Abstract
Objective
Recent availability of amyloid and tau positron emission tomography (PET) has provided us with a unique opportunity to measure the association of systemic vascular health with brain health after accounting for the impact of Alzheimer disease (AD) pathologies. We wanted to quantify early cerebrovascular health–related magnetic resonance imaging brain measures (structure, perfusion, microstructural integrity) and evaluate their utility as a biomarker for cerebrovascular health.
Methods
We used 2 independent samples (discovery, n = 390; validation, n = 1,035) of individuals, aged ≥ 60 years, along the cognitive continuum with imaging from the population‐based sample of Mayo Clinic Study of Aging. We ascertained vascular health by summing up recently existing cardiovascular and metabolic conditions (CMC) from health care records (hypertension, hyperlipidemia, cardiac arrhythmias, coronary artery disease, congestive heart failure, diabetes mellitus, and stroke). Using multiple regression models, we quantified associations between CMC and brain health after accounting for age, sex, education/occupation, and AD burden (from amyloid and tau PET).
Results
Systemic vascular health was associated with medial temporal lobe thinning, widespread cerebral hypoperfusion, and loss of microstructural integrity in several white matter tracts including the corpus callosum and fornix. Further investigations suggested that microstructural integrity of the genu of the corpus callosum was suitable for assessing prodromal cerebrovascular health, had similar distributions in the discovery and independent validation datasets, and predicted cognitive performance above and beyond amyloid deposition.
Interpretation
Systemic vascular health has significant impact on brain structure and function. Quantifying prodromal cerebrovascular health–related brain measures that are independent of AD pathology–related changes has great utility for cognitive aging. Ann Neurol 2018;84:713–724
Vascular health significantly declines with age, increasing the risk of cognitive impairment and dementia.1 Declining systemic vascular health can cause significant cerebrovascular injury, appearing as brain lesions on neuroimaging (infarctions, microbleeds, and white matter hyperintensities [WMH]).2 There is extensive literature establishing an association between worsening systemic vascular health and poor brain function and structure.3, 4, 5, 6 Recent work has also shown that these changes precede the appearance of overt brain lesions.7, 8 The inability to quantify the effect of systemic vascular health on early brain structural and functional changes in a meaningful way has hampered our understanding of the role of cerebrovascular disease in the elderly both as a standalone etiology and as a coexisting pathology along with other dementias, specifically Alzheimer disease (AD). We hypothesized that quantifying the impact of systemic vascular health on brain health after accounting for AD‐related changes will allow us to develop measures specific and sensitive to prodromal cerebrovascular health. Therefore, the goal of this work was to develop and evaluate magnetic resonance imaging (MRI)‐based biomarkers for cerebrovascular health. We considered 3 primary MRI‐based measures of brain health: T1‐weighted structural MRI (sMRI) as a surrogate of brain morphology/structure, arterial spin labeling (ASL) MRI as a surrogate of brain perfusion, and diffusion tensor imaging (DTI) as a surrogate of microstructural integrity.
Furthermore, we hypothesized that an ideal MRI‐based marker for cerebrovascular health would satisfy the following criteria: (1) regional independence—given that AD pathologies (amyloid and tau) and cerebrovascular measures are the two most common known processes that alter aging brain health, the biomarker would ideally capture brain changes that manifest in regions not affected by AD; (2) low measurement variability—although each imaging modality has been designed to quantify a specific physiological measure (eg, structure or perfusion), it is accompanied by measurement variability due to the noise and variability in the acquisition as well as biological variability; a robust measure ideally would have low measurement variability (especially related to the acquisition) to enable greater reproducibility across independent datasets and differing acquisitions; (3) sensitivity—the MRI‐based measure would be sensitive to early vascular health–related brain changes, that is, it would be indicative of prodromal disease even in the absence of infarctions and AD pathologies. Due to the increasing evidence that brain changes are seen prior to visible cerebrovascular injury on fluid‐attenuated inversion recovery (FLAIR; ie, WMH and macroscopic infarctions7, 8, 9, we focused on MRI measures that captured brain health and did not include FLAIR findings.
Subjects and Methods
Overall Experimental Design
The overall approach for quantification and evaluation of cerebrovascular health measures consisted of 3 steps:
In a discovery dataset, we first investigated the effect of systemic vascular health as measured by recently existing cardiovascular and metabolic conditions (CMC) on brain structure, perfusion, and diffusion after adjusting for the effects of AD pathologies (using amyloid and tau positron emission tomography [PET]), age, sex, and education/occupation composite score. Here we assumed that there is minimal to no mechanistic interaction between AD and cerebrovascular measures early in the disease process.10, 11
Next, we chose an MRI modality and specific regions that satisfied all 3 criteria mentioned above (regional independence, variability, and sensitivity) as a cerebrovascular health biomarker in the discovery dataset.
In an independent validation dataset, we finally evaluated the utility of the cerebrovascular health biomarker in providing independent information about cognition in addition to amyloidosis.
Selection of Participants
All participants were enrolled in the Mayo Clinic Study of Aging (MCSA), a population‐based study of Olmsted County, Minnesota residents. The Olmsted County population was enumerated using the Rochester Epidemiology Project (REP) medical records linkage system.12, 13 This allowed us to ascertain vascular risk factors from detailed health care records instead of relying on self‐report. The complete details of the MCSA study design and clinical diagnostic criteria were discussed by Petersen et al14 and Roberts et al.15 Standard protocol approvals, registrations, and patient consents were obtained; the study was approved by the Mayo Clinic Institutional Review Board, and informed consent was obtained from all participants or their surrogates.
Discovery Dataset
We included all 390 elderly individuals (aged ≥ 60 years) with vascular health indicators (discussed below) and concurrent imaging (Pittsburgh compound B [PiB] PET, tau PET, and all 3 MRI imaging sequences – sMRI, ASL, and DTI). At the time of the scans, 352 were cognitively unimpaired, 29 had mild cognitive impairment, 6 were diagnosed with a neurodegenerative disorder (3 Alzheimer clinical syndrome with dementia, 2 with mixed dementia, and 1 with vascular dementia), and 3 had a missing clinical diagnosis due to incomplete data.
Validation Dataset
We used all 1,035 unique (not included in the discovery dataset) elderly individuals (aged ≥ 60 years) with vascular health indicators and concurrent imaging (PiB PET and DTI scans), who were not part of the discovery dataset because they did not have tau PET and ASL imaging. At the time of the scans, 869 were cognitively unimpaired, 144 had mild cognitive impairment, 16 were diagnosed with a neurodegenerative disorder (12 Alzheimer clinical syndrome with dementia, 1 mixed dementia, 1 vascular dementia, 1 parkinsonism, and 1 progressive supranuclear palsy), and 6 had a missing clinical diagnosis due to incomplete data.
Demographics
Sex and years of education were obtained at the clinical visit. Age at the time of the MRI scan was used as a covariate. Education and occupation composite score was calculated as a combination of education and the major occupation in each participant's life.16
Indicator of Vascular Health
The REP diagnostic indices were searched in a 5‐year capture frame before the MCSA visit17, 18 to identify International Classification of Diseases, 9th Revision (ICD‐9) codes and 10th Revision (ICD‐10) codes associated with health care visits. Both ICD‐9 (through September 2015) and ICD‐10 (after October 2015) codes were pooled together by the REP under 7 cardiovascular and metabolic conditions proposed by the US Department of Health and Human Services in 2010 as indicators of vascular health19—hypertension, hyperlipidemia, cardiac arrhythmias, coronary artery disease, congestive heart failure, diabetes mellitus, and stroke—to compute a composite score (referred to as CMC), which represents the summation of the presence or absence of each of these conditions. The specific codes pooled together were published previously.20
AD Imaging Biomarkers—Amyloid and Tau Assessment from PET scans
The acquisition, processing, and summary measure details for amyloid PET and tau PET scans acquired on the MCSA study participants are discussed in Jack et. al.21 For amyloid PET, the global amyloid load was computed for each subject by calculating median uptake in the prefrontal, orbitofrontal, parietal, temporal, anterior cingulate, and posterior cingulate/precuneus regions of interest (ROIs) divided by the median uptake in the cerebellar crus gray matter ROI. For tau PET, a composite ROI was computed using median tau PET uptake in the entorhinal, amygdala, parahippocampal, fusiform, inferior temporal, and middle temporal ROIs divided by the median tau PET uptake from the cerebellar crus gray matter ROI.
MRI and Assessment
All MRI images were acquired on 3T GE MRI (GE Medical Systems, Milwaukee, WI).
sMRI
Cortical thickness measurements were made using a previously published method22 on standard structural magnetization‐prepared rapid acquisition gradient echo scans. We then computed median thickness of bilateral ROIs in an in‐house–modified version of the Automated Anatomical Labeling atlas.
ASL
Whole head perfusion MRI was acquired with pseudocontinuous 3‐dimensional fast spin echo ASL23 with spiral readouts consisting of 512 points and 8 arms, bandwidth = 62.5kHz, repetition time = 5 seconds, echo time = 16.4 milliseconds, with 3 signal averages. We positioned the ASL volume at the bottom of the cerebellum with slice thickness of 3mm and in plane resolution of 3 × 3mm; the time from labeling pulse to excitation (postlabeling delay) was 2,025 milliseconds. For quantification of the cerebral blood flow (CBF), a proton density (PD) reference image was also acquired. CBF maps were generated by pairwise subtraction of label and control images followed by conversion to an absolute CBF map on the basis of a 2‐compartment perfusion model from Alsop and Detre.24 The PD reference image was used to register the CBF map to the T1‐weighted scan, and pons scaled perfusion was computed in the same regions as sMRI.
DTI
The DTI acquisition protocol was a 2.7mm isotropic resolution spin echo sequence with 5 b = 0 volumes followed by 41 b = 1,000s/mm2 diffusion‐weighted volumes with directions evenly spread over the whole sphere. The k‐space data were reconstructed with zero padding. Preprocessing included Gibbs ringing correction,25 skull stripping, denoising,26 and debiasing.27 Head motion and eddy current distortion was corrected with FSL Software and echo‐planar imaging (EPI) distortion correction using BrainSuite.28 Diffusion tensors were then fit using nonlinear minimization, after which fractional anisotropy (FA) and mean diffusivity (MD) were computed. ANTs software29 was used to nonlinearly register an in‐house–modified version of the Johns Hopkins University Eve white matter (WM) atlas30 to each subject's FA image to compute regional median FA and MD. Voxels with MD > 2 × 10−3 or < 7 × 10−5mm2/s were excluded as mostly cerebrospinal fluid or air, respectively. ROIs with < 7 diffusion voxels in subject space were excluded as being too small to be reliably registered.
Ascertainment of WMH and Infarcts on FLAIR MRI
Brain infarctions were assessed by trained image analysts and confirmed by a clinician blinded to all clinical information. WMH were ascertained as previously described, and the masks were edited by trained image analysts.10
Cognitive Performance
The MCSA neuropsychological battery consists of 9 tests covering 4 cognitive domains, as previously described.14, 15 In this work, we used a global cognitive z score that was estimated from the z transformation of the average of the 4 domain z scores (executive function, language, memory, and visuospatial performance) as a cognitive outcome variable.
Statistical Methods
Step 1: Association of Systemic Vascular Health with Brain Health
To compare regression coefficients across modalities, we computed global mean and standard deviation across all ROIs and all subjects for each modality and standardized the raw region level values for each modality. For each modality (sMRI, ASL, and DTI measures), we fit separate multiple regression models for each ROI with the following predictors: age, sex, education/occupation composite score, AD biomarkers (global amyloid load and composite tau uptake), and CMC.
Step 2: Development of a Cerebrovascular Health Biomarker
To assess variability (criterion 2), we computed the coefficients of variation for unstandardized sMRI, ASL, and DTI measures in each ROI, across all subjects in the discovery dataset as well as a set of young individuals (30–49 years old). We used the coefficient of variation averaged across ROIs as a measure of variability of each modality. To assess sensitivity (criterion 3), we repeated step 1, that is, the regression models in a subset of individuals without brain infarcts (cortical or subcortical; n = 300), who were amyloid negative (n = 216), and in a subset of individuals who were amyloid and tau negative (n = 162). Based on regional patterns of association with CMC, these variability results, and the strength of associations of clusters with CMC, we selected one best overall MRI measure (structure, perfusion, FA, or MD) for the development of the signature. For the selected MRI measure, we performed hierarchical clustering based on squared Pearson correlations (using the varclus function in the R Hmisc package) to combine highly correlated regions, reduce the number of input features, and aid in the identification of a signature for cerebrovascular health. We verified the clustering using squared Spearman correlations, and found that the methods produced similar results.
Step 3: Evaluation of the Cerebrovascular Biomarker
We estimated the cerebrovascular biomarker in the validation dataset and assessed the reproducibility of the association of cognition with the biomarker in the discovery and validation datasets both by itself and in conjunction with amyloidosis. We fit regression models with global cognition as an outcome variable in both discovery and validation datasets with age, sex, education/occupation, and cycle number (ie, the number of times the cognitive battery has been administered to each specific subject to adjust for practice effects) as predictors. We tested for interactions of age with amyloid and genu FA, and interactions of amyloid with genu FA, in the discovery data. Interactions of age with amyloid (coefficient = −0.022, 95% confidence interval [CI] = −0.051 to 0.007, p = 0.137) and age with genu FA (coefficient = 0.105, 95% CI = −0.146 to 0.356, p = 0.415) were not significant in the discovery data. The interaction of amyloid with genu FA was significant in the discovery data (coefficient = 7.706, 95% CI = 3.341–12.070, p = 0.001). This interaction, however, did not replicate in the validation data (coefficient = −1.802, 95% CI = −5.110 to 1.506, p = 0.286). We thus went to the model without interactions, where we saw similar results in both datasets. Although future studies or analyses in other populations might detect interactions among age, amyloid, and genu FA, in these datasets we do not have sufficient consistent evidence to include them. Three different models were fit with either amyloid, cerebrovascular biomarker, or both as additional predictors of interest. To aid with interpretation, neither amyloid nor the cerebrovascular biomarker was standardized in these final models. We also described the marginal and joint distributions of genu FA and amyloid in both the discovery and validation datasets using bagplots.
Results
The characteristics of the discovery dataset and validation datasets are shown in Table 1.
Table 1.
Characteristics of Discovery and Validation Datasets with the Mean (SD) Listed for the Continuous Variables and Count (%) for the Categorical Variables
| Characteristic | Discovery, n = 390 | Validation, n = 1,035 | Difference (95% CI) | p |
|---|---|---|---|---|
| Age, yrs | 75.5 (8.5) | 75.6 (8.6) | −0.12 (−1.12, 0.88) | 0.81 |
| Males, n (%) | 215 (55%) | 552 (53%) | 1.8% (−4.0%, 7.6%) | 0.54 |
| APOE4 carrier, n (%) | 110 (28%) | 296 (29%) | −0.4% (−5.7%, 4.9%) | 0.89 |
| Education, yr | 14.6 (2.6) | 14.7 (2.8) | −0.06 (−0.38, 0.26) | 0.72 |
| Job score | 3.4 (1.4) | 3.3 (1.5) | 0.06 (−0.11, 0.23) | 0.51 |
| Education–occupation composite | 12.5 (2.5) | 12.5 (2.7) | 0.002 (−0.31, 0.31) | 0.99 |
| Cardiometabolic condition, CMC | 2.2 (1.5) | 2.3 (1.6) | −0.11 (−0.29, 0.07) | 0.24 |
| Amyloid deposition, PiB SUVR | 1.41 (0.17) | 1.58 (0.41) | −0.17 (−0.21, −0.13) | <0.001 |
| Global cognition z domain | 0.12 (1.07) | −0.14 (1.18) | 0.27 (0.13, 0.41) | <0.001 |
CI = confidence interval; CMC = cardiovascular and metabolic conditions; PiB = Pittsburgh compound B; SD = standard deviation; SUVR = standardized uptake value ratio.
Step 1: Association of Systemic Vascular Health with Brain Health
Within each modality (sMRI, ASL, DTI), the regression coefficients and their associated 95% CIs showing the association between CMC and brain health after accounting for age, sex, AD biomarkers, and education/occupation composite score are shown in Figures 1 and 2. Figure 1 shows the association of CMC with regional cortical thickness in the left panel and regional perfusion in the right panel. Poor vascular health (ie, greater CMC) was associated with decreased medial temporal lobe (entorhinal cortex and hippocampus) and middle temporal pole thicknesses (p < 0.05). We also found increased superior parietal lobe thickness associated with vascular risk but with a smaller effect compared to the effect on medial temporal lobe decrease (p < 0.05). Greater CMC was associated with decreased perfusion throughout the brain, except for most of the occipital lobe (p < 0.05). Figure 2 shows the CMC association with regional FA in the left panel and with regional MD in the right panel. Greater CMC was associated with decreased FA and increased MD in the corpus callosum and fornix tracts. Additionally, there were significant negative associations between CMC and FA in the cingulum tracts, postcentral WM, and anterior limb of the internal capsule, and positive associations between CMC and MD in the inferior occipital WM and supramarginal WM. Figure 3 summarizes the regional distribution of these associations for each modality.
Figure 1.
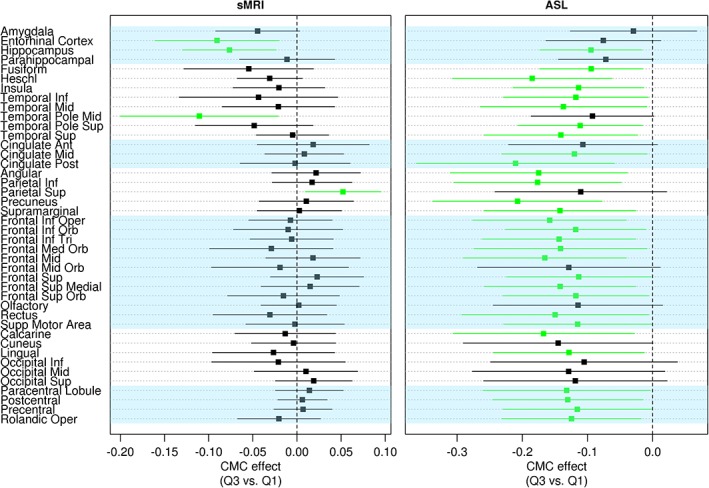
The association of vascular health with thickness (left panel) measured using structural magnetic resonance imaging (sMRI) and perfusion measured using arterial spin labeling (ASL) (right panel) after accounting for age, sex, education/occupation, global amyloid, and composite tau burden. Regions with p < 0.05 are shown in green. Ant = anterior; CMC = cardiovascular and metabolic conditions; Inf = inferior; Med = medial; Oper = operculum; Orb = orbital; Post = posterior; Sup = superior; Supp = Supplementary; Tri = triangularis.
Figure 2.
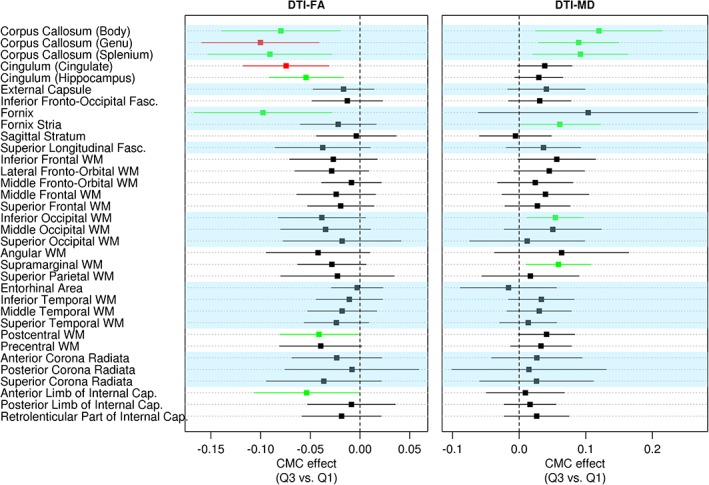
The association of vascular health with fractional anisotropy (FA; left panel) and mean diffusivity (MD; right panel) measured using diffusion tensor imaging (DTI) after accounting for age, sex, education/occupation, global amyloid, and composite tau burden. Regions with p < 0.05 are shown in green and p < 0.001 are shown in red. Cap. = capsule; CMC = cardiovascular and metabolic conditions; Fasc. = fasciculus; WM = white matter.
Figure 3.
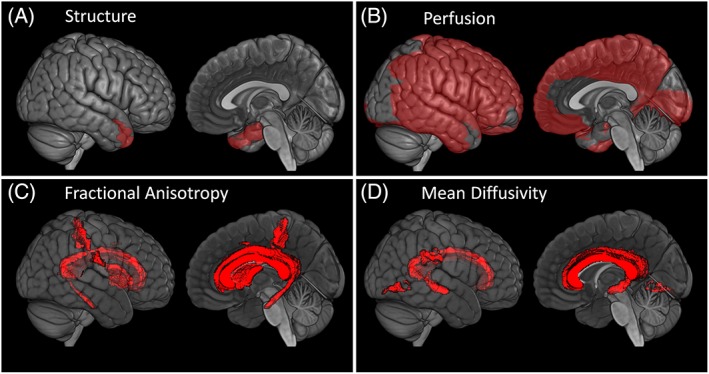
Images showing the association of vascular health with (A) structure, (B) perfusion, (C) fractional anisotropy, and (D) mean diffusivity measured using diffusion tensor imaging after accounting for age, sex, education/occupation, global amyloid, and composite tau burden (significant at p < 0.05).
Step 2: Development of a Cerebrovascular Health Biomarker
Based on Step 1, we found that sMRI did not clearly satisfy the regional independence criterion as stated above, because the most significant sMRI regions that exhibited associations with CMC were in the medial temporal lobe, which is also a key AD‐related region. In the 69 young cognitively normal individuals (30–49 years; who have lower biological variability in signal), we found that the mean coefficient of variation (CV) of cortical thickness was 0.110, CV of perfusion measure was 0.130, CV of FA measure was 0.062, and CV of MD measure was 0.034. When we assessed variability of the signal in the discovery set of subjects, who have greater biological variability than younger individuals due to aging and pathology, we found that the mean CVs of the cortical thickness measure, perfusion measure, FA measure, and MD measure were 0.138, 0.198, 0.088, and 0.070, respectively. Measurement variability is a combination of the noise and variability in the acquisition and biological variability, which can be separated using test–retest data that were not available in our case. Based on the CVs in both datasets, we found evidence that regional ASL measures had high variability or low signal to noise ratio compared to DTI measures. To assess sensitivity, we repeated Step 1 analyses in a subset of individuals without infarcts and again in a subset without significant amyloid deposition, based on PiB PET standardized uptake value ratio (SUVR) < 1.42 (amyloid negative individuals).21 In these sensitivity analyses, the effect sizes were similar to the main analyses, but several of the regions were nonsignificant due to the smaller sample sizes in the sensitivity analyses compared to the main analyses. Several regions for ASL and sMRI were now nonsignificant but remained significant for DTI FA and DTI MD, supporting the greater sensitivity of DTI measures to prodromal cerebrovascular health. In addition, we also performed sensitivity analyses in amyloid‐ and tau‐negative (based on tau PET SUVR < 1.23)21 individuals (n = 162). As expected given the substantial drop in sample size and power, CIs were wider and we did not see as many significant regions. However, the magnitudes of the CMC associations were generally similar. DTI FA had the greatest sensitivity (criterion 3) as well as low variability (criterion 2) compared to other MRI measures. In addition, it also satisfied the regional independence criterion (criterion 1), because in amyloid‐negative individuals most effects remained significant except for the lesser extent of effects on the cingulum tracts. Therefore, we focused on the development of a DTI FA biomarker in this step.
To reduce the number of input features and to combine highly correlated regions, we performed hierarchical clustering of the FA measure, which yielded several clusters. This analysis brought forward an important observation that FA values in different brain regions were highly correlated with each other, and there was no clear clustering of regions based on the variance of FA. Therefore, based on the strong association of FA in the genu of the corpus callosum in the overall cohort, in those without brain infarcts, and in those who were amyloid negative, we chose genu FA as a cerebrovascular biomarker. Genu was also the only ROI where FA survived Bonferroni correction at p < 0.05 in the overall cohort (see Fig 2) and in the sensitivity analyses without brain infarcts. In a sensitivity analysis, we found that genu FA significantly correlated with global WMH (Pearson correlation = 0.629). Furthermore, when we build 2 separate models with FA and WMH as outcomes, we found that both CMC and WMH were required to explain the variability in genu FA as an outcome, but only genu FA was sufficient to explain the variability in WMH as an outcome (results shown in Table 2). We would need longitudinal data to demonstrate, but this is consistent with our idea that DTI‐based biomarkers capture early or prodromal cerebrovascular changes and may be more sensitive to WM integrity loss than WMH measurements.
Table 2.
Models with Genu FA as an Outcome versus WMH as an Outcome
| Variable | Regression Coefficient (SE) | p |
|---|---|---|
| FA as an outcome | ||
| Intercept | −0.83 (0.10) | <0.0001 |
| WMH | 23.80 (2.60) | <0.0001 |
| CMC | 0.36 (0.13) | 0.007 |
| WMH*CMC | −7.97 (3.05) | 0.009 |
| WMH as an outcome | ||
| Intercept | 0.03 (0.002) | <0.0001 |
| FA score | 0.023 (0.003) | <0.0001 |
| CMC | 0.004 (0.003) | 0.216 |
| FA score*CMC | −0.003 (0.003) | 0.447 |
CMC = cardiovascular and metabolic conditions; FA = fractional anisotropy; WMH = white matter hyperintensities.
Step 3: Evaluation of the Cerebrovascular Biomarker
In the validation dataset, CMC was a significant predictor of genu FA (estimate = −0.003, standard error = 0.001, p = 0.004) after adjusting for age and sex. Figure 4 shows the 2‐dimensional boxplots or bagplots for genu FA and amyloid in both the datasets. The marginal and joint distributions of genu FA and amyloid appeared similar in both the discovery and validation datasets. Table 3 shows the regression results with amyloid alone, genu FA alone, or both in a model along with age, sex, education/occupation composite score, and cycle number. In all models, older age and being male were associated with lower cognitive performance. Higher cycle number and higher education/occupation composite score were associated with higher cognitive performance. In models with amyloid and genu FA alone (D1, D2, V1, V2), amyloid and genu FA each predicted cognitive performance. With both in the model (D3 and V3), the model fits based on R 2 were considerably higher, and both amyloid and genu FA significantly predicted cognitive performance. As an illustration of the utility of the cerebrovascular biomarker, a contour plot showing predicted cognition in model V3 with amyloid and genu FA and a plot of predicted cognition by age are shown in Figure 5.
Figure 4.
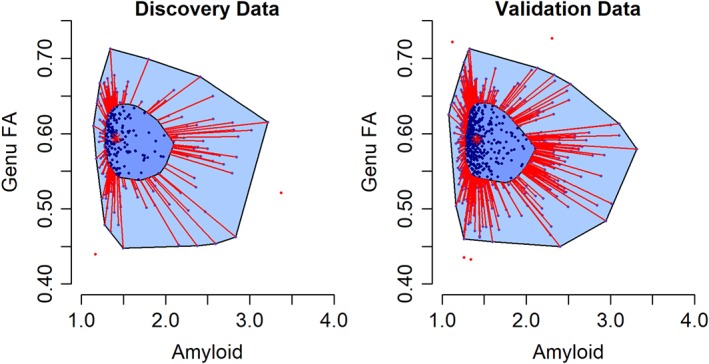
Bagplots (2‐dimensional boxplots) showing the joint distribution of fractional anisotropy (FA) in the genu of the corpus callosum and amyloid. The central asterisk marks the 2‐dimensional median. Fifty percent of the data lie in the dark blue polygon (bag). The bag is expanded by a factor of 3 to form the light blue polygon. Points outside this light blue polygon are considered outliers. FA and amyloid, in both the discovery and validation datasets, had similar variability, with ranges covering about 6 standard deviations.
Table 3.
Regression Models Evaluating the Utility of the Cerebrovascular Biomarker in Predicting Cognitive Performance
| Discovery Data | Validation Data | ||||||
|---|---|---|---|---|---|---|---|
| Model/R 2/Adjusted R 2 | Variable | Regression Coefficient (SE) | p | Model/R 2/Adjusted R 2 | Variable | Regression Coefficient (SE) | p |
| Models with amyloid alone | |||||||
| D1/0.324/0.313 | Intercept | 3.37 (0.58) | <0.0001 | V1/0.367/0.363 | Intercept | 3.62 (0.40) | <0.0001 |
| Age | −0.06 (0.01 | <0.0001 | Age | −0.06 (0.01) | <0.0001 | ||
| Sex, male | −0.28 (0.09) | <0.0001 | Sex, male | −0.31 (0.06) | <0.0001 | ||
| Educ/Occ | 0.13 (0.02) | <0.0001 | Educ/Occ | 0.14 (0.01) | <0.0001 | ||
| cycle number | 0.12 (0.03) | <0.0001 | Cycle number | 0.09 (0.02) | <0.0001 | ||
| PiB | −0.62 (0.12) | <0.0001 | PiB | −0.52 (0.09) | <0.0001 | ||
| Models with genu FA alone | |||||||
| D2/0.300/0.289 | Intercept | −0.25 (1.06) | 0.8132 | Intercept | 1.69 (0.67) | 0.0116 | |
| Age | −0.05 (0.01) | <0.0001 | V2/0.350/0.345 | Age | −0.07 (0.01) | <0.0001 | |
| Sex, male | −0.34 (0.10) | 0.0006 | Sex, male | −0.31 (0.07) | <0.0001 | ||
| Educ/Occ | 0.12 (0.02) | <0.0001 | Educ/Occ | 0.14 (0.01) | <0.0001 | ||
| Cycle number | 0.11 (0.03) | 0.0001 | Cycle number | 0.10 (0.02) | <0.0001 | ||
| Genu FA | 4.51 (1.17) | 0.0001 | Genu FA | 2.41 (0.71) | 0.0008 | ||
| Models with both amyloid and genu FA | |||||||
| D3/0.356/0.343 | Intercept | 0.08 (1.02) | 0.9396 | V3/0.377/0.372 | Intercept | 1.78 (0.65) | 0.0067 |
| Age | −0.05 (0.01) | <0.0001 | Age | −0.06 (0.01) | <0.0001 | ||
| Sex, male | −0.37 (0.10) | 0.0001 | Sex, male | −0.34 (0.06) | <0.0001 | ||
| Educ/Occ | 0.13 (0.02) | <0.0001 | Educ/Occ | 0.14 (0.12) | <0.0001 | ||
| Cycle number | 0.12 (0.03) | <0.0001 | Cycle number | 0.10 (0.02) | <0.0001 | ||
| PiB | −0.61 (0.12) | <0.0001 | PiB | −0.53 (0.09) | <0.0001 | ||
| Genu FA | 4.39 (1.13) | 0.0001 | Genu FA | 2.48 (0.70) | 0.0004 | ||
Educ = education; FA = fractional anisotropy; Occ = occupation; PiB = Pittsburgh compound B; SE = standard error.
Figure 5.
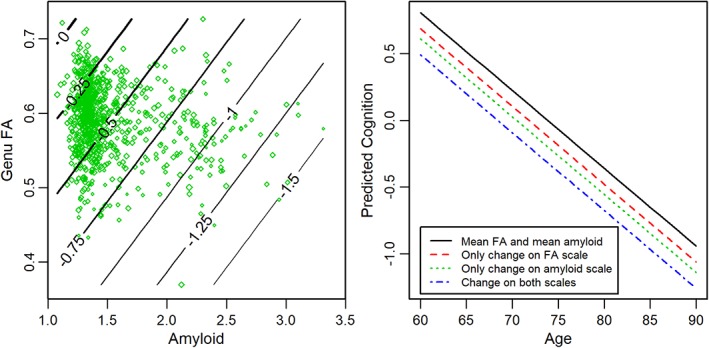
(Left) Contour plot showing predicted cognition given fractional anisotropy (FA) and amyloid. The lines on the contour plot are predicted global cognitive z scores for an 80‐year‐old man for the mean education/occupation score (12.7) and mean cycle number (4.5). The predicted z score lines cover a range from a high of 0 (mean) to a low of −1.5 (1.5 standard deviations below the mean). The green diamonds are observed values, with larger sizes indicating higher observed z scores. (Right) The predicted cognition plotted by age for a given FA and amyloid level. The lines in the plot on the right are predictions for a male participant with the same characteristics as above. The solid black line shows the prediction for both FA and amyloid at their mean values. The additional lines are after FA and/or amyloid move by 1 standard deviation in the “bad” direction.
Discussion
The major conclusions of this study were: (1) systemic vascular health has a significant impact on brain health; systemic vascular health measured as a composite of recent cardiovascular and metabolic chronic conditions was associated with medial temporal lobe thinning, widespread cerebral hypoperfusion, and poor microstructural integrity in several WM tracts, including the corpus callosum and fornix; (2) DTI‐based regional FA measures had lower variability and were sensitive to early changes even in the absence of brain infarcts and amyloid positivity; therefore, we chose genu FA as a cerebrovascular biomarker in the discovery dataset; and (3) using an independent validation dataset, we found that genu FA provided significant information about cognitive performance in addition to the information provided by amyloid deposition.
Systemic Vascular Health and sMRI
We found that the association of systemic vascular health with sMRI measures was greatest primarily in the medial temporal lobes. Medial temporal regions have increased vulnerability to aging independent of the impact of AD pathologies.31 Because the accumulation of chronic conditions (in this study, worsening vascular health) has been equated to accelerated aging, these results are as expected. There is evidence supporting the association between increased vascular risk and atrophy in regions vulnerable to AD.7, 32, 33, 34 Given that the associations of systemic vascular health and AD with sMRI are observed in the same regions, sMRI does not qualify as an independent prodromal biomarker for cerebrovascular health. In addition, the association of vascular health with medial temporal atrophy was significantly reduced in the sensitivity analyses, suggesting that it may be a less sensitive marker of early cerebrovascular disease. Across all 3 analyses (see Fig 1 and sensitivity analyses), vascular health was associated with increased superior parietal lobe thickness. This result of greater parietal lobe thickness, although surprising, has also been observed in other studies35, 36 and is suggested to be a compensatory response to early pathological changes in the brain, which needs further evaluation using longitudinal imaging studies.
Systemic Vascular Health and Cerebral Perfusion
Vascular health, specifically hypertension, causes both functional and morphological alterations to the cerebral blood vessels; therefore, it was not surprising to find that widespread cerebral hypoperfusion was associated with worsened vascular health. There is recent evidence that temporal lobe ASL changes may be a good indicator of vascular health.37 In this study, we found a stronger association of vascular health with ASL in parietal lobes after accounting for the effects of AD. Although there were significant associations between vascular health and ASL changes, we found that the variability of the regional ASL measures was at least twice that of the sMRI and DTI measures, owing to the low signal to noise ratio of the acquisition methodology.38 ASL signal relies on blood flow and the time it takes for the labeled spins to travel from the labeling plane to the imaged voxel in the tissue. In populations with vascular pathology, prolonged arterial transit time to tissue can cause artifacts, that is, increased signal in vascular regions, and spuriously reduced perfusion in tissue,39 which additionally adds variability to using ASL as a cerebrovascular biomarker.
Association of Systemic Vascular Health with Microstructural Integrity and Genu FA as a Cerebrovascular Health Biomarker
Vascular health has been shown to significantly impact microstructural organization of the WM tracts. Several studies have shown the impact of independent vascular risk factors on DTI changes including hypertension,40, 41, 42 diabetes,43, 44 hyperlipidemia,45 and obesity.46, 47 Among these studies, the most consistent finding has been the impact on corpus callosum, which has also been confirmed in longitudinal studies.48 Although there have been consistent corpus callosum/vascular risk findings in the literature, the anterior part (genu) of the corpus callosum may be most susceptible to vascular disease effects due to its small diameter or thin fibers compared to the posterior part (splenium) of the corpus callosum, where the fibers are larger in diameter. In addition, frontal lobes appear to be more susceptible to cerebrovascular disease, have greater burden of WM lesions, and are also a convergence point for several brain connections,6, 32, 49 supporting the use of genu FA as the cerebrovascular biomarker.
DTI has been shown to be a promising biomarker for small vessel disease50, 51 and has been tested for its utility in multicenter trials.52 Whereas MD is a marker of overall water diffusion and may be less specific, FA in tracts provides information about the directionality of the diffusion and may be more sensitive to small vessel disease.52 It is also a dimensionless, intrinsic quantity that does not need reference scaling or correction for head size. In this work, we showed that DTI has practical utility as a cerebrovascular biomarker because it has low measurement variability and provided similar genu FA values in 2 independent samples.
Another important reason for the use of DTI as a cerebrovascular biomarker is its sensitivity to early vascular disease–specific prodromal changes. In all of our regression analyses (overall cohort, individuals without infarcts, individuals without amyloidosis), genu FA was significantly associated with CMC. In addition, genu FA as an outcome captured the variability due to the presence of visible cerebrovascular injury (WMH) and a vascular health indicator (CMC).
Although there is considerable heterogeneity in cognitive aging, the 2 most commonly found neuropathologies are AD and cerebrovascular disease.53 Using genu FA as a measure of cerebrovascular health and global amyloid as a marker of amyloidosis (see Table 3), we found that they both independently predicted cognitive performance (D1, V1, D2, V2) and together had improved model prediction (D3, V3), supporting the utility of DTI as a cerebrovascular biomarker of cognitive aging. Figure 5 directly illustrates how our proposed biomarker can be used in clinical and research studies as a biomarker. Based on an individual's cerebrovascular biomarker in conjunction with their amyloid burden, we would be able to deduce the underlying etiology of cognitive impairment in impaired individuals and cerebrovascular health in those with preclinical disease.
We have found that partial volume correction can amplify noise and partial volume correction would imply incorporating information from sMRI into DTI and ASL. We wanted to evaluate each modality separately and therefore did not partial volume correct. We also acknowledge that our measurement of genu FA is not purely microstructural integrity and can be potentially influenced by volume changes. However we use the median voxel values of the region of interest to mitigate edge effects.
Strengths and Limitations
There are several strengths and weaknesses of this study. The key strength of this study was modeling the impact of vascular health on the brain after accounting for AD biomarkers. The information on systemic vascular health conditions in each individual based on their electronic health records through REP was extremely useful as a modeling variable in this work and avoided the need for self‐reports, which are much less accurate. A unique aspect of the study was the head‐to‐head comparison between the 3 main MRI‐based brain health measures. Although we evaluated different properties of the brain, the 3 criteria we used as guidelines allowed us to evaluate and compare these properties for clinical utility in cross‐sectional studies. The use of 2 independent discovery and validation sets further validated the generalizability of study findings. The simplistic approach we took with the use of global measures (CMC and global cognition) can be considered a weakness, but this allowed us to develop a cerebrovascular biomarker with broader utility. Further work is warranted to evaluate the performance of the proposed biomarker for longitudinal measurements, in different samples as well as among midlife participants. We also did not have data to use other diffusion MRI metrics that utilize multishell acquisitions and may provide information more sensitive to small vessel disease. This will be part of our future investigations.
Author Contributions
P.V. conceived and designed the study. All authors participated in data collection and analysis. P.V., T.G.L., and S.A.P. drafted the manuscript and figures.
Potential Conflicts of Interest
Nothing to report.
Acknowledgment
This work was supported by National Institute of Neurological Disorders and Stroke (NINDS) and National Institute on Aging (NIA) grants R01 NS097495 (principal investigator [PI]: P.V.), U01 AG06786 (PI: R.C.P.), R01 AG56366 (PI: P.V.), P50 AG16574/P1 (PI: P.V.), P50 AG16574 (PI: R.C.P.), R01 AG11378 (PI: C.R.J.), and R01 AG041851 (PIs: C.R.J. and D.S.K.); the GHR Foundation, The Millis Family, the Alexander Family Alzheimer's Disease Research Professorship of the Mayo Foundation, the Liston Award, the Elsie and Marvin Dekelboum Family Foundation, the Schuler Foundation, and Opus Building NIH grant C06 RR018898, and was made possible by the Rochester Epidemiology Project (R01 AG034676).
We thank Dr E. M. Arenaza‐Urquijo for helping with interpretation of the sMRI results; the study participants and staff at the Mayo Clinic Study of Aging, Mayo Alzheimer's Disease Research Center, and Aging Dementia Imaging Research Laboratory at the Mayo Clinic for making this study possible; and AVID Radiopharmaceuticals for their support in supplying AV‐1451 precursor, chemistry production advice and oversight, and US Food and Drug Administration regulatory cross‐filing permission and documentation needed for this work.
References
- 1. Kivipelto M, Ngandu T, Fratiglioni L, et al. Obesity and vascular risk factors at midlife and the risk of dementia and Alzheimer disease. Arch Neurol 2005;62:1556–1560. [DOI] [PubMed] [Google Scholar]
- 2. Wardlaw JM, Smith C, Dichgans M. Mechanisms of sporadic cerebral small vessel disease: insights from neuroimaging. Lancet Neurol 2013;12:483–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Knopman DS, Penman AD, Catellier DJ, et al. Vascular risk factors and longitudinal changes on brain MRI: the ARIC study. Neurology 2011;76:1879–1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Carmelli D, Swan GE, Reed T, et al. Midlife cardiovascular risk factors and brain morphology in identical older male twins. Neurology 1999;52:1119–1124. [DOI] [PubMed] [Google Scholar]
- 5. DeCarli C, Miller BL, Swan GE, et al. Cerebrovascular and brain morphologic correlates of mild cognitive impairment in the National Heart, Lung, and Blood Institute Twin Study. Arch Neurol 2001;58:643–647. [DOI] [PubMed] [Google Scholar]
- 6. Raz N, Rodrigue KM, Kennedy KM, Acker JD. Vascular health and longitudinal changes in brain and cognition in middle‐aged and older adults. Neuropsychology 2007;21:149–157. [DOI] [PubMed] [Google Scholar]
- 7. Werden E, Cumming T, Li Q, et al. Structural MRI markers of brain aging early after ischemic stroke. Neurology 2017;89:116–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Maillard P, Carmichael O, Harvey D, et al. FLAIR and diffusion MRI signals are independent predictors of white matter hyperintensities. AJNR Am J Neuroradiol 2013;34:54–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Maniega SM, Valdes Hernandez MC, Clayden JD, et al. White matter hyperintensities and normal‐appearing white matter integrity in the aging brain. Neurobiol Aging 2015;36:909–918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Vemuri P, Lesnick TG, Przybelski SA, et al. Vascular and amyloid pathologies are independent predictors of cognitive decline in normal elderly. Brain 2015;138(pt 3):761–771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chui HC, Zheng L, Reed BR, et al. Vascular risk factors and Alzheimer's disease: are these risk factors for plaques and tangles or for concomitant vascular pathology that increases the likelihood of dementia? An evidence‐based review. Alzheimers Res Ther 2012;4:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Rocca WA, Yawn BP, St Sauver JL, et al. History of the Rochester Epidemiology Project: half a century of medical records linkage in a US population. Mayo Clinic Proc 2012;87:1202–1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. St Sauver JL, Grossardt BR, Yawn BP, et al. Data resource profile: the Rochester Epidemiology Project (REP) medical records‐linkage system. Int J Epidemiol 2012;41:1614–1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Petersen RC, Roberts RO, Knopman DS, et al. Prevalence of mild cognitive impairment is higher in men. The Mayo Clinic Study of Aging. Neurology 2010;75:889–897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Roberts RO, Geda YE, Knopman DS, et al. The Mayo Clinic Study of Aging: design and sampling, participation, baseline measures and sample characteristics. Neuroepidemiology 2008;30:58–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Vemuri P, Lesnick TG, Przybelski SA, et al. Effect of lifestyle activities on alzheimer disease biomarkers and cognition. Ann Neurol 2012;72:730–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Vassilaki M, Aakre JA, Cha RH, et al. Multimorbidity and risk of mild cognitive impairment. J Am Geriatr Soc 2015;63:1783–1790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Rocca WA, Boyd CM, Grossardt BR, et al. Prevalence of multimorbidity in a geographically defined American population: patterns by age, sex, and race/ethnicity. Mayo Clinic Proc 2014;89:1336–1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Rocca WA, Gazzuola Rocca L, Smith CY, et al. Bilateral oophorectomy and accelerated aging: cause or effect? J Gerontol A Biol Sci Med Sci 2017;72:1213–1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Vemuri P, Lesnick TG, Przybelski SA, et al. Age, vascular health, and Alzheimer disease biomarkers in an elderly sample. Ann Neurol 2017;82:706–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Jack CR Jr, Wiste HJ, Weigand SD, et al. Defining imaging biomarker cut points for brain aging and Alzheimer's disease. Alzheimers Dement 2017;13:205–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Schwarz CG, Gunter JL, Wiste HJ, et al. A large‐scale comparison of cortical thickness and volume methods for measuring Alzheimer's disease severity. Neuroimage Clin 2016;11:802–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Dai W, Garcia D, de Bazelaire C, Alsop DC. Continuous flow‐driven inversion for arterial spin labeling using pulsed radio frequency and gradient fields. Magn Reson Med 2008;60:1488–1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Alsop DC, Detre JA. Reduced transit‐time sensitivity in noninvasive magnetic resonance imaging of human cerebral blood flow. J Cereb Blood Flow Metab 1996;16:1236–1249. [DOI] [PubMed] [Google Scholar]
- 25. Kellner E, Dhital B, Kiselev VG, Reisert M. Gibbs‐ringing artifact removal based on local subvoxel‐shifts. Magn Reson Med 2016;76:1574–1581. [DOI] [PubMed] [Google Scholar]
- 26. Veraart J, Novikov DS, Christiaens D, et al. Denoising of diffusion MRI using random matrix theory. Neuroimage 2016;142:394–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Koay CG, Ozarslan E, Basser PJ. A signal transformational framework for breaking the noise floor and its applications in MRI. J Magn Reson 2009;197:108–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Bhushan C, Haldar JP, Choi S, et al. Co‐registration and distortion correction of diffusion and anatomical images based on inverse contrast normalization. Neuroimage 2015;115:269–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Avants BB, Epstein CL, Grossman M, Gee JC. Symmetric diffeomorphic image registration with cross‐correlation: evaluating automated labeling of elderly and neurodegenerative brain. Med Image Anal 2008;12:26–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Oishi K, Faria A, Jiang H, et al. Atlas‐based whole brain white matter analysis using large deformation diffeomorphic metric mapping: application to normal elderly and Alzheimer's disease participants. Neuroimage 2009;46:486–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Fjell AM, McEvoy L, Holland D, et al. What is normal in normal aging?. Effects of aging, amyloid and Alzheimer's disease on the cerebral cortex and the hippocampus. Prog Neurobiol 2014;117:20–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Knopman DS, Griswold ME, Lirette ST, et al. Vascular imaging abnormalities and cognition: mediation by cortical volume in nondemented individuals: atherosclerosis risk in communities‐neurocognitive study. Stroke 2015;46:433–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Guzman VA, Carmichael OT, Schwarz C, et al. White matter hyperintensities and amyloid are independently associated with entorhinal cortex volume among individuals with mild cognitive impairment. Alzheimers Dement 2013;9(5 suppl):S124–S131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Villeneuve S, Reed BR, Madison CM, et al. Vascular risk and Abeta interact to reduce cortical thickness in AD vulnerable brain regions. Neurology 2014;83:40–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Fortea J, Vilaplana E, Alcolea D, et al. Cerebrospinal fluid beta‐amyloid and phospho‐tau biomarker interactions affecting brain structure in preclinical Alzheimer disease. Ann Neurol 2014;76:223–230. [DOI] [PubMed] [Google Scholar]
- 36. Johnson SC, Christian BT, Okonkwo OC, et al. Amyloid burden and neural function in people at risk for Alzheimer's disease. Neurobiol Aging 2014;35:576–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Jefferson AL, Liu D, Gupta DK, et al. Lower cardiac index levels relate to lower cerebral blood flow in older adults. Neurology 2017;89:2327–2334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Alsop DC, Detre JA, Golay X, et al. Recommended implementation of arterial spin‐labeled perfusion MRI for clinical applications: a consensus of the ISMRM perfusion study group and the European consortium for ASL in dementia. Magn Reson Med 2015;73:102–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Mutsaerts HJ, Petr J, Vaclavu L, et al. The spatial coefficient of variation in arterial spin labeling cerebral blood flow images. J Cereb Blood Flow Metab 2017;37:3184–3192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Maillard P, Seshadri S, Beiser A, et al. Effects of systolic blood pressure on white‐matter integrity in young adults in the Framingham Heart Study: a cross‐sectional study. Lancet Neurol 2012;11:1039–1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Gons RA, van Oudheusden LJ, de Laat KF, et al. Hypertension is related to the microstructure of the corpus callosum: the RUN DMC study. J Alzheimers Dis 2012;32:623–631. [DOI] [PubMed] [Google Scholar]
- 42. McEvoy LK, Fennema‐Notestine C, Eyler LT, et al. Hypertension‐related alterations in white matter microstructure detectable in middle age. Hypertension 2015;66:317–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Tan X, Fang P, An J, et al. Micro‐structural white matter abnormalities in type 2 diabetic patients: a DTI study using TBSS analysis. Neuroradiology 2016;58:1209–1216. [DOI] [PubMed] [Google Scholar]
- 44. Reijmer YD, Brundel M, de Bresser J, et al. Microstructural white matter abnormalities and cognitive functioning in type 2 diabetes: a diffusion tensor imaging study. Diabetes Care 2013;36:137–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Maillard P, Carmichael OT, Reed B, et al. Cooccurrence of vascular risk factors and late‐life white‐matter integrity changes. Neurobiol Aging 2015;36:1670–1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Stanek KM, Grieve SM, Brickman AM, et al. Obesity is associated with reduced white matter integrity in otherwise healthy adults. Obesity (Silver Spring) 2011;19:500–504. [DOI] [PubMed] [Google Scholar]
- 47. Bettcher BM, Walsh CM, Watson C, et al. Body mass and white matter integrity: the influence of vascular and inflammatory markers. PLoS One 2013;8:e77741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Bender AR, Raz N. Normal‐appearing cerebral white matter in healthy adults: mean change over 2 years and individual differences in change. Neurobiol Aging 2015;36:1834–1848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Tullberg M, Fletcher E, DeCarli C, et al. White matter lesions impair frontal lobe function regardless of their location. Neurology 2004;63:246–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Tu MC, Lo CP, Huang CF, et al. Effectiveness of diffusion tensor imaging in differentiating early‐stage subcortical ischemic vascular disease, Alzheimer's disease and normal ageing. PLoS One 2017;12:e0175143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Williams OA, Zeestraten EA, Benjamin P, et al. Diffusion tensor image segmentation of the cerebrum provides a single measure of cerebral small vessel disease severity related to cognitive change. Neuroimage Clin 2017;16:330–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Croall ID, Lohner V, Moynihan B, et al. Using DTI to assess white matter microstructure in cerebral small vessel disease (SVD) in multicentre studies. Clinical science (Lond) 2017;131:1361–1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Schneider JA, Aggarwal NT, Barnes L, et al. The neuropathology of older persons with and without dementia from community versus clinic cohorts. J Alzheimers Dis 2009;18:691–701. [DOI] [PMC free article] [PubMed] [Google Scholar]


