Abstract
Immunologic graft rejection is the main complication after corneal transplant into pathologically prevascularized so‐called high‐risk eyes. The aim of this study was to evaluate whether ultraviolet (UV) light crosslinking can regress pathologic corneal blood and lymphatic vessels and thereby improve subsequent graft survival. Using the murine model of suture‐induced corneal neovascularization, we found that corneal crosslinking with UVA light and riboflavin regressed both preexisting blood and lymphatic vessels significantly via induction of apoptosis in vascular endothelial cells. In addition, macrophages and CD45+ cell counts were significantly reduced. Consistently, corneal crosslinking reduced keratocyte density and corneal thickness without affecting corneal nonvascular endothelial cells, iris, and lens depending on the crosslinking duration. Furthermore, using the murine model of corneal transplant, long‐term graft survival was significantly promoted (P < .05) and CD4+ CD25+FoxP3+ T regulatory cells were upregulated (P < .01) in high‐risk eyes preoperatively treated with crosslinking. Our results suggest UV light crosslinking as a novel method to regress both pathologic corneal blood and lymphatic vessels and to reduce CD45+ inflammatory cells. Furthermore, this study demonstrates for the first time that preoperative corneal crosslinking in prevascularized high‐risk eyes can significantly improve subsequent graft survival and may become a promising novel therapy in the clinic.
Keywords: animal models: murine, basic (laboratory) research/science, corneal transplantation/ophthalmology, graft survival, immunohistochemistry, organ transplantation in general, pathology/histopathology, rejection, tolerance: experimental, translational research/science
Short abstract
Corneal crosslinking with ultraviolet‐A light and riboflavin can regress mature pathological corneal blood and lymphatic vessels, thereby promoting graft survival of a prevascularized high‐risk corneal transplantation.
Abbreviations
- BV
blood vessel
- CXL
corneal crosslinking
- DAPI
4’,6‐diamidino‐2‐phenylindole
- FACS
fluorescence‐activated cell sorting
- LV
lymphatic vessel
- LYVE‐1
lymphatic vessel endothelial hyaluronan receptor 1
- OCT
optical coherence tomography
- PDT
photodynamic therapy
- Treg
T regulatory cell
- TUNEL
terminal deoxynucleotidyl transferase dUTP nick end labeling
- UV
ultraviolet
- VEGF
vascular endothelial growth factor
1. INTRODUCTION
The healthy cornea, the transparent windscreen of the eye, is actively maintained in an avascular state by antiangiogenic and antilymphangiogenic factors and therefore is one of the few tissues of the human body devoid of both blood (BVs) and lymphatic vessels (LVs).1 This avascular privilege can be disturbed by several corneal disorders and hypoxic diseases, followed by the ingrowth of pathologic BVs and LVs into the physiologically transparent corneal center.2 These pathologic corneal BVs and LVs can deteriorate visual acuity and lead to corneal blindness. Furthermore, corneal neovascularization is a crucial risk factor for immune‐mediated corneal allograft rejection after therapeutic transplant and reduces graft survival significantly after penetrating keratoplasty (= corneal transplant).3 Clinically invisible LVs have been shown to play an important role in mediating immune responses after corneal transplant.4 The rejection rate of the corneal graft is much higher in high‐risk recipients with preexisting corneal BVs and LVs than in normal‐risk patients.5 Therefore, it is decisive to regress preexisting corneal BVs and LVs to improve the long‐term survival rate in high‐risk corneal transplant.
However, there is an unmet clinical need for an effective and easy applicable therapy to regress mature corneal BVs and LVs in pathologically prevascularized high‐risk eyes before transplant to promote subsequent graft survival. Although there are several possibilities to suppress actively outgrowing corneal BVs and LVs, such as the topical application of vascular endothelial growth factor (VEGF) inhibitors6, 7, 8, 9 and corticosteroids,10 as well as antisense oligonucleotide eye drops against insulin receptor substrate‐1,11 these methods cannot regress mature corneal vessels. For preexisting corneal BVs, only preclinical approaches like anti–VEGF‐B antibody fragment have been used,12 and fine needle diathermy united with anti‐VEGFs has been shown to regress mature BVs, while its effect on LVs is still unclear.13, 14, 15 Photodynamic therapy (PDT) with intrastromal verteporfin can regress mature corneal LVs.16 PDT with intravenous verteporfin can regress corneal vessels, but it requires systemic administration and light protection before treatment.17 These methods are either not clinically approved or not routinely used at the cornea.
Therefore, corneal crosslinking (CXL) is a promising option to regress preexisting corneal vessels and further improve graft survival. CXL with riboflavin application and ultraviolet (UV)A irradiation is a novel treatment for corneal ectatic diseases.18 CXL has been used for the treatment of progressive keratoconus since 2003 and received US Food and Drug Administration approval in 2016.19 In addition, this CXL method is also used in corneal ulcers, pellucid marginal degeneration, corneal melting, and iatrogenic keratectasia after laser eye surgery.20 Riboflavin is a photosensitizer and can be photoactivated by UV light to produce reactive oxygen radicals.21 Furthermore, UV radiation itself can damage both DNA and RNA of pathogens and is used as an antimicrobial procedure.22 Recently, CXL with the interaction of riboflavin and UV irradiation was reported to also be used in corneal infections.23, 24, 25 After the topical application of riboflavin and UVA light, the reaction causes the release of very reactive singlet oxygen and other reactive oxygen radicals, which leads to apoptosis of, for example, keratocytes in the cornea.26, 27, 28 It has been shown that CXL can lead to a reduction in corneal keratocytes.29 Thus, we hypothesized that the CXL treatment using riboflavin with UVA light may potentially also affect endothelial cells of pathologic corneal BVs and LVs, similar to corneal keratocytes.
Therefore, using a novel model of murine CXL, in this study we investigated whether CXL with local application of riboflavin and UVA can regress preexisting corneal BVs and LVs and subsequently improve graft survival after high‐risk corneal transplants, which would enable a novel therapeutic concept relevant for use in the clinic.
2. MATERIALS AND METHODS
2.1. Animals and anesthesia
All procedures involving animals were approved by the local animal care committee, and all mice were treated in compliance with the Association for Research in Vision and Ophthalmology “Statement for the Use of Animals in Ophthalmic and Vision Research.” Female BALB/c and C57BL/6 mice (aged 6‐8 weeks) were purchased from Charles River Laboratories (Sulzfeld, Germany) and were deeply anesthetized intraperitoneally before any surgical procedures.
2.2. Suture‐induced inflammatory corneal neovascularization assay
We used a suture‐induced inflammatory corneal neovascularization mouse model as previously described.1, 28, 30 Briefly, three 11‐0 nylon sutures were placed intrastromally for 2 weeks to acquire standardized combined angiogenic and lymphangiogenic responses. Sutures were then removed before further experiments.
2.3. CXL treatment with riboflavin and UVA rays
After corneal abrasion, the 6‐minutes CXL–treated group received a 6‐minutes topical application of 0.1% riboflavin in 20% dextran phosphate sodium (BerlinApotheke, Berlin, Germany) (1 drop every 3 minutes) followed by a 6‐minutes UVA irradiation (370 nm, 3 mW/cm2) using the CCL‐vario CXL system (AIVIMED GmbH, Wiesbaden, Germany). Similarly, in the 9‐minutes CXL–treated group, mice underwent a 9‐minutes topical riboflavin application followed by a 9‐minutes UVA irradiation. During UVA irradiation, limbal stem cells were protected by a black, light‐preventing, plastic shield. The controls received only riboflavin drops after epithelial abrasion without UVA irradiation.
2.4. Murine allogeneic corneal transplantation
Allogeneic corneal transplantation was performed as previously described31, 32 to compare the long‐term graft survival between the CXL group and controls. Twenty‐four female BALB/c mice were used as recipients, and 12 female C57BL/6 mice were used as corneal donors. At 4 days after the CXL procedure, donor corneas (diameter 2 mm) from C57BL/6 mice were transplanted into 9‐minutes CXL–treated or control BALB/c recipient beds (diameter 1.8 mm) that had prevascularization induced via suture placement. Grafts were evaluated for corneal opacity once weekly from 2 weeks posttransplant until week 8. In addition, the CXL‐treated and control groups were blinded before the first grading. The graft opacity was graded by using a standardized opacity grading (range 0 to 5+) to identify rejection as previously described.32 Grafts were considered to be rejected when the opacity scores were higher than +2.
2.5. Corneal immunohistochemistry assay
Corneal flatmounts were prepared and double stained as previously described.33, 34, 35 Corneal BVs were detected in green with the use of rat anti‐mouse FITC‐conjugated CD31 antibody (1:100; BD Biosciences, San Diego, CA), and LVs were stained in red with the use of rabbit anti‐mouse lymphatic vessel endothelial hyaluronan receptor 1 (LYVE‐1) antibody (1:200; AngioBio, Del Mar, CA) followed by Cy3‐conjugated goat anti‐rabbit secondary antibody (1:100; Jackson ImmunoResearch [Dianova], West Grove, PA) staining. In addition, corneas were stained with Alexa Fluor 488 rat anti‐mouse CD45 receptor antibody (BD Pharmingen, Heidelberg, Germany). Cell nuclei were labeled with 4’,6‐diamidino‐2‐phenylindole (DAPI). Nine to 12 digital pictures of double‐stained flatmounts were taken automatically with the use of a fluorescence microscope (BX53; Olympus, Hamburg, Germany) at magnification ×100 and assembled into a whole image (multi‐image alignment). Thereafter, the areas covered by BVs, LVs, LYVE‐1+ macrophages, or CD45+ cells were estimated with use of the Cell^F image analyzing program (Olympus, Münster, Germany) as described previously.30
2.6. TUNEL assay
Apoptosis of vascular endothelial cells was detected by using an In Situ Cell Death Detection Kit (fluorescein; RocheDiagnostics, Mannheim, Germany) with costaining of CD 31 (rat anti‐mouse; Acris GmbH, Herford, Germany) followed by Alexa Fluor 555 secondary antibody (goat anti‐rat; Thermo Fisher Scientific, Rockford, IL) or LYVE‐1 (rabbit anti‐mouse; AngioBio) and then by Cy3 secondary antibody (goat anti‐rabbit; Jackson ImmunoResearch, West Grove, PA) in cryosections. Terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL)‐positive control was performed by using DNase I (RocheDiagnostics) recombinant to induce DNA strand breaks before labeling procedures, and TUNEL‐negative control was accomplished without terminal transferase. Digital images were taken at magnification ×600.
2.7. Flow cytometry
Ipsilateral submandibular lymph nodes were excised 8 weeks after corneal transplant. Single‐cell suspensions were labeled with CD3, CD4, CD8a, CD11b, CD11c, CD45 (BioLegend, San Diego, CA), and CD25 (Thermo Fisher Scientific, San Diego, CA). In addition, FoxP3 intracellular staining was performed in accordance with FoxP3 kit (eBioscience, San Diego, CA) instructions. Thereafter, single‐cell suspensions were used for fluorescence‐activated cell sorting (FACS) with a FACSCantoTM II Flow Cytometer (BD, San Jose, CA). All antibodies were analyzed with the appropriate isotype controls. Further data analysis was performed with the use of FlowJo software (Tree Star, Inc., Ashland, OR).
2.8. Histological analysis of cornea
Three naïve eyes from BALB/c mice were excised and stained with hematoxylin–eosin in paraffin as normal histological controls and compared with 6‐minutes or 9‐minutes CXL–treated eyes at 1 day after CXL. For covalidation of the corneal thickness, cross‐sectional optical coherence tomography (OCT) images were acquired in vivo continuous before CXL, right after 6‐minutes and 9‐minutes CXL treatment (after corneal abrasion and riboflavin application) using an OCT system (HSM‐01; Optomedical Technologies GmbH, Lübeck, Germany). The numbers of keratocytes and corneal endothelial cells were counted in histological images of the central cornea, and corneal thickness was measured in both histological and OCT images from the top of stroma to the bottom of endothelial layer in ImageJ 1.50i (Wayne Rasband, National Institutes of Health, Bethesda, MD).
2.9. Measurement of epithelial defect size and corneal regularity
For 7 days, 9 mice per group were examined before and daily after CXL to assess the reepithelization process and the alteration of corneal regularity. Corneal epithelial defect size was determined via 0.1% fluorescein staining. Epithelial defect area was measured with use of the Cell^F software and then calculated in relation to the whole cornea. Complete reepithelialization was identified when there was no detectable fluorescein staining in the whole cornea.
Corneal regularity was evaluated in corneas with the use of a microscope (C‐PS160; Nikon, Tokyo, Japan) providing an illumination ring projected onto the corneal surface. A 5‐point scale was used to assess the irregularity of the corneal surface as previously described.36, 37
2.10. Statistical analysis
Data were presented as mean ± SD and analyzed via 1‐way ANOVA or Student t‐test. P < .05 was considered to be statistically significant. Prism 6 version 6.07 (GraphPad Software, San Diego, CA) was used to perform statistical analyses. Long‐term survival proportion of corneal allograft survival was analyzed with Kaplan–Meier survival curves and log‐rank test.
3. RESULTS
3.1. Effect of UV light crosslinking on mature corneal BVs and LVs
Existing corneal BVs and LVs, induced by the suture‐induced corneal inflammatory neovascularization model, can be regressed significantly by CXL with riboflavin and UVA light. Four days after CXL treatment, BVs showed 19% regression in the 6‐minutes CXL group (n = 5; P < .01) and 18.8% regression in the 9‐minutes CXL group (n = 5; P < .01), and LVs showed a significant regression of 54.3% in the 6‐minutes CXL group (n = 5; P < .05) and 59.8% regression in the 9‐minutes CXL group (n = 5; P < .01) (Figure 1). Corneas excised 8 days after CXL showed 16.0% regression of BVs (n = 5; P < .05) and 24.8% regression of LVs (n = 5; P > .05) in the 6‐minutes CXL group and 27.7% regression of BVs (n = 5; P < .01) and 54.4% regression of LVs (n = 5; P < .05) in the 9‐minutes CXL group compared with the controls (Figure 1). At 14 days after CXL treatment, there were fewer LVs in CXL‐treated groups with a significant difference in the 9‐minutes CXL group (n = 5; P < .05) compared with controls but no significant difference in corneal BVs (Figure 1). In addition, TUNEL‐positive cells were noted in both corneal blood and lymphatic vascular endothelia at 1 day after crosslinking in the 6‐minutes and 9‐minutes CXL groups (Figure 2). These data demonstrate for the first time that corneal CXL is an effective method to regress both mature corneal BVs and LVs via induction of apoptosis in vascular endothelial cells.
Figure 1.
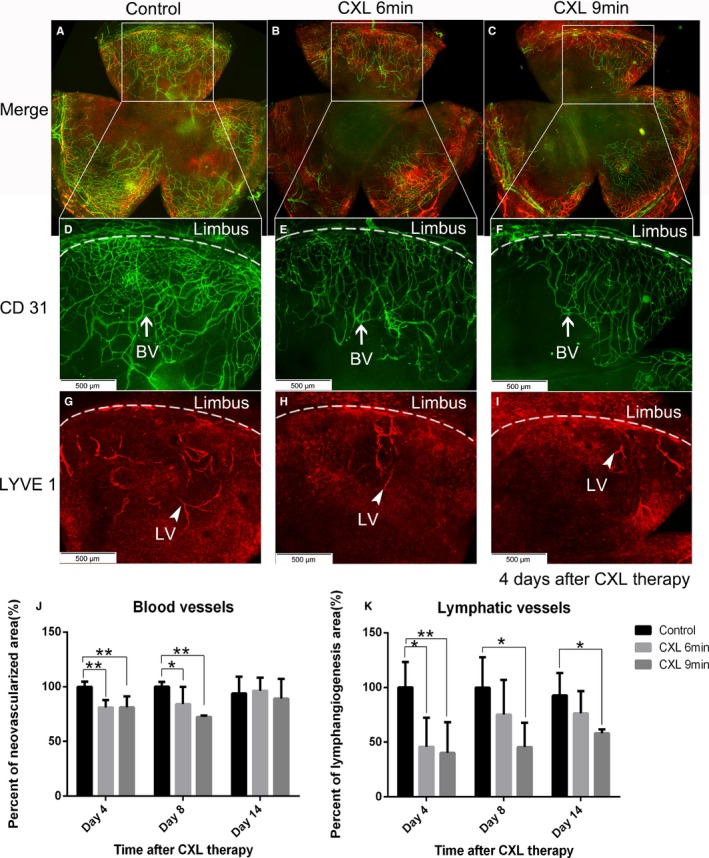
Regression of mature corneal blood and even more so lymphatic vessels after corneal crosslinking (CXL). Corneal whole mount staining was performed to quantify both corneal blood and lymphatic vessels via immunohistochemistry. Blood vessels were stained with FITC‐conjugated CD31 (D‐F: BV with white arrow in green) and lymphatic vessels were stained with lymphatic vessel endothelial hyaluronan receptor 1 (LYVE‐1) followed by Cy3‐conjugated secondary antibody (G‐I: LV with white arrowhead in red) (magnification: ×100; scale bar: 500 μm). Mature corneal blood vessels were regressed significantly on day 4 and day 8 after a 6‐minutes or 9‐minutes CXL treatment (J). Corneal lymphatic vessels in the 6‐minutes CXL–treated group showed a significant reduction on day 4 post CXL but not on day 8, while in the 9‐minutes CXL–treated group, lymphatic vessels were regressed significantly on both day 4 and day 8 after treatment (K) (n = 5; *P < .05, **P < .01). At 14 days after CXL treatment, corneal lymphatic vessels in the 9‐minutes CXL–treated group were still significantly reduced compared with the control group, while there was no significant difference in the 6‐minutes CXL–treated group (K). Corneal blood vessels were no longer significantly different (J). (n = 5; *P < .05)
Figure 2.
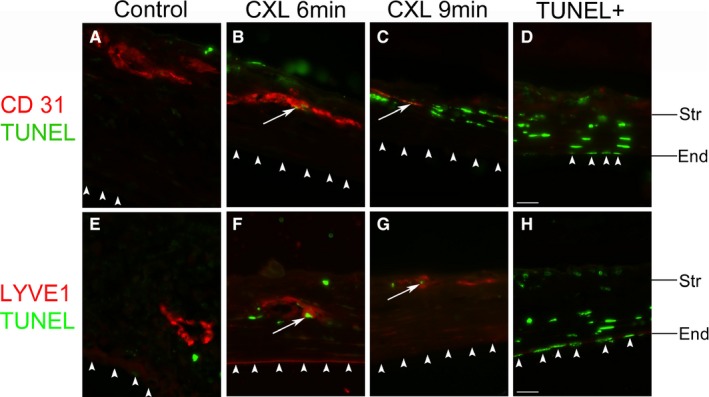
Apoptosis of vascular endothelial cells in corneas after ultraviolet (UV) light crosslinking. Representative terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) assay images of the central corneas from murine corneas 1 day after UV light crosslinking (CXL) for 6‐minutes (B,F) or 9‐minutes (C,G) and noncrosslinked control corneas (A,E). White arrows indicate TUNEL (green) and CD31+ (A‐D) or lymphatic vessel endothelial hyaluronan receptor 1 (LYVE1)+ (E‐H) (red) vascular endothelial cells in each panel. Corneal nonvascular endothelial cells are TUNEL negative in all groups; D,H: positive control of TUNEL staining (Str: stroma; End: nonvascular endothelium. Colocalization: white arrow. Corneal nonvascular endothelium: white arrowhead. magnification: ×600. scale bar: 20 μm). Corneal epithelium is missing in the treatment groups due to corneal abrasion
3.2. Corneal CXL reduces macrophages and CD45+ cells in inflamed corneas
Macrophages in inflammatory corneas were reduced by 31.3% in the 6‐minutes CXL group and by 30.8% in the 9‐minutes CXL group 4 days after crosslinking with a significant difference compared with controls (n = 5; P < .001) (Figure 3). Fewer macrophages were noted in inflammatory corneas 8 days after crosslinking (6‐minutes CXL: 15.7%; 9‐minutes CXL: 19.9%), and 16.5% more macrophages in the 6‐minutes CXL group and 11.2% fewer macrophages in the 9‐minutes CXL group were observed 14 days after CXL compared with control corneas, with a nonsignificant difference.
Figure 3.
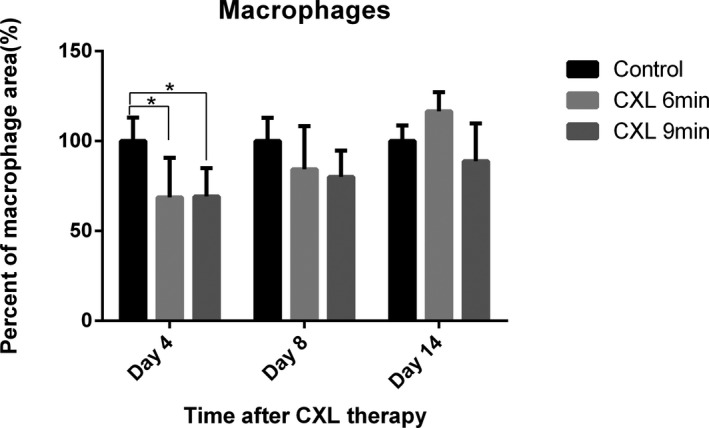
Analysis of macrophages in suture induced inflamed corneas after corneal crosslinking (CXL). Macrophages were stained with lymphatic vessel endothelial hyaluronan receptor 1 (LYVE‐1) followed by Cy3‐conjugated secondary antibody and quantified in inflamed corneas after CXL. At 4 days after CXL, macrophages in inflamed corneas decreased significantly to 68.7% in the 6‐minutes CXL–treated group and 69.2% in the 9‐minutes CXL–treated group compared with the control group. CXL‐treated corneas also had fewer macrophages (6‐minutes CXL: 15.7%; 9‐minutes CXL: 19.9%) at 8 days after CXL compared with the control group. At 14 days after CXL, there was 16.5% more macrophages in CXL 6‐minutes group and 11.2% less macrophages in CXL 9‐minutes group than in the controls (non‐significant; n = 5; *P < .05)
In addition, corneal CXL using riboflavin and UVA reduced CD45+ cell quantity on both days 4 and 8 posttreatment in inflamed corneas (Figure 4). At 4 days after CXL, the treated groups had significantly less CD45+ cells in suture‐induced inflammatory corneas, with a 61.7% reduction in the 6‐minutes CXL group and a 69.2% reduction in the 9‐minutes CXL group compared with control mice (n = 5; P < .0001). In addition, CD45+ cells in the corneas decreased significantly by 28.5% in the 6‐minutes CXL group (n = 5; P < .05) and 46.6% in the 9‐minutes CXL group (n = 5; P < .001) at 8 days after treatment, which is significantly different from the control.
Figure 4.
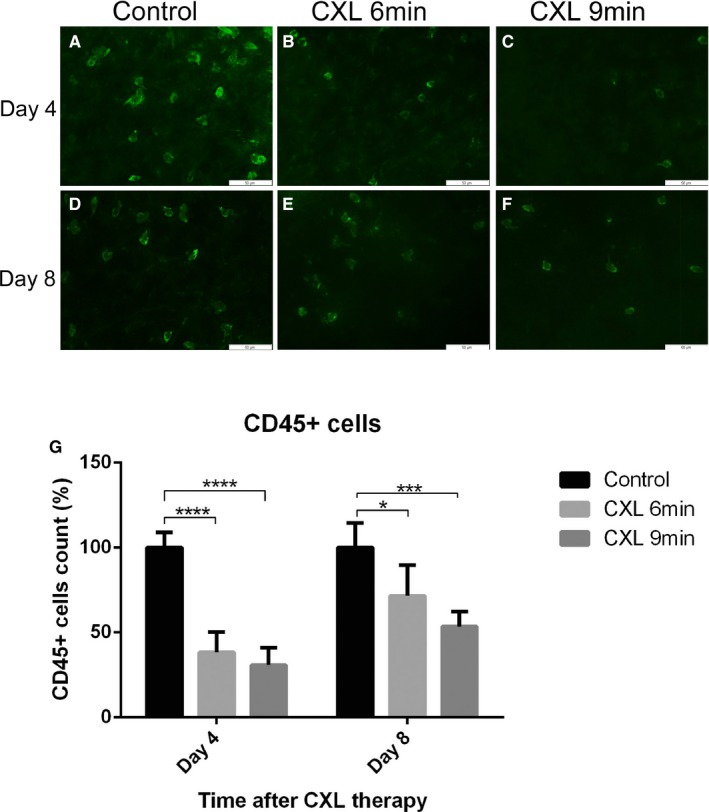
Reduction of CD45+ cells in inflamed corneas after coneal crosslinking (CXL). A‐F. Representative images of CD45+ cells in corneas excised 4 days or 8 days after CXL (magnification: ×400; scale bar: 50 μm). G. CD45+ cells in inflamed corneas decreased by 61.7% in 6‐minutes CXL–treated group and 69.2% in 9‐minutes CXL–treated group with a significant difference at 4 days post CXL compared with the control group. At 8 days after CXL, CD45+ cells in corneas also decreased significantly (6‐minutes CXL: 28.5%; 9‐minutes CXL: 46.6%) compared with the control group (n = 5; *P < .05, *** P < .001, **** P < .0001)
This result indicates that corneal CXL is a novel method to reduce leukocytes in inflamed corneas.
3.3. Promotion of corneal transplant tolerance in high‐risk keratoplasty with CXL therapy
As we observed the maximal regression of corneal LVs and CD45+ cells in the 9‐minutes CXL–treated groups on day 4 after treatment, we assumed that performing corneal transplant 4 days after a 9‐minutes CXL preoperative treatment should best promote subsequent graft survival in high‐risk eyes. Under these conditions, allograft survival proportion was significantly improved in preoperatively 9‐minutes CXL–treated, vascularized high‐risk mice at 8 weeks after corneal transplant (n = 12; P < .01). As shown in Figure 5A, the 9‐minutes CXL group achieved a 66.7% survival rate, while only 25.0% of the grafts survived in the control group, which received just 9‐minutes riboflavin treatment and no UVA irradiation. The promotion of long‐term graft survival may be due to the significant regression of preexisting corneal BVs and LVs (Figure 1) and the significant reduction in CD45+ cells in inflammatory corneas after CXL (Figure 4).
Figure 5.
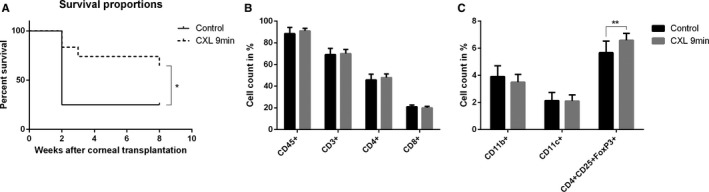
Promotion of graft survival and upregulation of T regulatory cells in vascularized high‐risk eyes after pretreatment with corneal crosslinking (CXL). A. The 9‐minutes CXL treatment before corneal transplant significantly promoted corneal allograft survival in pretreated high‐risk eyes 8 weeks after corneal transplant (control: 25.0% survived, 9‐minutes CXL: 66.7% survived; n = 12; *P < .05). B,C. There were significantly more CD4+ CD25+FoxP3+ cells in draining lymph nodes in the 9‐minutes CXL‐treated group than in the control group at 8 weeks post corneal transplant (n = 12; **P < .01). FACS analysis showed no statistical difference for CD3+, CD4+, CD8+, CD11b+, CD11c+, or CD45+ cells
In addition, 8 weeks after corneal transplant, CD4+CD25+FoxP3+ T regulatory cells (Tregs) of draining lymph nodes in the 9‐minutes CXL–treated group were significantly increased in comparison with the control group (Figure 5C) (n = 12; P < .01). Quantification of CD3+, CD4+, CD8+, and CD45+ lymphocytes demonstrated no change between the CXL‐treated group and the control group (Figure 5B). Furthermore, no alterations were detectable in the percentage of CD11b+ and CD11c+ dendritic cells in lymph nodes.
3.4. Decrease in keratocyte density and corneal thickness after CXL
Keratocytes and corneal endothelial cells were counted in the central corneal region (Figure 6A: black frame) in histological images. Corneal thickness was measured in both histological images and OCT images. Keratocytes in the central cornea were reduced significantly to 20.6 ± 1.9 cells/visual field in the 6‐minutes CXL group (P < .05) and 5.3 ± 1.8 cells/visual field in the 9‐minutes CXL group (P < .01) compared with naïve corneas (37.6 ± 7.1 cells/visual field) 1 day after treatment (Figure 6B‐D, N). There was no significant change in central corneal endothelial cell count in the 6‐ and 9‐minutes CXL–treated groups 1 day after the procedure compared with naïve corneas (Figure 6E‐G, O). In addition, no visible histological disorder in the iris or lens was observed in 6‐minutes or 9‐minutes CXL groups (Figure 6K‐M). In histological images, we observed that corneal stroma thickness in the center was decreased to 56.6% in the 6‐ minutes CXL group and to 41.2% in the 9‐minutes CXL group at 1 day post CXL compared with naïve corneas. All CXL‐treated groups showed significant differences in the thickness compared with naïve eyes (P < .05) (Figure 6P). In the representative OCT images (in vivo), the covalidation is consistent with the histological measurements; corneal thickness was reduced consistently during the 9‐minutes CXL procedure (6‐minutes CXL: decreased to 75.0%; 9‐minutes CXL: decreased to 55.8%, compared with before CXL) (Figure 6H‐J). CXL reduced keratocyte density and corneal thickness in the central cornea without a visible effect on corneal endothelium.
Figure 6.
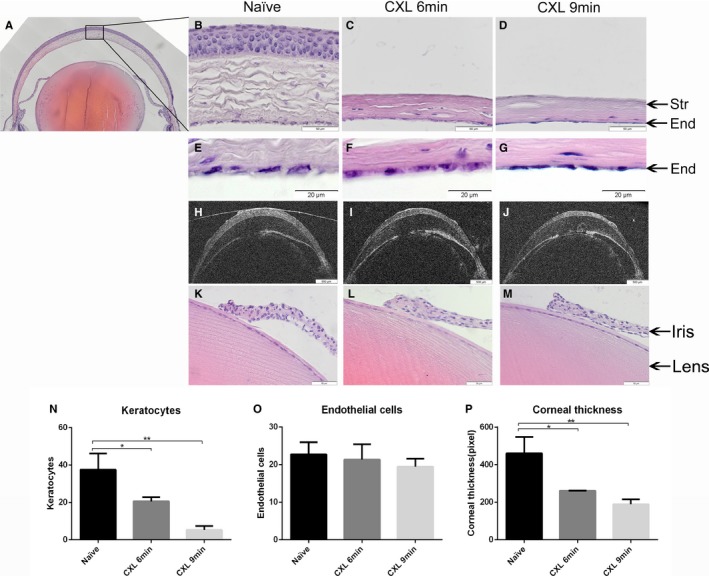
Corneal crosslinking (CXL) in mice reduces keratocyte density and corneal thickness without affecting corneal nonvascular endothelial cells, iris, or lens. Keratocytes, endothelial cells, and corneal thickness were measured in the central cornea, and the central corneal region was defined as the black frame in A. Keratocytes in mice treated for 6 and 9 minutes with CXL showed a significant reduction at 1 day after CXL compared with the naïve group (B‐D, N) (B‐D. magnification: ×400; scale bar: 50 μm). No significant differences in corneal endothelial cells were found in all CXL‐treated groups compared with naïve eyes (E‐G, O) (E‐G. magnification: ×600; scale bar: 500 μm). Representative OCT images demonstrate that corneal thickness decreased consistently during the CXL process (H‐J). Corneal thickness in all CXL‐treated groups decreased significantly 1 day after CXL but without any difference between 6‐minutes CXL and 9‐minutes CXL (P). No visible morphologic disorder in the iris or lens was noted in 6‐ or 9‐minutes CXL–treated eyes compared with naïve eyes (K‐M) (magnification: ×400; scale bar: 50 μm). (n = 3; *P < .05, **P < .01)
3.5. Effect of CXL on corneal reepithelization
Through corneal epithelial abrasion before CXL, a comparable initial epithelial defect without any damage of the limbus was created in all groups. After abrasion, riboflavin was applied in all groups and UVA irradiation was performed subsequently in CXL groups. On day 1 postabrasion, the average epithelial defect size was larger in the 6‐minutes UVA–treated group than in the control group (n = 5; P < .01) (Figure 7). In the 9‐minutes UVA–treated group, the reepithelialization process was slower on day 1 and day 2 postabrasion compared with the control group (day 1: P < .05, day 2: P < .0001) (Figure 7). Although corneal reepithelialization was mildly delayed on the first 2 days after CXL, there was no significant difference in the following healing process among the CXL groups and the control group (P > .05). Furthermore, although corneal reepithelialization was slightly delayed in this study, all the corneas in any groups achieved complete corneal epithelialization 7 days after corneal abrasion. CXL may slow down the reepithelialization process at the beginning but has no significant effect on achieving complete corneal epithelialization.
Figure 7.
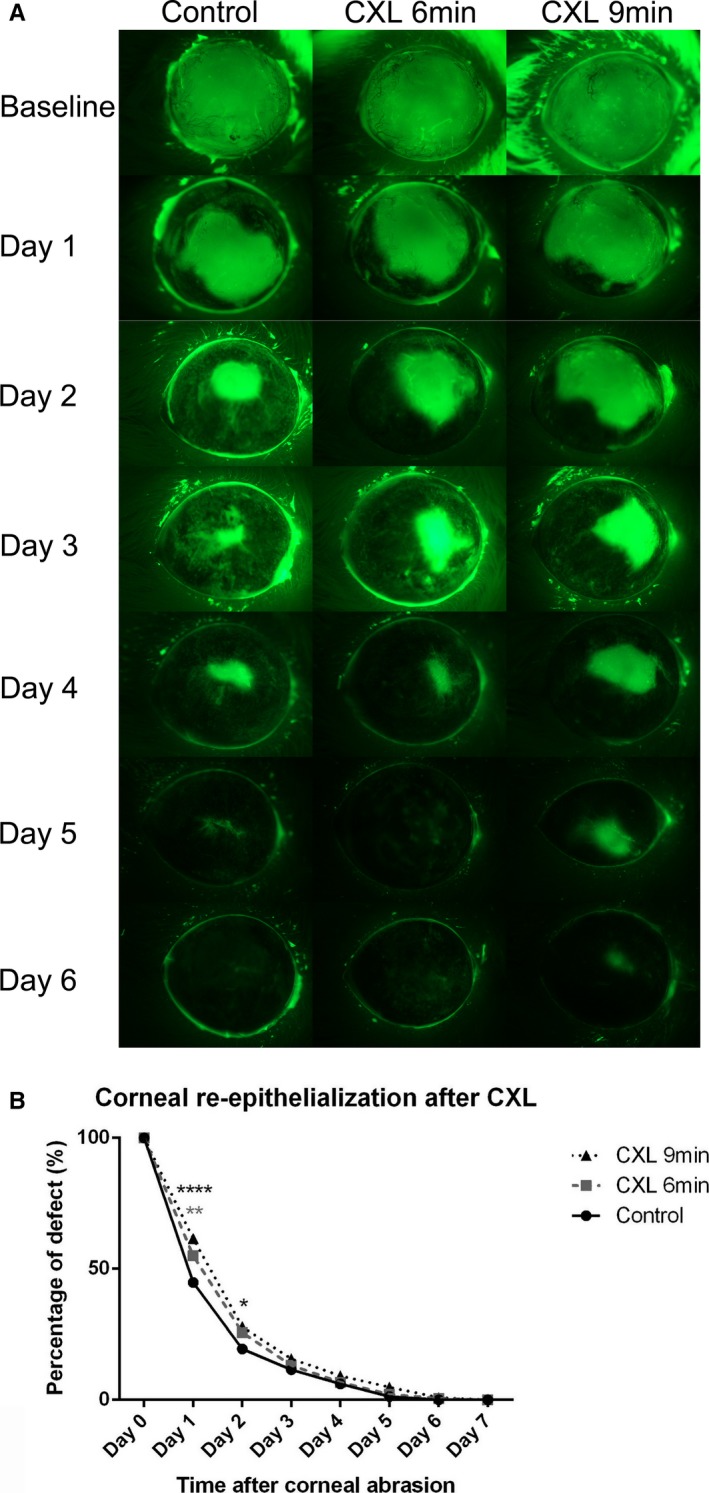
Corneal reepithelialization is minimally delayed after corneal crosslinking (CXL) treatment. A. Representative cases to illustrate reepithelialization after crosslinking. Representative images illustrate epithelial defect at baseline (at time of epithelial abrasion) and from day 1 to day 6 after abrasion among control, 6‐minutes CXL, and 9‐minutes CXL mice. The average epithelial defect size was measured by fluorescein staining in abraded corneas after CXL during 7 days of follow‐up. Corneal epithelial defect size was statistically larger on average in the 6‐minutes CXL–treated group on day 1 post corneal abrasion compared with the control and was significantly bigger in the 9‐minutes CXL–treated group than in controls on day 1 and day 2 post abrasion (n = 9; *P < .05, ** P < .01, **** P < .0001). B. All corneas achieved complete reepithelialization 7 days after corneal abrasion with no significant difference among the 3 groups from day 3 to day 7 (P > .05)
3.6. Corneal epithelial regularity after CXL
Corneal epithelial regularity was assessed by analyzing the regularity of a reflected, circular white light projected onto the corneal surface. The irregularity of corneal epithelium was graded by using a 5‐point scale based on the number of distorted quarters in the reflected ring as described previously.36, 37 In general, corneal epithelial regularity was comparable in all groups before abrasion, notably distorted 1 day after abrasion in all groups, and less distorted in the 9‐minutes CXL–treated group (P < .05) but progressively improved 2 days after corneal abrasion with no significant difference between the CXL group and the control group (Figure 8). In addition, at 6 days after corneal abrasion, no statistical difference of corneal epithelial regularity was observed in any group compared with before the abrasion. CXL had no notably effect on the restoration of corneal epithelial regularity.
Figure 8.
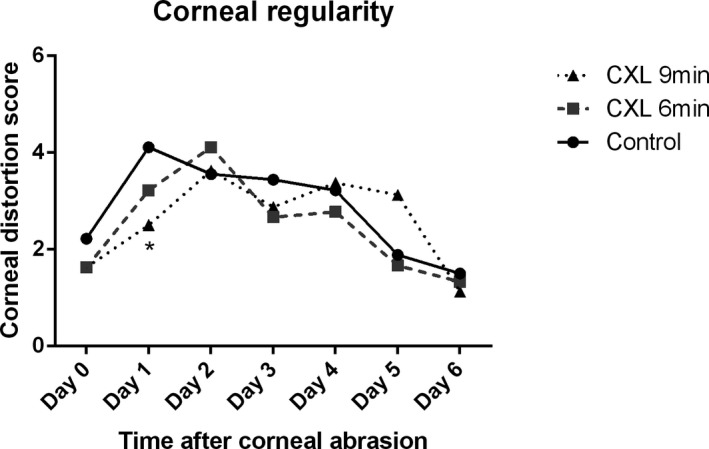
Restoration of corneal regularity after corneal crosslinking (CXL) treatment. The percentage of corneas that achieved corneal regularity showed no significant difference in 6‐minutes CXL–treated eyes compared with the control group during 6 days of follow‐up. Average corneal distortion score was significantly smaller in the 9‐minutes CXL–treated eyes than in the control on day 1 (n = 9; *P < .05) with no statistical difference from day 2 to day 6. Corneal regularity 6 days after corneal abrasion showed no difference with that before the abrasion in each group
4. DISCUSSION
This study demonstrates for the first time that CXL using riboflavin and UVA can lead to a regression of preexisting corneal BVs and LVs and to improvement of subsequent graft survival in high‐risk murine corneal transplants. This suggests the use of UVA crosslinking of prevascularized high‐risk recipient beds to become a novel therapeutic strategy in the clinic to promote corneal graft survival.
Several clinical and experimental studies have shown that pathological corneal hemangiogenesis and lymphangiogenesis before transplantation increase graft rejection after subsequent corneal transplantation. Both preexisting corneal BVs and LVs are important risk factors for immune‐mediated allograft rejection after high‐risk keratoplasty.4, 31, 38 Therefore, it is mandatory to regress preexisting corneal vessels in high‐risk eyes before transplantation, and mature BVs and LVs have become new therapeutic targets to enhance surgical success rates after corneal transplantation. Using our CXL strategy with topical riboflavin application and UVA irradiation, we induced apoptosis of both blood and lymphatic vascular endothelial cells in corneas and succeeded in regressing preexisting corneal BVs and LVs significantly in prevascularized eyes.
Consistent with previous studies,26, 27, 28 our CXL procedure reduced corneal keratocyte numbers in the stroma. After the topical application of the riboflavin solution and UVA irradiation, in addition to the biomechanical stiffening effect of the procedure, the reaction of riboflavin and UVA leads to the release of highly reactive singlet oxygen and other reactive oxygen radicals, which can cause apoptosis of keratocytes and endothelial cells in the cornea.26, 27, 28 Because corneal BVs and LVs mainly grow in the upper corneal stromal layer, the reaction of riboflavin and UVA should also induce similar apoptosis of endothelial cells in corneal vessels and thereafter lead to the regression. Thus, CXL is a promising therapeutic technique that has an angioregressive effect on both mature corneal BVs and LVs.
In addition, because this method requires only the topical use of riboflavin and local irradiation, it avoids potential systemic side effects—no systemic or intravenous therapy is needed. Normally, the human corneal stroma absorbs 32% of the UVA light. After riboflavin application, UVA absorption in the stroma increases to 95%.27, 39 The reason for the high absorption rate is that the wavelength range of UVA llight (315‐400 nm) corresponds to one of the riboflavin absorption maxima (ie, 370 nm).26 Because of the high absorption, the UVA irradiation dose that reaches the lens and retina is very small (0.22‐0.32 mJ/cm2), which may be comparable to the outdoor exposure to UVA sunlight for a day.40 Therefore, UVA irradiation afterward causes an effect only on the area where it is absorbed, without disturbing other adjacent tissues.40 By adjusting the duration of riboflavin application and UVA irradiation in the CXL procedure, keratocytes in the central cornea were reduced significantly in CXL groups compared with naïve corneas, which can reproduce with a repopulation process.41 Furthermore, via adjustment of the dose of applied UVA irradiation and riboflavin, in contrast to an earlier study,42 we were able to save corneal endothelial cells, which are essential to maintain corneal transparency and cannot be reproduced in the adult organism,43 and we succeeded in avoiding damage to the iris and lens. This CXL treatment may be a novel minimally invasive preconditioning procedure to reduce graft rejection reaction before high‐risk penetrating corneal transplant.
Interestingly, macrophages decreased in inflammatory corneas after CXL, which should contribute to the regression of pathologic BVs and LVs in corneas, as macrophages play a key role in the induction of angiogenesis44, 45 and lymphangiogenesis46, 47, 48, 49 under pathological conditions in the cornea and in the maintenance of LVs.50 Furthermore, we also observed significant reduction in CD45+ leukocytes in suture‐induced inflammation in corneas after CXL treatment using riboflavin and UVA. Because leukocytes are involved in preserving the immune system against foreign invaders, the decrease of leukocytes in recipient beds may also contribute to the tolerance induction of the graft after corneal transplant. Although the corneal epithelium was removed before CXL, all eyes achieved complete corneal reepithelialization. Our data indicate that CXL with riboflavin and UVA may slow reepithelialization on the first 2 days but had no notable effect on the whole healing process. All the aforementioned results suggest that CXL with riboflavin and UVA irradiation is an effective and safe method to regress mature corneal BVs and LVs, as well as to reduce macrophages and CD45+ leukocytes in inflammatory corneas.
Furthermore, the long‐term allograft survival rate significantly improved in prevascularized eyes after preoperative CXL treatment using riboflavin and UVA irradiation. The regression of corneal LVs after CXL should contribute to the promotion of graft survival in high‐risk eyes, as it was shown previously that LVs are key to induce immune responses after transplant.4 Interestingly, CXL induced only a temporary regression of both BVs and LVs. But even this temporary regression was obviously sufficient to promote graft survival. Furthermore, our data show that the predominant effect of CXL was on LVs, further adding to the growing body of evidence suggesting that LVs and not BVs are primarily responsible for graft rejections in high‐risk corneal transplant.4 Additionally, the reduction of CD45+ leukocytes in corneas after CXL treatment may also benefit the increase of graft survival rate, as leukocytes play an important role against foreign invaders. Moreover, 8 weeks after allogeneic corneal transplant, we noticed that CD4+CD25+FoxP3+ Tregs significantly increased in cervical draining lymph nodes in the preoperatively CXL‐treated mice. It is reported that CD4+CD25+FoxP3+ Tregs can be uniquely induced when orthotopic corneal allografts are transplanted to the eye.51, 52, 53 Furthermore, Tregs support long‐term corneal allograft survival.54, 55, 56, 57 Tregs can respond to interleukin‐17A (a proinflammatory cytokine), suppress the efferent arm of the immune response, and then enhance corneal allograft survival.55
Taken together, we established a novel murine model for CXL. This showed that CXL using riboflavin and UVA is a novel method to regress both preexisting corneal BVs and LVs, to reduce CD45+ cells, and to minimize the damages on corneal endothelium in corneas via adjustment of the dose of UVA and riboflavin. Moreover, we have shown for the first time that the long‐term allograft survival rate is highly promoted after preoperative CXL treatment using riboflavin and UVA in high‐risk corneal transplant. This suggests that CXL therapy with riboflavin and UVA is a promising option to treat prevascularized human corneas before transplant and thereby decrease the graft rejection rate after corneal transplant surgery in high‐risk eyes.
DISCLOSURE
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
ACKNOWLEDGMENTS
The authors would like to sincerely thank the support from German Research Foundation (DFG) FOR2240, European Cooperation in Science and Technology (EU COST BM1302); EU Horizon 2020 Advanced Regenerative and Restorative Therapies to combat corneal BLINDNESS (ARREST BLINDNESS), Center for Molecular Medicine Cologne, University of Cologne and China Scholarship Council.
Hou Y, Le VNH, Tóth G, et al. UV light crosslinking regresses mature corneal blood and lymphatic vessels and promotes subsequent high‐risk corneal transplant survival. Am J Transplant. 2018;18:2873–2884. 10.1111/ajt.14874
Funding information German Research Foundation (DFG) FOR2240 “(Lymph)angiogenesis and Cellular Immunity in Inflammatory Diseases of the Eye,” Cu 47/4‐2 (CC), Cu 47/6‐1 (CC), Cu 47/9‐1 (CC) (http://www.for2240.de); EU COST BM1302 (FBo, CC; http://www.biocornea.eu); EU Horizon 2020 ARREST BLINDNESS (CC; http://www.arrestblindness.eu); Center for Molecular Medicine Cologne, University of Cologne (FBo, CC; http://www.cmmc-uni-koeln.de/home/); China Scholarship Council (en.csc.edu.cn).
Felix Bock and Claus Cursiefen are co‐senior authors.
REFERENCES
- 1. Cursiefen C, Chen L, Saint‐Geniez M, et al. Nonvascular VEGF receptor 3 expression by corneal epithelium maintains avascularity and vision. Proc Natl Acad Sci U S A. 2006;103(30):11405‐11410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bock F, Maruyama K, Regenfuss B, et al. Novel anti(lymph)angiogenic treatment strategies for corneal and ocular surface diseases. Prog Retin Eye Res. 2013;34:89‐124. [DOI] [PubMed] [Google Scholar]
- 3. Maddula S, Davis DK, Maddula S, et al. Horizons in therapy for corneal angiogenesis. Ophthalmology. 2011;118(3):591‐599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Dietrich T, Bock F, Yuen D, et al. Cutting edge: lymphatic vessels, not blood vessels, primarily mediate immune rejections after transplantation. J Immunol. 2010;184(2):535‐539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Yamagami S, Dana MR, Tsuru T. Draining lymph nodes play an essential role in alloimmunity generated in response to high‐risk corneal transplantation. Cornea. 2002;21(4):405‐409. [DOI] [PubMed] [Google Scholar]
- 6. Bock F, Onderka J, Dietrich T, et al. Bevacizumab as a potent inhibitor of inflammatory corneal angiogenesis and lymphangiogenesis. Invest Ophthalmol Vis Sci. 2007;48(6):2545‐2552. [DOI] [PubMed] [Google Scholar]
- 7. Bucher F, Parthasarathy A, Bergua A, et al. Topical ranibizumab inhibits inflammatory corneal hem‐ and lymphangiogenesis. Acta Ophthalmol. 2014;92(2):143‐148. [DOI] [PubMed] [Google Scholar]
- 8. Cursiefen C, Bock F, Horn FK, et al. GS‐101 antisense oligonucleotide eye drops inhibit corneal neovascularization: interim results of a randomized phase II trial. Ophthalmology. 2009;116(9):1630‐1637. [DOI] [PubMed] [Google Scholar]
- 9. Cursiefen C, Viaud E, Bock F, et al. Aganirsen antisense oligonucleotide eye drops inhibit keratitis‐induced corneal neovascularization and reduce need for transplantation: the I‐CAN study. Ophthalmology. 2014;121(9):1683‐1692. [DOI] [PubMed] [Google Scholar]
- 10. Hos D, Saban DR, Bock F, et al. Suppression of inflammatory corneal lymphangiogenesis by application of topical corticosteroids. Arch Ophthalmol. 2011;129(4):445‐452. [DOI] [PubMed] [Google Scholar]
- 11. Lorenz K, Scheller Y, Bell K, et al. A prospective, randomised, placebo‐controlled, double‐masked, three‐armed, multicentre phase II/III trial for the Study of a Topical Treatment of Ischaemic Central Retinal Vein Occlusion to Prevent Neovascular Glaucoma – the STRONG study: study protocol for a randomised controlled trial. Trials. 2017;18(1):128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Irani YD, Scotney PD, Klebe S, et al. An anti‐VEGF‐B antibody fragment induces regression of pre‐existing blood vessels in the rat cornea. Invest Ophthalmol Vis Sci. 2017;58(9):3404‐3413. [DOI] [PubMed] [Google Scholar]
- 13. Pillai CT, Dua HS, Hossain P. Fine needle diathermy occlusion of corneal vessels. Invest Ophthalmol Vis Sci. 2000;41(8):2148‐2153. [PubMed] [Google Scholar]
- 14. Koenig Y, Bock F, Kruse FE, et al. Angioregressive pretreatment of mature corneal blood vessels before keratoplasty: fine‐needle vessel coagulation combined with anti‐VEGFs. Cornea. 2012;31(8):887‐892. [DOI] [PubMed] [Google Scholar]
- 15. Elbaz U, Mireskandari K, Shen C, et al. Corneal fine needle diathermy with adjuvant bevacizumab to treat corneal neovascularization in children. Cornea. 2015;34(7):773‐777. [DOI] [PubMed] [Google Scholar]
- 16. Bucher F, Bi Y, Gehlsen U, et al. Regression of mature lymphatic vessels in the cornea by photodynamic therapy. Br J Ophthalmol. 2014;98(3):391‐395. [DOI] [PubMed] [Google Scholar]
- 17. Hou Y, Le VNH, Clahsen T, et al. Photodynamic therapy leads to time‐dependent regression of pathologic corneal (lymph) angiogenesis and promotes high‐risk corneal allograft survival. Invest Ophthalmol Vis Sci. 2017;58(13):5862‐5869. [DOI] [PubMed] [Google Scholar]
- 18. Raiskup F, Spoerl E. Corneal crosslinking with riboflavin and ultraviolet A. I. Principles. Ocul Surf. 2013;11(2):65‐74. [DOI] [PubMed] [Google Scholar]
- 19. Wollensak G, Spoerl E, Seiler T. Riboflavin/ultraviolet‐A‐induced collagen crosslinking for the treatment of keratoconus. Am J Ophthalmol. 2003;135(5):620‐627. [DOI] [PubMed] [Google Scholar]
- 20. Kohlhaas M, Spoerl E, Speck A, et al. A new treatment of keratectasia after LASIK by using collagen with riboflavin/UVA light cross‐linking. Klin Monbl Augenheilkd. 2005;222(5):430‐436. [DOI] [PubMed] [Google Scholar]
- 21. Goodrich RP. The use of riboflavin for the inactivation of pathogens in blood products. Vox Sang. 2000;78(Suppl 2):211‐215. [PubMed] [Google Scholar]
- 22. Tabibian D, Richoz O, Hafezi F. PACK‐CXL: corneal cross‐linking for treatment of infectious keratitis. J Ophthalmic Vis Res. 2015;10(1):77‐80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Iseli HP, Thiel MA, Hafezi F, et al. Ultraviolet A/riboflavin corneal cross‐linking for infectious keratitis associated with corneal melts. Cornea. 2008;27(5):590‐594. [DOI] [PubMed] [Google Scholar]
- 24. Alio JL, Abbouda A, Valle DD, et al. Corneal cross linking and infectious keratitis: a systematic review with a meta‐analysis of reported cases. J Ophthalmic Inflamm Infect. 2013;3(1):47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Said DG, Elalfy MS, Gatzioufas Z, et al. Collagen cross‐linking with photoactivated riboflavin (PACK‐CXL) for the treatment of advanced infectious keratitis with corneal melting. Ophthalmology. 2014;121(7):1377‐1382. [DOI] [PubMed] [Google Scholar]
- 26. Spoerl E, Mrochen M, Sliney D, et al. Safety of UVA‐riboflavin cross‐linking of the cornea. Cornea. 2007;26(4):385‐389. [DOI] [PubMed] [Google Scholar]
- 27. Wollensak G, Spoerl E, Reber F, et al. Keratocyte cytotoxicity of riboflavin/UVA‐treatment in vitro. Eye (Lond). 2004;18(7):718‐722. [DOI] [PubMed] [Google Scholar]
- 28. Wollensak G, Spoerl E, Wilsch M, et al. Keratocyte apoptosis after corneal collagen cross‐linking using riboflavin/UVA treatment. Cornea. 2004;23(1):43‐49. [DOI] [PubMed] [Google Scholar]
- 29. Mencucci R, Marini M, Paladini I, et al. Effects of riboflavin/UVA corneal cross‐linking on keratocytes and collagen fibres in human cornea. Clin Exp Ophthalmol. 2010;38(1):49‐56. [DOI] [PubMed] [Google Scholar]
- 30. Bock F, Onderka J, Hos D, et al. Improved semiautomatic method for morphometry of angiogenesis and lymphangiogenesis in corneal flatmounts. Exp Eye Res. 2008;87(5):462‐470. [DOI] [PubMed] [Google Scholar]
- 31. Cursiefen C, Cao J, Chen L, et al. Inhibition of hemangiogenesis and lymphangiogenesis after normal‐risk corneal transplantation by neutralizing VEGF promotes graft survival. Invest Ophthalmol Vis Sci. 2004;45(8):2666‐2673. [DOI] [PubMed] [Google Scholar]
- 32. Sonoda Y, Streilein JW. Orthotopic corneal transplantation in mice – evidence that the immunogenetic rules of rejection do not apply. Transplantation. 1992;54(4):694‐704. [DOI] [PubMed] [Google Scholar]
- 33. Cursiefen C, Chen L, Borges LP, et al. VEGF‐A stimulates lymphangiogenesis and hemangiogenesis in inflammatory neovascularization via macrophage recruitment. J Clin Invest. 2004;113(7):1040‐1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Cursiefen C, Schlotzer‐Schrehardt U, Kuchle M, et al. Lymphatic vessels in vascularized human corneas: immunohistochemical investigation using LYVE‐1 and podoplanin. Invest Ophthalmol Vis Sci. 2002;43(7):2127‐2135. [PubMed] [Google Scholar]
- 35. Hos D, Bucher F, Regenfuss B, et al. IL‐10 indirectly regulates corneal lymphangiogenesis and resolution of inflammation via macrophages. Am J Pathol. 2016;186(1):159‐171. [DOI] [PubMed] [Google Scholar]
- 36. De Paiva CS, Corrales RM, Villarreal AL, et al. Apical corneal barrier disruption in experimental murine dry eye is abrogated by methylprednisolone and doxycycline. Invest Ophthalmol Vis Sci. 2006;47(7):2847‐2856. [DOI] [PubMed] [Google Scholar]
- 37. Tighe S, Moein HR, Chua L, et al. Topical cryopreserved amniotic membrane and umbilical cord eye drops promote re‐epithelialization in a murine corneal abrasion model. Invest Ophthalmol Vis Sci. 2017;58(3):1586‐1593. [DOI] [PubMed] [Google Scholar]
- 38. Bachmann B, Taylor RS, Cursiefen C. Corneal neovascularization as a risk factor for graft failure and rejection after keratoplasty: an evidence‐based meta‐analysis. Ophthalmology. 2010;117(7):1300‐1305.e1307. [DOI] [PubMed] [Google Scholar]
- 39. Tsubai T, Matsuo M. Ultraviolet light‐induced changes in the glucose‐6‐phosphate dehydrogenase activity of porcine corneas. Cornea. 2002;21(5):495‐500. [DOI] [PubMed] [Google Scholar]
- 40. Bottos KM, Dreyfuss JL, Regatieri CV, et al. Immunofluorescence confocal microscopy of porcine corneas following collagen cross‐linking treatment with riboflavin and ultraviolet A. J Refract Surg. 2008;24(7):S715‐S719. [DOI] [PubMed] [Google Scholar]
- 41. Hovakimyan M, Guthoff RF, Stachs O. Collagen cross‐linking: current status and future directions. J Ophthalmol. 2012;2012:406850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Armstrong BK, Lin MP, Ford MR, et al. Biological and biomechanical responses to traditional epithelium‐off and transepithelial riboflavin‐UVA CXL techniques in rabbits. J Refract Surg. 2013;29(5):332‐341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Takacs L, Toth E, Berta A, et al. Stem cells of the adult cornea: from cytometric markers to therapeutic applications. Cytometry A. 2009;75(1):54‐66. [DOI] [PubMed] [Google Scholar]
- 44. Li ZR, Li YP, Lin ML, et al. Activated macrophages induce neovascularization through upregulation of MMP‐9 and VEGF in rat corneas. Cornea. 2012;31(9):1028‐1035. [DOI] [PubMed] [Google Scholar]
- 45. Song HB, Park SY, Ko JH, et al. Mesenchymal stromal cells inhibit inflammatory lymphangiogenesis in the cornea by suppressing macrophage in a TSG‐6‐dependent manner. Mol Ther. 2018;26(1):162‐172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Maruyama K, Ii M, Cursiefen C, et al. Inflammation‐induced lymphangiogenesis in the cornea arises from CD11b‐positive macrophages. J Clin Invest. 2005;115(9):2363‐2372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Kerjaschki D, Huttary N, Raab I, et al. Lymphatic endothelial progenitor cells contribute to de novo lymphangiogenesis in human renal transplants. Nat Med. 2006;12(2):230‐234. [DOI] [PubMed] [Google Scholar]
- 48. Kataru RP, Jung K, Jang C, et al. Critical role of CD11b+ macrophages and VEGF in inflammatory lymphangiogenesis, antigen clearance, and inflammation resolution. Blood. 2009;113(22):5650‐5659. [DOI] [PubMed] [Google Scholar]
- 49. Maruyama K, Asai J, Ii M, et al. Decreased macrophage number and activation lead to reduced lymphatic vessel formation and contribute to impaired diabetic wound healing. Am J Pathol. 2007;170(4):1178‐1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Maruyama K, Nakazawa T, Cursiefen C, et al. The maintenance of lymphatic vessels in the cornea is dependent on the presence of macrophages. Invest Ophthalmol Vis Sci. 2012;53(6):3145‐3153. [DOI] [PubMed] [Google Scholar]
- 51. Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003;299(5609):1057‐1061. [DOI] [PubMed] [Google Scholar]
- 52. Vignali DA, Collison LW, Workman CJ. How regulatory T cells work. Nat Rev Immunol. 2008;8(7):523‐532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Wood KJ, Sakaguchi S. Regulatory T cells in transplantation tolerance. Nat Rev Immunol. 2003;3(3):199‐210. [DOI] [PubMed] [Google Scholar]
- 54. Chauhan SK, Saban DR, Lee HK, et al. Levels of Foxp3 in regulatory T cells reflect their functional status in transplantation. J Immunol. 2009;182(1):148‐153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Cunnusamy K, Chen PW, Niederkorn JY. IL‐17A‐dependent CD4+CD25+ regulatory T cells promote immune privilege of corneal allografts. J Immunol. 2011;186(12):6737‐6745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Cunnusamy K, Niederkorn JY. IFN‐gamma blocks CD4+CD25+ Tregs and abolishes immune privilege of minor histocompatibility mismatched corneal allografts. Am J Transplant. 2013;13(12):3076‐3084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Reyes NJ, Chen PW, Niederkorn JY. Allergic conjunctivitis renders CD4(+) T cells resistant to T regulatory cells and exacerbates corneal allograft rejection. Am J Transplant. 2013;13(5):1181‐1192. [DOI] [PMC free article] [PubMed] [Google Scholar]


