Abstract
Re-feeding syndrome is an uncommon clinical entity of fluid and electrolyte disorders that typically occurs after re-initiation of enteral nutrition following prolonged fasting. This disorder can be complicated by left ventricular (LV) dysfunction, arrhythmias, and death. Alcohol abuse and anorexia nervosa are independently associated with similar complications. The interaction between these diagnoses can result in significant, but reversible, LV dysfunction. We present the case of a 69-year-old woman with a history of significant alcohol abuse and anorexia nervosa. The patient was admitted to hospital for the management of re-feeding syndrome, which was complicated by significant LV dysfunction. Her LV function normalized following a combination of electrolyte replacement, re-institution of feeding, and abstinence from alcohol. Re-feeding syndrome, anorexia nervosa, and alcohol abuse are conditions that commonly co-exist. These conditions may have a synergistic relationship, potentially resulting in a profound cardiomyopathy. Careful monitoring and aggressive electrolyte replacement may be helpful in identifying this complication and minimizing its potential harm.
<Learning objective: Re-feeding syndrome can be complicated by significant myocardial dysfunction, particularly in patients with a history of alcohol abuse or anorexia nervosa, which independently cause cardiac dysfunction. Physicians should be aware of the risk of new cardiomyopathy in patients with these overlapping diagnoses. We review the case of a patient with these conditions who developed a significant reversible cardiomyopathy managed with re-institution of feeding and electrolyte replacement.>
Keywords: Re-feeding syndrome, Alcoholic cardiomyopathy, Anorexia
Introduction
Anorexia nervosa (AN) is a common cause of protein-calorie malnutrition with several potential cardiovascular complications including brady-arrhythmias, hypotension, and mitral valve prolapse [1]. The development of left ventricular (LV) dysfunction is relatively uncommon in patients with isolated AN [2], although reduced LV dimensions have been observed [3]. In addition to cardiovascular complications, patients with AN are prone to development of metabolic disturbances, collectively referred to as re-feeding syndrome, with re-initiation of enteral nutrition [4]. This syndrome typically occurs within 4 days of feeding and is characterized by cardiac, neurologic, pulmonary, neuromuscular, and hematologic effects. The cardiac effects of re-feeding syndrome, which are primarily attributed to hypophosphatemia and hypokalemia, may include arrhythmias, hypotension, LV dysfunction, and sudden cardiac death [4].
Chronic alcohol abuse increases risk of developing re-feeding syndrome, and is independently associated with hypophosphatemia and hypokalemia [4]. The co-existence of re-feeding syndrome and chronic alcohol abuse may have a potential negative synergistic relationship that enhances the risk and severity of cardiac complications, such as significant LV dysfunction, due to the abnormal metabolic milieu [4]. However, correction of the electrolyte abnormalities may result in reversibility [4]. We describe the case of a patient with re-feeding syndrome and chronic alcohol abuse, who developed a profound, but reversible, cardiomyopathy.
Case report
A 59-year-old woman was brought to the emergency department by her daughter for concerns regarding significant weight loss and immobility. The patient had been restricting her food intake for several weeks, resulting in a 10 kg weight loss over that same period. Her weight upon presentation to the emergency department was 38 kg with a body mass index of 14 kg/m2. Additionally, she had been unable to get out of bed for approximately 4 weeks with several recent falls. The current situation had been triggered by a recent divorce with her husband, with significant decline in her mood, inability to sleep, and decreased appetite.
Her past medical history was significant for AN, depression, and chronic macrocytic anemia. The patient's social history was significant for alcohol abuse with a 30-year history of drinking 6–7 ounces of rum per day. Prior to her current illness, the patient had no significant functional limitations or other cardiac symptoms. She did not endorse recent infectious symptoms, exposures to cardiotoxic drugs, and denied a family history of cardiomyopathy. The patient had no previous cardiac investigations.
The patient's initial laboratory investigations are outlined in Table 1. These investigations were significant for a potassium level of 2.6 mmol/L, a bicarbonate of 32 mmol/L, magnesium of 0.34 mmol/L, and phosphate 0.84 mmol/L. The patient's alcohol level was not detectable.
Table 1.
Laboratory results throughout the course of the hospitalization. – indicates no lab value obtained on that particular day.
| Normal values | Day 0 | Day 2 | Day 5 | Day 8 | |
|---|---|---|---|---|---|
| Hemoglobin (g/L) | 123–157 | 119 | 108 | 106 | 102 |
| Mean corpuscular volume (fL) | 80–100 | 107 | 109 | 108 | 108 |
| Sodium (mmol/L) | 135–145 | 137 | 142 | 138 | 139 |
| Potassium (mmol/L) | 3.5–5.0 | 2.6 | 4.8 | 4.4 | 4.8 |
| Bicarbonate (mmol/L) | 24–30 | 32 | 26 | 28 | 30 |
| Magnesium (mmol/L) | 0.75–0.95 | 0.34 | 0.89 | 0.63 | 0.60 |
| Phosphate (mmol/L) | 0.80–1.50 | 0.84 | – | 0.30 | 1.41 |
| Calcium (mmol/L) | 2.18–2.58 | 1.85 | – | 2.18 | 2.30 |
| Creatinine (μmol/L) | 50–90 | 38 | 38 | 46 | 37 |
| Random glucose (mmol/L) | 3.8–11.1 | 4.8 | 5.5 | 6.0 | 5.7 |
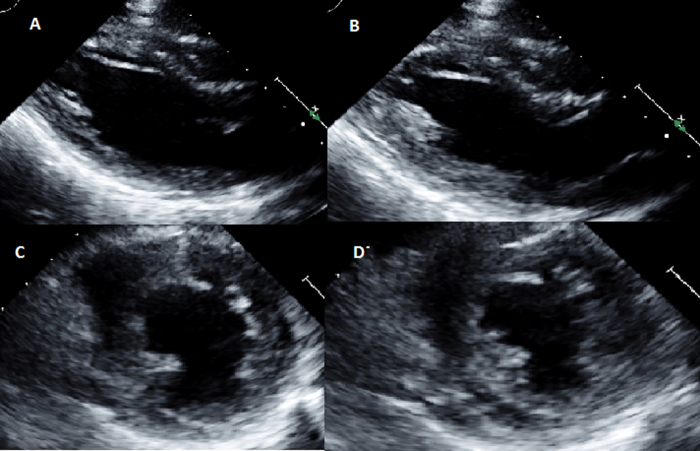
The patient was initially admitted to the hospital for re-institution of enteral nutrition. Due to persistent tachycardia an electrocardiogram (ECG) and transthoracic echocardiogram (TTE) were ordered. The ECG showed sinus tachycardia without other significant changes. The TTE, shown in Fig. 1 and Supplemental Video 1, was performed 2 days following admission and showed that the LV systolic function was moderately reduced with a LV ejection fraction of approximately 30–40%. The right ventricular (RV) size and function were grossly normal, and there was no significant valve disease. The LV wall thickness was small with intraventricular septal thickness of 0.57 cm (normal 0.6–1.0 cm). At that time, cardiology was consulted regarding the etiology of her LV dysfunction.
Fig. 1.
Initial transthoracic echocardiogram showing moderate left ventricular dysfunction. Para-sternal long-axis view during end-diastole (A) and end-systole (B) and para-sternal short-axis views during end-diastole (C) and end-systole (D).
Supplementary Video 1 related to this article can be found, in the online version, at http://dx.doi.org/10.1016/j.jccase.2016.04.006.
Her physical examination at time of cardiology consultation revealed a heart rate of 133 bpm, respiratory rate 18 breaths per minute, blood pressure 133/85 mmHg, and oxygen saturation of 94% on room air. Auscultation of the chest revealed crackles at the bases bilaterally. Jugular venous pressure was elevated at 5 cm above sternal angle with positive abdominal jugular reflux. Auscultation of the heart revealed normal first and second heart sounds, with the presence of a third heart sound. Repeat laboratory investigations at that time are outlined in Table 1. Of note, there was normal thyroid stimulating hormone, elevated transferrin saturation, and significantly reduced phosphate at 0.30 mmol/L, which was consistent with the development of re-feeding syndrome.
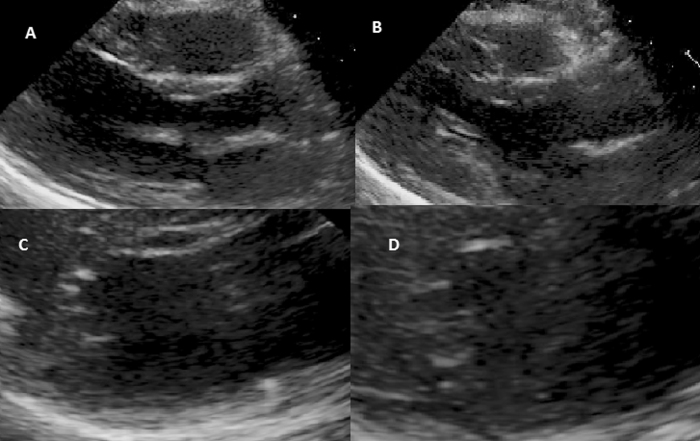
At that time, it was felt that there were several potential causes of the patients LV dysfunction. The leading diagnoses were alcoholic cardiomyopathy, re-feeding syndrome, Takotsubo cardiomyopathy, hemochromatosis, or other cause of pre-existing cardiac dysfunction with new symptoms related to increased intake. Cardiac magnetic resonance imaging was arranged to help elucidate the etiology of the LV dysfunction. This was completed 7 days following admission and revealed normal biventricular systolic function. Edema sequences were normal and there was no significant late gadolinium enhancement. These findings argue strongly against a diagnosis of previous myocarditis, ischemic cardiomyopathy, or infiltrative cardiomyopathy. Additionally, T2-star imaging was normal, excluding iron overload as the underlying cause of her cardiomyopathy. Repeat echocardiogram, 8 days after admission, confirmed resolution of the LV dysfunction (images shown in Fig. 2 and Supplemental Video 2).
Fig. 2.
Follow-up transthoracic echocardiogram showing normal left ventricular systolic function. Para-sternal long-axis view during end-diastole (A) and end-systole (B) and para-sternal short-axis views during end-diastole (C) and end-systole (D).
Supplementary Video 2 related to this article can be found, in the online version, at http://dx.doi.org/10.1016/j.jccase.2016.04.006.
The patient was hospitalized for a total of 28 days and at discharge she weighed 43 kg, with a body mass index (BMI) of 16 kg/m2. The remainder of the patient's hospitalization was uncomplicated and the patient was mobilizing well without any symptoms prior to discharge. As an outpatient, her weight has gradually been increasing and she has had no recurrence of heart failure symptomatology at 1 year of follow-up. Additionally, follow-up echocardiogram at 1-year showed preserved LV systolic function.
Discussion
Re-feeding syndrome refers to a group of metabolic disturbances that occurs within 4 days of reinstating nutrition in patients who are starved or severely malnourished [4]. There are several risk factors for developing re-feeding syndrome including: unintentional weight loss, low nutrient intake for more than 7 days, AN, chronic alcoholism, depression in the elderly, patients with cancer, chronic infectious diseases, homelessness, and social deprivation [4]. Current guidelines identify patients with lower BMI, significant unintentional weight loss, low nutritional intake for ≥5 days, or who have electrolyte abnormalities prior to initiating feeding as higher risk for developing re-feeding syndrome [5]. Our patient had a history of chronic alcohol abuse, depression, AN, and significant food restriction prior to hospitalizations, resulting in a high risk of developing re-feeding syndrome. In high-risk patients guidelines suggest initiating enteral nutrition at 10 kcal/kg/day and gradually increasing to meet full caloric needs over 4–7 days [5]. They also suggest supplementing potassium, phosphate, and magnesium in all patients unless the pre-feeding serum levels are high and daily monitoring of electrolytes in high-risk patients until serum levels are stable [5]. However, they recommend that it is not necessary to delay initiating enteral nutrition to correct electrolyte abnormalities [5]. Our patient's complications from re-feeding syndrome potentially could have been prevented by following the above management guidelines.
Re-feeding syndrome may be complicated by the development of LV dysfunction, for which there are several proposed mechanisms [6]. It is believed that the profound electrolyte abnormalities that accompanies re-feeding syndrome may play a causal role. Hypophosphatemia, a common complication of both chronic alcohol abuse and re-feeding syndrome, may be particularly important [7]. Darsee et al. reported a case series of three patients with severe LV dysfunction related to hypophosphatemia which improved following correction of hypophosphatemia [8]. Furthermore, myocardial performance is depressed relative to the degree of hypophosphatemia [9]. Finally, hypophosphatemia predisposes patients to cardiac arrhythmias which are reduced by correcting phosphate deficiencies [10]. Alternatively, severe hypoglycemic states may induce a state of catecholamine excess that results in myocardial damage similar to a Takotsubo cardiomyopathy [6]. Finally, hypokalemia has been associated with a reversible cardiomyopathy and was also present in our case [11]. Ultimately, it may be a combination of these abnormalities that results in the rapid development of significant LV dysfunction.
Chronic malnutrition also leads to important changes to cardiac structure in patients with AN and chronic alcohol abuse. Chronic malnutrition results in diminished protein synthesis, activation of calcium-dependent proteinases, mitochondrial swelling, and interstitial edema [6]. These processes result in myofibrillar atrophy with overall decreases in LV mass that may improve following weight gain [3], [6]. The association between alcohol consumption and LV dysfunction is less clear. Despite the known association between significant alcohol intake and dilated cardiomyopathy, the two largest trials looking at population effects of alcohol on LV function failed to show a clear association [12]. However, both of these studies suffered from the limitations of self-reported alcohol intake. The pathophysiology may also be related to direct toxic effects of alcohol and co-existing malnutrition [13]. Finally, both chronic alcohol abuse and AN may be associated with cardiomyopathy due to a predisposition to arrhythmias and associations with other cardiac risk factors [6]. Interestingly, the echocardiogram in our patient showed decreased LV wall thickness, which suggests the presence of some underlying chronic changes. The degree to which chronic abnormalities of cardiac structure predispose patients to developing acute cardiac changes during the course of re-feeding syndrome is unknown.
AN and chronic alcohol abuse commonly co-exist, and collectively increase the risk of re-feeding syndrome. The constellation of these disease entities can potentially result in a significant impairment of LV function. Careful monitoring and knowledge of potential complications is crucial when managing patients at high risk of developing re-feeding syndrome. Additionally, physicians should be aware of the association with LV dysfunction and have a low threshold for considering new onset cardiomyopathy in patients admitted with these conditions.
Conflicts of interest
There are no relevant conflicts of interest to declare.
References
- 1.Thurston J., Marks P. Electrocardiographic abnormalities in patients with anorexia nervosa. Br Heart J. 1974;36:719–723. doi: 10.1136/hrt.36.7.719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Swenne I., Larsson P.T. Heart risk associated with weight loss in anorexia nervosa and eating disorders: risk factors for QTc interval prolongation and dispersion. Acta Paediatr. 1999;88:304–309. doi: 10.1080/08035259950170079. [DOI] [PubMed] [Google Scholar]
- 3.Gottdiener J.S., Gross H.A., Henry W.L., Borer J.S., Ebert M.H. Effects of self-induced starvation on cardiac size and function in anorexia nervosa. Circulation. 1978;58:425–433. doi: 10.1161/01.cir.58.3.425. [DOI] [PubMed] [Google Scholar]
- 4.Stanga Z., Brunner A., Leuenberger M., Grimble R.F., Shenkin A., Allison S.P., Lobo D.N. Nutrition in clinical practice-the refeeding syndrome: illustrative cases and guidelines for prevention and treatment. Eur J Clin Nutr. 2008;62:687–694. doi: 10.1038/sj.ejcn.1602854. [DOI] [PubMed] [Google Scholar]
- 5.National Collaborating Centre for Acute Care; London: 2006. Nutrition support for adults: oral nutrition support, enteral tube feeding and parenteral nutrition. [PubMed] [Google Scholar]
- 6.Ono T., Kasaoka S., Fujita M., Yamashita S., Kumagai K., Kaneda K., Tsuruta R., Maekawa T. Complete recovery from severe myocardial dysfunction in a patient with anorexia nervosa. J Cardiol. 2009;54:480–484. doi: 10.1016/j.jjcc.2009.02.016. [DOI] [PubMed] [Google Scholar]
- 7.Brunelli S.M., Goldfarb S. Hypophosphatemia: clinical consequences and management. J Am Soc Nephrol. 2007;18:1999–2003. doi: 10.1681/ASN.2007020143. [DOI] [PubMed] [Google Scholar]
- 8.Darsee J.R., Nutter D.O. Reversible severe congestive cardiomyopathy in three cases of hypophosphatemia. Ann Intern Med. 1978;89:867–870. doi: 10.7326/0003-4819-89-6-867. [DOI] [PubMed] [Google Scholar]
- 9.Davis S.V., Olichwier K.K., Chakko S.C. Reversible depression of myocardial performance in hypophosphatemia. Am J Med Sci. 1988;295:183–187. doi: 10.1097/00000441-198803000-00004. [DOI] [PubMed] [Google Scholar]
- 10.Schwartz A., Brotfain E., Koyfman L., Kutz R., Gruenbaum S.E., Klein M., Zlotnik A. Association between hypophosphatemia and cardiac arrhythmias in the early stage of sepsis: could phosphorus replacement treatment reduce the incidence of arrhythmias? Electrolyte Blood Press. 2014;12:19–25. doi: 10.5049/EBP.2014.12.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kramer L.D., Cole J.P., Messenger J.C., Ellestad M.H. Cardiac dysfunction in a patient with familial hypokalemic periodic paralysis. Chest. 1979;75:189–192. doi: 10.1378/chest.75.2.189. [DOI] [PubMed] [Google Scholar]
- 12.Bryson C.L., Mukamal K.J., Mittleman M.A., Fried L.P., Hirsch C.H., Kitzman D.W., Siscovick D.S. The association of alcohol consumption and incident heart failure: the Cardiovascular Health Study. J Am Coll Cardiol. 2006;48:305–311. doi: 10.1016/j.jacc.2006.02.066. [DOI] [PubMed] [Google Scholar]
- 13.Root T., Pinheiro A.P., Thornton L., Strober M., Fernandez-Aranda F., Brandt H., Crawford S., Fichter M.M., Halmi K.A., Johnson C., Kaplan A.S., Klump K.L., La Via M., Mitchell J., Woodside D.B. Substance use disorders in women with anorexia nervosa. Int J Eat Disord. 2010;43:14–21. doi: 10.1002/eat.20670. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.