Abstract
Bovine tuberculosis (bTB) is a chronic disease of cattle that impacts productivity and represents a major public health threat. Despite the considerable economic costs and zoonotic risk consequences associated with the disease, accurate estimates of bTB prevalence are lacking in many countries, including India, where national control programmes are not yet implemented and the disease is considered endemic. To address this critical knowledge gap, we performed a systematic review of the literature and a meta‐analysis to estimate bTB prevalence in cattle in India and provide a foundation for the future formulation of rational disease control strategies and the accurate assessment of economic and health impact risks. The literature search was performed in accordance with PRISMA guidelines and identified 285 cross‐sectional studies on bTB in cattle in India across four electronic databases and handpicked publications. Of these, 44 articles were included, contributing a total of 82,419 cows and buffaloes across 18 states and one union territory in India. Based on a random‐effects (RE) meta‐regression model, the analysis revealed a pooled prevalence estimate of 7.3% (95% CI: 5.6, 9.5), indicating that there may be an estimated 21.8 million (95% CI: 16.6, 28.4) infected cattle in India—a population greater than the total number of dairy cows in the United States. The analyses further suggest that production system, species, breed, study location, diagnostic technique, sample size and study period are likely moderators of bTB prevalence in India and need to be considered when developing future disease surveillance and control programmes. Taken together with the projected increase in intensification of dairy production and the subsequent increase in the likelihood of zoonotic transmission, the results of our study suggest that attempts to eliminate tuberculosis from humans will require simultaneous consideration of bTB control in cattle population in countries such as India.
Keywords: bovine tuberculosis, buffaloes, cattle, control program, cows, India, meta‐analysis, prevalence, review
1. INTRODUCTION
Bovine tuberculosis (bTB) is a chronic granulomatous inflammatory disease that is predominantly caused by Mycobacterium bovis. While primarily affecting bovines, the pathogen has a broad host range that includes humans. It has been estimated that M. bovis causes ~10% of the total human TB cases in developing countries and subsequently poses a significant threat to global health (Olea‐Popelka et al., 2014) (Etchechoury et al., 2010) (“OIE, Bovine Tuberculosis: General Disease Information sheets,”). Prior to mandatory pasteurization of milk in many countries, M. bovis accounted for ~25% of all TB cases in children (Roswurm & Ranney, 1973). In addition to being a threat to public health, bTB is also a major economic concern, costing an estimated USD 3 billion worldwide annually due to losses from reduced cattle productivity, culling and movement and trade restrictions (Waters, Palmer, Buddle, & Vordermeier, 2012).
Bovine TB is well controlled in most developed countries where national control programmes have been implemented, although complete eradication and maintenance of bTB‐free status are challenging given the potential of spillover from wildlife reservoir hosts. Such control programmes for bTB were successfully adopted over a century ago in many developed countries by applying test and cull strategies, resulting in enormous benefits to human health and more than 10‐fold return on investment in animal productivity (Olmstead & Rhode, 2004). In contrast, bTB remains endemic in developing countries like India that lack disease control programmes because of the associated economic costs and social barriers to test and cull strategies. This current level of endemicity is likely to increase in the coming years due to a confluence of factors including the growing intensification of dairy and cattle farming and increased emphasis on improving animal productivity in these countries.
In conjunction with possessing the largest population of cattle in the world (nearly 300 million cows and buffaloes) (Basic Animal Husbandry and Fisheries Statistics, Government of India 2017), India's lack of a control programme poses a potential threat for bTB infection and transmission worldwide. In the absence of a national surveillance programme, accurate prevalence data are lacking and, to our knowledge, there has thus far not been a comprehensive review of the existing literature to determine an estimate of the overall prevalence of bTB in the country. Such an estimate will prove crucial in future efforts to accurately assess risk and inform policy for the development of effective control strategies. In this systematic review and meta‐analysis, we sought to address this critical gap and determine the overall prevalence of bTB in the cattle of India. This systematic review conforms to Preferred Reporting Items for Systematic Reviews and Meta‐analyses (PRISMA) guidelines (Liberati et al., 2009).
2. METHODS
2.1. Literature search strategy
A systematic search for published articles reporting prevalence data for bTB in cows and buffaloes in India was conducted on 11th September 2017. The four databases used in our search (CAB Direct, Web of Science, Web of Science Biological Abstracts and PubMed) were selected in order to comprehensively capture articles published in both international and local journals and minimize journal biases. After examining common MeSH terms for pre‐identified and relevant publications, the following search terms were used across all four databases: ((“mycobacterium bovis” OR tuberculosis) AND (cows OR cattle OR bovine) AND (epidemiolog* OR prevalen* OR inciden* OR surve*) AND (India)). No restrictions were placed on the date of publication. The citation software program EndNote X8 (Clarivate Analytics, Philadelphia, PA) was used to organize and remove duplicate articles between the databases. Additional articles were also identified manually from the reference lists of articles generated in the database search.
2.2. Study inclusion criteria
The inclusion/exclusion criteria for data extraction are detailed in Table 1. Included studies reported the prevalence of bTB in cows and/or buffaloes in India based on commonly accepted methods for the diagnosis of bTB. More specifically, studies whose main objectives were not to determine bTB prevalence but required a preliminary prevalence study for determining initial disease status were included as long as data were reported and animals were not pre‐selected for bTB symptoms. Prevalence studies that examined the effects of bTB control strategies were excluded in order to avoid the introduction of potential sampling bias, as the primary aims of these studies were to compare the effectiveness of control strategies. For instance, Dhanda et al. have reported an increase in prevalence in herds at Puri, Orissa, from 9.1% in 1937 to 84.7% in 1942 (Dhanda & Lall, 1959). The cattle populations that were tested were part of farms that did not practice any bTB control strategies. We believe that inclusion of studies conducted on pre‐selected herds as opposed to randomly sampled prevalence studies would not be truly representative of the prevailing prevalence in the region. Also, all other publications that did not precisely fit the main exclusion categories were excluded within the “Other” category. Finally, all included studies were cross‐sectional in nature.
Table 1.
Study inclusion/exclusion criteria
| Inclusion | Exclusion |
|---|---|
| Cross‐sectional prevalence study | Wrong type of study: not a cross‐sectional study or animals chosen for bTB symptoms |
| Study conducted in India | Study conducted elsewhere |
| Tested for Mycobacterium bovis using standard diagnostic tests | Study not addressing bTB |
| Any breed of cow or buffalo | Study neither performed on cow nor on buffalo |
| Reported the prevalence of bTB and the number of total animals screened | No statistics reported |
| In English | Language limitation: Not in English |
| Full text of publication obtained | Full text unavailable |
| Other |
Note. Criteria for study inclusion or exclusion to our systematic review on the prevalence of bTB in India.
2.3. Data extraction
Before beginning data extraction, a template was created based on population demographics and other conditions common to bTB prevalence studies. The data set recording general study characteristics included author, publication year, study period, location of study, diagnostic test used, criteria for positivity, sample size, prevalence by different production system, overall prevalence for cow and buffalo combined, overall prevalence for specific cattle breeds, and overall prevalence for male and female animals. Headings for prevalence data broken down by more specific characteristics were production system (organized farm, rural, Gaushala and other), cow breed (exotic, indigenous and cross‐bred), sex, age (younger or older than 6 months) and species (cow versus buffalo). Data extracted from studies’ individual farm‐level data by each of the three of the authors (SS, LE and BR) were assigned to different strata targeted in this study. The determination of bTB infection status was accepted as reported by the studies.
A pilot test on 20 randomly selected papers was performed in order to test the inclusion and exclusion criteria and finalize the data extraction form. For the formal review of all articles generated, an initial screening for inclusion was made based on the titles and abstracts, and publications that were clearly based on different species, countries or diseases were immediately excluded. Otherwise, full texts were read for any prevalence data that could be extracted. Three of the authors (SS, LE and BR) independently reviewed all publications before comparing their respective data forms. When discrepancies were found amongst the forms, the authors (SS, LE and BR) collectively discussed their reasoning before reaching a final consensus. All studies included and excluded are publicly available at https://doi.org/10.18113/d37s9x.
2.4. Statistical analysis
All quantitative analyses were performed in RStudio (version 1.0.143) (“R core team, R: A language and environment for statistical computing.,” R core team 2016) where the “meta” package was used to estimate models (Schwarzer, 2007) (Viechtbauer, 2010). Codes used for the statistical analyses are publicly available at https://doi.org/10.18113/d37s9x. The prevalence estimates from individual studies were logit‐transformed, and the pooled prevalence was estimated using meta‐analytic models. Cochran's Q statistic (Cochran, 1954) was computed to test for heterogeneity, and Higgin's statistic (Higgins, Thompson, Deeks, & Altman, 2003) (I 2 > 50% represents at least moderate heterogeneity) helped describe the variability in the pooled prevalence estimate due to heterogeneity between studies.
A univariate screen was used to select a parsimonious set of moderator factors for multivariate analysis. Diagnostic test type was excluded from this selection procedure and forced into the final model in order to adjust for the well‐known variability in sensitivity and specificity of diagnostic tests for bTB (Farnham, Norby, Goldsmith, & Wells, 2012). Univariable meta‐regression models were estimated using both the random‐effects (RE) and fixed‐effects (FE) models for each potential moderator variable. Analysis of variance (ANOVA) tests were run on all moderators to assess their significance when compared to the full model with all other variables included. For the purposes of variable selection, given the low power of these tests and precedence set in other systematic reviews and meta‐analyses, variables with a p‐value < 0.25 were retained for inclusion in the final model (Sibhat et al., 2017) (Asmare et al., 2016) (Dohoo, Martin, & Stryhn, 2009).
The fit of the resulting multivariable meta‐regression models and evidence of publication bias was assessed through funnel plots, Egger's asymmetry test (Egger, Davey Smith, Schneider, & Minder, 1997) and Begg's rank correlation (Begg & Mazumdar, 1944) test.
To visualize the prevalence of bTB in the different states of India, we generated a map utilizing an open‐source library called D3.js (Data‐Drive Documents) (Bostock, Ogievetsky, & Heer, 2011). This allowed us to plot data positions via the centroids of given shapefile locations represented in the map and control graphical elements based on their values (Cleveland & McGill, 1984). We utilized a continuous log scale for circle size to represent bTB prevalence and a power function for circle lightness to represent the confidence in the prevalence estimates of each state, ratifying values to visual variables on a linear scale (Bertin, 1983).
3. RESULTS
3.1. Characteristics of included studies
From the 285 publications screened, 44 articles were included in the systematic review (Figure 1). In the instance that a publication reported prevalence data for multiple states, years, cattle breeds, species or production systems, they were considered as separate strata level data. A total of 106 strata level data were extracted from the 44 articles for meta‐analysis. For example, as seen in https://doi.org/10.18113/d37s9x, the study by Iyer (1944) has been extracted into three strata level data, the strata being the three locations in which the study was performed. The same was done for other studies that included data on different production system, breed, species, etc. These studies included in the quantitative analyses spanned from 1942 to 2016 and provided bTB prevalence data for a total sample size of 82,419 of which 29,037 were buffaloes and 53,382 were cows (Table 2).
Figure 1.
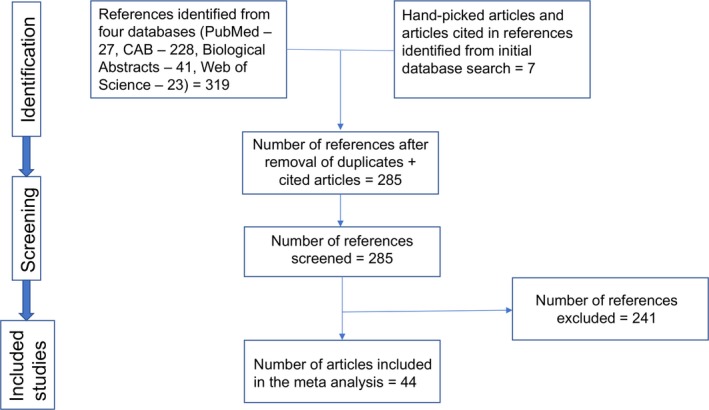
Schematic representation of the literature selection procedure for the systematic review of bTB prevalence in India [Colour figure can be viewed at http://wileyonlinelibrary.com]
Table 2.
Reported bTB prevalence for included studies
| Authors | Study location | Dx. Test | Sample size | Reported prevalence‡ (%) |
|---|---|---|---|---|
| Mallick, Aggarwal, and Dua (1942) | Punjab | DIT | 1217 | 23.2 |
| Iyer (1944) | Uttar Pradesh | PM Exam | 250 | 2.4 |
| Iyer (1944) | Maharashtra | PM Exam | 120 | 13.3 |
| Iyer (1944) | West Bengal | PM Exam | 130 | 2.3 |
| Taneja (1955) | Haryana | DIT | 102 | 26.5 |
| Dhanda and Lall (1959) | Gujarat | SIT | 25142 | 16.7 |
| Lall, Singh, and Sen Gupta (1969) | Uttarakhand | DIT | 128 | 0.0 |
| Lall et al. (1969) | Punjab | DIT | 111 | 13.5 |
| Lall et al. (1969) | Haryana | DIT | 1567 | 2.7 |
| Lall et al. (1969) | Bihar | DIT | 169 | 4.7 |
| Lall et al. (1969) | Uttar Pradesh | DIT | 1418 | 4.9 |
| Lall et al. (1969) | Rajasthan | DIT | 727 | 2.6 |
| Lall et al. (1969) | Telangana | DIT | 426 | 1.9 |
| Lall et al. (1969) | Maharashtra | DIT | 194 | 1.0 |
| Lall et al. (1969) | West Bengal | DIT | 65 | 0.0 |
| Lall et al. (1969) | Himachal Pradesh | DIT | 177 | 0.6 |
| Purohit and Mehrotra (1969) | Rajasthan | SICT | 1010 | 1.8 |
| Rawat and Kataria (1971) | Madhya Pradesh | DIT | 1830 | 2.4 |
| Nagaraja, Krishnaswamy, Adinarayanaiah, Murthy, and Nanjiah (1973) | Karnataka | DIT | 3250 | 5.2 |
| Joshi, Sharma, Dhillon, and Sodhi (1976) | Punjab | DIT | 1081 | 10.5 |
| Bali and Khanna (1979) | Haryana | SIT | 663 | 1.4 |
| Bali and Khanna (1979) | Haryana | SIT | 624 | 4.6 |
| Paily, Georgekutty, and Venugopal (1979) | Kerala | SIT | 608 | 0.8 |
| Appuswamy, Batish, Parkash, and Ranganathan (1980) | Haryana | Culture | 308 | 4.6 |
| Kulshreshtha, Jagjit, and Chandiramani (1980) | Haryana | SIT | 13089 | 2.5 |
| Bali and Singh (1980) | Haryana | SIT | 628 | 2.4 |
| Bala and Sidhu (1981) | West Bengal | NR | 475 | 41.5 |
| Bala and Sidhu (1981) | Haryana | NR | 712 | 1.1 |
| Bala and Sidhu (1981) | Uttar Pradesh | NR | 732 | 13.1 |
| Murti and Hazarika (1982) | Meghalaya | SICT | 302 | 8.9 |
| Sharma et al. (1985) | Uttar Pradesh | PM, ZN staining | 1268 | 13.3 |
| Bapat and Bangi (1985) | Maharashtra | SICT | 2043 | 1.2 |
| Maity, Deb and Pramanik (1992) | West Bengal | PM, ZN staining | 1571 | 0.4 |
| Sharma, Kwatra, Joshi, and Saharma (1994) | Punjab | SIT | 2623 | 4.0 |
| Rakesh Sisodia, Shuykla and Sisodia (1995) | Madhya Pradesh | SIT | 465 | 9.0 |
| Rajaram, Rao and Manickam (1996) | Tamil Nadu | SIT | 1339 | 14.6 |
| Mishra, Panda, and Panda (1997) | Orissa | SIT | 670 | 3.4 |
| Dev, Purohit, and Joshi (1998) | Rajasthan | SICT | 75 | 10.7 |
| Kumar, Sharma, Iyer, and Prasad (1998) | Uttar Pradesh | PM, ZN staining | 1435 | 9.8 |
| Aswathanarayana et al. (1998) | Karnataka | SIT | 1189 | 25.7 |
| Kumar and Parihar (1998) | Uttar Pradesh | PM Exam | 2028 | 0.8 |
| Chowdhury, Sarkar, Pal, Roy, and Chakraborty (2001) | West Bengal | PM, ZN staining | 1050 | 3.9 |
| Mukhopadhyay, Antony, and Pillai (2001) | Pondicherry | SICT | 41 | 51.2 |
| Shringi (2004) | Rajasthan | SIT | 353 | 4.8 |
| Singh, Gumber, Randhawa, Aradhana and Dhand (2004) | Punjab | SIT | 627 | 9.1 |
| Dali et al. (2004) | Maharashtra | NR** | 340 | 6.2 |
| Raval, Sunil, Belsare, Kanani and Patel (2006) | Gujarat | SIT | 164 | 1.8 |
| Raval et al. (2006) | Gujarat | SIT | 167 | 0.0 |
| Raval et al. (2006) | Gujarat | SIT | 172 | 0.0 |
| Raval et al. (2006) | Gujarat | SIT | 152 | 3.3 |
| Raval et al. (2006) | Gujarat | SIT | 161 | 1.9 |
| Ganesan (2006) | Tamil Nadu | SIT | 63 | 65.1 |
| Nishath and Ganesan (2006) | Tamil Nadu | SIT | 63 | 49.2 |
| Taggar and Bhadwal (2008) | Jammu and Kashmir | SIT | 40 | 37.5 |
| Phaniraja, Jayaramu, Jagadeesh and Kumar (2010) | Karnataka | SIT | 2668 | 2.4 |
| Aneesh, Mandeep, Katoch, Prasenjit, and Katoch (2010) | Himachal Pradesh | SIT | 440 | 14.3 |
| Trangadia, Rana and Srinivasan (2013) | Gujarat | SIT | 2310 | 2.3 |
| Trangadia et al. (2013) | Uttar Pradesh | SIT | 338 | 0.6 |
| Bhanu Rekha, Gunaseelan, Pawar, and Giri (2014) | Tamil Nadu | ELISA | 357 | 4.5 |
| Neeraja et al. (2014) | Karnataka | SIE | 45 | 26.7 |
| Ashish, Amit, and Deepak (2014) | Uttar Pradesh | SIT | 245 | 14.3 |
| Thakur, Sinha and Singh (2016) | Uttar Pradesh | SIT | 442 | 16.1 |
| Thakur et al. (2016) | Uttarakhand | SIT | 99 | 0.0 |
| Filia, Leishangthem, Mahajan, and Singh (2016) | Punjab | SICT | 121 | 14.0 |
The reported bTB prevalence for each included study on a state‐by‐state basis. Diagnostic techniques (Dx. Tests) used were single intradermal test (SIT), single intradermal comparative tuberculin test (SICT), double intradermal test (DIT), ELISA, interferon‐gamma release assay (IGRA), multiple tests that included SIT, IGRA, and ELISA (SIE), Ziehl–Neelsen (ZN) staining and detailed post‐mortem examinations (PM). While most studies reported which Dx. test was used, some were not reported (NR) or ‡were unconventional. **Confidence intervals were reported for only a few studies and thus not included in the table above.
Included studies used common diagnostic techniques for bTB testing including the single intradermal test (SIT), single intradermal comparative tuberculin test (SICT), double intradermal test (DIT), enzyme‐linked immunosorbent assay (ELISA), interferon‐gamma release assay (IGRA), Ziehl–Neelsen (ZN) staining and detailed post‐mortem (PM) examinations; some studies performed multiple tests that included SIT, IGRA and ELISA (SIE). While most studies followed OIE recommended guidelines for skin test positivity at ≥4 mm after 72 hr (“International Office of Epizootics (OIE),” OIE, 2006) (the cut‐off for both SIT and SICT tests), some studies defined their cut‐off point as ≥5 mm; however, a small number of publications did not report criteria for test positivity (NR). A few studies classified animals as “doubtful” if the increase in skin thickness was between 3 and 4 mm. We did not use any cut‐off values on the number of animals for classification of the various production systems. Most included publications explicitly mentioned the type of production system that was used in their studies. In the instance that a study did not specify the production system, we did not include that study under any production system strata. To examine any effect of time on the prevalence of bTB, the study periods were separated into four time intervals: 1941–1960; 1961–1980; 1981–2000; and, 2001–2016.
3.2. Meta‐analysis
To assess for potential publication bias, a funnel plot was constructed of the logit prevalence against standard error (Figure 2). The lack of symmetry in the funnel plot illustrates potential publication bias towards smaller studies with lower prevalence. Egger's asymmetry test was significant and showed presence of bias (p‐value < 0.001), while Begg's rank correlation test did not (p‐value > 0.05).
Figure 2.
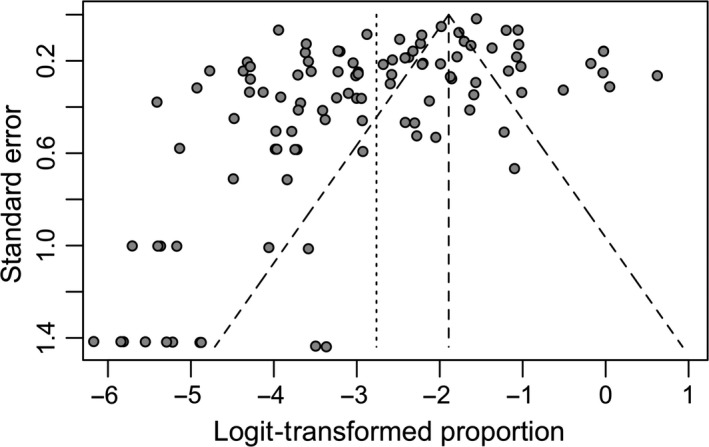
Funnel plot of standard error and logit‐transformed prevalence demonstrates potential publication bias
This evidence of publication bias suggests that RE model will be more appropriate for these data. To explore this further, we estimated both random‐ and fixed‐effects models and constructed funnel plots to compare their fit and look for evidence of systematic bias (Supporting information Figures [Link], [Link]a,b). The RE model demonstrated greater symmetry than the FE model comparatively, suggesting that the RE model is a better fit to the data. Visual inspection of the predicted versus empirical observations (Normal Q‐Q plot) also suggests that the RE model is a better fit to the data (Supporting information Figures [Link], [Link]a,b). As a final check, we constructed receiver operating characteristic (ROC) curves for the two models. The ROC analyses found no difference between the two models in terms of their classification ability (AUC ~ 0.74 for both models).
Given the evidence for publication bias and improved qualitative fit of the RE model, we focus on this model, which accounts for heterogeneity between individual studies, to estimate the prevalence of bTB in India from these data. The RE model was estimated from logit‐transformed prevalence rates from individual publications, and the pooled prevalence estimate of bTB in India was determined to be 7.3% (95% CI: 5.6, 9.5). Cochran's (Q) value (Q = 3939.85, df = 105 and p < 0.0001) and Higgins statistic (I 2 = 98.9%) were computed to test for heterogeneity. The meta‐analysis, and comparison to the RE model, is graphically summarized in a forest plot (Figure 3).
Figure 3.
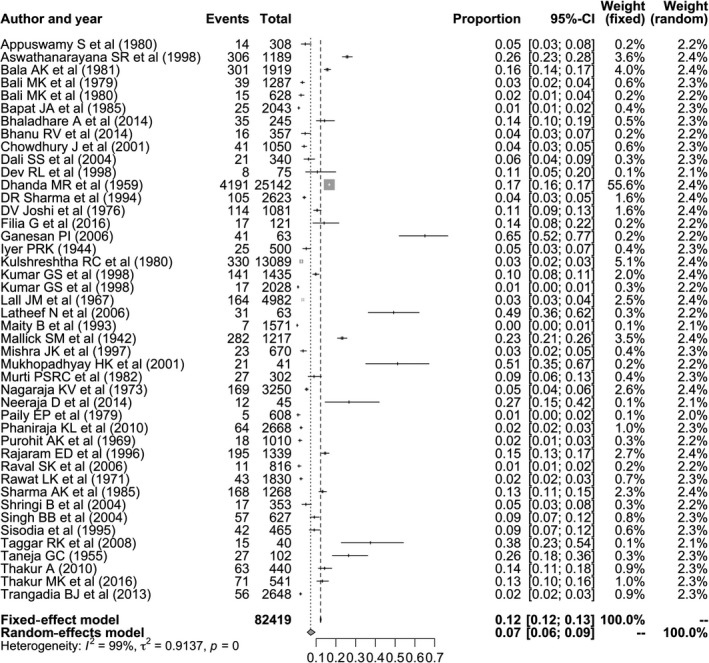
Forest plot visualizing the varying bTB prevalence reported for each included publication in the meta‐analysis. Weightage given to each included publication by both RE and FE models have been shown for rigorous comparison. “Total” refers to the number of animals in each publication, while “Events” refers to the number of bTB‐positive animals. “Proportion” reports the bTB prevalence for each publication
3.3. Meta‐regression
3.3.1. Univariable meta‐regression
Due to the presence of statistical heterogeneity, we conducted univariable meta‐regression in order to determine the effect of study‐level covariates on the estimates of cumulative prevalence. The moderators considered for the analyses were study period, study location, sample size, production system, species, cattle breed and diagnostic technique used.
As seen in Table 3, the proportion of each predictor variable's effect on heterogeneity (R 2) ranged from 0% to 16.5% in the RE model. Further, under the RE model, the highest value of R 2 was observed for study location while, diagnostic technique, and sample size exhibited no effect on heterogeneity (R 2 = 0%).
Table 3.
Univariable meta‐regression
| Predictors | Proportion (R 2) (%) | p value (RE) |
|---|---|---|
| Study period | 7.0 | 0.04 |
| Study location | 16.5 | 0.01 |
| Diagnostic technique | 0.0 | 0.70 |
| Species | 0.7 | 0.22 |
| Breed | 0.7 | 0.40 |
| Production system | 2.5 | 0.16 |
| Sample Size | 0.0 | 0.95 |
Proportion of effect of predictors on heterogeneity. All variables had a p < 0.01 in the FE model.
3.3.2. Multivariable meta‐regression
All moderators from the univariable meta‐regression were subjected to multivariable meta‐regression (Table 4), which showed that these moderators accounted for 31.4% of the observed heterogeneity. Hence, the significant variables included in our regression model explain only a fraction of the variability observed.
Table 4.
Multivariable meta‐regression
| Predictors | Categories | No. of studies | Odds ratio (95% CI) | p‐value (RE) |
|---|---|---|---|---|
| Study period | 1941–1960 | 7 | Reference | |
| 1961–1980 | 36 | 0.15 (0.04, 0.65) | 0.01 | |
| 1981–2000 | 29 | 0.21 (0.05, 1.01) | 0.05 | |
| 2001–2016 | 34 | 0.14 (0.03, 0.65) | 0.01 | |
| Production systems | Gaushala | 6 | Reference | |
| Organized | 71 | 0.34 (0.09, 1.20) | 0.09 | |
| Rural | 4 | 0.24 (0.04, 1.52) | 0.13 | |
| Semen station | 1 | 1.05 (0.03, 34.89) | 0.98 | |
| Slaughterhouse | 9 | 0.57 (0.06, 5.51) | 0.61 | |
| Species | Buffalo | 23 | Reference | |
| Cow | 83 | 0.60 (0.28, 1.27) | 0.16 | |
| Study location | Andhra Pradesh | 2 | Reference | |
| Bihar | 1 | 2.57 (0.13, 52.46) | 0.54 | |
| Gujarat | 10 | 0.33 (0.03, 3.59) | 0.36 | |
| Haryana | 15 | 0.51 (0.06, 4.48) | 0.54 | |
| Himachal Pradesh | 3 | 3.88 (0.30, 49.20) | 0.29 | |
| Jammu and Kashmir | 1 | 7.74 (0.27, 218.94) | 0.22 | |
| Karnataka | 7 | 1.82 (0.19, 17.33) | 0.60 | |
| Kerala | 2 | 0.22 (0.01, 5.80) | 0.36 | |
| Madhya Pradesh | 5 | 1.56 (0.14, 17.54) | 0.72 | |
| Maharashtra | 7 | 0.81 (0.08, 8.70) | 0.86 | |
| Meghalaya | 2 | 1.22 (0.06, 24.31) | 0.89 | |
| Orissa | 2 | 0.73 (0.03, 17.07) | 0.84 | |
| Pondicherry | 1 | 58.57 (2.16, 1595.91) | 0.01 | |
| Punjab | 12 | 2.12 (0.26, 17.49) | 0.48 | |
| Rajasthan | 6 | 1.89 (0.18, 19.82) | 0.58 | |
| Tamil Nadu | 5 | 8.17 (0.55, 121.89) | 0.12 | |
| Uttar Pradesh | 16 | 1.32 (0.15, 11.50) | 0.80 | |
| Uttarakhand | 2 | 0.13 (0.01, 3.21) | 0.21 | |
| West Bengal | 7 | 2.39 (0.23, 24.87) | 0.46 | |
| Diagnostic test | SIT | 46 | Reference | |
| Culture | 2 | 3.99 (0.53, 30.28) | 0.18 | |
| DIT | 25 | 0.69 (0.23, 2.10) | 0.52 | |
| ELISA | 2 | 0.71 (0.09, 5.52) | 0.75 | |
| PM Exam | 6 | 0.08 (0.01, 0.77) | 0.03 | |
| SICT | 11 | 0.69 (0.18, 2.65) | 0.59 | |
| SIE | 1 | 0.07 (0.00, 1.03) | 0.05 | |
| Breed | Cross‐bred | 19 | Reference | |
| Exotic | 10 | 1.08 (0.37, 3.18) | 0.88 | |
| Indigenous | 15 | 0.97 (0.39, 2.37) | 0.94 | |
| Sample size | 1.00 | < 0.0001 |
Multivariable meta‐regression of the selected predictors on the prevalence of bTB in India. (R 2 = 31.4%, n = 106).
Analysis of variance (ANOVA) tests indicated that five (study period, study location, species, diagnostic test and breed) of the seven moderators were significant (p < 0.25) when the other variables were included (Table 5).
Table 5.
ANOVA results
| Predictors | p‐value (RE) |
|---|---|
| Study period | 0.04* |
| Study location | 0.001* |
| Production system | 0.55 |
| Species | 0.16* |
| Diagnostic test | 0.12* |
| Breed | 0.13* |
| Sample size | 0.93 |
ANOVA results of individual predictors subjected to multivariable meta‐regression. All variables had a p < 0.01 in the FE model. *represents significance.
3.4. Effect of moderators on prevalence of bTB
Prevalence estimates using both the RE and FE models are reported in Table 6. As noted above, the values reported from RE model are likely more appropriate given the observed heterogeneity in the studies as the FE model is biased by studies with larger sample size. Based on the RE model, the prevalence of bTB in cows, 6.3% (95% CI: 4.9, 8.0), was marginally higher than the prevalence in buffaloes, 4.3% (95% CI: 2.7, 6.7). Amongst cows, prevalence by breed did not vary greatly as cross‐bred cows were found to have the highest prevalence with 8.1% (95% CI: 4.6, 13.8), followed by indigenous cows with 7.4% (95% CI: 4.0, 13.1), and exotic cows with 7.0% (95% CI: 3.7, 12.9). Unlike cattle breed, larger differences were seen amongst production systems as cattle housed in Gaushalas (protective shelters for unproductive or destitute cows in India) had a higher prevalence, 19.1% (95% CI: 13.0, 27.1) than those kept in organized farms, 5.1% (95% CI: 3.8, 6.7) and rural conditions, 4.4% (95% CI: 1.0, 16.5). The time period, 1941–1960, was found to have the highest prevalence, 13.8% (95% CI: 10.5, 17.9), while 1961–1980 was found to have the lowest, 3.6% (95% CI: 2.6, 4.9). A total of 28,073 animals had been tested during 1961–1980. The time period between 1981 and 2000 showed a prevalence of 7.0% (95% CI: 4.8, 10.2), and the prevalence of the most recent time period between 2001 and 2016 was determined to be 6.8% (95% CI: 4.3, 10.7) (Table 6).
Table 6.
Pooled prevalence estimates (derived from both RE and FE models) of the various predictors namely, cattle species, breed, production system and study period
| Predictors | Sample size | Prevalence (95% CI) (RE model) | Prevalence (95% CI) (FE model) | |
|---|---|---|---|---|
| Species | Buffalo | 29,037 | 4.3% (2.7, 6.7) | 16.0% (15.5, 16.4) |
| Cow | 53,382 | 6.3% (4.9, 8.0) | 10.2% (9.8, 10.5) | |
| Cattle breed | Exotic | 2,011 | 7.0% (3.7, 12.9) | 16% (14.1, 18.2) |
| Cross‐bred | 9,548 | 8.1% (4.6, 13.8) | 13.5% (12.7, 14.5) | |
| Indigenous | 4,169 | 7.4% (4.0, 13.1) | 15.5% (14.0, 17.1) | |
| Production systems | Gaushala | 576 | 19.1% (13.0, 27.1) | 18.7% (15.7, 22.3) |
| Organized farm | 43,847 | 5.1% (3.8, 6.7) | 8.4% (8.1, 8.7) | |
| Rural farm | 1,607 | 4.4% (1.0, 16.5) | 3.3% (2.2, 4.7) | |
| Study period | 1941–1960 | 26,961 | 13.8% (10.5, 17.9) | 17.0% (16.6, 17.5) |
| 1961–1980 | 28,073 | 3.6% (2.6, 4.9) | 3.9% (3.6, 4.2) | |
| 1981–2000 | 16,927 | 7.0% (4.8, 10.2) | 13.9% (13.2, 14.6) | |
| 2001–2016 | 10,458 | 6.8% (4.3, 10.7) | 9.2% (8.5, 10.0) |
3.5. Geographical distribution of included studies in India
Study reports from included publications encompassed 18 states and one union territory in India. No reports were found for Arunachal Pradesh, Assam, Chhattisgarh, Goa, Jharkhand, Manipur, Mizoram, Nagaland, Sikkim, Telangana, Tripura, Andaman and Nicobar Islands, Chandigarh, Dadra and Nagar Haveli, Daman and Diu, Delhi, and Lakshadweep, comprising a total of 11 states and six union territories. It can be observed from the map that the prevalence of bTB varied highly between states (Figure 4) (Table 7).
Figure 4.
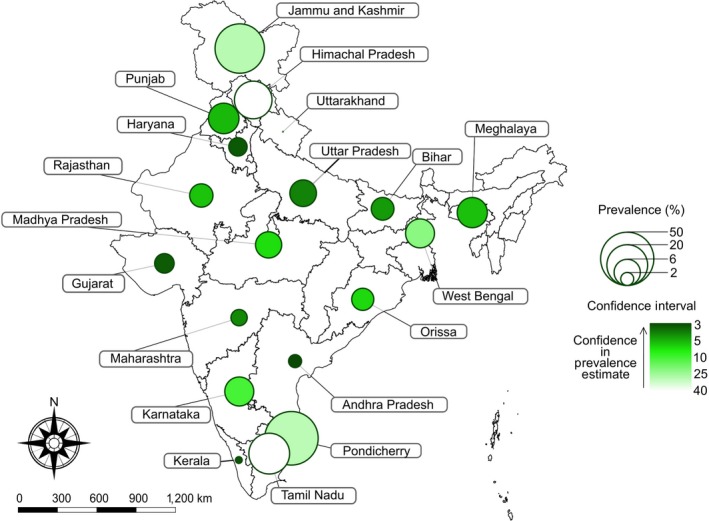
Geographical distribution and pooled prevalence estimates (RE model) of bTB in the different states of India. For the confidence intervals, a power scale was used to map colour lightness and represented as y = mxk + b, where k = 0.5. Prevalence was mapped to a log‐scale where data were uniformly corrected with allow for visual properties. Note that although scales were altered, the original data set is provided in Table 7 to afford accurate measures [Colour figure can be viewed at http://wileyonlinelibrary.com]
Table 7.
Pooled prevalence estimates (RE model) of bTB prevalence in India by state
| STATE | Sample size | Prevalence (95% CI) (RE model) |
|---|---|---|
| Andhra Pradesh | 426 | 2% (1.0, 3.9) |
| Bihar | 169 | 4.7% (2.4, 9.2) |
| Gujarat | 28,268 | 3.6% (2.2, 5.8) |
| Haryana | 17,693 | 3.3% (1.9, 5.4) |
| Himachal Pradesh | 617 | 15.4% (4.2, 43.4) |
| Jammu and Kashmir | 40 | 37.5% (24.0, 53.2) |
| Karnataka | 7,152 | 7.9% (3.0, 19.2) |
| Kerala | 608 | 1.0% (0.3, 3.6) |
| Madhya Pradesh | 2,295 | 6.3% (2.7, 14.00) |
| Maharashtra | 2,697 | 2.7% (1.0, 6.9) |
| Meghalaya | 302 | 8.7% (5.1, 14.3) |
| Orissa | 670 | 4.5% (1.5, 12.5) |
| Pondicherry | 41 | 51.2% (36.3, 66.0) |
| Punjab | 5,780 | 8.9% (5.5, 14.2) |
| Rajasthan | 2,165 | 5.0% (2.1, 11.5) |
| Tamil Nadu | 1,822 | 19.6% (6.6, 45.9) |
| Uttar Pradesh | 8,156 | 6.5% (4.3, 9.8) |
| Uttarakhand | 227 | 0.4% (0.1, 3.1) |
| West Bengal | 3,291 | 7.8% (2.1, 25.7) |
| Grand Total | 82,419 |
4. DISCUSSION
After screening of 285 publications, we extracted data from 44 cross‐sectional studies published in peer‐reviewed journals that report the prevalence of bTB in India and conducted meta‐analysis. The pooled prevalence estimate (RE model) for all of India was found to be 7.3% (95% CI: 5.6, 9.5). Despite being a disease of antiquity with significant animal and public health costs that have been controlled in most developed countries over a half‐century ago, bTB has a high and widespread prevalence in India as no national control strategies have been implemented in the country (Figure 4). These data suggest that India, as the world's largest producer of milk (~156 MMT), accounting for ~18.5% of the world's total milk production and the world's largest red meat exporter (~1.9 MMT), has an urgent and as yet unmet need for control of bTB for both economic and public health reasons (DADF, 2015).
Following the White Revolution (Bellur, Singh, Chaganti, & Chaganti, 1990) (Nair, 1985), a rural development programme in India that resulted in making India the largest producer of milk and milk products, organized dairy farming has expanded rapidly. These farms have comparatively high (and still increasing) animal densities, paving the way for higher probabilities of disease transmission. In contrast, rural farms are owned by small‐holder farmers and have much lower stocking densities, resulting in lower likelihoods of disease transmission. Our results show the prevalence of bTB in animals housed under organized farming systems to be 5.1% (95% CI: 3.8, 6.7) and rural conditions to be 4.4% (95% CI: 1.0, 16.5). The overlap of CI in prevalence between organized and rural settings as suggested by the RE model is curious given the prevailing dogma that organized farming poses higher risk of disease transmission, suggesting that further investigation is needed for clarification of this issue. Yet, amongst the three different production systems in which the animals were housed, Gaushalas had the highest prevalence of bTB, 19.1% (95% CI: 13.0, 27.1). Gaushalas are protective shelters for destitute or unproductive cows in India. There are over 5,100 of these “old age homes” for cows in India (DADF, G. o. I., 2015), which may account for the higher prevalence observed in this group of animals as bTB is a chronic infection. While noteworthy and in line with expectations of observing greater prevalence in such a high‐risk setting, the lower sample size for Gaushalas compared with sample sizes of other production systems must be kept in consideration and warrants further investigation.
Overall, the ordering of prevalence estimates determined using the FE model for different production systems follows the same trend as in the RE model (i.e., prevalence in Gaushala > Organized farms > Rural farms) (Table 6). However, given the observed heterogeneity in the studies, it is difficult to assess the validity of the FE model, and hence, further study is necessary to clarify the exact influence that each production system has on bTB prevalence before definitive conclusions can be made. We note that accurate estimates of prevalence rates for each production system are particularly important in the Indian context where the magnitude of animals housed in Gaushalas and the increasing population of cattle being reared under intensive conditions have the potential to considerably impact overall prevalence and influence assessment of bTB transmission rates and targeted interventions.
Regarding animal species (cow versus buffalo), the meta‐analysis (RE model) shows prevalence to be higher in cows [6.3% (95% CI: 4.9, 8.0)] than in buffaloes [4.3% (95% CI: 2.7, 6.7)]. However, we note that the prevalence in buffaloes determined using the FE model was 16.0% (95% CI: 15.5, 16.4) and that in cows was 10.2% (95% CI: 9.8, 10.5). The high prevalence observed in buffaloes using the FE model is most likely driven by a single study that sampled 21,592 buffaloes (of a total buffalo sample size of 29,037 included in this meta‐analysis) and recorded a prevalence of 17.4% (Dhanda & Lall, 1959). As per the Government of India's Department of Animal, Dairy and Fisheries (DADF) 2016–2017 Annual report, the share of milk contribution from buffaloes is 49% and that of cows is 48% (DADF, 2016). Assuming a conservative 10% loss in milk productivity due to bTB (Thoen, 2008) and the overall estimated bTB prevalence rates based on the RE model, the annual costs to farmers only from loss in milk production in cows and buffaloes in India are estimated to range from 375 to 544 million USD (Supporting information Table S1). We note that the need for intensification of dairy production to meet increased milk demand and national priorities for nutritional improvement and rural development is likely to significantly increase bTB disease prevalence as the disease is known to more easily spread amongst intensively reared cattle. With the inevitable increase in bTB prevalence, this already large economic cost will only continue to grow if no intervention measures are implemented.
Published studies on the influence of breed on genetic susceptibility to bTB showed that the native breed of cattle is more resistant to the disease than exotic breed (Vordermeier et al., 2012) (Soparkar, 1925) (Liston & Soparkar, 1917) (Sharma, Vanamayya, & Parihar, 1985), affirming a generally held and commonly disseminated dogma. In contrast, our results note no significant differences in bTB prevalence between cow breeds in either the RE or the FE models (Table 3). However, given the heterogeneity observed in the studies, rigorous investigations of the true differences in susceptibility amongst different cattle breeds to bTB will be essential for evidence‐based formulation of a rational approach to control this disease in India.
India has a cattle population of nearly 300 million, and we attempted a conservative estimation of the number of infected cattle in India. As per DADF, G. o. I., 2015 (Basic Animal Husbandry and Fisheries Statistics, 2015), there were 39.7 m exotic and cross‐bred cows, 151.2 m indigenous cows and 108.7 m buffaloes in 2015 (DADF, G. o. I., 2015). Applying bTB prevalence estimates (obtained from our meta‐analysis) of 7.3% (95% CI: 5.6, 9.5) across all cattle types, there are likely to be ~21.8 m (95% CI: 16.6, 28.4) bTB‐positive cattle in India, suggesting that India likely also has the highest burden of bTB‐infected animals in the world, exceeding even at the lower confidence interval the total number of dairy cattle in the United States (USDA, 2016). We note that M. bovis may not necessarily be the only causative agent of bTB in all reactor animals as isolation of M. tuberculosis from cattle samples has also been reported (Srivastava et al., 2008) (Sweetline Anne, Ronald, Kumar, Kannan, & Thangavelu, 2017).
The multivariable logistic regression model accounted for ~31% of the heterogeneity between studies, suggesting that additional factors not part of our model are also contributors to bTB prevalence. These factors may include animal age, sex and herd size that were not represented with enough frequency in the papers included in the systematic review to be subject to robust and rigorous meta‐analysis. Hence, future studies should strive to understand how these factors contribute to overall bTB prevalence.
We note that the findings of this study must be considered in conjunction with the limitations inherent in systematic reviews and meta‐analyses. For example, studies were limited to those included in the four databases used and no single study sample can provide a perfect representation of the cattle in a state or across the country. Further, our review consisted of only published studies written in English and did not capture any unpublished data, subjecting our analysis to publication bias (Figure 4). This potential publication bias is supported by the significant result of Egger's regression test, the test with greater statistical power (Hayashino, Noguchi, & Fukui, 2005).
In addition, our findings are limited by the variation in experimental design and methodology of each included article. Variation in the reporting details of each article also contributes to variations in study quality. It is important to note that the pooled prevalence estimate of 7.3% (95% CI: 5.6, 9.5) was derived from studies that, despite efforts during the screening process to exclude studies with biased sampling methods, may not have been entirely random surveys and needs to be refined with proper cross‐sectional national level surveys using internationally recognized and well‐standardized methods. In addition, acceptance of each study's reported number of positive animals as truly positive is also a limiting but unavoidable reality of lacking access to the original data and an inability to know the sensitivity, specificity and other performance characteristics of the tests used. In addition, while most studies’ objectives were to determine bTB prevalence in the study population, studies differed in their examination or reporting of specific moderators of interest.
Our analysis also indicated the presence of temporal heterogeneity (R 2 = 7.04%) over the 74‐year time frame (1942–2016) represented by the included studies (Table 3). While the specific source(s) of this heterogeneity is unclear, contributors may include differences in environmental conditions over time (Humblet et al., 2010) (Bekara, Azizi, Bénet, & Durand, 2016), the number of studies within each time interval, animals tested, test operators’ skills/methods and the diagnostic tests themselves. Recent studies have also shown that the quality, origin and source of tuberculin used are variable within tuberculin‐based tests, highlighting a lack of standardization (Bakker et al., 2005). In addition to such variation within individual tests, the performance, sensitivity and specificity vary across tuberculin‐based tests making comparisons difficult and imprecise (Hartnack & Torgerson, 2012) (Varello et al., 2008) (Cousins & Florisson, 2005) (Ameni, Miörner, Roger, & Tibbo, 2000). While most tests are tuberculin‐based, there are potential causes for heterogeneity that remain to be explored. Thus, combined with the existing limitations of non‐standardized and varying performance characteristics of current diagnostic tests, we underscore the need for a national surveillance programme using a single, well‐standardized skin test performed by independent, well trained operators using OIE approved protocols and well‐standardized tuberculin antigen to enable accurate monitoring of bTB prevalence over time and the impacts of any potential intervention or control programme.
Several previous studies have reported prevalence of bTB in farms that used control strategies for the disease. Although test and slaughter of reactor animals as a control strategy are practically impossible in developing countries like India due to both economic and social considerations, the above‐mentioned studies provide preliminary evidence that even test, and segregate approaches have the potential to help reduce the prevalence of bTB in India, at least in intensively farmed animals that are regularly tested using well‐standardized tests (Dhanda & Lall, 1959) (Krishnaswamy, Nagaraja, Keshavamurthy, Nanjiah, & Adinarayanaiah, 1973; Mukherjee, 2006).
Taken together, the meta‐analysis highlights a critical and hitherto unmet need for the development of a national surveillance programme and the implementation of an effective strategy for control of bTB in India—a need that will only continue to grow in conjunction with India's increasing cattle population and demands on milk production and an inability to cull potentially diseased cows. Given the likely inability of implementing a test and cull programme at any scale due to social and economic considerations, the need for a vaccine that can reduce the burden of infection and transmission is critical. In this context, we note that recent reports suggest that the century‐old BCG vaccine may have considerable utility in this regard (Ameni, Vordermeier, Aseffa, Young, & Hewinson, 2010) (Ameni et al., 2017), but requires further study to evaluate its ability in reducing onward transmission. However, if effective, there is also an unmet need for a validated and accepted fit‐for‐purpose DIVA (Differentiating Infected from Vaccinated Animals) diagnostic test for the detection of bTB‐infected cattle that can be used in conjunction with a vaccine programme.
Mycobacterium bovis has also been isolated from milk samples of tuberculous cattle (Aswathanarayana, Rao, Krishnappa, Ramanatha, & Raghavan, 1998) (Veerasami et al., 2012). Given the fact that over 70% of the milk in India is sold unpasteurized (FAO/OIE/WHO, 1993), this raises concerns regarding the potential for zoonotic transmission of bTB and continued spread of human tuberculosis (India has the world's largest burden of human TB) (Thoen, LoBue, & de Kantor, 2006). In May 2014, the World Health Assembly adopted a new strategy to attain an ambitious goal of ending the global TB epidemic by 2035: the End TB strategy (Uplekar et al., 2015). Given the prevalence of bTB and the potential for zoonotic transmission, particularly to children and others who consume unpasteurized or unprocessed milk from infected cows, there is a critical need for a national bTB control programme in India and other developing countries as attempts to eradicate the disease from humans without eradicating it from cattle are likely to prove futile. Importantly, implementation of a national control programme would not only enable accurate temporal trends and estimates of bTB prevalence, risk and economic costs, but would equally importantly improve the health and productivity of cattle in India.
5. CONCLUSION
Overall, the results of our systematic review and meta‐analysis conducted on 44 publications indicate high and widespread bTB prevalence in India of 7.3% (95% CI: 5.6, 9.5). Further study is necessary to obtain more robust state‐by‐state prevalence estimates and explore other moderators of risk (including herd size, animal sex, and age, amongst others) that are likely to impact development and implementation of a rational and effective bTB control strategy. Taken together with the expected dairy farm intensification, growing demands for increased milk production and the zoonotic nature of M. bovis, the results of our current studies highlight the importance of developing and implementing a national bTB control programme that will need to include a national surveillance plan using (a) well‐standardized method(s) and evidence‐based intervention(s) that are likely to work in India and other developing country settings.
CONFLICT OF INTEREST
The authors have no conflict of interest.
Supporting information
ACKNOWLEDGEMENTS
The authors thank Ms. Helen Smith (The Pennsylvania State University, USA) for her expertise and guidance in defining database search strategy and search terms. The authors also gratefully acknowledge Douwe Bakker (the Netherlands), Stefan Berg (APHA, UK), Premanshu Dandapat (ICAR‐Indian Veterinary Research Institute, India), Kumanan Kathaperumal (TANUVAS, India), Dhinakar Raj (TANUVAS, India), Maroudam Veerasami (TANUVAS, India), Martin Vordermeier (APHA, UK) and James Wood (Cambridge, UK) for their careful reading and numerous very helpful suggestions for improving the study. We owe them a debt of gratitude. This work was supported by a grant [OPP1176950] from the Bill and Melinda Gates Foundation in partnership with the United Kingdom's Department for International Development.
Srinivasan S, Easterling L, Rimal B, et al. Prevalence of Bovine Tuberculosis in India: A systematic review and meta‐analysis. Transbound Emerg Dis. 2018;65:1627–1640. 10.1111/tbed.12915
REFERENCES
- Ameni, G. , Miörner, H. , Roger, F. , & Tibbo, M. (2000). Comparison between comparative tuberculin and gamma‐interferon tests for the diagnosis of bovine tuberculosis in Ethiopia. Tropical Animal Health and Production, 32(5), 267–276. 10.1023/A:1005271421976 [DOI] [PubMed] [Google Scholar]
- Ameni, G. , Tafess, K. , Zewde, A. , Eguale, T. , Tilahun, M. , Hailu, T. , … Vordermeier, H. M. (2017). Vaccination of calves with Mycobacterium bovis Bacillus Calmette‐Guerin reduces the frequency and severity of lesions of bovine tuberculosis under a natural transmission setting in Ethiopia. Transboundary and Emerging Diseases, 65(1), 96–104. 10.1111/tbed.12618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ameni, G. , Vordermeier, M. , Aseffa, A. , Young, D. B. , & Hewinson, R. G. (2010). Field evaluation of the efficacy of Mycobacterium bovis bacillus Calmette‐Guérin against bovine tuberculosis in neonatal calves in Ethiopia. Clinical and Vaccine Immunology, 17(10), 1533–1538. 10.1128/CVI.00222-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aneesh, T. , Mandeep, S. , Katoch, V. C. , Prasenjit, D. , & Katoch, R. C. (2010). A study on the prevalence of bovine tuberculosis in farmed dairy cattle in Himachal Pradesh. Veterinary World, 3(9), 409–414. [Google Scholar]
- Appuswamy, S. , Batish, V. K. , Parkash, O. , & Ranganathan, B. (1980). Prevalence of mycobacteria in raw milk sampled in Karnal India. Journal of Food Protection, 43(10), 778–781. [DOI] [PubMed] [Google Scholar]
- Ashish, B. , Amit, K. , & Deepak, S. (2014). Single nucleotide polymorphism in cattle and its association with susceptibility to bovine tuberculosis. Indian Journal of Field Veterinarians, 10(1), 1–4. [Google Scholar]
- Asmare, K. , Sheferaw, D. , Aragaw, K. , Abera, M. , Sibhat, B. , Haile, A. , … Wieland, B. (2016). Gastrointestinal nematode infection in small ruminants in Ethiopia: A systematic review and meta‐analysis. Acta Tropica, 160(Supplement C), 68–77. 10.1016/j.actatropica.2016.04.016 [DOI] [PubMed] [Google Scholar]
- Aswathanarayana, S. R. , Rao, M. S. , Krishnappa, G. , Ramanatha, K. R. , & Raghavan, R. (1998). Isolation of typical mycobacteria from milk of tuberculin test positive cows. Mysore Journal of Agricultural Sciences, 32(1), 71–74. [Google Scholar]
- Bakker, D. , Eger, A. , McNair, J. , Riepema, K. , Willemsen, P. T. J. , Haagsma, J. , … Pollock, J. M. (2005). Comparison of commercially available PPDs: practical considerations for diagnosis and control of bovine tuberculosis. 4th International Conference on Mycobacterium bovis (poster). Dublin, Ireland 22‐26th August, 2005.
- Bala, A. K. , & Sidhu, N. S. (1981). Studies on disease resistance vis‐a‐vis susceptibility in cattle and buffalo. 2. Genetic group difference in the incidence of tuberculosis. Indian Journal of Animal . Health, 20(1), 25–29. [Google Scholar]
- Bali, M. K. , & Khanna, R. N. S. (1979). Prevalence of tuberculosis among cattle [breed effect]. Indian Journal of Dairy Science, 32(3), 316–318. [Google Scholar]
- Bali, M. K. , & Singh, R. P. (1980). Incidence of tuberculosis and paratuberculosis of dairy cattle in Hau Dairy Farm. Haryana Agricultural University Journal of Research, 10(1), 157–158. [Google Scholar]
- Bapat, J. A. , & Bangi, M. I. (1985). Tuberculosis and Johne's disease amongst cattle and buffaloes in Maharashtra State. Indian Journal of Animal Sciences, 55(12), 1022–1023. [Google Scholar]
- Basic Animal Husbandry and Fisheries Statistics, Government of India (2017). Retrieved from http://dahd.nic.in/Division/statistics/animal-husbandry-statistics-division
- Basic Animal Husbandry and Fisheries Statistics . (2015). Basic Animal Husbandry and Fisheries Statistics, India.
- Begg, C. B. , & Mazumdar, M. (1944). Operating characteristics of a rank correlation test for publication bias. Biometrics, 50(4), 1088–1101. [PubMed] [Google Scholar]
- Bekara, M. E. A. , Azizi, L. , Bénet, J. J. , & Durand, B. (2016). Spatial‐temporal variations of bovine tuberculosis incidence in France between 1965 and 2000. Transboundary and Emerging Diseases, 63(1), 101–113. 10.1111/tbed.12224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellur, V. V. , Singh, S. P. , Chaganti, R. , & Chaganti, R. (1990). The white revolution—How Amul brought milk to India. Long Range Planning, 23(6), 71–79. 10.1016/0024-6301(90)90104-C [DOI] [Google Scholar]
- Bertin, J. (1983). Semiology of graphics: diagrams, networks, maps. Madison, WI: The University of Wisconsin Press. [Google Scholar]
- Bhanu Rekha, V. , Gunaseelan, L. , Pawar, G. R. , & Giri, T. (2014). Recombinant antigen based ELISA for diagnosis of bovine tuberculosis in organized and unorganized dairy units of Tamil Nadu. Indian Journal of Animal Health, 53(2), 128–134. [Google Scholar]
- Bostock, M. , Ogievetsky, V. , & Heer, J. (2011). D³ data‐driven documents. IEEE Transactions on Visualization and Computer Graphics, 17, 2301–2309. 10.1109/TVCG.2011.185 [DOI] [PubMed] [Google Scholar]
- Chowdhury, J. , Sarkar, S. , Pal, N. K. , Roy, N. , & Chakraborty, M. (2001). Bovine tuberculosis ‐ a slaughter house based assessment. Indian Journal of Animal Health, 40(1), 41–44. [Google Scholar]
- Cleveland, W. S. , & McGill, R. (1984). Graphical perception: Theory, experimentation, and application to the development of graphical methods. Journal of the American statistical association, 79(387), 531–554. 10.1080/01621459.1984.10478080 [DOI] [Google Scholar]
- Cochran, W. G. (1954). The combination of estimates from different experiments. Biometrics, 10(1), 101–129. 10.2307/3001666 [DOI] [Google Scholar]
- Cousins, D. V. , & Florisson, N. (2005). A review of tests available for use in the diagnosis of tuberculosis in non‐bovine species. Revue Scientifique et Technique, 24(3), 1039–1059. 10.20506/rst.24.3.1635 [DOI] [PubMed] [Google Scholar]
- DADF, G. o. I. (2015). Department of Animal Husbandry, Dairying & Fisheries, Ministry of Agriculture.
- DADF, G. o. I. (2016. ‐2017). Annual report, Department of Animal Husbandry, Dairying & Fisheries, Ministry of Agriculture.
- Dali, S. S. , Waskar, V. S. , Paturkar, A. M. , Sherikar, A. A. , Patil, V. M. , & Wagh, N. V. (2004). Seroprevalence of tubercle bacilli in cattle and buffaloes in and around Mumbai city. Journal of Bombay Veterinary College, 12(1/2), 62. [Google Scholar]
- Dev, R. L. , Purohit, S. K. , & Joshi, R. (1998). Prevalence of bovine tuberculosis in cattle domesticated by human tubercular patients. Indian Veterinary Medical Journal, 22(2), 137–138. [Google Scholar]
- Dhanda, M. R. , & Lall, J. M. (1959). Systematic control and eradication of tuberculosis among cattle and buffaloes in self‐contained herds. Indian Veterinary Journal, 36(10), 467–472. [Google Scholar]
- Dohoo, I. , Martin, W. S. , & Stryhn, H. (2009). Veterinary epidemiologic research, 2nd ed. (pp. 739–766). Charlottetown, Prince Edward Island: AVC Inc. [Google Scholar]
- Egger, M. , Davey Smith, G. , Schneider, M. , & Minder, C. (1997). Bias in meta‐analysis detected by a simple, graphical test. British Medical Journal, 315(7109), 629–634. 10.1136/bmj.315.7109.629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etchechoury, I. , Valencia, G. E. , Morcillo, N. , Sequeira, M. D. , Imperiale, B. , López, M. , … Romano, M. I. (2010). Molecular typing of Mycobacterium bovis isolates in Argentina: First description of a person‐to‐person transmission case. Zoonoses and Public Health, 57(6), 375–381. 10.1111/j.1863-2378.2009.01233.x [DOI] [PubMed] [Google Scholar]
- FAO/OIE/WHO . (1993). FAO/OIE/WHO Animal health year book.
- Farnham, M. W. , Norby, B. , Goldsmith, T. J. , & Wells, S. J. (2012). Meta‐analysis of field studies on bovine tuberculosis skin tests in United States cattle herds. Preventive Veterinary Medicine, 103(2), 234–242. 10.1016/j.prevetmed.2011.08.009 [DOI] [PubMed] [Google Scholar]
- Filia, G. , Leishangthem, G. D. , Mahajan, V. , & Singh, A. (2016). Detection of Mycobacterium tuberculosis and Mycobacterium bovis in Sahiwal cattle from an organized farm using ante‐mortem techniques. Veterinary World, 9(4), 383–387. 10.14202/vetworld.2016.383-387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganesan, P. I. (2006). Excretion of mycobacteria in cattle. Indian Veterinary Journal, 83(10), 1112–1113. [Google Scholar]
- Hartnack, S. , & Torgerson, P. R. (2012). The accuracy of the single intradermal comparative skin test for the diagnosis of bovine tuberculosis‐estimated from a systematic literature search. Journal Mycobacterial Diseases, 2, 120 10.4172/2161-1068.1000120 [DOI] [Google Scholar]
- Hayashino, Y. , Noguchi, Y. , & Fukui, T. (2005). Systematic evaluation and comparison of statistical tests for publication bias. Journal of Epidemiology, 15, 235–243. 10.2188/jea.15.235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins, J. P. T. , Thompson, S. G. , Deeks, J. J. , & Altman, D. G. (2003). Measuring inconsistency in meta‐analyses. British Medical Journal, 327(7414), 557–560. 10.1136/bmj.327.7414.557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humblet, M. F. , Gilbert, M. , Govaerts, M. , Fauville‐Dufaux, M. , Walravens, K. , & Saegerman, C. (2010). New assessment of bovine tuberculosis risk factors in Belgium based on nationwide molecular epidemiology. Journal of Clinical Microbiology, 48(8), 2802–2808. 10.1128/JCM.00293-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- International Office of Epizootics (OIE) . (2006). Manual of Diagnostic Tests and Vaccines for Terrestrial Animals 2004, Paris.
- Iyer, P. R. K. (1944). Tuberculosis in buffaloes. Indian Journal of Veterinary Science, 14, 100–105. [Google Scholar]
- Joshi, D. V. , Sharma, D. R. , Dhillon, S. S. , & Sodhi, S. S. (1976). Prevalence of tuberculosis in animals of organised farms in Punjab. Indian Journal of Dairy Science, 29(3), 217–219. [Google Scholar]
- Krishnaswamy, S. , Nagaraja, K. V. , Keshavamurthy, B. S. , Nanjiah, R. D. , & Adinarayanaiah, C. L. (1973). Control of bovine tuberculosis by tuberculin testing and segregation in an organised dairy herd. Mysore Journal of Agricultural Sciences, 7(4), 615–620. [Google Scholar]
- Kulshreshtha, R. C. , Jagjit, S. , & Chandiramani, N. K. (1980). A study on the prevalence of tuberculosis and Johne's disease in cattle and buffaloes in Haryana State. Haryana Veterinarian, 19(2), 139–141. [Google Scholar]
- Kumar, G. S. , & Parihar, N. S. (1998). Isolation of mycobacteria from suspected cases of pulmonary tuberculosis in buffaloes slaughtered for food. Indian Journal of Animal Sciences, 68(6), 555–556. [Google Scholar]
- Kumar, G. S. , Sharma, A. K. , Iyer, P. K. R. , & Prasad, M. C. (1998). Tuberculosis in crossbred dairy cattle. Indian Journal of Veterinary Pathology, 22(1), 11–15. [Google Scholar]
- Lall, J. M. , Singh, G. , & Sen Gupta, B. R. (1969). Incidence of tuberculosis among cattle and buffaloes in India. Indian Journal of Animal Sciences, 39, 51–58. [Google Scholar]
- Liberati, A. , Altman, D. G. , Tetzlaff, J. , Mulrow, C. , Gøtzsche, P. C. , Ioannidis, J. P. A. , … Moher, D. (2009). The PRISMA statement for reporting systematic reviews and meta‐analyses of studies that evaluate healthcare interventions: explanation and elaboration. PLoS Medicine, 6(7), e1000100 10.1371/journal.pmed.1000100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liston, W. G. , & Soparkar, S. M. B. (1917). The susceptibility of Indian milch cattle to tuberculosis. Indian Journal of Medical Research, 5(1), 19–71. [Google Scholar]
- Maity, B. , Deb, P. , & Pramanik, A. K. (1992). Pulmonary tuberculosis in cattle: Abattoir survey. Indian Journal of Veterinary Pathology, 16(1), 38. [Google Scholar]
- Mallick, S. M. , Aggarwal, H. , & Dua, R. L. (1942). An investigation into the incidence and type of tuberculous infection in cattle at Amritsar with special reference to human infections. Indian Medical Gazette, 77(11), 668–672. [PMC free article] [PubMed] [Google Scholar]
- Mishra, J. K. , Panda, S. N. , & Panda, H. K. (1997). Prevalence of bovine tuberculosis among farm cattle in Orissa. Indian Veterinary Journal, 74(3), 195–198. [Google Scholar]
- Mukherjee, F. (2006). Comparative prevalence of tuberculosis in two dairy herds in India. Revue Scientifique Et Technique‐Office International Des Epizooties, 25(3), 1125–1130. 10.20506/rst.25.3.1717 [DOI] [PubMed] [Google Scholar]
- Mukhopadhyay, H. K. , Antony, P. X. , & Pillai, R. M. (2001). Prevalence of tuberculosis and Johne's disease in an organized cattle farm at Pondicherry. Indian Journal of Animal Health, 40(2), 185–186. [Google Scholar]
- Murti, P. S. R. C. , & Hazarika, G. C. (1982). Studies on the incidence of tuberculosis in cattle and pigs in Meghalaya. Journal of Research, Assam Agricultural University, 3(2), 151–153. [Google Scholar]
- Nagaraja, K. V. , Krishnaswamy, S. , Adinarayanaiah, C. L. , Murthy, B. S. K. , & Nanjiah, R. D. (1973). Incidence of bovine tuberculosis in Mysore State. Indian Veterinary Journal, 50(2), 200–202. [Google Scholar]
- Nair, K. N. (1985). White revolution in India: Facts and issues. Economic and Political Weekly, 20(25/26), A89–A95. [Google Scholar]
- Neeraja, D. , Veeregowda, B. M. , Rani, M. S. , Rathnamma, D. , Bhaskaran, R. , Leena, G. , … Chakraborty, S (2014). Comparison of single intradermal test, gamma interferon assay and indirect ELISA for the diagnosis of tuberculosis in a dairy farm. Asian Journal of Animal and Veterinary Advances, 9(9), 593–598. [Google Scholar]
- Nishath, L. , & Ganesan, P. I. (2006). Haematological and biochemical parameters in tuberculin reactor and non reactor cattle. Indian Veterinary Journal, 83(8), 918–919. [Google Scholar]
- OIE . Bovine Tuberculosis: General Disease Information sheets.
- Olea‐Popelka, F. , Muwonge, A. , Perera, A. , Dean, A. S. , Mumford, E. , Erlacher‐Vindel, E. , … Fujiwara, P. I. (2014). Zoonotic tuberculosis in human beings caused by Mycobacterium bovis ‐ a call for action. The Lancet Infectious Diseases, 17(1), e21–e25. 10.1016/S1473-3099(16)30139-6 [DOI] [PubMed] [Google Scholar]
- Olmstead, A. L. , & Rhode, P. W. (2004). An impossible undertaking: The eradication of bovine tuberculosis in the United States. The Journal of Economic History, 64(3), 734–772. [Google Scholar]
- Paily, E. P. , Georgekutty, P. T. , & Venugopal, K. (1979). Incidence of tuberculosis in cattle and buffaloes in the livestock farms of the Kerala Agricultural University. Kerala Journal of Veterinary Science, 10(2), 339–341. [Google Scholar]
- Phaniraja, K. L. , Jayaramu, G. M. , Jagadeesh, S. , & Kumar, G. S. N. (2010). Incidence of tuberculosis in and around Banglore. Veterinary World, 3(4), 161–164. [Google Scholar]
- Purohit, A. K. , & Mehrotra, P. N. (1969). Incidence of tuberculosis in cattle in Rajasthan. Indian Veterinary Journal, 46(1), 5–12. [PubMed] [Google Scholar]
- R core team . (2016). R: A language and environment for statistical computing. Vienna, Austria: R Core Team. [Google Scholar]
- Rajaram, E. D. , Rao, V. N. A. , & Manickam, R. (1996). Certain epizootiological features of bovine tuberculosis. Indian Veterinary Journal, 73(4), 435–438. [Google Scholar]
- Rakesh Sisodia, R. , Shuykla, P. C. , & Sisodia, R. S. (1995). Incidence of tuberculosis and Johne's disease in cattle in Malwa region of Madhya Pradesh. Indian Veterinary Journal, 72(4), 370–373. [Google Scholar]
- Rawat, L. K. , & Kataria, R. S. (1971). Incidence of bovine tuberculosis in Madhya Pradesh as determined by double intradermal tuberculin test. Indian Veterinary Journal, 48(9), 974–975. [Google Scholar]
- Raval, S. K. , Sunil, T. , Belsare, V. P. , Kanani, A. N. , & Patel, P. R. (2006). Diagnosis of tuberculosis in animal and man. Intas Polivet, 7(2), 247–250. [Google Scholar]
- Roswurm, J. D. , & Ranney, A. F. (1973). Sharpening the attack on bovine tuberculosis. American Journal of Public Health, 63(10), 884–886. 10.2105/ajph.63.10.884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarzer, G. (2007). meta: {A}n {R} package for meta‐analysis. R News, 7, 40–45. [Google Scholar]
- Sharma, D. R. , Kwatra, M. S. , Joshi, D. V. , & Saharma, D. K. (1994). Prevalence of tuberculosis under rural and organised farm conditions. Indian Journal of Comparative Microbiology, Immunology and Infectious Diseases, 15(3/4), 5–7. [Google Scholar]
- Sharma, A. K. , Vanamayya, P. R. , & Parihar, N. S. (1985). Tuberculosis in cattle: A retrospective study based on necroscopy. Indian Journal of Veterinary Pathology, 9, 17–18. [Google Scholar]
- Shringi, B. N. (2004). Bovine tuberculosis in organised farms. Indian Veterinary Journal, 81(12), 1394–1395. [Google Scholar]
- Sibhat, B. , Asmare, K. , Demissie, K. , Ayelet, G. , Mamo, G. , & Ameni, G. (2017). Bovine tuberculosis in Ethiopia: A systematic review and meta‐analysis. Preventive Veterinary Medicine, 147(Supplement C), 149–157. 10.1016/j.prevetmed.2017.09.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh, B. B. , Gumber, S. , Randhawa, S. S. , Aradhana, & Dhand, N. K. (2004). Prevalence of bovine tuberculosis and paratuberculosis in Punjab. Indian Veterinary Journal, 81(11), 1195–1196. [Google Scholar]
- Soparkar, M. B. (1925). The relative susceptibility of Indian milch cattle of various breeds to tuberculosis. Indian Journal of Medical Research, 9, 17–18. [Google Scholar]
- Srivastava, K. , Chauhan, D. S. , Gupta, P. , Singh, H. B. , Sharma, V. D. , Yadav, V. S. , & Katoch, V. M. (2008). Isolation of Mycobacterium bovis & M‐tuberculosis from cattle of some farms in north India ‐ Possible relevance in human health. Indian Journal of Medical Research, 128(1), 26–31. [PubMed] [Google Scholar]
- Sweetline Anne, N. , Ronald, B. S. , Kumar, T. M. , Kannan, P. , & Thangavelu, A. (2017). Molecular identification of Mycobacterium tuberculosis in cattle. Veterinary Microbiology, 198, 81–87. 10.1016/j.vetmic.2016.12.013 [DOI] [PubMed] [Google Scholar]
- Taggar, R. K. , & Bhadwal, M. S. (2008). Incidence of tuberculosis in a heterogenous cattle herd. North‐East Veterinarian, 8(1), 14–15. [Google Scholar]
- Taneja, G. C. (1955). A note on the incidence of tuberculosis in zebu (Hariana) cattle in the Punjab (India). Indian Veterinary Journal, 31, 249–259. [Google Scholar]
- Thakur, M. K. , Sinha, D. K. , & Singh, B. R. (2016). Evaluation of complementary diagnostic tools for bovine tuberculosis detection in dairy herds from India. Vet World, 9(8), 862–868. 10.14202/vetworld.2016.862-868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thoen, C. O. (2008). Mycobacterum bovis infection in animals and human.
- Thoen, C. , LoBue, P. , & de Kantor, I. (2006). The importance of Mycobacterium bovis as a zoonosis. Veterinary Microbiology, 112(2), 339–345. 10.1016/j.vetmic.2005.11.047 [DOI] [PubMed] [Google Scholar]
- Trangadia, B. J. , Rana, S. K. , & Srinivasan, V. A. (2013). Prevalence of bovine tuberculosis in organized dairy farm. Indian Journal of Veterinary Pathology, 37(1), 72–74. [Google Scholar]
- Uplekar, M. , Weil, D. , Lonnroth, K. , Jaramillo, E. , Lienhardt, C. , Dias, H. M. , … Raviglione, M. (2015). WHO's new End TB strategy. The Lancet, 385(9979), 1799–1801. 10.1016/S0140-6736(15)60570-0 [DOI] [PubMed] [Google Scholar]
- USDA . (2016). Milk cows and production by State and region.
- Varello, K. , Pezzolato, M. , Mascarino, D. , Ingravalle, F. , Caramelli, M. , & Bozzetta, E. (2008). Comparison of histologic techniques for the diagnosis of bovine tuberculosis in the framework of eradication programs. Journal of Veterinary Diagnostic Investigation, 20(2), 164–169. 10.1177/104063870802000204 [DOI] [PubMed] [Google Scholar]
- Veerasami, M. , Reddy, D. S. , Sugumar, P. , Naidu, S. S. , Bahekar, V. , Mahesh Kumar, E. K. , … Srinivasan, V. A. (2012). Multi‐antigen print immunoassay for seroepidemiological surveillance of bovine tuberculosis on Indian cattle farms. Veterinaria Italiana, 48(3), 253–267. [PubMed] [Google Scholar]
- Viechtbauer, W. (2010). Conducting meta‐analyses in R with the metafor package. Journal of Statistical Software, 36(3), 48 10.18637/jss.v036.i03 [DOI] [Google Scholar]
- Vordermeier, M. , Ameni, G. , Berg, S. , Bishop, R. , Robertson, B. D. , Aseffa, A. , … Young, D. B. (2012). The influence of cattle breed on susceptibility to bovine tuberculosis in Ethiopia. Comparative Immunology, Microbiology and Infectious Diseases, 35(3), 227–232. 10.1016/j.cimid.2012.01.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters, W. R. , Palmer, M. V. , Buddle, B. M. , & Vordermeier, H. M. (2012). Bovine tuberculosis vaccine research: Historical perspectives and recent advances. Vaccine, 30(16), 2611–2622. 10.1016/j.vaccine.2012.02.018 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


