Abstract
Background and purpose
In randomized trials magnesium supplementation did not improve clinical outcome after aneurysmal subarachnoid haemorrhage (aSAH) on handicap scales. After aSAH, many patients have cognitive problems that may not translate into handicap. The effect of magnesium on cognitive outcome after aSAH was studied.
Methods
In total, 209 patients who had been included in the Magnesium for Aneurysmal Subarachnoid Haemorrhage (MASH‐2) trial in the University Medical Centre of Utrecht were studied. Patients had been randomized to 64 mmol magnesium sulfate daily or placebo during hospitalization. Three months after aSAH patients underwent a neuropsychological examination (NPE) consisting of six neuropsychological tests or a brief cognitive assessment. Poisson and linear regression analyses were used to analyse the effect of magnesium on cognition.
Results
In the magnesium group 53 (49.5%) of the 107 patients and in the placebo group 51 (50.0%) of the 102 patients scored lower than the median cognitive score [relative risk 0.99, 95% confidence interval (CI) 0.76–1.30]. Linear regression analyses showed no significant relationship between intervention and cognition (B = 0.05, 95% CI −0.15 to 0.33).
Conclusions
Treatment with magnesium has no effect on cognitive outcome after aSAH.
Keywords: aneurysm, cognition, intervention, subarachnoid haemorrhage
Introduction
Despite positive results from preclinical and phase II studies, large randomized clinical trials established that treatment with magnesium does not improve clinical outcome after aneurysmal subarachnoid haemorrhage (aSAH) 1, 2. The outcome in these trials was assessed by means of handicap scales. However, cognitive problems often hamper aSAH survivors and may not be detected with handicap scales 3. The aim of this study was to assess the effect of magnesium on cognition after SAH.
Methods
Study design and patients
Patients admitted in the University Medical Centre of Utrecht (UMCU) who had been included in the randomized controlled trial Magnesium for Aneurysmal Subarachnoid Haemorrhage (MASH‐2, registered ISRCTN 68742385, EudraCT 2006‐003523‐36) 1 were studied. In the UMCU all patients discharged home or to a rehabilitation institution are invited for our routine outpatient clinic 3 months post‐aSAH including neuropsychological examination (NPE). Patients in whom the aneurysm was not proven by computed tomography, magnetic resonance or conventional angiography and patients with more than 30% missing tests on the NPE were excluded. A part of the data in our study was derived from the MASH‐2 trial which complies with the Declaration of Helsinki and good clinical practice guidelines. All patients provided written and oral informed consent for this trial. The use of the additional neuropsychological data in the present study was approved by the UMCU Medical Ethics Committee. These data were derived from a prospective data collection according to clinical care as usual; therefore no additional informed consent was used.
MASH‐2
In the double‐blinded MASH‐2 study patients were randomized to 64 mmol magnesium or a matching placebo (saline) 1. Treatment was started within 4 days after the aSAH and continued 20 days after haemorrhage onset, or until hospital discharge or death if it occurred sooner. Functional outcome was measured by the modified Rankin Scale (mRS) 3 months after the aSAH.
Neuropsychological examination
Between November 2006 and August 2008, the NPE consisted of six standard neuropsychological tests covering memory, attention, executive functioning and visuospatial functioning. From September 2008 to March 2011, the NPE protocol was changed into a brief cognitive assessment. This assessment consisted of 18 items evaluating memory, language, attention, executive functioning, visuospatial functioning and orientation on a score ranging from 0 (unimpaired) to 2 (severely impaired). Overall cognitive functioning was measured with a sum score of all 18 items. (More information about both NPEs is presented in Data S1).
Analyses
Scores on both the NPE and the brief cognitive assessment were transformed into z‐scores based on means and standard deviations of all patients per type of assessment. The z‐scores of the individual tests of the NPE were summarized in a mean z‐score to parallel the z‐score of the brief cognitive assessment. The mean z‐scores of both assessments were grouped into one overall z‐score. Cognition was analysed both as a continuous (overall z‐score) and dichotomous variable (dichotomized by the median of the overall z‐score). The effect of magnesium was assessed by comparing patients who received magnesium with those on placebo with linear and Poisson regression analyses. Moreover, a multiple regression analysis was performed including adjustments for other possible determinants of cognition that changed the magnitude of the B (linear regression) or relative risk (RR) (Poisson regression) by >5%. These determinants were age, sex, educational level [using the Dutch Verhage classification system ranging from 1 (did not complete primary school) to 7 (university degree)] 4, clinical condition on admission measured with the World Federation of Neurosurgeons SAH grading scale 5, method of aneurysm treatment (clipping or endovascular) and the neurological complications delayed cerebral ischaemia and hydrocephalus. Because there was only one patient with aneurysmal rebleeding, this neurological complication was not included as a possible determinant. After the multiple regression analyses, a subgroup analysis was performed according to the type of cognitive assessment.
Results
In total, 209 patients were included (Fig. 1); their baseline characteristics are listed in Table 1. The mean interval for the NPE was 12 weeks after aSAH.
Figure 1.
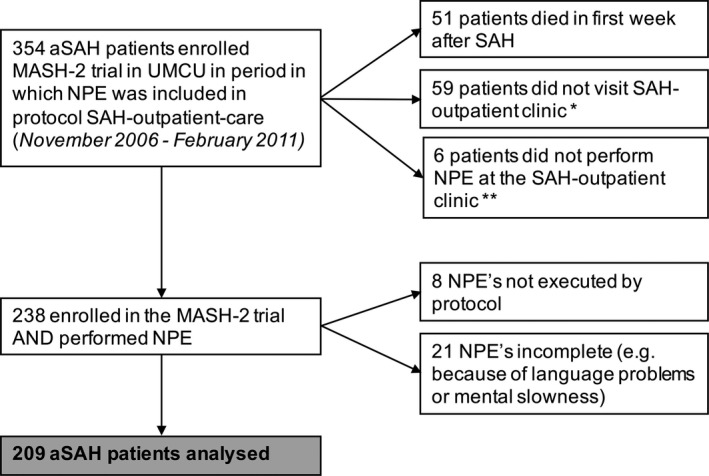
Patient inclusion. MASH, Magnesium for Aneurysmal Subarachnoid Haemorrhage; UMCU, University Medical Centre Utrecht; aSAH, aneurysmal subarachnoid haemorrhage; NPE, neuropsychological examination. *Reasons varied from being hospitalized, living abroad to no‐show. **Because of visual problems, already performed NPE elsewhere or patient's refusal.
Table 1.
Characteristics of aSAH patients (n = 209)
| Magnesium (n = 107) | Placebo (n = 102) | |||
|---|---|---|---|---|
| n | % | n | % | |
| Demographic characteristics | ||||
| Women | 84 | 79 | 81 | 79 |
| Mean age in years (SD) | 53.7 (11.7) | 55.7 (11.5) | ||
| Educational level | ||||
| Low–moderate (Verhage 1–5) | 82 | 77 | 81 | 79 |
| aSAH characteristics | ||||
| WFNS | ||||
| I–III, GCS 13–15 | 92 | 86 | 87 | 85 |
| Aneurysm treatmenta | ||||
| Clipping | 45 | 42 | 45 | 44 |
| Coiling | 61 | 57 | 57 | 56 |
| Neurological complications | ||||
| Rebleeding | 0 | 0 | 1 | 1 |
| DCI | 19 | 18 | 20 | 20 |
| Hydrocephalus | 21 | 20 | 16 | 16 |
| Outcome 3 months after aSAH | ||||
| Poor functional outcome | ||||
| Slight/moderate disability (mRS 2) | 45 | 42 | 46 | 45 |
| Moderate/severe disability (mRS 4) | 6 | 6 | 7 | 7 |
| Cognitive outcome | ||||
| Median (range) overall z‐score | 0.1 (−3.0 to 1.3) | 0.1 (−3 to 1.2) | ||
| Overall z‐score lower than median | 53 | 50 | 51 | 50 |
aSAH, aneurysmal subarachnoid haemorrhage; DCI, delayed cerebral ischaemia; GCS, Glasgow coma score; mRS, modified Rankin Scale; WFNS, World Federation of Neurosurgeons. aOne patient was not treated for a basilar top aneurysm because both posterior cerebral arteries originated from this aneurysm and both carotid arteries were occluded.
Neuropsychological examination
Patients who performed the NPE did not differ substantially from patients who completed the brief cognitive assessment with respect to the distribution of the intervention and the demographic and aSAH characteristics (Table 1). For the distribution and median split of the z‐scores of both NPEs see Data S2.
Analyses
In the magnesium group 53 (49.5%) of the 107 patients and in the placebo group 51 (50.0%) of the 102 patients scored lower than the median cognitive score (RR = 0.99, 95% CI 0.76–1.30). No significant relationship was found between magnesium and cognition in the linear regression analyses (mean overall z‐scores: magnesium group 0.05, placebo group −0.04, B = 0.09, 95% CI −0.15 to 0.33). Upon adjustment the RR estimate hardly changed whereas B was influenced by age (B = 0.05, 95% CI −0.18 to 0.28), level of education (B = 0.08, 95% CI 0.16–0.30), delayed cerebral ischaemia (B = 0.08, 95% CI −0.15 to 0.31) and hydrocephalus (B = 0.11, 95% CI 0.12– 0.35) yielding a multivariable estimate of 0.05, 96% CI −0.17 to 0.26. Subgroup analyses showed no differences between the effect of magnesium when analysing patients with an NPE (B = −0.06, 95% CI −0.32 to 0.20; RR = 1.04, 95% CI 0.70–1.54) or the brief cognitive assessment (B = 0.11, 95% CI −0.23 to 0.44; RR = 0.98; 95% CI 0.67–1.43).
Discussion
Magnesium does not influence cognitive outcome after aSAH. To our knowledge this is the first study that used cognition as the outcome measure in a randomized trial of magnesium in aSAH patients. Patients were retrieved from the MASH‐2 study 1, which is the largest randomized controlled trial investigating magnesium in aSAH patients to date. Not all patients of the MASH‐2 trial met the inclusion criteria of the current study but, given the criterion that patients had to be discharged home or to a rehabilitation institution, a large study population with relatively good outcome remained.
A limitation of our study is that two different measures of cognitive outcome were used. Cognitive data were derived from usual clinical care in which halfway through the study period a change was made from a formal NPE to a brief cognitive assessment. A subgroup analysis, however, showed no differences between the effects of magnesium when analysing the two measures of cognitive outcome separately.
Conclusions
This study shows that magnesium has no effect on cognitive outcome after aSAH.
Disclosure of conflicts of interest
The authors declare no financial or other conflicts of interest.
Supporting information
Data S1. Measures of cognition.
Data S2. Distribution of cognitive scores.
Acknowledgements
MASH‐2 was funded by the Netherlands Heart Foundation (grant number 2005BO16).
References
- 1. Dorhout Mees SM, Algra A, Vandertop WP, et al Magnesium for aneurysmal subarachnoid haemorrhage (MASH‐2): a randomised placebo‐controlled trial. Lancet 2012; 380: 44–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wong GK, Boet R, Poon WS, et al Intravenous magnesium sulphate for aneurysmal subarachnoid hemorrhage: an updated systemic review and meta‐analysis. Crit Care 2011; 15: 52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. de Haan R, Limburg M, Bossuyt P, et al The clinical meaning of Rankin ‘handicap’ grades after stroke. Stroke 1995; 26: 2027–2030. [DOI] [PubMed] [Google Scholar]
- 4. Verhage F. Intelligentie en leeftijdonderzoek bij Nederlanders van twaalf tot zevenenzeventig jaar [Intelligence and age: a study among Dutch people from age 12 to 77] Assen, 1964.
- 5. Teasdale GM, Drake CG, Hunt W, et al A universal subarachnoid hemorrhage scale: report of a committee of the World Federation of Neurosurgical Societies. J Neurol Neurosurg Psychiatry 1988; 51: 1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1. Measures of cognition.
Data S2. Distribution of cognitive scores.


