Abstract
Objective:
It is well known that patients with type 2 diabetes mellitus (T2DM) have a high risk of atrial fibrillation (AF). The current study was designed to determine the relationship between long-term glycemic variability and incidence of new-onset AF in T2DM patients.
Methods:
Between January 2008 and December 2009, we conducted a retrospective cohort study in patients with T2DM referred to our hospital. In 505 consecutive patients without any medical history of AF at baseline, the relationship between hemoglobin A1c (HbA1c) variability and future AF incidence was evaluated, with adjustments for other possible confounding factors. HbA1c variability was determined by standard deviation (SD) and coefficient of variation (CV).
Results:
Over a median of 6.9-year follow-up period, 48 patients (9.5%) developed incident AF. Multiple cox regression revealed that higher HbA1c-SD (HR: 1.726, 95% CI: 1.104–1.830, p=0.001) or HbA1c-CV (HR: 1.241, 95% CI: 1.029–1.497, p=0.024) remained the remarkable predictor of new-onset AF after adjusting for age, body mass index, left ventricular mass index, and left atrium diameter. Receiver operating curve analysis identified thresholds for HbA1c-SD (0.665%, sensitivity 71.4%, specificity 54.9%) and HbA1c-CV (8.970%, sensitivity 73.8%, specificity 47.1%) to detect new-onset AF development.
Conclusion:
In patients with T2DM, higher HbA1c variability is significantly associated with future AF development.
Keywords: hemoglobin A1c variability, type 2 diabetes mellitus, atrial fibrillation
Introduction
Type 2 diabetes mellitus (T2DM) is one of the most concerning public health problem worldwide with an estimated prevalence of 2.8% in 2000, which is projected to increase to approximately 4.5% by 2030 (1, 2). Atrial fibrillation (AF) is the most common arrhythmia in clinical practice, and its associations with ischemic stroke, heart failure, and overall mortality call for further study of preventable risk factors. It is estimated that the presence of T2DM, regardless of coexisting comorbidities, increases the risk of new-onset AF by approximately 1.5-fold (3). Moreover, the presence of diabetes mellitus has a poor prognosis for AF, increasing risk of thromboembolic stroke, mortality, and other cardiovascular events (3).
Considering the increasing epidemiological correlation of T2DM and AF, early predictors of AF in T2DM recognition are of great significance to further ameliorate the prevention and treatment strategies to reduce morbidity and mortality risk, especially in this high-risk population. Previous studies have recognized a positive linear relationship between long-term glycemic variability and incidence of cardiovascular disease (CVD) and all-cause mortality (4-6). The primary objective of this retrospective study was to examine the correlation between hemoglobin A1c (HbA1c) variability and risk of new-onset AF in patients with T2DM.
Methods
Study design and data sources
This was a retrospective cohort study in patients with T2DM performed at our hospital to determine the role of long-term glycemic variability in recognizing patients at high risk of new-onset AF. We used data from hospital medical record database, which contains information on hospitalization, outpatient services, and emergency care (7, 8). In this study, exclusion criteria included (1) ischemic heart disease, cardiomyopathy, congenital heart disease, or chronic heart failure; (2) moderate-to-severe valvular heart diseases; (3) atrial fibrillation or atrial flutter; (4) a history of pacemaker or implantable cardioverter defibrillator; (5) patients with alcohol abuse, cirrhosis, overt nephropathy, and cancer. Patients who were on antiarrhythmic drugs were also excluded. The study protocol was approved by the ethics committee of our institution.
Between January 2008 and December 2009, 1150 consecutive patients with T2DM first visited our hospital. On the basis of the inclusion and exclusion criteria, 385 patients were excluded. Further, 160 patients who had not been followed up for at least 2 years or had not undergone four or more HbA1c determinations were also excluded from analyses. The final study population comprised 505 patients. Participants were followed for new-onset AF through December 2015.
HbA1c variability
High-performance liquid chromatography (DCCT-aligned methods) was adopted to HbA1c measurement (Tosoh-G8, Tosoh, Tokyo, Japan). The average level of successive HbA1c measurements was calculated for each patient as the intraindividual mean (HbA1c-mean). HbA1c variability was determined as the standard deviation of serial HbA1c measurements (HbA1c-SD) as well as the coefficient of variation of HbA1c (HbA1c-CV). This was a retrospective study and the time interval for HbA1c measurement was not regular for each participant. Usually, HbA1c measurement is recommended every 6 months in our clinical practice.
Transthoracic tissue Doppler echocardiography
Echocardiography was performed with the Cardiovascular Ultrasound System (GE VIVIDT, GE Healthcare, LaMarquel, TX, USA) as previously described (7,8). In brief, the frequency of the ultrasonic probe was 2.5 MHz. The structure and function of heart were evaluated in the M-mode guided by two-dimensional imaging to acquire echocardiographic variables. Left ventricular mass index (LVMI) was computed using the following formula: LVMI=left ventricular mass/body surface area. Biplane-modified Simpson’s measurements were used to determine left ventricular ejection fraction (LVEF). Tissue Doppler was implemented to acquire the mitral annulus velocities in the apical four-chamber view. The sample was located at the junction of the left ventricular lateral wall with the mitral annulus as well as the junction of the posterior interventricular septum with the mitral annulus; both the early (E’) diastolic mitral annulus velocities and E/E’ ratio were evaluated.
Diagnosis of incident AF
During follow-up, participants were diagnosed with AF if AF or atrial flutter appeared on a standard ECG or Holter, which was obtained from a routine clinical examination or from hospital medical record database. Furthermore, AF was categorized as clinical if symptomatic and silent if asymptomatic or with unclear symptoms. Generally, clinical examination was performed every month and patients received ECG and Holter examination if necessary. Patients were also asked to record their ECG and Holter when they had symptoms indicating AF onset.
Statistical analysis
Statistical analysis was performed using SPSS Statistical Software, version 16.0 (SPSS Inc., Chicago, IL, USA). Normally distributed and skewed continuous data were presented as mean±SD and median±interquartile range, respectively, whereas percentages were used for categorical data. Differences in baseline clinical and echocardiographic characteristics among patients stratified by their status of incident AF at follow-up were tested with the unpaired Student’s t-test for normally distributed variables, Mann–Whitney U test for non-normally distributed variables, and chi-squared test for categorical variables. Cox proportional hazards regression model was performed to explore the association between risk factors and the risk of new-onset AF. All variables with a p value of <0.10 by means of univariate regression were entered into the multiple cox model. Relative risks were expressed as hazard ratios (HRs) with 95% confidence intervals (CIs). New-onset AF-free survival at 6 years was analyzed with Kaplan–Meier statistics, and differences between the survival curves were assessed using the log-rank test. The predictive value of HbA1c variability for the risk of new-onset AF was analyzed using receiver operating characteristic (ROC) curve. All the above analyses were considered significant at a p-value of <0.05.
Results
Baseline clinical characteristics of patients stratified by their status of incident AF during follow-up were shown in Table 1. At baseline, patients who developed AF during follow-up tended to be older (p=0.093) and have higher body mass index (BMI) (p=0.070) compared with those who did not. Moreover, sex, diabetes mellitus duration, HbA1c-mean, blood pressure, hypertension, dyslipidemia, smoking, and medical treatment were comparable between the two groups. Table 1 also shows baseline echocardiographic features of participants. At baseline, patients who developed AF during follow-up had higher LVMI and larger left atrium diameter (LAD) than those who did not. LVEF and E/E’ ratio were comparable between the two groups. Interestingly, patients with new-onset AF had markedly higher HbA1c variability, as indicated by HbA1c-SD or HbA1c-CV. The number of visits per patient was 80.3±7.2 and 78.2±7.5, respectively (p=0.065). Moreover, the average number of detection by Holter per patient was 1.7 during the follow-up. More Holter examinations were performed in the group of new-onset AF (4.2±1.9 vs. 1.5±1.0, p<0.0001). This was mainly due to more symptoms in the new-onset AF group.
Table 1.
Baseline characteristics
| New-onset AF at follow-up (n=48) | No AF at follow-up (n=457) | P | |
|---|---|---|---|
| Sociodemographics | |||
| Female,% | 23(47.9) | 180(29.4) | 0.252 |
| Age, years | 69.6±5.5 | 67.8±7.2 | 0.093 |
| Clinical | |||
| SBP, mm Hg | 131.0±12.3 | 132.6±12.2 | 0.388 |
| DBP, mm Hg | 78.1±9.6 | 80.4±9.5 | 0.112 |
| eGFR, mL/min/1.73m2 | 78.4±9.1 | 79.1±9.1 | 0.612 |
| BMI, kg/m2 | 25.1±1.9 | 24.5±2.2 | 0.070 |
| HbA1c-mean, % | 7.2±0.6 | 7.1±0.6 | 0.273 |
| HbA1C measure times | 11.3±2.9 | 11.6±2.8 | 0.482 |
| Duration of HbA1c tests, months | 82.3±4.6 | 83.6±5.6 | 0.121 |
| HbA1c-SD | 0.69±0.08 | 0.64±0.10 | 0.0009 |
| HbA1c-CV | 9.57±1.19 | 9.04±1.47 | 0.0161 |
| Comorbidities duration of T2DM, years | 8.4±2.8 | 8.1±2.9 | 0.494 |
| Hypertension | 26(54.2) | 289 (63.2) | 0.217 |
| Smoking | 17(35.4) | 131(28.7) | 0.328 |
| Dyslipidemia | 16(33.3) | 130(28.4) | 0.477 |
| Medical treatment | |||
| Calcium blocker | 24(50.0) | 208(45.5) | 0.553 |
| ACEI/ARB | 29(60.4) | 222(47.5) | 0.119 |
| Beta-blockers | 8(16.7) | 120(26.3) | 0.146 |
| Statin | 13(27.1) | 89(19.5) | 0.212 |
| Oral anti-diabetic drugs | 31(64.6) | 271(59.3) | 0.478 |
| İnsulin | 10(20.8) | 94(20.6) | 0.966 |
| Echocardiographic variables | |||
| LAD, mm | 39.9±2.1 | 38.9±2.2 | 0.003 |
| LVMI, g/m2 | 131.0±15.9 | 125.0±15.0 | 0.009 |
| E/E’ | 8.8±1.6 | 9.0±1.4 | 0.354 |
| LVEF, % | 61.4±5.5 | 60.2±5.1 | 0.124 |
Data are presented as mean±SD or number (%) of subjects.
ACEI/ARB-angiotensin-converting enzyme inhibitor/angiotensin II receptor blocker; BMI-body mass index; DBP-diastolic blood pressure; E/E’-E and E’ wave ratio; eGFR- estimated glomerular filtration rate; HbA1C- hemoglobin A1c; HbA1c-CV-coefficient of variation of hemoglobin A1c; HbA1C-SD-standard deviation of hemoglobin A1c; LAD-left atrium diameter; LVEF-left ventricular ejection fraction; LVMI-left ventricular mass index; SBP-systolic blood pressure; T2DM- type 2 diabetes mellitus
After a median follow-up of 6.9 years, 48 out of 505 patients (9.5%) experienced new-onset AF detected by ECG (29/48, 60.4%) and Holter (19/48, 39.6%). The majority of patients experienced clinical AF (87.5% compared with 12.5% of patients with silent AF). The number of paroxysmal AF and persistent AF was 34 and 14, respectively.
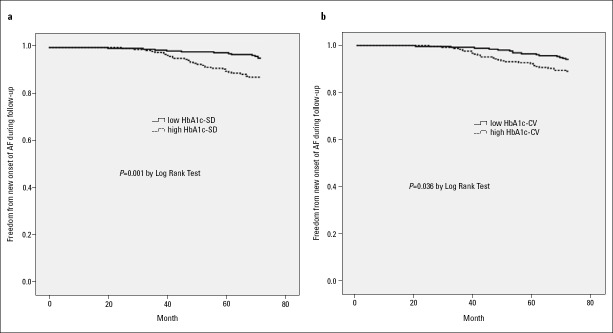
As there is no existing cut-off value for indices of HbA1c variability, we divided these subjects into two groups based on the median value of each HbA1c variability index: lower HbA1c variability group (HbA1c-SD ≤ 0.66%, HbA1c-CV ≤ 9.12%) and higher HbA1c variability group (HbA1c-SD > 0.66%, HbA1c-CV > 9.12%). Kaplan–Meier plot for new-onset AF at 6 years was presented in Figure 1; higher HbA1c-SD as well as higher HbA1c-CV level significantly increased the risk of new-onset AF.
Figure 1.
Kaplan–Meier curves of freedom from new-onset AF for low and high HbA1c-SD groups (a) as well as low and high HbA1c-CV groups (b) after a 6-year follow-up
For multiple regression analysis in model 1, variables [age, sex, systolic blood pressure, diastolic blood pressure, estimated glomerular filtration rate (eGFR), BMI, HbA1c-mean, HbA1c-SD, duration of T2DM, hypertension, smoking, dyslipidemia, medical treatment, and echocardiographic variables] were entered into the univariate regression analysis, and variables with p-value of <0.10 (age, BMI, HbA1c-SD, LAD, and LVMI) were further entered into the multiple cox regression model. The result indicated that the elevation of HbA1c-SD (HR: 1.726, 95% CI: 1.104–1.830, p=0.001), LVMI (HR: 1.025, 95% CI: 1.004–1.047, p=0.018), and larger LAD (HR: 1.168, 95% CI: 1.044–1.306, p=0.007) were associated with an increased risk of new-onset AF (Table 2). Results were similar [HbA1c-CV (HR: 1.241, 95% CI: 1.029–1.497, p=0.024), LAD (HR: 1.159, 95% CI: 1.036–1.296, p=0.010), and LVMI (HR: 1.026, 95% CI: 1.005–1.047, p=0.013)] when using HbA1c-CV instead of HbA1c-SD in model 2 (Table 2).
Table 2.
Multiple cox analysis for the new onset of AF
| HR (model 1) | 95% Confidence interval | P | HR (model 2) | 95% Confidence interval | P | |
|---|---|---|---|---|---|---|
| HbA1c-SD | 1.726 | 1.251-2.381 | 0.001 | not included | – | – |
| HbA1c-CV | not included | – | – | 1.241 | 1.029–1.497 | 0.024 |
| Age | 1.042 | 0.999–1.086 | 0.057 | 1.042 | 0.999–1.087 | 0.054 |
| LAD | 1.168 | 1.044–1.306 | 0.007 | 1.159 | 1.036–1.296 | 0.010 |
| LVMI | 1.025 | 1.004–1.047 | 0.018 | 1.026 | 1.005–1.047 | 0.013 |
| BMI | 1.108 | 0.977–1.257 | 0.109 | 1.122 | 0.991–1.270 | 0.068 |
BMI-body mass index; E/E’-E and E’ waves ratio; HbA1c-CV-coefficient of variation of hemoglobin A1c; HbA1C-SD-standard deviation of hemoglobin A1c; LAD-left atrium diameter; LVMI-left ventricular mass index
The optimum HbA1c variability threshold for identifying new-onset AF was subsequently determined using ROC curve from 6-year censored survival data (Fig. 2). The area under the HbA1c-SD ROC curve was 0.642, the optimum HbA1c-SD threshold that generated the highest Youden index was 0.665%, and sensitivity and specificity of HbA1c-SD cut-off value were 71.4% and 54.9%, respectively, at this value. Moreover, the area under the HbA1c-CV ROC curve was 0.610, the optimum HbA1c-CV threshold and corresponding sensitivity, specificity were 8.970%, 73.8%, and 47.1%, respectively
Figure 2.
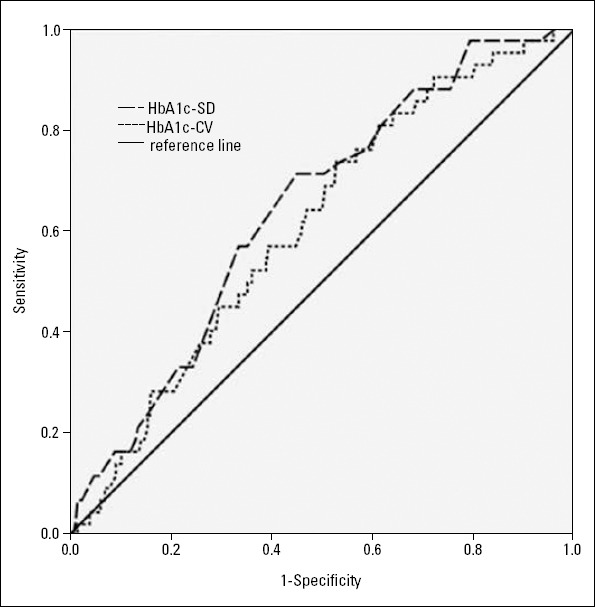
Receiver operating characteristic curve of HbA1c variability for the detection of future development of AF
Discussion
The main finding of this retrospective study was that higher HbA1c variability was related to an incremental risk of new-onset AF over a median follow-up of 6.9 years in T2DM patients. More importantly, this correlation was independent of a variety of clinical and echocardiographic risk factors. This finding indicates that long-term glycemic fluctuation is among the early predictors that make this particularly high-risk population more susceptible to subsequent AF development.
Numerous studies have shown that diabetes mellitus is independently related to new-onset AF (9-14). Framingham Heart Study indicated that diabetes mellitus is remarkably associated with risk for AF in both men and women (12). VHAH study also reported that diabetes mellitus is a powerful and independent risk factor of AF (13). Moreover, PROACTIVE study reported that the cumulative incidence of AF in patients with T2DM and macrovascular disease was 2.5% after an average follow-up of 34.5 months (14). Although the definite pathophysiological mechanisms implicating diabetes mellitus in AF development have not been fully elucidated, some potential factors, such as autonomic, electrical, electromechanical and structural remodeling, connexin remodeling, and oxidative stress, might play important roles (15). Diabetes mellitus-associated atrial fibrosis leads to prolongation of atrial activation time and cycle length as well as reduction of local atrial electrogram voltages, thus contributing to the occurrence of arrhythmia (16).
Previous studies have reported discordant results about the association between glycemic control and AF (11, 17-20). It was reported that increased HbA1c is still the factor in association with AF after adjusting for potential confounding factors (age, sex, vascular risk factors, cardiac disease, and eGFR) (11, 17, 18). A recent meta-analysis also suggested that elevated serum HbA1c levels were associated with an increased risk of AF, and therefore, serum HbA1c levels may be viewed as a potential biomarker to predict AF and as a tool for AF prevention (19). However, compared with standard strategy targeting an HbA1c level of 7.0%–7.9%, intensive glycemic control (HbA1c<6.0%) did not influence the incidence of new-onset AF (20). In the present study, HbA1c level was also not associated with new-onset AF in patients with T2DM. Recently, it has been suggested that AF initiation in diabetes mellitus is due to glycemic fluctuations rather than to the hyperglycemic state itself (16, 21, 22). In experimental models, hypoglycemia was associated with increased susceptibility to AF (21). It was also reported that sustained AF was more common under hypoglycemia than hyperglycemia, and the atrial refractory period of the left atrium was the shortest under hypoglycemia and that of the right atrium was the longest under normoglycemia or hyperglycemia (21). Moreover, glucose fluctuations were shown to contribute to the increased incidence of AF by enhancing cardiac fibrosis in a diabetic rat model (16). Increased reactive oxygen species levels induced by upregulation of thioredoxin-interacting protein and NADPH oxidase expression may be a potential mechanism, whereby glucose fluctuations result in atrial fibrosis (16). Furthermore, as a hypoglycemic complication, AF was reported in a diabetic patient, which successfully reverted to sinus rhythm after intravenous infusion of glucose (22).
In clinical practice, AF may be asymptomatic and is usually diagnosed after an adverse event. For these reasons, AF is thought to be a huge medical challenge associated with increased economic and social costs. Early identification of high-risk population (such as those with diabetes mellitus) for new-onset AF might help to prevent some AF-associated complications. Our present study indicates that HbA1c variability is a significant predictor of new-onset AF in patients with T2DM. HbA1c-SD of ≥0.665% (or HbA1c-CV ≥ 8.970%) provides an important diagnostic marker for predicting future AF.
Moreover, it has been reported that glycemic variability might be an indicator of irregular treatment or poor compliance to therapy due to various reasons (poor health education, insufficient awareness of disease severity) (23). Higher long-term glycemic variability is associated with other adverse risk factors, such as unhealthy lifestyle (smoking), elevated blood pressure, peripheral neuropathy, and peripheral vascular disease (24). It is interesting to note that some interventions (α1-glucosidase inhibitor or sodium-glucose cotransporter 2 inhibitors) that ameliorate glycemic variability have been found to reduce CVD compared with therapeutics with less effect on glycemic variability (25-27).
Study limitations
Some limitations in the present study merit attention. First, this report is a retrospective longitudinal analysis of patients referred to our center; therefore, selection bias cannot be fully excluded. Second, relatively small amount of clinical events was observed (48 cases of incident AF, 9.5%) during the follow-up period; therefore, we should interpret the results of our multivariate regression analyses with some caution. Third, each participant did not routinely receive ECG or 24-h Holter examination, the case of AF identified in this study might have been incomplete, especially in patients with some episodes of asymptomatic paroxysmal AF. Finally, some special circumstances, such as acute infection, severe anemia, and hemoglobin variants, might have influenced the HbA1c results.
Conclusion
In summary, the HbA1c variability might provide additional valuable information as a latent predictor of new-onset AF in patients with T2DM, and those with higher HbA1c-SD (or HbA1c-CV) should be carefully examined for AF for early prevention of thromboembolism.
Acknowledgements
This study was supported by National Nature Science Foundation of China (81670293) and research projects from Shanghai Shenkang hospital development center (16CR2034B) and Shanghai municipal commission of health and family planning (2014ZYJB0501).
Footnotes
Disclosure statement: The authors confirm that there are no conflicts of interest.
Conflict of interest: The authors confirm that there are no conflicts of interest.
Peer-review: Externally peer-reviewed.
Authorship contributions: Concept – J.G., Y.Q.F.; Supervision – C.Q.W.; Fundings – A.M.; Materials and Data collection &/or processing – J.G., Y.Q.F.; Analysis &/or interpretation and Literature search – J.F.Z.; Writing – J.G., J.F.Z.; Critical review – C.Q.W.

Ayla Bağ, from EFSAD’s collections.
References
- 1.Danaei G, Finucane MM, Lu Y, Singh GM, Cowan MJ, Paciorek CJ, et al. Global Burden of Metabolic Risk Factors of Chronic Diseases Collaborating Group (Blood Glucose) National, regional, and global trends in fasting plasma glucose and diabetes prevalence since 1980: systematic analysis of health examination surveys and epidemiological studies with 370 country-years and 2.7 million participants. Lancet. 2011;378:31–40. doi: 10.1016/S0140-6736(11)60679-X. [DOI] [PubMed] [Google Scholar]
- 2.van Dieren S, Beulens JW, van der Schouw YT, Grobbee DE, Neal B. The global burden of diabetes and its complications: an emerging pandemic. Eur J Cardiovasc Prev Rehabil. 2010;17(Suppl 1):S3–8. doi: 10.1097/01.hjr.0000368191.86614.5a. [DOI] [PubMed] [Google Scholar]
- 3.Kirchhof P, Benussi S, Kotecha D, Ahlsson A, Atar D, Casadei B, et al. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur Heart J. 2016;37:2893–962. doi: 10.1093/eurheartj/ehw210. [DOI] [PubMed] [Google Scholar]
- 4.Yang HK, Kang B, Lee SH, Yoon KH, Hwang BH, Chang K, et al. Association between hemoglobin A1c variability and subclinical coronary atherosclerosis in subjects with type 2 diabetes. J Diabetes Complications. 2015;29:776–82. doi: 10.1016/j.jdiacomp.2015.04.008. [DOI] [PubMed] [Google Scholar]
- 5.Wan EY, Fung CS, Fong DY, Lam CL. Association of variability in hemoglobin A1c with cardiovascular diseases and mortality in Chinese patients with type 2 diabetes mellitus - A retrospective population-based cohort study. J Diabetes Complications. 2016;30:1240–7. doi: 10.1016/j.jdiacomp.2016.05.024. [DOI] [PubMed] [Google Scholar]
- 6.Takao T, Matsuyama Y, Yanagisawa H, Kikuchi M, Kawazu S. Association between HbA1c variability and mortality in patients with type 2 diabetes. J Diabetes Complications. 2014;28:494–9. doi: 10.1016/j.jdiacomp.2014.02.006. [DOI] [PubMed] [Google Scholar]
- 7.Gu J, Fan YQ, Bian L, Zhang HL, Xu ZJ, Zhang Y, et al. Long-term prescription of beta-blocker delays the progression of heart failure with preserved ejection fraction in patients with hypertension: A retrospective observational cohort study. Eur J Prev Cardiol. 2016;23:1421–8. doi: 10.1177/2047487316636260. [DOI] [PubMed] [Google Scholar]
- 8.Gu J, Fan YQ, Han ZH, Fan L, Bian L, Zhang HL, et al. Association between long-term prescription of aldosterone antagonist and the progression of heart failure with preserved ejection fraction in hypertensive patients. Int J Cardiol. 2016;220:56–60. doi: 10.1016/j.ijcard.2016.06.190. [DOI] [PubMed] [Google Scholar]
- 9.Schoen T, Pradhan AD, Albert CM, Conen D. Type 2 diabetes mellitus and risk of incident atrial fibrillation in women. J Am Coll Cardiol. 2012;60:1421–8. doi: 10.1016/j.jacc.2012.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huxley RR, Filion KB, Konety S, Alonso A. Meta-analysis of cohort and case-control studies of type 2 diabetes mellitus and risk of atrial fibrillation. Am J Cardiol. 2011;108:56–62. doi: 10.1016/j.amjcard.2011.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Iguchi Y, Kimura K, Shibazaki K, Aoki J, Sakai K, Sakamoto Y, et al. HbA1c and atrial fibrillation: a cross-sectional study in Japan. Int J Cardiol. 2012;156:156–9. doi: 10.1016/j.ijcard.2010.10.039. [DOI] [PubMed] [Google Scholar]
- 12.Benjamin EJ, Levy D, Vaziri SM, D'Agostino RB, Belanger AJ, Wolf PA. Independent risk factors for atrial fibrillation in a population-based cohort. The Framingham Heart Study. JAMA. 1994;271:840–4. [PubMed] [Google Scholar]
- 13.Movahed MR, Hashemzadeh M, Jamal MM. Diabetes mellitus is a strong, independent risk for atrial fibrillation and flutter in addition to other cardiovascular disease. Int J Cardiol. 2005;105:315–8. doi: 10.1016/j.ijcard.2005.02.050. [DOI] [PubMed] [Google Scholar]
- 14.Pfister R, Michels G, Cairns R, Schneider CA, Erdmann E. Incidence of new onset bundle branch block and atrial fibrillation in patients with type 2 diabetes and macrovascular disease: an analysis of the PROactive study. Int J Cardiol. 2011;153:233–4. doi: 10.1016/j.ijcard.2011.09.049. [DOI] [PubMed] [Google Scholar]
- 15.Goudis CA, Korantzopoulos P, Ntalas IV, Kallergis EM, Liu T, Ketikoglou DG. Diabetes mellitus and atrial fibrillation: Pathophysiological mechanisms and potential upstream therapies. Int J Cardiol. 2015;184:617–22. doi: 10.1016/j.ijcard.2015.03.052. [DOI] [PubMed] [Google Scholar]
- 16.Saito S, Teshima Y, Fukui A, Kondo H, Nishio S, Nakagawa M, et al. Glucose fluctuations increase the incidence of atrial fibrillation in diabetic rats. Cardiovasc Res. 2014;104:5–14. doi: 10.1093/cvr/cvu176. [DOI] [PubMed] [Google Scholar]
- 17.Dublin S, Glazer NL, Smith NL, Psaty BM, Lumley T, Wiggins KL, et al. Diabetes mellitus, glycemic control, and risk of atrial fibrillation. J Gen Intern Med. 2010;25:853–8. doi: 10.1007/s11606-010-1340-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang YF, Zhu WQ, Cheng K, Chen QX, Xu Y, Pang Y, et al. Elevated glycated hemoglobin levels may increase the risk of atrial fibrillation in patients with diabetes mellitus. Int J Clin Exp Med. 2015;8:3271–80. [PMC free article] [PubMed] [Google Scholar]
- 19.Qi W, Zhang N, Korantzopoulos P, Letsas KP, Cheng M, Di F, et al. Serum glycated hemoglobin level as a predictor of atrial fibrillation: A systematic review with meta-analysis and meta-regression. PLoS One. 2017;12:e0170955. doi: 10.1371/journal.pone.0170955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fatemi O, Yuriditsky E, Tsioufis C, Tsachris D, Morgan T, Basile J, et al. Impact of intensive glycemic control on the incidence of atrial fibrillation and associated cardiovascular outcomes in patients with type 2 diabetes mellitus (from the Action to Control Cardiovascular Risk in Diabetes Study) Am J Cardiol. 2014;114:1217–22. doi: 10.1016/j.amjcard.2014.07.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vardas PE, Vemmos K, Sideris DA, Moulopoulos SD. Susceptibility of the right and left canine atria to fibrillation in hyperglycemia and hypoglycemia, J Electrocardiol. 1993;26:147–53. doi: 10.1016/0022-0736(93)90007-z. [DOI] [PubMed] [Google Scholar]
- 22.Çelebi S, Çelebi OO, Aydoğdu S, Diker E. A peculiar medical cardioversion of atrial fibrillation with glucose infusion--a rare cause of atrial fibrillation: hypoglycemia. Am J Emerg Med. 2011;29:134e1–3. doi: 10.1016/j.ajem.2010.02.012. [DOI] [PubMed] [Google Scholar]
- 23.Muggeo M, Zoppini G, Bonora E, Brun E, Bonadonna RC, Moghetti P, et al. Fasting plasma glucose variability predicts 10-year survival of type 2 diabetic patients: the Verona Diabetes Study. Diabetes Care. 2000;23:45–50. doi: 10.2337/diacare.23.1.45. [DOI] [PubMed] [Google Scholar]
- 24.Cheng D, Fei Y, Liu Y, Li J, Xue Q, Wang X, et al. HbA1c variability and the risk of renal status progression in diabetes mellitus: a meta-analysis. PLoS One. 2014;9:e115509. doi: 10.1371/journal.pone.0115509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chiasson JL, Josse RG, Gomis R, Hanefeld M, Karasik A, Laakso M, et al. Acarbose treatment and the risk of cardiovascular disease and hypertension in patients with impaired glucose tolerance: the STOP-NIDDM trial. JAMA. 2003;290:486–94. doi: 10.1001/jama.290.4.486. [DOI] [PubMed] [Google Scholar]
- 26.Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373:2117–28. doi: 10.1056/NEJMoa1504720. [DOI] [PubMed] [Google Scholar]
- 27.Rosenstock J, Hansen L, Zee P, Li Y, Cook W, Hirshberg B, et al. Dual add-on therapy in type 2 diabetes poorly controlled with metformin monotherapy: a randomized double-blind trial of saxagliptin plus dapagliflozin addition versus single addition of saxagliptin or dapagliflozin to metformin. Diabetes Care. 2015;38:376–83. doi: 10.2337/dc14-1142. [DOI] [PubMed] [Google Scholar]