Abstract
Intravenous arsenic trioxide (ATO) has been adopted as the first‐line treatment for acute promyelocytic leukemia (APL). Another arsenic compound named the Realgar‐Indigo naturalis formula (RIF), an oral traditional Chinese medicine containing As4S4, has been shown to be highly effective in treating adult APL. In the treatment of pediatric APL, the safety and efficacy of RIF remains to be confirmed. This randomized, multicenter, and noninferiority trial was conducted to determine whether intravenous ATO can be substituted by oral RIF in the treatment of pediatric APL. From September 2011 to January 2017, among 92 patients who were 16 years old or younger with newly diagnosed PML‐RARa positive APL, 82 met eligible criteria and were randomly assigned to ATO (n = 42) or RIF (n = 40) group. The remaining 10 patients did not fulfilled eligible criteria because five did not accept randomization, four died and one had hemiplegia prior to arsenic randomization due to intracranial hemorrhage or cerebral thrombosis. Induction and consolidation treatment contained ATO or RIF, all‐trans‐retinoic acid and low intensity chemotherapy. End points included event‐free survival (EFS), adverse events and hospital days. After a median 3‐year follow‐up, the estimated 5‐year EFS was 100% in both groups, and adverse events were mild. However, patients in the RIF group had significantly less hospital stay than those in the ATO group. This interim analysis shows that oral RIF is as effective and safe as intravenous ATO for the treatment of pediatric APL, with the advantage of reducing hospital stay. Final trial analysis will reveal mature outcome data.
1. INTRODUCTION
Acute promyelocytic leukemia (APL) has become a highly curable disease with contemporary treatment. With the advent of arsenic trioxide (ATO), the long‐term survival rate of APL in all risk groups is exceeding 90% with combination treatment with ATO, all‐trans‐retinoic acid (ATRA) and anthracycline‐based chemotherapy (CHT).1, 2, 3, 4, 5, 6, 7, 8, 9 Moreover, ATO and ATRA‐based therapy can abrogate the negative prognostic impacts of FLT3‐ITD mutations and high initial white blood cell (WBC) count.6, 7, 8, 9, 10, 11
However, ATO is administered intravenously and patients need long hospital stay. Besides ATO, another arsenic compound, As4S4, has recently been proved highly effective in the treatment of adult APL. The Realgar‐Indigo naturalis formula (RIF), a traditional Chinese medicine which can be taken orally, contains realgar (As4S4) as well as Indigo naturalis, Radix salviae miltiorrhizae and Radix pseudostellariae.12 The components of RIF yield synergy anti‐leukemia effects in murine APL model in vivo and an APL cell line in vitro.12 A multicenter clinical trial in China has confirmed the efficacy and safety of RIF in adults with APL.13 More recently, a multicenter study conducted by the Chinese APL Cooperative Group demonstrated the noninferiority of RIF and ATO when in combination with ATRA and CHT in terms of efficacy and safety in adult patients,14 and the estimated event‐free survival (EFS) rates were similar between the two groups after long‐term follow‐up of 7 years (93.7% vs 89.4%, P = .37).5 Moreover, oral RIF can be administered outside the hospital, reducing the number of hospital days compared to intravenous ATO.15, 16
There is limited data on the role of RIF in the treatment of pediatric patients. Some small retrospectively studies including ours suggested that RIF is also safe and effective in children with APL,17, 18 with less cardiac toxicity compared to ATO.18 These, however, remain to be confirmed. South China Children Leukemia Group (SCCLG) conducted a randomized study to compare the efficacy, safety and the number of hospital days between RIF‐ and ATO‐based therapies for treating pediatric APL. The ultimate aim of the study is to determine whether intravenous ATO can be substituted by oral RIF in the treatment of pediatric APL.
2. PATIENTS AND METHODS
2.1. Study design
The SCCLG‐APL study was a prospective, randomized, multicenter, open‐label, noninferiority trial. It was designed to show that the combination of RIF plus ATRA and CHT is not inferior to ATO plus ATRA and CHT in terms of EFS at 5 years. The study was started in September 2011 and included nine participating hospitals, and was joined by another five hospitals thereafter until the deadline in September 2016 for enrolling new hospital in the study. The trial was retrospectively registered in February 2014 at http://www.clinicaltrials.gov as NCT02200978.
Eligible patients were 16 years old or younger with newly diagnosed APL with confirmation of PML‐RARa by RT‐PCR assay ± fluorescence in situ hybridization (FISH), and randomly assigned by computer‐generated codes to ATO or RIF group. The excluded patients were those who had one of the following events occurring before genetic diagnosis and randomization: death from any cause, or coma, convulsion, paralysis due to intracranial hemorrhage, cerebral thrombosis or central nervous system leukemia; or who had prolonged QT syndrome because of the risk of QT interval prolongation during arsenic therapy; or who did not accept randomization. The study received institutional review board approval. Patients' families and/or patients provided informed consent in accordance with the Declaration of Helsinki.
The primary end point was the rate of EFS at 5 years. Secondary end points were safety and the number of accumulated hospital days during induction and consolidation therapy.
2.2. Treatment protocol
The details of treatment protocols are shown in Figure 1. Patients were treated with a risk‐adapted protocol. In particular, patients received oral ATRA if they were diagnosed with APL based on morphologic features according to the FAB criteria. Mitoxantrone was administrated on day 3 (10 mg/m2) or on days 2‐4 (7 mg/m2/day) of ATRA treatment for non‐high‐risk (diagnostic WBC count ≤10 × 109/L) or high‐risk (diagnostic WBC count >10 × 109/L) patients respectively. When the diagnosis was genetically confirmed (5‐6 days later from the day of morphological diagnosis), Patients were randomly assigned by computer‐generated codes to ATO or RIF group. For those assigned to the ATO group, ATO was administrated at a dose of 0.16 mg/kg/day (≯10 mg/day) intravenously over 12 hours because longer time infusion of ATO reduces differentiation and promotes apoptosis of APL cells.19 Patients who achieved hematologic complete remission (HCR) or HCR with incomplete platelet recovery (HCRp)20, 21 received three courses of consolidation therapy containing ATRA, ATO and low‐intensity chemotherapy with mitoxantrone, and addition of cytarabine for high‐risk patients. Intrathecal injection of cytarabine and dexamethasone was administrated on day one of each consolidation therapy (Figure 1, Supporting Information Table S1). Patients assigned to the RIF group received the same treatment as those assigned to the ATO group, but ATO was replaced by RIF (270 mg per pill) which contains Realgar (30 mg per pill), Indigo naturalis (125 mg per pill), Radix salviae miltiorrhizae (50 mg per pill), Radix pseudostellariae (45 mg per pill), and garment film (20 mg per pill). RIF was given at a dose of 135 mg/kg/day (≯30 pills/day) orally three times daily.
Figure 1.
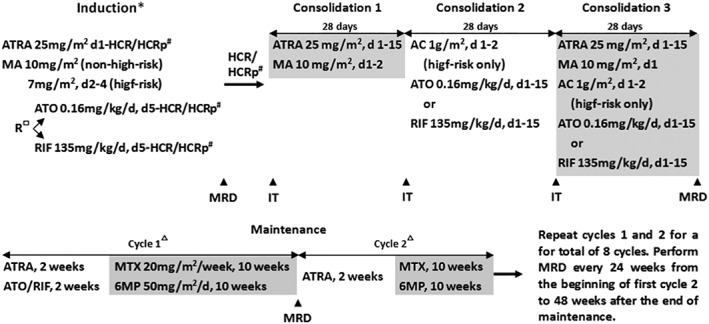
The SCCLG‐APL regimen. Abbreviations: ATRA, all‐trans retinoic acid; MA, mitoxantrone; R, randomization; ATO, arsenic trioxide; RIF, Realgar‐Indigo naturalis formula; HCR, haematologic complete remission; HCRp, HCR with incomplete platelet recovery; AC, cytarabine; MRD, minimal residual disease; IT, intrathecal injection of cytarabine and dexamethasone; MTX, methotrexate; 6MP, 6‐mercaptopurine. *Patients received ATRA if morphologically diagnosed with APL. #Bone marrow examination is considered for evaluating the achievement of HCR or HCRp when no leukemic cell was found in peripheral blood. □Patients were randomly assigned to ATO or RIF group when the diagnosis was genetically confirmed (5‐6 days later), and then ATO or RIF was started and ATRA was continued until HCR/HCRp or for a maximum of 42 days. ▵The doses of 6MP and MTX were adjusted to maintain the WBC of patients between 2 and 3.5 × 109/L
RT‐PCR for determining PML‐RARa expression was performed for genetic diagnosis of APL and monitoring of minimal residual disease after treatment (Figure 1).
2.3. Supportive therapy
At the initiation of and during induction therapy, when patients' WBC count was over 10 × 109/L, hydroxyurea (100 mg/kg/day) was administered until WBC <10 × 109/L. Dexamethasone (0.3 mg/kg/day) was given if differentiation syndrome or ATRA‐associated pseudotumor cerebri was suspected. The use of heparin or low‐molecular weight heparins for management of coagulopathy was not mandatory but encouraged because ATRA might increase thrombosis risk.22, 23 Heparin was given at a low dose of 0.5‐1 mg/kg intravenously for 24 hours daily if indicated until the coagulopathy resolved.
Transfusions of platelet, and fresh‐frozen plasma, cryoprecipitate and/or human fibrinogen, were given for the aims of maintaining platelet counts greater than 30 × 109/L, and fibrinogen greater than 1.5 g/L, respectively.
2.4. Statistical analysis
The Kaplan‐Meier method was used to estimate overall survival (OS) (time from date of randomization to death) and EFS (time from date of randomization to last follow‐up or first event including failure to achieve HCR/HCRp, hematologic and/or molecular relapse including extramedullary disease infiltrates, secondary malignancy or death of any cause). Patients lost to follow‐up were censored at their date of last known contact. The significance of predictor variables was tested by the log‐rank statistic for OS and EFS. Mean ± SD was used to describe normally distributed variables, while skewed variables were expressed as median (range). The two independent samples t test and Mann‐Whitney U test were used for normally distributed data and skewed data to comparing between groups separately. The comparison between categorical variables was evaluated by the Chi‐square (χ2) test. All statistical analyses were conducted using IBM SPSS statistics 21.0.
The primary endpoint was the 5‐year EFS. Assuming a 95% rate of EFS for patients with APL treated on arsenic‐ATRA‐CHT protocol,1, 14 a noninferiority margin of −10%, 5% type I error (onesided) and 90% power, 82 evaluable patients per group (164 in total) were required to draw a noninferiority conclusion. Noninferiority was to be concluded if the lower limit of the 95% CI for the rate difference of EFS was greater than −10% noninferiority margin. Analysis was per protocol. Interim analysis of the results was to be performed after enrolling 50% of evaluable patients treated for at least 1 year.
3. RESULTS
3.1. Enrollment and patient characteristics
The enrollment period of this interim study was from September 2011 to January 2017. The present analysis was performed in February 2017. A total of 94 patients were morphologically diagnosed with APL, of whom 12 were excluded. The excluded patients included two who were negative for PML‐RARa, five who did not accept randomization, and five who met the exclusion criteria because they had one of the following events occurring before genetic diagnosis and randomization: death in four due to intracranial hemorrhage and left hemiplegia in one because of cerebral thrombosis. There were 82 eligible patients, of whom 42 were assigned to ATO group and 40 assigned to RIF group (Figure 2). The main demographic, clinical, and laboratory features of the 82 patients are shown in Table 1. There were no significant differences in the baseline characteristics between the two groups except for a lower median age in the ATO group than that in the RIF group (7.8 vs 9.9 years, P = .028).
Figure 2.
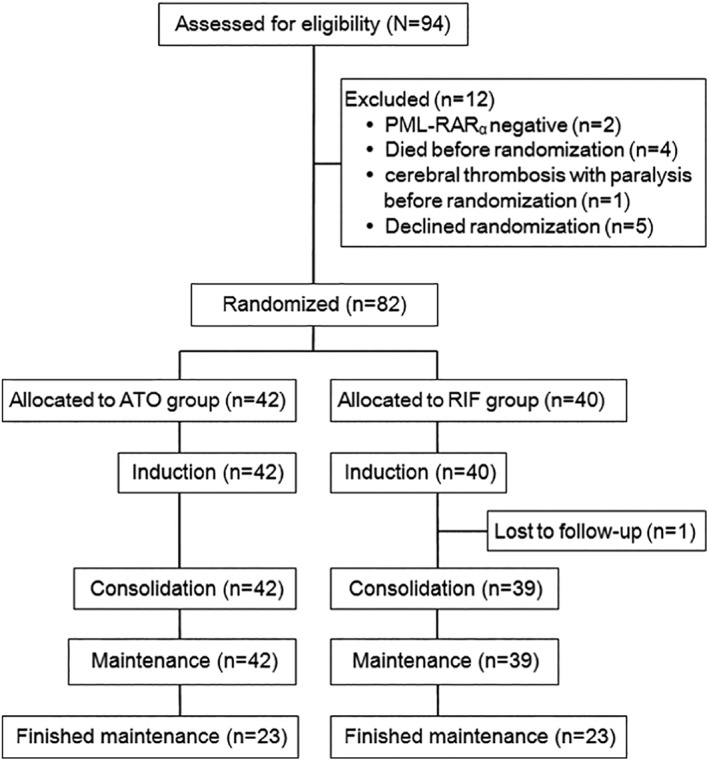
CONSORT diagram of enrolled patients. ATO, arsenic trioxide; RIF, Realgar‐Indigo naturalis formula
Table 1.
Patient characteristics
| Characteristic | Total | ATO group | RIF group | P |
|---|---|---|---|---|
| n = 83 | n = 42 | n = 40 | ||
| Median age, years (range) | 9.4 (1‐16) | 7.8 (1‐13) | 9.9 (2.1‐16) | .028 |
| Sex, n (%) | ||||
| Male | 51 (62.2) | 29 (69.0) | 22(55.0) | .190 |
| Female | 31 (37.8) | 13 (31.0) | 18 (45.0) | |
| Median WBC, ×109/L (range) | 5.0 (0.3‐227.9) | 5.6 (0.3‐227.9) | 3.6 (1.4‐48.0) | .180 |
| Median platelets, ×109/L (range) | 19 (4‐226) | 23 (4‐82) | 17 (4‐226) | .989 |
| Sanz risk, n (%) | ||||
| Non‐high‐risk | 60 (73.2) | 28 (66.7) | 32 (80.0) | .173 |
| High‐risk | 22 (26.8) | 14 (33.3) | 8 (20.0) |
Abbreviations: ATO, arsenic trioxide; ATRA, all‐trans‐retinoic acid.
3.2. Response data
All of the 82 eligible patients achieved HCR/HCRp after induction therapy. The median time to HCR/HCRp was 22.0 (range, 10.0‐42.0) days in the ATO group and 24.5 (range, 14.0‐46.0) days in the RIF group (P = .168). One patient with non‐high‐risk APL in the RIF group did not comply with the medical order and came back late for bone marrow examination on day 46 of induction therapy, and later was withdrawn from the study in consolidation phase and lost to follow‐up. The remaining 81 patients, 42 in the ATO group and 39 in the RIF group, achieved molecular remission (PML‐RARa not detected by RT‐PCR) at the end of consolidation.
For the total of 82 eligible patients (including the one who was withdrawn from the study in consolidation phase), the median follow‐up was 3.0 (range, 0.5‐6.4) years. The estimated 5‐year OS and EFS rates were 100% in both the two groups (Figure S1).
3.3. Toxicity data
Treatment‐related adverse effects are listed in Table 2. Toxic effects were graded according to the National Cancer Institute's Common Toxicity Criteria. Although mitoxantrone was given at a dose of 10 mg/m2 for 1 day to non‐high‐risk patients and 7 mg/m2 for 3 days to high‐risk patients during induction therapy, 22 (36.7%) of the non‐high‐risk patients, 10 (35.7%) in the ATO and 12 (37.5%) in the RIF groups (P = .886), developed leukocytosis greater than 10 × 109/L; and 10 (45.5%) of the high‐risk patients, 6 (42.9%) in the ATO and 4 (50.0%) in the RIF groups (P = .746), experienced a WBC count increase by more than 30% as compared with that at diagnosis, respectively. The leukocytosis was successfully managed in all patients as per protocol recommendation, and the incidence of differentiation syndrome, including moderate and severe forms,24 was low in our cohort. The incidence rates of infection, fever of unknown origin and headache were lower in the RIF group than in the ATO group. In addition, coagulopathy events newly occurring during induction phase were not commonly seen in the enrolled patients (Table 2, Supporting Information Table S2).
Table 2.
Toxicity profile
| Toxicity | High‐risk, n = 22 | Non‐high‐risk, n = 60a | ||||
|---|---|---|---|---|---|---|
| ATO group | RIF group | ATO group | RIF group | |||
| n = 14 | n = 8 | P | n = 28 | n = 32a | P | |
| During induction, n (%) | ||||||
| Liver (grade 3‐4) | 0 (0) | 0 (0) | 1 (3.6) | 2 (6.3) | .635 | |
| Renal (grade 3‐4) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | ||
| Cardiac (grade 3‐4) | 1 (7.1) | 0 (0) | .439 | 0 (0) | 0 (0) | |
| Infection and FUO | 14 (100) | 7 (87.5) | .176 | 17 (60.7) | 19 (59.4) | .916 |
| Headache | 3 (21.4) | 3 (37.5) | .416 | 12 (42.9) | 13 (40.6) | .816 |
| Differentiation syndrome | ||||||
| Moderate | 2 (14.3) | 1 (12.5 | .907 | 1 (3.6) | 0 (0) | .218 |
| Severe | 1 (7.1) | 0 (0) | .493 | 0 (0) | 0 (0) | |
| Coagulopathy events | ||||||
| Hemorrhage | 1 (7.1) | 0 (0) | .412 | 1 (3.6) | 4 (12.9) | .199 |
| Thrombosis | 0 (0) | 0 (0) | 0 (0) | 0 (0) | ||
| During consolidation, n (%) | ||||||
| Liver (grade 3–4) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | ||
| Renal (grade 3–4) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | ||
| Cardiac (grade 3‐4) | 0 (0) | 0 (0) | 1 (3.6) | 0 (0) | .289 | |
| Infection and FUO | 11 (78.6) | 3 (37.5) | .054 | 14 (50.0) | 7 (22.6) | .028 |
| Headache | 8 (57.1) | 1 (12.5 | 0.040 | 7 (25.0) | 5 (16.1) | .398 |
Abbreviations: ATO, arsenic trioxide; ATRA, all‐trans‐retinoic acid; FUO, fever of unknown origin.
One non‐high‐risk patient in the RIF group was withdrawn from the study in consolidation phase and lost to follow‐up.
3.4. Hospital days
The mean accumulated days required for inpatient management during induction and consolidation therapy was 67.8 ± 22.4 and 43.9 ± 19.3 for non‐high‐risk patients in the ATO and RIF groups (P = .000), and 68.1 ± 19.6 and 48.1 ± 18.6 for high‐risk patients in the ATO and RIF groups (P = .029), respectively (Supporting Information Table S2). As expected, peripherally inserted central catheter line was placed less frequently in patients in the RIF group than in the ATO group (22.5% vs 92.9% (P = .000).
4. DISCUSSION
We here present our findings from a randomized clinical trial comparing the efficacy and safety of oral RIF and intravenous ATO in children with APL in all risk groups. After a median follow‐up of 3 years, this interim analysis shows that the estimated rate of EFS and OS at 5 years is not deferent between the pediatric patients in the two groups, when RIF/ATO is used in combination with ATRA and low‐intensity chemotherapy.
The rates of treatment‐related adverse events were similar in the two groups, except for the incidences of infection and fever of unknown origin which were significantly lower in the RIF group. RIF can be taken orally in an outpatient setting when the disease was stable, which decrease the risk of cross infection in hospital and iatrogenic infection due to PICC line placement, all together resulting in shorter hospital stays.
There is no difficulty encountered with children taking RIF. For young children to make RIF easier to be taken, the pill is triturated with water, and with addition of sugar if necessary because RIF tastes bland with a little bit of bitter if triturated.
To our knowledge, only one study group (Chinese APL Cooperative Group) has reported prospective studies investigating the role of RIF in adult APL till now.5, 14, 15, 16 In one of the studies, 20 adults with non‐high‐risk APL were treated with a chemotherapy‐free protocol with oral RIF and ATRA and remained molecular remission after 14‐month median follow‐up, and half of the patients completed induction therapy in an outpatient setting.15 This “largely home‐based treatment protocol” may be a promising new treatment approach for non‐high‐risk APL, although a long‐term observation in a larger number of patients is still needed. In a randomized clinical trial conducted by the same study group,14 adults with all risk APL (except those with WBC > 50 × 109/L) received ATRA with ATO or RIF for induction and maintenance therapies, between which patients received three sequential courses of intensive chemotherapy containing homoharringtonine, cytarabine, daunorubicin, and mitoxantrone for consolidation. Both the rates of adverse events and estimated EFS at 7 years (93.7% vs 89.4%, P = .37) were similar between the two groups.5
Although APL in children shares many features with APL in adults, there are important distinctions between these age groups, and the efficacy and safety data from clinical trial restricted to adult patients needs to be proven in children.25 There have been two reports including ours, which are small retrospective studies,17, 18 demonstrating the treatment role of RIF in children with APL and suggesting RIF was effective with mild toxicity or possibly less cardiac toxicity compared to ATO. The present prospective and randomized study demonstrates that RIF and ATO have similar efficacy in the treatment of childhood APL, with less infections in the RIF group.
The Chinese APL Cooperative Group mentioned above used a uniform protocol containing ATO or RIF for all risk groups of adult patients and achieved outstanding outcomes.5, 14 However, that treatment protocol also contains three sequential courses of post‐remission consolidation with intensive chemotherapy. It is concerned that the non‐high‐risk patients, especially the elderly, may received excessive chemotherapy when treated with a non‐risk‐adapted protocol. In the present study, we used a risk‐adapted protocol for the treatment of childhood APL. Both the non‐high‐risk and high‐risk children received low cumulative doses of mitoxantrone (40 mg/m2 vs 51 mg/m2; in terms of daunorubicin,26 200 mg/m2 vs 255 mg/m2). Only the high‐risk children received additional short course of intermediate‐dose cytarabine (1 g/m2, q12h for 2 days) during consolidations 2 and 3, respectively. We also obtained an excellent survival rate in our cohort.
The issue of how best to treat patients with APL remains to be answered. Non‐high‐risk APL in adults has been recently reported to be successful treated by a chemotherapy‐free combination of ATRA and arsenic compound (RIF or ATO).2, 15, 27 However, there is concern that the use of the two differentiating agents without chemotherapy may result in an increasing risk of leukocytosis and differentiation syndrome.28 In fact, 35%‐47% of non‐high‐risk adult patients treated on that chemotherapy‐free induction therapy developed leukocytosis (>10 × 109/L),15, 27, 29 compared with 24% of those on ATRA‐idarubicin induction.28 In pediatric counterpart, the risk of leukocytosis may be much higher and the incidence was 84%‐100%.9, 30 In the present study, although there was an addition of low‐dose mitoxantrone to induction therapy with ATRA plus RIF or ATO, 36.7% of the non‐high‐risk children experienced leukocytosis, and 45.5% of the high‐risk children experienced WBC count increase by more than 30%, respectively. Therefore, it is likely that conventional use of cytotoxic drug is rational in induction therapy for pediatric APL, including non‐high‐risk APL. The role of cytarabine in treating APL has remained controversial.31, 32 There have been evidences showing that with the addition of cytarabine, ATRA and anthracycline‐based therapy yielded better outcomes for adult patients with high‐risk APL.33, 34, 35 Using cytarabine during consolidation therapy for high‐risk APL has been generally recommended.31, 32 Nevertheless, when the combination of ATRA and arsenic compound is used for first‐line therapy of APL, there are grounds for testing a chemotherapy‐free treatment in post‐remission induction phase for non‐high‐risk children, and a treatment with further reduction of chemotherapy for high‐risk children.
Although molecular remission after consolidation therapy has been considered a therapeutic objective in APL treatment, our study support that prolonged molecular monitoring may be unnecessary in view of the cost‐benefit ratio, because of the extremely low risk of relapse in patients with APL receiving arsenic‐based regimens.36 This is important in resource‐limited countries.
There are limitations of this interim study. A longer follow‐up time is needed to reveal if the treatment efficacy is maintained over time, as well as to evaluate if there are long‐term negative effects of ATO and RIF on cardiac function and childhood growth and development. Moreover, there were five PML‐RARa positive patents in our cohort excluded from our study according to the eligibility criteria, because they had severe intracranial hemorrhage or cerebral thrombosis before randomization. Of the five patients, four with intracranial hemorrhage died and one with cerebral thrombosis remained in continuous CR with left hemiplegia for 3 years on our ATO protocol. The risk of hemorrhagic death during the induction period remains at about 5% in the clinical trial setting,37 and the mortality rate before diagnosis is still unknown. If the survival rate including those patients who die early, it will be lower.
In conclusion, this interim analysis shows that oral RIF is effective and safe and might not be inferior to intravenous ATO as first‐line treatment of pediatric APL, at least when combined with ATRA and low intensity chemotherapy. Final trial analysis will reveal mature outcome data. When combined with previous studies on adult APL, this data indicates that substitution of RIF for ATO has the advantage of significantly reducing hospital days and may be a new standard future strategy for treatment of APL patients in all age groups including children.
CONFLICT OF INTEREST
The authors declare that they have no competing interests.
Supporting information
Figure S1 The estimated 5‐year EFS rates were 100% in both the ATO and RIF groups, after a median follow‐up was 3.0 years. The survival curves were not included the noneligible patients including 4 who died early in induction prior to arsenic randomization.
Table S1 Intrathecal injection.
Table S2. Coagulopathy events newly occurring during induction phase in the patients enrolled.
Table S3. Accumulated hospital days during induction and consolidation therapy.
ACKNOWLEDGMENTS
We thank Qing‐Xiao Ye for assistance in data collection. We thank the patients and their families for their willingness to participate in this trial, and all participants and research staff of all centers within SCCLG.
AUTHORSHIP CONTRIBUTIONS
Conception and design: X‐Q L, L‐B H, W‐Q W, K H, L‐H Y, H‐R M, H‐Q C, X‐F S, R‐Y L and G‐H C. Collection and assembly of data: J‐S L, M‐H Y, W‐Q W, M‐C Zh, K H, L‐H Y, H‐R M, H‐Q C, X‐F S, J L, R‐Y L, G‐H C, Z‐Y K and X F. Data analysis and interpretation: X‐Q L, L‐B H and Y‐L T. Statistical analysis: B L and J‐S L. Manuscript writing and final approval of manuscript: All authors.
SUPPORTING INFORMATION
Additional Supporting Information may be found online in the supporting information tab for this article.
FUNDING INFORMATION
This work was supported in China by the Science and Technology Planning Project of Guangdong Province (Grant No. 2014A020221008), the Science and Technology Planning Project of Guangzhou (Grant No. 201604020128), and Sun Yat‐Sen University Clinical Research 5010 Program (Grant No. 2010001).
Yang M‐H, Wan W‐Q, Luo J‐S, et al. Multicenter randomized trial of arsenic trioxide and Realgar‐Indigo naturalis formula in pediatric patients with acute promyelocytic leukemia: Interim results of the SCCLG‐APL clinical study. Am J Hematol. 2018;93:1467–1473. 10.1002/ajh.25271
Funding information Sun Yat‐Sen University Clinical Research 5010 Program, Grant/Award Number: 2010001; The Science and Technology Planning Project of Guangdong Province, China, Grant/Award Number: 2014A020221008; The Science and Technology Planning Project of Guangzhou, China, Grant/Award Number: 201604020128
Contributor Information
Yan‐Lai Tang, Email: tangyanlai@126.com.
Li‐Bin Huang, Email: 469882975@qq.com.
Xue‐Qun Luo, Email: l-xuequn@126.com.
REFERENCES
- 1. Shen ZX, Shi ZZ, Fang J, et al. All‐trans retinoic acid/As2O3 combination yields a high quality remission and survival in newly diagnosed acute promyelocytic leukemia. Proc Natl Acad Sci. 2004;101:5328‐5335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Burnett AK, Russell NH, Hills RK, et al. Arsenic trioxide and all‐trans retinoic acid treatment for acute promyelocytic leukaemia in all risk groups (AML17): results of a randomised, controlled, phase 3 trial. Lancet Oncol. 2015;16:1295‐1305. [DOI] [PubMed] [Google Scholar]
- 3. Powell BL, Moser B, Stock W, et al. Arsenic trioxide improves event‐free and overall survival for adults with acute promyelocytic leukemia: north American leukemia intergroup study C9710. Blood. 2010;116:3751‐3757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kutny MA, Alonzo TA, Gerbing RB, et al. Arsenic trioxide consolidation allows anthracycline dose reduction for pediatric patients with acute promyelocytic leukemia: report from the Children's oncology group phase III historically controlled trial AAML0631. J Clin Oncol. 2017;35:3021‐3029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zhu HH, Wu DP, Jin J, et al. Long‐term survival of acute promyelocytic leukaemia patients treated with arsenic and retinoic acid. Br J Haematol. 2016;174:820‐822. [DOI] [PubMed] [Google Scholar]
- 6. Iland HJ, Collins M, Bradstock K, et al. Use of arsenic trioxide in remission induction and consolidation therapy for acute promyelocytic leukaemia in the Australasian Leukaemia and lymphoma group (ALLG) APML4 study: a non‐randomised phase 2 trial. Lancet Haematol. 2015;2:e357‐e366. [DOI] [PubMed] [Google Scholar]
- 7. Lou Y, Ma Y, Suo S, et al. Prognostic factors of patients with newly diagnosed acute promyelocytic leukemia treated with arsenic trioxide‐based frontline therapy. Leuk Res. 2015;39:938‐944. [DOI] [PubMed] [Google Scholar]
- 8. Hu J, Liu YF, Wu CF, et al. Long‐term efficacy and safety of all‐trans retinoic acid/arsenic trioxide‐based therapy in newly diagnosed acute promyelocytic leukemia. Proc Natl Acad Sci USA. 2009;106:3342‐3347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zhang L, Zou Y, Chen Y, et al. Role of cytarabine in paediatric acute promyelocytic leukemia treated with the combination of all‐trans retinoic acid and arsenic trioxide: a randomized controlled trial. BMC Cancer. 2018;18:374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cicconi L, Divona M, Ciardi C, et al. PML‐RARα kinetics and impact of FLT3‐ITD mutations in newly diagnosed acute promyelocytic leukaemia treated with ATRA and ATO or ATRA and chemotherapy. Leukemia. 2016;30:1987‐1992. [DOI] [PubMed] [Google Scholar]
- 11. Wang LN, Tang YL, Zhang YC, et al. Arsenic trioxide and all‐trans‐retinoic acid selectively exert synergistic cytotoxicity against FLT3‐ITD AML cells via co‐inhibition of FLT3 signaling pathways. Leuk Lymphoma. 2017;58:2426‐2438. [DOI] [PubMed] [Google Scholar]
- 12. Wang L, Zhou GB, Liu P, et al. Dissection of mechanisms of Chinese medicinal formula Realgar‐indigo naturalis as an effective treatment for promyelocytic leukemia. Proc Natl Acad Sci USA. 2008;105:4826‐4831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. The Cooperation Group of Phase II Clinical Trial of Compound Huangdai Tablet . Phase II clinical trial of compound Huangdai tablet in newly diagnosed acute promyelocytic leukemia. Chin J Hematol. 2006;27:801‐804. [Google Scholar]
- 14. Zhu HH, Wu DP, Jin J, et al. Oral tetra‐arsenic tetra‐sulfide formula versus intravenous arsenic trioxide as first‐line treatment of acute promyelocytic leukemia: a multicenter randomized controlled trial. J Clin Oncol. 2013;31:4215‐4221. [DOI] [PubMed] [Google Scholar]
- 15. Zhu HH, Huang XJ. Oral arsenic and retinoic acid for non‐high‐risk acute promyelocytic leukemia. N Engl J Med. 2014;371:2239‐2241. [DOI] [PubMed] [Google Scholar]
- 16. Jiang H, Liang GW, Huang XJ, et al. Reduced medical costs and hospital days when using oral arsenic plus ATRA as the first‐line treatment of acute promyelocytic leukemia. Leuk Res. 2015;39:1319‐1324. [DOI] [PubMed] [Google Scholar]
- 17. Luo XQ, Ke ZY, Huang LB, et al. Improved outcome for Chinese children with acute promyelocytic leukemia: a comparison of two protocols. Pediatr Blood Cancer. 2009;53:325‐328. [DOI] [PubMed] [Google Scholar]
- 18. Wang J, Huang JB, Liu ZL, Zhang BH, Xu HG, Xue HM, Chen C Comparison of Curative Effect between Fu Fang Huang Dai Pian and Arsenic Trioxide in Treatment of 45 Patients with Acute Promyelocytic Leukaemia. Zhongguo Shi Yan Xue Ye Xue Za Zhi 2017;25:1605–1610. [DOI] [PubMed] [Google Scholar]
- 19. Zhou J, Meng R, Sui X, Meng L, Jia J, Yang B. Effects of administration styles of arsenic trioxide on intracellular arsenic concentration, cell differentiation and apoptosis. Haematologica. 2005;90:1277‐1279. [PubMed] [Google Scholar]
- 20. Ossenkoppele G, Schuurhuis GJ. MRD in AML: does it already guide therapy decision‐making? Hematol Am Soc Hematol Educ Program. 2016;2016:356‐365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Benjamini O, Dumlao TL, Kantarjian H, et al. Phase II trial of hyper CVAD and dasatinib in patients with relapsed Philadelphia chromosome positive acute lymphoblastic leukemia or blast phase chronic myeloid leukemia. Am J Hematol. 2014;89:282‐287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Levi M, Toh CH, Thachil J, Watson HG. Guidelines for the diagnosis and management of disseminated intravascular coagulation. Br J Haematol. 2009;145:24‐33. [DOI] [PubMed] [Google Scholar]
- 23. Barbui T, Finazzi G, Falanga A. The impact of all‐trans‐retinoic acid on the coagulopathy of acute promyelocytic leukemia. Blood. 1998;91:3093‐3102. [PubMed] [Google Scholar]
- 24. Montesinos P, Bergua JM, Vellenga E, et al. Differentiation syndrome in patients with acute promyelocytic leukemia treated with all‐trans retinoic acid and anthracycline chemotherapy: characteristics, outcome, and prognostic factors. Blood. 2009;113:775‐783. [DOI] [PubMed] [Google Scholar]
- 25. Kutny MA, Gregory J Jr, Feusner JH. Treatment of paediatric APL: how does the therapeutic approach differ from adults? Best Pract Res Clin Haematol. 2014;27:69‐78. [DOI] [PubMed] [Google Scholar]
- 26. Children's Oncology Group . Long‐term follow‐up guidelines for survivors of childhood, adolescent, and young adult cancers, Version 4.0. Monrovia, CA: Children's Oncology Group; 2013. Available from: http://www.survivorshipguidelines.org/ [Google Scholar]
- 27. Platzbecker U, Avvisati G, Cicconi L, et al. Improved outcomes with retinoic acid and arsenic trioxide compared with retinoic acid and chemotherapy in non‐high‐risk acute Promyelocytic leukemia: final results of the randomized Italian‐German APL0406 trial. J Clin Oncol. 2017;35:605‐612. [DOI] [PubMed] [Google Scholar]
- 28. Abedin S, Altman JK. Acute promyelocytic leukemia: preventing early complications and late toxicities. Hematology Am Soc Hematol Educ Program. 2016;2016:10‐15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lo‐Coco F, Avvisati G, Vignetti M, et al. Retinoic acid and arsenic trioxide for acute promyelocytic leukemia. N Engl J Med. 2013;369:111‐121. [DOI] [PubMed] [Google Scholar]
- 30. Creutzig U, Dworzak MN, Bochennek K, et al. First experience of the AML‐Berlin‐Frankfurt‐Münster group in pediatric patients with standard‐risk acute promyelocytic leukemia treated with arsenic trioxide and all‐trans retinoid acid. Pediatr Blood Cancer. 2017;64 10.1002/pbc.26461, [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 31. Sanz MA, Lo‐Coco F. Modern approaches to treating acute promyelocytic leukemia. J Clin Oncol. 2011;29:495‐503. [DOI] [PubMed] [Google Scholar]
- 32. Lo‐Coco F, Cicconi L, Breccia M. Current standard treatment of adult acute promyelocytic leukaemia. Br J Haematol. 2016;172:841‐854. [DOI] [PubMed] [Google Scholar]
- 33. Sanz MA, Montesinos P, Rayón C, et al. Risk‐adapted treatment of acute promyelocytic leukemia based on all‐trans retinoic acid and anthracycline with addition of cytarabine in consolidation therapy for high‐risk patients: further improvements in treatment outcome. Blood. 2010;115:5137‐5146. [DOI] [PubMed] [Google Scholar]
- 34. Lo‐Coco F, Avvisati G, Vignetti M, et al. Front‐line treatment of acute promyelocytic leukemia with AIDA induction followed by risk‐adapted consolidation for adults younger than 61 years: results of the AIDA‐2000 trial of the GIMEMA group. Blood. 2010;116:3171‐3179. [DOI] [PubMed] [Google Scholar]
- 35. Adés L, Sanz M, Chevret S, et al. Treatment of newly diagnosed acute promyelocytic leukemia (APL): a comparison of French‐Belgian‐Swiss and PETHEMA results. Blood 2008;111:1078–1084. [DOI] [PubMed] [Google Scholar]
- 36. Cicconi L, Lo‐Coco F. Current management of newly diagnosed acute promyelocytic leukemia. Ann Oncol. 2016;27:1474‐1481. [DOI] [PubMed] [Google Scholar]
- 37. Mantha S, Tallman MS, Soff GA. What's new in the pathogenesis of the coagulopathy in acute promyelocytic leukemia? Curr Opin Hematol. 2016;23:121‐126. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 The estimated 5‐year EFS rates were 100% in both the ATO and RIF groups, after a median follow‐up was 3.0 years. The survival curves were not included the noneligible patients including 4 who died early in induction prior to arsenic randomization.
Table S1 Intrathecal injection.
Table S2. Coagulopathy events newly occurring during induction phase in the patients enrolled.
Table S3. Accumulated hospital days during induction and consolidation therapy.


