Abstract
Spontaneous coronary artery dissection (SCAD) is a rare disease associated with high mortality rate, whose etiology and pathogenesis has been poorly understood to date. The management of these patients is still controversial. A young, otherwise healthy woman, without known underlying conditions leading to SCAD, was admitted to our Intensive Cardiology Care Unit; she had history of intense psychological stress. She was managed with a conservative approach based on watchful waiting and medical therapy. She had an uneventful course. This is a rare case of SCAD where stable hemodynamics allowed us to adopt a conservative approach.
<Learning objective: Spontaneous coronary artery dissection (SCAD) is a rare disease associated with high mortality rate, whose etiology and pathogenesis has been poorly understood to date. The management of spontaneous coronary artery dissection (SCAD) is still debated due to the rarity of cases; to treat or not to treat invasively? In our case, the hemodynamic stability of the patient suggested to wait and watch with serial angiographies and medical therapy. The uneventful course ruled in favor of our choice. Thus, the take home message is that not all cases of SCAD deserve to be treated with invasive procedures.>
Keywords: Spontaneous coronary artery dissection, Psychological stress, Medical therapy
Introduction
Spontaneous coronary artery dissection (SCAD) is historically considered a rare disease, involving mainly young healthy women of childbearing age without cardiac risk factors [1]. In most cases, SCAD involves a single coronary artery (mainly left descending artery, LAD), causing an acute coronary syndrome (ACS); rarely the disease causes sudden death [2]. In order to choose between a conservative or interventional approach, some features should be taken into account such as clinical presentation, site, and extent of SCAD [1].
In the present case, the patient was clinically stable, so a conservative approach was adopted.
Case report
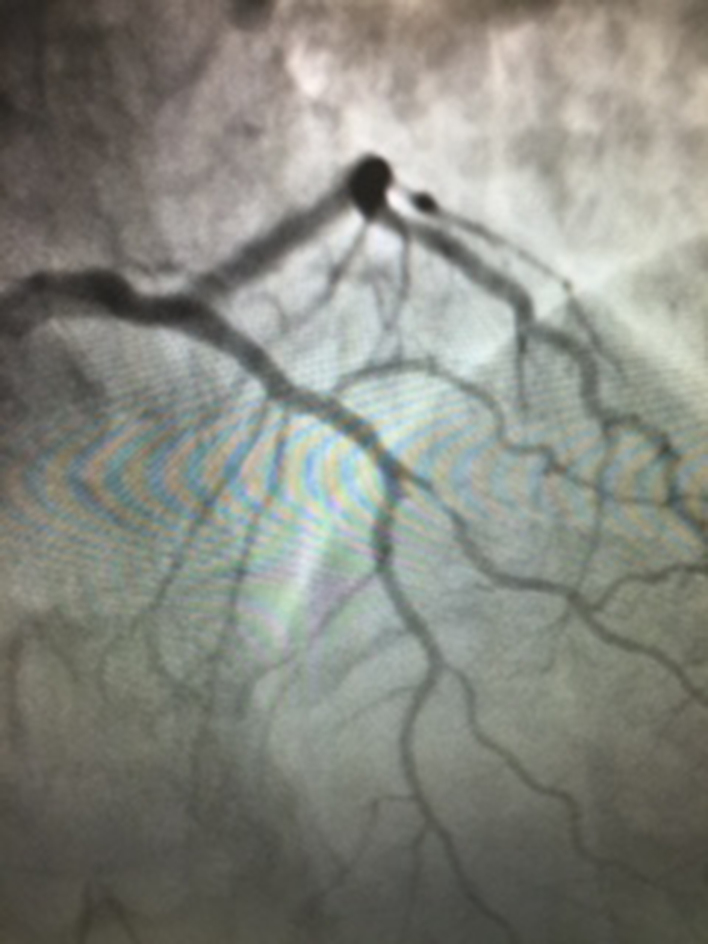
A 42-year-old woman was admitted to our Intensive Cardiology Care Unit due to prolonged chest pain and alterations at electrocardiogram (ECG): complete left bundle branch block (LBBB), never recorded before. Blood tests showed an increase of high sensitivity troponin (hsT) and creatine-kinase muscle and brain (CK-MB); Echocardiography showed limited apical hypokinesia. Because of chest pain persistence, the patient was submitted to coronary angiography that showed, after infusion of nitrates (1 mg), a moderate stenosis of the proximal left anterior descending artery (LAD) and sub-occlusive stenosis at the origin of diagonal branch, in absence of significant hemodynamic lesions of remaining coronary vessels (Fig. 1).
Fig. 1.
First coronary angiography: there was a moderate stenosis of the proximal left anterior descending artery and sub-occlusive stenosis at the origin of diagonal branch, in the absence of significant hemodynamic lesions of remaining coronary vessels.
Considering the small size of diagonal artery, whose diameter did not change after nitrate infusion, we opted for a conservative treatment: medical therapy and re-evaluation with new pre-discharge coronary angiography. The patient was asymptomatic, the ECG showed sinus tachycardia with an intermittent LBBB and the echocardiography showed a slight and limited apical hypokinesia. On the fourth day, coronary angiography was repeated, showing double contour compatible with coronary dissection starting from the middle of the anterior LAD, involving diagonal branch, sub-occluded at the previous angiography and extended up to the middle-distal portion of the vessel (Fig. 2).
Fig. 2.
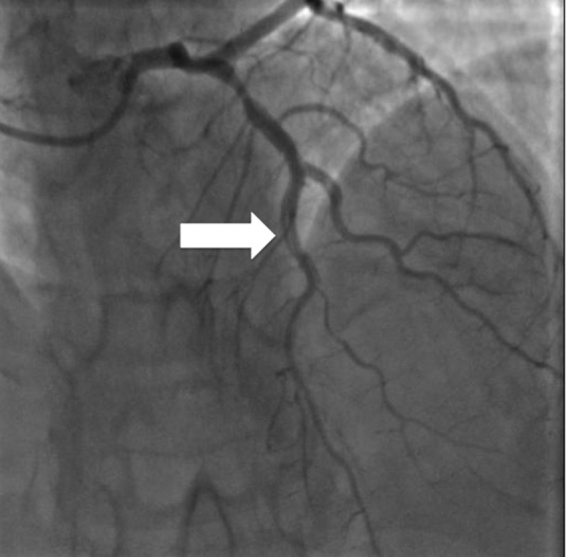
Second coronary angiography: double contour image compatible with coronary dissection starting from the middle of the left anterior descending artery, involving diagonal branch (white arrow).
Considering the lack of symptoms and stable hemodynamics, we preferred to carry on with conservative treatment and repeat an additional angiographic control, 10 days afterwards. In the remaining course, the patient was still asymptomatic on medical therapy (aspirin, statin, and bisoprolol). The third angiographic control showed a fully patent LAD and diagonal branch [thrombolysis in myocardial infarction (TIMI) flow 3], with complete regression of the previous double contour image (Fig. 3).
Fig. 3.
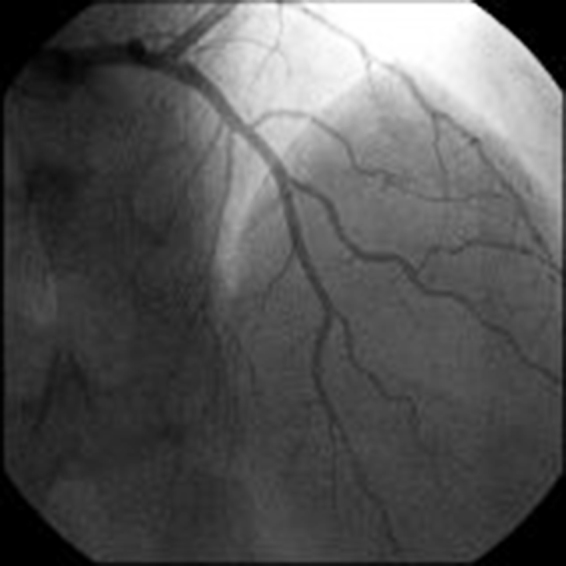
Third coronary angiography: a fully patent left anterior descending and diagonal branch (thrombolysis in myocardial infarction flow 3), with complete regression of the previous double contour image.
At discharge the patient was asymptomatic, the ECG showed a sinus rhythm with the regression of LBBB and the echocardiogram showed a normal left ventricular systolic function without kinetic anomalies.
A weekly clinical follow up was planned over the next 4 weeks along with an angiographic control at 1 month. The latter confirmed the presence of a normal coronary system, without images related to coronary dissection in the middle-distal LAD and diagonal branch.
Discussion
SCAD is a rare disease whose etiology is not fully understood. It often affects healthy women with no coronary risk factors [2]. The incidence of SCAD evaluated with coronary angiography varies from 0.1% to 1.1% [3].
There are two proposed mechanisms for SCAD: (1) intimal tear formation (from internal), (2) rupture of vasa vasorum (from external). Moreover, SCAD can be distinguished into atherosclerosis and non-atherosclerosis. In the latter case, SCAD is typically a culmination of predisposing diseases: Predisposing arteriopathy, fibromuscular dysplasia; pregnancy (history of multiple pregnancy, peri-partum); connective tissue disorder (Marfan's syndrome, Ehler Danlos syndrome, cystic medial necrosis, fibromuscular dysplasia); systemic inflammation (systemic lupus erythematosus, Crohn's disease, polyarteritis nodosa, sarcoidosis); hormonal therapy; coronary artery spasm; idiopathic. There are then some precipitating events that can trigger SCAD: intense exercise (aerobic or isometric), intense emotional stress, delivery; intense Valsalva-type activities (e.g. severe repetitive coughing, retching/vomiting, bowel movement), cocaine, amphetamines, met-amphetamines, beta-human chorionic gonadotropin [4], [5].
Among the various proposed underlying mechanisms, the most reliable was the increase in vessel wall stress (shear stress) in the presence of connective tissue disorders, cystic medionecrosis, localized vasculitis eosinophilic infiltration [4].
More recently, arterial tortuosity was identified as adding markers of arterial fragility.
In most cases dissection involves a single vessel and often it is the LAD, however in a small percentage a wider involvement of all the coronary system has been reported.
Optimal management of SCAD is not clearly defined; the possible options include conventional medical therapy or interventional procedures such as percutaneous coronary intervention (PCI) coronary artery bypass grafting (CABG). Thrombolysis, sometimes used in the past, resulted in deleterious effects, causing an extension of dissection [6], [7].
Yip and Saw [4] identified 3 types of SCAD on the basis of angiographic features. In type 1, the evident arterial wall strain allows to promptly identify the presence of SCAD. Conversely, types 2 and 3 are less frequent and more demanding to be identified due to the presence of atherosclerosis stenosis (type 2 diffuse stenosis; type 3 mimic atherosclerosis). Unfortunately, the present case was initially a type 3 which is infrequent (<5% of all SCAD), so difficult to address as SCAD. Then it changed to type 1, where angiography was enough to define it as SCAD. In both type 2 and 3 either optical coherence tomography (OCT) or intravascular ultrasound (IVUS) should be used to promptly identify SCAD [4]. One of the limitations of this case was the lack of these investigation tools.
The choice between conservative medical or revascularization treatment depends on clinical status, extension of dissection, and involved myocardium [1]. Medical therapy may be an option in the SCAD of middle-distal segments with a luminal narrowing less than 50% and a TIMI flow 3; PCI is the treatment of choice in case of SCAD of limited to a small proximal segment; CABG is mandatory in case of extensive SCAD involving the proximal LAD or common left main trunk [7]. Many important studies showed a good prognosis of SCAD in patients treated with a conservative strategy, especially when patients were carefully selected [7], [8], [9]. One study analyzed the outcome of 45 consecutive patients with SCAD initially treated with conservative medical therapy, where a further revascularization was performed only in 2 cases of recurrent ischemia [8]; only 1 patient died due to heart failure, but no patient had new SCAD, myocardial infarction, or sudden death. Survival was 92% and the spontaneous resolution of dissection was demonstrated in at least half of the patients [8].
In conclusion, the management of these rare cases remains anecdotal and addressed more by individual characteristics of the patient than by guidelines. In our case the good control of symptoms using an optimized medical therapy allowed a conservative approach and this avoided revascularization of a coronary dissection with a spontaneous resolution.
Conflict of interest
All the authors disclosure to have no conflict of interest.
References
- 1.Hyemoon C., Sung-Joo L., Jong-Kwan P., In Suk C., Ho Yeon W., Sohee K., Jung-Joon C., Byoung K.L. Spontaneous coronary artery dissection mimicking coronary spasm diagnosed by intravascular ultrasonography. Korean Circ J. 2013;43:491–496. doi: 10.4070/kcj.2013.43.7.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shamloo B.K., Chintala R.S., Nasur A., Ghazvini M., Shariat P., Diggs J.A., Singh S.N. Spontaneous coronary artery dissection: aggressive vs. conservative therapy. J Invasive Cardiol. 2010;22:222–228. [PubMed] [Google Scholar]
- 3.Lunebourg A., Letovanec I., Eggenberger P., Lehr H.A. Images in cardiovascular medicine. Sudden cardiac death due to triple vessel coronary dissection. Circulation. 2008;117:2038–2040. doi: 10.1161/CIRCULATIONAHA.107.729228. [DOI] [PubMed] [Google Scholar]
- 4.Yip A., Saw J. Spontaneous coronary artery dissection—a review. Cardiovasc Diagn Ther. 2015;5:37–48. doi: 10.3978/j.issn.2223-3652.2015.01.08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Almeda F.Q., Barkamullah S., Kavinsky C.J. Spontaneous coronary artery dissection. Clin Cardiol. 2004;27:377–380. doi: 10.1002/clc.4960270702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Glamore M.J., Garcia-Covarrubias L., Harrison L.H., Jr., Moreno N.L. Spontaneous coronary artery dissection. J Card Surg. 2012;27:56–59. doi: 10.1111/j.1540-8191.2011.01354.x. [DOI] [PubMed] [Google Scholar]
- 7.Eddinger J., Dietz W.A. Recurrent spontaneous coronary artery dissection. Catheter Cardiovasc Interv. 2005;66:566–569. doi: 10.1002/ccd.20543. [DOI] [PubMed] [Google Scholar]
- 8.Vanzetto G., Berger-Coz E., Barone-Rochette G., Chavanon O., Bouvaist H., Hacini R., Blin D., Machecourt J. Prevalence, therapeutic management and medium-term prognosis of spontaneous coronary artery dissection: results from a database of 11,605 patients. Eur J Cardiothorac Surg. 2009;35:250–254. doi: 10.1016/j.ejcts.2008.10.023. [DOI] [PubMed] [Google Scholar]
- 9.Alfonso F., Paulo M., Lennie V., Dutary J., Bernardo E., Jiménez-Quevedo P., Gonzalo N., Escaned J., Bañuelos C., Pérez-Vizcayno M.J., Hernández R., Macaya C. Spontaneous coronary artery dissection: long-term follow-up of a large series of patients prospectively managed with a “conservative” therapeutic strategy. JACC Cardiovasc Interv. 2012;5:1062–1070. doi: 10.1016/j.jcin.2012.06.014. [DOI] [PubMed] [Google Scholar]