Summary
Nitrous oxide (N2O) is a potent, globally important, greenhouse gas, predominantly released from agricultural soils during nitrogen (N) cycling. Arbuscular mycorrhizal fungi (AMF) form a mutualistic symbiosis with two‐thirds of land plants, providing phosphorus and/or N in exchange for carbon. As AMF acquire N, it was hypothesized that AMF hyphae may reduce N2O production.
AMF hyphae were either allowed (AMF) or prevented (nonAMF) access to a compartment containing an organic matter and soil patch in two independent microcosm experiments. Compartment and patch N2O production was measured both before and after addition of ammonium and nitrate.
In both experiments, N2O production decreased when AMF hyphae were present before inorganic N addition. In the presence of AMF hyphae, N2O production remained low following ammonium application, but increased in the nonAMF controls. By contrast, negligible N2O was produced following nitrate application to either AMF treatment.
Thus, the main N2O source in this system appeared to be via nitrification, and the production of N2O was reduced in the presence of AMF hyphae. It is hypothesized that AMF hyphae may be outcompeting slow‐growing nitrifiers for ammonium. This has significant global implications for our understanding of soil N cycling pathways and N2O production.
Keywords: agriculture, arbuscular mycorrhizal fungi (AMF), greenhouse gas, hyphosphere, N cycle, nitrification, nitrogen (N), nitrous oxide (N2O)
Introduction
Agricultural soils are a major source of the globally important greenhouse gas, nitrous oxide (N2O), a gaseous product of the nitrogen (N) cycle (Singh et al., 2010; Hartmann et al., 2013). In fact, the marked global N2O atmospheric concentration increases between 1940 and 2005 were predominantly a result of increased use of N‐based fertilizers in agricultural systems (Park et al., 2012). N2O also has a long pertubation lifetime of 121 yr (Hartmann et al., 2013), and thus it is essential that we understand the soil‐derived fluxes of N2O, as, unlike shorter‐lived greenhouse gases (e.g. CH4; Hartmann et al., 2013), any changes in the atmospheric concentration of N2O will have long‐term effects. Consequently, N2O is viewed as an immediate target to achieve greenhouse gas reductions (Wuebbles & Hayhoe, 2002; Reay et al., 2012). However, in order to achieve such reductions, an enhanced understanding of the major sources and sinks of N2O is urgently required.
In recent years, our understanding of N2O production in soil systems has significantly improved, mostly as a result of the development of isotopic methods for tracing the sources of N2O (Baggs, 2008; Kool et al., 2011a; Ostrom & Ostrom, 2011). The rate of N2O production is predominantly controlled by the availability of the inorganic N source (Hino et al., 2010), O2 (Bollmann & Conrad, 1998), and other factors that influence microbial activity (e.g. temperature, carbon (C) availability and pH; Bollmann & Conrad, 1998; Prosser, 2007; Thomson et al., 2012). In addition, recent evidence has revealed that N2O reduction is not only confined to denitrifers. Other commonly occurring soil bacteria and archaea may also utilize exogenous N2O, including under aerobic conditions, even though they lack the preceding steps in the denitrification pathway (Sanford et al., 2012; Jones et al., 2014). Therefore, it follows that the net N2O emitted from soils will be influenced by the presence of microorganisms.
Arbuscular mycorrhizal fungi (AMF) are a key group of soil microorganisms that form symbiotic associations with the majority of land plants (Smith & Read, 2008). Moreover, it is now widely acknowledged that these fungi play a previously unrecognized role in nitrogen (N) cycling, and can both aquire N for their host plant (Barrett et al., 2011; Herman et al., 2012) and have a substantial N requirement themselves (Hodge & Fitter, 2010). There is also evidence of reduced nitrate (NO3 −) leaching in the presence AMF (Asghari & Cavagnaro, 2012; Cavagnaro et al., 2015; Köhl & van der Heijden, 2016). Alongside NO3 −, a major output of the N cycle is the potent greenhouse gas, N2O. Therefore, it might be expected that these fungi might influence the availability of N substrates (ammonium (NH4 +) and NO3 −) for N2O production. AMF have been shown to be able to acquire both NH4 + and NO3 −, although it appears they may prefer the more energetically attractive NH4 + (Govindarajulu et al., 2005; Hodge & Storer, 2015). If these fungi compete effectively with other microorganisms for these inorganic N forms then this could reduce the availability of N substrates for N2O producers, leading to a reduction in N2O emissions. There is some circumstantial evidence to suggest this may be the case. For example, Bender et al. (2014) found a reduction in N2O fluxes from soils influenced by AMF‐colonized roots when compared with soils influenced by roots alone. N2O fluxes are also reduced when rice plants in draining paddies are arbuscular mycorrhizal (Zhang et al., 2015). Collectively, these studies suggest that AMF may alter N2O emissions in conventional agricultural soils but, thus far, it has not been determined if this is mediated through physiological changes in the AMF‐colonized roots, or as a direct result of the AMF themselves. If AMF hyphae can directly reduce N2O production, this could have significant implications for global N2O production and our understanding of soil N cycling.
Arbuscular mycorrhizal fungi hyphae have previously been demonstrated to proliferate in organic matter patches (e.g. Hodge et al., 2001; Barrett et al., 2014; Hodge, 2014) and have been shown to take up and transfer N in the inorganic form from these patches to their host plant (Leigh et al., 2009; Hodge & Fitter, 2010). The two studies described here followed a similar experimental design to that of Hodge & Fitter (2010) using dried, milled Zea mays L. leaves mixed with an agricultural soil (which had a high N2O production rate; Storer, 2013), to create organic matter ‘patches’. These organic matter patches represent ‘N2O hotspots’ which commonly occur in natural systems (Cowan et al., 2015). Both experiments tested the hypothesis that AMF hyphae would reduce N2O production from the organic matter patches, while the second experiment further examined the hypothesis that a reduction in N2O production was a consequence of reduced nitrification rates in the presence of AMF hyphae.
Materials and Methods
Microcosm design and growth media
To test the hypothesis that N2O production was reduced in the presence of AMF hyphae, two experiments were established under glasshouse conditions using compartmented microcosm units. Expt 1 was designed to determine the impact of AMF hyphae on N2O production, whereas Expt 2 was designed to determine whether AMF hyphae affected N2O produced by nitrification and/or denitrification. Organic matter patches were used to create ‘hotspots’ of N2O production, a commonly observed phenomenon under natural conditions.
Expt 1
Microcosm units (Fig. 1a) were constructed by joining two 1 l plastic containers (each 145 × 145 × 70 mm3) via a double‐mesh membrane of either 20 μm (John Stanier & Co., Whitefield, Manchester, UK) or 0.45 μm pore size (Osmonics Inc., Minnetonka, MN, USA). These size membranes either allowed (AMF) or denied (nonAMF) AMF hyphal access between the two compartments. In all cases, roots were prevented from passing between the compartments. There were three 6 mm drainage holes in the base of each compartment. In one compartment (the ‘planted’ compartment) a single Z. mays seedling (Incredible F1; Mr Fothergills, Newmarket, UK) inoculated with Rhizophagus irregularis (PlantWorks Ltd, Kent, UK) was placed, whereas the other compartment contained no plant (the ‘unplanted’ compartment).
Figure 1.
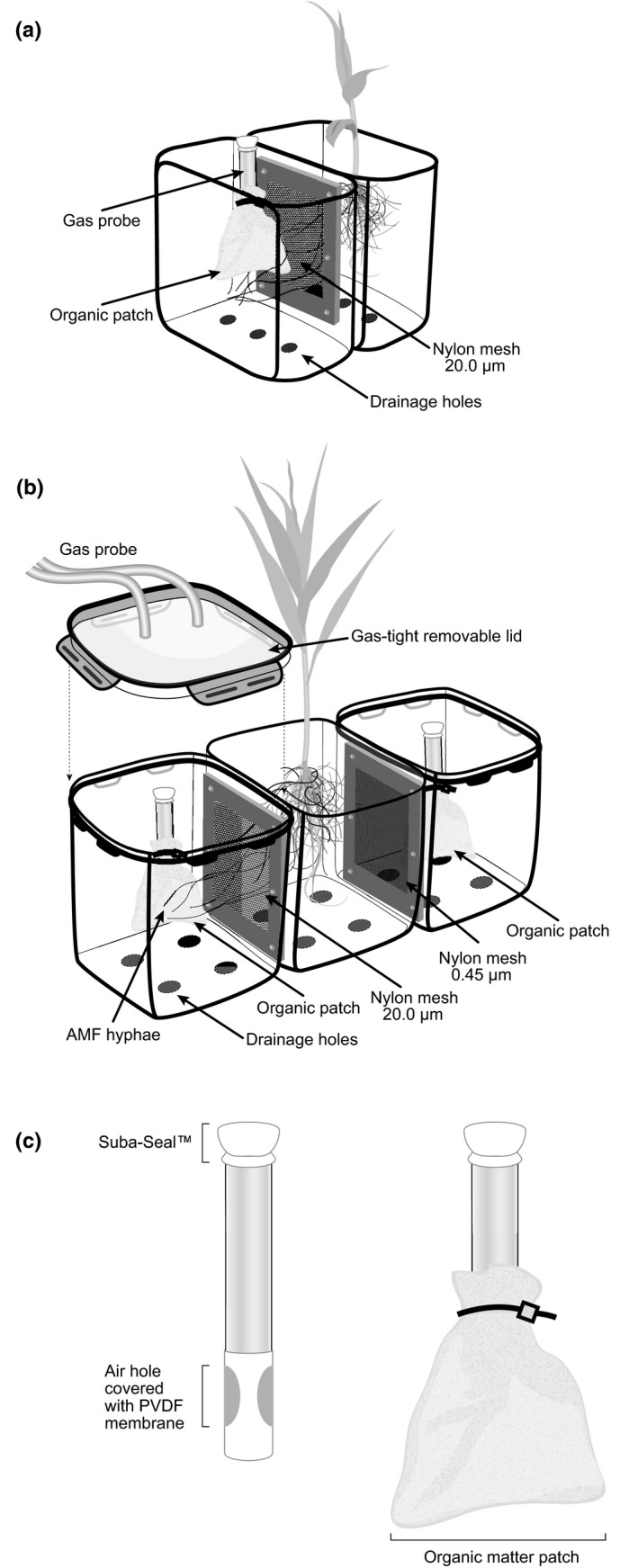
The microcosm units used in Expt 1 (a) and Expt 2 (b) and the organic matter patches and gas probes used in both experiments (c). In Expt 1 the planted compartment was planted with a single Zea mays plant and contained the arbuscular mycorrhizal fungal (AMF) inoculum, and the unplanted compartment either allowed or prevented AMF hyphal access. In Expt 2 the central compartment was also planted with a single Z. mays plant and contained the AMF inoculum. From the central, planted compartment, the AMF hyphae could access one outer, unplanted compartment (AMF) but not the other (nonAMF). The gas probe was placed within a mesh bag (the ‘organic matter patch’) which contained a mix of dried, milled Z. mays leaves and agricultural soil (c). The gas probe and organic matter patch designs were used in both experiments. PVDF, polyvinylidene difluoride.
Expt 2
Three compartment microcosm units were used (Fig. 1b). Each microcosm consisted of a central ‘planted’ compartment (volume, 2 l; dimensions, 150 × 150 × 150 mm; Thumbs Up Ltd, Bury, UK), containing a single Z. mays plant inoculated with R. irregularis, and on either side of the central planted compartment, two unplanted compartments, separated from the central compartment by a nylon mesh membrane as in Expt 1 (volume, 2.6 l; dimensions, 140 × 140 × 160 mm; Lock & Lock, Australia PTY Ltd, Blacktown, NSW, Australia). The mesh window either allowed AMF hyphal access (AMF; 20 μm mesh) or prevented AMF hyphal access (nonAMF; 0.45 μm mesh) from the central planted to the outer unplanted compartments. A supporting stainless steel mesh (0.25 mm aperture; Mesh Direct, Hanscan Ltd, Burslem, UK) was placed inside the plant compartment over the nylon meshes (0.45 and 20 μm) as a precautionary measure to protect the finer meshes from possible root damage. Thus, each unit had one AMF outer compartment and one nonAMF outer compartment, creating a paired design. The unplanted compartments were covered with a foil layer when the lids were not attached to prevent them from drying out.
Expts 1 and 2: growth media
In both experiments, the planted and unplanted compartments contained a mix (1 : 1 v/v) of sand and Agsorb® (Agsorb®; Oil‐Dri, Chicago, IL, USA; a calcined attapulgite clay soil conditioner) that had been rinsed thoroughly in deionized water to remove any excess soluble N and/or P. The planted compartments also had 50 g (Expt 1) or 90 g (Expt 2) of a fresh R. irregularis inoculum (Plantworks Ltd, Sittingbourne, Kent, UK) and 0.25 g l−1 bonemeal (a complex N and P source to encourage mycorrhizal development; 3.5% N, 8.7% P; Vitax, Coalville, Leicestershire, UK). Three pregerminated Z. mays seeds were added to each planted compartment for both experiments on 25 June 2012 and thinned to one per pot after 11 d (Expt 1) or 14 d (Expt 2). A sterile centrifuge tube (Expt 1, 15 cm3; Expt 2, 50 cm3) was added to each of the unplanted compartments to create a hole into which the organic matter patches and gas probes could be added at a later date (see ‘Organic matter patches and gas probes’ section).
Growth conditions
Microcosm units were placed in a randomized block design in a heated, lit glasshouse. The Experiments ran for 78 d between 25 June and 10 September (Expt 1), and 103 d between 25 June and 5 October 2012 (Expt 2). Photosynthetically active radiation (PAR) was measured weekly for both experiments at plant level in the centre of each block and averaged (± SEM) 141 ± 15 (Expt 1) and 251 ± 45 μmol m−2 s−1 (Expt 2). Overhead lights were used to extend the photoperiod to 16 h d−1 and the mean daily temperatures over the experimental period were 21.9 ± 0.02°C (Expt 1) and 21.5 ± 0.3°C (Expt 2). The planted and unplanted compartments for all microcosm units were watered daily as required. After 2 wk of plant growth, the planted compartments received 50 cm3 of a reduced N and P nutrient solution as described by Leigh et al. (2009) once a wk (Expts 1 and 2). This was increased to twice weekly at 49 d after planting in Expt 2 and to full N at 55 d after planting as the plants were starting to show symptoms of N deficiency. In Expt 2, at 76 d, the plants began to show P‐deficiency symptoms, so a 3/10 P, full N solution was used once a wk in addition to two 1/10 N and P additions. In total the plants received either 1.74 or 11.97 kg N ha−1 in Expts 1 and 2, respectively, over the duration of the experiments (11 and 14 wk, respectively).
Organic matter patches and gas probes
Organic matter patches
Organic matter patch material comprised 13 g DW equivalent agricultural soil (sandy loam; 53°92′N, −1°00′E, pH 6.6 in 0.01 M CaCl2 (following Allen, 1974)) mixed with 2 g DW milled Z. mays leaves, all enclosed in a 20 μm mesh bag (70 × 60 mm). The mean (± SEM) C and N contents of the mixed organic patches were 1435 ± 182 and 116 ± 15 mg (Expt 1) or 1200 ± 79 and 99 ± 15 mg (Expt 2), respectively, with a C : N ratio of 12 : 1 in both experiments. Each patch contained a gas probe (described in the next section) in the centre (Fig. 1c).
Gas probes
A stainless steel tube (9 cm long, outer diameter 1 cm, wall thickness 1 mm; Coopers Needle Works Ltd, Birmingham, UK) was welded at the base to form an air‐tight seal (Fig. 1c). Two diametrically opposed holes, of 6 mm, were drilled through each tube 13 mm from the base. These holes were covered in a polyvinylidene difluoride (PVDF) membrane (0.2 μm; Bio‐Rad) that was air‐permeable but impermeable to water. This fine PVDF membrane was then housed in a supporting silicone tube (wall thickness 0.8 mm, outer diameter 8 mm; Silex Ltd, Lindford, Bordon, Hampshire, UK) with access holes exposing the membrane covering the holes. The stainless steel tube was then sealed at the top with a white rubber Suba‐Seal® (no. 13; Sigma‐Aldrich) to form a gas sampling port. The total internal volume of the gas probe was c. 4.5 cm3.
A single organic matter patch and gas probe were placed into the preformed holes in the unplanted compartments 2 cm from the mesh window, 7 cm from the surface and covered with sand and Agsorb® media at 29 or 28 d (Expt 1 or 2, respectively) post‐planting.
Inorganic nitrogen addition
In Expt 1, half of the organic matter patches were injected with 7 cm3 of 30 mM NH4NO3 and the other half with 7 cm3 of deionized water (n = 6 in each case) at 44 d after patch addition. Consequently, the treatments were: AMF + NH4NO3, AMF + water, nonAMF + NH4NO3 and nonAMF + water. In Expt 2, at 62 d after organic patch addition (90 d after planting) each patch was injected with one of 7 cm3 of 15 mM (NH4)2SO4 (NH4 treatment), 30 mM KNO3 (NO3 treatment), 15 mM K2SO4 (K2SO4 treatment) or deionized water (water treatment), where the N treatments were equivalent to 0.196 mg N g−1 DW patch (n = 10 in each case). In both experiments, two 3.5 cm3 aliquots of solution were injected into each organic patch with a 1 h gap between each addition to reduce spread into the surrounding sand/Agsorb®.
Gas sampling and analysis
Expt 1
The air in the gas probes was sampled before N addition at 44 d after patch addition. The NH4NO3 and water addition treatments were then added and the gas probes were sampled again at 24, 48 and 96 h after NH4NO3 addition. Before sample removal, 1 cm3 of N2 was added to the probe via the Suba‐Seal, taking care not to disturb the surrounding media. This was left for 10 s before a 1 cm3 sample was slowly removed from the gas probe, waiting for a further 5 s to allow the sample to mix inside the syringes before removing the syringe. Each gas sample was then stored in a prefilled 3 cm3 Exetainer (Labco Ltd, Lampeter, Ceredigion, UK) (with 6 cm3 N2), overpressuring the sample to 7 cm3 in total. All gas samples were analysed using a gas chromatograph (GC) which quantified the concentration of N2O. The concentration (ppm) values for each sample were calculated by comparing with certified standards that were diluted in parallel in a 1 cm3 standard: 6 cm3 N2 ratio and correcting for this dilution. The concentration values were also corrected for dilution from addition of N2 to the gas probe just before gas sample removal.
Expt 2
Gas sampling was carried out using both gas probes (as described for Expt 1) and continuous flow loop sampling with an attached Los Gatos Isotopic N2O analyser (LGR N2O; Los Gatos Research Inc., San Jose, CA, USA) which provided an N2O concentration once per second. A gas‐tight lid (Fig. 1b) was attached to each of the 80 unplanted compartments in block sequence for a minimum of 5 min, with a minimum of 2 min flushing the system with air between each compartment measurement. Gas sampling using both methods was carried out before N addition (58–59 and 61 d after patch addition), and at 48, 96 and 192 h after N addition (64, 66 and 70 d after patch addition, respectively).
When using the LGR N2O analyser, the headspace in the microcosm unit (0.6 l), volume of connecting tubing (0.274 l) and internal volume of the N2O analyser (0.850 l) along with the surface area of the soil sampled (0.024 m−2) were used in the regression calculation of the N2O flux rate in mg m−2 h−1. These fluxes were calculated using values measured between 200 and 280 s after the cover‐box lid was attached. All regressions were calculated using Sas v.9.3 (SAS institute Inc., Cary, NC, USA).
Post‐harvest analyses
At harvest, above‐ground material was removed at the soil surface and separated into stalk, flowers, ear, and leaf material. Roots were extracted from the sand/Agsorb® media and washed, and FW and DW of all plant material were recorded. In Expt 1, the dried leaves (green leaves only, defined as > 50% green) were milled and analysed for C and N content using an elemental combustion system (Costech Analytical Technologies Inc., Valencia, CA, USA). The gravimetric water content (g g−1 DW) of soil, sand/Agsorb® and patches for each compartment were measured and the AMF extraradical mycorrhizal hyphae (ERM) were extracted from two 5 g (FW) samples from the organic patches and the surrounding growth medium in the unplanted compartments using a modified membrane filter technique (see Staddon et al., 1999) and acid fuchsin stain. Hyphal lengths were assessed using the gridline intercept method (Miller & Jastrow, 1992) for a minimum of 50 fields of view at ×125 magnification (using a square grid of 1 cm side length split into 10 × 10 grid sections; Graticules Ltd, Tonbridge, Kent, UK). These hyphal lengths were then converted to ERM length densities (m hyphae g−1 soil DW).
Data analysis
Data were first tested for normality and equality of variance using Kolmogorov–Smirnov and Levene's equality of variance tests, respectively. Statistical analyses were carried out in either Sas v.9.3 or Genstat v.16 (VSN International Ltd, Hemel Hempstead, UK). The pre‐N addition fluxes or concentrations were subtracted from the post‐N addition fluxes or concentrations, respectively, to obtain the change in N2O flux or concentration following N addition (referred to ∆N2O).
In Expt 1, where N2O concentration and ERM length density data did not fulfil normality or equality of variance assumptions, they were log10‐transformed. All gas concentration, plant and AMF data were analysed using a two‐way ANOVA, including block, with Duncan's post hoc tests. However, transformations on changes in N2O concentration following N addition failed to normalize the data, and nonparametric equivalent Friedman's two‐way ANOVAs, including block, with Wilcoxon post hoc tests were used. Where N2O concentrations were measured over time, repeated‐measures ANOVA, including treatment and block, was used on log10‐transformed data. Pearson's product‐moment correlations were used to determine the relationship between variables. Where variables were not normally distributed, Spearman's rank order correlations were used. Untransformed data are presented in all figures.
In Expt 2, differences among treatments were analysed using a two‐way ANOVA including block with Duncan's post hoc tests. ERM length density data were log10‐transformed before analysis. Where the data failed normality or equality of variance assumptions, nonparametric tests were used. A one‐sample t‐test or a Wilcoxon signed‐rank test was used to compare absolute values or differences to zero.
In Expt 2, the ∆N2O data were not normally distributed and therefore a Friedman's nonparametric two‐way ANOVA, controlling for block, with Mann–Whitney U‐test (unpaired data) or Wilcoxon signed rank (paired data) post hoc test and an applied false discovery rate correction were used. Where comparisons in ∆N2O flux or ∆N2O concentration data were made over time, a nonparametric Friedman's repeated‐measures analysis was used. The relationships between the ∆N2O flux and ∆N2O concentration for each gas sample following N addition (48, 96 and 192 h post‐N addition) were determined using a Spearman rank order correlation.
There was hyphal breakthrough in one of the nonAMF compartments (treatment: nonAMF, K2SO4) and therefore this microcosm was excluded from the subsequent data analyses. In addition, the N2O concentration for one experimental unit in the AMF treatment (treatment: (NH4)2SO4) was out of range on the GC for the sample taken 48 h post‐N addition and therefore these AMF and nonAMF N2O concentration values were also omitted.
Results
In the AMF treatments, R. irregularis colonized the organic matter patches successfully in both experiments, with ERM length densities of 1.23 ± 0.25 m g−1 DW in Expt 1 (nonAMF, 0.31 ± 0.05 m g−1 DW; F 1,12 = 30.77, P = 0.0001) and 0.88 ± 0.08 m g−1 in Expt 2 (nonAMF, 0.35 ± 0.04 m g−1 DW; t 39 = 8.993, P < 0.0001).
Pre‐N addition N2O production
Before inorganic N addition there was a greater concentration of N2O in the nonAMF patches than in the AMF patches in both experiments (Fig. 2; Expt 1, F 1,12 = 6.46, P = 0.026; Expt 2, S 38 = −186, P = 0.0076). A similar trend (at the P < 0.1 level) was found for the N2O fluxes in Expt 2, with greater N2O fluxes measured from the nonAMF compartments than from the AMF compartments (S 38 = −128, P = 0.074). In Expt 2, N2O fluxes measured by continuous flow loop sampling were positively correlated with the patch N2O concentrations measured using gas probes (r s = 0.7495, P < 0.0001). As N2O production is inherently variable, this degree of consistency both between and within experiments is striking, particularly because it was observed in the absence of any additional applied inorganic N. In both experiments, there was no significant correlation between the pre‐N addition AMF treatment N2O concentration or fluxes and the ERM lengths (P > 0.05 in each case).
Figure 2.
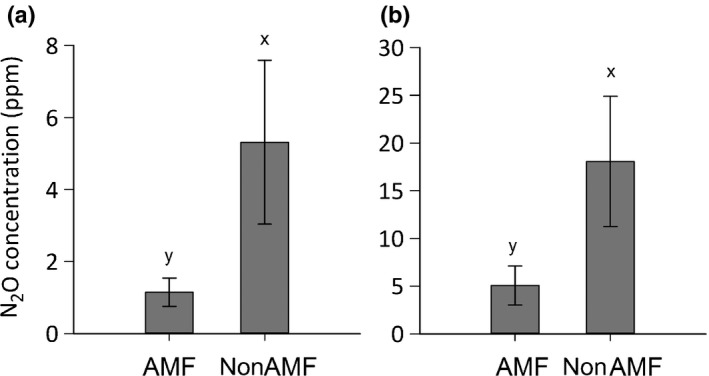
Mean N2O concentration (ppm) in arbuscular mycorrhizal fungal access (AMF) and no AMF access (nonAMF) organic matter patches at 43 d after patch addition in Expt 1 (a) and at 58 d after patch addition in Expt 2 (b). Error bars are ± SEM (a, n = 12; b, n = 39). Different letters represent significant differences at P < 0.05 as determined using: (a) two‐way ANOVAs; and (b) by comparing the ∆AMF value with zero (Wilcoxon signed‐rank test).
Post‐N addition and harvest
Expt 1
In Expt 1 the highest patch N2O concentrations were observed 24 h after the application of inorganic N or water in all treatments except AMF + water, demonstrating the rapid response of N2O producers to treatment application. The patch N2O concentrations of the nonAMF and AMF + NH4NO3 treatments subsequently decreased over time. By contrast, the AMF + water patch N2O concentration remained low. Consequently, there was a significant effect of both time and treatment on patch N2O concentration in addition to a significant interaction between these two factors (Fig. 3; time, F 2,30 = 4.37, P = 0.023; treatment, F 3,15 = 5.67, P = 0.0084; time × treatment, F 6,30 = 3.23, P = 0.015). These results therefore demonstrate how rapidly N2O production rates can change over time and emphasize the requirement for repeated measurements following inorganic N application. Two‐way ANOVAs at each time point showed that the N2O concentration of the AMF + water treatment was lower than all other treatments at 24 h post‐treatment application (Fig. 3; F 3,15 = 4.44, P = 0.020). This effect decreased by the 48 h sample, although the nonAMF + water and nonAMF + NH4NO3 treatments still had a higher N2O concentration than that of the AMF + water treatment (F 3,15 = 4.95, P = 0.014). At 96 h post‐treatment application, the AMF patch N2O concentrations were not significantly different from each other but were significantly lower than those of the nonAMF patches (F 3,15 = 7.25, P = 0.0031). At 24 h post‐treatment application, the ∆N2O concentration was higher in both the AMF + NH4NO3 and nonAMF + NH4NO3 treatments than in the AMF + water treatment (Q 3 = 8.2, P = 0.042). However, the nonAMF + water treatment was not significantly different from the AMF + NH4NO3 treatment or nonAMF + NH4NO3 treatment.
Figure 3.
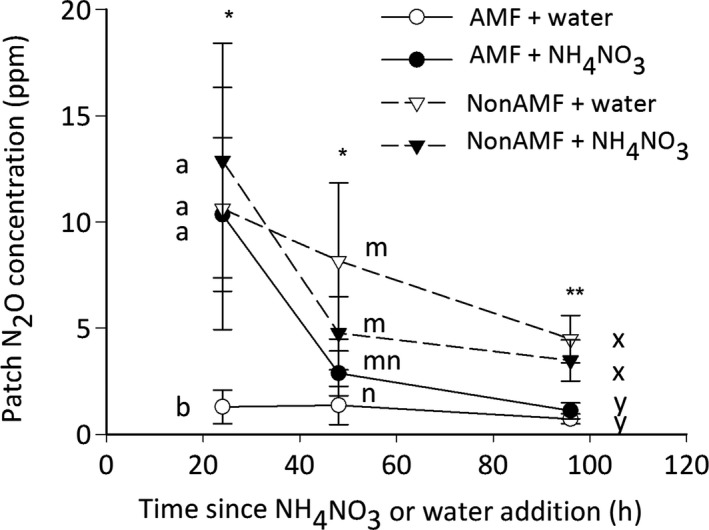
Mean patch nitrous oxide (N2O) concentration at 24, 48 and 96 h after addition of inorganic nitrogen (N) (NH 4 NO 3: closed symbols) or water (open symbols) for arbuscular mycorrhizal fungal access patches (AMF; solid lines) and no AMF access patches (nonAMF; dashed lines) shown over time. Error bars are ± SEM (n = 6). Asterisks represent a significant difference among treatments within each sample period (*, P < 0.05; **, P < 0.01) as determined using a two‐way ANOVA. Different letters within each sample timing represent significant differences between treatments for that sample timing (P < 0.05).
There was no relationship between the AMF ERM length densities and N2O concentration in the AMF patches at any point (P > 0.05 in each case) and the moisture contents of the organic patches did not differ among treatments at harvest (Q3 = 0.707, P = 0.871). Additionally, there was no significant difference (P > 0.05) in total plant DW or the DW of the various plant tissues (i.e. leaf, total shoot, stalk, total root, root weight ratio, tassel) between the AMF and nonAMF treatments (see Supporting Information Table S1). The addition of NH4NO3 or water had no effect on the leaf C and N content or concentrations or on the C : N ratios (P > 0.05 in each case), and therefore these data were combined for comparison of the AMF with the nonAMF treatments. Leaf C content did not differ between AMF and nonAMF plants (Table 1; F 1,12 = 0.30, P = 0.595), although the leaf C concentrations were lower in the AMF than in the nonAMF treatments (Table 1; F 1,12 = 5.37, P = 0.039). Both the N content (Table 1; F 1,12 = 14,18, P = 0.0023) and concentration (F 1,12 = 20.06, P = 0. 0008) of the leaves were higher in the AMF than in the nonAMF treatments. Consequently, the C : N ratio of the leaves was lower in the AMF than in the nonAMF treatments (Table 1; F 1,12 = 18.51, P = 0.001). However, the organic patch N2O concentration was not significantly related to the leaf C or N content or concentration, or to the leaf C : N ratio, either before or after N addition, for both the AMF and nonAMF treatments (P > 0.05 in each case).
Table 1.
Mean (± SEM) leaf nitrogen (N) and carbon (C) total content and concentration, and C : N ratio of Zea mays leaves from arbuscular mycorrhizal fungi (AMF) and nonAMF treatments in Expt 1 (n = 12)
| AMF | NonAMF | ||
|---|---|---|---|
| Leaf N | Total content (mg) | 13.8 ± 0.8 a | 10.2 ± 0.9 b |
| Concentration (mg g−1 DW) | 11.3 ± 0.6 f | 8.8 ± 0.5 g | |
| Leaf C | Total content (mg) | 503.2 ± 19.9 j | 488.1 ± 27.2 j |
| Concentration (mg g−1 DW) | 413.4 ± 2.8 m | 422.9 ± 3.7 n | |
| Leaf C : N ratio | 37.6 ± 2.0 x | 50.0 ± 3.0 y |
Different letters within rows represent significant differences at P = 0.05 (in bold) as determined using two‐way ANOVAs.
Expt 2
There was a significant difference in ∆N2O fluxes between the inorganic N and water application treatments at 48 h post‐application (Fig. 4; Q 7 = 44.85, P < 0.0001). In both the AMF and nonAMF patches, more N2O was produced following addition of NH4 + than for any other treatment. Strikingly, however, c. 2.5 times more N2O was produced from the nonAMF than from the AMF treatment (Fig. 4; S 9 = −26.5, P = 0.0084). These differences then declined by the 96 h sample and were no longer significant at the 192 h sample, again illustrating the transient nature of N2O release and the importance of following the fluxes over discrete timescales (Table 2). There was no significant difference in the % moisture content of either the patch or sand/Agsorb® medium between the AMF and nonAMF treatments at destructive harvest (patch, t 39 = −0.26, P = 0.799; sand/Agsorb®, S 39 = −47, P = 0.519).
Figure 4.
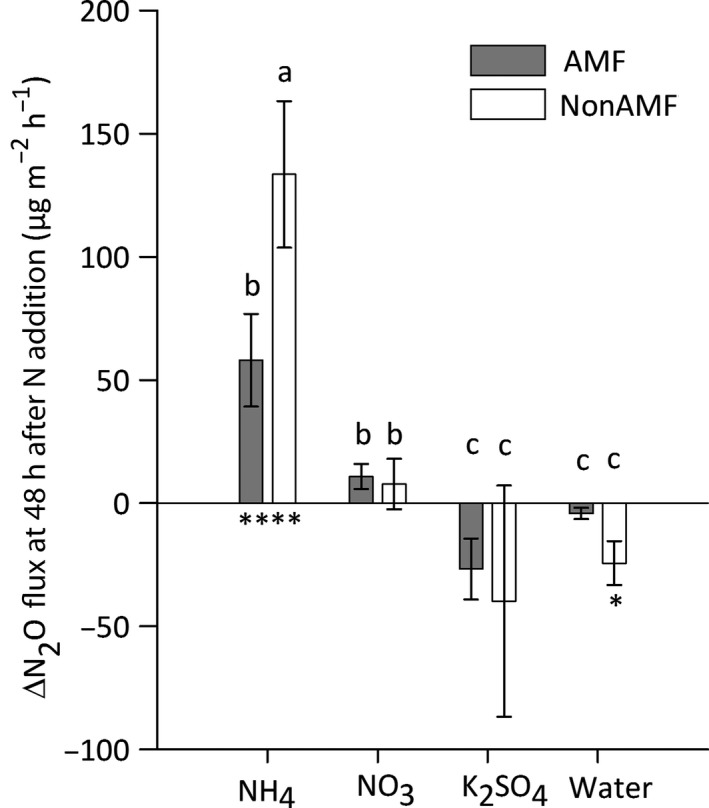
Mean difference between 48 h post‐nitrogen (N) addition (64 d after patch addition) and pre‐N addition (61 d after patch addition) nitrous oxide (N2O) fluxes (∆N2O flux) for arbuscular mycorrhizal fungal access (AMF; closed bars) and no AMF access (nonAMF; open bars) treatments, split by N‐addition treatment. The N‐addition treatments were (NH 4)2 SO 4 (labelled as NH 4), KNO 3 (labelled as NO 3), K2 SO 4 or water. Bars with different letters are significant at P = 0.0018 as determined by Mann–Whitney U or Wilcoxon signed‐rank post hoc tests with a false discovery rate correction applied. Asterisks below the bars indicate significant differences from zero (*, P < 0.05; **, P < 0.01). Error bars are ± SEM (n = 10).
Table 2.
Expt 2: Friedman's test statistics controlling for block comparing the post‐nitrogen (N) minus pre‐N (61 d post‐patch addition) patch nitrous oxide (N2O) concentrations (∆N2O concentrations) or compartment N2O fluxes (∆N2O fluxes) among N‐addition treatments, for each of the gas sampling events
| Time since N addition | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| 48 h | 96 h | 192 h | |||||||
| Q | d.f. | P | Q | d.f. | P | Q | d.f. | P | |
| Patch ∆N2O concentration | 28.89 | 7 | 0.0002 *** | 14.35 | 7 | 0.045 * | 3.79 | 7 | 0.804 |
| Compartment ∆N2O flux | 44.85 | 7 | < 0.0001 *** | 25.63 | 7 | 0.0006 *** | 4.80 | 7 | 0.684 |
Q, Friedman's test statistic; d.f., degrees of freedom; n = 10. Significant results are indicated in bold at P = 0.05 (*, P < 0.05; ***, P < 0.001).
Discussion
This is the first study to show that N2O production is reduced as a direct consequence of the presence of AMF hyphae. Moreover, this reduction was demonstrated in both the presence and, notably, the absence of applied inorganic N, indicating that this is a persistent effect. Studies to date have indicated that AMF may influence soil N2O production, but this has always been in the presence of plant roots and additional inorganic N (Lazcano et al., 2014; Bender et al., 2015). Critically, the finding that N2O production was reduced when AMF hyphae, but not plant roots, were present was consistent between the two independent experiments reported here.
Previous studies have applied inorganic N and assessed the N2O flux from the mycorrhizosphere (i.e. the soil influenced by AM colonized roots and AMF hyphae), often only at a single time point after N application, potentially masking cumulative effects (Bender et al., 2015). AMF hyphae can extend far beyond the plant roots alone, with the ERM being 10 times larger, in biomass terms, than the intraradical mycelium (Olsson et al., 1999). Thus, the influence of AMF hyphae on soils (in the ‘hyphosphere’) will extend beyond the zone of influence of roots alone, and studies to date have not explored this widespread zone of hyphal influence on N2O production in isolation. Furthermore, as the mycorrhizosphere includes both AMF‐colonized plant roots and AMF hyphae, it is impossible to know whether any effect is a consequence of the AMF hyphae or roots, or both. Rhizodeposition differs between AM and nonAM plants (Jones et al., 2004), while C exudation from AMF hyphae may also result in quantitative and qualitative changes in the total C flux into the soil (Toljander et al., 2007). Moreover, AMF hyphae influence N cycling through the capture of N and subsequent transfer of at least some of this N to their associated host plant (Leigh et al., 2009; Thirkell et al., 2016). C and N are key controls of denitrification and nitrification rates (Bollmann & Conrad, 1998; Hino et al., 2010). It is not possible, therefore, to separate AMF and root control of N2O fluxes in the mycorrhizosphere without first separating the AMF hyphae from the plant roots.
Nevertheless, there is some evidence of AMF interacting with soil N2O production in the mycorrhizosphere, although results have been inconsistent. Bender et al. (2015) found that the N2O flux was lower following the application of NO3 − in the AM mycorrhizosphere when compared with the rhizosphere of a nonAM control. By contrast, Cavagnaro et al. (2012) found no effect of AM plants on N2O production, whereas Lazcano et al. (2014) found a reduction in N2O in the mycorrhizosphere of AM plants. Thus, there is support for AMF resulting in reduced N2O production in the mycorrhizosphere, but the cause of this reduction has so far been poorly understood, probably because of confounding effects of the host plant root system also being present. Hypotheses for the decreased N2O production in the mycorrhizosphere included a reduction in denitrification (Bender et al., 2015) and increased water use by AM plants (Lazcano et al., 2014).
In this study, the finding of reduced N2O production in the presence of AMF hyphae was evident even before inorganic N application. There was also evidence for an increase in both leaf N content and concentration when the AMF had access to the organic matter patches. This suggests that the AMF were supplying their host plant with additional N, presumably from the organic matter patch, as all planted compartments received the same quantity of nutrient solution. Whilst there is a wide range in reported contribution of AMF to plant N (reviewed by Hodge & Storer, 2015), the findings in this study are in agreement with previous investigations using 15N that substantial quantities of N can be transferred from the patch to the plant via AMF hyphae (Leigh et al., 2009; Thirkell et al., 2016).
The inorganic N applications here were used as a tool to identify the pathway of N2O production being influenced by the AMF hyphae. The addition of NO3 − did not result in increased N2O production from any treatment, suggesting that in this study, denitrification was not a key factor in controlling N2O production. There was also no significant difference in gravimetric water content of the organic matter patches, or the surrounding sand/Agsorb® medium at harvest. Thus, these factors were not important controls of N2O production in the present study. Instead, we found direct evidence for a reduction in N2O produced via nitrification in the presence of AMF hyphae. This is a critical finding and may help to explain variable N2O fluxes under field conditions. As agricultural soils are one of the largest sources of N2O, it is highly relevant that the soil used here was agricultural in origin, and the plant material for the organic matter patches was Z. mays, a globally important crop (Leff et al., 2004).
The soil N2O fluxes in this study were predominantly controlled by the availability of NH4 +. These fluxes were monitored at intervals up to 192 h after inorganic N application, by which point the N2O peak declined back to pre‐N application values, thus ensuring that the full response period was recorded. There was a significantly greater N2O flux in response to NH4 + addition in the nonAMF than in the AMF treatment, indicating reduced N2O production via nitrification in the presence of AMF hyphae. The current understanding of the main pathways of N2O production in soils (as described in Baggs, 2011; Zhu et al., 2013) are shown in Fig. 5 together with the potential mechanisms by which AMF may interact with N2O production. If NH4 + elicits N2O production but NO3 − application does not, by process of elimination the pathway involved in N2O production must be a nitrification pathway.
Figure 5.
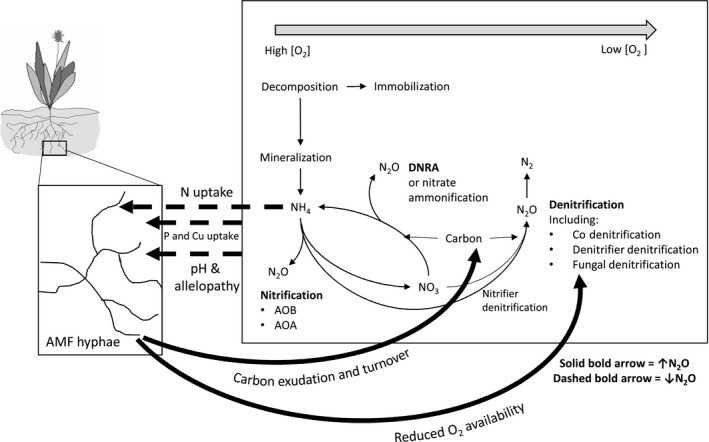
Summarized potential interactions between arbuscular mycorrhizal fungal (AMF) hyphae and soil nitrous oxide (N2O)‐producing processes as described in Baggs (2011) and Zhu et al. (2013). The solid and dashed bold lines represent AMF effects that could result in an increase and decrease in N2O production, respectively. AMF can affect the availability of nitrogen (N), phosphorus (P), copper (Cu) and iron (Fe) in soils, as well as potentially changing soil pH. Nitrifier nitrification is generally carried out by ammonia‐oxidizing bacteria (AOB) and archaea (AOA). Dissimilatory reduction of nitrate to ammonium (DNRA) may produce N2O as a side product. DNRA is also known as nitrate ammonification. There are various pathways and organisms capable of carrying out these roles, but, for simplicity, they are grouped by factors affecting the rate of N2O production (i.e. availability of O2, or carbon).
The links between AMF presence and reduced nitrification rates are in broad agreement with a series of one field‐based and three mesocosm‐based studies by Veresoglou et al. (2011). The potential nitrification rates were lower in the mycorrhizospheres of AM plants than in those from weakly AM mycorrhizospheres (Veresoglou et al., 2011). The nirK gene, responsible for N2O production has also been shown to be negatively correlated with AMF abundance (Bender et al., 2014). Thus, the presence of AM plants may reduce N2O production by reducing nitrification rates. Our present study demonstrates, for the first time, that AMF hyphae have a direct and limiting influence on soil N2O produced via nitrification, independent of any plant root influence.
The main ‘nitrification’ pathways in soil potentially resulting in N2O release are nitrifier nitrification, and nitrifier denitrification. Nitrifier nitrification is an aerobic process and can be carried out by ammonia‐oxidizing bacteria (AOB), archaea (AOA), and organisms capable of complete ammonia oxidation (comammox) (Daims et al., 2015; van Kessel et al., 2015). AOB and AOA have also been shown to produce N2O (Jiang & Bakken, 1999; Jung et al., 2014). Nitrifier denitrification is also carried out by autotrophic nitrifiers, and can be a significant source of N2O (Wrage et al., 2001; Kool et al., 2011b). Thus, there are various pathways by which the N2O in this study may have been produced following the application of NH4 + and consequently reduced by the presence of AMF hyphae (Fig. 5).
Regardless of the process, the response to NH4 + application in the AMF treatments suggests that there was either a reduction in N2O production, through reduced function or number of nitrifiers, or that nitrifier activity was masked by an increase in activity of N2O reducers, which can cause some soils to become N2O sinks (Domeignoz‐Horta et al., 2017). It is also feasible that the presence of AMF hyphae modified the microbial community, shifting it away from N2O‐producing nitrifiers or nitrifier denitrifiers, perhaps towards organisms capable of complete nitrification (van Kessel et al., 2015) or N2O reduction (Sanford et al., 2012; Jones et al., 2014; Domeignoz‐Horta et al., 2017).
Domeignoz‐Horta et al. (2017) found that N2O hotspots were predominantly controlled by changes in the microbial communities, whereas lower N2O‐producing areas were more likely to be controlled by variation in soil properties. Using similar organic patches as in the present study, Nuccio et al. (2013) found that while there was no overall change in bacterial diversity, the presence of AMF hyphae significantly modified the bacterial community. Interestingly, Gemmatimonadetes and Deltaproteobacteria were two of four bacterial phylum that had a higher relative abundance in response to the presence of AMF hyphae in the litter (Nuccio et al., 2013). Both the Gemmatimonadetes and Deltaproteobacteria have subsequently been found to posses nosZ genes, and can thus utilize exogenous N2O as an electron acceptor (Jones et al., 2013; Park et al., 2017). AMF abundance has also been found to positively correlate with nosZ gene abundance (Bender et al., 2014). This, together with the large export of N from the patch by the AMF hyphae and the resulting modifications in the physicochemical environment in the decomposing litter patch, may contribute to a reduction in N2O emissions.
Given the evidence that AMF are known to have a high N demand (Hodge & Fitter, 2010), one hypothesis could be that AMF hyphae were eliciting a longer‐term control on the nitrifying community, as nitrifiers are inherently slow‐growing, taking from 8 h up to a number of days to double in number (Belser & Schmidt, 1980; Woldendorp & Laanbroek, 1989; Prosser, 2007; Prosser & Nicol, 2012). AMF hyphae are thought predominantly to take up inorganic N in the form of NH4 + (Govindarajulu et al., 2005; Tanaka & Yano, 2005), and AOB are generally thought to be poor competitors for NH4 + (Verhagen et al., 1995; Bollmann et al., 2002). The AMF hyphae may therefore have reduced the amount of available NH4 + in the hyphosphere, resulting in a reduction in the population of active AOB. If AOB were the main N2O producers, this may explain the reduced N2O production before inorganic N application when the AMF hyphae were present. It may also explain the lack of N2O production in the presence of AMF hyphae when NH4 + was applied, i.e. the AOB population may have been small and too slow‐growing to respond to the inorganic NH4 + supplied, which may have, instead, been taken up by the N‐rich AMF hyphae.
While AMF may increase or decrease the pH of surrounding media, thought to be a consequence of NO3 − or NH4 + uptake, respectively (Li et al., 1991; Bago et al., 1996), the relative importance of pH effects on N2O production if C, NH4 + or NO3 − are limiting is not clear (reviewed by Šimek & Cooper, 2002), with both increased and decreased nitrification‐derived N2O production reported under low‐pH conditions (Mørkved et al., 2007; Cheng et al., 2013). The patch pH was not measured in this study, and potential changes in pH cannot be fully discounted. However, the implications of N, and more importantly the form of N, exported by AMF on the local physicochemical properties, including pH, warrant further attention. This may also aid in explaining the differing impacts reported for AMF on decomposition processes, and their importance not only for N, but also for C cycling and stabilization processes (Hodge, 2001; Hodge et al., 2001; Cheng et al., 2012).
In order to fully understand the mechanism for the reduction in N2O production via nitrification observed in the presence of AMF hyphae found in this study, further research should focus upon gene expression and the responses of the microbial community, including nitrifier communities, AOA, AOB and potential nondenitrifying N2O reducers. Monitoring would also help to establish if nitrifier populations were suppressed by the presence of AMF hyphae, as we suggest. Furthermore, field‐based studies using a wider range of soil types and environmental conditions are an essential next step to determine the global scale and significance of this interaction in both natural and agricultural systems.
In conclusion, using two independent glasshouse‐based experiments, we have found that the presence of AMF hyphae reduced the production of the globally important greenhouse gas, N2O. Cropped agricultural soils cover a significant proportion of land area, representing 28.4% of agricultural land, or 10.9% of the total global land area in 2011 (FAO, 2017). The diversity of AMF is reduced in agricultural soils (Helgason et al., 1998), and these soils are one of the largest contributors to N2O emissions. This study suggests that a reduction in the presence of AMF may contribute to further increases in N2O production. This could have significant implications for better management of agricultural soils in the future. Given the ubiquity of the AM association, including under agricultural situtations, these findings have global implications not only for our fundamental understanding of the mechanisms of soil N cycling, but also for greenhouse gas management and climate change mitigation.
Author contributions
K.S., P.I. and A.H. designed the research; K.S. performed the research and conducted all data analysis; A.C. performed practical work for Expt 1; and K.S. and A.H. wrote the manuscript.
Data accessibility
Data created during this research are available by request from the University of York Data Catalogue. https://doi.org/10.15124/67decab3-9ea6-4cde-812e-3c762eba2ec6.
Supporting information
Please note: Wiley Blackwell are not responsible for the content or functionality of any Supporting Information supplied by the authors. Any queries (other than missing material) should be directed to the New Phytologist Central Office.
Table S1 Mean plant biomass parameters from AMF and nonAMF treatments in Expt 1
Acknowledgements
K.S. was supported by a PhD studentship funded by the Biotechnology and Biological Sciences Research Council, UK (grant BB/GO16801/1), and A.C.'s student placement was supported by the British Mycological Society.
References
- Allen SE. 1974. Chemical analysis of ecological materials. Oxford, UK: Blackwell Scientific Publications. [Google Scholar]
- Asghari HR, Cavagnaro TR. 2012. Arbuscular mycorrhizas reduce nitrogen loss via leaching. PLoS ONE 7: e29825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baggs EM. 2008. A review of stable isotope techniques for N2O source partitioning in soils, recent progress, remaining challenges and future considerations. Rapid Communications in Mass Spectrometry 22: 1664–1672. [DOI] [PubMed] [Google Scholar]
- Baggs EM. 2011. Soil microbial sources of nitrous oxide, recent advances in knowledge, emerging challenges and future direction. Current Opinion in Environmental Sustainability 3: 321–327. [Google Scholar]
- Bago B, Vierheilig H, Piché Y, Azcón‐Aguilar C. 1996. Nitrate depletion and pH changes induced by the extraradical mycelium of the arbuscular mycorrhizal fungus Glomus intraradices grown in monoxenic culture. New Phytologist 133: 273–280. [DOI] [PubMed] [Google Scholar]
- Barrett G, Campbell CD, Fitter AH, Hodge A. 2011. The arbuscular mycorrhizal fungus Glomus hoi can capture and transfer nitrogen from organic patches to its associated host plant at low temperature. Applied Soil Ecology 48: 102–105. [Google Scholar]
- Barrett G, Campbell CD, Hodge A. 2014. The direct response of the external mycelium of arbuscular mycorrhizal fungi to temperature and the implications for nutrient transfer. Soil Biology & Biochemistry 78: 109–117. [Google Scholar]
- Belser LW, Schmidt EL. 1980. Growth and oxidation kinetics of three genera of ammonia oxidising nitrifiers. FEMS Microbiology Letters 7: 213–216. [Google Scholar]
- Bender SF, Conen F, van der Heijden MGA. 2015. Mycorrhizal effects on nutrient cycling, nutrient leaching and N2O production in experimental grassland. Soil Biology and Biochemistry 80: 283–292. [Google Scholar]
- Bender SF, Plantenga F, Neftel A, Jocher M, Oberholzer HR, Köhl L, Giles M, Daniell TJ, van der Heijden MGA. 2014. Symbiotic relationships between soil fungi and plants reduce N2O emissions from soil. ISME Journal 8: 1336–1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bollmann A, Bär‐Gilissen MJ, Laanbroek HJ. 2002. Growth at low ammonium concentrations and starvation response as potential factors involved in niche differentiation among ammonia‐oxidizing bacteria. Applied and Environmental Microbiology 68: 4751–4757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bollmann A, Conrad R. 1998. Influence of O2 availability on NO and N2O release by nitrification and denitrification in soils. Global Change Biology 4: 387–396. [Google Scholar]
- Cavagnaro TR, Barrios‐Masias FH, Jackson LE. 2012. Arbuscular mycorrhizas and their role in plant growth, nitrogen interception and soil gas efflux in an organic production system. Plant and Soil 353: 181–194. [Google Scholar]
- Cavagnaro TR, Bender SF, Asghari HR, van der Heijden MGA. 2015. The role of arbuscular mycorrhizas in reducing soil nutrient loss. Trends in Plant Science 20: 283–290. [DOI] [PubMed] [Google Scholar]
- Cheng L, Booker FL, Tu C, Burkey KO, Zhou L, Shaw HD, Rufty TW, Hu S. 2012. Arbuscular mycorrhizal fungi increase organic carbon decomposition under elevated CO2 . Science 337: 1084–1087. [DOI] [PubMed] [Google Scholar]
- Cheng Y, Wang J, Mary B, Zhang JB, Cai ZC, Chang SX. 2013. Soil pH has contrasting effects on gross and net nitrogen mineralizations in adjacent forest and grassland soils in central Alberta, Canada. Soil Biology and Biochemistry 57: 848–857. [Google Scholar]
- Cowan NJ, Norman P, Famulari D, Levy PE, Reay DS, Skiba UM. 2015. Spatial variability and hotspots of soil N2O fluxes from intensively grazed grassland. Biogeosciences 12: 1585–1596. [Google Scholar]
- Daims H, Lebedeva EV, Pjevac P, Han P, Herbold C, Albertsen M, Jehmlich N, Palatinszky M, Vierheilig J, Bulaev A et al 2015. Complete nitrification by Nitrospira bacteria. Nature 528: 504–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domeignoz‐Horta LA, Philippot L, Peyrard C, Bru D, Breuil MC, Bizouard F, Justes E, Mary B, Léonard J, Spor A. 2017. Peaks of in situ N2O emissions are influenced by N2O –producing and reducing microbial communities across arable soils. Global Change Biology. doi: 10.1111/gcb.13853. [DOI] [PubMed] [Google Scholar]
- FAO . 2017. FAOSTAT Agri‐environmental indicators: land. Accessed January 2017.
- Govindarajulu M, Pfeffer PE, Jin HR, Abubaker J, Douds DD, Allen JW, Bücking H, Lammers PJ, Shachar‐Hill Y. 2005. Nitrogen transfer in the arbuscular mycorrhizal symbiosis. Nature 435: 819–823. [DOI] [PubMed] [Google Scholar]
- Hartmann DL, Klein Tank AMG, Rusticucci M, Alexander LV, Brönnimann S, Charabi Y, Dentener FJ, Dlugokencky EJ, Easterling DR, Kaplan A et al 2013. Observations: atmosphere and surface In: Stocker TF, Qin D, Plattner G‐K, Tignor M, Allen SK, Boschung J, Nauels A, Xia Y, Bex V, Midgley PM, eds. Climate change 2013: the physical science basis. Contribution of working group i to the fifth assessment report of the intergovernmental panel on climate change. Cambridge, UK and NY, USA: Cambridge University Press. [Google Scholar]
- Helgason T, Daniell TJ, Husband R, Fitter AH, Young JPW. 1998. Ploughing up the wood‐wide web? Nature 394: 431. [DOI] [PubMed] [Google Scholar]
- Herman DJ, Firestone MK, Nuccio E, Hodge A. 2012. Interactions between an arbuscular mycorrhizal fungus and a soil microbial community mediating litter decomposition. FEMS Microbiology Ecology 80: 236–247. [DOI] [PubMed] [Google Scholar]
- Hino T, Matsumoto Y, Nagano S, Sugimoto H, Fukumori Y, Murata T, Iwata S, Shiro Y. 2010. Structural basis of biological N2O generation by bacterial nitric oxide reductase. Science 330: 1666–1670. [DOI] [PubMed] [Google Scholar]
- Hodge A. 2001. Arbuscular mycorrhizal fungi influence decomposition of, but not plant nutrient capture from, glycine patches in soil. New Phytologist 151: 725–734. [DOI] [PubMed] [Google Scholar]
- Hodge A. 2014. Interactions between arbuscular mycorrhizal fungi and organic material substrates. Advances in Applied Microbiology, 89: 47–99. [DOI] [PubMed] [Google Scholar]
- Hodge A, Campbell CD, Fitter AH. 2001. An arbuscular mycorrhizal fungus accelerates decomposition and acquires nitrogen directly from organic material. Nature 413: 297–299. [DOI] [PubMed] [Google Scholar]
- Hodge A, Fitter AH. 2010. Substantial nitrogen acquisition by arbuscular mycorrhizal fungi from organic material has implications for N cycling. Proceedings of the National Academy of Sciences, USA 107: 13754–13759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodge A, Storer K. 2015. Arbuscular mycorrhiza and nitrogen, implications for individual plants through to ecosystems. Plant and Soil 386: 1–19. [Google Scholar]
- Jiang Q‐Q, Bakken LR. 1999. Nitrous oxide production and methane oxidation by different ammonia‐oxidising bacteria. Applied and Environmental Microbiology 65: 2879–2884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones CM, Graf DRH, Bru D, Philippot L, Hallin S. 2013. The unaccounted yet abundant nitrous oxide‐reducing microbial community: a potential nitrous oxide sink. ISME Journal 7: 417–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones DL, Hodge A, Kuzyakov Y. 2004. Plant and mycorrhizal regulation of rhizodeposition. New Phytologist 163: 459–480. [DOI] [PubMed] [Google Scholar]
- Jones CM, Spor A, Brennan FP, Breuil M‐C, Bru D, Lemanceau P, Griffiths B, Hallin S, Philippot L. 2014. Recently identified microbial guild mediates soil N2O sink capacity. Nature Climate Change 4: 801–805. [Google Scholar]
- Jung M‐Y, Well R, Min D, Giesemann A, Park S‐J, Kim J‐G, Rhee S‐K. 2014. Isotopic signatures of N2O produced by ammonia oxidising archaea from soils. ISME Journal 8: 1115–1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Kessel MAHJ, Speth DR, Albertsen M, Nielsen PH, Op den Camp HJM, Kartal B, Jetten MSM, Lücker S. 2015. Complete nitrification by a single microorganism. Nature 528: 555–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Köhl L, van der Heijden MGA. 2016. Arbuscular mycorrhizal fungal species differ in their effect on nutrient leaching. Soil Biology and Biochemistry 94: 191–199. [Google Scholar]
- Kool DM, Dolfing J, Wrage N, van Groenigen JW. 2011b. Nitrifier denitrification as a distinct and significant source of nitrous oxide from soil. Soil Biology and Biochemistry 43: 174–178. [Google Scholar]
- Kool DM, Van Groenigen JW, Wrage N. 2011a. Source determination of nitrous oxide based on nitrogen and oxygen isotope tracing, dealing with oxygen exchange. Methods in Enzymology 496: 139–160. [DOI] [PubMed] [Google Scholar]
- Lazcano C, Barrios‐Masias FH, Jackson LE. 2014. Arbuscular mycorrhizal effects on plant water relations and soil greenhouse gas emissions under changing moisture regimes. Soil Biology and Biochemistry 74: 184–192. [Google Scholar]
- Leff B, Ramankutty N, Foley JA. 2004. Geographic distribution of major crops across the world. Global Biogeochemical Cycles 18: 1–27. [Google Scholar]
- Leigh J, Hodge A, Fitter AH. 2009. Arbuscular mycorrhizal fungi can transfer substantial amounts of nitrogen to their host plant from organic material. New Phytologist 181: 199–207. [DOI] [PubMed] [Google Scholar]
- Li XL, George E, Marschner H. 1991. Phosphorus depletion and pH decrease at the root‐soil and hyphae‐soil interfaces of VA mycorrhizal white clover fertilized with ammonium. New Phytologist 119: 397–404. [Google Scholar]
- Miller RM, Jastrow JD. 1992. Extraradical hyphal development of vesicular‐arbuscular mycorrhizal fungi in a chronosequence of prarie restoration In: Read DJ, Lewis DH, Fitter AH, Alexander IJ, eds. Mycorrhizas in ecosystems. Wallingford, UK: CAB International, 171–176. [Google Scholar]
- Mørkved PT, Dörsch P, Bakken LR. 2007. The N2O product ratio of nitrification and its dependence on long‐term changes in soil pH. Soil Biology and Biochemistry 39: 2048–2057. [Google Scholar]
- Nuccio EE, Hodge A, Pett‐Ridge J, Herman DJ, Weber PK, Firestone MK. 2013. An arbuscular mycorrhizal fungus significantly modifies the soil bacterial community and nitrogen cycling during litter decomposition. Environmental Microbiology 15: 1870–1881. [DOI] [PubMed] [Google Scholar]
- Olsson PA, Thingstrup I, Jakobsen I, Bååth E. 1999. Estimation of the biomass of arbuscular mycorrhizal fungi in a linseed field. Soil Biology and Biochemistry 31: 1879–1887. [Google Scholar]
- Ostrom NE, Ostrom PH. 2011. The isotopomers of nitrous oxide, analytical considerations and application to resolution of microbial production pathways In: Baskaran M, ed. Handbook of environmental isotope geochemistry. Berlin, Germany: Springer Heidelberg, 453–476. [Google Scholar]
- Park S, Croteau P, Boering KA, Etheridge DM, Ferretti D, Fraser PJ, Kim K‐R, Krummel PB, Langenfelds RL, van Ommen TD, et al 2012. Trends and seasonal cycles in the isotopic composition of nitrous oxide since 1940. Nature Geoscience 5: 261–265. [Google Scholar]
- Park D, Kim H, Yoon S. 2017. Nitrous oxide reduction by an obligate aerobic bacterium Gemmatimonas aurantiaca T‐27. Applied and Environmental Microbiology 83: e00502–e00517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prosser JI. 2007. The ecology of nitrifying bacteria In: Bothe H, Ferguson S, Newton WE, eds. Biology of the nitrogen cycle. Oxford, UK: Elsevier Science, 223–243. [Google Scholar]
- Prosser JL, Nicol GW. 2012. Archaeal and bacterial ammonia‐oxidisers in soil: the quest for niche specialisation and differentiation. Trends in Microbiology 11: 523–531. [DOI] [PubMed] [Google Scholar]
- Reay DS, Davidson EA, Smith KA, Smith P, Melillo JM, Dentener F, Crutzen PJ. 2012. Global agriculture and nitrous oxide emissions. Nature Climate Change 2: 410–416. [Google Scholar]
- Sanford RA, Wagner DD, Wu Q, Chee‐Sanford JC, Thomas SH, Cruz‐García C, Rodríguez G, Massol‐Deyá A, Krishnani KK, Ritalahti KM et al 2012. Unexpected nondenitrifier nitrous oxide reductase gene diversity and abundance in soils. Proceedings of the National Academy of Sciences, USA 109: 19709–19714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staddon PL, Fitter AH, Graves JD. 1999. Effect of elevated atmospheric CO2 on mycorrhizal colonization, external mycorrhizal hyphal production and phosphorus inflow in Plantago lanceolata and Trifolium repens in association with the arbuscular mycorrhizal fungis Glomus mosseae . Global Change Biology 5: 347‐358. [Google Scholar]
- Šimek M, Cooper JE. 2002. The influence of soil pH on denitrification: progress towards the understanding of this interaction over the last 50 years. European Journal of Soil Science 53: 345–354. [Google Scholar]
- Singh BK, Bardgett RD, Smith P, Reay DS. 2010. Microorganisms and climate change: terrestrial feedbacks and mitigation options. Nature Reviews Microbiology 8: 779–790. [DOI] [PubMed] [Google Scholar]
- Smith SE, Read DJ. 2008. Mycorrhizal symbiosis. Cambridge, UK: Academic Press. [Google Scholar]
- Storer KE. 2013. Interactions between arbuscular mycorrhizal fungi and soil greenhouse gas fluxes. PhD thesis, University of York, UK.
- Tanaka Y, Yano K. 2005. Nitrogen delivery to maize via mycorrhizal hyphae depends on the form of N supplied. Plant, Cell & Environment 28: 1247–1254. [Google Scholar]
- Thirkell TJ, Cameron DD, Hodge A. 2016. Resolving the ‘nitrogen paradox’ of arbuscular mycorrhizas: fertilization with organic matter brings considerable benefits for plant nutrition and growth. Plant, Cell & Environment 39: 1683–1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson AJ, Giannopoulos G, Pretty J, Baggs EM, Richardson DJ. 2012. Biological sources and sinks of nitrous oxide and strategies to mitigate emissions. Philosophical Transactions of the Royal Society B 367: 1157–1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toljander JF, Lindahl BD, Paul LR, Elfstrand M, Finlay RD. 2007. Influence of arbuscular mycorrhizal mycelial exudates on soil bacterial growth and community structure. FEMS Microbiology Ecology 61: 295–304. [DOI] [PubMed] [Google Scholar]
- Veresoglou SD, Sen R, Mamolos AP, Veresoglou DS. 2011. Plant species identity and arbuscular mycorrhizal status modulate potential nitrification rates in nitrogen‐limited grassland soils. Journal of Ecology 99: 1339–1349. [Google Scholar]
- Verhagen FJM, Laanbroek HJ, Woldendrop JW. 1995. Competition for ammonium between plant roots and nitrifying and heterotrophic bacteria and the effects of protozoan grazing. Plant and Soil 170: 241–250. [Google Scholar]
- Woldendorp JW, Laanbroek HJ. 1989. Activity of nitrifiers in relation to nitrogen nutrition of plants in natural ecosystems. Plant and Soil 115: 217–228. [Google Scholar]
- Wrage N, Velthof GL, van Beusichem ML, Oenema O. 2001. Role of nitrifier denitrification in the production of nitrous oxide. Soil Biology and Biochemistry 33: 1723–1732. [Google Scholar]
- Wuebbles DJ, Hayhoe K. 2002. Atmospheric methane and global change. Earth‐Science Reviews 57: 177–210. [Google Scholar]
- Zhang X, Wang L, Ma F, Shan D. 2015. Effects of arbuscular mycorrhizal fungi on N2O emissions from rice paddies. Water Air and Soil Pollution 226: 1–10. [Google Scholar]
- Zhu X, Burger M, Doane TA, Horwath WR. 2013. Ammonia oxidation pathways and nitrifier denitrification are significant sources of N2O and NO under low oxygen availability. Proceedings of the National Academy of Sciences, USA 110: 6328–6333. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Please note: Wiley Blackwell are not responsible for the content or functionality of any Supporting Information supplied by the authors. Any queries (other than missing material) should be directed to the New Phytologist Central Office.
Table S1 Mean plant biomass parameters from AMF and nonAMF treatments in Expt 1
Data Availability Statement
Data created during this research are available by request from the University of York Data Catalogue. https://doi.org/10.15124/67decab3-9ea6-4cde-812e-3c762eba2ec6.


