to the treatment of Sjogren's syndrome. J Rheumatol Suppl. 2000;61:15‐21. [PubMed] [Google Scholar]
- 7. Barone F, Campos J, Bowman S, Fisher BA. The value of histopathological examination of salivary gland biopsies in diagnosis, prognosis and treatment of Sjogren's Syndrome. Swiss Med Wkly. 2015;145:w14168. [DOI] [PubMed] [Google Scholar]
- 8. Choi BD, Cai M, Bigner DD, Mehta AI, Kuan CT, Sampson JH. Bispecific antibodies engage T cells for antitumor immunotherapy. Expert Opin Biol Ther. 2011;11:843‐853. [DOI] [PubMed] [Google Scholar]
- 9. Mason DY, Stein H, Gerdes J, et al. Value of monoclonal anti‐CD22 (p135) antibodies for the detection of normal and neoplastic B lymphoid cells. Blood. 1987;69:836‐840. [PubMed] [Google Scholar]
- 10. Shiboski CH, Shiboski SC, Seror R, et al., International Sjogren's Syndrome Criteria Working G . 2016 American College of Rheumatology/European League Against Rheumatism Classification Criteria for Primary Sjogren's Syndrome: a consensus and data‐driven methodology involving three International Patient Cohorts. Arthritis Rheumatol 2017;69:35‐45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Larsson A, Bredberg A, Henriksson G, Manthorpe R, Sallmyr A. Immunohistochemistry of the B‐cell component in lower lip salivary glands of Sjogren's syndrome and healthy subjects. Scand J Immunol. 2005;61:98‐107. [DOI] [PubMed] [Google Scholar]
- 12. vonBultzingslowen I , Sollecito TP, Fox PC, et al. (2007) Salivary dysfunction associated with systemic diseases: systematic review and clinical management recommendations. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 103 (Suppl S57) e51‐15. [DOI] [PubMed] [Google Scholar]
- 13. Coll J, Gambus G, Corominas J, Tomas S, Esteban JI, Guardia J. Immunohistochemistry of minor salivary gland biopsy specimens from patients with Sjogren's syndrome with and without hepatitis C virus infection. Ann Rheum Dis. 1997;56:390‐392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Christodoulou MI, Kapsogeorgou EK, Moutsopoulos HM. Characteristics of the minor salivary gland infiltrates in Sjogren's syndrome. J Autoimmun. 2010;34:400‐407. [DOI] [PubMed] [Google Scholar]
- 15. Groom J, Kalled SL, Cutler AH, et al. Association of BAFF/BLyS overexpression and altered B cell differentiation with Sjogren's syndrome. J Clin Invest. 2002;109:59‐68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Le Pottier L, Devauchelle V, Fautrel A, et al. Ectopic germinal centers are rare in Sjogren's syndrome salivary glands and do not exclude autoreactive B cells. J Immunol. 2009;182:3540‐3547. [DOI] [PubMed] [Google Scholar]
- 17. Jin L, Yu D, Li X, et al. CD4 + CXCR5 + follicular helper T cells in salivary gland promote B cells maturation in patients with primary Sjogren's syndrome. Int J Clin Exp Pathol. 2014;7:1988‐1996. [PMC free article] [PubMed] [Google Scholar]
- 18. Pijpe J, Meijer JM, Bootsma H, et al. Clinical and histologic evidence of salivary gland restoration supports the efficacy of rituximab treatment in Sjogren's syndrome. Arthritis Rheum. 2009;60:3251‐3256. [DOI] [PubMed] [Google Scholar]
- 19. Fisher BA, Jonsson R, Daniels T, et al., Sjogren's histopathology workshop group from E ; (2016) Standardisation of labial salivary gland histopathology in clinical trials in primary Sjogren's syndrome. Ann Rheum Dis 2017;76:1161‐1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hamel KM, Liarski VM, Clark MR. Germinal center B‐cells. Autoimmunity. 2012;45:333‐347. [DOI] [PubMed] [Google Scholar]
- 21. Radfar L, Kleiner DE, Fox PC, Pillemer SR. Prevalence and clinical significance of lymphocytic foci in minor salivary glands of healthy volunteers. Arthritis Rheum. 2002;47:520‐524. [DOI] [PubMed] [Google Scholar]
- 22. Brito‐Zeron P, Ramos‐Casals M, Bove A, Sentis J, Font J. Predicting adverse outcomes in primary Sjogren's syndrome: identification of prognostic factors. Rheumatology (Oxford). 2007;46:1359‐1362. [DOI] [PubMed] [Google Scholar]
- 23. Tishler M, Yaron I, Shirazi I, Levartovsky D, Yaron M. Salivary and serum soluble interleukin‐2 receptor in primary Sjogren's syndrome. Arch Oral Biol. 1999;44:305‐308. [DOI] [PubMed] [Google Scholar]
- 24. Tomas S, Coll J, Palazon X. Soluble interleukin‐2 receptor in primary and secondary Sjogren's syndrome. Br J Rheumatol. 1997;36:194‐197. [DOI] [PubMed] [Google Scholar]
- 25. Akiyama M, Sasaki T, Kaneko Y, et al. (2018) Serum soluble interleukin‐2 receptor is a useful biomarker for disease activity but not for differential diagnosis in IgG4‐related disease and primary Sjogren's syndrome adults from a defined population. Clin Exp Rheumatol [Epub ahead of print]. [PubMed] [Google Scholar]


