Abstract
Objectives
The aim of this study was to compare three‐dimensional alterations following the use of autogenous versus allogeneic onlay grafts for augmentation at single tooth defects.
Materials and methods
Alveolar bone width at specific implant sites were assessed using sagittal and cross‐sectional CBCT images prior grafting and at three subsequent time points. Twenty‐one patients received autogenous bone blocks harvested from the retromolar region and another 21 patients received freeze‐dried cancellous allogeneic bone blocks.
Results
The vertical and horizontal dimensions did not significantly differ between autogenous and allogeneic bone grafts at any time point. In addition, there were no statistically significant differences in graft remodeling rates between autogenous (mean shrinkage rate after 12 months: 12.5% ± 7.8%) and allogeneic onlay grafts (mean shrinkage rate after 12 months: 14.4% ± 9.8%).
Conclusions
Freeze‐dried cancellous allogeneic bone blocks showed equivalent volumetric shrinkage rates as autogenous bone blocks when used for treating circumscribed bone defects classified as Type‐II to Type‐IV according to the ITI‐treatment guide categories. Therefore, it is not necessary to over‐contour the alveolar ridge when using allogeneic blocks for treating single tooth defects, but to apply the same procedure as when using autogenous blocks.
Keywords: allogeneic, alveolar ridge defect, augmentation, autogenous, dental implants, graft remodeling
1. INTRODUCTION
Despite the development of diameter‐reduced and short implants, challenging situations for proper implant placement due to advanced bone loss are a regular occurrence. In these situations, guided bone regeneration with the purpose of increasing the alveolar ridge dimensions to enable a successful dental implant installation remains inevitable. The most widely used material for this kind of surgery is autogenous bone, which is currently considered the gold standard due to its osteoconductivity, osteoinductivity, and osteogenecity (Jensen & Sindet‐Pedersen, 1991; Laurencin, Khan, & El‐Amin, 2006; Listrom & Symington, 1988). Autogenous bone grafts can be harvested intraorally from the mandibular ramus and the chin, whereas the iliac crest represents the most frequently used extraoral donor site (Khoury & Hanser, 2015; Khoury, Keller, & Keeve, 2017; Nkenke, Schultze‐Mosgau, Radespiel‐Troger, Kloss, & Neukam, 2001; Sakkas, Wilde, Heufelder, Winter, & Schramm, 2017).
However, harvesting an autogenous bone block is associated with the risk of donor site morbidity, postoperative pain, increased blood loss, and infections. Further disadvantages include a limited bone quantity and unpredictable bone quality (Nissan, Marilena, Gross, Mardinger, & Chaushu, 2011; Nkenke & Stelzle, 2009). In addition, autografts might also be subject to excessive remodeling after surgery, especially when no barrier membranes are used (Daugela, Cicciu, & Saulacic, 2016). Mean graft resorption of onlay grafts harvested from the iliac crest was reported to range between 15% (Dreiseidler et al., 2016) and 25% (Mertens et al., 2013). Based on these reports, it is recommended to over‐contour the ridge with grafting material in order to ensure sufficient ridge volume for adequate support of the implant body and prevent the threat of exposure (Hanser & Khoury, 2016; Nkenke & Neukam, 2014).
An ideal grafting material for ridge augmentation should be easy to handle and apply, should have excellent biocompatibility in order to enable graft integration or remodeling and be a supply highly osteoconductive scaffold for osteogenic cells (Aslan et al., 2016). Bone allografts have been demonstrated to excel other grafting materials with respect to the aforementioned criteria. The benefits of allogeneic bone blocks include unlimited supply, decreased operative trauma and blood loss, absence of donor site morbidity, and extremely low antigenic potential (Blume et al., 2017; Chavda & Levin, 2017; Corbella, Taschieri, Francetti, Weinstein, & Del Fabbro, 2017; Laino, Iezzi, Piattelli, Lo Muzio, & Cicciu, 2014).
Preliminary publications on the successful clinical application of processed decellularized block allografts for alveolar ridge augmentation by Keith and colleagues have been followed by a multitude of reports underscoring their great clinical performance (Keith, 2004; Keith et al., 2006; Petrungaro & Amar, 2005). Although a large volume of data on the efficacy of processed allogeneic bone blocks can be derived from these publications, patient collectives and the level of evidence in most individual publications is rather low so that there is an unmet need for further verification of this material (Araujo et al., 2013; Elangovan, Barwacz, Antonious, Swenson, & Avila‐Ortiz, 2017). As mineralized bone allograft represents the only grafting material which has been demonstrated to function analogous to traditional autogenous block grafting by multiple authors, comparative studies on their clinical performance in horizontal and vertical bone augmentation are of high scientific relevance (Al‐Abedalla et al., 2015; Amorfini, Migliorati, Signori, Silvestrini‐Biavati, & Benedicenti, 2014).
The purpose of this study is the retrospective evaluation of a novel, cancellous allogeneic bone block derived from the bone of femoral heads of living human donors with respect to horizontal and vertical bone gain and volume stability. These findings are compared to results achieved with autogenous bone blocks in alveolar ridge augmentation. We compared the 12‐month remodeling rate of cancellous allogeneic bone blocks to the remodeling rate of cortical autogenous bone blocks harvested from the retromolar region. To the best of our knowledge, this is the first longitudinal clinical study comparing freeze‐dried cancellous allogeneic with autogenous bone blocks for vertical and horizontal alveolar ridge augmentation.
2. MATERIALS AND METHODS
2.1. Patients
The study was performed in accordance with the STROBE guidelines. A total of 42 patients with one tooth missing and insufficient bone quantity for direct implant placement were enrolled into this retrospective study and underwent autogenous or allogeneic bone block augmentation procedures. All patients were fully informed about the surgical procedures and treatment alternatives. The inclusion criterion was the presence of a clinically relevant bone atrophy of the alveolar ridge in the predominantly horizontal and/or vertical plane as identified by cone beam computed tomography (CBCT) para‐axial reconstruction images. The minimum defect size for inclusion was a Type‐II bone defect, defined according to the ITI‐treatment guide categories (Chen & Buser, 2008). Exclusion criteria consisted of a history of radiotherapy in the head and neck region, systemic disease that would contraindicate oral surgery, uncontrolled periodontal disease, bruxism, a smoking habit or alcoholism, pregnancy, psychiatric problems, and/or use of medications known to alter bone healing.
Prior to surgery, patients were presented with the two available procedures (autogenous or allogeneic bone block augmentation). The patients chose one of the two procedures themselves. In order to reduce potential sources of bias, patients were selected for each of the two study groups so that they did not differ in demographic, anamnestic or therapeutic characteristics (Table 1). After screening the available clinical data, 21 patients with comparable demographic characteristics were allocated to each group. A total of 21 patients received a cortical autogenous block graft and 21 patients received a cancellous allogeneic block graft for alveolar ridge augmentation. The study population comprised of 14 males and 28 females, in total 42 patients.
Table 1.
Demographic characteristics of the patient groups
| Autogenous bone | Allogeneic bone | |
|---|---|---|
| Number of patients | 21 | 21 |
| Gender | 5 males; 16 females | 9 males; 12 females |
| Age (mean ± standard deviation) | 46.9 ± 12.1 | 48.9 ± 15.3 |
| Source of bone material | Harvested from retromolar | Freeze‐dried bone block |
| Defect classification |
Type‐II: n = 9 Type‐III: n = 11 Type‐IV: n = 1 |
Type‐II: n = 14 Type‐III: n = 6 Type‐IV: n = 1 |
| Implant diameters (mm; median and range) | 4.1 (3.3–4.8) | 4.1 (3.3–4.8) |
2.2. Surgical procedure
The partially edentulous patients underwent a thorough initial periodontal examination, including the assessment of plaque, gingivitis, and probing depth. Immediately before the operation, the patients were instructed to rinse their mouth with 0.2% chlorhexidine mouthwash for 1 min. A two‐stage approach with implant placement after six months of healing with surgical intervention performed under infiltration of local anesthetics (Ultracain DS forte, Sanofi‐Aventis, Vienna, Austria) was used for all patients. The muco‐periosteal flap was prepared by a crestal incision together with a vertical releasing incision and gently elevated from the native bone tissue to allow complete visualization of the defect and surrounding bone. Any soft tissue remnants were removed from the bone surface and the native bone was perforated with drills during irrigation with saline to ensure vascularization of the block graft and the recipient site.
The autogenous and allogeneic bone blocks were both used as full blocks. The bone blocks were adapted to the defect morphology and fixated onto the host bone site using 1.5 mm osteosynthesis screws (Komet, Germany). Resorbable collagen membranes made from porcine pericardium (Jason® membrane, botiss biomaterials GmbH, Berlin, Germany) were used for coverage of the augmentation sites. The muco‐periosteal flaps were repositioned with mattress and interrupted non‐resorbable sutures. An example of the surgical procedure is illustrated in Figure 1.
Figure 1.
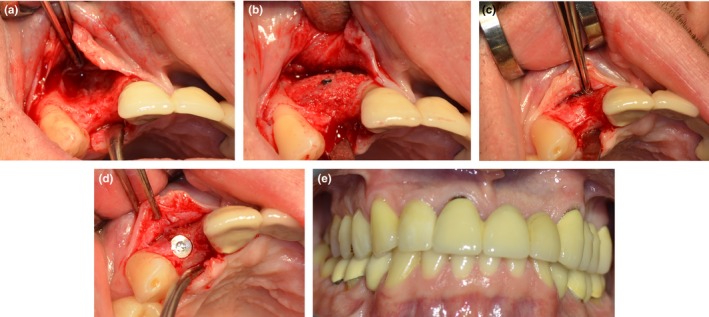
Example of an allogeneic bone augmentation in a patient with a Type‐III bone defect of the alveolar ridge. (a) Situation before augmentation; (b) Situation directly after augmentation; (c) Situation before implantation, 6 months after augmentation; (d) Situation after implantation; (e) Final situation, 12 months after implantation
Routine postoperative care included administration of amoxicillin and clavulanic acid (625 mg, administered orally, three times a day for 4 days), ibuprofen (600 mg, administered orally, every 6 hr as needed), and mouthwash (0.2% chlorhexidine, three times daily for 7 days).
The patients were recalled at monthly intervals for a period of 6 months to detect possible complications, such as infection, pain, discomfort, graft exposure, and graft mobility. Graft stability was assessed at the time of dental implant placement. At re‐entry of the surgical site, a crestal incision was placed along the initial incision line in order to prevent the formation of additional scar tissue as a result of installing the implant.
2.3. Autogenous bone augmentation
Bone blocks were harvested from the external oblique line of the mandible with piezo surgery. At the donor site, a paramarginal incision was made in the molar area if teeth were present, or on top of the alveolar crest in the case of an edentulous ridge. A full‐thickness flap was elevated, exposing the external oblique ridge and the lateral aspect of the ramus as well as the lateral aspect of the mandibular body.
The osteotomy cuts were prepared with a piezoelectric instrument (Piezomed, W&H Dentalwerk, Bürmoos, Austria). The block was removed with a straight, thin chisel without the need for hammering. The flap was sutured using single sutures. The autogenous bone blocks were adapted to the defect site and then grafted in combination with autogenous bone particles scraped from the same lamina (Khoury & Hanser, 2015; Khoury et al., 2017).
2.4. Allogeneic bone augmentation
maxgraft® allogeneic bone blocks (botiss biomaterials GmbH) are made from cancellous bone from explanted femoral heads provided by living donors subjected to hip arthroplasty treatment. Following an assessment of the donor's health status, the femoral heads are forwarded to a tissue bank where they are split, cleaned, degreased in an ultrasonic bath, and wet‐chemically purified in order to remove all living cells and potential contaminants. Subsequent lyophilization of the bone material ensures long‐term storability at room temperature, while gamma irradiation is applied for the terminal sterilization. Due to the molecular and morphological similarities to the patient's own bone, the final allograft product, which consists of mineralized human collagen, exhibits ideal osteoconductive capacities that qualify this material for the application in guided bone regeneration (GBR) procedures (Otto, Kleye, Burian, Ehrenfeld, & Cornelius, 2017). The grafting procedure with allogeneic bone grafts was done analogous to that with autogenous bone blocks so that the cancellous allogeneic blocks were also adapted to the defect situation and fixed onto the ridge with osteosynthesis screws.
2.5. Implants
Every patient received one titanium implant per augmented region. The inserted implants were from Straumann (n = 35; Type SLActive®; Straumann Holding AG, Basel, Switzerland), bredent (n = 6; blueSKY; bredent medical GmbH & Co.KG, Senden, Germany) and BIOMET 3i (n = 1; BIOMET 3i; Munich; Germany).
2.6. Radiographic analyses
Every patient was subjected to three‐dimensional x‐ray diagnostics (CBCT), followed by computer‐aided planning of the augmentation and subsequent implantation. In total four CBCTs were recorded for each patient, one before treatment, one directly after augmentation, one after 6 months of healing, and one after 12 months. At each time point, the alveolar bone levels were measured in their height, width, and depth at the cervical level, the middle height of the defect, and at the apical level. An illustration of the measured regions is shown in Figure 2.
Figure 2.

Radiographic evaluation of an alveolar ridge defect before (a) and 6 months after (b) augmentation. The green line at the left side corresponds to the vertical height of the bone in the defect area before augmentation. The green lines at the right side correspond to the defect depth at the apical, at the middle, and at the cervical level before augmentation. The same measurements were taken after augmentation, at 6 and at 12 months after surgery
The CBCT machine used to acquire all images was the Carestream CS 9300 (Carestream Health Inc.). The imaging parameters were set with a dose of 120 mGy cm2, tube current of 18.66 mAs, and a voxel size of 90 μm × 90 μm × 90 μm. The selected field of view was 5 cm × 5 cm. Data from the scans were saved in the Digital Imaging and Communications in Medicine (DICOM) format and reconstructed with the Carestream implant planning software program.
All patients needed single‐site or single‐implant treatments. All measurements were made on parasagittal sections perpendicular to the longitudinal axis of the adjacent teeth. The CBCT was oriented transversally through the teeth neighboring the defect in such a way that the nerve canal of the tooth, which was mesial to the defect region, was clearly visible. The nerve canal of the mesial tooth was defined as an anatomic reproducible landmark and a straight line was drawn through the middle of the defect region between the two neighboring teeth. The mesial tooth was used as a reference for the apical and the crestal bone levels. The distances were obtained using a software ruler. The same anatomic landmarks and distances were used for measurements on CBCT at the defined time intervals. The following measurements were taken (Figures 3 and 4):
Defect height (mm): distance between the apical and crestal bone level in the middle of the defect region; represented by line “h” in Figure 4.
Apical defect width (mm): distance between the apical root tips of the neighboring teeth; represented by line “e” in Figure 4.
Defect width in the middle zone (mm): distance between the roots of the neighboring teeth in the middle of the defect height; represented by line “d” in Figure 4.
Cervical defect width (mm): distance between the crestal bone levels of the neighboring teeth; represented by line “a” in Figure 4.
Apical defect depth (mm): distance between the labial/buccal and palatal edges of the jaw crest at the level of the apical tips of the neighboring teeth, but in the middle of the defect area; represented by line “f” in Figure 4.
Defect depth in the middle zone (mm): distance between the labial/buccal and palatal edges of the jaw crest at the level of the middle zone; represented by line “c” in Figure 4.
Cervical defect depth (mm): distance between the labial/buccal and palatal edges of the jaw crest at the cervical level; represented by line “b” in Figure 4.
Figure 3.
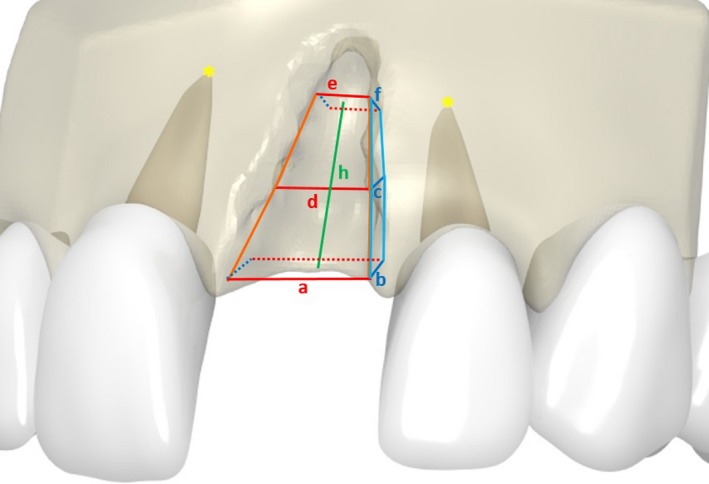
3D‐Model for visualizing our mathematical approach for calculating the volume of the defect
Figure 4.
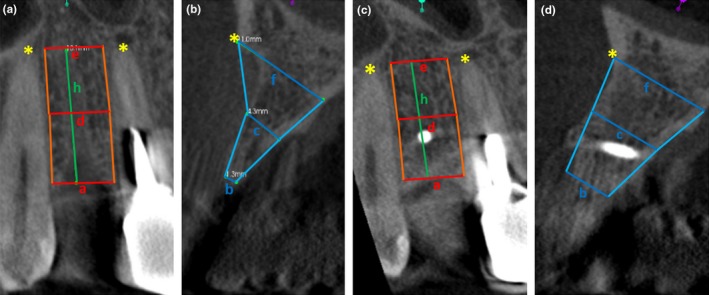
2‐D Model for visualizing our mathematical approach for calculating the volume of the defect. The lines correspond to the defect widths and depths as described in the section “Radiographic analyses”
2.7. Mathematics and statistics
Based on the radiographic measurements, the graft volume was inferred as the sum of the volumes of two superimposed four‐sided rectangular frustums of pyramids. The formula for obtaining the volume of one pyramid trunk is: , where g is the height of the truncated pyramid, B is the base area, and P is the peak area.
The two pyramid trunks are depicted in Figure 4. The formula for obtaining the volume of the entire defect was therefore:
Statistical analyses were performed with IBM SPSS (version 23; International Business Machines Corp., Armonk, NY, USA). For a graphical condensation of data, the software GraphPad Prism (La Jolla, CA, USA) was applied. For descriptive statistics, mean values and standard deviations were calculated. For a statistical power calculation, we made the following assumptions: the average resorption rate for autogenous grafts from the calvarium was 8.44% (SD 3.64), while the average resorption rate for grafts from the iliac crest was 24.16% (SD 8.47) (Mertens et al., 2013). Since the allogeneic material that we used was cancellous bone from explanted femoral heads, and since cancellous bone is also harvested from the iliac crest for autogenous bone augmentation, we assumed that the resorption rate of allogeneic material used in this study was similar to the resorption rate from the iliac crest. With these assumptions, we had statistical power of 100% to detect clinically relevant differences between the two study groups with 21 patients each and with a level of significance of 5%.
Pearson's chi‐squared test was applied to sets of unpaired categorical data to evaluate the likelihood that any observed difference between the sets was due to chance. An independent sample t test was used when two separate sets of independent and identically distributed samples were obtained, and their population means were compared to each other.
For determining the intra‐rater reliability, 70 distance measurements as described in the section “Radiographic analyses” were repeated six months after the first measurement. To assess the intra‐rater reliability in this test–retest setting, the Pearson's product‐moment correlation coefficient was determined. In addition, a paired sample t test was applied to compare the results from the first measurement to the results from the second measurement.
In order to validate our approach of inferring the graft volume, we also determined the volume of the graft in the area of the augmentation site using the program CoDiagnostix9 (Dental Wings, Straumann Holding AG) for 10 randomly selected patients. The segmentation of the area was based on the inclusion of all Hounsfield Units in the area and led to the determination of the volume in ml. The region of interest had to be selected manually. The volume was then determined in ml by the program CoDiagnostix9.
For comparing the two different procedures of determining the graft volume, and for an assessment of concordance of intraindividual measurement values, we generated a scatterplot of the measured points and a Bland–Altman Plot (Bland & Altman, 1986, 1999 ) and calculated the bias‐corrected concordance correlation coefficient (Koch & Spörl, 2007). A one‐sample t test was applied for testing the null hypothesis that the mean value of the difference between the two measurement techniques was equal to 0.
3. RESULTS
All patients were followed for up to 12 months. There were no signs of infection, wound dehiscence, block graft exposure, or other postoperative complications during the healing period following bone augmentation. At the time of implant placement, all autogenous and allogeneic bone blocks were successfully integrated into the recipient site. In all patients, the grafted bone remained stable during drilling and implant placement, without graft separation, and all implants were successfully stabilized and restored three months after implant placement. All patients received a fixed implant‐supported single crown. No implant was lost after loading during follow‐up.
The intra‐rater reliability, assessed as Pearson's product‐moment correlation coefficient, was 0.964 (p < 0.001). The average difference between first and second measurement was 0.11 ± 0.87 mm (p = 0.268), corresponding to an average intra‐rater error of 1.6%.
There was a good agreement between the two measurement techniques for assessing the graft volume (the pyramid trunk approach and the Hounsfield units approach), as can be seen in Figure 5. The bias‐corrected concordance correlation coefficient between the two different measurement techniques was 0.756, indicating a very strong heuristic agreement (Koch & Spörl, 2007). The mean difference between the two different measurement techniques was −0.1414 (95%‐confidence interval of the difference: −0.237 to −0.045), as can be seen in Figure 6. The mean value of the difference differed significantly from 0 on the basis of a one‐sample t test, thereby indicating the presence of a fixed bias.
Figure 5.

Scatterplot of measurement pairs with the graft volume in ml, assessed with the pyramid trunk approach, on the x‐axis and with the graft volume in ml, assessed with the Hounsfield units approach, on the y‐axis. The line corresponds to the regression with the Hounsfield units approach as depending and the pyramid trunk approach as independent variable
Figure 6.
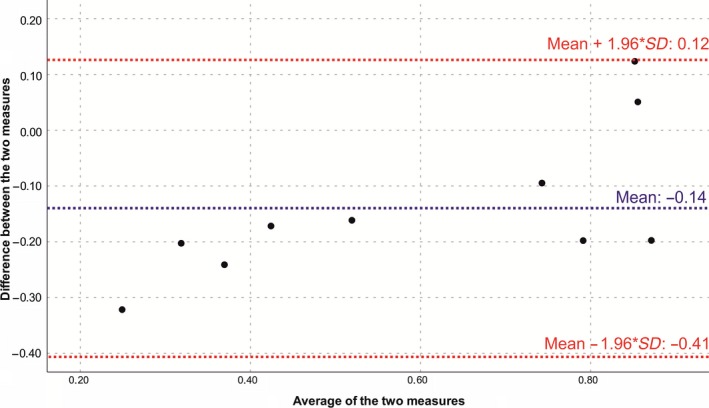
Bland–Altman plot for the comparison of the two measurement techniques for assessing the graft volume. The differences between the two techniques were plotted against the averages of the two techniques. Horizontal lines were drawn at the mean difference, and at the limits of agreement, which were defined as the mean difference plus and minus 1.96 times the standard deviation of the differences
3.1. Demographics
The demographic characteristics of the study population are summarized in Table 1. Gender was distributed evenly between the two study groups (chi‐squared test; p = 0.326). The bone defects were classified as Type‐II (non‐self‐containing dehiscence defect with bone eminences next to adjacent teeth) for 23 patients, as Type‐III (combined horizontal and vertical defect) for 17 patients, and as Type‐IV (through‐and‐through‐defect) for two patients according to the ITI‐treatment guide categories (Chen & Buser, 2008). There was no significant difference in the distribution of bone defects between the two study groups (chi‐squared test; p = 0.278). The patients were on average 48.0 ± 13.7 years old. There was no significant difference in age between the two study populations (t test, p = 0.642). The implants varied in diameter between 3.3–4.8 mm, with an average diameter of 4.0 mm and no significant difference in diameter size between the two study populations (t test, p = 0.464).
3.2. Vertical gain
The mean vertical increase directly after augmentation was 1.4 ± 2.2 mm for autogenous blocks and 0.7 ± 1.4 mm for allogeneic blocks. There were no statistically significant differences between autogenous and allogeneic bone grafts in the vertical gain after augmentation, after 6 months or 12 months (Table 2). The vertical dimensions did not differ significantly between autogenous and allogeneic bone grafts at any point in time (Figure 7 and Supporting Information Table S1).
Table 2.
Vertical gain in relation to the defect before therapy at three time points
| Vertical gain (mm) compared to the defect height before therapy | Autogenous bone | Allogeneic bone | p‐Value |
|---|---|---|---|
| Vertical gain after augmentation | 1.4 ± 2.2 | 0.7 ± 1.4 | 0.184 |
| Vertical gain after 6 months | 1.0 ± 1.9 | 0.2 ± 1.2 | 0.110 |
| Vertical gain after 12 months | 0.8 ± 1.9 | 0.1 ± 1.2 | 0.110 |
Figure 7.
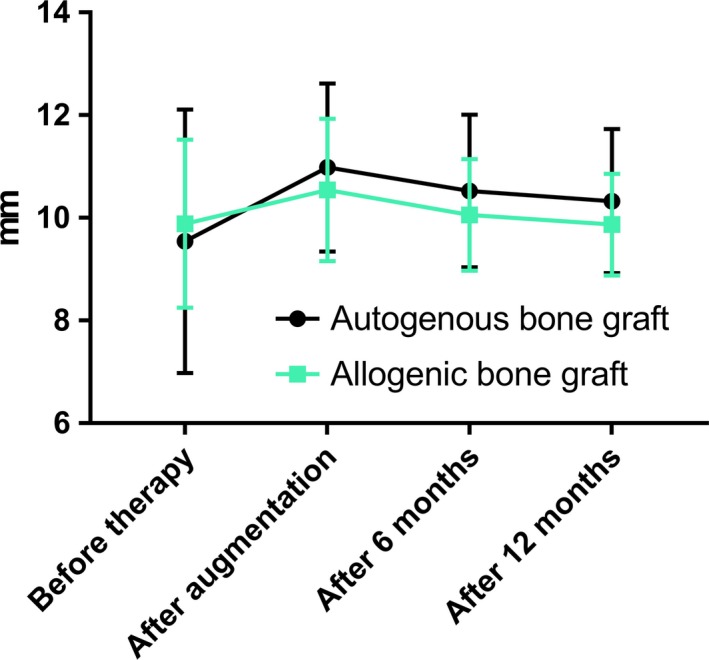
Comparison of defect heights before therapy, post augmentation, after 6 months and after 12 months
3.3. Horizontal gain
The mean horizontal gain at the apical level after augmentation was 1.6 ± 1.5 mm for autogenous grafts and 2.6 ± 2.5 mm for allogeneic blocks. There were no statistically significant differences between autogenous and allogenic bone grafts in the horizontal gain at the apical level after augmentation, after 6 months or after 12 months (Table 3).
Table 3.
Horizontal gain compared to the apical defect depth before therapy at three time points
| Horizontal apical gain (mm) | Autogenous bone | Allogeneic bone | p‐Value |
|---|---|---|---|
| Apical gain after augmentation | 1.6 ± 1.5 | 2.6 ± 2.5 | 0.092 |
| Apical gain after 6 months | 1.2 ± 1.4 | 2.1 ± 2.4 | 0.147 |
| Apical gain after 12 months | 1.0 ± 1.4 | 1.9 ± 2.4 | 0.128 |
The mean horizontal gain at the cervical level after augmentation was 5.6 ± 1.5 mm for autogenous grafts and 5.5 ± 1.3 mm for allogeneic blocks. There were no statistically significant differences between autogenous and allogeneic bone grafts in the horizontal gain at the cervical level after augmentation, after 6 months or after 12 months (Table 4) and horizontal dimensions did not differ significantly between autogenous and allogeneic bone grafts at any point in time (Figure 8, Supporting Information Table S3–S8).
Table 4.
Horizontal gain compared to the cervical defect depth before therapy at three time points
| Horizontal cervical gain (mm) | Autogenous bone | Allogeneic bone | p‐Value |
|---|---|---|---|
| Cervical gain after augmentation | 5.6 ± 1.5 | 5.5 ± 1.3 | 0.928 |
| Cervical gain after 6 months | 5.2 ± 1.6 | 5.2 ± 1.4 | 0.975 |
| Cervical gain after 12 months | 5.1 ± 1.5 | 5.2 ± 1.4 | 0.931 |
Figure 8.
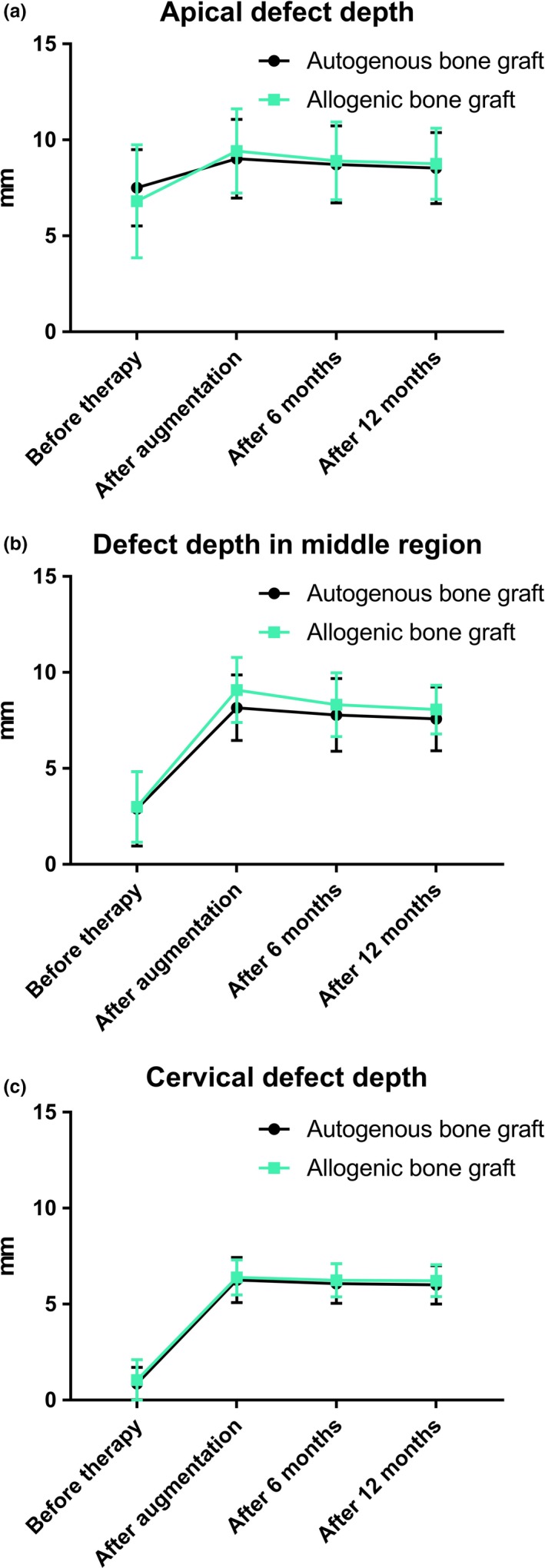
Comparison of horizontal defect depths before therapy, post augmentation, after 6 and 12 months at the apical level (a), in the middle of the vertical defect height (b), and at cervical level (c)
3.4. Remodeling
The overall volume gain after augmentation was 543.9 ± 431.5 mm3 for autogenous grafts and 508.1 ± 187.3 mm3 for allogeneic blocks. There were no statistically significant differences between autogenous and allogeneic bone grafts in the overall volume gain after augmentation, after 6 or 12 months (Table 5).
Table 5.
Volume gain in relation to the defect before therapy at three time points
| Volume gain (mm3) compared to the defect volume before therapy | Autogenous bone | Allogeneic bone | p‐Value |
|---|---|---|---|
| Volume gain after augmentation | 543.9 ± 431.5 | 508.1 ± 187.3 | 0.729 |
| Volume gain after 6 months | 455.7 ± 354.4 | 411.1 ± 159.4 | 0.602 |
| Volume gain after 12 months | 423.4 ± 326.8 | 385.9 ± 147.3 | 0.635 |
After 6 months, there was no significant difference in the vertical graft remodeling rate between autogenous grafts (mean percentage of graft shrinkage: 3.9% ± 5.6%) and allogeneic blocks (mean percentage of graft shrinkage: 4.2% ± 5.5%) and in the horizontal graft remodeling rate (autogenous: 5.3% ± 6.2%; allogeneic: 4.4% ± 7.1%) (Table 6). Also after 12 months, there was no significant difference in the vertical graft remodeling rate between autogenous blocks (mean percentage of graft shrinkage: 5.7% ± 5.6%) and allogeneic blocks (mean percentage of graft shrinkage: 5.9% ± 6.1%) or in the horizontal graft remodeling rate (autogenous: 6.3% ± 6.2%; allogeneic: 4.8% ± 7.2%) (Table 6).
Table 6.
Average percentages of graft shrinkage
| Graft shrinkage | Autogenous bone | Allogeneic bone | p‐Value |
|---|---|---|---|
| Vertical graft shrinkage at 6 months | 3.9% ± 5.6% | 4.2% ± 5.5% | 0.850 |
| Vertical graft shrinkage at 12 months | 5.7% ± 5.6% | 5.9% ± 6.1% | 0.877 |
| Horizontal cervical graft shrinkage at 6 months | 5.3% ± 6.2% | 4.4% ± 7.1% | 0.902 |
| Horizontal cervical graft shrinkage at 12 months | 6.3% ± 6.2% | 4.8% ± 7.2% | 0.706 |
| Overall graft shrinkage at 6 months | 9.1% ± 7.3% | 11.4% ± 9.7% | 0.390 |
| Overall graft shrinkage at 12 months | 12.5% ± 7.8% | 14.4% ± 9.8% | 0.486 |
Based on the volume of the bone graft that was assessed immediately after augmentation and on the volumes that were measured after 6 and 12 months (Figure 9 and Supporting Information Table S2), the mean percentage of graft shrinkage was calculated. After 6 months, there was no significant difference in the volumetric graft remodeling rate between autogenous blocks (mean percentage of graft shrinkage: 9.1% ± 7.3%) and allogeneic blocks (mean percentage of graft shrinkage: 11.4% ± 9.7%) (Table 6). Also after 12 months, there was no significant difference in the three‐dimensional graft remodeling rate between autogenous blocks (mean percentage of graft shrinkage: 12.5% ± 7.8%) and allogeneic blocks (mean percentage of graft shrinkage: 14.4% ± 9.8%) (Table 6).
Figure 9.
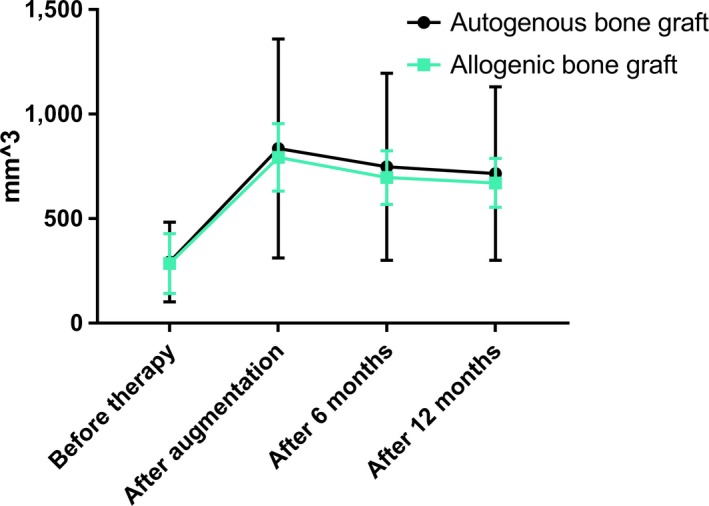
Comparison of the defect volumes before therapy, post augmentation, after 6 and 12 months
4. DISCUSSION
The present study evaluated horizontal and vertical bone gain and bone remodeling rates at 6 and at 12 months after alveolar ridge augmentation with both autogenous bone blocks harvested from the external oblique line of the mandible and with allogeneic bone blocks.
Remodeling of the alveolar ridge is a multifactorial process resulting from a combination of anatomical, metabolic, genetic, and biomechanical factors (Larsson et al., 2016; Masaki, Nakamoto, Mukaibo, Kondo, & Hosokawa, 2015; Sundar, Jayesh, & Hussain, 2015). These factors vary from person to person, and the numerous contributions from multiple different factors account for the differences in remodeling between individuals (Lyford, Mills, Knapp, Scheyer, & Mellonig, 2003). Alveolar bone augmentation includes measures for maintaining and protecting the residual crest and increasing the height and/or width of the alveolar bone. According to the latest research, several bone block augmentation techniques have been described to enable appropriate implant positioning (Iglhaut et al., 2014; Roccuzzo, Savoini, Dalmasso, & Ramieri, 2017; Schwarz, Mihatovic, Ghanaati, & Becker, 2017; Spin‐Neto et al., 2015; Strietzel, Reichart, & Graf, 2007).
Although autogenous bone block grafting yields satisfactory results, this technique is associated with disadvantages such as prolonged operation times, limited graft acquisition, risk for damage to adjacent teeth, neurosensory deficits, donor area flap exposure, bleeding, and infection (Esposito et al., 2009a, 2009b ). Because of these disadvantages, there is an increased need for alternative grafting materials that show lower morbidity and easy application. Since their introduction into dentistry, allogeneic bone blocks have been demonstrated to be capable of overcoming many of the disadvantages of autogenous block grafting, especially complications associated with the donor site (Esposito et al., 2009a; Horowitz, Holtzclaw, & Rosen, 2012).
Aslan and colleagues reported a mean percentage of vertical graft resorption of 5.4% following the use of a cortical block allograft for augmentation of the alveolar ridge after 5 months of healing (Aslan et al., 2016). This percentage is nearly exactly the same value as found in our analysis for vertical graft shrinkage. Pereira and colleagues found that the mean horizontal bone resorption of cortico‐cancellous fresh‐frozen allogeneic bone blocks between the augmentation procedure and re‐entry for implantation was approximately 7.1% (Pereira et al., 2015). Spin‐Neto and colleagues reported an average horizontal graft resorption of 8.3% at 6–8 months after cortico‐cancellous fresh‐frozen block bone allograft placement (Spin‐Neto et al., 2015). These values are considerably higher than the values we found for the freeze‐dried cancellous allogeneic bone blocks (2.0%) and the cortical autogenous bone blocks (3.1%).
Concerning the low vertical graft resorption rate observed here, it is necessary to highlight that the amount of vertical bone gain in our study was rather limited (~1 mm). This means that the percentage of vertical bone resorption has to be interpreted with caution. Resorption of vertical bone augmentations with larger heights of ~5 mm will probably show far higher resorption rates (Mertens et al., 2013).
The application of cancellous freeze‐dried bone blocks, however, has also been associated with complications such as incision line opening, or perforations of the mucosa, leading in some cases to infections accompanied by partial or total block loss (Chaushu, Mardinger, Peleg, Ghelfan, & Nissan, 2010). These incidences have already been described in a preliminary study on allogeneic block grafting by Keith and colleagues, who reported seven block allograft failures and seven dehiscences in 82 augmentation sites, whereby the authors noted that the leading cause for graft failure was improper graft adaptation leading to mucosal irrigation and infections (Keith et al., 2006). Several authors reported higher rates of complications regarding allogeneic block augmentations in the posterior mandible (Nissan, Ghelfan, Mardinger, Calderon, & Chaushu, 2011; Novell et al., 2012). It is worth mentioning that within the limitation of the present study, no surgical complications have been observed, neither with autogenous blocks, nor when using allogeneic blocks. Though this is rather an ideal than a regular situation in clinical practice, overall complication rates with the application of allogeneic bone blocks found in the literature are also very low and concordant with excellent success and survival rates (Araujo et al., 2013; Monje et al., 2014; Motamedian, Khojaste, & Khojasteh, 2016; Nissan, Ghelfan, et al., 2011). Additionally, the long‐term experience of the practitioner in guided bone regeneration as well as the predominantly horizontal augmentation in the present study may have contributed to reducing the risk of postoperative complications (Esposito et al., 2009a, 2009b ).
To the best of our knowledge, our study is the first to compare the overall three‐dimensional remodeling rate of allogeneic and autogenous bone blocks for alveolar ridge augmentation over a period of 12 months. Despite the fact that the freeze‐dried allogeneic bone blocks were of cancellous bone structure, while the autogenous onlay grafts were of cortical bone structure, the remodeling rates were equal over the study period. This observation has two important consequences for alveolar ridge augmentation: first, we could demonstrate that freeze‐dried cancellous allogeneic bone blocks are comparable in their mid‐term performance to autogenous bone blocks. Secondly, we conclude that it is not necessary to massively over‐contour the augmentation site when using allogeneic bone blocks for treating single tooth gaps, but to apply the same procedure of contouring as when using autogenous cortical bone blocks.
One limitation of our study is that we compared cancellous allogeneic bone blocks with cortical autogenous bone blocks, while a comparison of cortical autogenous bone blocks and cortical allogeneic bone blocks would have been more appropriate with respect to the materials used. Nowadays, cancellous bone blocks predominantly being used in European countries, since they can be obtained from living donors, whereas the cortical fraction of femoral heads is insufficient for cortical blocks, so that multi‐organ donors would be required. Histomorphometric studies on fresh‐frozen allogeneic cortical bone blocks indicated a high percentage of avital tissue within these grafts (Spin‐Neto et al., 2015), whereas histomorphometric evaluations of cancellous allogenic bone blocks showed excellent new tissue formation and fast remodeling (Blume et al., 2017; Chaushu et al., 2010; Nissan, Marilena, et al., 2011).
Another limitation of our study is that we performed predominantly horizontal augmentation procedures. Defects spanning a singular tooth are less prone to complications and can be successfully treated with a variety of techniques and materials. However, a recently published meta‐analysis, which evaluated the bone gain with respect to the applied materials (autogenous, allogeneic, xenogeneic, alloplastic) and their form (granules vs. blocks), reported a mean horizontal bone gain for all granules of 3.7 ± 1.2 mm, whereas the mean horizontal bone gain for bone blocks was 4.5 ± 1.2 mm (Troeltzsch et al., 2016). The gain in horizontal dimensions in our study was above 5 mm for each group at each measurement point. This substantiates the decision for using allogenic bone blocks, as clinical evidence demonstrated these to be the best alternative to autogenous block harvesting regarding the defect dimensions presented here. Although other materials and techniques like titan meshes or split jaw approaches are also qualified for extensive augmentations, these procedures are notably more invasive and require the application of autogenous bone granules in most instances.
A possible weakness of our study is that we did not exactly determine the defect volume, but approximated it. Our approach of representing the defect volume as the sum of two truncated pyramids is derived from a generalization of the Stone‐Weierstrass approximation theorem. In mathematical analysis, the Weierstrass approximation theorem states that every continuous function defined on a closed interval [a, b] can be uniformly approximated as closely as desired by a polynomial function. In this sense, we measured three points (at the cervical level, in the middle of the defect and at the apical level) for the contour of the defect on four edges, and then connected these three points to a polygon. This results in a shape that can be represented as two stacked pyramid stumps. Since the defects were very small in height (on average 1 mm), only a very small error is made by the piecewise linearization of the actual function.
In order to validate our method, we selected ten patients and measured the volume also with the computer program CoDiagnostix9. The two measurement methods showed a high agreement, indicating that the two procedures could be applied in an exchangeable manner. The pyramid approach gave a volume that was on average 0.14 ml less than the volume obtained with the Hounsfield approach. This difference was systematic. However, since all defect volumes were calculated in the same way in both study groups, it can be assumed that we did not introduce a bias that affected the main outcome of the study.
5. CONCLUSION
The quintessence of the study presented here is that freeze‐dried cancellous allogeneic bone blocks are equivalent to autogenous bone blocks regarding their volumetric graft remodeling rates for treating single tooth defects classified as Type‐II to Type‐IV according to the ITI‐treatment guide categories. However, the long‐term effects need further systematic evaluation. Finally, the avoidance of donor site morbidity and unlimited availability is an undisputed advantage of the application of allogeneic bone blocks.
CONFLICT OF INTEREST
None declared.
Supporting information
Kloss FR, Offermanns V, Kloss‐Brandstätter A. Comparison of allogeneic and autogenous bone grafts for augmentation of alveolar ridge defects—A 12‐month retrospective radiographic evaluation. Clin Oral Impl Res. 2018;29:1163–1175. 10.1111/clr.13380
REFERENCES
- Al‐Abedalla, K. , Torres, J. , Cortes, A. R. , Wu, X. , Nader, S. A. , Daniel, N. , & Tamimi, F. (2015). Bone augmented with allograft onlays for implant placement could be comparable with native bone. Journal of Oral and Maxillofacial Surgery, 73(11), 2108–2122. 10.1016/j.joms.2015.06.151 [DOI] [PubMed] [Google Scholar]
- Amorfini, L. , Migliorati, M. , Signori, A. , Silvestrini‐Biavati, A. , & Benedicenti, S. (2014). Block allograft technique versus standard guided bone regeneration: A randomized clinical trial. Clinical Implant Dentistry and Related Research, 16(5), 655–667. 10.1111/cid.12040 [DOI] [PubMed] [Google Scholar]
- Araujo, P. P. , Oliveira, K. P. , Montenegro, S. C. , Carreiro, A. F. , Silva, J. S. , & Germano, A. R. (2013). Block allograft for reconstruction of alveolar bone ridge in implantology: A systematic review. Implant Dentistry, 22(3), 304–308. 10.1097/ID.0b013e318289e311 [DOI] [PubMed] [Google Scholar]
- Aslan, E. , Gultekin, A. , Karabuda, C. , Mortellaro, C. , Olgac, V. , & Mijiritsky, E. (2016). Clinical, histological, and histomorphometric evaluation of demineralized freeze‐dried cortical block allografts for alveolar ridge augmentation. Journal of Craniofacial Surgery, 27(5), 1181–1186. 10.1097/SCS.0000000000002548 [DOI] [PubMed] [Google Scholar]
- Bland, J. M. , & Altman, D. G. (1986). Statistical methods for assessing agreement between two methods of clinical measurement. Lancet, 1(8476), 307–310. S0140-6736(86)90837-8[pii] [PubMed] [Google Scholar]
- Bland, J. M. , & Altman, D. G. (1999). Measuring agreement in method comparison studies. Statistical Methods in Medical Research, 8(2), 135–160. 10.1177/096228029900800204 [DOI] [PubMed] [Google Scholar]
- Blume, O. , Hoffmann, L. , Donkiewicz, P. , Wenisch, S. , Back, M. , Franke, J. , … Barbeck, M. (2017). Treatment of severely resorbed maxilla due to peri‐implantitis by guided bone regeneration using a customized allogenic bone block: a case report. Materials (Basel), 10(10), 10.3390/ma10101213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaushu, G. , Mardinger, O. , Peleg, M. , Ghelfan, O. , & Nissan, J. (2010). Analysis of complications following augmentation with cancellous block allografts. Journal of Periodontology, 81(12), 1759–1764. 10.1902/jop.2010.100235 [DOI] [PubMed] [Google Scholar]
- Chavda, S. , & Levin, L. (2017). Human studies of vertical and horizontal alveolar ridge augmentation comparing different types of bone graft materials: A systematic review. Journal of Oral Implantology, 10.1563/aaid-joi-D-17-00053 [DOI] [PubMed] [Google Scholar]
- Chen, S. T. , & Buser, D. (2008). ITI Treatment Guide Vol 3: Implants in extraction sockets In Buser D., Belser U., & Wismeijer D. (Eds.), Implants in post‐extraction sites: A literature update (pp. 9–16). Berlin, Germany: Quintessense Publishing Co Ltd. [Google Scholar]
- Corbella, S. , Taschieri, S. , Francetti, L. , Weinstein, R. , & Del Fabbro, M. (2017). Histomorphometric results after postextraction socket healing with different biomaterials: A systematic review of the literature and meta‐analysis. International Journal of Oral and Maxillofacial Implants, 32(5), 1001–1017. 10.11607/jomi.5263 [DOI] [PubMed] [Google Scholar]
- Daugela, P. , Cicciu, M. , & Saulacic, N. (2016). Surgical regenerative treatments for peri‐implantitis: meta‐analysis of recent findings in a systematic literature review. Journal of Oral and Maxillofacial Research, 7(3), e15 10.5037/jomr.2016.7315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreiseidler, T. , Kaunisaho, V. , Neugebauer, J. , Zoller, J. E. , Rothamel, D. , & Kreppel, M. (2016). Changes in volume during the four months' remodelling period of iliac crest grafts in reconstruction of the alveolar ridge. British Journal of Oral and Maxillofacial Surgery, 54(7), 751–756. 10.1016/j.bjoms.2016.04.024 [DOI] [PubMed] [Google Scholar]
- Elangovan, S. , Barwacz, C. , Antonious, M. , Swenson, R. , & Avila‐Ortiz, G. (2017). Limited evidence for a guided bone regeneration procedure commonly performed in contemporary clinical practice. Clinical Advances in Periodontics, 7(2), 105–113. 10.1902/cap.2016.160054 [DOI] [Google Scholar]
- Esposito, M. , Grusovin, M. G. , Felice, P. , Karatzopoulos, G. , Worthington, H. V. , & Coulthard, P. (2009a). The efficacy of horizontal and vertical bone augmentation procedures for dental implants ‐ a Cochrane systematic review. European Journal of Oral Implantology, 2(3), 167–184. [PubMed] [Google Scholar]
- Esposito, M. , Grusovin, M. G. , Felice, P. , Karatzopoulos, G. , Worthington, H. V. , & Coulthard, P. (2009b). Interventions for replacing missing teeth: Horizontal and vertical bone augmentation techniques for dental implant treatment. Cochrane Database Systematic Review, 4, CD003607 10.1002/14651858.CD003607.pub4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanser, T. , & Khoury, F. (2016). Alveolar ridge contouring with free connective tissue graft at implant placement: A 5‐year consecutive clinical study. International Journal of Periodontics & Restorative Dentistry, 36(4), 465–473. 10.11607/prd.2730 [DOI] [PubMed] [Google Scholar]
- Horowitz, R. , Holtzclaw, D. , & Rosen, P. S. (2012). A review on alveolar ridge preservation following tooth extraction. Journal of Evidence Based Dental Practice, 12(Suppl. 3), 149–160. 10.1016/S1532-3382(12)70029-5 [DOI] [PubMed] [Google Scholar]
- Iglhaut, G. , Schwarz, F. , Grundel, M. , Mihatovic, I. , Becker, J. , & Schliephake, H. (2014). Shell technique using a rigid resorbable barrier system for localized alveolar ridge augmentation. Clinical Oral Implants Research, 25(2), e149–e154. 10.1111/clr.12078 [DOI] [PubMed] [Google Scholar]
- Jensen, J. , & Sindet‐Pedersen, S. (1991). Autogenous mandibular bone grafts and osseointegrated implants for reconstruction of the severely atrophied maxilla: A preliminary report. Journal of Oral and Maxillofacial Surgery, 49(12), 1277–1287. 10.1016/0278-2391(91)90303-4 [DOI] [PubMed] [Google Scholar]
- Keith Jr, J. D. (2004). Localized ridge augmentation with a block allograft followed by secondary implant placement: A case report. International Journal of Periodontics & Restorative Dentistry, 24(1), 11–17. [PubMed] [Google Scholar]
- Keith Jr, J. D. , Petrungaro, P. , Leonetti, J. A. , Elwell, C. W. , Zeren, K. J. , Caputo, C. , … Warner, M. M. (2006). Clinical and histologic evaluation of a mineralized block allograft: Results from the developmental period (2001–2004). International Journal of Periodontics & Restorative Dentistry, 26(4), 321–327. [PubMed] [Google Scholar]
- Khoury, F. , & Hanser, T. (2015). Mandibular bone block harvesting from the retromolar region: A 10‐year prospective clinical study. International Journal of Oral and Maxillofacial Implants, 30(3), 688–697. 10.11607/jomi.4117 [DOI] [PubMed] [Google Scholar]
- Khoury, F. , Keller, P. , & Keeve, P. L. (2017). Stability of grafted implant placement sites after sinus floor elevation using a layering technique: 10‐year clinical and radiographic results. International Journal of Oral and Maxillofacial Implants, 32(5), 1086–1096. 10.11607/jomi.5832 [DOI] [PubMed] [Google Scholar]
- Koch, R. , & Spörl, E. (2007). Statistical methods for comparison of two measuring procedures and for calibration: Analysis of concordance, correlation and regression in the case of measuring intraocular pressure. Klin Monbl Augenheilkd, 224(1), 52–57. 10.1055/s-2006-927278 [DOI] [PubMed] [Google Scholar]
- Laino, L. , Iezzi, G. , Piattelli, A. , Lo Muzio, L. , & Cicciu, M. (2014). Vertical ridge augmentation of the atrophic posterior mandible with sandwich technique: Bone block from the chin area versus corticocancellous bone block allograft–clinical and histological prospective randomized controlled study. BioMed Research International, 2014, 982104 10.1155/2014/982104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsson, L. , Decker, A. M. , Nibali, L. , Pilipchuk, S. P. , Berglundh, T. , & Giannobile, W. V. (2016). Regenerative medicine for periodontal and peri‐implant diseases. Journal of Dental Research, 95(3), 255–266. 10.1177/0022034515618887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurencin, C. , Khan, Y. , & El‐Amin, S. F. (2006). Bone graft substitutes. Expert Review of Medical Devices, 3(1), 49–57. 10.1586/17434440.3.1.49 [DOI] [PubMed] [Google Scholar]
- Listrom, R. D. , & Symington, J. M. (1988). Osseointegrated dental implants in conjunction with bone grafts. International Journal of Oral and Maxillofacial Surgery, 17(2), 116–118. 10.1016/S0901-5027(88)80163-2 [DOI] [PubMed] [Google Scholar]
- Lyford, R. H. , Mills, M. P. , Knapp, C. I. , Scheyer, E. T. , & Mellonig, J. T. (2003). Clinical evaluation of freeze‐dried block allografts for alveolar ridge augmentation: A case series. International Journal of Periodontics & Restorative Dentistry, 23(5), 417–425. [PubMed] [Google Scholar]
- Masaki, C. , Nakamoto, T. , Mukaibo, T. , Kondo, Y. , & Hosokawa, R. (2015). Strategies for alveolar ridge reconstruction and preservation for implant therapy. Journal of Prosthodontic Research, 59(4), 220–228. 10.1016/j.jpor.2015.04.005 [DOI] [PubMed] [Google Scholar]
- Mertens, C. , Decker, C. , Seeberger, R. , Hoffmann, J. , Sander, A. , & Freier, K. (2013). Early bone resorption after vertical bone augmentation – A comparison of calvarial and iliac grafts. Clinical Oral Implants Research, 24(7), 820–825. 10.1111/j.1600-0501.2012.02463.x [DOI] [PubMed] [Google Scholar]
- Monje, A. , Pikos, M. A. , Chan, H. L. , Suarez, F. , Gargallo‐Albiol, J. , Hernandez‐Alfaro, F. , … Wang, H. L. (2014). On the feasibility of utilizing allogeneic bone blocks for atrophic maxillary augmentation. BioMed Research International, 2014, 814578 10.1155/2014/814578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motamedian, S. R. , Khojaste, M. , & Khojasteh, A. (2016). Success rate of implants placed in autogenous bone blocks versus allogenic bone blocks: A systematic literature review. Annals of Maxillofacial Surgery, 6(1), 78–90. 10.4103/2231-0746.186143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nissan, J. , Ghelfan, O. , Mardinger, O. , Calderon, S. , & Chaushu, G. (2011). Efficacy of cancellous block allograft augmentation prior to implant placement in the posterior atrophic mandible. Clinical Implant Dentistry and Related Research, 13(4), 279–285. 10.1111/j.1708-8208.2009.00219.x [DOI] [PubMed] [Google Scholar]
- Nissan, J. , Marilena, V. , Gross, O. , Mardinger, O. , & Chaushu, G. (2011). Histomorphometric analysis following augmentation of the posterior mandible using cancellous bone‐block allograft. Journal of Biomedical Materials Research Part A, 97(4), 509–513. 10.1002/jbm.a.33096 [DOI] [PubMed] [Google Scholar]
- Nkenke, E. , & Neukam, F. W. (2014). Autogenous bone harvesting and grafting in advanced jaw resorption: Morbidity, resorption and implant survival. European Journal of Oral Implantology, 7(Suppl. 2), S203–S217. [PubMed] [Google Scholar]
- Nkenke, E. , Schultze‐Mosgau, S. , Radespiel‐Troger, M. , Kloss, F. , & Neukam, F. W. (2001). Morbidity of harvesting of chin grafts: A prospective study. Clinical Oral Implants Research, 12(5), 495–502. 10.1034/j.1600-0501.2001.120510.x [DOI] [PubMed] [Google Scholar]
- Nkenke, E. , & Stelzle, F. (2009). Clinical outcomes of sinus floor augmentation for implant placement using autogenous bone or bone substitutes: A systematic review. Clinical Oral Implants Research, 20(Suppl. 4), 124–133. 10.1111/j.1600-0501.2009.01776.x [DOI] [PubMed] [Google Scholar]
- Novell, J. , Novell‐Costa, F. , Ivorra, C. , Farinas, O. , Munilla, A. , & Martinez, C. (2012). Five‐year results of implants inserted into freeze‐dried block allografts. Implant Dentistry, 21(2), 129–135. 10.1097/ID.0b013e31824bf99f [DOI] [PubMed] [Google Scholar]
- Otto, S. , Kleye, C. , Burian, E. , Ehrenfeld, M. , & Cornelius, C. P. (2017). Custom‐milled individual allogeneic bone grafts for alveolar cleft osteoplasty – A technical note. Journal of Cranio‐Maxillo‐Facial Surgery, 45(12), 1955–1961. 10.1016/j.jcms.2017.09.011 [DOI] [PubMed] [Google Scholar]
- Pereira, E. , Messias, A. , Dias, R. , Judas, F. , Salvoni, A. , & Guerra, F. (2015). Horizontal resorption of fresh‐frozen corticocancellous bone blocks in the reconstruction of the atrophic maxilla at 5 months. Clinical Implant Dentistry and Related Research, 17(Suppl. 2), e444–e458. 10.1111/cid.12268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrungaro, P. S. , & Amar, S. (2005). Localized ridge augmentation with allogenic block grafts prior to implant placement: Case reports and histologic evaluations. Implant Dentistry, 14(2), 139–148. 10.1097/01.id.0000163805.98577.ab [DOI] [PubMed] [Google Scholar]
- Roccuzzo, M. , Savoini, M. , Dalmasso, P. , & Ramieri, G. (2017). Long‐term outcomes of implants placed after vertical alveolar ridge augmentation in partially edentulous patients: A 10‐year prospective clinical study. Clinical Oral Implants Research, 28(10), 1204–1210. 10.1111/clr.12941 [DOI] [PubMed] [Google Scholar]
- Sakkas, A. , Wilde, F. , Heufelder, M. , Winter, K. , & Schramm, A. (2017). Autogenous bone grafts in oral implantology‐is it still a "gold standard"? A consecutive review of 279 patients with 456 clinical procedures International Journal of Implant Dentistry, 3(1), 23 10.1186/s40729-017-0084-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz, F. , Mihatovic, I. , Ghanaati, S. , & Becker, J. (2017). Performance and safety of collagenated xenogeneic bone block for lateral alveolar ridge augmentation and staged implant placement. A monocenter, prospective single‐arm clinical study. Clinical Oral Implants Research, 28(8), 954–960. 10.1111/clr.12902 [DOI] [PubMed] [Google Scholar]
- Spin‐Neto, R. , Stavropoulos, A. , Coletti, F. L. , Pereira, L. A. , Marcantonio Jr, E. , & Wenzel, A. (2015). Remodeling of cortical and corticocancellous fresh‐frozen allogeneic block bone grafts – A radiographic and histomorphometric comparison to autologous bone grafts. Clinical Oral Implants Research, 26(7), 747–752. 10.1111/clr.12343 [DOI] [PubMed] [Google Scholar]
- Strietzel, F. P. , Reichart, P. A. , & Graf, H. L. (2007). Lateral alveolar ridge augmentation using a synthetic nano‐crystalline hydroxyapatite bone substitution material (Ostim): Preliminary clinical and histological results. Clinical Oral Implants Research, 18(6), 743–751. 10.1111/j.1600-0501.2007.01416.x [DOI] [PubMed] [Google Scholar]
- Sundar, S. S. , Jayesh, S. R. , & Hussain, S. (2015). Association of matrix metalloproteinase 1 gene promoter mutation and residual ridge resorption in edentulous patients of South Indian origin. Journal of Pharmacy and Bioallied Sciences, 7(Suppl. 2), S652–S655. 10.4103/0975-7406.163591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troeltzsch, M. , Troeltzsch, M. , Kauffmann, P. , Gruber, R. , Brockmeyer, P. , Moser, N. , … Schliephake, H. (2016). Clinical efficacy of grafting materials in alveolar ridge augmentation: A systematic review. Journal of Cranio‐Maxillo‐Facial Surgery, 44(10), 1618–1629. 10.1016/j.jcms.2016.07.028 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


