Abstract
Background
Systemic lupus erythematosus (SLE) is associated with cognitive deficit but the exact neural mechanisms remain unclear.
Purpose
To explore sequential brain activities using functional magnetic resonance imaging (fMRI) during the performance of a decision‐making task, and to determine whether serum or clinical markers can reflect the involvement of the brain in SLE.
Subjects
Sixteen female SLE patients without overt clinical neuropsychiatric symptoms and 16 healthy controls were included.
Field Strength/Sequence
1.5T, T1‐weighted anatomic images, gradient‐echo echo‐planar imaging sequence, and 3D images.
Assessment
The computer‐based Iowa Gambling Task (IGT) for assessing decision‐making was performed by SLE patients and 16 matched controls; brain activity was recorded via blood oxygen level‐dependent (BOLD) fMRI. The amplitudes of the average BOLD responses were calculated for each individual subject, and activation data from fMRI experiments were compared between the two groups.
Statistical Tests
Two‐sample t‐test; repeated‐measures analysis of variance (ANOVA); linear regression analyses.
Results
Imaging revealed activity in a distributed network of brain regions in both groups, including the ventromedial prefrontal cortex (vmPFC), the orbitofrontal cortex (OFC), the dorsolateral prefrontal cortex (dlPFC), the anterior cingulate cortex (ACC), the posterior cingulate cortex (PCC), and the striatum, as well as the insular, parietal, and occipital cortices. Compared to controls, SLE patients showed lower activation in a convergence zone and the limbic system, namely, the OFC, vmPFC, ACC, and PCC, but greater activation in memory, emotion, and behavior systems involving the dlPFC, the insular cortex and the striatum. Furthermore, brain activation in the vmPFC was positively correlated with IGT scores (r = 0.63, P < 0.001), but inversely related to disease activity (r = −0.57, P < 0.01).
Data Conclusion
The dynamics among the aforementioned neural systems (some hyperfunctioning, others hypofunctioning) may shed some light on the pathologic mechanisms underlying SLE without overt clinical neuropsychiatric symptoms. In addition, disease activity may potentially be used as an effective biomarker reflecting cerebral involvement in SLE.
Level of Evidence: 1
Technical Efficacy: Stage 3
J. Magn. Reson. Imaging 2018;48:1508–1517
Keywords: functional imaging, systemic lupus erythematosus, decision‐making, Iowa Gambling Task, cognitive deficit
Systemic lupus erythematosus (SLE) is a female‐dominant autoimmune‐mediated collagen disorder characterized by multisystem involvement.1 Neuropsychiatric involvement is, arguably, the least understood aspect of SLE and is associated with a more complex clinical presentation.1 Neuropsychiatric manifestations in patients with SLE (NPSLE) include overt disorders like stroke and seizures, and milder manifestations such as cognitive dysfunction.2 The diagnosis of NPSLE is generally based on the American College of Rheumatology (ACR) criteria,2 but the reported prevalence of cognitive impairment in SLE varies widely, ranging from 14–75%, largely depending on the methodology used for diagnosis.3, 4 Recently, it has been shown that cognitive dysfunction can also be identified in SLE patients without clinically overt neuropsychiatric symptoms, but the pathogenesis of this impairment is yet to be elucidated.3, 5
Cognitive dysfunction in SLE can affect any or all of the following functions: working memory, attention, reasoning, executive function, visual–spatial processing, and language.2 Currently, multiple neurocognitive testing is considered the standard for diagnosing cognitive dysfunction in SLE.6 Accumulating behavioral studies have identified numerous differences between SLE and matched controls; in particular, disturbances in executive and attention functions and impairment of the general capacity to appreciate other's mental states. Nonetheless, the etiology and complications of cognitive dysfunction remain elusive and continue to be a hot research question.
Numerous studies have revealed structural white and gray matter abnormalities in SLE patients using structural imaging and spectrometry,7, 8 but they have failed to elucidate the pathophysiology underlying cognitive dysfunction in SLE. Blood oxygen level‐dependent functional magnetic resonance imaging (BOLD‐fMRI) is a promising, noninvasive method for exploring human cognitive function, which can map brain activation patterns related to specific cognitive tasks. BOLD‐fMRI has already been applied in SLE patients, using a variety of populations, tasks, and methods of analysis, to identify activation patterns related to cognitive dysfunction.
In past neuroimaging studies, altered brain function in NPSLE patients, including decreased or increased brain activity during resting conditions or during the performance of cognitive tasks, has been widely reported.9, 10 Dysfunction of certain brain regions may impair cognitive function (ie, attention, executive function, spatial working memory, motor function, and learning).11, 12, 13 Behaviorally, a recent study demonstrated that SLE patients display poorer decision‐making,14 but the brain alterations responsible for this outcome, visualized using fMRI, have not been reported.
The most frequently used paradigm to assess decision‐making abilities, by simulating a real‐life situation, is the Iowa Gambling Task (IGT),15 which was originally developed for use in patients with focal brain lesions. Multiple studies have demonstrated that people with a variety of neuropsychiatric impairments (eg, substance dependence, Parkinson's disease, and pathological gamblers) show poor decision‐making compared to controls, as measured by the IGT.16, 17, 18 Investigating the brain regions involved in performing the IGT task is of great importance for gaining insight into the neural mechanisms underlying the decision‐making deficit observed in a wide range of clinical and neurological conditions.17, 18 To date, fMRI studies with the IGT have been theoretically guided by the neural network of the Somatic Marker Hypothesis (SMH),19 and it has been shown that several neural structures are key parts of the neural circuitry underlying SMH.20
Therefore, in this pilot study the temporal profile of neural activity during implicit decision‐making was investigated via fMRI, in patients with SLE without clinical neuropsychiatric symptoms. In addition, the relationship between brain activity and cognitive testing, and serum or clinical markers, was explored. Specifically, we tested the hypothesis that SLE patients display poorer decision‐making during IGT compared to controls, due to altered brain activity in important parts of the somatic marker neural circuitry.
Materials and Methods
Participants
Sixteen SLE patients were included in this study. The patients who satisfied the American College of Rheumatology classification criteria for the disease21 were recruited from the Department of Rheumatology, the First Affiliated Hospital of Shantou University Medical College and Shantou Chaonan Minsheng Hospital. The study was restricted to females to ensure a homogenous population. Inclusion criteria included SLE patients without overt clinical neuropsychiatric symptoms, normal vision, or good corrected visual acuity. Controls included 16 healthy female subjects recruited from the community to be similar in age, handedness, education, and duration of education. All subjects had a prescreening visit, and the exclusion criteria were psychiatric problems or any history of pathology that might have affected their cognitive functions, neuropsychiatric diseases, or taking psychotropic medication, or MRI scan contraindications. All subjects completed behavior tests before fMRI scanning. The MRI appointment was completed within 1 week of the behavior testings.
All subjects provided written informed consent that was approved by the Medical Ethics Committee of Shantou University Medical College before any testing or neuropsychological evaluation.
Demographic, Clinical, and Neuropsychological Data
All subjects had clinical and standard assessments including disease activity (Systemic Lupus Erythematosus Disease Activity Index [SLEDAI]) 22 and the Systemic Lupus International Collaborating Clinics/ACR Damage Index (SLICC/DI) 23 on the day of the MRI scan. Serum assay indices (C3 and C4) and anti‐dsDNA antibodies were measured at the clinical laboratory in the hospital.
The Montreal Cognitive Assessment (MOCA) 24 is a 30‐item assessment tool commonly used to detect mild cognitive impairment in a clinical setting; this 10‐minute researcher‐administered test assesses several cognitive domains including visuospatial/executive, naming, memory, attention, language, abstraction, delayed recall, and orientation function. The total possible score is 30; persons scoring 26 points or greater are considered normal cognitive function, below 26 points are considered mild cognitive impairment, and <19 points are considered dementia.25 This test was conducted by two qualified and certified psychologists from the Shantou University Medical College.
fMRI Tasks: Iowa Gambling Task(IGT) Modified for fMRI
All subjects performed a computerized version of the IGT26 that was modified for BOLD‐fMRI. The IGT15 task simulates real‐life decision‐making by testing the ability of participants to sacrifice immediate rewards in spite of long‐term gain. During the IGT task (Fig. 1), participants were instructed to select a card from any of the four decks of cards (A, B, C, D). Participants were also told that when they picked a card they would win some money but occasionally they would lose some money. They can select any deck and the ultimate goal is to win as much as possible. Decks A and B provided large gains and large losses, whereas the other decks provided small gains and small losses. Decks A and B are disadvantageous since they lead to a net loss with time, whereas decks C and D lead to a net gain and are advantageous. Performance on the decision‐making task was measured by a net score, and the IGT net score was calculated by the total number of cards selected from the advantage decks (C + D) minus the total number of cards selected from the disadvantage decks (A + B).
Figure 1.
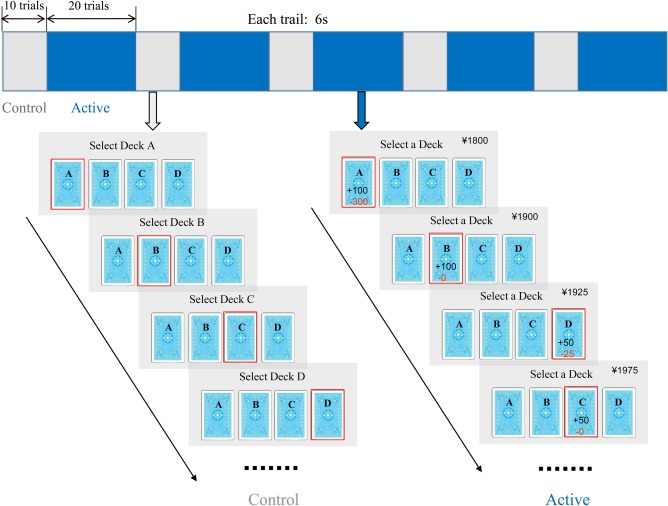
An illustration of the experimental tasks. Each run contained five blocks of the IGT (blue) and five blocks of the control task (gray). Each trial lasted 6 seconds. In the IGT task, an ongoing monetary score showed on the upper right‐hand side of the screen across all the trials.
The control task (Fig. 1) was designed to be identical to the IGT regarding visual and motor demands. There were no gains and losses between decks compare to the active task. Subjects were instructed to select a card sequentially in the fixed order of A–B–C–D–A–B–C–D, etc. Card selection, consequently, did not require complex decision‐making.
Design
The task employed a blocked periodic design incorporating alternating five blocks of the IGT task as well as five blocks of the control task. The IGT block consisted of 20 card selection trials and the control block consisted of 10 trials. Participants were given 3 seconds to make a choice, followed immediately by feedback of 3 seconds duration (Fig. 1). The task was programmed and presented using E‐Prime Software.
Image Acquisition and Analysis
Imaging was performed with a 1.5T General Electric (Milwaukee, WI) MRI scanner. T1‐weighted anatomic images were acquired using conventional SE sequences as follows: pixel matrix 256 × 256, field of view (FOV) 230 × 230 mm, 6 mm section thickness, and 1 mm gap. A gradient‐echo echo‐planar imaging sequence was applied for functional imaging with the following parameters: repetition time (TR) 3000 msec, flip angle 90°, FOV 230 × 230 mm, 6 mm section thickness, and pixel matrix 64 × 64. 3D images were collected with a fast low‐angle radiofrequency pulse sequence, using the following parameters: TR = 30 msec, echo time (TE) = 3.0 msec, flip angle 30°, FOV = 230 × 230 mm, matrix = 256 × 256, 1.3 mm thickness, 1.3 mm section thickness, no gap, and 120 slices.
Image analysis was conducted using Analysis of Functional NeuroImages (http://afni.nimh.nih.gov/afni/doc/manual/AlphaSim), including data preprocessing, statistical analysis, and result display. In data preprocessing, all functional datasets were processed to remove any linear drift, correct motion, be normalized to the stereotaxic coordinates of Talairach and Tournoux, and be spatially smoothed with a Gaussian filter (full‐width half‐maximum = 6 mm). Any scan in which the head motion was larger than 2 mm or rotation larger than 2° was excluded from further analysis.
Data analysis included group and individual analysis. At the group level, correlational analysis based on the direct contrast between the IGT task versus control task was carried out to generate the activation map for each group (cluster size >20 voxels). These activation maps were used to locate the regions of interest (ROIs). For each subject, correlation analysis was performed on the functional data to generate two activation maps and a combined activation map by the logical "OR" of these maps (cluster size >20 voxels). The amplitudes of the average BOLD responses were calculated for each subject (IGT task vs. control task). All the thresholds of statistical analysis were set at P < 0.05 (false discovery rate correction).
Data Analysis
Clinical and neuropsychological data were compared between groups using the independent two‐sample t‐test. The IGT scores were calculated per block. Repeated‐measures analysis of variance (ANOVA) was used on the IGT net score to assess the learning effect, with the block as the within‐subject factor, and the group (patients vs. controls) as the between‐subject factor. The associations between the activation intensity of individual patients and their respective IGT score, MOCA‐CA score, C3, C4, anti‐dsDNA, SLEDAI, SLICC/DI, disease duration, age, and daily prednisone dose were evaluated using linear regression analyses. All statistical analyses were performed using SPSS 19.0 (Chicago, IL). Descriptive participant characteristics and performance of IGT are reported as means ± standard deviation (SD). P < 0.05 was considered statistically significant.
Results
Clinical, Demographic, and Neuropsychological Data
There were no significant differences between groups in terms of age (P = 0.36), education background (P = 0.38), and sex. Upon recruitment, the majority of the SLE patients had low serum levels of C3 and C4, and a high serum level of anti‐dsDNA. Overall, 8 out of 16 patients had active SLE (SLEDAI >4). With regard to performance on the MOCA, SLE patients had significantly lower scores in contrast to controls (P < 0.05). In the SLE group, five patients scored <26 (two 22, two 23, one 24), whereas all control subjects scored >26. In addition, there were significant differences between the two groups for the memory subtests (P < 0.05), as well as for visuospatial/executive and total scores (P < 0.01). The demographic, clinical data, and MOCA performance data of the study participants are summarized in Table 1.
Table 1.
Means (SDs) and P‐values for Demographic, Clinical, and Neuropsychological Data
| SLE patients N = 16 | Controls N = 16 | P‐value | |
|---|---|---|---|
| Demographic and clinical characteristics | |||
| Age (yrs) | 26.88 (3.69) | 25.88 (2.19) | 0.36 |
| Education (yrs) | 13.20 (1.22) | 12.81 (1.80) | 0.38 |
| Sex | 16 female | 16 female | |
| Disease duration (month) | 31.56 (13.72) | ||
| SLEDAI | 5.19 (2.64) | ||
| SLICC/DI | 0.43 (0.63) | ||
| C3 (mg/dL, normal range 79‐152) | 97.50 (37.81) | ||
| C4 (mg/dL, normal range 16‐38) | 14.50 (8.44) | ||
| Anti‐dsDNA antibody titer | 8.49 (16.13) | ||
| Prednisolone (mg/d) | 9.84 (5.88) | ||
| MOCA performance | |||
| Visuospatial/executive | 3.06 (0.10) | 5.00 (0.00) | <0.001a |
| Naming | 3.00 (0.00) | 3.00 (0.00) | |
| Memory | 3.06 (1.10) | 4.19 (0.75) | 0.001a |
| Attention | 4.64 (1.09) | 5.25 (0.77) | 0.07 |
| Language | 2.19 (0.40) | 2.12 (0.34) | 0.64 |
| Abstraction | 1.63 (0.50) | 1.88 (0.09) | 0.11 |
| Orientation | 5.62 (0.50) | 5.81 (0.40) | 0.25 |
| Total score | 23.19 (1.80) | 27.25 (1.13) | <0.001a |
SLE: systemic lupus erythematosus; MOCA: Montreal Cognitive Assessment; SLEDAI: SLE Disease Activity Index; SLICC/DI: Systemic Lupus International Collaborating Clinics/American College of Rheumatology Damage Index.
Significant P‐value.
IGT Performance of SLE Patients and Controls
For the IGT, a 2 × 5 repeated‐measures ANOVA yielded a significant effect of group (F = 217.75, P < 0.001), a significant effect of IGT block (F = 166.13, P < 0.001), and a significant group*IGT block interaction (F = 63.52, P < 0.001). Although SLE patients and controls had a similar performance in the first and second blocks (first 40 cards), there was a marked preference in later blocks (Fig. 2).
Figure 2.
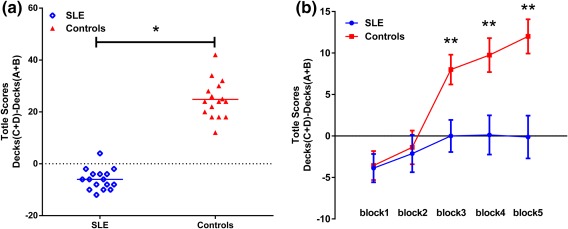
Performance on the IGT between groups. (a) Performance differences between SLE and controls on the IGT, based on the mean performance scores (net score = number of cards from the advantage decks minus number of cards from the disadvantage decks). (b) IGT performance over time in SLE and controls across five blocks expressed as mean (SE) (*P < 0.01; **P < 0.001).
Brain Activation in the Control and SLE Patients During Performance of the IGT
Brain activities during the active versus control conditions from both SLE patients and controls are shown in Fig. 3A,B. During the IGT task‐related fMRI paradigm, both SLE patients and controls exhibited similar task‐related activation in the ventromedial prefrontal cortex (vmPFC), orbitofrontal cortex (OFC), dorsolateral prefrontal cortex (dlPFC), anterior cingulate cortex (ACC), posterior cingulate cortex (PCC), and striatum, as well as in the insular, parietal and occipital cortices.
Figure 3.
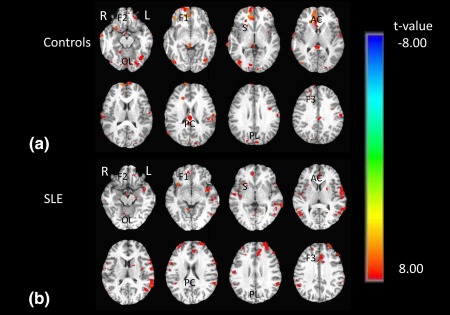
Whole‐brain analysis of task‐related brain activity during decision‐making (IGT‐control task). T‐statistic maps of healthy control subjects (a) and SLE patients (b). Red/yellow areas indicate activation; green/blue areas indicate deactivation. R: right hemisphere; L: left hemisphere; F1: ventromedial prefrontal cortex; F2: orbitofrontal cortex; F3: dorsolateral prefrontal cortex; AC: anterior cingulate; PC: posterior cingulate; S: striatum; I: insular; PL: parietal lobe; OL: occipital lobe.
Differences in Brain Activity Between the Two Groups During Performance of the IGT
Statistical comparison of activation maps for SLE patients and controls demonstrated lower activation in the OFC, vmPFC, cingulate, and occipital cortex in SLE patients relative to controls. There were several regions showing increased activation in SLE patients compared to controls, namely, dlPFC, insular cortex, ventral striatum, and parietal cortex (Table 2).
Table 2.
Regional Differences in Brain Activation During Decision‐Making (IGT‐Control Task) in SLE Patients Versus Controls
| Regional activations | Side | Talairach | BA | Voxel T | t‐value | ||
|---|---|---|---|---|---|---|---|
| X | Y | Z | |||||
| SLE<controls | |||||||
| Orbitofrontal cortex | L | −8 | 45 | −3 | 10 | 30 | −2.83 |
| Ventromedial prefrontal cortex | R | 56 | 20 | 17 | 45 | 68 | −4.57 |
| Anterior cingulate cortex | R | 26 | −19 | 38 | 32 | 38 | −3.01 |
| Posterior cingulate cortex | L/R | −22 | −57 | 20 | 30 | 21 | −2.54 |
| Occipital cortex | L | 20 | 38 | −3 | 17 | 21 | −2.54 |
| R | 17 | −58 | 44 | 17 | 24 | −3.67 | |
| SLE>controls | |||||||
| Dorsolateral prefrontal cortex | L | 52 | 10 | −22 | 46 | 47 | 6.93 |
| R | 31 | 64 | 2 | 46 | 24 | 3.52 | |
| Insula | L | 29 | −10 | −10 | − | 40 | 3.40 |
| R | 32 | −24 | 12 | − | 24 | 2.67 | |
| Striatum | L | 11 | −1 | −1 | − | 23 | 2.58 |
| Parietal cortex | L | 53 | −28 | 38 | 7 | 33 | 3.31 |
| R | 52 | −34 | −23 | 7 | 21 | 2.54 | |
SLE, systemic lupus erythematosus; BA, Brodmann area.
Correlational Analysis
Significant positive correlations were found between the IGT score and activation only in vmPFC (r = 0.63, P < 0.001) (Fig. 4), and no relationship was found between activation intensity and MOCA‐CA score.
Figure 4.
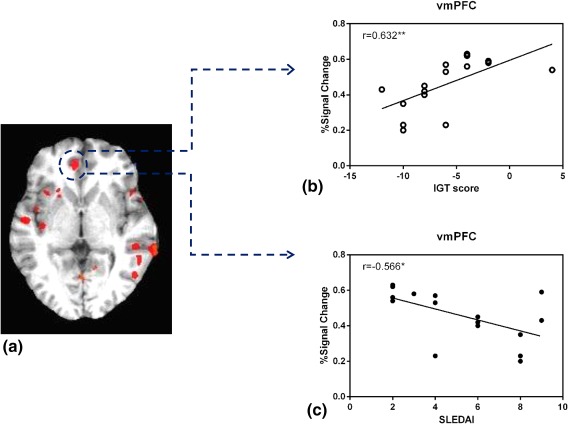
Functional correlation. Regions in the vmPFC were positively correlated with IGT scores but inversely related to disease activity (a). Scatterplots of correlations between IGT scores and percentage BOLD signal change in the vmPFC (b). Scatterplots of correlations between disease activity and percentage BOLD signal change in the vmPFC (c) (*P < 0.01; **P < 0.001).
We found a correlation between SLEDAI score and BOLD response of active block related to the control block. The results, shown in Fig. 4, revealed that the higher SLEDAI score correlated negatively with activity in the vmPFC (r = –0.57, P < 0.01). However, no correlation was noted between the activation intensity and other serum biomarkers or clinical indices (all P > 0.05).
Discussion
Consistent with our hypotheses, even in the absence of overt clinical neurological or psychiatric symptoms, SLE patients showed neurocognitive and functional brain abnormalities. We found that the MOCA‐CA executive, working memory, and total scores of the SLE patients were lower than those of the control subjects, confirming that the cognitive abilities of SLE patients were mildly impaired, which is consistent with prior studies.3, 4, 27
As expected, our results confirmed that SLE is associated with compromised decision‐making abilities. SLE patients showed a less learning effect than the controls, which indicates that the SLE patients may be hypersensitive to immediate rewards, less sensitive to losses, and slow to learn from previous mistakes. Consequently, we can infer that SLE patients without clinical neuropsychiatric manifestations have dysfunctional decision‐making, which is in agreement with a previous study using a similar task.14
When measuring the BOLD signal, both groups showed common, largely overlapping patterns of task‐induced BOLD activations. Although both groups activated a relatively similar set of regions, SLE patients showed decreased activation in the OFC, vmPFC, cingulate, and occipital cortex, as well as greater activation in dlPFC, insular cortex, ventral striatum, and parietal cortex. Moreover, brain activation in the vmPFC was positively correlated with IGT scores, but inversely related with disease activity.
The pattern of brain activity during the IGT task generally matches the theoretical neural framework of affective decision‐making. Decision‐making is a complex process that relies on an extensive brain network, including systems for memory, emotion/affect, and feeling. To elaborate, the IGT requires the interaction of several systems: a memory system (dlPFC), emotional system (insula), limbic system (ACC and PCC), a convergence zone for the aforementioned systems (vmPFC and OFC), and a motor/behavioral system (striatum).20 In the present study, sensory and visual systems (parietal and occipital lobes) were also involved. Of note, no significant activity was detected in the amygdala. A possible explanation is that the amygdala ceases to be actively engaged once a strategy is implemented.28 Indeed, the amygdala plays a critical role in processing the affective significance of a stimulus that is considered in formulating a strategic approach.29 Once the strategic approach is devised, the amygdala may become less involved. Another explanation may be the experimental paradigm designed in fMRI.20
SLE patients showed lower activation mainly in two prefrontal regions, including the vmPFC and OFC. This compromised activity may predict that SLE patients have functional abnormalities in prefrontal neural networks involved in decision‐making. It has been repeatedly indicated that patients with OFC or vmPFC lesions cannot perform the IGT successfully.15, 29 A study has shown that activation in the vmPFC and OFC in healthy individuals is associated with the development of "somatic markers" of autonomic arousal, which are believed to aid in the evaluation of emotional material during decision‐making.15 This is supported by the relationship between dynamic activity in these regions and successful performance of the IGT.30, 31 This link between vmPFC activation and task performance was shown in our SLE patients, which implies that the functional role of the vmPFC in reward processing was preserved. In addition, decreased activation of the cingulate cortex during decision‐making could reflect a deficit limbic system. A previous study has shown the importance of the limbic pathway in the development of depression in SLE patients.32 This suggests that the dysfunction of the limbic system can potentially lead to depression. Nevertheless, the relationship between limbic system dysfunction and depression in patients with SLE should be investigated further.
Under the present conditions, increased activity in the dlPFC in SLE patients replicates many previous findings in the dlPFC during the performance of the n‐back task.33, 34 The dlPFC is critical for planning and working memory, both of which are required to perform the IGT. Based on the present and previous findings, the dlPFC appears to be an important component of the prefrontal neural network that is significantly affected in SLE patients. The dlPFC is recruited during the continuous updating of working memory, and has been identified as part of the neuronal circuitry underlying decision‐making in fMRI studies on healthy individuals.20, 35 We can deduce that the dlPFC is involved in working memory, which is needed to perform the IGT adequately.
The IGT paradigm also produced stronger activation in the SLE patients, with significant clusters in striatum and insular regions. de Jong et al found that an increased metabolism in the striatum in SLE, which supports the presence of a direct immune response against neuronal tissue in SLE, is similar to the crossreaction against inhibitory components in striatal tissue provoked by streptococcal antigens.36 In particular, the nucleus accumbens within the ventral striatum has a significant role in reward anticipation. A study on mice has shown that the progression of SLE impaired motivated behavior by producing a lesion in the dopaminergic reward system.37 The increased activation in insula appears to be of great importance for integrating cognitive and affective information, which agrees with a study in SLE patients.12 Mak et al12 detected increased activation in the insula of SLE patients during the Wisconsin Card Sorting Test (WCST), in contrast to healthy controls. The increased activation was seen in patients without overt clinical neuropsychiatric symptoms, which suggests that this impairment may be an early and common compensatory phenomenon and may serve as a mechanism to overcome subtle neuronal damage.33, 34
Many studies have shown an increased brain activity dynamic in SLE patients, suggesting a compensatory mechanism to overcome cognitive deficit; however, a clinical cognitive deficit and decreased activation may occur when the compensatory pattern becomes impaired.11, 33, 34 The dynamics among multiple neural systems (hyperactivity in memory, emotional and motor systems, but hypoactivity in the convergence zone and the limbic systems) may shed new light on the pathologic mechanism underlying SLE without clinical neuropsychiatric symptoms. Under the complex IGT task, which engages several systems, diminished activation in some main brain areas will happen if the compensatory pattern is impaired.
Remarkably, correlation analysis between regional activity and clinical data revealed that activity in the vmPFC correlated negatively with disease activity, whereas no area correlated with C3, C4, disease duration, and daily prednisone dose dosages. The finding could suggest that the vmPFC, as part of an executive control network, could be involved in the pathogenesis of SLE. The increase of SLEDAI is associated with decreased regional 18FDG uptakes in the gray matter volume, which may have a significant impact of lowering SLE disease activity early on, due to the enhancement of executive functioning.38 Hou et al found that disease activity positively correlated with functional connectivity strength in the frontal‐parietal cortex in SLE patients compared with healthy controls. They have also shown that the relatively decreased activity in SLE patients might be transient, and it is probably dependent on disease activity, as the activity will increase after treatment and changes in patients progressing from active to inactive disease.39 The underlying mechanism of transient alteration in our lupus patients deserve further discussion. The role of disease activity in cerebral metabolic activity is also supported by previous studies that have demonstrated changes in cerebral vascularity in SLE patients with active disease, with elevated cerebral blood flow and volume compared to healthy controls.40 The results indicate that greater attention must be paid to the involvement of the central nervous system in SLE, even in patients without clinically overt neuropsychiatric symptoms. However, it is worth noting that none of the serum or clinical biomarkers were related to brain activity. Further investigation examining fMRI and other biomarkers for cognitive dysfunction in SLE is warranted.
This study is not without its limitations. The sample size was relatively small. However, sample size of this scale has systematically demonstrated sensitivity of BOLD signals in achieving statistical significance in the literature despite a relatively small sample size.10, 11, 13 In addition, the possible effects of corticosteroids treatment on BOLD signals may confound the interpretation of our results in SLE patients. Since the majority of patients who underwent fMRI scanning were newly diagnosed, the effect of treatment on BOLD signals should be very low.
In conclusion, our results demonstrated that even without clinically neuropsychiatric symptoms, SLE patients had subclinical cognitive dysfunction and decision‐making deficit. By combining decision‐making tasks, we could extend our understanding of this illness in three important ways. First, there is a mild cognitive and decision‐making deficit in SLE patients; consequently, physicians may need to be aware of patients' adherence to treatment plans, as well as difficulties in making the necessary changes and in adapting to new information, for the optimization of disease control. Second, under the complex IGT task, that compensatory pattern becomes impaired, resulting in different activation dynamics in multiple neural systems; thus, fMRI may be useful for elucidating the neuropathology of cognitive deficit in SLE patients. Last, the negative correlation between disease activity and brain activity supports the proposal that the general disease state has an impact on the cerebral function of SLE patients, which may potentially be used as an indicator of cerebral involvement in SLE.
Acknowledgments
Contract grant sponsor: National Natural Science Foundation of China; contract grant number: 81774395, 81373745, 81072905; Contract grant sponsor: Science and Technology Planning Project of Guangdong Province of China; contract grant number: 2017A020215060, 2009B030801323, 2010B031600023; Contract grant sponsor: Natural Science Foundation of Guangdong Province of China; contract grant number: S2011010005019, 10151503102000015; Contract grant sponsor: Shantou Technology Bureau Science Foundation of China, Shantou Government Technology; contract grant number: [2011] 46; [2006] 85
We thank the patients and healthy controls who agreed to be recruited for this study. All authors contributed to the conception or design of the work. Wu, Beibei, Xie, Lei, and Ma, Ye were involved in the design of the study. Wu, Beibei, Huang, Jinzhuang, Sun, Zongbo, Lin, Zhirong, Xie, Yao, Zhu, Chunmin, Sun, Danmiao, and Guo, Ruiwei participated in the experiment. Sun, Danmiao and Hou, Zhiduo collected the data. Wu, Beibei and Ma, Ye analyzed the data; Wu, Beibei, Ma, Ye, and Xie, Lei contributed to the discussion. All authors reviewed the article. Ma, Shuhua supervised the project.
The first three authors contributed equally to this work.
References
- 1. Rahman A, Isenberg DA. Systemic lupus erythematosus. N Engl J Med 2008;358:929–939. [DOI] [PubMed] [Google Scholar]
- 2.[No authors listed.] The American College of Rheumatology nomenclature and case definitions for neuropsychiatric lupus syndromes. Arthritis Rheum 1999;42:599–608. [DOI] [PubMed] [Google Scholar]
- 3. Kozora E, Ellison MC, West S. Reliability and validity of the proposed American College of Rheumatology neuropsychological battery for systemic lupus erythematosus. Arthritis Rheum 2004;51:810–818. [DOI] [PubMed] [Google Scholar]
- 4. Hanly JG, Fisk JD, Sherwood G, et al. Cognitive impairment in patients with systemic lupus erythematosus. J Rheumatol 1992;19:562–567. [PubMed] [Google Scholar]
- 5. Kozora E, Arciniegas DB, Filley CM, et al. Cognitive and neurologic status in patients with systemic lupus erythematosus without major neuropsychiatric syndromes. Arthritis Rheum 2008;59:1639–1646. [DOI] [PubMed] [Google Scholar]
- 6. Mikdashi JA, Esdaile JM, Alarcon GS, et al. Proposed response criteria for neurocognitive impairment in systemic lupus erythematosus clinical trials. Lupus 2007;16:418–425. [DOI] [PubMed] [Google Scholar]
- 7. Bosma GP, Steens SC, Petropoulos H, et al. Multisequence magnetic resonance imaging study of neuropsychiatric systemic lupus erythematosus. Arthritis Rheum 2004;50:3195–3202. [DOI] [PubMed] [Google Scholar]
- 8. Sarbu N, Toledano P, Calvo A, et al. Advanced MRI techniques: biomarkers in neuropsychiatric lupus. Lupus 2017;26:510–516. [DOI] [PubMed] [Google Scholar]
- 9. Lin Y, Zou QH, Wang J, et al. Localization of cerebral functional deficits in patients with non‐neuropsychiatric systemic lupus erythematosus. Hum Brain Mapp 2011;32:1847–1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Fitzgibbon BM, Fairhall SL, Kirk IJ, et al. Functional MRI in NPSLE patients reveals increased parietal and frontal brain activation during a working memory task compared with controls. Rheumatology (Oxford, England) 2008;47:50–53. [DOI] [PubMed] [Google Scholar]
- 11. Ren T, Ho RC, Mak A. Dysfunctional cortico‐basal ganglia‐thalamic circuit and altered hippocampal‐amygdala activity on cognitive set‐shifting in non‐neuropsychiatric systemic lupus erythematosus. Arthritis Rheum 2012;64:4048–4059. [DOI] [PubMed] [Google Scholar]
- 12. Mak A, Ren T, Fu EH, Cheak AA, Ho RC. A prospective functional MRI study for executive function in patients with systemic lupus erythematosus without neuropsychiatric symptoms. Semin Arthritis Rheum 2012;41:849–858. [DOI] [PubMed] [Google Scholar]
- 13. Zhu CM, Ma Y, Xie L, et al. Spatial working memory impairment in patients with non‐neuropsychiatric systemic lupus erythematosus: A blood‐oxygen‐level dependent functional magnetic resonance imaging study. J Rheumatol 2017;44:201–208. [DOI] [PubMed] [Google Scholar]
- 14. Montero‐Lopez E, Santos‐Ruiz A, Navarrete‐Navarrete N, et al. The effects of corticosteroids on cognitive flexibility and decision‐making in women with lupus. Lupus 2016;25:1470–1478. [DOI] [PubMed] [Google Scholar]
- 15. Bechara A, Damasio AR, Damasio H, Anderson SW. Insensitivity to future consequences following damage to human prefrontal cortex. Cognition 1994;50:7–15. [DOI] [PubMed] [Google Scholar]
- 16. Benussi A, Alberici A, Cantoni V, et al. Modulating risky decision‐making in Parkinson's disease by transcranial direct current stimulation. Eur J Neurol 2017;24:751–754. [DOI] [PubMed] [Google Scholar]
- 17. Nakamura M, Nestor PG, Levitt JJ, et al. Orbitofrontal volume deficit in schizophrenia and thought disorder. Brain 2008;131:180–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Tanabe J, Thompson L, Claus E, et al. Prefrontal cortex activity is reduced in gambling and nongambling substance users during decision‐making. Hum Brain Mapp 2007;28:1276–1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bechara A, Damasio AR. The somatic marker hypothesis: A neural theory of economic decision. Games Econ Behav 2005;52:336–372. [Google Scholar]
- 20. Li X, Lu ZL, D'Argembeau A, Ng M, Bechara A. The Iowa Gambling Task in fMRI images. Hum Brain Mapp 2010;31:410–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Tan EM, Cohen AS, Fries JF, et al. The 1982 revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum 1982;25:1271–1277. [DOI] [PubMed] [Google Scholar]
- 22. Bombardier C, Gladman DD, Urowitz MB, Caron D, Chang CH. Derivation of the SLEDAI. A disease activity index for lupus patients. The Committee on Prognosis Studies in SLE. Arthritis Rheum 1992;35:630–640. [DOI] [PubMed] [Google Scholar]
- 23. Gladman DD, Goldsmith CH, Urowitz MB, et al. The Systemic Lupus International Collaborating Clinics/American College of Rheumatology (SLICC/ACR) Damage Index for Systemic Lupus Erythematosus International Comparison. J Rheumatol 2000;27:373–376. [PubMed] [Google Scholar]
- 24. Nasreddine ZS, Phillips NA, Bedirian V, et al. The Montreal Cognitive Assessment, MoCA: A brief screening tool for mild cognitive impairment. J Am Geriatr Soc 2005;53:695–699. [DOI] [PubMed] [Google Scholar]
- 25. Pan W, Xi Z, Bo Z. The application of Montreal cognitive assessment (Chinese version) in diagnosing and assessing cognitive changes of mild cognitive impairment. Chin J Contemp Neurol Neurosurg 2012;12:193–197. [Google Scholar]
- 26. Bechara A, Tranel D, Damasio H. Characterization of the decision‐making deficit of patients with ventromedial prefrontal cortex lesions. Brain 2000;123(Pt 11):2189–2202. [DOI] [PubMed] [Google Scholar]
- 27. Emori A, Matsushima E, Aihara O, et al. Cognitive dysfunction in systemic lupus erythematosus. Psychiatry Clin Neurosci 2005;59:584–589. [DOI] [PubMed] [Google Scholar]
- 28. Ernst M. Decision‐making in a risk‐taking task. A PET study. Neuropsychopharmacology 2002;26:682–691. [DOI] [PubMed] [Google Scholar]
- 29. Bechara A, Damasio H, Damasio AR, Lee GP. Different contributions of the human amygdala and ventromedial prefrontal cortex to decision‐making. J Neurosci 1999;19:5473–5481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Northoff G, Grimm S, Boeker H, et al. Affective judgment and beneficial decision making: ventromedial prefrontal activity correlates with performance in the Iowa Gambling Task. Hum Brain Mapp 2006;27:572–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Christakou A, Brammer M, Giampietro V, Rubia K. Right ventromedial and dorsolateral prefrontal cortices mediate adaptive decisions under ambiguity by integrating choice utility and outcome evaluation. J Neurosci 2009;29:11020–11028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Katzav A, Solodeev I, Brodsky O, et al. Induction of autoimmune depression in mice by anti‐ribosomal P antibodies via the limbic system. Arthritis Rheum 2007;56:938–948. [DOI] [PubMed] [Google Scholar]
- 33. Kozora E, Ulug AM, Erkan D, et al. Functional magnetic resonance imaging of working memory and executive dysfunction in systemic lupus erythematosus and antiphospholipid antibody‐positive patients. Arthritis Care Res 2016;68:1655–1663. [DOI] [PubMed] [Google Scholar]
- 34. Mackay M, Bussa MP, Aranow C, et al. Differences in regional brain activation patterns assessed by functional magnetic resonance imaging in patients with systemic lupus erythematosus stratified by disease duration. Mol Med 2011;17:1349–1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Heekeren HR, Marrett S, Bandettini PA, Ungerleider LG. A general mechanism for perceptual decision‐making in the human brain. Nature 2004;431:859–862. [DOI] [PubMed] [Google Scholar]
- 36. de Jong BM, Pruim J, Sinnige LG, et al. Regional specific changes of cerebral metabolism in systemic lupus erythematosus identified by positron emission tomography. Eur Neurol 1999;41:187–193. [DOI] [PubMed] [Google Scholar]
- 37. Anderson KK, Ballok DA, Prasad N, Szechtman H, Sakic B. Impaired response to amphetamine and neuronal degeneration in the nucleus accumbens of autoimmune MRL‐lpr mice. Behav Brain Res 2006;166:32–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Zhang Z, Wang Y, Shen Z, et al. The neurochemical and microstructural changes in the brain of systemic lupus erythematosus patients: A multimodal MRI study. Sci Rep 2016;6:19026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Hou J, Lin Y, Zhang W, et al. Abnormalities of frontal‐parietal resting‐state functional connectivity are related to disease activity in patients with systemic lupus erythematosus. PLoS One 2013;8:e74530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Wang PI, Cagnoli PC, McCune WJ, et al. Perfusion‐weighted MR imaging in cerebral lupus erythematosus. Acad Radiol 2012;19:965–970. [DOI] [PMC free article] [PubMed] [Google Scholar]


