Abstract
Aim
To evaluate the persistence with oral antidiabetic drug (OAD) treatment characterized by drug class, patient characteristics and severity of renal impairment (RI) in patients with type 2 diabetes (T2DM) in Japan.
Materials and Methods
This retrospective, observational study extracted data from a large‐scale hospital database (April 2008 to September 2016). Patients with T2DM aged ≥40 years on the day of their first prescription (index date) of any OAD (biguanides [BGs], thiazolidinediones [TZDs], sulphonylureas [SUs], glinides, dipeptidyl peptidase‐4 [DPP‐4] inhibitors, or α‐glucosidase inhibitors [α‐GIs]) available between January 1, 2014 and September 30, 2016 were identified. Sodium‐glucose co‐transporter‐2 inhibitors were not available at study initiation. Treatment persistence was assessed by Kaplan–Meier survival curves. Patients were also categorized by RI status using estimated glomerular filtration rate: ≥90 mL/min/1.73 m2 (G1); 60 to <90 mL/min/1.73 m2 (G2); 30 to <60 mL/min/1.73 m2 (G3); and <30 mL/min/1.73 m2 (G4+).
Results
We identified 206 406 index dates from 162 116 eligible patients. The largest number of index dates (91634) was observed for DPP‐4 inhibitors, followed by BGs, SUs, α‐GIs, glinides and TZDs. Treatment persistence was longest for DPP‐4 inhibitors (median 17.0 months, 95% confidence interval [CI] 16.4‐17.5) and BGs (median 17.3 months, 95% CI 16.6‐18.2), and shortest for α‐GIs (median 5.6 months, 95% CI 5.4‐5.9) and SUs (median 4.3 months, 95% CI 4.2‐4.6). Persistence was longest with DPP‐4 inhibitors at all RI stages (G1–G4+), followed by BGs at stages G1/G2.
Conclusions
The longest OAD persistence was observed for BGs and DPP‐4 inhibitors at RI stages G1/G2, and for DPP‐4 inhibitors at RI stages G3/G4+.
Keywords: antidiabetic drug, database research, DPP‐4 inhibitor, observational study, pharmacoepidemiology, type 2 diabetes
1. INTRODUCTION
The worldwide prevalence of diabetes is rising, and Japan ranks among the top 10 countries in terms of the number of adults with diabetes.1 The Japan Ministry of Health, Labour and Welfare estimated that in 2014, 3.2 million patients had diabetes and were receiving continuous treatment,2 equating to 1.22 trillion yen in annual medical expenditure.3 Based on a systematic sub‐analysis of the Global Burden of Disease Study 2015, diabetes was the 28th leading cause of death and 14th leading cause of disability‐adjusted life years in Japan in 2015.4
The Japan Diabetes Society recommends oral antidiabetic drugs (OADs), insulin and glucagon‐like peptide‐1 receptor agonists for patients with non‐insulin‐dependent type 2 diabetes mellitus (T2DM) with poorly controlled blood glucose levels after 2 to 3 months of diet and exercise.5 The choice of OADs depends on disease status, with consideration of the characteristics and side effects of each drug. Based on the underlying cause of T2DM, patients may be prescribed insulin‐sensitizing agents (biguanides [BGs] or thiazolidinediones [TZDs]), insulin secretagogues (sulphonylureas [SUs], glinides or dipeptidyl peptidase‐4 [DPP‐4] inhibitors), or carbohydrate absorption/excretion‐modulating agents (α‐glucosidase inhibitors [α‐GIs] or sodium‐glucose co‐transporter‐2 [SGLT2] inhibitors). Notably, although no recommended guidelines exist for their concurrent use, DPP‐4 inhibitors were the most frequently prescribed OAD class during the last decade in Japan.6
In patients with T2DM and renal impairment (RI), all drugs, including OADs, and particularly those that affect renal metabolism and excretion, should be used according to their prescribing information with due consideration to the patient's glomerular filtration rate (GFR).5 In general, all OADs can be used in patients with mild RI; however, treatment options for patients with moderate‐to‐severe chronic kidney disease or end‐stage renal disease (ESRD) are limited because reduced GFR may lead to drug or metabolite accumulation and subsequent side effects.7
Because T2DM requires long‐term treatment, patients must remain adherent to and persistent with their prescribed OADs to optimize clinical benefits.8 According to the World Health Organization, non‐adherence to long‐term medications for diseases such as hypertension, dyslipidaemia and diabetes is common, leading to compromised clinical outcomes and major economic consequences.9 Patients' likelihood of maintaining adherence to and persistence with treatment is important, therefore, when choosing from a complex array of OADs.8, 9, 10, 11 According to a literature review of six retrospective observational studies from the United States, Canada and Europe between January 2000 and November 2005, the mean estimated OAD persistence over 6 to 24 months was 56% (95% confidence interval [CI] 46‐66), with estimates ranging from 41% to 81%.12 Although treatment adherence and persistence may be assessed as part of rigorously controlled clinical trials involving specific patient populations with diabetes,13 reports of real‐world OAD adherence and persistence rates are limited, particularly in Japan.14, 15
To our knowledge, this is the first large‐scale, real‐world evaluation of treatment persistence, patient characteristics and severity of RI among classes of OADs in T2DM patients in Japan.
2. MATERIALS AND METHODS
2.1. Study objectives
The study objectives were to analyse treatment persistence and patient characteristics stratified by OAD class, individual DPP‐4 inhibitors, RI category at index date, and lines of therapy (1, 2 and 3+).
2.2. Study design and data source
This retrospective, observational cohort study collected data from a large‐scale hospital database provided by Medical Data Vision (Tokyo, Japan) to determine the persistence with OADs in patients with T2DM in Japan. The details of the database are provided in the Supporting Information. The study period for individual patients consisted of a look‐back period (6 months before the index date) for history of drug prescription,16 index date (date of first OAD prescription), and administration/observation period (assessment of persistence). The study protocol was reviewed and approved by the Keio University Faculty of Pharmacy Ethics Committee for Research Involving Humans (170217‐1) and was registered with http://clinicaltrials.gov (NCT03092752).
2.3. Patient eligibility
Eligible patients had a diagnosis of T2DM (International Classification of Diseases 10th Revision [ICD‐10] code E11–E14),17 had received any OAD prescription, and had data available during the look‐back period. Index date for an OAD was defined as the date of first prescription of that OAD for a patient and was determined between January 1, 2014 and September 30, 2016. January 1, 2014, was selected as the start date for data retrieval in this study to enable assessment of all seven DPP‐4 inhibitors, which could be prescribed starting from a similar time point. In Japan, prescriptions are limited to 2 weeks for a period of 1 year after product launch; therefore, patients who visit the hospital every 2 weeks are able to access the necessary prescription. Seven DPP‐4 inhibitors had entered the Japanese market in succession by July 2013. Multiple OADs in the first OAD prescription contributed to multiple index dates. Additional eligibility criteria were age ≥40 years at the time of T2DM diagnosis, no diagnosis of type 1 diabetes mellitus (ICD‐10 code E10), no prescription for the OAD during the look‐back period, and average of <92 days between hospital visits (excluding periods of inpatient hospitalization). Detailed information regarding the data source used in this study, study drugs prescribed to patients, and determination of index dates for comparison of drugs are provided in the Appendix S1.
2.4. Analyses and statistical methods
Treatment persistence was defined as the duration of time from the index date to the first occurrence of discontinuation for each OAD class. OAD treatment was considered to be discontinued when there was a treatment gap of ≥30 days between two subsequent visits and days of drug supply.
For between‐OAD class analysis, continuation was defined as continuing the same drug or switching drugs within a class; switching to another drug class was defined as discontinuation. Estimated GFR (eGFR) was calculated based on sex, age at index date, and the most recent serum creatinine value at the index date or during the look‐back period using the following formula recommended by the Japanese Society of Nephrology,18 where Cr is serum creatinine (mg/dL).
Categories of eGFR/RI were defined by the following stages: G1 (eGFR ≥90 mL/min/1.73 m2; normal or high kidney function), G2 (60 to <90 mL/min/1.73 m2; normal or mildly decreased kidney function), G3a (45 to <60 mL/min/1.73 m2; mildly or moderately decreased kidney function), G3b (30 to <45 mL/min/1.73 m2; moderately to severely decreased kidney function), G4 (15 to <30 mL/min/1.73 m2; severely decreased kidney function), and G5 (<15 mL/min/1.73 m2; ESRD). Comorbidities were defined by ICD‐10 code (Table S1, Appendix S1) and categorized by baseline RI at the index date (Table S2, Appendix S1).
Descriptive statistics were used for patient demographics and characteristics stratified by OAD class and RI stage. Treatment persistence for each OAD class by RI stage, and for RI stage by OAD class, was determined using Kaplan–Meier survival curves. The median survival time representing median persistence was estimated from the Kaplan–Meier plot. Sensitivity analyses were performed by re‐evaluating baseline patient characteristics for OAD class and individual DPP‐4 inhibitors by reducing the duration of the look‐back period from 6 to 3 months, thereby increasing the number of index dates, and by re‐evaluating OAD persistence by modifying the definition of discontinuation from a treatment gap of ≥30 to ≥60 days. Data analyses were performed using Excel 2010 (Microsoft, Redmond, Washington) and SAS Version 9.4 (SAS Institute Inc., Cary, North Carolina).
3. RESULTS
3.1. Between‐OAD class analyses: patient characteristics and OAD prescribing patterns
Of 523 585 patients with a T2DM diagnosis identified from the database, 162 116 met the eligibility criteria (Figure 1). Overall, 206 406 OAD index dates were identified, most commonly for DPP‐4 inhibitors (91 634; 44%), followed by BGs, SUs, α‐GIs, glinides and TZDs (Table 1). Among SUs, 24 101 index dates were identified for glimepiride, 2903 for gliclazide, 1202 for glibenclamide, and five for tolbutamide.
Figure 1.
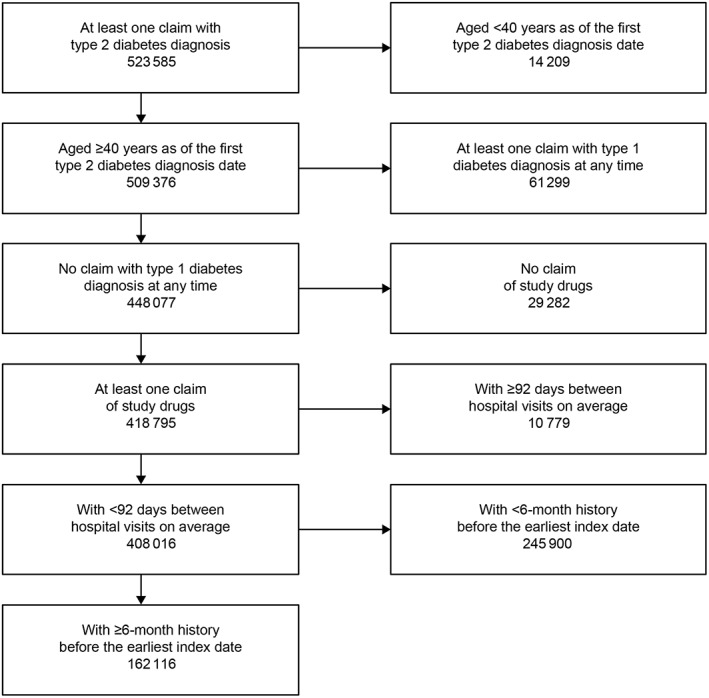
Flowchart of patient eligibility criteria
Table 1.
Characteristics of patients categorized by index dates in each oral antidiabetic drug class
| Total | DPP‐4 inhibitors | BGs | SUs | α‐GIs | Glinides | TZDs | |
|---|---|---|---|---|---|---|---|
| Number of index dates | 206 406 | 91 634 | 33 238 | 28 211 | 26 192 | 16 787 | 10 344 |
| Men, % | 61 | 61 | 62 | 61 | 62 | 61 | 63 |
| Age at index date | |||||||
| Mean ± SD age, years | 70.7 ± 11.2 | 71.6 ± 11.0 | 66.5 ± 11.0 | 72 ± 11.1 | 71.4 ± 10.8 | 71.4 ± 10.8 | 69.7 ± 11.4 |
| ≤64 years, % | 27 | 24 | 39 | 23 | 24 | 24 | 30 |
| 65 to 74 years, % | 33 | 33 | 36 | 31 | 33 | 34 | 33 |
| ≥75 years, % | 40 | 43 | 24 | 45 | 43 | 42 | 37 |
| Mean ± SD follow‐up duration, days | 363 ± 282 | 360 ± 283 | 370 ± 281 | 357 ± 286 | 361 ± 281 | 361 ± 270 | 383 ± 284 |
| Mean ± SD HbA1c, % | 7.5 ± 1.5 | 7.4 ± 1.5 | 7.7 ± 1.6 | 7.6 ± 1.5 | 7.5 ± 1.5 | 7.6 ± 1.5 | 7.7 ± 1.6 |
| eGFR at index date | |||||||
| Mean ± SD eGFR, mL/min/1.73 m2 | 65.2 ± 28.1 | 63 ± 28.8 | 74 ± 23.9 | 67.4 ± 26.4 | 60.1 ± 29.4 | 59.5 ± 30.2 | 69.7 ± 23.7 |
| G1 (≥90 mL/min/1.73 m2), n (%) | 3868 (15) | 1592 (14) | 838 (19) | 530 (16) | 429 (14) | 252 (13) | 227 (17) |
| G2 (60 to <90 mL/min/1.73 m2), n (%) | 11 188 (44) | 4877 (43) | 2373 (55) | 1446 (44) | 1175 (37) | 691 (37) | 626 (48) |
| G3 (30 to <60 mL/min/1.73 m2), n (%) | 7776 (31) | 3566 (31) | 1045 (24) | 1090 (33) | 1055 (34) | 610 (32) | 410 (31) |
| G4+ (<30 mL/min/1.73 m2), n (%) | 2554 (10) | 1411 (12) | 67 (2) | 208 (6) | 488 (16) | 337 (18) | 43 (3) |
| Comorbidities at index date, % | |||||||
| Hypertension | 68 | 69 | 66 | 66 | 70 | 73 | 68 |
| Ischaemic heart disease | 30 | 30 | 27 | 29 | 33 | 33 | 28 |
| Myocardial infarction | 14 | 14 | 13 | 14 | 15 | 14 | 12 |
| Heart failure | 24 | 25 | 19 | 23 | 27 | 28 | 19 |
| Stroke | 25 | 26 | 22 | 26 | 26 | 27 | 25 |
| RI | 18 | 19 | 10 | 14 | 23 | 26 | 11 |
| Diabetic foot | 3 | 4 | 3 | 3 | 4 | 5 | 3 |
| Previous treatmenta, % | |||||||
| DPP‐4 inhibitors | 1 | 0 | 3 | 2 | 1 | 3 | 8 |
| BGs | 1 | 2 | 0 | 1 | 2 | 3 | 2 |
| SUs | 4 | 3 | 2 | 0 | 3 | 19 | 2 |
| α‐GIs | 2 | 2 | 2 | 1 | 0 | 8 | 1 |
| Glinides | 1 | 1 | 1 | 3 | 2 | 0 | 0 |
| TZDs | 1 | 2 | 2 | 1 | 1 | 1 | 0 |
| Insulin | 9 | 9 | 7 | 12 | 10 | 12 | 8 |
| Concomitant treatment at index dateb, % | |||||||
| DPP‐4 inhibitors | 23 | 3 | 40 | 32 | 37 | 56 | 34 |
| BGs | 10 | 9 | 0 | 11 | 12 | 20 | 19 |
| SUs | 10 | 9 | 17 | 1 | 13 | 4 | 21 |
| α‐GIs | 8 | 8 | 9 | 6 | 2 | 16 | 10 |
| Glinides | 3 | 2 | 3 | 1 | 4 | 4 | 4 |
| TZDs | 3 | 2 | 4 | 2 | 3 | 6 | 1 |
| Insulin | 27 | 27 | 23 | 25 | 31 | 37 | 24 |
| Number of concomitant treatments at index dateb in class, % | |||||||
| 0 | 45 | 55 | 38 | 45 | 38 | 20 | 38 |
| 1 | 34 | 33 | 37 | 37 | 34 | 37 | 29 |
| 2+ | 21 | 12 | 26 | 19 | 28 | 43 | 34 |
Abbreviations: α‐GI, α‐glucosidase inhibitor; BG, biguanide; DPP‐4, dipeptidyl peptidase‐4; eGFR, estimated glomerular filtration rate; HbA1c, glycated haemoglobin; RI, renal impairment; SU, sulphonylurea; TZD, thiazolidinedione.
Previous treatment was defined as medications prescribed before, but not after, the index date.
Concomitant treatments were defined as medications prescribed with the first oral antidiabetic drug prescription at the index date or prescribed during the administration and were analysed as individual drugs.
Most patients (61%) were men, and the proportions of men were similar (61%‐63%) across all OAD classes. The mean (SD) age of patients at the index date was 70.7 (11.2) years; 73% of patients were aged ≥65 years and 40% were aged ≥75 years. Patients who were prescribed BGs were younger than those prescribed other OAD classes (mean [SD] 66.5 [11] years vs 69.7 [11.4] to 72 [11.1] years). The mean (SD) follow‐up duration was 363 (282) days. The mean (SD) glycated haemoglobin (HbA1c) levels were similar between classes. The mean (SD) eGFR was 65.2 (28.1) mL/min/1.73 m2 at the index date; eGFR was highest in patients prescribed BGs (74 [23.9] mL/min/1.73 m2) and lowest in those prescribed α‐GIs (60.1 [29.4] mL/min/1.73 m2) and glinides (59.5 [30.2] mL/min/1.73 m2). The percentages of patients with index dates for DPP‐4 inhibitors, BGs, SUs, α‐GI, glinides and TZDs among patients with RI stage G4+ (12%, 2%, 6%, 16%, 18% and 3%) were lower than for those with RI stage G3 (31%, 24%, 33%, 34%, 32%, and 31%), respectively. The most common comorbidity at the index date was hypertension (68%); 18% of patients had RI. Comorbidities were more common among patients who were prescribed glinides and α‐GIs, and less common among those prescribed BGs and TZDs. For example, compared with those prescribed other OADs, fewer patients prescribed BGs or TZDs had comorbid heart failure (23%‐28% vs 19%) and RI (14%‐26% vs 10% and 11%).
Previous treatment was noted in 0% to 19% of index dates categorized by drug class (Table 1); 19% of glinide prescriptions resulted from switching from SUs. Overall, 8% to 12% of all OAD prescriptions resulted from switching from insulin. The relative frequency of prescribing a concomitant treatment in each OAD class was similar to the relative number of index dates; DPP‐4 inhibitors were the highest (23% overall) and were prescribed for 56% of patients taking glinides and 40% of patients taking BGs. The rate of added use of concomitant treatments was lowest for DPP‐4 inhibitors (45%) and SUs (55%) and was highest for glinides (80%); glinides also contributed to the highest concomitant treatments with ≥2 OAD classes for 43% of patients, followed by TZDs (34%).
3.2. Within‐OAD class analyses: patient characteristics and DPP‐4 inhibitor prescribing patterns
In total, 91 634 DPP‐4 inhibitor index dates were identified, most commonly for sitagliptin, followed by linagliptin, vildagliptin, teneligliptin and alogliptin. Anagliptin and saxagliptin, the last two DPP‐4 inhibitors to be launched, were not commonly prescribed (Table 2). The mean age and ratio of men to women were similar across different DPP‐4 inhibitor groups; however, within each group, prescribing patterns differed by age, level of RI, and rates of comorbidities. For example, almost half of the patients who were prescribed linagliptin were aged ≥75 years with eGFR <60 mL/min/1.73 m2 (G3/G4+), whereas almost half the patients prescribed other DPP‐4 inhibitors had eGFR ≥60 mL/min/1.73 m2 (G1/G2). Also, patients prescribed linagliptin showed the highest rate of comorbidities and concomitant insulin use. DPP‐4 inhibitors were the most commonly prescribed previous treatment for each individual drug in this class. The proportion of patients switching from another DPP‐4 inhibitor was consistent with the timing of the launch of these new drugs, except for vildagliptin and saxagliptin.
Table 2.
Characteristics of patients categorized by index dates for each generic DPP‐4 inhibitor
| DPP‐4 inhibitor | |||||||
|---|---|---|---|---|---|---|---|
| Sitagliptin | Vildagliptin | Alogliptin | Linagliptin | Teneligliptin | Anagliptin | Saxagliptin | |
| Number of index dates | 39 576 | 22 592 | 10 930 | 31 353 | 18 284 | 2051 | 3134 |
| Men, % | 60 | 60 | 61 | 62 | 61 | 60 | 60 |
| Age at index date | |||||||
| Mean ± SD age, years | 71.7 ± 11.0 | 70.3 ± 11.2 | 71 ± 10.9 | 72.5 ± 10.8 | 70.9 ± 10.9 | 68.6 ± 10.8 | 69.3 ± 10.5 |
| ≤64 years, % | 24 | 28 | 26 | 22 | 26 | 33 | 30 |
| 65 to 74 years, % | 33 | 33 | 34 | 31 | 34 | 34 | 37 |
| ≥75 years, % | 43 | 39 | 40 | 47 | 40 | 32 | 33 |
| Mean ± SD HbA1c, % | 7.4 ± 1.5 | 7.4 ± 1.3 | 7.3 ± 1.3 | 7.2 ± 1.4 | 7.3 ± 1.3 | 7.5 ± 1.4 | 7.4 ± 1.4 |
| eGFR at index date | |||||||
| Mean ± SD eGFR mL/min/1.73 m2 | 69.3 ± 26.2 | 62.5 ± 30.8 | 64.2 ± 26.4 | 50.9 ± 29.2 | 60.6 ± 28.2 | 69 ± 21.5 | 61.7 ± 25.5 |
| G1 (≥90 mL/min/1.73 m2), n (%) | 800 (16) | 324 (14) | 262 (14) | 323 (8) | 239 (12) | 44 (15) | 52 (12) |
| G2 (60 to <90 mL/min/1.73 m2), n (%) | 2496 (50) | 966 (41) | 845 (46) | 1104 (29) | 764 (40) | 148 (51) | 203 (47) |
| G3 (30 to <60 mL/min/1.73 m2), n (%) | 1479 (30) | 774 (33) | 538 (29) | 1411 (37) | 636 (33) | 94 (32) | 127 (29) |
| G4+ (<30 mL/min/1.73 m2), n (%) | 223 (4) | 311 (13) | 205 (11) | 985 (26) | 276 (14) | 7 (2) | 54 (12) |
| Comorbidities at index date, % | |||||||
| Hypertension | 66 | 70 | 70 | 77 | 75 | 72 | 75 |
| Ischaemic heart disease | 27 | 31 | 32 | 37 | 34 | 31 | 33 |
| Myocardial infarction | 13 | 14 | 14 | 17 | 15 | 12 | 14 |
| Heart failure | 22 | 26 | 26 | 34 | 30 | 25 | 25 |
| Stroke | 26 | 25 | 26 | 29 | 27 | 26 | 25 |
| RI | 12 | 20 | 16 | 31 | 24 | 11 | 22 |
| Diabetic foot | 3 | 4 | 3 | 5 | 4 | 2 | 4 |
| Previous treatmenta, % | |||||||
| DPP‐4 inhibitors | 6 | 28 | 15 | 20 | 32 | 42 | 32 |
| BGs | 1 | 5 | 2 | 3 | 2 | 1 | 1 |
| SUs | 3 | 3 | 3 | 4 | 4 | 3 | 3 |
| α‐GIs | 2 | 2 | 2 | 2 | 3 | 3 | 3 |
| Glinides | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| TZDs | 1 | 1 | 5 | 2 | 2 | 2 | 1 |
| Insulin | 9 | 7 | 7 | 8 | 6 | 3 | 5 |
| Concomitant treatment at index dateb, % | |||||||
| DPP‐4 inhibitors | 1 | 2 | 2 | 2 | 2 | 3 | 2 |
| BGs | 10 | 18 | 16 | 11 | 16 | 32 | 21 |
| SUs | 10 | 18 | 15 | 13 | 19 | 24 | 20 |
| α‐GIs | 7 | 12 | 11 | 11 | 14 | 17 | 14 |
| Glinides | 2 | 4 | 3 | 3 | 4 | 7 | 6 |
| TZDs | 3 | 4 | 4 | 3 | 4 | 7 | 4 |
| Insulin | 26 | 27 | 20 | 31 | 25 | 15 | 22 |
| Launch | Dec 2009 | Apr 2010 | Jun 2010 | Sep 2011 | Sep 2012 | Nov 2012 | Jul 2013 |
Abbreviations: α‐GI, α‐glucosidase inhibitor; BG, biguanide; DPP‐4, dipeptidyl peptidase‐4; eGFR, estimated glomerular filtration rate; HbA1c, glycated haemoglobin; RI, renal impairment; SU, sulphonylurea; TZD, thiazolidinedione.
Previous treatment was defined as medications prescribed before, but not after, the index date.
Concomitant treatments were defined as medications prescribed with the first oral antidiabetic drug prescription at the index date or prescribed during the administration and were analysed as individual drugs.
In the sensitivity analyses with the look‐back period shortened from 6 to 3 months, the number of index dates increased by 23 820 (10%), with DPP‐4 inhibitors accounting for 7784 (33%) of the total increase (Table S3, Appendix S1). No remarkable differences were observed against the primary analysis in patient characteristics, comorbidities and relative distribution of index dates in each OAD class, proportion of previous treatments, or proportion of concomitant treatments. Across DPP‐4 inhibitors, the number of index dates increased relative to the timing of new drug launches, with sitagliptin—the earliest DPP‐4 inhibitor to be released—accounting for over half of the new index dates (4157/7784; 53% [Table S4, Appendix S1]).
3.3. RI stage analyses: patient characteristics
The mean (SD) age of patients at RI stages G1, G2, G3 and G4+ was 65.6 (12.2), 69.5 (10.7), 75.0 (9.8) and 73.2 (10.8) years, respectively. In general, HbA1c level decreased and the proportions of patients with each comorbidity increased with severity of RI (Table S2, Appendix S1). Among patients without available serum creatinine data, age and proportions of patients with each comorbidity were in the range of values for patients at RI stages G2 and G3, except for myocardial infarction and diabetic foot.
3.4. Between‐OAD class analyses: treatment persistence
Treatment persistence was longest for DPP‐4 inhibitors (median 17.0 [95% CI 16.4‐17.5] months) and BGs (median 17.3 months [95% CI 16.6‐18.2]) and shortest for α‐GIs (median 5.6 months [95% CI 5.4‐5.9]) and SUs (median 4.3 months [95% CI 4.2‐4.6]; Figure 2A). In the sensitivity analyses with a less stringent definition of persistence, treatment persistence was extended by ~70% for all OAD classes; the relative order of persistence rates among drug classes was maintained (Figure S1, Appendix S1). Irrespective of the number of concomitant drugs (or line of treatment) at the index date, treatment persistence was longest for DPP‐4 inhibitors and BGs and shortest for α‐GIs and SUs (Figure S2, Appendix S1).
Figure 2.
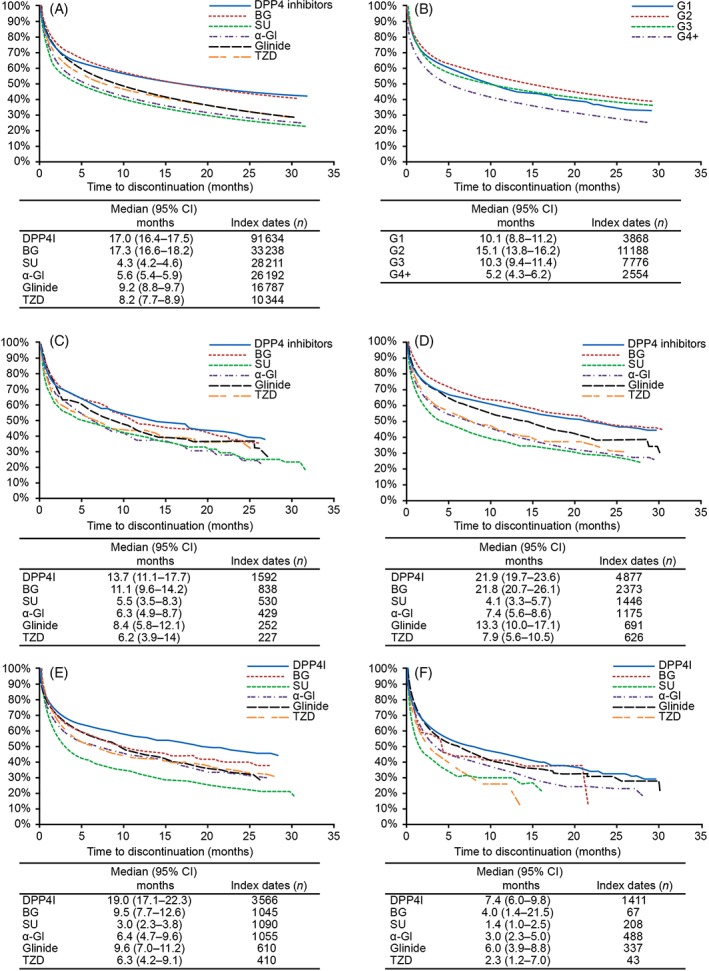
Persistence rates A, by oral antidiabetic drug (OAD) class in all patients, B, by estimated glomerular filtration rate (eGFR) stage of renal impairment (RI) and by OAD class for each eGFR stage of RI, C, stage G1, D, stage G2, E, stage G3 and F, stage G4+. α‐GI, α‐glucosidase inhibitor; BG, biguanide; CI, confidence interval; DPP‐4, dipeptidyl peptidase‐4; SU; sulphonylurea; TZD, thiazolidinedione
3.5. RI stage analyses: treatment persistence
In general, treatment persistence (assessed as time to discontinuation) for OADs was longest in patients at RI stage G2, similar between patients in stages G1 and G3, and shortest at stage G4+ (Figure 2B). Persistence with each drug class varied by RI stage, and was longest for DPP‐4 inhibitors at all RI stages, followed by BGs at RI stages G1 and G2 (Figure 2C‐F).
4. DISCUSSION
The results of the present retrospective, real‐world database analysis show that, among the OADs evaluated, DPP‐4 inhibitors and BGs were the most frequently prescribed OAD classes, with the longest treatment persistence. These findings are similar to those from previous reports in Japan.6, 19, 20 TZDs and glinides were the least frequently prescribed OADs, and treatment persistence was shortest for α‐GIs and SUs. We believe treatment persistence is a result of several factors, including a balance of effectiveness, tolerability, safety, superior utility and cost. Specifically, the increased risk of hypoglycaemia associated with SUs5, 21 and reduced quality of life linked to α‐GIs in comparison with DPP‐4 inhibitors13, 22 may have been related to the poor persistence observed for these OADs.
When treatment persistence was evaluated by severity of RI at the index date, persistence was shortest in patients at RI stage G4+ and varied for each OAD drug class by the RI stage. The longest persistence was observed with DPP‐4 inhibitors across all RI stages (G1‐G4+), followed by BGs at stages G1 and G2, and subsequently by glinides across all stages (G1‐G4+). The number of index dates for BGs (208), SUs (67) and TZDs (43) was lowest for RI stage G4+, suggesting that OADs are generally prescribed properly by physicians, in accordance with the Guidelines for the Treatment of Diabetes in Japan.5
Considering patient baseline characteristics, the increasing severity of RI was consistent with ageing and higher rates of comorbidities.23, 24 The younger average age of patients at RI stage G1 could explain why persistence appears lower than for those at stage G2 in our analysis15; however, in general, treatment persistence rates declined with increasing severity of RI. This could be attributed to switching to other drugs without prescription restrictions in patients with increasing severity of RI, a reduced requirement for OADs because of a decline in blood sugar, and an increased risk of hypoglycaemia.25 Although we observed similar (but longer) persistence for DPP‐4 inhibitors and BGs compared with other OAD classes at RI stages G1 and G2, a longer persistence was observed for DPP‐4 inhibitors than for BGs at RI stages G3 and G4+.
Evaluation of prescribing patterns showed that no concomitant OADs were prescribed with DPP‐4 inhibitors 55% of the time at the index date, suggesting that DPP‐4 inhibitors were most frequently prescribed as first‐line monotherapy. This observation is consistent with a study concluding that DPP‐4 inhibitors were the most frequently prescribed OAD class during the last decade in Japan.6 In the present study, BGs—which are widely endorsed as first‐line treatment in Western countries26, 27—were used in younger patients with relatively stable renal function (G3, 24%; G4+, 2%). BGs such as metformin are contraindicated in patients with eGFR <30 mL/min/1.73 m2, and their use should be avoided in patients with eGFR between 30 and 45 mL/min/1.73 m2 according to the Japan Diabetes Society28 and the US Food and Drug Administration.29 Patients who were prescribed SUs had better renal function despite their older age, suggesting that patients who had previously been prescribed SUs switched to glinides when their renal function declined. Similarly, α‐GIs were prescribed to patients who were older, had reduced renal function, and more comorbidities. Many patients who were prescribed glinides at the index date had concomitant treatments, implying a tendency to prescribe this OAD class for patients with poor blood sugar control.30 Notably, 19% of patients prescribed BGs and TZDs had comorbid heart failure despite contraindications.5
Within the class of DPP‐4 inhibitors, linagliptin was preferentially prescribed for patients at RI stage G4+ and for those who were older, had higher complication rates, and concomitant insulin use. Among patients prescribed other DPP‐4 inhibitors at the index date, differences in baseline characteristics were negligible, indicating the potential for interchangeability of DPP‐4 inhibitors in the sample. Further, more patients who were prescribed linagliptin as first‐line treatment had baseline cardiac, renal and urinary disorders, an observation that was also reported in a previous post‐marketing surveillance study.31 Linagliptin is excreted via the biliary route rather than the renal route like other DPP‐4 inhibitors; therefore, it can be prescribed regardless of RI stage.32 Teneligliptin, which has the second highest rate of biliary excretion (34%),33 was also frequently prescribed in patients with RI stage G4+. Notably, teneligliptin‐treated patients had higher average eGFRs than linagliptin‐treated patients.
For patients with DPP‐4 inhibitor prescriptions at the index date, previous treatment with other DPP‐4 inhibitors was common (15%‐42%) except for sitagliptin (6%), which was the first DPP‐4 inhibitor launched in Japan in December 2009. A high rate of previous treatment with other DPP‐4 inhibitors was observed for drugs that were launched later.
Since prescription of DPP‐4 inhibitors has increased rapidly in Japan, the study period is likely to impact this type of real‐world data analysis. Tanabe et al14 reported that DPP‐4 inhibitors were stopped for 2 years in ~80% of cases in a study using the same database (2008‐2013) in 7108 patients with T2DM. Although those data cannot be compared with our results because of differences in data analysis methods, it might be worth noting that DPP‐4 inhibitors became available in Japan in December 2009 with a 2‐week prescription limitation until December 2010, indicating that only a limited number of patients could have been assessed for 2 years until the end of their study period in March 2013.
The present study has several strengths, including its real‐world setting, the data evaluation from a recognized, large database covering 300 Japanese hospitals providing care for acute‐phase diseases to >20 million patients,34 and a distribution of age of patients with T2DM similar to national patient statistics provided by the statistics bureau of the Ministry of Health, Labour and Welfare.2 Some limitations are also worth noting. The treatment persistence observed in this study might be underestimated, as persistence could only be measured within the same hospital in the database and patients who changed hospitals were not followed up. Factors affecting treatment persistence, such as changes in biometric values (e.g. body weight), reasons for starting or terminating a treatment, duration of diabetes, social background, and compliance with diet, exercise and drug treatment could not be evaluated given the lack of these data in the study database. Furthermore, the results of the sensitivity analysis showed an extension in persistence with a prescription gap of ≥60 days vs ≥30 days; this indicates continuation of treatment beyond the prescribed days of supply, possibly because of poor adherence and consequent leftover tablets, which we did not consider. Because the relative persistence between OAD classes was maintained in the sensitivity analysis, we considered our comparisons of treatment persistence to be valid. Data from Diagnosis Procedure Combination hospitals included patients with multiple moderate‐to‐severe diseases and may not reflect the general patient population with T2DM in Japan, resulting in limited generalizability. Additionally, the 6‐month look‐back period may have enabled time for patients to be evaluated for other diseases, leading to higher rates of comorbidities, especially myocardial infarction, heart failure and stroke, than those in previous reports.35, 36 Differences between patient characteristics among the OAD classes were not adjusted for using propensity‐score matching; however, stratification by baseline renal function was applied for persistence analysis. Our results reflect the treatment status of patients mostly at RI stages G2 to G4+, including those without serum creatinine values categorized by comorbidity. Factors affecting treatment persistence, such as changes in biometric values (e.g. body weight), reasons for starting or terminating a treatment, duration of diabetes, social background and compliance with diet, exercise and drug treatment, could not be evaluated given the source of data. Patients with available serum creatinine data to calculate eGFR were limited; consequently, analyses based on RI included fewer data points than the other analyses. Also, the durability/persistence of each drug or drug combination as first‐ and second‐line therapy was not evaluated. The post‐discontinuation treatment pattern was also not evaluated. Finally, DPP‐4 inhibitors are widely used in Japan37; however, their use may be restricted in other countries. This might be partly attributable to differences in approach while formulating treatment guidelines,38 the health insurance reimbursement system, as well as differences in diabetes pathophysiology.39, 40
Nevertheless, the results reflect a long‐term, real‐world scenario in a large number of patients from numerous centres and provide a comprehensive description of the characteristics of present treatment with OADs in Japanese patients with T2DM.
Overall, DPP‐4 inhibitors were the most frequently prescribed OAD class, accounting for half of the index dates, followed by BGs, SUs, α‐GIs, glinides and TZDs. Treatment persistence was longest for DPP‐4 inhibitors and BGs. Notably, persistence was highest with DPP‐4 inhibitors in patients with severely decreased renal function. Most OADs were prescribed appropriately by practitioners in Japan; however, contrary to recommendations, BGs were prescribed for patients at RI stage G3 and G4, and SUs and TZDs for patients at stage G4. We believe that the appropriate use of OADs can be improved to achieve the goals of long‐term, effective treatment of patients with T2DM. An open question is whether the higher persistence seen for certain classes was associated with improvements in health outcomes.
Supporting information
Table S1. Definition of comorbidities by ICD‐10 code.
Table S2. Comorbidities by renal impairment category.
Table S3. Baseline characteristics of patients categorized by index dates for each OAD class (3‐month look‐back period).
Table S4. Baseline characteristics of patients categorized by index dates for each generic DPP‐4I (3‐month look‐back period).
Figure S1. Persistence rate by OAD drug class from sensitivity analysis.
Figure S2. Treatment persistence categorized by number of concomitant drugs at the index date.
ACKNOWLEDGMENTS
This study was funded by Nippon Boehringer Ingelheim Co., Ltd and Eli Lilly Japan K.K. Editorial support, in the form of medical writing, assembling tables and creating high‐resolution images based on the authors' detailed directions, collating author comments, copy editing, fact checking and referencing, was provided by Annirudha Chillar MD, PhD, and Maribeth Bogush, MCI, PhD, of Cactus Communications and Dr Tomomi Takeshima of Milliman, and was funded by Nippon Boehringer Ingelheim Co., Ltd and Eli Lilly Japan K.K.
Conflict of interest
T.K. has received: payment from Nippon Boehringer Ingelheim Co., Ltd and Eli Lilly Japan K.K for composing the study protocol, reviewing results, and travel to meetings for study concept discussion and review of results; grants from Kowa Pharmaceutical Co., Ltd, Mitsubishi Tanabe Pharma Corporation, Novo Nordisk Pharma Ltd, Ono Pharmaceutical Co., Ltd, Takeda Pharmaceutical Co., Ltd, Astellas Pharma Inc., Kissei Pharmaceutical Co., Ltd, Sumitomo Dainippon Pharma Co., Ltd, Daiichi Sankyo Co., Ltd, Novartis Pharma K.K., Sanwa Kagaku Corp, Sanofi K.K., Taisho Toyama Pharmaceutical Co., Ltd, and Kyowa Hakko Kirin Co., Ltd; lecture fees from Kowa Pharmaceutical Co., Ltd, Mitsubishi Tanabe Pharma Corporation, MSD K.K., Nippon Boehringer Ingelheim Co., Ltd, Novo Nordisk Pharma Ltd, Ono Pharmaceutical Co., Ltd, Takeda Pharmaceutical Co., Ltd, Astellas Pharma, AstraZeneca K.K., Eli Lilly Japan K.K, Kissei Pharmaceutical Co., Ltd, and Sumitomo Dainippon Pharma Co., Ltd; laboratory funds as a donation from Kowa Pharmaceutical Co., Ltd, Mitsubishi Tanabe Pharma Corporation, MSD K.K., Nippon Boehringer Ingelheim Co., Ltd, Novo Nordisk Pharma Ltd, Ono Pharmaceutical Co., Ltd, and Takeda Pharmaceutical Co., Ltd; and funds for contracted or collaborative research from Takeda Pharmaceutical Co., Ltd, Daiichi Sankyo Co., Ltd, Novartis Pharma K.K., and Sanwa Kagaku Corp. N.S. and T.H. are employees of Nippon Boehringer Ingelheim Co., Ltd. K.T. is an employee of Eli Lilly Japan K.K. K.I. is an employee of Milliman, which has received consultancy fees from Nippon Boehringer Ingelheim Co., Ltd. H.U. has received research grants from CAC Croit Corporation and does consultancy work for Eisai Co., Ltd and Nippon Boehringer Ingelheim Co., Ltd.
Author contributions
All named authors met the International Committee of Medical Journal Editors (ICMJE) criteria for authorship, take responsibility for the integrity of the work as a whole, and provided the final approval for the version to be published.
Kadowaki T, Sarai N, Hirakawa T, Taki K, Iwasaki K, Urushihara H. Persistence of oral antidiabetic treatment for type 2 diabetes characterized by drug class, patient characteristics and severity of renal impairment: A Japanese database analysis. Diabetes Obes Metab. 2018;20:2830–2839. 10.1111/dom.13463
Funding information This study was funded by Nippon Boehringer Ingelheim Co., Ltd and Eli Lilly Japan K.K.
REFERENCES
- 1. International Diabetes Foundation . Diabetes Atlas. 7th ed. Brussels, Belgium: International Diabetes Federation; 2015. http://www.diabetesatlas.org. Accessed August 7, 2017. [Google Scholar]
- 2. Ministry of Health, Labour and Welfare . Overview of patient survey as of 2014. http://www.mhlw.go.jp/toukei/saikin/hw/kanja/14/index.html (Japanese). Accessed August 7, 2017.
- 3. Ministry of Health, Labour and Welfare . Overview of national medical expenditure as of 2014. http://www.mhlw.go.jp/toukei/saikin/hw/k-iryohi/14/ (Japanese). Accessed August 7, 2017.
- 4. Nomura S, Sakamoto H, Glenn S, et al. Population health and regional variations of disease burden in Japan, 1990‐2015: a systematic subnational analysis for the Global Burden of Disease Study 2015. Lancet. 2017;390:1521‐1538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. The Japan Diabetes Society; Treatment Guide for Diabetes 2014–2015. http://www.jds.or.jp/modules/en/index.php?content_id=1#guide. Accessed August 7, 2017. [Google Scholar]
- 6. Kohro T, Yamazaki T, Sato H, et al. Trends in antidiabetic prescription patterns in Japan from 2005 to 2011. Int Heart J. 2013;54:93‐97. [DOI] [PubMed] [Google Scholar]
- 7. Nogueira C, Souto SB, Vinha E, Braga DC, Carvalho D. Oral glucose lowering drugs in type 2 diabetic patients with chronic kidney disease. Hormones. 2013;12:483‐494. [DOI] [PubMed] [Google Scholar]
- 8. McGovern A, Tippu Z, Hinton W, Munro N, Whyte M, de Lusignan S. Systematic review of adherence rates by medication class in type 2 diabetes: a study protocol. BMJ Open. 2016;6:e010469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. World Health Organization . Adherence to long‐term therapies: evidence for action, 2003. http://www.who.int/chp/knowledge/publications/adherence_full_report.pdf?ua=1. Accessed August 7, 2017.
- 10. Taitel M, Fensterheim L, Kirkham H, Sekula R, Duncan I. Medication days' supply, adherence, wastage, and cost among chronic patients in Medicaid. Medicare Medicaid Res Rev. 2012;2:E1‐E13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cramer JA, Benedict A, Muszbek N, Keskinaslan A, Khan ZM. The significance of compliance and persistence in the treatment of diabetes, hypertension and dyslipidaemia: a review. Int J Clin Pract. 2008;62:76‐87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Iglay K, Cartier SE, Rosen VM, et al. Meta‐analysis of studies examining medication adherence, persistence, and discontinuation of oral antihyperglycemic agents in type 2 diabetes. Curr Med Res Opin. 2015;31:1283‐1296. [DOI] [PubMed] [Google Scholar]
- 13. Ishii H, Hayashino Y, Akai Y, Yabuta M, Tsujii S. Dipeptidyl peptidase‐4 inhibitors as preferable oral hypoglycemic agents in terms of treatment satisfaction: results from a multicenter, 12‐week, open label, randomized controlled study in Japan (PREFERENCE 4 study). J Diabetes Investig. 2018;9:137‐145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Tanabe M, Motonaga R, Terawaki Y, Nomiyama T, Yanase T. Prescription of oral hypoglycemic agents for patients with type 2 diabetes mellitus: a retrospective cohort study using a Japanese hospital database. J Diabetes Investig. 2017;8:227‐234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kurtyka K, Nishikino R, Ito C, Brodovicz K, Chen Y, Tunceli K. Adherence to dipeptidyl peptidase‐4 inhibitor therapy among type 2 diabetes patients with employer‐sponsored health insurance in Japan. J Diabetes Investig. 2016;7:737‐743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Girman CJ, Faries D, Ryan P, et al. Pre‐study feasibility and identifying sensitivity analyses for protocol pre‐specification in comparative effectiveness research. J Comp Eff Res. 2014;3:259‐270. [DOI] [PubMed] [Google Scholar]
- 17. Ministry of Health, Labour and Welfare (Japan) . Statistical classification of diseases and cause of death [in Japanese]. http://www.mhlw.go.jp/toukei/sippei/. Accessed May 29, 2017.
- 18. Japanese Society of Nephrology . Evidence‐based Clinical Practice Guideline for CKD 2013. Clin Exp Nephrol. 2014;18:346‐423. [Google Scholar]
- 19. Fujihara K, Igarashi R, Matsunaga S, et al. Comparison of baseline characteristics and clinical course in Japanese patients with type 2 diabetes among whom different types of oral hypoglycemic agents were chosen by diabetes specialists as initial monotherapy (JDDM 42). Medicine. 2017;96:e6122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Yabe D, Kuwata H, Nishikino R, et al. Use of the Japanese health insurance claims database to assess durability of DPP‐4 inhibitors in patients with diabetes: comparison with other anti‐diabetic drugs. Diabetologia. 2015;58:S1‐S607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Shorr RI, Ray WA, Daugherty JR, Griffin MR. Antihypertensives and the risk of serious hypoglycemia in older persons using insulin or sulfonylureas. JAMA. 1997;278:40‐43. [PubMed] [Google Scholar]
- 22. Tanaka M, Nishimura T, Sekioka R, Itoh H. Dipeptidyl peptidase‐4 inhibitor switching as an alternative add‐on therapy to current strategies recommended by guidelines: analysis of a retrospective cohort of type 2 diabetic patients. J Diabetes Metab. 2016;7:701. [Google Scholar]
- 23. Sarnak MJ, Levey AS, Schoolwerth AC, et al. Kidney disease as a risk factor for development of cardiovascular disease: a statement from the American Heart Association Councils on Kidney in Cardiovascular Disease, High Blood Pressure Research, Clinical Cardiology, and Epidemiology and Prevention. Hypertension. 2003;42:1050‐1065. [DOI] [PubMed] [Google Scholar]
- 24. Sharma AM. Renal involvement in hypertensive cardiovascular disease. Eur Heart J. 2003;5:F12‐F18. [Google Scholar]
- 25. Alsahli M, Gerich JE. Hypoglycemia in patients with diabetes and renal disease. J Clin Med. 2015;4:948‐964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Inzucchi SE, Lipska KJ, Mayo H, Bailey CJ, McGuire DK. Metformin in patients with type 2 diabetes and kidney disease: a systematic review. JAMA. 2014;312:2668‐2675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. American Diabetes Association . Standards of medical care in diabetes ‐ 2017. Diabetes Care. 2017;40:S1‐S135.27979885 [Google Scholar]
- 28. The Japan Diabetes Society . Recommendation for appropriate use of metformin [in Japanese], Revised in 2016. http://www.fa.kyorin.co.jp/jds/uploads/recommendation_metformin.pdf. Accessed May 10, 2017.
- 29. U.S. Food and Drug Administration . FDA Drug Safety Communication: FDA revises warnings regarding use of the diabetes medicine metformin in certain patients with reduced kidney function, 2016. https://www.fda.gov/downloads/Drugs/DrugSafety/UCM494140.pdf. Accessed May 10, 2017.
- 30. Cryer PE. Glycemic goals in diabetes: trade‐off between glycemic control and iatrogenic hypoglycemia. Diabetes. 2014;63:2188‐2195. [DOI] [PubMed] [Google Scholar]
- 31. Sarai N, Farsani SF, Taniguchi A, et al. Linagliptin preferential prescribing identified in expanded post‐marketing surveillance in Japan. J Jpn Diabetes Soc. 2017;609(suppl): S‐278. [Google Scholar]
- 32. Graefe‐Mody U, Friedrich C, Port A, et al. Effect of renal impairment on the pharmacokinetics of the dipeptidyl peptidase‐4 inhibitor linagliptin(*). Diabetes Obes Metab. 2011;13:939‐946. [DOI] [PubMed] [Google Scholar]
- 33. Kishimoto M. Teneligliptin: a DPP‐4 inhibitor for the treatment of type 2 diabetes. Diabetes Metab Syndr Obes. 2013;6:187‐195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Medical Data Vision. Introducing MDV database https://www.mdv.co.jp/solution/pharmaceutical/english/. Accessed 29 January, 2018.
- 35. Cheng LJ, Chen JH, Lin MY, et al. A competing risk analysis of sequential complication development in Asian type 2 diabetes mellitus patients. Sci Rep. 2015;5:15687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Tanaka S, Tanaka S, Iimuro S, et al. Cohort profile: the Japan diabetes complications study: a long‐term follow‐up of a randomised lifestyle intervention study of type 2 diabetes. Int J Epidemiol. 2014;43:1054‐1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Seino Y, Kuwata H, Yabe D. Incretin‐based drugs for type 2 diabetes: focus on East Asian perspectives. J Diabetes Investig. 2016;7(suppl 1):102‐109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Tajima N, Noda M, Origasa H, et al. Evidence‐based practice guideline for the treatment for diabetes in Japan 2013. Diabetol Int. 2015;6:151‐187. [Google Scholar]
- 39. Cho YM. Incretin physiology and pathophysiology from an Asian perspective. J Diabetes Investig. 2015;6:495‐507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Yabe D, Seino Y, Fukushima M, Seino S. β cell dysfunction versus insulin resistance in the pathogenesis of type 2 diabetes in East Asians. Curr Diab Rep. 2015;15:602. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Definition of comorbidities by ICD‐10 code.
Table S2. Comorbidities by renal impairment category.
Table S3. Baseline characteristics of patients categorized by index dates for each OAD class (3‐month look‐back period).
Table S4. Baseline characteristics of patients categorized by index dates for each generic DPP‐4I (3‐month look‐back period).
Figure S1. Persistence rate by OAD drug class from sensitivity analysis.
Figure S2. Treatment persistence categorized by number of concomitant drugs at the index date.


